Abstract
Background
Penthorum chinense Pursh (Penthoraceae, PCP), a well-known Miao ethnomedicine, has been traditionally used to treat several liver-related diseases, such as jaundice and viral hepatitis. The aims of the present study were to evaluate the probable properties of the aqueous extract of PCP on carbon tetrachloride (CCl4)—induced acute liver injury in mice.
Methods
C57BL/6 mice were orally administered an aqueous extract of PCP (5.15 and 10.3 g/kg BW) or silymarin (100 mg/kg) once daily for 1 week prior to CCl4 exposure. Silymarin serves as a positive drug to validate the effectivenes of PCP.
Results
A single dose of CCl4 exposure caused severe acute liver injury in mice, as evidenced by the elevated serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alanine phosphatase (ALP), and the increased TUNEL-positive cells in liver, which were remarkably ameliorated by the pretreatment of PCP. PCP was also found to decrease the levels of malondialdehyde (MDA), restore the glutathione (GSH) and enhance the activities of superoxide dismutase (SOD) and catalase (CAT) in the liver. In addition, the pretreatment of PCP inhibited the degradation of hepatic cytochrome P450 2E1 (CYP2E1), up-regulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its target proteins in CCl4-treated mice.
Conclusion
Results indicated that the pretreatment of PCP (10.3 g/kg BW) effectively protected against CCl4-induced acute liver injury, which was comparable to efficacy of silymarin (100 mg/kg). This hepatoprotective effects might be attributed to amelioration of CCl4-induced oxidative stress via activating Nrf2 signaling pathway.
Electronic supplementary material
The online version of this article (10.1186/s13020-017-0153-x) contains supplementary material, which is available to authorized users.
Keywords: Carbon tetrachloride, Nuclear factor E2-related factor 2, Hepatotoxicity, Oxidative stress, Penthorum chinense Pursh
Background
Oxidative stress is well known to be involved in the pathogenesis of acute or chronic liver injury [1]. The over generation of reactive oxygen species (ROS) may be induced by various hepatotoxicants, including heavy metals, alcohol and carbon tetrachloride (CCl4) [2]. As a chemical inducer, CCl4 has been extensively used to assess the protection of natural products on liver injury in experimental cellular and animal models [3]. CCl4 is metabolized in the liver by cytochrome P450 2E1 (CYP2E1), and mainly yields the highly-reactive trichloromethyl radicals, which disturbs the redox homeostasis and causes oxidative stress. These free radicals may cause cellular DNA damage and the increased lipid peroxidation by reacting with cellular unsaturated lipids, leading to hepatocyte apoptosis and necrosis, which ultimately results in liver injury [4].
Antioxidant defense system, including non-enzymatic and enzymatic mechanisms, is principally responsible for protecting living organism from oxidative stress [5]. Among them, superoxide dismutase (SOD), catalases (CAT) and glutathione peroxidase (GSH-Px) serve as three main classes of antioxidant defense-related enzymes, which are modulated by nuclear factor-erythroid 2-related factor-2 (Nrf2) [6]. Normally, Nrf2 is restrained in cytosol through interacting with Kelch-like ECH-associated protein 1 (Keap1), a specific repressor [7]. Upon oxidative stress, Nrf2 translocates to the nucleus after the dissociation with Keap1, and regulates the expressions of antioxidant-related genes, including haem oxygenase 1 (HO-1) and glutamate cysteine ligase (GCL) [8]. Thus, chemicals or natural products that can activate Nrf2 signaling pathway might be used to prevent CCl4-induced liver injury.
Penthorum chinense Pursh (Penthoraceae, PCP), has been traditionally used as the Miao ethnomedicine and folk remedy in the treatment of liver-related diseases, including jaundice and viral hepatitis for a long time [9]. PCP is widely cultivated in Gulin county, Sichuan Province, China, around where there are a lot of liquor factories. The tea made from the aerial of PCP became more popular in local residents who often drink liquor, and the bartenders who work in liquor factories. In the recent years, a number of studies have demonstrated that PCP or its ingredients possess the diverse bioactivities, including antioxidant, anti-complement, anti-hyperglycemic, and anti-hepatocarcinoma [10]. Our previous studies have also indicated that the aqueous extract of PCP could protect against both acute [11] and chronic alcohol-induced liver injury [12]. However, the impacts of PCP against CCl4-induced liver injury has not been adequately addressed. Therefore, in this study, we aimed to evaluate the probable protective properties of PCP against acute liver injury induced by CCl4, and further elucidate its underlying mechanisms with respect to Nrf2-mediated antioxidant response.
Methods
Materials and sample preparation
Aerial part of PCP was provided by Sichuan New Lotus Traditional Chinese Herb Limited Company (Chengdu, China). Its botanical origin was identified by Dr. Chun-Feng Qiao from our university. The aqueous extract of PCP was prepared as previously described [11, 12]. The dried powder of PCP (150 g) was decocted three times with 1500 mL water for 2 h each. After combination and filtration, the decoction was lyophilized by a freeze dryers (VirTis BenchTop Pro, SP Scientific, Warminster, PA, USA). The freeze-dried extract was re-constructed by the distilled water for the present animal study. To assure the repeatability of the pharmacological study, pinocembrin-7-O-β-d-glucoside, the chemical marker, was measured as 3.49 mg/g in raw PCP by HPLC–UV. The voucher specimen of PCP sample (No. GHX201401) was stored at Institute of Chinese Medical Sciences, University of Macau, Macau.
