Abstract
Objectives
Intrapartum-related complications are the second leading cause of neonatal death worldwide. We estimate the community-level risk and burden of intrapartum-related fetal/neonatal mortality and morbidity associated with non-cephalic and multiple birth in rural Sarlahi District, Nepal.
Design
Community-based prospective cohort study.
Setting
Rural Sarlahi District, Nepal.
Participants
Pregnant women residing in the study area.
Methods
We collected data on maternal background characteristics, conditions during labour and delivery, fetal presentation and multiple birth during home visits. We ran log-binomial regression models to estimate the associations between non-cephalic/multiple births and fresh stillbirth, early neonatal mortality and signs of neonatal encephalopathy, respectively, and calculated the per cent attributable fraction. To better understand the context under which these adverse birth outcomes are occurring, we also collected data on maternal awareness of non-cephalic presentation and multiple gestation prior to delivery.
Primary outcome measures
Risk of experiencing fresh stillbirth, early neonatal encephalopathy and early neonatal mortality associated with non-cephalic and multiple birth, respectively.
Results
Non-cephalic presentation had a particularly high risk of fresh stillbirth (aRR 12.52 (95% CI 7.86 to 19.95), reference: cephalic presentation). 20.2% of all fresh stillbirths were associated with non-cephalic presentation. For multiple births, there was a fourfold increase in early neonatal mortality (aRR: 4.57 (95% CI 1.44 to 14.50), reference: singleton births). 3.4% of early neonatal mortality was associated with multiple gestation.
Conclusions
Globally and in Nepal, a large percentage of stillbirths and neonatal mortality is associated with intrapartum-related complications. Despite the low incidence of non-cephalic and multiple birth, a notable proportion of adverse intrapartum-related outcomes is associated with these conditions. As the proportion of neonatal deaths attributable to intrapartum-related complications continues to rise, there is a need to investigate how best to advance diagnostic capacity and management of these conditions.
Trial registration number
NCT01177111; pre-results.
Keywords: multiple gestation, non-cephalic presentation, breech, Nepal, neonatal mortality, stillbirth
Strengths and limitations of this study.
Contrary to many previous studies conducted in tertiary facility-based settings, this study provides a representative, population-based estimate of adverse intrapartum-related outcomes associated with non-cephalic and multiple birth in rural Nepal.
Despite the low incidence of non-cephalic and multiple birth, we show that the two conditions are associated with a notable proportion of adverse intrapartum-related birth outcomes.
We collected data during pregnancy and immediately following birth (84% of women visited at home within 3 days of birth), allowing us to minimise recall bias.
The outcome of neonatal encephalopathy was based on clinical signs as reported by the mother. This outcome variable will be impacted by subjectivity.
We were not able to collect information on indications for caesarean section; thus, caesarean section births were excluded from the fetal presentation analysis.
Background
It is estimated that 662 000 neonatal deaths and1 1.3 million stillbirths2 occur annually due to intrapartum-related complications, or complications during labour and delivery. Of the 303 000 maternal deaths3 that occur each year, 20.6% occur from intrapartum and postpartum haemorrhage, 2.8% from complications during labour and delivery, and another 2.8% due to obstructed labour.4 Deaths and morbidities combined, intrapartum-related complications account for the loss of 74.6 million disability-adjusted life years (excludes stillbirths) according to 2012 estimates.5 Early identification and management of childbirth complications, such as obstructed labour or malpresentation, and access to a skilled birth attendant are effective in reducing intrapartum-related mortality.6 An estimated 60 million women still deliver at home globally, of whom over 85% deliver without a skilled birth attendant present.7
Intrapartum-related complications can occur without warning among the healthiest mothers, but several risk factors are identifiable antenatally. Non-cephalic presentation and multiple gestation are two such conditions. These conditions can lead to adverse birth outcomes by causing prolonged labour and limiting fetal oxygenation, or by causing fetal entrapment.8 While the association between these conditions and adverse birth outcomes in low and middle income countries (LMICs) has been reported previously in tertiary facility-based settings,9–12 the study population is unlikely to be representative. Very few studies13 14 have reported on the community-level risks in a context where home delivery rates are still high.
We conducted a prospective cohort study in rural Nepal to examine non-cephalic presentation and multiple gestation as potential risk factors for adverse intrapartum-related fetal and neonatal outcomes. Understanding the burden of mortality and morbidity associated with these conditions and the current standard of care could provide more generalisable evidence to justify improved diagnostic capacity, better quality of care at birthing facilities and increased access to quality, comprehensive emergency obstetric care in low-resource settings.
Methods
The study was conducted from March 2014 to March 2015 in rural Sarlahi District, Nepal. Sarlahi District is located in the southern terai plains of the country, bordering the Indian state of Bihar on the south. The data were collected as a part of the Nepal Oil Massage Study, a community-based cluster-randomised controlled trial examining the impact of sunflower seed oil massage on neonatal mortality and morbidity, compared with traditional mustard seed oil massage (Clinicaltrials.gov NCT01177111). The intervention of the trial occurred after the birth of the infant; thus, we do not expect any impact on the results of this study, which looks at outcomes proximate to the time of delivery. While the main study began enrolment in November 2010, data on fetal presentation were collected only starting March 2014.
