Abstract
Background
Antibody mediated rejection (AMR) has been associated with increased mortality and cardiac allograft vasculopathy (CAV). Early studies suggested that late AMR was rarely associated with graft dysfunction while recent reports have demonstrated an association with increased mortality. We sought to investigate the timing of AMR and its association with graft dysfunction, mortality, and CAV.
Methods
This retrospective cohort study identified all adult heart transplant recipients at Columbia University Medical Center from 2004–2013 (689 patients). There were 68 primary cases of AMR, which were stratified by early (<1 year post-OHT) or late (>1-year post-OHT) AMR. Kaplan-Meier survival analysis and modeling was performed with multivariable logistic regression and Cox proportional hazards regression.
Results
From January 1, 2004 through October 1, 2015 43 patients had early AMR (median 23 days post-OHT) and 25 had late AMR (median 1084 days post-OHT). Graft dysfunction was less common with early compared with late AMR (25.6% vs. 56%, p=0.01). Patients with late AMR had decreased post-AMR survival compared with early AMR (1-year 80% vs. 93%, 5-year 51% vs. 73%, p<0.05). When stratified by graft dysfunction, only those with late AMR and graft dysfunction had worse survival (30-day 79%, 1-year 64%, and 5-year 36%, p<0.006). The association remained irrespective of age, sex, DSA, LVAD use, reason for OHT, and recovery of graft function. Similarly, those with late AMR and graft dysfunction had accelerated development of de-novo CAV (50% at 1 year, HR 5.42, p=0.009), while all other groups were all similar to the general transplant population.
Conclusion
Late AMR is frequently associated with graft dysfunction. When graft dysfunction is present in late AMR there is an early and sustained increased risk of mortality and rapid development of de-novo CAV despite aggressive treatment.
Introduction
Survival following orthotopic heart transplantation (OHT) has steadily improved over the last four decades, with a median survival that is presently over 11 years. Improved post-transplant survival has been driven by increased survival during the first year post-transplant, with little change in annual risk thereafter. Improved immunosuppressive regimens have decreased the prevalence of rejection during the same period, yet still over 40% of patients will require hospitalization for allograft rejection by four years post-OHT (1). Originally known as humoral or vascular rejection, a 2004 conference formalized the term antibody mediated rejection (AMR) (2). AMR has been demonstrated to have significant sequelae, resulting in increased graft loss, cardiac allograft vasculopathy (CAV), and mortality (3–5). In 2006 the International Society of Heart and Lung Transplantation (ISHLT) convened a task force that described the pathologic and serologic evidence for AMR and biopsy grading criteria (4). The consensus paper cited prior studies that showed early AMR was associated with graft dysfunction in 68% of patients, but in late AMR (months to years after OHT) only 13% was hemodynamically significant. Two recent single center analyses (15 and 20 patients respectively) of patients with late AMR (>1 year post-transplant) demonstrated that one-year mortality was 50–53% (6, 7). In this retrospective cohort study we sought to investigate the timing of AMR and its association with graft dysfunction, mortality, and CAV.
Methods
All adult (age >18 years) patients transplanted at Columbia University Medical Center from January 1, 2004-December 31, 2013 were identified. A total of 689 transplants occurred during the study period and follow-up was recorded through October 1, 2015. During the study period there were 68 patients who experienced AMR. Early rejection was defined as the initial rejection occurring within one year of primary OHT. Patients were grouped based according to the timing of their initial episode of AMR.
Endomyocardial Biopsy
Routine surveillance endomyocardial biopsies (EMBs) were performed weekly in the first month after transplantation, then every 2 weeks for 2 months, monthly for 3 months, every 2 months for 6 months, every 3 month for 6 months, and then every 6 to 12 months. The frequency of EMB was annual by the third year after transplantation unless clinical rejection was suspected. With each EMB, 4 to 5 pieces of the right ventricular myocardium were obtained. Seventy four percent of biopsies were performed as part of regularly scheduled care. When stratified by timing of AMR, 98% were protocol biopsies for the cases of early AMR and 32% were protocol biopsies for late AMR.