Animals and treatments
Mice (C57BL/6, 8–9 weeks old) were housed in institutional individually ventilated cage (IVC) system. All animals were assigned randomly to five groups (n = 10, half males and half females in each group), i.e. control group, CCl4 group, silymarin-treated group (100 mg/kg BW, as the positive control), two PCP-treated groups (5.15 and 10.3 g/kg BW). The dose of PCP (10.3 g/kg) was calculated from the usage of Gan-Su-Ke-Li (WS3-B-2526-97), a drug approved by China Food and Drug Administration (CFDA), which was made from the aqueous extract of PCP for the management of viral hepatitis. Mice were gavaged silymarin or PCP once daily for 1 week prior to CCl4 challenge in treatment group. 24 h after last dosing, animals were intraperitoneally injected with 10% CCl4 diluted in olive oil (v/v, 2 mL/kg) to induce acute liver injury [13], mice in the control group were treated with same volume of vehicle (i.p.). After fasting for 12 h, all mice were anesthetized, and serum samples and the entire liver tissues were immediately collected. Animal protocol was conducted according to the animal procedure approved by Animal Ethics Committee, Institute of Chinese Medical Sciences, University of Macau (ICMS-AEC-2015-05). The Minimum Standards of Reporting Checklist (Additional file 1) contains details of the experimental design, and statistics, and resources used in this study.
Measurements of haematological parameters
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alanine phosphatase (ALP) were examined through enzymatic colorimetric methods by their respective commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. For the measurements of AST and ALT, serum was mixed well with corresponding matrix solution, and then reacted with 2,4-dinitro-phenylhydrazine for 20 min. NaOH solution (4 mol/L) was added to terminate the reaction. Results were measured by SpectraMax® M5 Multi-Mode Microplate Reader (Waltham, MA, USA).
Histopathological analysis
The right lobe of the liver tissue was fixed in 10% (v/v) phosphate-buffered formalin overnight, and embedded in paraffin. The cryostat sections were stained with hematoxylin and eosin according to a standard protocol [14]. The histopathological alterations of liver were observed by an Olympus CX-31 light microscopy with CCD camera (Olympus Crop, Tokyo, Japan).
Parameter measurements for oxidative stress in the liver
Partial liver tissues were homogenized in 9 volumes of cold RIPA (Beyotime Institute of Biotechnology, Nanjing, China) on ice. The liver homogenates (10%) were centrifuged, and the final supernatants were subjected to measure the levels of malondiadehyde (MDA), reduced glutathione (GSH), oxidized glutathione (GSSG), the activities of superoxide dismutase (SOD) and catalase (CAT) by their respective assay kits (Jiancheng Bioengineering). Protein content in the homogenates was determined using a Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL, USA). The results were normalized per gram total protein.
TUNEL assay
The apoptotic cells in liver cryostat section were evaluated by a commercial ApopTag® Plus In Situ Apoptosis Fluorescein Detection Kit (EMD Millipore Corporation, Billerica, MA, USA). Briefly, liver sections (4 μM) were fixed in 1% paraformaldehyde solution, then incubated in green fluorescein labelled dUTP solution at 37 °C for 1 h. After washing, the sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific Inc., Rockford, IL, USA). The apoptotic cells were visualized on Zeiss Axio Imager A2 microscope (Carl Zeiss, Oberkochen, Germany).
RT-PCR analysis
The transcriptional expressions of CYP2E1, Keap1, HO-1 and GCLC in liver were determined by qPCR as previously described [5, 11]. In brief, total RNA was extracted from liver by a TRIzol® reagent, and subjected to cDNA synthesis using TaqMan Reverse Transcription Reagents Kit (Life Technologies, Carlsbad, CA). The primers (Table 1) were synthesized by Invitrogen Life Technologies (Shanghai, China). qPCR was conducted on a Mx3005P qPCR system (Agilent Technologies) by SYBR® Green PCR Master Mix (Life Technologies). The mRNA expression was normalized to β-actin.
Table 1.
