Abstract
While trastuzumab is firmly established as the cornerstone of therapy for both early and advanced breast cancer expressing human epidermal growth factor receptor 2 (HER2), many patients either do not respond to trastuzumab treatment or progress following therapy. Improved understanding of breast cancer biology, particularly the complex signaling interactions managed by the HER family of receptors, have resulted in development of several novel HER2-directed therapies and combinations. This article will review the novel approaches to HER2 targeting that have been developed in recent years, with particular focus on results from these approaches in early breast cancer, and will discuss strategies to improve the tolerability of HER2-directed therapies, including prevention of cardiac toxicity and diarrhea.
Keywords: Breast cancer, HER2, Trastuzumab, T-DM1, Neratinib, Pertuzumab, Lapatinib
1. Introduction
Overexpression of the human epidermal growth factor receptor 2 (HER2) is found in approximately 17% of patients with breast cancer and is associated with aggressive tumor behavior, reduced responses to traditional therapies, and decreased survival (Slamon et al., 1987; Choritz et al., 2011). The introduction of trastuzumab, a humanized HER2-targeting monoclonal antibody, revolutionized the treatment of patients with HER2-positive breast cancer. Compared to chemotherapy alone, the historic treatment for this group of patients, the addition of trastuzumab in patients with advanced disease resulted in prolonged progression-free survival (PFS) and a 5-month improvement in median overall survival (OS) (Slamon et al., 2001). Since the initial studies of trastuzumab in metastatic breast cancer, its use has been extended to both the adjuvant and neoadjuvant settings in early breast cancer, leading to improved survival and increased rates of complete pathologic response in the breast and regional lymph nodes in the two disease settings respectively (Piccart-Gebhart et al., 2005; Slamon et al., 2011; Perez et al., 2014; Gianni et al., 2014).
Despite the efficacy of trastuzumab in treating patients with HER2-positive breast cancer, some patients do not respond to treatment, while others will eventually progress. Although the exact mechanism behind this resistance is not known, much research in recent years has focused on elucidating the biology underlying HER2 signaling and developing strategies to overcome HER2 resistance and thereby improve outcomes in both early and metastatic breast cancer. This has resulted in the emergence of several novel HER2-targeting agents, including the monoclonal antibody pertuzumab, which inhibits HER2 dimerization, the antibody-drug conjugate trastuzumab emtansine (T-DM1), and the tyrosine kinase inhibitors (TKIs) lapatinib and neratinib, which target the signal transduction pathway downstream from HER2 (Swain et al., 2015; Verma et al., 2012; Baselga et al., 2012a; Chan, 2016b). An overview of these agents and their targets can be seen in Fig. 1. Each of these agents brings its own unique benefit to the treatment of HER2-positive breast cancer, and some have resulted in significant improvements over trastuzumab across multiple stages of disease. In addition to new agents, novel strategies, such as dual targeted HER2 blockade, pan-HER inhibition, and antibody-drug conjugates that deliver targeted chemotherapy, have resulted in a multitude of opportunities to capitalize on the biology of HER2-positive breast cancer and ultimately improve responses to HER2-targeted therapy.
Fig. 1.
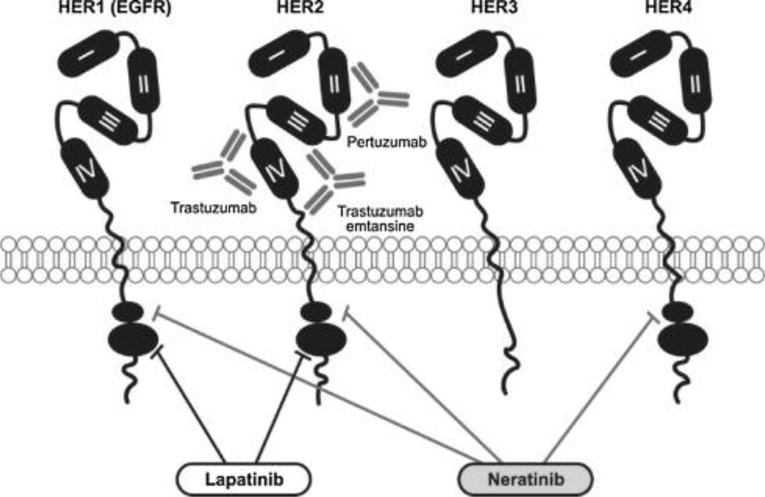
Targets of novel HER2 inhibitors.
2. Biological insights into improved HER2 targeting
2.1. Inhibition of HER family dimerization
HER2 is a member of a family of receptors that includes the epidermal growth factor receptor (EGFR), also known as HER1, HER3, and HER4. Dimerization of HER family members is a known mechanism of resistance to anti-HER2 therapy (Baselga, 2002). All members of the HER family can form dimers together, but HER2 and HER3 form a particularly potent heterodimer that is important in breast cancer development and growth. While HER3 does not exert tyrosine kinase activity on its own, dimerization with HER2 drastically increases downstream signaling activity, (Tzahar et al., 1996; Lee-Hoeflich et al., 2008) and provides a mechanism of escape from HER2 inhibition (Baselga, 2002; Menendez et al., 2015; Franklin et al., 2004).
Pertuzumab is a humanized monoclonal antibody that binds to the dimerization domain of HER2, serving both as an inhibitor of HER2/HER3 dimerization and a target for antibody-dependent cellular cytoxicity (ADCC) (Scheuer et al., 2009). While pertuzumab has some clinical activity on its own, when used concomitantly with trastuzumab, the dual binding to HER2 leads to synergy (Scheuer et al., 2009; Nahta et al., 2004). When 808 patients with metastatic breast cancer that had not previously received anti-HER2 therapy for metastatic disease were treated with the combination of pertuzumab and trastuzumab with docetaxel on the phase III CLEOPATRA trial, PFS was improved by 6 months (HR 0.65, P < 0.001) and there was a 10.8% improvement in objective response rate (Baselga et al., 2012a,b). The combination also resulted in a marked improvement in OS of almost 16 months compared to standard trastuzumab plus docetaxel (HR 0.68, P < 0.001) (Swain et al., 2015). Since this study, pertuzumab plus trastuzumab has replaced trastuzumab as the standard of care for first-line metastatic breast cancer, and this highly active combination has been investigated in early breast cancer.
2.2. Delivering HER2-targeted chemotherapy
Antibody-drug conjugates have emerged in recent years across a number of different tumor types as a mechanism for delivery of toxic chemotherapy in a highly targeted way. One such example is in breast cancer, where the antibody-drug conjugate T-DM1, a combination of the HER2-targeting antibody trastuzumab with the microtubule-inhibiting chemotherapeutic agent DM1 (emtansine), has been used to deliver cytotoxic therapy directly to breast cancer cells while avoiding the severe adverse event profile often associated with chemotherapy (Verma et al., 2012). In patients with metastatic breast cancer who were resistant to trastuzumab, T-DM1 significantly prolonged time to progression and resulted in an improvement in OS of 4 months compared to lapatinib and capecitabine (29.9 months vs 25.9 months; HR 0.75) (Diéras et al., 2017). T-DM1 is now approved as a treatment for metastatic breast cancer that has progressed on first-line trastuzumab-based therapy.
While T-DM1 has demonstrated efficacy as a rescue therapy for patients with metastatic breast cancer who become resistant to trastuzumab, it is not more effective than trastuzumab and chemotherapy as a first-line treatment. In the phase III MARIANNE trial, neither T-DM1 alone nor in combination with pertuzumab improved PFS compared with trastuzumab and chemotherapy (Perez et al., 2017).