Antibody Mediated Rejection & Graft Dysfunction
An instance of AMR was defined according to the 2013 ISHLT guidelines (8) (EMB with histologic findings consistent with AMR using either immunofluorescence (one case prior to 2010) or immunohistochemistry (C4d) technique). Graft dysfunction was defined as at least two of the following: 33% decrease in cardiac index and a cardiac index (CI) of less than 2.2 L/min/m2, 33% increase in pulmonary capillary wedge pressure (PCWP), or 33% decrease in left ventricular ejection fraction (EF).
Baseline Immunosuppression
Induction therapy with daclizumab was used prior to 2009 and subsequently basiliximab was used unless a contraindication existed (infection, bleeding, or retransplantation). Standard immunosuppressive regimens included prednisone, a calcineurin inhibitor (tacrolimus or cyclosporine), and mycophenolate mofetil. Patients who developed renal insufficiency (creatinine >2.5 mg/dL) or CAV received sirolimus or everolimus.
Donor Specific Antibodies
With each EMB, serum was collected for analysis. Prior to 2010, complement-dependent cytotoxic (CDC) enzyme-linked immunoassay analysis was used to detect the presence of anti-HLA antibodies. The HLA reference panel consists of approximately 70 cells type for class I (A, B, C) and class II (DR, DQ, DP) HLA antigens. Since 2010, CDC analysis has been used in concert with a solid phase assay, Luminex LABScreen Single Antigen (One Lambda, Canoga Park, CA). This microbead technology allows for detection of individual class I and class II anti-HLA antibodies and the intensity of the reactions using median fluorescent intensity.
Treatment of AMR
Treatment was at the discretion of the individual treating provider. Treatments included corticosteroids, plasmapheresis, cyclophosphamide, IVIG, rituximab, and thymoglobulin. Among the entire cohort, 91.2% of episodes of AMR were treated (Table 1).
Table 1.
Baseline characteristics
| All | Early | Late | p-value | |
|---|---|---|---|---|
| n | 68 | 43 | 25 | |
| Mean Age (y) | 52.2 ± 13.3 | 53.7 ± 11.8 | 49.7 ± 15.5 | 0.24 |
| Gender | 0.09 | |||
| Female (%) | 28 (41.2) | 21 (48.9) | 7 (28) | |
| Male (%) | 40 (58.8) | 22 (51.1) | 18 (72) | |
| Time from OHT to AMR (d) | <0.0001 | |||
| Median | 123 | 23 | 1084 | |
| Interquartile Range | 16.75–787.75 | 9–112 | 708–1573 | |
| LVAD Bridge (%) | 26 (38.2) | 19 (44.9) | 7 (28.0) | 0.19 |
| Etiology of HF | 0.66 | |||
| Ischemic | 21 | 11 | 10 | |
| Non-ischemic | 39 | 26 | 13 | |
| Congenital | 7 | 5 | 2 | |
| Retransplant | 1 | 1 | 0 | |
| Presence of DSA (%) | 29 (42.6) | 22 (51.1) | 7 (28.0) | 0.06 |
| Graft Dysfunction with AMR (%) | 25 (36.8) | 11 (25.6) | 14 (56.0) | 0.01 |
| Treatment (%) | 62 (91.2) | 39 (90.7) | 23 (92.0) | 1 |
| Treatment Type (%) | 0.06 | |||
| Corticosteroids | 62 (91.2) | 39 (90.7) | 23 (92.0) | |
| IVIG | 40 (58.8) | 29 (67.4) | 11 (44.0) | |
| Plasmapheresis | 42 (61.8) | 23 (53.5) | 19 (76.0) | |
| Cyclophosphamide | 33 (48.5) | 19 (44.2) | 14 (56.0) | |
| Rituximab | 24 (35.3) | 8 (18.6) | 16 (64.0) | |
| Thymoglobulin | 6 (8.8) | 3 (7.0) | 3 (12.0) | |
| AMR Diagnosis (%) | 0.24 | |||
| pAMR 1h | 4 (5.9) | 4 (9.3) | 0 (0) | |
| pAMR 1i | 51 (75.0) | 32 (74.4) | 19 (76.0) | |
| pAMR 2 | 13 (19.1) | 7 (16.3) | 6 (24.0) | |
| pAMR 3 | 0 (0) | 0 (0) | 0 (0) | |
| Mixed: Cellular and AMR (%) | 15 (22.1) | 11 (25.6) | 4 (16.0) | 0.54 |
| CAV Diagnosis | 0.64 | |||
| CAV1 | 11 | 5 | 6 | |
| CAV2 | 1 | 0 | 1 | |
| CAV3 | 6 | 3 | 3 |
P-values represent early vs. late AMR comparison. LVAD=Left ventricular assist device, DSA=Donor-specific antibodies, IVIG=Intravenous immunoglobulin, CAV=Cardiac allograft vasculopathy
Cardiac Allograft Vasculopathy
Screening for cardiac allograft vasculopathy (CAV) was performed at one year post-OHT and then every other year thereafter with coronary angiograms. A small subset of patients also underwent intracoronary imaging with intravascular ultrasound or optical coherence tomography. Dobutamine stress echocardiography (DSE) was used to screen for CAV during non-angiogram years. If patients had a creatinine above 2.0 mg/dL, DSE was used in place of angiography. Additional angiograms were performed if there was unexplained graft dysfunction. Angiograms were all graded based on the 2010 ISHLT CAV recommended nomenclature (9).