Primers used for the quantitative RT-PCR analysis
| Gene | Forward primer | Reverse primer |
|---|---|---|
| CYP2E1 | 5′-CGTTGCCTTGCTTGTCTGGA-3′ | 5′-AAGAAAGGAATTGGGAAAGGTCC-3′ |
| Keap1 | 5′-TGCCCCTGTGGTCAAAGTG-3′ | 5′-GGTTCGGTTACCGTCC TGC-3′ |
| HO-1 | 5′-AAGCCGAGAATGCTGAGTTCA-3′ | 5′-GCCGTGTAATATGGTACAAGGA-3′ |
| GCLC | 5′-GGGGTGACGAGGTGGAGTA-3′ | 5′-GTTGGGGTTTGTCCTCTCCC-3′ |
| β-actin | 5′-GGCTGTATTCCCCTCCATCG-3′ | 5′-GTTGGGGTTTGTCCTCTCCC-3′ |
Immunoblot analysis
The total protein was isolated from the left lobe of the liver tissues by the cold RIPA lysis buffer containing 1% phosphatase inhibitor cocktail (Beyotime Institute of Biotechnology). Approximately 60 μg of total proteins were loaded onto 10% SDS-PAGE, subsequently transblotted onto PVDF membranes (Bio-Rad Laboratories Inc., Hercules, CA, USA). After blocking with 5% nonfat dry milk in TBST (0.1% Tween-20 in Tris-buffered saline), the membranes were incubated with primary antibodies for 24 h at 4 °C, including CYP2E1 (1:1000, Cell Signaling Technology, Danvers, MA, USA), Keap-1 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Nrf2 (1:200, Santa Cruz Biotechnology), HO-1 (1:250, Abcam, Cambridge, MA, USA), GCLC (1:1000, Abcam) and GAPDH (1:1000, Cell Signaling), then incubated with secondary antibodies conjugated to horseradish peroxidase (HRP) for 1 h. The proteins were visualized by Amersham ECL Select Western Blotting Detection Reagent (GE Healthcare BioSciences, Piscataway, NJ, USA).
Statistical analysis
The value was expressed as mean ± SD. After verifying the distribution of the data by Kolmogorov–Smirnov test, the between-group comparison was conducted by ordinary one-way analysis of variance (ANOVA) using GraphPad 5.0 Software (San Diego, CA, USA).
Results
Effects of PCP on serum parameters
The serum levels of AST, ALT and ALP, the commonly-used biomarkers of liver damage in the clinics [15], were measured by colorimetrical methods. A single dose of CCl4 exposure caused severe hepatotoxicity in mice (Fig. 1), the serum levels of AST, ALT and ALP in the CCl4 group were dramatically increased by 11.5-fold (46.6 ± 17.8 vs. 610.0 ± 95.6 U/L), 42.2-fold (6.02 ± 3.61 vs. 260.3 ± 60.0 U/L) and 63.1% (101.2 ± 17.3 vs. 165.1 ± 23.8 U/L), respectively, compared with control group. However, these elevations were decreased significantly (p < 0.05) by pre-treatments of PCP at the doses of both 5.15 and 10.3 g/kg BW, and silymarin, a positive control, as well.
Fig. 1.
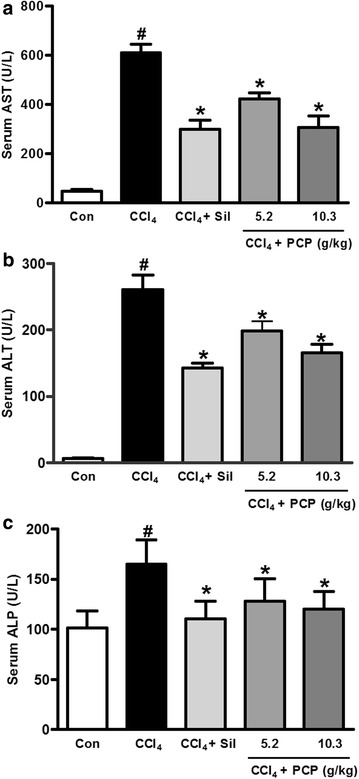
Effects of PCP on serum activities of a aspartate aminotransferase (AST), b alanine aminotransferase (ALT), and c alanine phosphatase (ALP). Value represents mean ± SD (n = 7–10), # p < 0.05 vs. control group, *p < 0.05 vs. CCl4 group
Effects of PCP on CCl4-induced histopathological alteration
Histological observations were performed to examine the pathological changes in liver. As shown in Fig. 2, the liver tissues from the control group showed normal architectures. CCl4 challenge caused marked histopathological changes in the liver, characterized by apparent microvesicular and macrovesicular steatosis, massive inflammatory cells infiltration, and extensive hepatocyte necrosis. These histopathological changes were remarkably ameliorated by the pre-treatments of silymarin and PCP (10.3 g/kg BW), which were consistent with the results of serum parameters.
Fig. 2.