2.3. Targeting HER2/ER crosstalk
Although HER2 inhibition is highly effective in improving outcomes in HER2-positive patients, those whose tumors also express the estrogen receptor (ER) have poorer responses to targeted therapy against either receptor and are more likely to relapse (Cameron et al., 2017; Andre et al., 2014; De Laurentiis et al., 2005). Current theories suggest that crosstalk between HER2 and ER, most likely via phosphoinositide 3-kinase (PI3K), may be a key mechanism of trastuzumab resistance in patients with HER2-positive/ER-positive tumors. Considerable crosstalk exists between the HER2 and ER pathways, and in preclinical studies in HER2-positive/ER-positive tumor models, inhibition of HER2 results in an increase in ER signaling (Xia et al., 2006).
PI3K is a key member of the HER2 signaling pathway (Hellyer et al., 1998), and is known to play an important role in regulating ER expression in breast cancer. Activation of the PI3K pathway, either through activating mutations in PIK3CA or through cell surface receptor engagement, causes suppression of ER transcription (Toska et al., 2017). Conversely, inhibition of PI3K signaling activity induces a switch in the transcriptome, moving the tumor toward a more luminal, ER-driven phenotype and increases ER gene expression (Bosch et al., 2015). This change is hypothesized to be due to chromatin remodeling at the ER gene, which occurs in the absence of PI3K signals, allowing for increased transcription of ER RNA (Toska et al., 2017).
Based on the observed impact of HER2 and/or PI3K signaling inhibition on ER expression, a proposed solution to the issue of HER2/ER crosstalk is to combine HER2 inhibition with ER inhibition, blocking both mechanisms. This strategy has been shown to be effective in preclinical models of ER-positive tumors with PIK3CA mutations, where coadministration of PI3K inhibitors with hormone therapy increased responses (Bosch et al., 2015).
In HER2-positive breast cancer, the clinical benefit of adding an ER inhibitor to HER2 inhibition has been variable. In some trials, the combination of HER2 and ER inhibition improved outcomes over inhibition over either pathway alone (Johnston et al., 2009; Kaufman et al., 2009; Rimawi et al., 2013), while in the neoadjuvant setting, targeting both pathways simultaneously did not impact on response (Harbeck et al., 2016). Recently, results from the ongoing phase II PERTAIN study showed a benefit for the combination of an aromatase inhibitor with pertuzumab and trastuzumab in metastatic breast cancer, with a median PFS of 18.9 months for patients receiving the triplet therapy and 15.8 months for patients receiving trastuzumab plus hormone therapy (Arpino et al., 2016). Further studies are currently underway to clarify the potential of this method for improving responses to HER2-targeted therapy in ER-positive patients, including a phase II trial examining the combination of neratinib and fulvestrant [NC-T01670877] (National Institutes of Health, 2017a).
2.4. Mutated HER2 as a target in HER2-non amplified breast cancer
Because therapies like trastuzumab, pertuzumab, and T-DM1 target surface HER2, their use is limited to patients in which HER2 expression is amplified. In recent years, however, multiple activating mutations in the HER2 gene have been found to be present in tumors that do not over-express HER2 (Lee et al., 2006; Bose et al., 2013). These polymorphisms occur at low levels in multiple tumor types, including breast cancer, where they are estimated to occur in approximately 4000 patients annually in the United States (Bose et al., 2013). Somatic mutations in the HER2 gene cause activation of the HER2 signaling pathway independently from receptor dimerization, resulting in constitutive kinase signaling, activation of survival pathways, oncogenic transformation, and enhanced tumor growth (Lee et al., 2006). While antibodies that target cell surface receptors are ineffective against this form of HER2 activation, agents that target downstream HER2 signaling may be useful in blocking mutant HER2 activity.
In recent years, several TKIs have been developed that target the signal transduction pathway downstream from HER2, including lapatinib and neratinib (Fig. 1). Lapatinib has been shown to be effective against HER2 amplification when given in combination with trastuzumab in both early and metastatic breast cancer (Baselga et al., 2012b; Blackwell et al., 2012). However, in preclinical studies most HER2 somatic mutations were resistant to lapatinib (Bose et al., 2013). A second HER2-targeting TKI, neratinib has emerged as a potent inhibitor of HER2 activity. Neratinib is an irreversible pan-inhibitor of HER2 and HER1/EGFR that has been shown to be more effective than lapatinib at blocking HER2 activation (Rabindran et al., 2004; Sánchez-Martín and Pandiella, 2012). In preclinical trials in tumor cell lines expressing HER2 somatic mutations, neratinib led to potent inhibition of intracellular signaling, cell proliferation, and colony formation (Bose et al., 2013).
The phase II SUMMIT trial examined the safety and efficacy of neratinib alone or in combination with fulvestrant in patients with solid tumors, including patients with breast cancer that had somatic mutations in HER2 and HER3 (Hyman et al., 2017; Hyman et al., 2016). While no clinical activity was observed in patients with HER3 mutations, 8 of the 25 (32%) patients with HER2-mutated breast cancer receiving neratinib monotherapy responded by week 8 (Fig. 2). Neratinib monotherapy was associated with a 40% clical benefit rate in patients with HER2-mutated breast cancer (Hyman et al., 2017). Of the 12 evaluable patients with breast cancer who received neratinib in combination with fulvestrant, 5 (41.7%) experienced an objective response at week 8, and the clinical benefit rate for the combination was 58.3% (Hyman et al., 2016). Studies of neratinib in this rare but important subset of patients with breast cancer are ongoing.
Fig. 2.
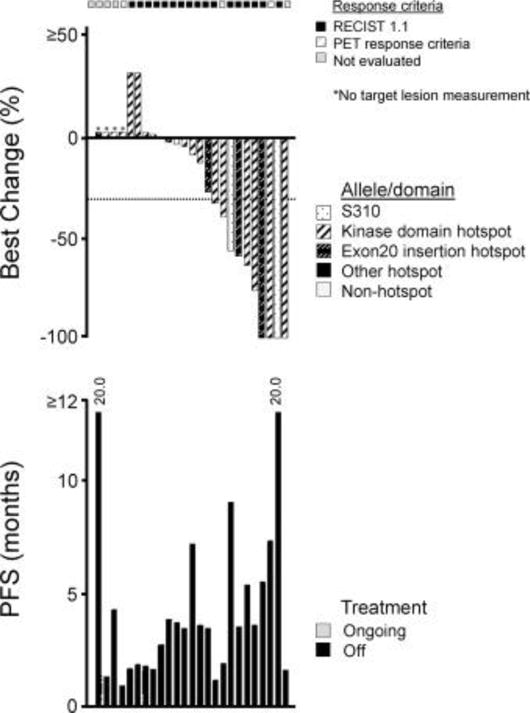
Response to neratinib in patients with HER2-mutated breast cancer on the SUMMIT trial. PFS, progression-free survival (Hyman et al., 2017).
3. Optimizing HER2-targeted therapy for early breast cancer
3.1. Current standards in HER2-positive early breast cancer
The current standard of care for HER2-positive early breast cancer includes trastuzumab (with or without pertuzumab) and chemotherapy given either before or after surgery. This is based on results from several phase III trials demonstrating a significant benefit for trastuzumab over standards of care in both the adjuvant and neoadjuvant settings (Piccart-Gebhart et al., 2005; Cameron et al., 2017; Perez et al., 2014; Slamon et al., 2011; Gianni et al., 2014). In the phase III HERA trial, 1 year of adjuvant trastuzumab was associated with significant improvements in both 10-year rates of disease-free survival (DFS; 69% vs 63%; HR 0.76) and 12-year rates of OS (79% vs 73%; HR 0.74) compared to observation. (Piccart-Gebhart et al., 2005; Cameron et al., 2017). In the phase III neoadjuvant NOAH trial, HER2-positive patients treated with trastuzumab plus chemotherapy had a significantly higher rate of pathologic complete response (pCR) than patients who only received chemotherapy (38.5% vs 19.5%; HR 0.29, P = 0.0135) and these patients remained event-free longer (5-year event-free survival [EFS] 58% vs 43%; HR 0.64, P = 0.016) (Gianni et al., 2014).