Field workers initially surveyed our study area to identify all married women of reproductive age (15–40 years of age). Subsequently, these women were visited at home every 5 weeks to identify any new pregnancies. A pregnancy test was provided if the women reported no menstrual period since the prior visit. Positive tests led to recruitment and consent to study participation, followed by the collection of the woman's reproductive and pregnancy history, anthropometric measurements and socioeconomic status. Households were instructed to notify the study staff of delivery as soon as possible. On the first home visit following delivery, data on labour and delivery conditions and neonatal vital status and anthropometry were collected. Neonatal and maternal vital status and morbidity were ascertained on home visits on days 1, 3, 7, 14, 21 and 28.
The main exposures of interest were non-cephalic presentation and multiple birth. We categorised maternal report of fetal presentation as cephalic (head presenting first), non-cephalic (feet, umbilical cord, arm or buttocks presenting first) or caesarean section (C-section). For those presenting in cephalic presentation, we did not seek further clarification regarding face or brow presentation. Those who underwent C-section were excluded from the analysis on fetal presentation, as we did not collect information on the indications for C-section. For fetal presentation, a sensitivity analysis was conducted excluding and including multiple births in the analysis. Any pregnancy with more than one fetus was categorised as multiple birth (whether live or stillborn), and those missing data on fetal presentation (including C-sections) were included in the multiple birth analysis.
We examined outcomes that are most proximate to the time of labour and delivery: fresh stillbirth, defined as a stillbirth with maternal self-report of the fetal skin not being pulpy (assumed to have occurred within 12 hours prior to delivery), first-day neonatal mortality and early neonatal mortality (within the first 7 days of life). We also examined neonatal encephalopathy as an adverse outcome. Neonatal encephalopathy is identified and staged by severity using clinical and EEG criteria.15 However, owing to lack of medical equipment access and the use of non-clinicians for data collection, we applied a basic clinical sign-based definition: report of convulsions/seizure or two of the following: lethargy, poor suck or respiratory rate <40 breaths/min.16 The signs were based on maternal report except respiratory rate, which was measured by a trained staff member. Only those who had morbidity data collected at least once in the early neonatal period were categorised for neonatal encephalopathy, including those who later experienced early neonatal death. We also created an aggregate ‘any adverse outcome’ variable of having any of the aforementioned outcomes. Maternal deaths as an outcome were also examined, but were excluded due to data limitations and small sample size. Ten maternal deaths occurred during the data collection period, with corresponding fetal / neonatal outcomes of three miscarriages, three fresh stillbirths and four live births. Fetal presentation data were not collected for maternal death cases. The findings on stillbirths and live births in relation to fetal presentation were based on data from singleton pregnancies. Data on single or multiple gestation were not collected for miscarriages.
On the first data collection visit following delivery, we also asked women who gave non-cephalic or multiple birth whether they were aware of the condition prior to delivery, how they found out and whether any birth preparations were made in the light of their knowledge.
Analysis
We summarised the rates of fresh stillbirth, first-day mortality, early neonatal mortality, neonatal encephalopathy and any of the above outcomes, comparing cephalic versus non-cephalic births, and singleton versus multiple births, respectively. Using those outcomes as dependent variables, we estimated unadjusted and adjusted risk ratios (RR) using log-binomial regression for fetal presentation and log-binomial regression with generalised estimating equations17 to account for clustering within twin pairs for multiple birth. For the outcome of ‘any of the above’, the Poisson regression model with robust variance was used for both risk factors due to convergence issues.18 We included the following prespecified potential confounders in the models: preterm (defined as gestational age <37 completed weeks), facility birth (reference: home birth), maternal height <145 cm, neonatal weight <2500 g (excluded for fresh stillbirth and the aggregate ‘any adverse outcome’, as weight was not collected for stillbirths, and only included among neonates whose weights were taken within 72 hours of birth), primiparity, maternal education and maternal age at marriage, the last two as indicators of socioeconomic status.
Gestational age was calculated using the date of the last menstrual period, which was collected from maternal recall at enrolment. Using the estimated RRs and incidence data, we also calculated the per cent attributable fractions (PAF) of the outcomes that are associated with non-cephalic presentation and multiple birth, respectively. PAFs were calculated only for outcomes with statistically significant RRs; the counterfactual was the RR for non-cephalic presentation or multiple birth being set to one (equivalent to no increased risk) while the incidence remained the same. The following equation was used:
 |
1 |
Where, Pi is the proportion of population at exposure level i; RR, the risk ratio at exposure level i; RR’i, the risk ratio at exposure level i, counterfactual or ideal level of risk; n, the number of exposure levels.
We tabulated the results regarding prior knowledge and birth preparation among mothers who experienced non-cephalic and multiple births, respectively. We used Stata V.13.0 (Stata Corp.) for analysis.
We obtained ethical approval from the Institutional Review Boards of the Johns Hopkins Bloomberg School of Public Health in the USA and the Tribhuvan University Institute of Medicine in Nepal, respectively.