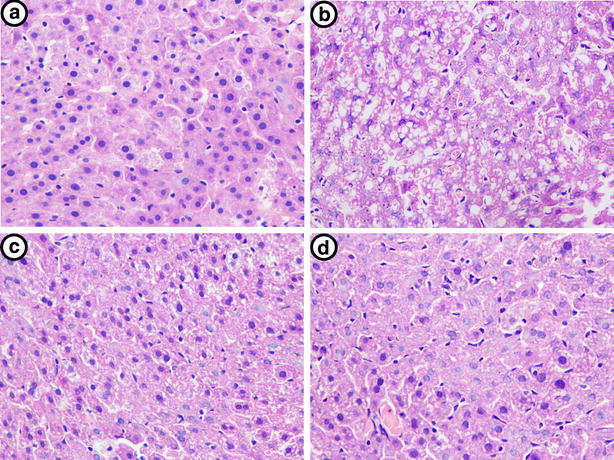
Representative H&E staining of liver tissues. a Control, b CCl4, c CCl4 + Silymarin (100 mg/kg), and d CCl4 + PCP (10.3 g/kg BW)
Effects of PCP on CCl4-induced hepatocyte apoptosis
As hepatocyte apoptosis also reflects the extent of CCl4-induced liver injury [16], a TUNEL assay was performed. As shown in Fig. 3, after 12 h of CCl4 challenge, the number of TUNEL-positive cells in liver section was obviously increased over the control group, the apoptotic cells were greatly decreased in the pre-treatment of PCP (10.3 g/kg BW) or silymarin.
Fig. 3.
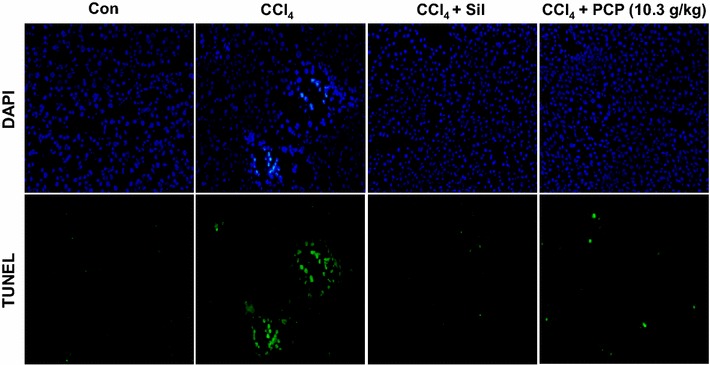
Effects of PCP on CCl4-induced hepatocyte apoptosis
Effects of PCP on CCl4-induced oxidative stress
To assess the protective effects of PCP on hepatic oxidative stress induced by CCl4 exposure, the levels of MDA, GSH and GSSG, and the activities of SOD and CAT were examined in liver. As an end-product of lipid peroxidation (LPO), MDA was considered to be a useful marker of oxidative stress [1]. As shown in Fig. 4a, the hepatic MDA level was significantly elevated upon a single dose of CCl4 exposure, which was decreased in both silymarin and PCP (10.3 g/kg BW)—treated groups. PCP (5.15 g/kg BW) showed a decreased concentration in hepatic MDA, but with no significant difference. CCl4 exposure also depleted endogenous antioxidants, as indicated by the level of GSH, and the activities of SOD and CAT in CCl4 group were significantly decreased to 50.5, 22.4 and 58.7%, respectively, compared to control group. These depletions were notably ameliorated by the pretreatment of silymarin and PCP (10.3 g/kg BW) (Fig. 4b–d). Compared to control group, one single dose of CCl4 challenge significantly elevated the hepatic GSSG level (129 ± 35 vs. 284 ± 48 n mol/mg protein), leading to the decrease in GSH/GSSG ratio (0.77 ± 0.23 vs. 0.25 ± 0.08) (Fig. 4e, f). These alterations were greatly ameliorated by silymarin (100 mg/kg BW) and PCP (10.3 g/kg BW), and their protective efficacies were comparable.
Fig. 4.
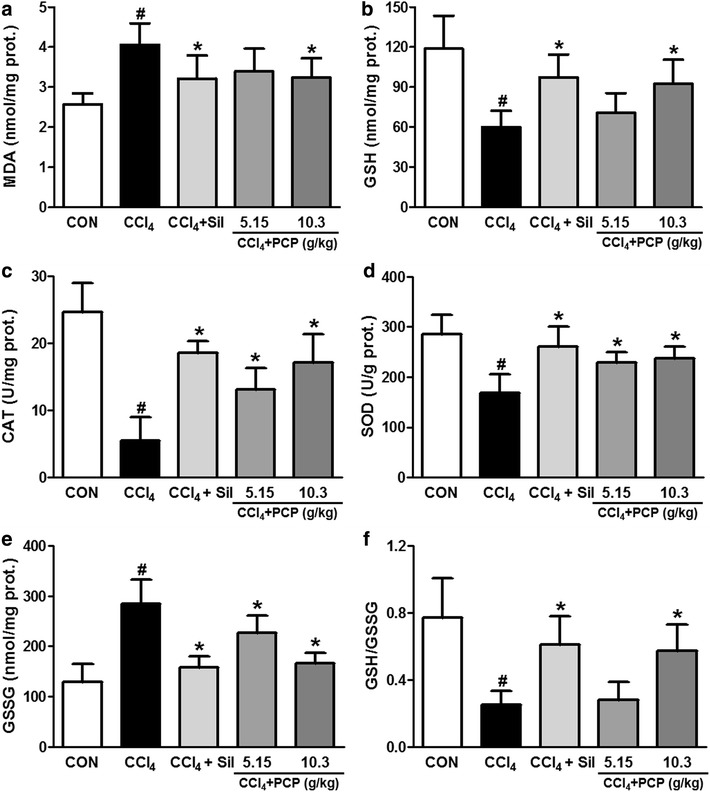
Effects of PCP on CCl4-induced oxidative stress in liver. a malondialdehyde (MDA); b reduced glutathione (GSH); c catalase (CAT); d superoxide dismutase (SOD); e glutathione disulfide (GSSG); f the ratio of GSH to GSSG. Value represents mean ± SD (n = 7–10), # p < 0.05 vs. control group, *p < 0.05 vs. CCl4 group
Effects of PCP on CYP2E1 expression
As shown in Fig. 5, CCl4 challenge dramatically decreased the both mRNA and protein expressions of CYP2E1 in the liver. The pre-treatment of either PCP (10.3 g/kg BW) or silymarin significantly reversed the expression of CYP2E1 in the protein level, but not the transcriptional level.