Despite the demonstrated efficacy of trastuzumab in early breast cancer, a significant proportion of patients will eventually progress. At 10-years follow-up on the HERA trial, 28.8% of patients treated with trastuzumab experienced disease progression (Cameron et al., 2017). Similarly, at only 5 years post-treatment on the NOAH trial, 42% of patients treated with trastuzumab had experienced a disease event (Gianni et al., 2014). While more recent trials have seen improvements in outcomes for patients receiving adjuvant trastuzumab, with a 5-year DFS of over 85% (Slamon et al., 2011; Piccart-Gebhart et al., 2016), there’s still an unmet medical need in this population. Furthermore, HER2-positive breast cancer is a heterogeneous disease, and while trastuzumab with chemotherapy is highly effective in many patients, there are subgroups where recurrence rates are still high, such as those with node-positive disease (Slamon et al., 2011). Clearly, for many patients with HER2-positive early breast cancer there is still significant unmet need, and research in recent years has focused on identifying novel approaches to adjuvant and neoadjuvant therapy that can improve outcomes for these patients.
3.2. Novel approaches to HER2-targeted adjuvant therapy
Several novel HER2-targeting agents have been studied as potential adjuvant therapy for patients with HER2-positive breast cancer. Results from key phase III trials are summarized in Table 1.
Table 1.
Disease-free survival and overall survival rates for phase III adjuvant trials of novel HER2 inhibitors.
| Agent/trial | Disease setting | Regimen | N | Follow-up | DFS | OS |
|---|---|---|---|---|---|---|
| Lapatinib | ||||||
| ALTTO | Stage I–III adjuvant therapy | • Lapatinib trastuzumab+ | 8381 | 6 years | 85% (HR 0.86) | 93% (HR 0.86) |
| Moreno-Aspitia et al. (2017) | • Lapatinib (34 w) → Trastuzumab (12 w) | 84% (HR 0.93) | 92% (HR 0.88) | |||
| Piccart-Gebhart et al. (2016) | • Trastuzumab | 82% | 91% | |||
| • Lapatinib | 82% (HR 1.34) | 93% (HR 1.36) | ||||
| TEACH | Stage I–III delayed adjuvant therapy. Prior trastuzumab unless contraindicated | • Lapatinib | 3147 | 47.4 mo | 87% (HR 0.83) | 94% (HR 0.99) |
| Goss et al. (2013) | • Placebo | 48.3 mo | 83% | 94% | ||
| Neratinib | ||||||
| ExteNET | Stage I–III delayed adjuvant therapy. Prior trastuzumab | • Neratinib | 2840 | 5 years | 90.2% (HR 0.73)* | Not mature |
| (Martin et al., 2017) | • Placebo | 87.7%* | ||||
| Pertuzumab | ||||||
| APHINITY | Stage II–III adjuvant therapy | • Pertuzumab (plus chemo & trastuzumab) | 4805 | 36 mo | 94.1% (HR 0.81)* | 97.7% (HR 0.89) |
| Von Minckwitz et al. (2017) | • Placebo (plus chemo & trastuzumab) | 93.2%* | 97.7% |
Chemo, chemotherapy; DFS, disease-free survival; ET, endocrine therapy; mo, months; OS, overall survival.
Invasive DFS.
3.2.1. Lapatinib
Two phase III trials have examined the HER2-targeting TKI lapatinib as a potential adjuvant therapy for HER2-positive breast cancer, either alone or in combination with trastuzumab. In the TEACH trial, which compared lapatinib to placebo in women with HER2-positive early breast cancer who had previously received adjuvant chemotherapy but not trastuzumab, single-agent lapatinib failed to demonstrate a significant DFS benefit over placebo (HR 0.83, 95% CI 0.70–1.00), though there was a marginal benefit for patients with HER2-positive disease confirmed by central fluorescence in situ hybridization (Goss et al., 2013).
In the ALTTO trial, patients who received single-agent lapatinib had poorer outcomes than patients who received trastuzumab (HR 1.34) (Piccart-Gebhart et al., 2016). The combination of lapatinib and trastuzumab appeared to improve outcomes, as patients who received both lapatinib and trastuzumab, either in combination (L + T) or in sequence (T → L), had better DFS rates than patients who received single-agent lapatinib (L + T: 88% and T → L: 87% vs L: 82%). However, with 5 years of follow-up, the combination did not sufficiently improve either DFS (L + T: HR 0.86, 95% CI 0.74–1.0; T → L: HR 0.93, [95% CI 0.81–1.08]) or OS (L + T: HR 0.86, [95% CI 0.70–1.06]; T → L: HR 0.88, [95% CI 0.71–1.08]) compared to single-agent trastuzumab, to enable recommendation of lapatinib in the adjuvant disease setting (Moreno-Aspitia et al., 2017). Currently lapatinib is not being further investigated in the adjuvant setting, either alone or in combination with trastuzumab.
3.2.2. Neratinib
Results with the pan-HER TKI neratinib in the adjuvant setting have been promising. In the phase III ExteNET trial, which compared 1 year of neratinib (240 mg daily) to placebo in women with stage I–III HER2-positive breast cancer who had completed neoadjuvant and adjuvant trastuzumab up to 2 years before randomization, neratinib improved invasive DFS at 2 years by 2.3% over placebo (HR 0.67, P = 0.009) (Chan et al., 2016a). This improvement was most pronounced in patients with hormone receptor (HR)-positive tumors (HR 0.51, P = 0.001), suggesting neratinib might provide an additional treatment option for this subset of patients who have an ongoing, long-term, and fairly constant risk of relapse on trastuzumab (Chan et al., 2016a). At 5-years follow-up, the benefit with neratinib was maintained with a 2.5% absolute improvement in invasive DFS (90.2% vs 87.7%; HR 0.73, P = 0.008) for the whole population (Martin et al., 2017). This improvement in invasive DFS was most apparent in HR-positive patients (91.2% vs 86.8%; HR 0.60, P = 0.002). Patients who had completed adjuvant trastuzumab less than 1 year prior to the start of study showed the most benefit with neratinib (Martin et al., 2017). On the strength of these results, neratinib has been approved by the US Food and Drug Administration, and is under review by the European Medicines Agency.
While patients treated with neratinib, in the absence of anti-diarrhea prophylaxis, also experienced high rates of grade 3 diarrhea, this did not appear to impact treatment efficacy. Prophylactic regimens have since been developed to reduce the incidence and severity of neratinib-associated diarrhea and will be discussed in the next section.
3.2.3. Pertuzumab
The combination of pertuzumab and trastuzumab with chemotherapy is approved as first-line treatment of metastatic breast cancer. The phase III APHINITY study examined the same combination as adjuvant treatment compared to standard trastuzumab plus chemotherapy in 4805 patients with HER2-positive early breast cancer (Von Minckwitz et al., 2017). While this trial met its primary endpoint of improved invasive disease-free survival at 3 years (94.1% vs 93.2%; HR 0.81, P = 0.045), the magnitude of the benefit was modest and there was no associated improvement in OS (97.7% in both groups; HR 0.89, P = 0.467). Subgroup analysis suggested a somewhat greater effect in ER-negative tumors (HR 0.76, P = 0.085). Pertuzumab treatment was associated with an increase in grade ≥3 diarrhea (9.8% vs 3.7%). Long-term analysis of this trial is ongoing to determine the role of pertuzumab as adjuvant therapy more precisely (Von Minckwitz et al., 2017).