Statistical Analyses
The primary endpoint was freedom from death or retransplantation following the episode of early versus late AMR. The secondary endpoints were freedom from CAV following late and early AMR. Both analyses were performed with sub-stratification with graft dysfunction. Demographic and clinical variables were summarized with standard descriptive statistics and expressed as mean±SD for normally distributed continuous variables, median (with interquartile range) for skewed continuous variables, and count (with percentage) for categorical variables. Group comparisons were made with Chi-squared, t-test, Fisher’s exact test, and Mood’s median test where appropriate. Kaplan-Meier survival analysis was performed to determine survival statistics. A multivariable logistic regression model was fit for post-AMR survival and post-AMR CAV. For each model, timing of AMR was always included and covariates that were analyzed included age, gender, donor specific antibodies (DSA), LVAD use, reason for OHT, recovery of graft function, and graft dysfunction. Co-variates whose univariate regression p-value was <0.2 (10) were included in a multivariable logistic regression model. Cox proportional-hazards regression was used to model risk of mortality or retransplant and CAV after AMR among the four groups of patients based on timing and graft dysfunction. All statistical tests were 2-tailed with statistical significance defined to be at the 0.05 level. Analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, North Carolina).
Results
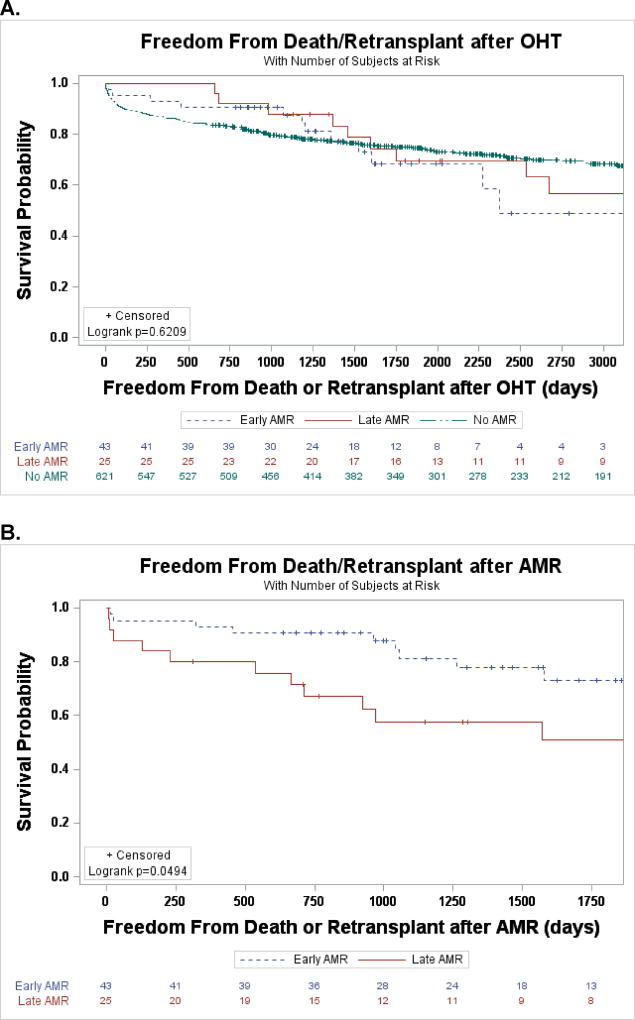
There were 68 patients (9.9% of all adult transplants during the study period) who experienced an episode of AMR from January 1, 2004 through October 1, 2015 at CUMC, with 43 patients having early AMR and 25 having late AMR. The cohort was middle aged, predominantly male, the etiology of heart failure was predominantly non-ischemic, roughly half had DSA, nearly all patients were treated for the episode of AMR, about 40% of patients had an LVAD bridge, and graft dysfunction was present in 37% of patients (Table 1). Nearly one quarter (22%) of patients experienced mixed cellular rejection (at least ISHLT 1R/1B) and AMR, however there was no difference in post-AMR survival between those with mixed rejection and AMR alone (p=0.37). Among patients with graft dysfunction, 11 met three criteria and 14 met two (92% had decreased EF, 84% had decreased CI, and 68% had increased PCWP). When early versus late AMR was compared (Table 1), the notable intergroup differences were that the late group was more male (72.0% vs. 51.1%, p=0.09), the median time from transplant was longer (23 days vs. 1084 days, p<0.0001, Figure 1), and there was more graft dysfunction in the late group (56.0% vs. 25.6%, p=0.01).