Results
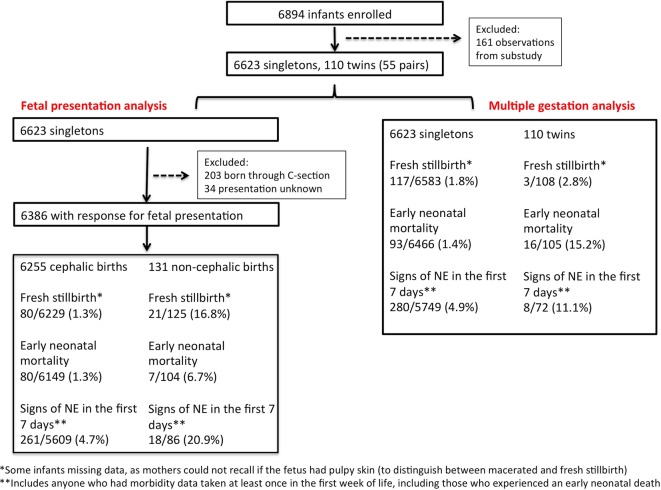
Data from 6894 births were collected between March 2014 and March 2015. One hundred and sixty-one births (2.3%) were excluded as their mothers were enrolled in a separate study that provided home-based obstetric ultrasound exams in the third trimester,19 leaving 6623 singletons and 110 twins (55 pairs). The ultrasound study operated for a total of 14 weeks out of the 1-year data collection period for this study, rotating through 7 predesignated VDCs out of 34 total VDCs in the parent study. Using an estimated 3% incidence of non-cephalic birth, along with a type I error of 0.05 (two-sided test), a power of 0.90, a 3% loss to follow-up and assuming a 5% perinatal mortality rate among cephalic births, we had the power to detect a risk ratio of 2.19 of perinatal mortality, comparing non-cephalic with cephalic births.
Maternal characteristics are provided in table 1, and a data flow chart is available in figure 1. The mean gestational age at which maternal background data were collected was 21.9 weeks (IQR: 12.6–30.9 weeks). Of the enrolled mothers, 84% received their first postdelivery data collection visit within 72 hours of delivery and 86% within 1 week of delivery.
Table 1.
Characteristics of study participants (n=6705, includes C-sections)
| By fetal presentation (singleton, vaginal births only) |
By gestation |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cephalic birth |
Non-cephalic birth |
Single gestation |
Multiple gestation |
|||||
| Characteristic | n | % or mean (SD) | n | % or mean (SD) | n | % or mean (SD) | n* | % or mean (SD) |
| Maternal age at delivery | ||||||||
| <18 years old | 529 | 8.5 | 14 | 10.7 | 561 | 8.5 | 1 | 1.8 |
| 18−<35 years old | 5555 | 88.8 | 113 | 86.3 | 5879 | 88.8 | 52 | 84.6 |
| ≥35 years old | 170 | 2.7 | 4 | 3.1 | 182 | 2.8 | 2 | 3.6 |
| Maternal gravidity (excluding this pregnancy) | ||||||||
| 0 | 1773 | 28.4 | 48 | 36.6 | 1911 | 28.9 | 6 | 10.9 |
| 1–2 | 2892 | 46.2 | 62 | 47.3 | 3060 | 46.2 | 25 | 45.5 |
| 3–4 | 1210 | 19.3 | 14 | 10.7 | 1254 | 18.9 | 18 | 32.7 |
| ≥5 | 380 | 6.1 | 7 | 5.3 | 398 | 6.0 | 6 | 10.9 |
| Facility delivery rate | 6253 | 43.4 | 131 | 53.4 | 6618 | 45.4 | 54 | 46.3 |
| Location of delivery | ||||||||
| At home | 2461 | 39.4 | 35 | 26.7 | 2511 | 37.9 | 17 | 31.5 |
| At her maternal home | 938 | 15.0 | 17 | 13.0 | 957 | 14.5 | 10 | 18.5 |
| At a health post/clinic | 1941 | 31.0 | 37 | 28.2 | 2042 | 30.9 | 15 | 27.8 |
| At a hospital | 774 | 12.4 | 33 | 25.2 | 959 | 14.5 | 10 | 18.5 |
| On the way to a facility | 132 | 2.1 | 9 | 6.9 | 142 | 2.2 | 2 | 3.7 |
| Outdoors | 7 | 0.1 | 0 | 0.0 | 7 | 0.1 | 0 | 0 |
| Maternal education | 2.5 (4.0) | 2.0 (3.7) | ||||||
| No years | 4292 | 68.6 | 96 | 73.3 | 4511 | 68.1 | 44 | 80.0 |
| 1–9 years | 1265 | 20.2 | 26 | 19.9 | 1348 | 20.4 | 8 | 14.6 |
| ≥10 years | 697 | 11.1 | 9 | 6.9 | 763 | 11.5 | 3 | 5.5 |
| Ethnicity | ||||||||
| Madheshi | 6016 | 96.5 | 129 | 98.5 | 6359 | 96.3 | 54 | 98.2 |
| Pahadi | 218 | 3.5 | 2 | 1.5 | 243 | 3.7 | 1 | 1.8 |
| Maternal height | 150.7 (5.6) | 150.5 (6.1) | ||||||
| 145–<150 cm | 881 | 14.1 | 22 | 16.8 | 937 | 14.2 | 7 | 12.7 |
| 150–<155 cm | 1857 | 29.7 | 36 | 27.5 | 1958 | 29.6 | 10 | 18.2 |
| 150–155 cm | 2151 | 34.4 | 42 | 32.1 | 2278 | 34.4 | 24 | 43.6 |
| ≥155 cm | 1358 | 21.7 | 31 | 23.7 | 1442 | 21.8 | 14 | 25.5 |
| Birth outcome | n | % or mean (SD) | n | % or mean (SD) | n | % or mean (SD) | n† | % or mean (SD) |
| Birth weight‡: low birth weight (<2500 g) | 5349 | 2706 (413): 29.7 | 77 | 2437 (400): 57.1 | 5448 | 2702 (414): 30.1 | 62 | 2042 (511): 85.5 |
| Gestational age§: preterm birth (<37 weeks) | 6159 | 39.2 (2.7): 25.4 | 126 | 38.3 (3.6): 23.9 | 6519 | 39.2 (2.8): 17.0 | 108 | 36.3 (3.4): 57.4 |
| Small-for-gestational-age¶ | 4925 | 41.6 | 71 | 60.6 | 5016 | 41.9 | 62 | 58.1 |
| Non-cephalic presentation (excluding C-sections and unknowns) | 6386 | 2.1 | 82 | 15.9 | ||||
*Unit=mother.