3.2.4. T-DM1
Two key phase III trials are currently ongoing examining the efficacy of the antibody-drug conjugate T-DM1 in the adjuvant setting, though no results are yet available from these trials. The KATHERINE trial compares 1 year of treatment with T-DM1 to trastuzumab in women who have residual disease in the breast or axillary lymph nodes following neoadjuvant therapy [NCT01772472] (National Institutes of Health, 2017b). The KAITLIN trial is examining the combination of T-DM1 and pertuzumab to trastuzumab, pertuzumab, and a taxane component of chemotherapy following 3 cycles of anthracycline-based chemotherapy [NCT01966471] (National Institutes of Health, 2017c).
3.3. Duration of therapy
Several studies have looked at the optimal duration of adjuvant therapy. The phase III HERA trial compared 1 year versus 2 years of adjuvant trastuzumab. Extending adjuvant trastuzumab to 2 years did not improve outcomes, but resulted in increased adverse events and cardiotoxicity (Goldhirsch et al., 2013). Since this trial, 1 year of adjuvant therapy has been considered standard. However, shorter durations of trastuzumab have been investigated, including a 9-week schedule developed by Joensuu and colleagues (Joensuu et al., 2009), and a 6-month schedule investigated by Pivot and colleagues (Pivot et al., 2013). Comparison of 6 months versus 12 months of trastuzumab was unable to exclude non-inferiority of the shorter duration of treatment (Pivot et al., 2013), but still the interest in de-escalation of treatment has persisted. More recently, the multicenter Italian phase III Short-HER trial has further examined the question of adjuvant therapy duration, comparing the standard 1-year course of adjuvant trastuzumab to a shorter 9-week course (Conte et al., 2017). While the study did not meet its primary endpoint of noninferiority of 9 weeks to 1 year (HR 1.15 [90% CI 0.91–1.46]), subgroups were identified in which a shorter course of trastuzumab provides optimal outcomes. In particular, patients with a lower disease burden (stage I or II; N0 or N1) fared at least as well on the 9-week trastuzumab course. Importantly, cardiac toxicity was significantly reduced in patients receiving 9 weeks of trastuzumab (HR 0.32, P < 0.0001) (Conte et al., 2017), suggesting that 9 weeks of adjuvant trastuzumab may be an option for patients with low risk of relapse or high risk of cardiac toxicity.
3.4. Neoadjuvant therapy for HER2-positive breast cancer
Although initial trials of HER2-targeted agents were performed in the adjuvant setting, in recent years there has been a large push to investigate these agents prior to surgery, as this improves rates of breast conservation and may provide insights into risk of recurrence (Von Minckwitz et al., 2013). In high-risk patients, such as those on the NOAH trial, rates of disease progression continue to be high following trastuzumab treatment (Gianni et al., 2014), suggesting a need for continued improvement in this setting. Furthermore, in the neoadjuvant setting pCR has been shown to be predictive of long-term outcomes, allowing for the conduct of small, informative clinical trials (Von Minckwitz et al., 2013). An overview of key phase II and III trials in this setting can be seen in Table 2. Of agents investigated thus far, only pertuzumab in combination with trastuzumab have been approved in this setting.
Table 2.
pCR from key phase II/III neoadjuvant trials of novel HER2 inhibitors.
| Trial/agent | Phase | Regimen | N | pCR (All HER2 +) |
pCR (HER2+ HR +) |
pCR (HER2+ HR−) |
|---|---|---|---|---|---|---|
| Lapatinib | ||||||
| GeparQuinto | III | Chemo + lapatinib | 615 | 22.7% | NR | NR |
| Untch et al.(2012) | Chemo + trastuzumab | 30.3% | ||||
| NeoALTTO | III | Chemo + lapatinib | 455 | 24.7% | 16.1% | 33.7% |
| Baselga et al.(2012b) | Chemo + trastuzumab | 29.5% | 22.7% | 36.5% | ||
| Chemo + lapatinib + trastuzumab | 51.3% | 41.6% | 61.3% | |||
| CHER-LOB | II | Chemo + lapatinib | 121 | 26.3% | NR | NR |
| Guarneri et al.(2012) | Chemo + trastuzumab | 25.0% | ||||
| Chemo + lapatinib + trastuzumab | 46.7% | |||||
| NSABP B-41 | III | Chemo + lapatinib | 519 | 53.2% | 48.0% | 60.6% |
| Robidoux et al.(2013) | Chemo + trastuzumab | 52.5% | 46.7% | 65.5% | ||
| Chemo + lapatinib + trastuzumab | 62.0% | 55.6% | 73.0% | |||
| Neratinib | ||||||
| NSABP FB-7 | II | Chemo + neratinib | 126 | 33.3% | 27.6% | 46.2% |
| Jacobs et al.(2015) | Chemo + trastuzumab | 38.1% | 29.6% | 57.1% | ||
| Chemo + neratinib + trastuzumab | 50.0% | 30.4% | 73.7% | |||
| I-SPY 2 (HER2+ cohort) | II | Chemo + neratinib | 87 | 39% | 30% | 56% |
| Park et al.(2016) | Chemo + trastuzumab | 23% | 17% | 33% | ||
| Pertuzumab | ||||||
| NeoSphere | II | Chemo + trastuzumab | 417 | 29.0% | 20.0% | 36.8% |
| Gianni et al., (2012) | Chemo + pertuzumab + trastuzumab | 45.8% | 26.0% | 63.2% | ||
| Pertuzumab + trastuzumab | 16.8% | 5.9% | 27.3% | |||
| Chemo + pertuzumab | 24.0% | 17.4% | 30.0% | |||
| TRYPHAENA | II | Pertuzumab + trastuzumab + chemo (FEC → docetaxel) | 225 | 61.6% | 46.2% | 79.4% |
| Schneeweiss et al.(2013) | FEC → pertuzumab + trastuzumab + docetaxel | 57.3% | 48.6% | 65.0% | ||
| Chemo + pertuzumab + trastuzumab | 66.2% | 50.0% | 83.8% | |||
| T-DM1 | ||||||
| KRISTINE | III | Chemo + pertuzumab + trastuzumab | 444 | 55.7% | 44.8% | 72.4% |
| Hurvitz et al.(2016) | ||||||
| T-DM1 + pertuzumab | 44.4% | 37.9% | 53.8% | |||
| ADAPT | II | T-DM1 | 375 | NR | 41.0% | NR |
| Harbeck et al. (2016) | T-DM1 + ET | 41.5% | ||||
| Trastuzumab + ET | 15.1% |
Chemo, chemotherapy; ET, endocrine therapy; FEC, 5-fluorouracil, epirubicin, cyclophosphamide; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; NR, not reported; pCR, pathologic complete response; T-DM1, trastuzumab emtansine.
3.4.1. Pertuzumab
Pertuzumab given in combination with trastuzumab and chemotherapy is now considered a standard of care for neoadjuvant therapy in patients with HER2-positive breast cancer. This is based on the NeoSphere trial, which demonstrated significant improvements in the pCR rate with this combination over either trastuzumab and chemotherapy or pertuzumab and chemotherapy (Gianni et al., 2012). In the long-term follow-up of the NeoSphere trial, the improved pCR rates associated with the pertuzumab and trastuzumab combination were found to associate with improvements in survival. At 5 years, 86% of patients who received the combinations were alive and disease free, compared to 81% of patients who received trastuzumab plus chemotherapy (Gianni et al., 2015). Similar results were seen in the phase II cardiac safety study TRYPHAENA, where the pertuzumab and trastuzumab combination resulted in a pCR ranging from 57.3% to 66.2% depending on dose and schedule (Schneeweiss et al., 2013).