Figure 1.
A. Freedom from death or retransplant after OHT stratified by timing of AMR B. Freedom from death or retransplant after AMR stratified by timing of AMR
In the study cohort a total of 19 patients (28.8%) were sensitized prior to transplantation (data was unavailable on one). The risk of AMR in the first 30 days after transplant was 2.12 times greater (95% CI 1.21–3.70) for those who were sensitized compared with those who were not. Patients in the early AMR cohort were more likely to be sensitized (34.9% vs. 17.4%, p=0.16) and had a higher risk of being sensitized pre-transplant (HR 2.01, 95% CI 0.75–5.34), but neither finding was statistically significant.
Treatment
Among all patients diagnosed with AMR, 91.2% were treated with a median of three treatments. There was no significant difference in the number of treatments used between early and late AMR (3.12 vs. 3.44, p=0.07). All patients with graft dysfunction received treatment and on average received more treatments than those with preserved function (3.5 vs. 2.8, p<0.05). AMR with graft dysfunction was more frequently treated with plasmapheresis (80% vs. 51%, p=0.02) and ATG (24% vs. 0%, p=0.002) and there was a trend towards increased use of rituximab (48% vs. 28%, p=0.095) and steroids (100% vs. 86%, p=0.051). There was comparable use of cyclophosphamide (48% vs. 49%, p=0.99) and IVIG (56% vs. 60%, p=0.72).
Mortality and Retransplantation
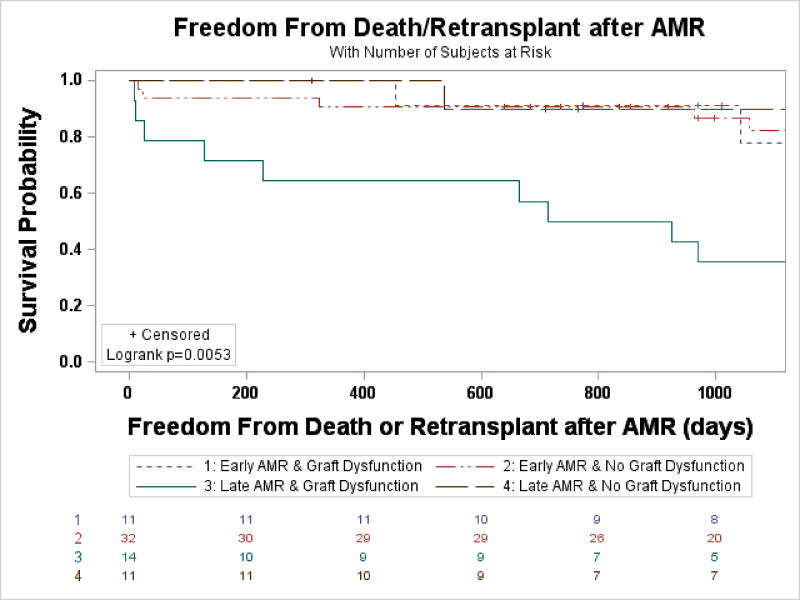
Post-transplant survival for adult patients transplanted during the study period did not significantly differ for those with AMR and those without AMR (95.6% vs. 86.5% at 1 year, 67.9% vs. 74.9% at 5 years, p=0.38). Looking specifically at AMR stratified by timing, freedom from death and retransplant post-OHT was no different (Figure 1A), however post-AMR it was significantly decreased among those with late AMR (30-day 88% vs. 95%, 1-year 80% vs. 93%, 5-year 51% vs. 73%, p<0.05, Figure 1B). When the early and late AMR cohorts were stratified by the presence of graft dysfunction, late AMR with graft dysfunction had an early and sustained decreased event free post-AMR survival (30-day 79%, 1-year 64%, and 5-year 36%, p<0.006, Figure 2). Patients