Abstract
Introduction
Resection of perihilar cholangiocarcinoma (PHC) entails high-risk surgery with substantial postoperative mortality reported up to 18%, even in specialized centers. The aim of this study was to compare outcomes of PHC patients who underwent associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) to patients with a small functional liver remnant who underwent resection without ALPSS.
Methods
All patients who underwent ALPPS for PHC were identified from the international ALPPS registry and matched controls were selected from a standard resection cohort from two centers based on future remnant liver size. Outcomes included morbidity, mortality, and overall survival.
Results
Of the 37 patients who had undergone ALPPS for PHC in the registry, 29 had sufficient data for analyses. ALPPS for PHC was associated with a 48% (14/29) 90-day mortality and median OS of 6 months. A total of 257 patients underwent major liver resection for PHC without ALPPS. The 90-day mortality was 13% and median OS 46 months. The 29 ALPPS patients were matched to 29 patients resected without ALPPS, with similar future liver remnant volume (P=0.480). Mortality in the matched control group was 24% (P=0.100) and median OS was 27 months (P = 0.064).
Discussion
Outcomes of ALPPS for PHC appear inferior when compared to standard extended resections in high-risk patients. Considering these outcomes, portal vein embolization should remain the preferred method to increase future remnant liver volume in PHC patients. ALPPS is not recommended for PHC due to the 48% 90-day mortality in expert centers.
Introduction
Perihilar cholangiocarcinoma (PHC) is a tumor of biliary origin that originates between the second-order bile ducts and insertion of the cystic duct.(1) This location usually necessitates combined hepatic and bile duct resection to obtain tumor-free margins, which offers patients the chance of long-term survival.(1, 2) The tumor typically obstructs main bile ducts causing obstructive cholestasis and jaundice,(3) thereby postoperative compromising function and the regenerative capacity of the liver(4) and thereby, outcomes.(5) Preoperative biliary drainage is considered essential to perform safe liver especially in patients who require extended liver resections.(7) These surgery,(6) render resection of PHC factors high-risk surgery with postoperative mortality rates ranging from 5–18%, even in specialized centers.(8–10) In patients requiring extended resections with a future liver remnant (FLR) share below 30%, mortality rates are reported up to 29%.(11) Therefore, portal vein embolization is now the accepted standard to increase remnant liver volume in patients with a small FLR, in order to reduce the risks associated with major liver resection.(2, 12)
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) has been introduced as a new technique in liver surgery that induces rapid FLR hypertrophy measured in liver volume and thereby allows extended resections. The initially reported series of 25 patients illustrated the potential of ALPPS to provide curative resection for advanced primary or secondary hepatic tumors, however, mortality was considerable at 12%.(13) The small series with heterogeneous diagnoses spiked major interest but also criticism regarding the benefits and safety of the procedure.(13, 14) The first report of the international ALPPS registry followed and demonstrated a reduction of mortality to 9% in 202 patients.(15) In this report, diagnoses other than colorectal liver metastases were an independent predictor of mortality. Especially in the 11 patients who underwent ALPPS for PHC, mortality was considerable at 27%.(15) These high mortality rates in ALPPS for PHC led to the discussion at the expert meeting held in Hamburg in February 2015, whether ALPPS should be contraindicated in patients with PHC.(16, 17)
The reported mortality rates in ALPPS are high, but these patients are most likely high-risk patients with usually very small initial FLRs. Outcomes of ALPPS in PHC patients should be put into perspective by comparison with appropriate control patients who underwent standard resection for PHC. Therefore, the aim of the present study was to analyze the outcomes of all patients who underwent ALPPS for PHC in the international ALPPS registry and compare these outcomes to patients who underwent standard resection for PHC matched by extent of resection and FLR size.