Fig. 5.
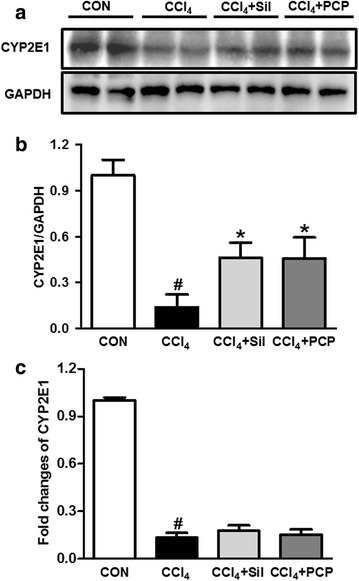
Effects of PCP (10.3 g/kg BW) on CYP2E1 expression. a Immunoblot analysis of CYP2E1; b its densitometric analysis; c qPCR analysis of CYP2E1. Value represents mean ± SD (n = 3–4), # p < 0.05 vs. control group, *p < 0.05 vs. CCl4 group
Effects of PCP on Nrf2-mediated oxidative stress response pathway
To elucidate the molecular mechanisms underlying the protection of PCP against CCl4-induced oxidative stress, the Nrf2 signaling pathway was measured by using immunoblot. Compared to control group, one single dose of CCl4 exposure decreased the protein expressions of Nrf2 in total, cytosol and nucleus, the pre-treatment of silymarin and PCP (10.3 g/kg BW) significantly normalized these reductions in Nrf2 expressions induced by CCl4 exposure (Fig. 6). Additionally, Keap-1 and the downstream regulated genes of Nrf2 were also examined in the liver by qPCR and immunoblot analysis. As shown in Fig. 7, the pretreatment of silymarin and PCP (10.3 g/kg BW) remarkably increased the expressions of Keap-1, HO-1 and GCLC in terms of both mRNA and protein levels, over the CCl4 group. A lower protein expression of Keap-1 in the CCl4 group was found compared to the control group. However, no significant difference in either mRNA or protein expression of HO-1 and GCLC was observed between the control and the CCl4 groups.
Fig. 6.
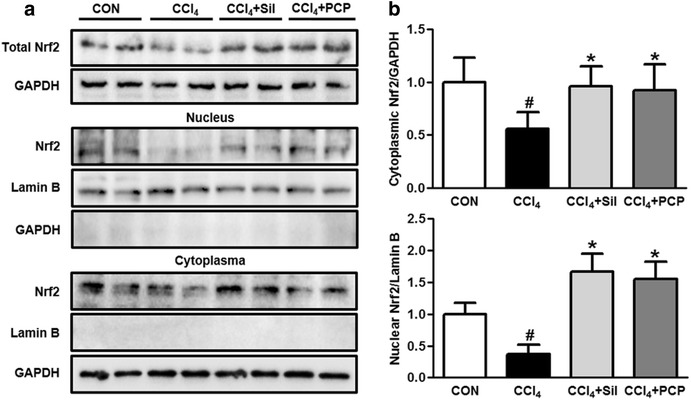
Effects of PCP (10.3 g/kg BW) on protein expression of Nrf2. a Immunoblot analysis of Nrf2 in total, nucleus and cytosol; b their results of the densitometric analysis. Value represents mean ± SD (n = 3–4), # p < 0.05 vs. control group, *p < 0.05 vs. CCl4 group
Fig. 7.