with early AMR irrespective of graft dysfunction and late AMR without graft dysfunction had survival similar to the overall cohort at 30 days and 1-year (Figure 2). On univariate analysis prior LVAD use, late AMR, and graft dysfunction were the only co-variates that were significant (Table 2). When the significant co-variates were modeled, only patients with graft dysfunction continued to have worse outcomes (OR 3.88, 95% CI 1.27–11.90), while no other categories were statistically significant (Table 2). There was a trend in the data that suggested patients with graft dysfunction who did not recover function had increased early post-AMR mortality compared with those with graft recovery (1-year survival 33% vs. 91.3%, p=0.08). Similarly, those patients had a 4.5 times greater odds of mortality, but again this was not significant (p=0.21).
Figure 2.
Freedom from death or retransplant stratified by timing of AMR and graft dysfunction
Table 2.
Univariate and Multivariable Logistic Regression
| Mortality or Retransplant |
CAV | Mortality or Retransplant |
CAV | |||||
|---|---|---|---|---|---|---|---|---|
| OR | p-value | OR | p-value | OR | p-value | OR | p-value | |
| Age at AMR (y) | 0.99 | 0.88 | 1.02 | 0.38 | ||||
| Female Gender | 0.71 | 0.51 | 1.16 | 0.78 | ||||
| DSA at AMR | 0.842 | 0.74 | 0.69 | 0.48 | ||||
| LVAD Bridge | 0.491 | 0.19 | 0.256 | 0.03 | 0.60 | 0.38 | 0.35 | 0.12 |
| Late AMR | 3.7 | 0.01 | 3.24 | 0.031 | 2.53 | 0.11 | 2.25 | 0.2 |
| Graft Dysfunction | 4.95 | 0.003 | 4.375 | 0.008 | 3.88 | 0.02 | 2.99 | 0.08 |
| Recovery of Function | 0.22 | 0.21 | 0.91 | 0.93 | ||||
| Etiology (vs. ischemic) | 0.21 | 0.75 | ||||||
| Congenital | 3.5 | 0.21 | <0.001 | 0.95 | ||||
| Non-ischemic | 3.818 | 0.02 | 0.52 | 0.27 | ||||
| Retransplant | 999 | 0.99 | 999 | 0.98 | ||||
The cause of mortality or retransplantation was predominantly cardiac (52%) for the study cohort. Similarly, in both the early (45%) and late AMR (57%) cohorts cardiac causes were the most common cause of event. Infection was uncommon as the cause of death in both cohorts, representing 18% in early AMR and 14% in late AMR. Malignancy was the cause of death for one patient from the early AMR group, while multi-organ failure and neurologic disease were approximately the same in each group. While treatment with aggressive immunosuppression increases the risk of infection, in the first year after treatment for AMR only one of eight events were due to infection (six cardiac and one neurologic).