Materials and Methods
AMC-MSKCC cohort of perihilar cholangiocarcinoma resections
Data were analyzed from a prospective database containing all consecutive patients who underwent major resection for suspected PHC between January 2000 and December 2015 at the Academic Medical Center (AMC) in Amsterdam or between January 2000 and March 2014 at the Memorial Sloan Kettering Cancer Center (MSKCC) in New York. Preoperative work-up and optimization of patients have been described in detail elsewhere.(11) Resection type was scored according to the operative report and confirmed by the number of resected segments. An extended left hepatectomy was defined as standard left hepatectomy with additional anatomic resection of segments 1, 5 and 8. An extended right hepatectomy was defined as standard right hepatectomy with additional anatomic resection of segments 1 and 4. Major liver resection was defined as resection of at least 3 Couinaud liver segments. FLR share was defined as the percentage FLR volume of the total liver volume, and the body surface area standardized FLR share (sFLR) was calculated as described by Ribero and Vauthey et al.(18) Preoperative portal vein embolization was considered when FLR share was below 30% while ALPPS was not performed in the study period.
ALPPS patients
Data on the ALPPS patients were obtained from the international ALPPS registry, which is described in detail elsewhere.(15) All patients in the ALPPS registry with PHC were selected for analysis. Patients, in whom 90-day survival status was not reported, were excluded from the analyses. Tumor staging was reported according to DeOliveira et al.(19) Complications were reported separately after stage 1 and 2, and for comparison with standard resection, overall major morbidity after both stages. The indication to perform ALPPS in the reported patients was not specified in the registry.
Standard resection controls
ALPPS patients were matched to patients from the AMC-MSKCC patient cohort. Matched patients (1:1 matching) were selected based on the extent FLR share. For each patient in the ALPSS registry a patient was selected in the AMC-MSKCC cohort with a similar FLR share. Patients characteristics and outcomes of both groups were compared.
Study endpoints
All complications were scored and graded according to the Clavien-Dindo classification, with complications of at least grade IIIa defined as major complication.(20) Liver failure was graded according to the International Study Group of Liver Surgery (ISGLS) criteria,(21) with grade B and C considered as clinically relevant liver failure. Liver failure in the ALPPS group was scored when reported by the physician as postoperative complication with no known classification or definition. Mortality was defined as death within 90 days after surgery. Overall survival (OS) was defined as the time between resection and the time of death or last follow-up visit. Overall survival after standard resection of selected patients was compared to survival in the ALPPS patients. For the ALPPS cohort, OS was defined as the time between stage one and death or last follow-up visit.
Statistical analysis
Data were presented as mean and standard deviation (SD) for variables that follow a normal distribution and as median and interquartile range (IQR) for not normally distributed variables. Categorical variables were analyzed using Fisher’s exact of chi-square tests. Normally distributed variables were analyzed using t-test’s and not normally distributed data using Mann-Whitney U-tests. Survival was presented using Kaplan-Meier curves and differences between survival curves were analyzed using log-rank tests. All statistical analyses were performed using IBM SPSS Statistics (Version 23, SPSS Inc., Chicago, IL).
Results
Resection of PHC without ALPPS
A total of 299 patients underwent resection without ALPPS of suspected PHC at the AMC and MSKCC within the study period of which 257 were major liver resections. A total of 138 patients (54%) experienced at least one Clavien-Dindo grade IIIa or higher complication. Mortality was 13% in the entire cohort. Morbidity and mortality by subgroup are shown in table 1. These morbidity and mortality rates illustrate high risk subgroups in the cohort of patients resected without ALPPS, and the reported 13% overall mortality does not apply to every subgroup.
Table 1.