†Unit=child.
‡Limited to weights taken within 72 hours of birth.
§Limited to feasible GA, between 24 and 46 weeks.
¶Intergrowth standard, cut-offs available for 24–<43 weeks GA.
Figure 1.
Flow diagram of enrolment and follow-up of participants for adverse pregnancy outcomes among non-cephalic and multiple birth in rural Nepal.
For singletons, 6387 births had a valid response regarding fetal presentation after excluding women who underwent a C-section (n=202 out of 6705, 3.0%) and those reporting unknown fetal presentation (n=34 out of 6705, 0.5%). Of singleton fetuses, 2.1% were reported as being in non-cephalic presentation at the time of birth. Compared with term (≥37 weeks) infants, the rate of non-cephalic presentation was statistically significantly higher among preterm (<37 weeks) than term births (3.1% vs 1.8%, p<0.001).
The adverse outcome rates were as follows for singleton non-cephalic infants: a fresh stillbirth rate of 168/1000 births compared with 13/1000 births among cephalic infants, an early neonatal mortality rate of 67/1000 live births compared with 13/1000 live births and a neonatal encephalopathy rate of 20.9% compared with 4.7%. Among the 111 non-cephalic singleton births with data on stillbirth and mortality and morbidities through the first week of life, 39.6% experienced an adverse outcome; among the 5720 cephalic singleton births, 7.0% experienced an adverse outcome (table 2).
Table 2.
Crude fetal and neonatal mortality/morbidity rates, by fetal presentation and singleton/multiple birth status
| Fetal presentation at delivery (singleton, vaginal births only) |
Multiple births* |
|||||
|---|---|---|---|---|---|---|
| Cephalic | Non-cephalic | p Value | Singleton | Twins | p Value | |
| Fresh stillbirth | 80/6229 (13 per 1000 births) | 21/125 (168 per 1000 births) | <0.001 | 117/6583 (18 per 1000 births) | 3/108 (28 per 1000 births) | 0.437 |
| First-day mortality | 42/6149 (7 per 1000 live births) | 5/104 (48 per 1000 live births) | <0.001 | 53/6466 (8 per 1000 live births) | 5/105 (48 per 1000 live births) | <0.001 |
| Early neonatal mortality | 80/6149 (13 per 1000 live births) | 7/104 (67 per 1000 live births) | <0.001 | 93/6466 (14 per 1000 live births) | 16/105 (152 per 1000 live births) | <0.001 |
| Showing symptoms of neonatal encephalopathy† | 261/5609 (4.7% of live births) | 18/86 (20.9% of live births) | <0.001 | 280/5749 (4.9% of live births) | 8/72 (11.1% of live births) | 0.015 |
| Any of the above outcomes‡ | 402/5720 (7.0% of births) | 44/111 (39.6% of births) | <0.001 | 469/5906 (7.9% of births) | 24/83 (28.9% of births) | <0.001 |
*There were no triplets in our study.
†Must have had at least one assessment of clinical signs of neonatal encephalopathy prior to day 7. About 7.6% of cephalic births and 11.3% of non-cephalic births alive at day 7 had no assessment prior to day 7. About 9.8% of singletons and 19.1% of twins alive at day 7 had no assessment prior to day 7.
‡Includes births with data on stillbirth status and mortality/morbidity through day 7. About 91.8% of all cephalic births and 88.8% of all non-cephalic births contributed data. About 89.7% of all singleton births and 76.9% of all multiple births contributed data.
The twinning rate was 8.2 out of 1000 pregnancies. No triplets were reported. The adverse outcome rates were as follows for multiple births: a fresh stillbirth rate of 28/1000 births compared with 18/1000 births among singleton infants, an early neonatal mortality rate of 152/1000 live births compared with 14/1000 live births among singleton infants and a neonatal encephalopathy rate of 11.1% compared with 4.9% among singleton infants. For the 83 twins with data on stillbirth and mortality and morbidities through the first week of life, 28.9% experienced an adverse outcome; among the 5906 singleton births, 7.9% experienced an adverse outcome (table 2).
The non-cephalic presentation rate was 13.5% for first twins and 20.0% for second twins. Twins who were non-cephalic had an adverse outcome rate of 41.7% compared with twins who were cephalic with a rate of 22.6%.
Fetal presentation among singletons
We estimated a fivefold increased risk of adverse outcomes for non-cephalic singletons compared with cephalic singletons (aRR 4.85 (95% CI 3.72 to 6.32)). When including multiple births and their individual presentation at birth into the analysis, the association did not change significantly (aRR 4.74 (95% CI 3.69 to 6.08)). Non-cephalic presentation had the strongest association with fresh stillbirth, with a 13-fold increase in risk (aRR 12.52 (95% CI 7.86 to 19.95)) (table 3). About 20.2% of fresh stillbirths, 4.6% of neonatal encephalopathy and 7.8% of adverse outcomes were associated with non-cephalic presentation.
Table 3.