Freedom from Cardiac Allograft Vasculopathy
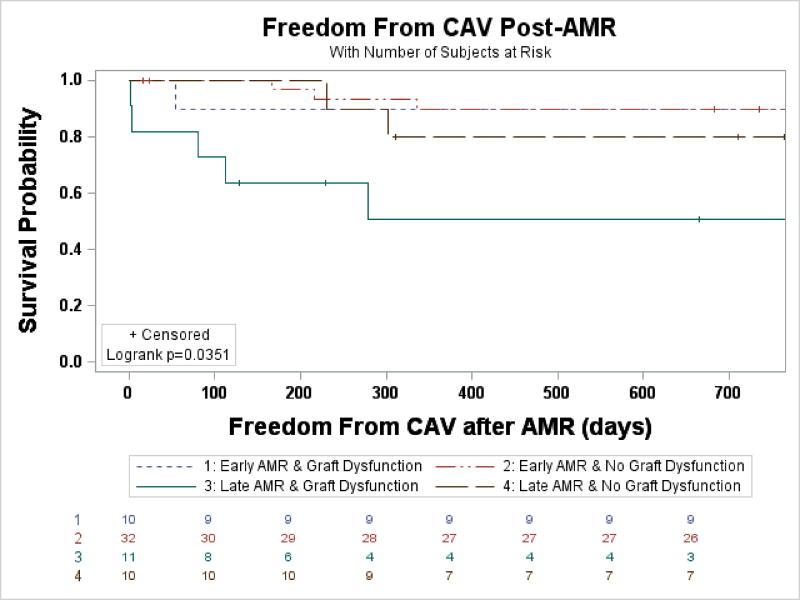
The prevalence of CAV was not significantly different among patients with early and late AMR (38% early vs. 46% late, p=0.51). Freedom from CAV after OHT was not significantly different between groups. Freedom from CAV after AMR (censoring for pre-existing CAV) was not statistically different either. However, when stratified by the presence of graft dysfunction, freedom from CAV after AMR was significantly decreased for those with late AMR with graft dysfunction (1-year 50%, HR 5.93, p=0.009) compared with all other groups (1-year 80%–90%, Figure 3 & Table 3). Univariate analysis identified late AMR, graft dysfunction, and lack of LVAD use as risk factors for developing CAV after AMR. However, none were significant when included in the multivariable logistic regression model.
Figure 3.
Freedom from CAV after AMR, stratified by timing and presence of graft dysfunction. Patients with pre-existing CAV were censored.
Table 3.
Cox proportional hazards regression for timing and graft dysfunction
| Mortality or Retransplant |
De-Novo CAV After AMR |
|||
|---|---|---|---|---|
| HR | p-value | HR | p-value | |
| Early AMR & Graft Dysfunction* | 1.47 | 0.54 | 3.42 | 0.083 |
| Late AMR & No Graft Dysfunction* | 0.986 | 0.98 | 2.18 | 0.312 |
| Late AMR & Graft Dysfunction* | 4.16 | 0.004 | 5.93 | 0.009 |
Reference for comparison was Early AMR & No Graft Dysfunction
Discussion
In this retrospective cohort study we have investigated the association between the timing of AMR and graft dysfunction, survival and CAV. Late AMR was found to be more frequently associated with symptoms, graft dysfunction, and a decreased post-AMR survival, but only in those patients with graft dysfunction. Patients with early AMR with or without graft dysfunction and patients with late AMR with preserved graft function, when treated, had post-AMR survival that was similar to the general post-transplant population. Patients with late AMR and graft dysfunction also developed rapidly progressive CAV.