Mortality and morbidity among risk groups following resection without ALPPS.
| Mortality, n (%) | P-value | Morbidity, n (%) | P-value | |
|---|---|---|---|---|
|
| ||||
| Age, years | 0.026 | 0.646 | ||
| - < 55 (n=53) | 3 (6) | 29 (55) | ||
| - 55–64 (n=83) | 8 (10) | 40 (48) | ||
| - 65–74 (n=82) | 13 (16) | 46 (56) | ||
| - ≥ 75 (n=39) | 10 (26) | 23 (59) | ||
|
| ||||
| Resection type | 0.219 | 0.172 | ||
| - Left hepatectomy (n=102) | 8 (8) | 45 (44) | ||
| - Extended left hepatectomy (n=23) | 5 (22) | 14 (61) | ||
| - Right hepatectomy (n=44) | 8 (18) | 27 (61) | ||
| - Extended right hepatectomy (n=85) | 13 (15) | 50 (59) | ||
| - Central hepatectomy (n=3) | 0 (0) | 2 (67) | ||
|
| ||||
| FLR V share | 0.040 | 0.019 | ||
| - < 30 % (n=45) | 12 (27) | 33 (73) | ||
| - 30–39 % (n=38) | 6 (16) | 18 (47) | ||
| - ≥ 40 % (n=143) | 16 (11) | 73 (51) | ||
|
| ||||
| Preoperative cholangitis | <0.001 | <0.001 | ||
| - Yes (n=79) | 19 (24) | 57 (72) | ||
| - No (n=175) | 15 (9) | 79 (45) | ||
Abbreviations: FLRV, future liver remnant volume.
ALPPS registry data
At the time of analysis, the international ALPPS registry contained 741 patients of which a total of 37 patients (5%) had undergone ALPPS for PHC (between 2010 and 2015). The 37 patients were operated in 23 centers with a median (IQR) of 9 (3–17) ALPPS registry procedures per center and a median of 1 (range 1–5) patient undergoing ALPPS for PHC per center. 13 of the 37 PHC patients were operated in 7 centers with experience of at least 16 ALPPS procedures. Of these 37 patients, 29 had a reported 90-day survival status and were included for further analyses.
Of the 29 included ALPPS patients, 13 patients had a major complication after stage one (Table 2). Among them were 4 patients who died after stage 19 patients suffered at least one subsequent major one.. Of the 25 patients who underwent stage two, complication, and 10 of the 25 patients died after stage two. This resulted in an overall 90-day mortality after ALPPS of 48% (14/29 patients). In comparison of patients with and without a complication after stage one, 10 of the 13 patients with a major complication after stage one died within 90 days, whereas 4 of the 16 patients without a major complication after stage one died within 90 days (P < 0.01).
Table 2.
Patient, disease and operative characteristics of ALPPS patients and matched controls of patients resected without ALPPS.
| ALPPS (n=29) | Standard resection (n=29) | P-value | |
|---|---|---|---|
|
| |||
| Age, years, median (IQR) | 65 (48–76) | 61 (52–70) | 0.290 |
|
| |||
| Male gender, n (%) | 14 | 20 | 0.182 |
|
| |||
| BMI, 2 kg/m, median (IQR) | 24 (18–30) | 25 (24–27) | 0.290 |
|
| |||
| Bismuth stage, n (%) | 0.09 | ||
| - Left or right duct | – | 1 | |