Adjusted risk ratios (aRR) between non-cephalic presentation/potential confounding variables and adverse intrapartum-related fetal/neonatal outcomes among singletons
| Fresh stillbirth aRR (95% CI) | Early neonatal mortality aRR (95% CI) | Signs of neonatal encephalopathy (early neonatal period) aRR (95% CI) | Any of the aforementioned conditions aRR (95% CI) | |
|---|---|---|---|---|
| Non-cephalic presentation | 12.52 (7.86 to 19.95) | 1.42 (0.20 to 10.21) | 3.20 (1.87 to 5.46) | 4.85 (3.72 to 6.32) |
| Preterm | 1.87 (1.22 to 2.87) | 2.04 (0.96 to 4.33) | 0.82 (0.57 to 1.18) | 1.43 (1.16 to 1.76) |
| Facility delivery (reference: home delivery) | 0.93 (0.62 to 1.41) | 0.98 (0.46 to 2.08) | 1.62 (1.25 to 2.10) | 1.53 (1.27 to 1.84) |
| Maternal stature <145 cm | 1.25 (0.75 to 2.09) | 1.95 (0.89 to 4.29) | 1.52 (1.11 to 2.07) | 1.42 (1.14 to 1.78) |
| Low birth weight (<2500 g)* | — | 5.11 (2.19 to 11.89) | 1.02 (0.77 to 1.34) | — |
| Primiparous | 1.27 (0.83 to 1.95) | 1.06 (0.48 to 2.37) | 1.43 (1.09 to 1.88) | 1.34 (1.10 to 1.63) |
| Education (reference: no education) | ||||
| 1–9 years | 1.28 (0.79 to 2.09) | 0.58 (0.17 to 1.97) | 0.80 (0.58 to 1.12) | 0.87 (0.69 to 1.10) |
| ≥10 years | 0.69 (0.29 to 1.65) | 1.47 (0.41 to 5.28) | 0.70 (0.44 to 1.12) | 0.61 (0.43 to 0.88) |
| Mother's age at marriage (reference: ≥18 years) | ||||
| <18 years | 1.11 (0.69 to 1.80) | 2.57 (0.86 to 7.70) | 1.01 (0.76 to 1.35) | 1.03 (0.84 to 1.27) |
*Newborn weight is not included for stillbirth-related outcomes.
Multiple births
Multiple birth was not statistically significantly associated with fresh stillbirth, but had a close to fivefold increased risk of early neonatal mortality (aRR 4.57 (95% CI 1.44 to 14.50)). Multiple births had a marginally significant increased risk of experiencing signs of neonatal encephalopathy (aRR 2.26 (95% CI 0.97 to 5.26)). Multiple birth was associated with 3.4% of early neonatal mortality and 2.0% of adverse outcomes (table 4).
Table 4.
Adjusted risk ratios between multiple birth/potential confounding variables and adverse intrapartum-related fetal/neonatal outcomes, accounting for clustering within twin pairs
| Fresh stillbirth aRR (95% CI) | Early neonatal mortality aRR (95% CI) | Signs of neonatal encephalopathy (early neonatal period) aRR (95% CI) | Any of the aforementioned conditions aRR (95% CI) | |
|---|---|---|---|---|
| Multiple birth | 1.20 (0.39 to 3.71) | 4.57 (1.44 to 14.50) | 2.26 (0.97 to 5.26) | 3.01 (1.90 to 4.78) |
| Preterm | 2.17 (1.46 to 3.23) | 1.93 (0.93 to 3.99) | 0.85 (0.60 to 1.21) | 1.59 (1.31 to 1.94) |
| Facility delivery (reference: home delivery) | 1.17 (0.80 to 1.71) | 0.89 (0.43 to 1.83) | 1.66 (1.29 to 2.14) | 1.62 (1.36 to 1.94) |
| Maternal stature <145 cm | 1.42 (0.89 to 2.27) | 1.63 (0.75 to 3.53) | 1.46 (1.07 to 2.00) | 1.44 (1.16 to 1.79) |
| Low birth weight (<2500 g)* | — | 5.42 (2.35 to 12.49) | 1.07 (0.82 to 1.40) | — |
| Primiparous | 1.17 (0.77 to 1.76) | 1.00 (0.46 to 2.20) | 1.43 (1.09 to 1.88) | 1.33 (1.09 to 1.61) |
| Education (reference: no education) | ||||
| 1–9 years | 1.07 (0.68 to 1.70) | 0.52 (0.15 to 1.73) | 0.77 (0.56 to 1.08) | 0.83 (0.66 to 1.04) |
| ≥10 years | 0.57 (0.25 to 1.28) | 1.89 (0.60 to 5.95) | 0.72 (0.46 to 1.13) | 0.59 (0.42 to 0.84) |
| Mother’s age at marriage (reference: ≥18 years) | ||||
| <18 years | 1.11 (0.71 to 1.72) | 2.43 (0.88 to 6.69) | 1.04 (0.78 to 1.39) | 1.05 (0.86 to 1.29) |
*Newborn weight is not included for stillbirth-related outcomes.
For the outcome of first-day mortality, adjusted RRs were not estimated for either risk factor due to the low rate of the outcome.