Early studies did not appreciate that AMR can occur years following cardiac transplant. It was generally believed that late AMR was uncommon and rarely associated with graft dysfunction. In 2007 the Columbia group reported on the bimodal distribution of AMR, which was subsequently validated (11). Two recent studies have demonstrated poor outcomes with late AMR (6, 7). This study further supports these findings, however late AMR without graft dysfunction, when treated, has outcomes similar to all transplant patients. Patients with graft dysfunction and late AMR have poor outcomes despite aggressive treatment. This group also had accelerated development CAV, as 50% of those who survive to one year develop de-novo CAV. These associations remained after multivariable logistic regression controlling for typical risk factors for both AMR and CAV including DSA. While the association between AMR and CAV has long been presumed, the mechanisms linking these two complications have not been elucidated. It is particularly unclear whether DSA directly contribute to the development of CAV and graft dysfunction. The most supportive evidence comes from a recent study by Loupy et al. (12), reporting the high frequency of subclinical, usually unrecognized, AMR prior to CAV. Most cases of CAV following these cryptic AMR episodes appear to show histological evidence of antibody-mediated tissue damage such as endothelitis and microvascular inflammation. Subclinical AMR is likely to be more frequent in patients with late AMR when routine screening biopsies are less frequent and usually symptom driven (68% in the present study). While purely speculative, untreated asymptomatic or subclinical AMR may contribute to worse patient outcomes in detected late AMR with graft dysfunction. Furthermore, our group has also begun a retrospective examination of explanted failed cardiac allografts with CAV at time of re-transplantation. Virtually all cases had evidence of B cell infiltrates in the epicardial tissue surrounding the coronary arteries (unpublished data). These latter findings, together with previously published reports suggest a continuum between AMR and CAV. It remains unknown, however, if all AMR cases are equal and would result in CAV.
This study demonstrates that late AMR with graft dysfunction has poor outcomes despite aggressive treatment. Late AMR with graft dysfunction needs to be treated differently as the current therapies are inadequate. Patients who have late AMR without graft dysfunction or early AMR of any type have survival that is analogous to all transplant patients when treated aggressively for AMR. This is encouraging, but it generates additional questions: Are these different disease processes? Do patients without graft dysfunction require treatment? Whether treatment of asymptomatic AMR is warranted remains unclear. In our practice, several additional factors are considered as to whether treatment should be initiated. These factors include the presence of donor specific antibodies, left and right ventricular dysfunction on echocardiogram, abnormal hemodynamic measurements, and history of prior rejection. A patient with normal cardiac function, no donor specific antibodies, and no prior episodes of rejection will be monitored. In contrast, if donor specific antibodies are present, treatment with high dose steroids and IVIG may be administered despite normal graft function. Patients with asymptomatic AMR but evidence of graft dysfunction (echocardiographic or by right heart catheterization) are treated aggressively with steroids, plasmapheresis, rituximab, and/or cyclophosphamide. Modification of chronic immunosuppression was performed if the patient was receiving cyclosporine or azathioprine. These patients were transitioned to tacrolimus and mycophenolate mofetil. Our initial therapeutic strategy did not include the addition of a proliferation signal inhibitor unless we demonstrated early that CAV was present.
AMR has been identified as a risk factor for developing CAV. In this study, patients who had late AMR with graft dysfunction had accelerated development of CAV, with 50% having de novo CAV within one year of AMR. This was not true for all other groups. For these patients, we believe angiography should be considered earlier than during annual following late AMR with graft dysfunction. This would allow the introduction of a proliferation signal inhibitor (everolimus or sirolimus), which have been demonstrated to limit CAV progression and development (13–16). It is only in the last year that proliferation signal inhibitors have been substituted for mycophenolate mofetil following detection of AMR. The number of cases where this approach was used is too small to know if there are differences in the rate of recurrence of AMR or outcome.
We acknowledge that this study has a number of limitations, beginning with the single-center and retrospective nature. The single-center aspect did allow for a more detailed and comprehensive analysis however. This study, while a sizable series for cardiac AMR, is small in absolute numbers, which limits statistical analyses (such as analysis of other co-morbidities & covariates), makes sub-group analyses potentially problematic, and can limit the generalizability of some findings. Another limitation is that the treatment regimen for each patient was not uniform. Although each patient was treated aggressively with three to four treatments, the regimens were not consistent. Further, since our program has aggressively treated AMR, it was not possible in this study to compare outcomes among patients who were not treated. This warrants further prospective study. Next, the monitoring of DSA was only with CDC until 2010 and as such it is possible that DSA were missed with the less sensitive technique. As we are comparing groups who vary in their time period after transplant, the possibility of lead time bias is introduced. We attempted to limit this bias by having a comparison group within each time period during analysis stratified by timing and graft dysfunction. Similarly, the possibility of lead time bias exists in the diagnosis of CAV after the episode of AMR. Some patients with graft dysfunction will undergo an angiogram earlier than their usual annual screening and this could contribute to increased diagnosis in the first year after the episode of AMR. Lastly, the study employed a retrospective cohort design with each patient classified according to the timing of their initial episode of AMR. As such, 17 patients had subsequent episodes of AMR; five of these were in the early AMR group but had a second AMR episode after one year. This crossover between groups may bias the results towards the null hypothesis.