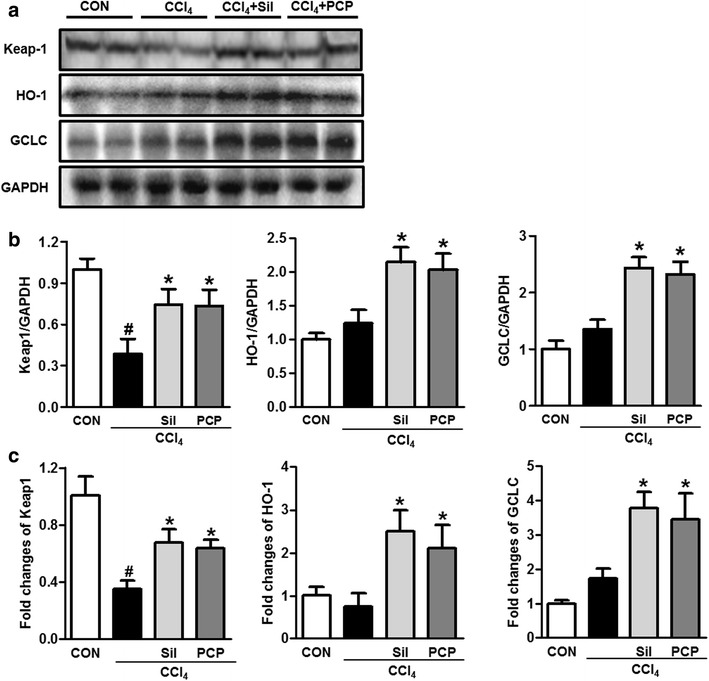
Effects of PCP (10.3 g/kg BW) on Nrf2-mediated oxidative stress response. a Immunoblot analysis of Keap1, HO-1 and GCLC; b their results of the densitometric analysis; c qPCR analysis of Keap1, HO-1 and GCLC. Value represents mean ± SD (n = 3–4), # p < 0.05 vs. control group, *p < 0.05 vs. CCl4 group
Discussion
CCl4, a well-known hepatotoxicant, has been commonly used in cellular and animal models to evaluate the protective effects of natural products on liver injury [17]. Serum levels of AST and ALT are the preferred indicators for evaluation of liver function, they usually reflect the altered permeability of hepatocellular membrane and the damaged structural integrity of hepatocytes [1]. The elevated ALP level in serum was commonly observed in the patients with extrahepatic, intrahepatic biliary obstruction, and infiltrative liver diseases [18]. In our study, CCl4 challenge induced a profound elevation in serum levels of ALT, AST and ALP, indicating the acute hepatotoxicity caused by CCl4. However, these increments were effectively alleviated by the pretreatment with PCP and silymarin. The effects of PCP (10.30 g/kg BW) were comparable to silymarin (100 mg/kg BW), a positive control which is a herbal remedy for liver treatment with anti-inflammatory and antioxidant properties [19]. These results demonstrated that PCP effectively protects against acute liver injury induced by a single dose of CCl4. This observation was also verified by histopathological examination and TUNEL assay as well.
CCl4 is metabolized by CYP2E1 to produce the CCl4-derived free radical in the liver [4]. These highly-reactive species can irreversibly oxidize biological macromolecules, such as DNA, proteins and lipids, resulting in lipid peroxidation, oxidative stress, hepatocyte apoptosis, leading ultimately to hepatotoxicity [20, 21]. CCl4-induced oxidative stress also depletes the endogenous antioxidants, including the non-enzymatic group, such as GSH, and enzymatic antioxidants, such as SOD and CAT. It was documented that GSH is an important antioxidant in eliminating toxic free radicals and reactive toxic CCl4 metabolites [22, 23]. The sulfhydryl residues of GSH molecule is easily oxidized to GSSG, in turn, GSSG can be converted back to GSH by the aid of glutathione reductase (GR). Thus, the redox ratio of GSH/GSSG is often used as a useful indicator of oxidative stress [24]. In this study, CCl4 exposure caused severe oxidative stress in the liver where CCl4 is primarily metabolized, as evidenced by the increased hepatic levels of MDA and GSSG, the decreased GSH level, GSH/GSSG ratio and antioxidant enzyme activities of CAT and SOD. However, several compounds were identified from PCP, including flavonoids, flavonoid glycosides, polyphenols, steroids [25]. Among these compounds, flavonoids and polyphenols exhibited anti-oxidative effects and other pharmacological bioactivity [26, 27], which may mainly contributed to the hepatoprotection effects of PCP. Our data showed that PCP effectively attenuated CCl4-induced oxidative stress by not only reducing hepatic MDA level but also enhancing endogenous non-enzymatic and enzymatic antioxidants.
Hepatic CYP2E1 is mainly responsible for the metabolism of CCl4 to produce highly-reactive trichloromethyl free radicals [28]. Thus, CYP2E1 plays a vital role in the regulation of CCl4-induced oxidative stress. CYP2E1 deficient mice are resistant to CCl4-induced hepatoxicity [29], CYP2E1 inhibitors and the antibodies specific for CYP2E1 reduced liver injury induced by CCl4 exposure in rats [30]. While, CCl4-induced hepatotoxicity can be enhanced by the pretreatment of alcohol, a CYP2E1 inducer [31]. However, a large body of studies has also indicated that CCl4 challenge decreased the expression and activity of CYP2E1 [32–34]. A most plausible explanation is that CCl4 might labilize and inactivate CYP2E1, and enhance its degradation, which reveals ongoing oxidative damage [34]. Our data showed that a remarkable decrease in both mRNA and protein expression of CYP2E1 in the liver was observed after CCl4 exposure, and the pretreatment of PCP at the doses of either 5.15 or 10.3 g/kg BW significantly elevated the CCl4-induced decrease of CYP2E1 expression in the protein level, but not in the transcriptional level, suggesting that PCP efficiently reduces the degradation of CYP2E1 induced by CCl4.