Awareness of condition prior to start of labour
Among the mothers who experienced a singleton non-cephalic delivery (n=131), only 25.4% were aware of their condition prior to the start of labour. Among these, a majority (67.6%) knew through an ultrasound examination, 17.6% through self-examination and the remaining through physical examination by a health worker (multiple responses were allowed). Among the 34 who knew, 23.5% did not make any particular birth preparation. When including the mothers who were not aware of fetal presentation prior to the start of labour, only 19.8% made birth preparations (see online supplementary table S1). The facility delivery rate was higher (70.6% vs 49.0%, p=0.029) and the rate of adverse outcomes was lower among those who knew the presentation prior to delivery compared with those who did not (21.4% vs 45.8%, p=0.023). The aRR of adverse outcomes for non-cephalic presentation was 2.74 (95% CI 1.40 to 5.36) among women who knew and 4.99 (95% CI 3.91 to 6.38) among those who did not, both compared with cephalic presentation.
bmjopen-2016-013099supp001.pdf (68KB, pdf)
Among mothers who had multiple births (n=55), only 36.4% were aware of the condition before labour started. Among these, 80.0% knew through an ultrasound examination. Among the 20 individuals who knew, 35.0% did not make any birth preparation. When including the mothers who were not aware prior to the start of labour, only 23.6% made preparations for multiple birth (see online supplementary table S2).
Discussion
Our population-based study reported a high risk of adverse birth outcomes associated with non-cephalic presentation and multiple gestation, respectively, in a rural area with poor access to emergency obstetric facilities. Despite the low incidence of these conditions, a substantial proportion of adverse intrapartum-related outcomes are associated with these conditions; we estimated that 20.2% of fresh stillbirths are associated with non-cephalic presentation and 3.4% of early neonatal mortality is associated with multiple gestation. The proportion of neonatal mortality attributable to intrapartum-related complications has been on the rise around the globe,1 and if this trend continues, the conditions we focused on here will become even more important targets for intervention.
Most LMIC studies exploring the risks associated with these conditions have been conducted in tertiary facility-based settings. The results we present here are more reflective of the true population-level burden, especially in a context like rural Nepal where about half of the women still deliver at home.20 Also, our findings are likely to be an underestimate of the health burden associated with these two conditions. Developed country data suggest that there could be additional morbidities that we were unable to capture in our data, such as fractures and spinal cord injury for the fetus/neonate and genital tract lacerations for the mother.21 We had few reports of maternal deaths in our study; we expected low numbers in relation to our sample size, given the maternal mortality ratio of 258 (year 2015) in Nepal.22 Other studies have reported high maternal mortality among those experiencing non-cephalic presentation or multiple gestation. For example, a Nigerian study reported a maternal mortality ratio of 895 maternal deaths per 100 000 live births associated with twinning.12
The associations we present between non-cephalic/multiple birth and adverse pregnancy outcomes may not all be directly causal. For instance, congenital abnormalities and placenta previa are associated with non-cephalic birth, and the causal mechanism may be operating through those conditions. Similarly, non-cephalic and multiple births are associated with preterm birth. We attempted to control for preterm birth in our regression models. While acknowledging this potential misattribution of causation, these two conditions may serve as signs for both direct and indirect causal mechanisms that would benefit greatly from timely referral and facility-based care.
One important step to addressing these conditions would be to improve diagnostic capacity and access so women and her household members have timely awareness of the acute risk. We identified low awareness of non-cephalic presentation or multiple gestation prior to the time of delivery among women who reported experiencing one or the other. These two conditions are only accurately diagnosable by ultrasound. Abdominal palpation methods have shown variable results in accuracy.23–25 None of the public birthing centres in Sarlahi District currently have sonographic capacity and the dearth of this capacity can be attributable to human resource and equipment cost. Several studies from developing countries have shown success with shifting sonographic diagnostic tasks to non-radiologist clinicians.26–28 A study conducted at the same study site showed that auxiliary nurse midwives, who are high school graduates with 18 months of midwifery training, can accurately diagnose non-cephalic presentation and multiple gestation using ultrasonography, with just 2 weeks of training.19 Groups are also exploring whether sonographic technology can be altered to meet the cost and staffing barriers in low-resource settings.29 Expanding ultrasonographic access will require appropriate regulation. Currently, many unregulated private clinics operate in South Asia, leading to concerns of inaccurate diagnoses and illegal fetal sex determination. Better incorporation of ultrasonography into the formal healthcare system may help with regulation, but there will still need to be political will at the national, district and community level to systematically address this issue and to allow appropriate clinical use of the technology.
For risk screening to improve health outcomes, access to and quality of facility-based care needs to be improved as well. Major barriers to accessing facility-based intrapartum care remain in low-income countries, such as cost, transport and cultural acceptability. Reduction or elimination of user fees is one type of intervention that has been tested in LMICs and has succeeded in increasing facility delivery rates.30 In Nepal, in addition to the elimination of user fees for antenatal and intrapartum care, a conditional cash transfer system awards cash to women who attend four antenatal care visits and also to women who have a facility delivery.31 A study conducted in a nearby district has shown, however, that these programmes have inequitably benefited the wealthier strata of society.32 The costs incurred prior to receiving the cash, such as that of the woman or household members getting to and staying near a facility, remain prohibitive for many.
We expect these barriers to be more pronounced when trying to reach higher-level facilities that can handle complications and have C-section capacity. Detection and management of breech births using C-section has previously been identified as an intervention that could reduce neonatal mortality rates in LMICs.33 Of those in our study who had a facility delivery, a large majority delivered in a health post or a health clinic. Health posts and clinics should have skilled birth attendants, but do not have the capacity to handle complicated deliveries. The nearest referral facility with C-section capacity from our study area is located 3–4 hours away by car. This level of access fails to meet the recommended ratio of one comprehensive emergency obstetric care (CEmOC) facility per 500 000 population.