| - Bismuth I | 2 | 2 | |
| - Bismuth II | 3 | 5 | |
| - Bismith IIIa | – | 16 | |
| - Bismuth IIIb | 5 | 2 | |
| - Bismuth IV | 8 | 3 | |
| - Missing | 11 | – | |
|
| |||
| Portal vein embolization*, n (%) | 1 | 2 | 1.000 |
|
| |||
| FLRV share, %, median (IQR) | 20 (16–25) (n=17) | 24 (18–28) | 0.480 |
|
| |||
| sFLRV share, %, median (IQR) | 20 (16–26) (n=26) | 25 (19–29) | 0. 0 79 |
|
| |||
| Duration stage one, min, median (IQR) | 401 (296–558) | – | |
|
| |||
| FLRV share after stage one, %, median (IQR) | 31 (25–40) | ||
|
| |||
| sFLRV share after stage one, %, median (IQR) | 32 (26–42) | – | |
|
| |||
| FLR increase, %, median (IQR) | 68 (50–134) | – | |
|
| |||
| Interval between stages, days, median (IQR) | 8 (7–14) | – | |
|
| |||
| Duration stage two, min, median (IQR) | 178 (136–228) | – | |
|
| |||
| Laparoscopic procedure, n (%) | 1 | 0 | 1.000 |
|
| |||
| Type of Resection | 0.428 | ||
| - Left hepatectomy | |||
| - Extended left hepatectomy | 1 | 1 | |
| - Right hepatectomy | 5 | 8 | |
| - Extended right hepatectomy | 22 | 20 | |
| - Unknown | 1 | – | |
|
| |||
| Resection margin, n (%) | 1.000 | ||
| - R0 | 19 | 23 | |
| - R1 | 4 | 6 | |
| - Missing | 6 | 0 | |
|
| |||
| Morbidity, ≥ Clavien-Dindo grade IIIa, n (%) | 0.747 | ||
| - Overall | 24 | 22 | |
| - Stage one | 13 | ||
| - Stage two | 19 (n=25) | ||
|
| |||
| Liver failure, n (%) | n/a | ||
| - Physician reported | 13 | – | |
| - ISGLS grade B/C | – | 10 | |
|
| |||
| 90-day mortality, n (%) | 14 | 7 | 0.100 |
FLR volumetry was performed after portal vein embolization in both the ALPPS and standard resection groups. Abbreviations: BMI, body mass index; FLRV, future liver remnant volume; sFLRV, standardized future liver remnant volume; FLR, future liver remnant; ISGLS, International Study Group of Liver Surgery.
There were 23 patients with both pre- and post-stage one volumetry data available. The FLR volume in these patients increased from a median of 289 mL (IQR 250–380) to 531 mL (IQR 404–635), which corresponds to a median 68% increase (range 50–134%) in 6 days (range 3–11) (Figure 1A).
Figure 1.
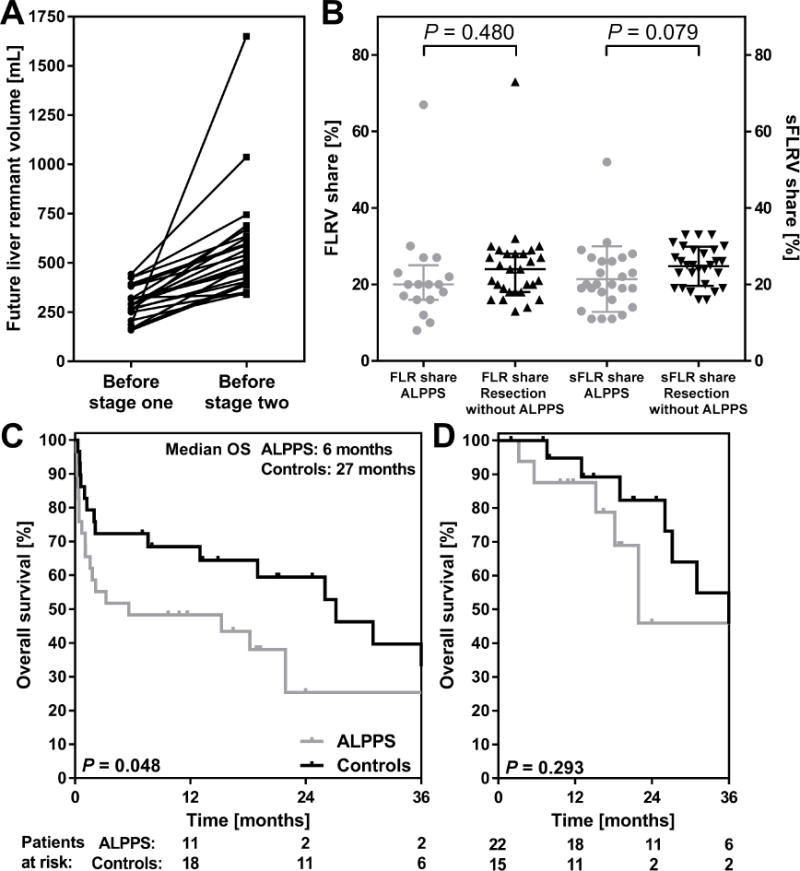
A: Future remnant liver volume before stage one and before stage two of all ALPPS patients in whom both volumes were available (n=23). B: Future remnant liver volume share before stage one of ALPPS patients (n=17) and before standard resection (n=29) and standardized future remnant liver volume share in ALPPS patients before stage one (n=26) and before resection without ALPPS (n=29). C: Overall survival in the selected high risk controls who underwent standard resection of PHC black curve) and ALPPS patients (grey curve). D: Overall survival following exclusion of 90-day mortality.