3.4.2. Neratinib
Neoadjuvant neratinib has been examined in two phase II trials in HER2-positive breast cancer, FB-7 and I-SPY2. The FB-7 study was a 3 arm trial comparing neratinib, trastuzumab, or the combination of neratinib and trastuzumab, all given with chemotherapy (Jacobs et al., 2015). In this study, there was no benefit for neratinib plus chemotherapy over trastuzumab plus chemotherapy (pCR 33.3% vs 38.1%). However, the combination of neratinib and trastuzumab resulted in a nearly 12% improvement in pCR over trastuzumab and chemotherapy. Best results with neratinib were seen in the HER2-positive/HR-negative cohort, where the combination of neratinib, trastuzumab, and chemotherapy resulted in a 73.7% pCR. Unlike in the adjuvant ExteNET trial, there was no benefit for neratinib in the HR-positive subgroup, either alone or in combination with trastuzumab (Jacobs et al., 2015).
Interestingly, while the FB-7 study only found benefit for neratinib in combination with trastuzumab, neratinib outperformed trastuzumab in the I-SPY 2 study (Park et al., 2016). In this trial, the combination of neratinib and chemotherapy resulted in a higher rate of pCR than trastuzumab and chemotherapy (39% vs 23%). This benefit was most pronounced in the HR-negative subset of patients, in whom neratinib was associated with a 56% pCR, compared to 33% with trastuzumab. Based on these results, the predicted probability of success of neratinib in a phase III trial in HER2-positive/HR-negative patients is 79% (Park et al., 2016). Investigation of neratinib in the neoadjuvant setting is ongoing.
3.4.3. T-DM1
Trials of T-DM1 in the neoadjuvant setting have been disappointing thus far. The phase III KRISTINE trial examined the combination of T-DM1 and pertuzumab compared to pertuzumab and trastuzumab with chemotherapy. While treatment-related adverse events occurred less frequently in the T-DM1 arm, there was no benefit in pCR. Only 44.4% of patients in the T-DM1 arm achieved pCR, compared with 55.7% of patients receiving pertuzumab and trastuzumab. These results were consistent regardless of hormone receptor status (Hurvitz et al., 2016). Interestingly, a phase II trial in HR-positive patients demonstrated that both single-agent T-DM1 and T-DM1 in combination with endocrine therapy improved pCR rates over trastuzumab plus endocrine therapy. However, in this trial patients did not receive chemotherapy, resulting in a low pCR rate of 15.1% in the trastuzumab plus endocrine therapy arm (Harbeck et al., 2016). It is likely that the addition of chemotherapy to the trastuzumab and endocrine therapy arm would have negated the benefit seen with T-DM1.
3.4.4. Lapatinib
As with the adjuvant setting, the efficacy of lapatinib has been investigated in the neoadjuvant setting both as a single agent and in combination with trastuzumab. In the phase III NeoALTTO study, lapatinib plus chemotherapy failed to improve pCR rates over trastuzumab plus chemotherapy (24.7% vs 29.5%). However, the combination of lapatinib and trastuzumab over chemotherapy resulted in a significant benefit, with 51.3% achieving pCR (Baselga et al., 2012b). Similar results with the combination were seen in the phase II CHER-LOB study, but the same magnitude of benefit was not observed in the phase III NSABP B-41 study (Guarneri et al., 2012; Robidoux et al., 2013). Unfortunately, there was no significant improvement in either OS or event-free survival with lapatinib plus trastuzumab on the NeoALTTO trial (de Azambuja et al., 2014). Given the high toxicity rates seen with the lapatinib and trastuzumab combination, the lack of a consistent benefit in pCR, and absence of an impact on long-term survival, there is little interest in developing this combination further in the neoadjuvant setting.
4. Minimizing the impact of treatment-related adverse events
4.1. Management of trastuzumab-associated cardiotoxicity
With the introduction of any novel therapy or treatment regimen, careful consideration must be made of the risks and benefits of treatment, particularly in terms of treatment-related adverse events (AEs). Many HER2-directed therapies are associated with challenging AE profiles that, if not properly managed, can significantly impair patient quality of life. However, when appropriate proactive management and prevention strategies are in place, these AEs can be controlled, minimizing their impact on the patient and treatment regimen.
When trastuzumab was initially introduced, it was associated with high rates of cardiotoxicity, with 27% of patients receiving trastuzumab in combination with an anthracycline and cyclophosphamide experiencing cardiac dysfunction (all grades) and 16% experiencing New York Heart Association class III or IV cardiac dysfunction (Slamon et al., 2001). Cardiac toxicity associated with trastuzumab is a mechanism-related AE. In its naturally functioning state, HER2 is responsible for key cell survival pathways that regulate the heart’s response to stress. These responses include increased cellular transcription pathways, increased production of nitric oxide, and inhibition of reactive oxygen species (Kuramochi et al., 2004; Zhao et al., 1998; Lemmens et al., 2007). When HER2 function is blocked by trastuzumab, the key cell survival pathways are also blocked, inhibiting the heart’s ability to respond to stress. Combination of trastuzumab with anthracycline chemotherapy, a known cause of cardiac stress (Smith et al., 2010), leads to an increased incidence of congestive heart failure.
While rates of cardiac dysfunction were high in early trials of trastuzumab, monitoring and prevention strategies have since been enacted that have reduced these rates and converted cardiac dysfunction into a rare and manageable side effect of trastuzumab. These strategies include obtaining a full medical history, performing a physical examination, including evaluation for edema and hepatomegaly, obtaining an echocardiogram, and assessing the left ventricular ejection fraction (LVEF) before starting trastuzumab. Trastuzumab is not recommended in patients with a LVEF of < 50% (Slamon et al., 2005). Such patients should be seen by a cardiologist and their cardiac function optimized with appropriate medication. If a repeat measurement of the LVEF is > 50%, adjuvant trastuzumab can be reconsidered. During trastuzumab treatment, regular assessment of heart rate increase of > 15%, body weight increase ≥2 kg/week (AGO Website, 2017), cardiac signs and symptoms, and repeat measurements of the LVEF (every 3 months) should be performed (Dang et al., 2016). When following these recommendations, rates of cardiac toxicity have been decreased in modern trials of trastuzumab and cardiac toxicity may no longer be considered prohibitive to trastuzumab use (Dang et al., 2016). Additionally, while the more recently developed HER2-targeted antibodies pertuzumab and T-DM1 carry similar risks of cardiotoxicity, the prevention and management strategies developed for trastuzumab have been successfully used to mitigate this risk (Swain et al., 2013; Verma et al., 2012).
4.2. Management of neratinib-associated diarrhea
Similar to trastuzumab, neratinib is associated with a mechanism-based AE that, when left uncontrolled, can severely impair patient quality of life. In the phase III ExteNET trial, treatment with neratinib without prophylactic anti-diarrhea medication was associated with diarrhea in 95.4% of patients, with 39.8% experiencing grade 3 diarrhea and 1 patient (0.1%) experiencing a grade 4 event (Chan et al., 2016a). High rates of treatment-related diarrhea have been consistent across all early-stage trials of neratinib, with rates of grade ≥3 events ranging from 21% to 40% (Chan, 2016b). Severe diarrhea occurs most frequently in the first month of neratinib treatment, with low rates observed in later months. The median duration of diarrhea in the ExteNET trial was 5 days. The impact on quality of life is also limited to early in treatment. While patients treated with neratinib experienced a decrease in quality of life due to diarrhea in month 1 of treatment, this decrease had been completely corrected by month 3 (Chan et al., 2016a).