Even after reaching the facility, quality of care is a major concern, posing the very critical question of whether a referral in actuality gives the woman and her child better chances of survival. A review indicated that among women admitted into a facility for complications in LMICs, the mean wait time was ∼24 hours, with major causes of delays being shortage of treatment materials, surgery facilities and qualified staff.34 In addition to the clinical quality of care, disrespectful care serves as a major deterrent. A landscape review conducted on respectful care highlighted seven types of disrespect and abuse women and their family members incur at facilities during childbirth: physical abuse, non-consented care, non-confidential care, non-dignified care, discrimination, abandonment of care and detention in facilities.35 In a separate qualitative study conducted in our study area, many female focus group participants reported hearing of physically abusive intrapartum care (Under review). These quality issues put into question the ethics of referral, taking into account the great burden posed on families to complete such referrals. For referrals to successfully improve health outcomes, the complexity of household-side and facility-side barriers needs to be acknowledged and addressed.
There are several limitations to our study. Some of our variables are based on maternal recall. For the outcome of neonatal encephalopathy, we most likely overestimate the incidence with the use of non-specific clinical signs, as we could not use neuroimaging equipment for diagnosis in this context. Furthermore, not all children had a morbidity assessment conducted within the first 7 days of life and clinical signs such as poor suck and lethargy may be subject to reporting bias and are also subjective. Given the poor record keeping in facilities and the fact that about half of the women had a facility delivery, we were unable to validate any of the self-reported variables against clinical records. For self-report of fetal presentation, we believe there is minimal miscategorisation. Family members who were present at the time of delivery were able to provide insight during the data collection interview, and a separate study on health indicator validity from Mozambique reported high validity of maternal recall of fetal presentation.36 We did not collect data on the indications for C-section. Several women may have undergone the C-section because of non-cephalic or multiple birth. By excluding those cases, we may be overestimating the risk of non-cephalic birth or multiple birth and underestimating the incidence of these conditions, although it is unlikely to be of a large magnitude, given that only 3% of deliveries were by C-section. We used the date of the last menstrual period to calculate gestational age. We expect the error to be small due to the five-weekly home visits that were made to identify missed menstrual periods, but we still expect some misclassification of preterm births. We were unable to confirm gestational age using any other clinical or ultrasonographic methods in this setting.
Conclusions
Non-cephalic and multiple births have significantly increased the risk of adverse intrapartum-related pregnancy outcomes. Despite the low incidence, the per cent of adverse pregnancy outcomes attributable to these two factors is considerable. These findings may justify investments in screening programmes to identify these women prior to the time of delivery and to appropriate improvements to quality of and access to tertiary care. Subsequent studies must explore if and how early diagnosis impacts institutional delivery rates and subsequent health outcomes.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the staff of Nepal Nutrition Intervention Project—Sarlahi (NNIPS).
Footnotes
Contributors: NK made primary contributions to the design, conduct, analysis and interpretation of the research. JK and LCM contributed to the study design, data analysis plan and interpretation of the results. SKK, JMT and SCL contributed to the conduct of the research and interpretation of the results.
Funding: The study was supported by the National Institutes of Health/National Institute of Child Health and Human Development (1R01HD060712-01), the Bill and Melinda Gates Foundation (OPP1084399) and the Children's Prize.
Disclaimer: The funders had no involvement in the design or implementation of the study.
Competing interests: None declared.
Ethics approval: Johns Hopkins Bloomberg School of Public Health, Tribhuvan University Institute of Medicine.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data belong to an ongoing trial and are not available at this time.
References
- 1.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430–40. 10.1016/S0140-6736(14)61698-6 [DOI] [PubMed] [Google Scholar]
- 2.Lawn JE, Blencowe H, Waiswa P, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet 2016;387:587–603. 10.1016/S0140-6736(15)00837-5 [DOI] [PubMed] [Google Scholar]
- 3.Alkema L, Chou D, Hogan D, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet 2016;387:462–74. 10.1016/S0140-6736(15)00838-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014;2:e323–33. 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 5.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 6.Lawn JE, Lee AC, Kinney M, et al. Two million intrapartum-related stillbirths and neonatal deaths: where, why, and what can be done? Int J Gynaecol Obstet 2009;107(Suppl 1):S5–18, S19 10.1016/j.ijgo.2009.07.016 [DOI] [PubMed] [Google Scholar]
- 7.UNICEF. State of the World's Children 2009. New York, 2009. [Google Scholar]
- 8.Wall SN, Lee AC, Carlo W, et al. Reducing intrapartum-related neonatal deaths in low- and middle-income countries-what works? Semin Perinatol 2010;34:395–407. 10.1053/j.semperi.2010.09.009 [DOI] [PubMed] [Google Scholar]