Matched controls
The 29 ALPPS patients with follow-up data were compared to 29 patients from the AMC-MSKCC cohort with the smallest FLR shares who underwent standard resection (Table 2). Patient characteristics including median FLR volumes were similar between groups (Table 2) Postoperative morbidity and 90-day mortality was twice as high in the ALPPS group (14/29 patients versus 7/29 patients) but this failed to reach statistical significance (P = 0.100). Median OS was 6 months in the ALPPS patients and 29 months in the matched controls (P = 0.048, Figure 1C). After exclusion of perioperative mortality, survival was comparable between groups (Figure 1D).
Audit of mortality after ALPPS for PHC
In total, 14 (48%) of the 29 ALPPS died within 90 days after stage one or two. Four patients died after stage one, three from liver failure and one patient due to cardiac complications(Table 3). The other 10 patients died after stage two, which was performed 1 to 14 days after stage one in these patients (Table 3). Of the patients who died after stage 2, 3 patients had a FLR and/or sFLR share below 30% at stage two. Five patients had ongoing hyperbiliribinemia and 3 patients had elevated INR of at least 1.5. Of the 6 patients with a bilirubin >50 μmol/L before stage two 5 died compared to 4 of 18 patients with bilirubin levels below 50 μmol/L before stage two (P = 0.02). However, mortality was comparable in patients with FRLV share above and below 30% (6/17 and 3/6, P = 0.64) and a sFLRV share above and below 30% (7/18 and 3/7, P = 1.00).
Table 3.
Audit of mortality after ALPPS.
| Stage one parameters | Parameters directly before stage two | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mortality after stage | Cause of death | Resection type | Complication grade | Inter-stage interval (days) | FLR V (%)share | sFLR (%)V share | Bilirubin μmol/L | INR | |
| 1 | one | Cardiac | R | V | |||||
| 2 | one | Liver failure | Ext. R | V | |||||
| 3 | one | Liver failure | Ext. R | V | |||||
| 4 | one | Liver failure | Ext. R | V | |||||
| 5 | two | Unknown | Ext. R | None | 14 | 24 | 21 | 9 | 1.2 |
| 6 | two | Biliary | Ext. R | None | 9 | 25 | 31 | 14 | 1.0 |
| 7 | two | MOF | Ext. R | None | 7 | n/a | 32 | 114 | 1.7 |
| 8 | two | Unknown | Unknown | II | 13 | n/a | 31 | 10 | 1.1 |
| 9 | two | Liver failure | Ext. R | IIIa | 8 | 57 | n/a | 52 | 1.4 |
| 10 | two | Infection | R | IIIb | 7 | n/a | 37 | 13 | 1.5 |
| 11 | two | Liver failure | Ext. R | IIIb | 9 | 19 | 19 | 385 | 1.4 |
| 12 | two | Liver failure | Ext. R | IIIb | 7 | 35 | 45 | 123 | 1.2 |
| 13 | two | MOF | Ext. R | IVb | 1 | n/a | n/a | n/a | n/a |
| 14 | two | MOF | Ext. R | IVb | 12 | 38 | 45 | 571 | 1.2 |
Abbreviations: MOF, multiple organ failure; R, right hepatectomy; Ext. R, extended right hepatectomy; FLRV, future liver remnant volume; sFLRV, standardized future liver remnant volume; INR, international normalized ratio.