In conclusion, late AMR is frequently associated with graft dysfunction. When graft dysfunction is present with late AMR, there is a significantly increased mortality and rapid development of CAV despite aggressive treatment when compared with all other groups of AMR. Clinicians must be vigilant in caring for these patients and further prospective study is needed to identify an ideal treatment regimen.
Acknowledgments
Dr. Clerkin is supported by National Institutes of Health Grant T32 HL007854-16.
Footnotes
No other authors have disclosures.
References
- 1.Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Heart Transplantation Report-2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant. 2015;34:1244–54. doi: 10.1016/j.healun.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Takemoto SK, Zeevi A, Feng S, et al. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4:1033–41. doi: 10.1111/j.1600-6143.2004.00500.x. [DOI] [PubMed] [Google Scholar]
- 3.Michaels PJ, Espejo ML, Kobashigawa J, et al. Humoral rejection in cardiac transplantation: risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant. 2003;22:58–69. doi: 10.1016/s1053-2498(02)00472-2. [DOI] [PubMed] [Google Scholar]
- 4.Reed EF, Demetris AJ, Hammond E, et al. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25:153–9. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Kfoury AG, Renlund DG, Snow GL, et al. A clinical correlation study of severity of antibody-mediated rejection and cardiovascular mortality in heart transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2009;28:51–7. doi: 10.1016/j.healun.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Coutance G, Ouldamar S, Rouvier P, et al. Late antibody-mediated rejection after heart transplantation: Mortality, graft function, and fulminant cardiac allograft vasculopathy. The Journal of Heart and Lung Transplantation. 2015;34:1050–1057. doi: 10.1016/j.healun.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Hodges AM, Lyster H, McDermott A, et al. Late antibody-mediated rejection after heart transplantation following the development of de novo donor-specific human leukocyte antigen antibody. Transplantation. 2012;93:650–6. doi: 10.1097/TP.0b013e318244f7b8. [DOI] [PubMed] [Google Scholar]
- 8.Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2013;32:1147–62. doi: 10.1016/j.healun.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2010;29:717–27. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. American journal of epidemiology. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 11.Almuti K, Haythe J, Dwyer E, et al. The changing pattern of humoral rejection in cardiac transplant recipients. Transplantation. 2007;84:498–503. doi: 10.1097/01.tp.0000278094.41131.9f. [DOI] [PubMed] [Google Scholar]
- 12.Loupy A, Toquet C, Rouvier P, et al. Late Failing Heart Allografts: Pathology of Cardiac Allograft Vasculopathy and Association With Antibody-Mediated Rejection. American Journal of Transplantation. 2016;16:111–120. doi: 10.1111/ajt.13529. [DOI] [PubMed] [Google Scholar]
- 13.Eisen HJ, Tuzcu EM, Dorent R, et al. Everolimus for the Prevention of Allograft Rejection and Vasculopathy in Cardiac-Transplant Recipients. New England Journal of Medicine. 2003;349:847–858. doi: 10.1056/NEJMoa022171. [DOI] [PubMed] [Google Scholar]
- 14.Jin YP, Valenzuela NM, Ziegler ME, Rozengurt E, Reed EF. Everolimus inhibits anti-HLA I antibody-mediated endothelial cell signaling, migration and proliferation more potently than sirolimus. Am J Transplant. 2014;14:806–19. doi: 10.1111/ajt.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobashigawa JA, Pauly DF, Starling RC, et al. Cardiac Allograft Vasculopathy by Intravascular Ultrasound in Heart Transplant Patients Substudy From the Everolimus Versus Mycophenolate Mofetil Randomized, Multicenter Trial. JACC: Heart Failure. 2013;1:389–399. doi: 10.1016/j.jchf.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Mancini D, Pinney S, Burkhoff D, et al. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation. 2003;108:48–53. doi: 10.1161/01.CIR.0000070421.38604.2B. [DOI] [PubMed] [Google Scholar]