Although the diarrhea associated with neratinib appears to be self-limiting, it is a challenging and potentially serious side effect. Similar to AEs in other malignancies, management algorithms have been developed to control and decrease the severity of neratinib-associated diarrhea. These strategies include both supportive care and pharmacological interventions. Supportive care methods recommended to manage diarrhea associated with cancer treatment include increasing fluid intake to at least 2 liters per day, removing all lactose-containing products from the diet, following a low-fat diet enriched with bananas, rice, apple sauce, and toast (BRAT diet), and engaging a specialty nursing team to support and educate the patient from the beginning of treatment (Ustaris et al., 2015). In addition to these dietary changes, diarrhea should be proactively managed with loperamide up to a maximum of 16 mg/day. If loperamide alone is not sufficient to manage diarrhea (grades 3/4), additional therapies include short-acting octreotide at 150 ug (with dose escalation up to 500 ug), diphenoxylate hydrochloride plus atropine sulfate 2.5 mg every 6–8 h, and prophylactic antibiotics. Stool cultures may also be necessary to rule out infection as a cause of diarrhea. Once the diarrhea has resolved, the dose of loperamide should be reduced to 4 mg/day and continued as pro phylaxis (Ustaris et al., 2015).
The best strategy for managing neratinib-associated diarrhea is to prevent its occurrence entirely. Since the early trials of neratinib performed without routine diarrhea prophylaxis, efforts have focused on identifying proactive methods to decrease rates of treatment-associated diarrhea and minimize the impact of this AE, as was done with trastuzumab-associated cardiotoxicity. To that end, prophylaxis strategies have been developed that significantly decrease the rates of diarrhea. Current recommendations for diarrhea prophylaxis with neratinib include loperamide given concurrently with neratinib from day 1. Loperamide is given at 16 mg on day 1 of cycle 1 (given as 4 doses of 4 mg). The dose is lowered to 12 mg/day for days 2–3, and then 6 mg to 8 mg per day for day 4 through the end of the cycle. Following cycle 1, loperamide is given on an as needed basis (Ustaris et al., 2015).
Prophylaxis with loperamide is effective at reducing rates of neratinib-associated diarrhea. In the NSABP phase I trial examining neratinib in combination with paclitaxel and trastuzumab, none of the patients who received loperamide prophylaxis (n = 6) experienced grade 3 diarrhea (grade 1/2 in 83%), while 53% of the patients who did not receive prophylaxis experienced grade 3 diarrhea (Ustaris et al., 2015; Jankowitz et al., 2013). In the 10–005 trial examining the combination of neratinib and temsirolimus, only 7 of the 41 patients who received prophylaxis (17%) experienced grade 3 diarrhea, and the average duration of treatment-emergent diarrhea was 2 days. In the same trial, patients who received either no or suboptimal prophylaxis experienced a 32% rate of grade 3 diarrhea, with an average duration of 14 days (Ustaris et al., 2015; Gajria et al., 2015).
The ongoing phase II CONTROL trial is prospectively examining the impact of different loperamide-based prophylaxis regimens on neratinib-induced diarrhea (Ibrahim et al., 2017). Women with HER2-positive early-stage breast cancer who had completed adjuvant therapy with trastuzumab received neratinib daily for 1 year along with prophylaxis comprising either loperamide alone, loperamide with the locally-acting corticosteroid budesonide, or loperamide with the bile acid sequestrant colestipol. At the interim analysis, loperamide alone was associated with a 30.7% rate of grade 3 diarrhea, while loperamide in combination with budesonide or colestipol was associated with 23.4% and 11.5% rates of grade 3 diarrhea, respectively (Table 3). Use of any prophylaxis was associated with a shortened duration of diarrhea of 2–3 days (Ibrahim et al., 2017). Compared to the ExteNET trial (Chan et al., 2016a; Chan, 2016b), rates of grade 3 diarrhea were decreased in patients receiving loperamide prophylaxis and the duration of diarrhea events shortened. While the exact approach to diarrhea prevention and management continues to be refined, these early trial results demonstrate that prophylactic regimens are highly effective at preventing and reducing the incidence, severity, and duration of neratinib-associated diarrhea.
Table 3.
Rates and duration of neratinib-associated diarrhea with prophylaxis on the CONTROL trial (Ibrahim et al., 2017).
| Study | CONTROL | ExteNET | ||
|---|---|---|---|---|
|
| ||||
| Antidiarrheal prophylaxis | Loperamide (original + modified) | Budesonide + loperamide | Colestipol + loperamide | Loperamide pm |
| N (at data cut-off) | 137 | 64 | 26 | 1408 |
| Diarrhea, % | ||||
| Any grade | 77.4 | 79.7 | 57.7 | 95.4 |
| Grade 1 | 24.1 | 26.6 | 30.8 | 22.9 |
| Grade 2 | 22.6 | 29.7 | 15.4 | 32.5 |
| Grade 3 | 30.7 | 23.4 | 11.5 | 39.8 |
| Grade 4 | 0 | 0 | 0 | 0.1 |
| Median cumulative duration of diarrhea, days | ||||
| Any grade | 12.0 | 10.0 | 8.0 | 59.0 |
| Grade ≥2 | 4.0 | 3.0 | 2.0 | 10.0 |
| Grade ≥3 | 3.0 | 2.0 | 2.0 | 5.0 |
| Median episodes of diarrhea per patient, n | ||||
| Any grade | 2.0 | 4.0 | 3.0 | 8.0 |
| Grade ≥2 | 2.0 | 2.0 | 2.0 | 3.0 |
| Grade ≥3a | 1.0 | 1.0 | 2.0 | 2.0 |
| Median duration of neratinib treatment, months | 10.6 | 5.1 | 1.7 | 11.6 |
No grade 4 events in the CONTROL study; one grade 4 event in the ExteNET study.
4.3. Financial toxicity
While improvements to HER-targeted treatment strategies have resulted in increased remission rates and prolonged survival, there is an unwanted effect associated with many treatment options: increased cost. Cost of care for patients with breast cancer is already quite high, and the addition of a second biologic to adjuvant trastuzumab, as in an extended or dual blockade strategy, results in a significant increase in treatment costs, although these costs are somewhat offset by the significant treatment costs associated with metastatic disease that may be avoided. Moving forward, cost of treatment will need to be carefully balanced with patient need. Furthermore, the potential introduction of lower-cost trastuzumab biosimilars may offset some of the added costs. Another option may be shorter durations of trastuzumab treatment in patients with very good risk factors or an approach that reserves extended therapy or dual blockade for patients with node-positive disease.
5. Conclusion
HER2-targeted therapy has transformed the outlook for both early and metastatic HER2-positive breast cancer, beginning with trastuzumab and continuing through the development of several novel HER2-targeting agents. The biology of HER2-positive breast cancer is complex, with multiple interactions between signaling pathways. These interactions promote resistance to trastuzumab and can impact on the chances of successful treatment, but they also provide opportunities for new methods of treating HER2-positive disease. Newly developed agents and treatment strategies, such as the combination of pertuzumab and trastuzumab, and extended HER2 inhibition with trastuzumab followed by neratinib, have further improved upon results seen with trastuzumab, reducing the relapse rate in HER2-positive early breast cancer. With the introduction of any new therapy or regimen, careful attention must be given to the risks versus benefits of therapy. When a novel treatment approach offers a significant improvement on current standards, some additional toxicity may be tolerated. We must take care, however, to ensure that these advances do not come at the cost of significantly impairing patient quality of life. Careful attention should be paid to patient education, prophylaxis against adverse events, and proactive management in order to minimize any unwanted effects associated with HER2-targeted therapy.