All 7 patients who died within 90 days after standard resection for PHC in the matched group, died of liver failure. These seven patients all had an FLR share of 30% or less at the time of resection, and none underwent portal vein embolization.
Discussion
In the present study we demonstrated that resection of PHC using ALPPS resulted in a 48% 90-day mortality rate. Resection of PHC without ALPPS is high-risk surgery with substantial morbidity and mortality, especially in several subgroups of patients. Matched patients who underwent resection without ALPPS had 28% mortality compared to 48% in the ALPPS patients, however, the difference did not reach statistical significance. OS was 6 months in the ALPPS patients compared to 29 months in the matched controls which was significantly less.
Mortality following resection of PHC has been reported to vary from 5 to 18% in larger series.(8–10) However, the case-mix in these series often includes local extrahepatic bile duct resections and minor liver resections, so mortality is probably higher in patients after major liver resection. This was described in detail in a recent study that found a mortality rate of 28% among patients who underwent resection with a FLR volume below 30%.(11) These patients are at considerably higher risk while other predictors such as age and preoperative cholangitis further increase postoperative mortality in these patients. Despite these risks, median OS is 40 months following resection of PHC,(22) while prognosis remains poor for unresectable patients with a median OS of 8–13 months.(23)
Since its introduction, ALPPS has generated major interest over the world with both positive and critical opinions. Whereas the initial series reported 12% mortality in a heterogeneous cohort of 25 patients, the first report of the international ALPPS registry reported a reduced mortality of 8% in 202 patients.(15) However, in the subgroup of 11 patients with PHC, ALPPS resulted in 27% mortality.(15) Although these figures fueled the discussion whether ALPPS should be contraindicated in PHC patients,(16, 17, 24) ALPPS is employed in patients with very small FLRs, usually in connection with extended right hemihepatectomy.(15) The latter two factors have also been associated with substantial mortality following resection of PHC. Therefore, PHC patients who were managed with ALPPS should be considered as very high-risk patients and should be compared to the appropriate high-risk controls. Whereas the median OS of 6 months following ALPPS is determined by the perioperative mortality, after 3 months the survival curve appears comparable to standard resection (Figure 1D). This suggests that ALPPS potentially results in an adequate oncological outcome when R0 resection is achieved, however, the data is insufficient in both quantity and quality to confirm the hypothesis, as adequate staging, tumor extent and pathology are missing in the registry.
Portal vein embolization (PVE) is the gold standard procedure to increase FLR volume in patients scheduled for major liver resection.(25) A FLR share below 40% is often used as indication for PVE in patients with PHC.(26) In a systematic review containing 836 patients, PVE increased FLR volume by a mean 34% in 2 to 4 weeks with the downside of 1% major complications and 0.09% mortality.(26) PVE is therefore considered as a safe procedure to reduce postoperative risks in PHC patients. However, the reported FLR increase might be insufficient for some patients with very small FLRs. The observed median 68% (50–134%) FLR increase after ALPPS might be useful in these patients. However, considering the high risks of ALPPS versus the low risks of PVE, it might be better to perform a controlled PVE as initial step instead of upfront ALPPS. In the case of insufficient hypertrophy, ALPPS may be considered as last resort while ALPPS-induced hypertrophy does not seem to be affected by previous PVE (often termed salvage ALPPS).(27) Furthermore, PHC patients have mostly suffered from cholestasis which hampers the regenerative capacity.(4) Therefore the high regenerative response induced by ALPPS theoretically could benefit PHC patients. A drawback of PVE in the context of PHC is that permanent embolization does not allow an intra-operative change of resection strategy, i.e. right to left hepatectomy or right to left depending on intra-operative findings.(24) ALPPS has the advantage that the ultimate decision to proceed can be taken during the operation. However, the functional value of the rapid increase in liver volume seen after stage 1 in ALPPS needs furher clinical assessment.