To understand how PCP reduces the oxidative stress induced by CCl4 exposure, the expressions of Nrf2 and its downstream genes in the liver were measured. Nrf2 plays a critical role in regulating antioxidant defense system in response to oxidative stress [12]. Nrf2-null mice are more susceptible to hepatotoxicity and oxidative stress induced by various chemicals, including CCl4, ethanol, acetaminophen, pyrazole and arsenic [35]. In response to stress signals, the redox sensitive protein Keap1 undergoes oxidation, leading to the stabilization of Nrf2 and its nuclear translocation [36]. The activation of Nrf2 by binding to ARE triggers the expression of downstream genes, including HO-1 and GCLC. HO-1 is considered as a strong antioxidant and improves hepatocyte survival. GCLC is a rate-limiting enzyme in GSH biosynthesis in the liver [37]. In the present study, compared with the CCl4 group, the hepatic Nrf2 expressions in total, cytosol and nucleus were significantly increased in PCP-treated group. As expected, the PCP-treated group showed higher expressions of Keap-1, HO-1 and GCLC in the liver.
Conclusion
Collectively, the pre-treatment of the aqueous extract of PCP (10.3 g/kg BW) can effectively protected against CCl4-induced acute liver injury, which was closely similar to efficacy of silymarin (100 mg/kg BW). These hepatoprotective effects might be associated with ameliorating CCl4-induced oxidative stress via activation of Nrf2 signaling pathway (Fig. 8).
Fig. 8.
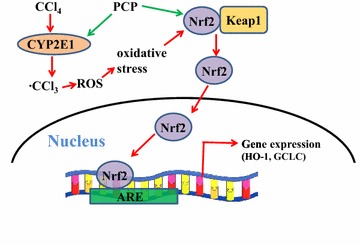
Schematic diagram of the potential mechanisms underlying the protective effects of PCP on the CCl4-induced liver injury
Authors’ contributions
JBW conceived and designed the study; MW and XJZ performed the experiments and drafted the manuscript; RF conducted the western blot of Nrf2; YJ and DYZ. provided the herb; CH and PL revised the manuscript and provided essential comments. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
All of authors consent to publication of this study in Journal of Chinese Medicine.
Ethics approval and consent to participate
Animal protocol was conducted according to the animal procedure approved by Animal Ethics Committee, Institute of Chinese Medical Sciences, University of Macau (ICMS-AEC-2015-05).
Funding
This work was financially supported by the grants from the Research Committee of the University of Macau (MYRG2016-00042-ICMS-QRCM to JB Wan), and from National Natural Science Foundation of China (NSFC, No. 81503288).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ALP
alanine phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CAT
catalase
- CCl4
tetrachloride
- CFDA
China Food and Drug Administration
- CYP2E1
cytochrome P450 2E1
- GCL
glutamate cysteine ligase
- GR
glutathione reductase
- GSH
reduced glutathione
- GSH-Px
glutathione peroxidase
- GSSG
oxidized glutathione
- HO-1
haem oxygenase 1
- IVC
individually ventilated cage
- Keap1
Kelch-like ECH-associated protein 1
- LPO
lipid peroxidation
- MDA
malondialdehyde
- Nrf2
nuclear factor erythroid 2-related factor 2
- PCP
Penthorum chinense Pursh
- SOD
superoxide dismutase
- ROS
reactive oxygen species
Additional file
Additional file 1. The Minimum Standards of Reporting Checklist.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13020-017-0153-x) contains supplementary material, which is available to authorized users.
Meng Wang and Xiao-Jiao Zhang contributed equally to this work
Contributor Information
Meng Wang, Email: wangmeng198610@126.com.
Xiao-Jiao Zhang, Email: shiyizhang@126.com.
Ruibing Feng, Email: fengruibing128@126.com.
Yun Jiang, Email: jiangyun133@163.com.
Da-Yong Zhang, Email: zdy@xinhehua.com.
Chengwei He, Email: chengweihe@umac.mo.
Peng Li, Email: pengli@umac.mo.
Jian-Bo Wan, Phone: +853-8822 4680, Email: jbwan@umac.mo.