Acknowledgments
The authors would like to thank Chelsey Goins, PhD, and Sanneke Koekkoek, RN, for their assistance with the manuscript, and Christi Gray for her editorial assistance. This manuscript was funded by an independent medical education grant from Puma Biotechnology who had no input on the content of the manuscript at any stage in the writing process.
Footnotes
Conflicts of interest
José Baselga has disclosed that he has received consulting fees from Eli Lilly, Grail Inc., and Novartis. He also has ownership interest in Grail, Inc., Infinity Pharmaceuticals, Juno Therapeutics, and PMV Pharma. He has also disclosed leadership roles in Infinity Pharmaceuticals and Varian Medical Systems.
Robert E. Coleman has disclosed that he has performed contracted research for Bayer and Celgene, and he has given expert testimony for Novartis.
Javier Cortés has disclosed that he has received consulting fees from AstraZeneca, Biothera, Celgene, Cellestia Biotech, Roche. He has also received fees for non-CME services directly from Celgene, Eisai, Novartis, Pfizer, and Roche.
Wolfgang Janni has disclosed that he has received consulting fees from Amgen, AstraZeneca, Eisai, MSD, Novartis, Pfizer, and Roche. He has also performed contracted research for Amgen, AstraZeneca, Eisai, MSD, Novartis, Pfizer, and Roche.
References
- AGO Website. Guidelines of the AGO Breast Committee: Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer. 2017 http://www.agoonline.de/fileadmin/downloads/leitlinien/mamma/2017-03/AGO_englisch/PDF_Gesamtdatei_englisch/Updated%20Guidelines_2017.pdf (Accessed July 1, 2017)
- Andre F, O’Regan R, Ozguroglu M, Toi M, Xu B, Jerusalem G, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:580–591. doi: 10.1016/S1470-2045(14)70138-X. [DOI] [PubMed] [Google Scholar]
- Arpino G, Gerrero JM, de la Haba-Rodriguez J, Easton V, Schuhmacher C, Restuccia E, et al. 2016 San Antonio Breast Cancer Symposium. San Antonio, TX: 2016. Dec 6–10, Primary analysis of PERTAIN: A randomized, two-arm, open-label, multicentre phase II trial assessing the efficacy and safety of pertuzumab given in combination with trastuzumab plus an aromatase inhibitor in first-line patients with HER2-positive and hormone receptor-positive metastatic or locally advanced breast cancer. Abstract S3–04. [Google Scholar]
- Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012a;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomized, open-label, multicenter, phase 3 trial. Lancet. 2012b;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J. A new anti-ErbB2 strategy in the treatment of cancer: prevention of ligand-dependent ErbB2 receptor heterodimerization. Cancer Cell. 2002;2:93–95. doi: 10.1016/s1535-6108(02)00098-3. [DOI] [PubMed] [Google Scholar]
- Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900. J Clin Oncol. 2012;30:2585–2592. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med. 2015;7:283ra51. doi: 10.1126/scitranslmed.aaa4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicenter, randomized, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016a;17:367–377. doi: 10.1016/S1470-2045(15)00551-3. [DOI] [PubMed] [Google Scholar]
- Chan A. Neratinib in HER-2-positive breast cancer: results to date and clinical usefulness. Ther Adv Med Oncol. 2016b;8:339–350. doi: 10.1177/1758834016656494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choritz H, Büsche G, Kreipe H. Quality assessment of HER2 testing by monitoring of positivity rates. Virchows Arch. 2011;459:283–289. doi: 10.1007/s00428-011-1132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte PF, Bisagni G, Frassoldati A, Brandes A, Anselmi E, Giotta F, et al. 9 weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: results of the phase III multicentric Italian Short-HER study. J Clin Oncol. 2017;35(suppl) doi: 10.1093/annonc/mdy414. Abstract 501. [DOI] [PubMed] [Google Scholar]
- Dang CT, Yu AF, Jones LW, Liu J, Steingart RM, Argolo DF, et al. Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol. 2016;34:1030–1033. doi: 10.1200/JCO.2015.64.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azambuja E, Holmes AP, Piccart-Gebhart M, Holmes E, Di Cosimo S, Swaby RF, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15:1137–1146. doi: 10.1016/S1470-2045(14)70320-1. [DOI] [PubMed] [Google Scholar]
- De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11:4741–4748. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- Diéras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomized, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- Gajria D, Modi S, Saura C, Sakr R, Solano K, Won H, et al. 2015 San Antonio Breast Cancer Symposium. San Antonio, TX: 2015. Dec 8–12, A Phase I/II Study of Neratinib Plus Temsirolimus in HER2+ Metastatic Breast Cancer Reveals Ongoing HER2 Pathway Dependence in Many Patients Despite Several Lines of HER2-targeted Therapy. Abstract P5-19-04. [Google Scholar]
- Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomized multicenter, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15:640–647. doi: 10.1016/S1470-2045(14)70080-4. [DOI] [PubMed] [Google Scholar]
- Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, et al. Five-year analysis of the phase II NeoSphere trial evaluating four cycles of neoadjuvant docetaxel (D) and/or trastuzumab (T) and/or pertuzumab (P) J Clin Oncol. 2015:33. Abstract 505. [Google Scholar]
- Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, de Azambuja E, Procter M, Suter TM, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomized controlled trial. Lancet. 2013;382:1021–1028. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- Goss PE, Smith IE, O’Shaughnessy J, Ejlertsen B, Kaufmann M, Boyle F, et al. Adjuvant lapatinib for women with early-stage HER2-positive breast cancer: a randomized, controlled, phase 3 trial. Lancet Oncol. 2013;14:88–96. doi: 10.1016/S1470-2045(12)70508-9. [DOI] [PubMed] [Google Scholar]
- Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2- positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30:1989–1995. doi: 10.1200/JCO.2011.39.0823. [DOI] [PubMed] [Google Scholar]
- Harbeck N, Gluz O, Christgen M, Braun M, Kuemmel S, Schumacher C, et al. 2016 San Antonio Breast Cancer Symposium. San Antonio, TX: 2016. Dec 6–10, Final analysis of WSG-ADAPT HER2+/HR+ phase II trial: efficacy, safety, and predictive markers for 12-weeks of neoadjuvant TDM1 with or without endocrine therapy versus trastuzumab + endocrine therapy in HER2-positive hormone receptor-positive early breast cancer. Abstract S5-03. [Google Scholar]
- Hellyer NJ, Cheng K, Koland JG. ErbB3 (HER3) interaction with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochem J. 1998;333:757–763. doi: 10.1042/bj3330757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurvitz SA, Martin M, Symmans WF, Jung KH, Huang CS, Thompson AM, et al. Pathologic complete response rates after neoadjuvant trastuzumab emtansine + pertuzumab vs docetaxel + carboplatin + trastuzumab + pertuzumab treatment in patients with HER2-positive early breast cancer (KRISTINE) J Clin Oncol. 2016;34(suppl) [Google Scholar]
- Hyman DM, Piha-Paul S, Saura C, Arteaga C, Mayer I, et al. 2016 San Antonio Breast Cancer Symposium. San Antonio, TX: 2016. Dec 6–10, Neratinib plus fulvestrant in ERBB2-mutant, HER2-non-amplified, estrogen receptor-positive, metastatic breast cancer: preliminary analysis from the phase II SUMMIT trial. Abstract PD2-08. [Google Scholar]
- Hyman DM, Piha-Paul SA, Rodon J, Saura C, Shapiro GI, Quinn DI, et al. 2017 American Association for Cancer Research Annual Meeting. Washington, DC: 2017. Apr 1–5, Neratinib in HER2-or HER3-mutant solid tumors: SUMMIT, a global, multi-histology, open-label, phase 2 basket study. Abstract 2017; CT001. [Google Scholar]