It was previously shown that the inter-stage course is a major determinant of post-stage two outcomes, for instance the occurrence of biliary leakage.(28) Therefore in PHC, there might be an advantage to perform the biliary reconstruction at the second stage of ALPPS to reduce inter-stage morbidity and possibly improve overall outcomes. Current data is insufficient to support this hypothesis. In addition, when examining the 14 fatalities, not all patients had a FLR or sFLR share above 30% at stage two and not all patients had normalized bilirubin and INR levels. These patients might have benefited from a delay in stage two, which should most likely only be performed when FLR has increased sufficiently and both bilirubin and INR have normalized. If these recommendations are followed, outcomes of ALPPS might improve and the incidence of liver failure reduced.
The current study has several limitations. Firstly, the ALPPS registry is a database designed for all ALPPS patients and does not contain PHC specific parameters such as status of biliary drainage. Secondly, registration of patients is voluntary and not all registered patients have complete data. Therefore reporting bias could be a major issue. However, ALPPS for PHC has been performed 37 times across 23 centers according to the current registry data. While the outcomes of ALPPS for PHC based on data from a single center obviously are not informative, the use of these preliminary analyses obtained from the registry provide a more reliable estimation of outcomes.
In conclusion, the analyses of initial results of ALPPS for PHC demonstrate poor outcomes with 48% perioperative mortality and median OS of 6 months. Although these outcomes did not differ statistically from high-risk PHC patients subjected to standard resection, the study is most likely underpowered, and outcomes of ALPPS are likely worse compared to standard resection of PHC. If morbidity and mortality after ALPPS can be reduced, ALPPS might increase resectability of PHC, but for now, PVE should remain the gold standard to augment FLR volumes preoperatively which is usually recommended when FLRV share is below 40%.(26) When ALPPS is considered for PHC, these high-risk procedures should be performed in specialized centers with large experience in both resection of PHC, as well as ALPPS for other indications. However, based on the current data, PHC for ALPPS is not recommended.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–29. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258(1):129–40. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]
- 3.Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–69. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama Y, Nagino M, Nimura Y. Mechanism of impaired hepatic regeneration in cholestatic liver. J Hepatobiliary Pancreat Surg. 2007;14(2):159–66. doi: 10.1007/s00534-006-1125-1. [DOI] [PubMed] [Google Scholar]
- 5.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191(1):38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 6.Iacono C, Ruzzenente A, Campagnaro T, Bortolasi L, Valdegamberi A, Guglielmi A. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: highlights and drawbacks. Ann Surg. 2013;257(2):191–204. doi: 10.1097/SLA.0b013e31826f4b0e. [DOI] [PubMed] [Google Scholar]
- 7.Farges O, Regimbeau JM, Fuks D, Le Treut YP, Cherqui D, Bachellier P, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013;100(2):274–83. doi: 10.1002/bjs.8950. [DOI] [PubMed] [Google Scholar]
- 8.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755–62. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser GM, Paul A, Sgourakis G, Molmenti EP, Dechene A, Trarbach T, et al. Novel prognostic scoring system after surgery for Klatskin tumor. Am Surg. 2013;79(1):90–5. [PubMed] [Google Scholar]