- 9.Stringer EM, Vwalika B, Killam WP, et al. Determinants of stillbirth in Zambia. Obstet Gynecol 2011;117:1151–9. 10.1097/AOG.0b013e3182167627 [DOI] [PubMed] [Google Scholar]
- 10.Geetha T, Chenoy R, Stevens D, et al. A multicentre study of perinatal mortality in Nepal. Paediatr Perinat Epidemiol 1995;9:74–89. 10.1111/j.1365-3016.1995.tb00120.x [DOI] [PubMed] [Google Scholar]
- 11.Jahn A, Dar Iang M, Shah U, et al. Maternity care in rural Nepal: a health service analysis. Tropical Med Int Health 2000;5:657–65. 10.1046/j.1365-3156.2000.00611.x [DOI] [PubMed] [Google Scholar]
- 12.Sunday-Adeoye I, Twomey ED, Egwuatu VE. A 20-year review of twin births at Mater Misericordiae Hospital, Afikpo, South Eastern Nigeria. Niger J Clin Pract 2008;11:231–4. [PubMed] [Google Scholar]
- 13.Bjerregaard-Andersen M, Lund N, Jepsen FS, et al. A prospective study of twinning and perinatal mortality in urban Guinea-Bissau. BMC Pregnancy Childbirth 2012;12:140 10.1186/1471-2393-12-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matendo RM, Engmann CM, Ditekemena JD, et al. Challenge of reducing perinatal mortality in rural Congo: findings of a prospective, population-based study. J Health Popul Nutr 2011;29:532–40. 10.3329/jhpn.v29i5.8908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AC, Kozuki N, Blencowe H, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res 2013;74(Suppl 1):50–72. 10.1038/pr.2013.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee AC, Mullany LC, Tielsch JM, et al. Incidence of and risk factors for neonatal respiratory depression and encephalopathy in rural Sarlahi, Nepal. Pediatrics 2011;128:e915–24. 10.1542/peds.2010-3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang KY, Zeger SL. Longitudinal data-analysis using generalized linear-models. Biometrika 1986;73:13–22. 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- 18.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 19.Kozuki N, Mullany LC, Khatry SK, et al. Accuracy of home-based ultrasonographic diagnosis of obstetric risk factors by primary-level health workers in rural Nepal. Obstet Gynecol 2016;128:604–12. 10.1097/AOG.0000000000001558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Government of Nepal Central Bureau of Statistics, UNICEF, MICS. Multiple Indicator Cluster Survey 2014—Final Report Kathmandu, 2014. [Google Scholar]
- 21.Cunningham FG, Leveno KJ, Bloom S, et al. Williams obstetrics. 23rd edn: The McGraw-Hill Companies, 2010. [Google Scholar]
- 22.WHO, UNICEF, UNFPA, et al. Trends in maternal mortality: 1990 to 2015. Geneva: WHO, 2015. 54. [Google Scholar]
- 23.Thorp JM Jr, Jenkins T, Watson W. Utility of Leopold maneuvers in screening for malpresentation. Obstet Gynecol 1991; 78(3 Pt 1):394–6. [PubMed] [Google Scholar]
- 24.Lydon-Rochelle M, Albers L, Gorwoda J, et al. Accuracy of Leopold maneuvers in screening for malpresentation: a prospective study. Birth 1993;20:132–5. 10.1111/j.1523-536X.1993.tb00437.x [DOI] [PubMed] [Google Scholar]
- 25.Watson WJ, Welter S, Day D. Antepartum identification of breech presentation. J Reprod Med 2004;49:294–6. [PubMed] [Google Scholar]
- 26.Shah SP, Epino H, Bukhman G, et al. Impact of the introduction of ultrasound services in a limited resource setting: rural Rwanda 2008. BMC Int Health Hum Rights 2009;9:4 10.1186/1472-698X-9-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geerts L, Theron AM, Grove D, et al. A community-based obstetric ultrasound service. Int J Gynaecol Obstet 2004;84:23–31. 10.1016/S0020-7292(03)00310-2 [DOI] [PubMed] [Google Scholar]
- 28.Rijken MJ, Lee SJ, Boel ME, et al. Obstetric ultrasound scanning by local health workers in a refugee camp on the Thai-Burmese border. Ultrasound Obstet Gynecol 2009;34:395–403. 10.1002/uog.7350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunette W, Gerard W, Hicks MA, et al. Portable antenatal ultrasound platform for village midwives. Proceedings of the First ACM Symposium on Computing for Development 2010. https://homes.cs.washington.edu/~rea/papers/Ultrasound-ACMDEV2010.pdf This is not a traditional journal article.
- 30.Sharma G, Mathai M, Dickson KE, et al. Quality care during labour and birth: a multi-country analysis of health system bottlenecks and potential solutions. BMC Pregnancy Childbirth 2015;15(Suppl 2):S2 10.1186/1471-2393-15-S2-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell-Jackson T, Morrison J, Tiwari S, et al. The experiences of districts in implementing a national incentive programme to promote safe delivery in Nepal. BMC Health Serv Res 2009;9:97 10.1186/1472-6963-9-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell-Jackson T, Neupane BD, Tiwari S, et al. The impact of Nepal's national incentive programme to promote safe delivery in the district of Makwanpur. Adv Health Econ Health Serv Res 2009;21:221–49. [PubMed] [Google Scholar]
- 33.Darmstadt GL, Bhutta ZA, Cousens S, et al. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet 2005;365:977–88. 10.1016/S0140-6736(05)71088-6 [DOI] [PubMed] [Google Scholar]
- 34.Cavallaro FL, Marchant TJ. Responsiveness of emergency obstetric care systems in low- and middle-income countries: a critical review of the ‘third delay’. Acta Obstet Gynecol Scand 2013;92:496–507. 10.1111/aogs.12071 [DOI] [PubMed] [Google Scholar]
- 35.Bowser D, Hill K. Exploring evidence for disrespect and abuse in facility-based childbirth. Washington, DC, 2010. [Google Scholar]
- 36.Stanton CK, Rawlins B, Drake M, et al. Measuring coverage in MNCH: testing the validity of women's self-report of key maternal and newborn health interventions during the peripartum period in Mozambique. PLoS ONE 2013;8:e60694 10.1371/journal.pone.0060694 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-013099supp001.pdf (68KB, pdf)