- Ibrahim E, Tripathy D, Wilkinson M, Hurvitz S, Iannotti N, Kellum A, et al. 2017 American Association for Cancer Research Annual Meeting. Washington, DC: 2017. Apr 1–5, Effects of adding budesonide or colestipol to loperamide prophylaxis on neratinib-associated diarrhea in patients with HER2+ early-stage breast cancer: the CONTROL trial. Abstract CT128. [Google Scholar]
- Jacobs SA, Robidoux A, Garcia JMPMP, Abraham J, La Verde N, Orcutt JMM, et al. 2015 San Antonio Breast Cancer Symposium. San Antonio, TX: 2015. Dec 8–12, NSABP FB-7: a phase II randomized trial evaluating neoadjuvant therapy regimens with weekly paclitaxel plus trastuzumab or neratinib or trastuzumab and neratinib followed by doxorubicin and cyclophosphamide with postoperative trastuzumab in women with locally advanced HER2-positive breast cancer. Abstract PD5-04. [Google Scholar]
- Jankowitz RC, Abraham J, Tan AR, Limentani SA, Tierno MB, Adamson LM, et al. Safety and efficacy of neratinib in combination with weekly paclitaxel and trastuzumab in women with metastatic HER2-positive breast cancer: an NSABP Foundation Research Program phase I study. Cancer Chemother Pharmacol. 2013;72:1205–1212. doi: 10.1007/s00280-013-2262-2. [DOI] [PubMed] [Google Scholar]
- Joensuu H, Bono P, Kataja V, Alanko T, Kokko R, Asola R, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer trial. J Clin Oncol. 2009;27:5685–5692. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- Johnston S, Pippen J, Pivot X, Lichinitser M, Sadeghi S, Dieras V, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R, et al. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem. 2004;279:51141–51147. doi: 10.1074/jbc.M408662200. [DOI] [PubMed] [Google Scholar]
- Lee JW, Soung YH, Seo SH, Kim SY, Park CH, Wang YP, et al. Somatic mutations of ERBB2 kinase domain in gastric, colorectal, and breast carcinomas. Clin Cancer Res. 2006;12:57–61. doi: 10.1158/1078-0432.CCR-05-0976. [DOI] [PubMed] [Google Scholar]
- Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for the therapy of heart failure. Circulation. 2007;116:954–960. doi: 10.1161/CIRCULATIONAHA.107.690487. [DOI] [PubMed] [Google Scholar]
- Martin M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, et al. Neratinib after trastuzumab-based adjuvant therapy in early-stage HER2-positive breast cancer: 5-year analysis of the phase III ExteNET trial. Ann Oncol. 2017;28(Suppl 5) Abstract 1490. [Google Scholar]
- Menendez JA, Schroeder B, Peirce SK, Vellon L, Papadimitropoulou A, Espinoza I, et al. Blockade of a key region in the extracellular domain inhibits HER2 dimerization and signaling. J Natl Cancer Inst. 2015;107:djv090. doi: 10.1093/jnci/djv090. [DOI] [PubMed] [Google Scholar]
- Moreno-Aspitia A, Holmes E, Jackisch C, de Azambuja E, Boyle F, Hillman DW, et al. Updated results from the phase III ALTTO trial (BIG NCCTG/Alliance N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T → L) or their combination (L + T) in the adjuvant treatment of HER2-positive early breast cance. J Clin Oncol. 2017;35(suppl):2–06. Abstract 502. [Google Scholar]
- Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Neratinib +/? Fulvestrant in Metastatic HER2 Non-amplified but HER2 Mutant Breast Cancer. 2017a https://clinicaltrials.gov/ct2/show/NCT01670877 (Accessed July 1, 2017)
- National Institutes of Health. A Study of Trastuzumab Emtansine Versus Trastuzumab as Adjuvant Therapy in Patients with HER2-positive Breast Cancer Who Have Residual Tumor in the Breast or Axillary Lymph Nodes Following Preoperative Therapy (KATHERINE) 2017b https://clinicaltrials.gov/ct2/show/NCT01772472 (Accessed July 1, 2017)
- National Institutes of Health. A Study of Trastuzumab Emtansine Plus Pertuzumab Following Anthracyclines in Comparison with Trastuzumab Plus Pertuzumab and a Taxane Following Anthracyclines as Adjuvant Therapy in Participants with Operable HER2-positive Primary Breast Cancer. 2017c https://clinicaltrials.gov/ct2/show/NCT01966471 (Accessed July 1, 2017)
- Park JW, Liu MC, Yee D, Yau C, van’t Veer LJ, Symmans WF, et al. Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375:11–22. doi: 10.1056/NEJMoa1513750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez EA, Barrios C, Eiermann W, Toi M, Im YH, Conte P, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35:141–148. doi: 10.1200/JCO.2016.67.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Holmes E, Baselga J, de Azambuja E, Dueck AC, Vialeet G, et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol. 2016;34:1034–1042. doi: 10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomized phase 3 trial. Lancet Oncol. 2013;14:741–748. doi: 10.1016/S1470-2045(13)70225-0. [DOI] [PubMed] [Google Scholar]
- Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- Rimawi MF, Mayer IA, Forero A, Nanda R, Goetz MP, Rodriguez AA, et al. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006. J Clin Oncol. 2013;31:1726–1731. doi: 10.1200/JCO.2012.44.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robidoux A, Tang G, Rastogi P, Geyer CE, Azar CA, Atkins JN, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomized, phase 3 trial. Lancet Oncol. 2013;14:1183–1192. doi: 10.1016/S1470-2045(13)70411-X. [DOI] [PubMed] [Google Scholar]
- Sánchez-Martín M, Pandiella A. Differential action of small molecule HER kinase inhibitors on receptor heterodimerization: therapeutic implications. Int J Cancer. 2012;131:244–252. doi: 10.1002/ijc.26358. [DOI] [PubMed] [Google Scholar]
- Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24:2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Eiermann W, Robert N, et al. Phase iii randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (ac → t) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (ac → th) with docetaxel, carboplatin and trastuzumab (tch) in her2 positive early breast cancer patients: bcirg 006 study. Breast Cancer Res Treat. 2005;94(suppl 1) Abstract 1. [Google Scholar]
- Slamon DJ, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systemic review and meta-analysis of randomized controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Ewer MS, Cortés J, Amadori D, Miles D, Clark E, et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist. 2013;18:257–264. doi: 10.1634/theoncologist.2012-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toska E, Osmanbeyoglu HU, Castel P, Chan C, Hendrickson RC, Elkabets M, et al. PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science. 2017;355:1324–1330. doi: 10.1126/science.aah6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, et al. A hierarchical network of interreceptor interactions determines signal transduction by neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JW, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomized phase 3 trial. Lancet Oncol. 2012;13:135–144. doi: 10.1016/S1470-2045(11)70397-7. [DOI] [PubMed] [Google Scholar]
- Ustaris F, Saura C, Di Palma J, Bryce R, Moran S, Neuman L, et al. Effective management and prevention of neratinib-induced diarrhea. Am J Hematol Oncol. 2015;11:13–22. [Google Scholar]
- Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eiermann H, Eirmann W, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013;31:3623–3630. doi: 10.1200/JCO.2012.45.0940. [DOI] [PubMed] [Google Scholar]
- Von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017 doi: 10.1056/NEJMoa1703643. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Bacus S, Hegde P, Husain I, Strum J, Liu L, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci USA. 2006;103:7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, et al. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]