- 10.Olthof PB, Coelen RJ, Wiggers JK, Besselink MG, Busch OR, van Gulik TM. External biliary drainage following major liver resection for perihilar cholangiocarcinoma: impact on development of liver failure and biliary leakage. HPB (Oxford) 2016;18(4):348–53. doi: 10.1016/j.hpb.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiggers JK, Koerkamp BG, Cieslak KP, Doussot A, van Klaveren D, Allen PJ, et al. Postoperative Mortality after Liver Resection for Perihilar Cholangiocarcinoma: Development of a Risk Score and Importance of Biliary Drainage of the Future Liver Remnant. J Am Coll Surg. 2016 doi: 10.1016/j.jamcollsurg.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243(3):364–72. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, et al. Right portal vein ligation combined with in situ splitting induces rapid extended right hepatic resection in small-for-size left lateral liver lobe hypertrophy enabling 2-staged settings. Ann Surg. 2012;255(3):405–14. doi: 10.1097/SLA.0b013e31824856f5. [DOI] [PubMed] [Google Scholar]
- 14.de Santibanes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann Surg. 2012;255(3):415–7. doi: 10.1097/SLA.0b013e318248577d. [DOI] [PubMed] [Google Scholar]
- 15.Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014;260(5):829–36. doi: 10.1097/SLA.0000000000000947. discussion 36–8. [DOI] [PubMed] [Google Scholar]
- 16.Donati M, Basile F, Oldhafer KJ. Present status and future perspectives of ALPPS (associating liver partition and portal vein ligation for staged hepatectomy) Future Oncol. 2015;11(16):2255–8. doi: 10.2217/fon.15.145. [DOI] [PubMed] [Google Scholar]
- 17.Oldhafer KJ, Stavrou GA, van Gulik TM. core g. ALPPS-Where Do We Stand, Where Do We Go?: Eight Recommendations From the First International Expert Meeting. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001633. [DOI] [PubMed] [Google Scholar]
- 18.Ribero D, Chun YS, Vauthey JN. Standardized liver volumetry for portal vein embolization. Semin Intervent Radiol. 2008;25(2):104–9. doi: 10.1055/s-2008-1076681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deoliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus P, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53(4):1363–71. doi: 10.1002/hep.24227. [DOI] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149(5):713–24. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Groot Koerkamp B, Wiggers JK, Gonen M, Doussot A, Allen PJ, Besselink MG, et al. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol. 2015;26(9):1930–5. doi: 10.1093/annonc/mdv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruys AT, van Haelst S, Busch OR, Rauws EA, Gouma DJ, van Gulik TM. Long-term survival in hilar cholangiocarcinoma also possible in unresectable patients. World J Surg. 2012;36(9):2179–86. doi: 10.1007/s00268-012-1638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donati M, Stavrou GA, van Gulik TM, Oldhafer KJ. Associating liver partition and portal vein ligation for staged hepatectomy for Klatskin tumours: hinc sunt leones! ANZ J Surg. 2015;85(1–2):3–4. doi: 10.1111/ans.12893. [DOI] [PubMed] [Google Scholar]
- 25.Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nagino M. Portal vein embolization before extended hepatectomy for biliary cancer: current technique and review of 494 consecutive embolizations. Dig Surg. 2012;29(1):23–9. doi: 10.1159/000335718. [DOI] [PubMed] [Google Scholar]
- 26.Higuchi R, Yamamoto M. Indications for portal vein embolization in perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2014;21(8):542–9. doi: 10.1002/jhbp.77. [DOI] [PubMed] [Google Scholar]
- 27.Bjornsson B, Gasslander T, Sandstrom P. In situ split of the liver when portal venous embolization fails to induce hypertrophy: a report of two cases. Case Rep Surg. 2013;2013:238675. doi: 10.1155/2013/238675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Truant S, Scatton O, Dokmak S, Regimbeau JM, Lucidi V, Laurent A, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): impact of the inter-stages course on morbi-mortality and implications for management. Eur J Surg Oncol. 2015;41(5):674–82. doi: 10.1016/j.ejso.2015.01.004. [DOI] [PubMed] [Google Scholar]