References
- 1.Ding RB, Tian K, Huang LL, He CW, Jiang Y, Wang YT, Wan JB. Herbal medicines for the prevention of alcoholic liver disease: a review. J Ethnopharmacol. 2012;144:457–465. doi: 10.1016/j.jep.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 2.Jadeja RN, Urrunaga NH, Dash S, Khurana S, Saxena NK. Withaferin-A reduces acetaminophen-induced liver injury in mice. Biochem Pharmacol. 2015;97:122–132. doi: 10.1016/j.bcp.2015.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee IC, Kim SH, Baek HS, Moon C, Kang SS, Kim SH, Kim YB, et al. The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride-induced hepatic oxidative damage and inflammatory response in rats. Food Chem Toxicol. 2014;63:174–185. doi: 10.1016/j.fct.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Su C, Xia X, Shi Q, Song X, Fu J, Xiao C, Chen H, et al. Neohesperidin dihydrochalcone versus CCl4-induced hepatic injury through different mechanisms: the implication of free radical scavenging and Nrf2 activation. J Agric Food Chem. 2015;63:5468–5475. doi: 10.1021/acs.jafc.5b01750. [DOI] [PubMed] [Google Scholar]
- 5.Ding RB, Tian K, Cao YW, Bao JL, Wang M, He C, Hu Y, et al. Protective effect of panax notoginseng saponins on acute ethanol-induced liver injury is associated with ameliorating hepatic lipid accumulation and reducing ethanol-mediated oxidative stress. J Agric Food Chem. 2015;63:2413–2422. doi: 10.1021/jf502990n. [DOI] [PubMed] [Google Scholar]
- 6.Copple IM, Goldring CE, Kitteringham NR, Park BK. The Nrf2-Keap1 defence pathway: role in protection against drug-induced toxicity. Toxicology. 2008;246:24–33. doi: 10.1016/j.tox.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 8.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Q, Jiang M-H, Jiang J-G, Zhang R-F, Zhang M-W. Isolation and identification of compounds from Penthorum chinense Pursh with antioxidant and antihepatocarcinoma properties. J Agric Food Chem. 2012;60:11097–11103. doi: 10.1021/jf303755w. [DOI] [PubMed] [Google Scholar]
- 10.Wang A, Lin L, Wang Y. Traditional Chinese herbal medicine Penthorum chinense Pursh: a phytochemical and pharmacological review. AmJ Chinese Med. 2015;43:601–620. doi: 10.1142/S0192415X15500378. [DOI] [PubMed] [Google Scholar]
- 11.Cao YW, Jiang Y, Zhang DY, Zhang XJ, Hu YJ, Li P, Su H, et al. The hepatoprotective effect of aqueous extracts of Penthorum chinense Pursh against acute alcohol-induced liver injury is associated with ameliorating hepatic steatosis and reducing oxidative stress. Food Funct. 2015;6:1510–1517. doi: 10.1039/C5FO00098J. [DOI] [PubMed] [Google Scholar]
- 12.Cao YW, Jiang Y, Zhang DY, Wang M, Chen WS, Su H, Wang YT, et al. Protective effects of Penthorum chinense Pursh against chronic ethanol-induced liver injury in mice. J Ethnopharmacol. 2015;161:92–98. doi: 10.1016/j.jep.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, Gao B. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X-J, He C, Li P, Su H, Wan J-B. Ginsenoside Rg1, a potential JNK inhibitor, protects against ischemia/reperfusion-induced liver damage. J Funct Foods. 2015;15:580–592. doi: 10.1016/j.jff.2015.04.010. [DOI] [Google Scholar]
- 15.Wang M, Zhang XJ, Feng K, He C, Li P, Hu YJ, Su H, et al. Dietary alpha-linolenic acid-rich flaxseed oil prevents against alcoholic hepatic steatosis via ameliorating lipid homeostasis at adipose tissue-liver axis in mice. Sci Rep. 2016;6:26826. doi: 10.1038/srep26826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng R, Wang M, Yan C, Li P, Chen M, He C, Wan JB. Endogenous n-3 fatty acids alleviates carbon tetrachloride-induced acute liver injury in fat-1 transgenic mice. Oxid Med Cell Longev. 2016;2016:7962948. doi: 10.1155/2016/7962948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Recknagel RO, Glende EA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 18.Field KM, Dow C, Michael M. Part I: liver function in oncology: biochemistry and beyond. Lancet Oncol. 2008;9:1092–1101. doi: 10.1016/S1470-2045(08)70279-1. [DOI] [PubMed] [Google Scholar]
- 19.Surai PF. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants. 2015;4:204–247. doi: 10.3390/antiox4010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plaa GL. Chlorinated methanes and liver injury: highlights of the past 50 years. Annu Rev Pharmacol. 2000;40:43–65. doi: 10.1146/annurev.pharmtox.40.1.43. [DOI] [PubMed] [Google Scholar]
- 21.Ismail AF, Salem AA, Eassawy MM. Hepatoprotective effect of grape seed oil against carbon tetrachloride induced oxidative stress in liver of gamma-irradiated rat. J Photochem Photobiol B. 2016;160:1–10. doi: 10.1016/j.jphotobiol.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Ray G, Husain SA. Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol. 2002;40:1213–1232. [PubMed] [Google Scholar]
- 23.Boll M, Weber LW, Becker E, Stampfl A. Mechanism of carbon tetrachloride-induced hepatotoxicity. Hepatocellular damage by reactive carbon tetrachloride metabolites. Z Naturforsch C. 2001;56:649–659. doi: 10.1515/znc-2001-7-826. [DOI] [PubMed] [Google Scholar]
- 24.Aoyama K, Nakaki T. Glutathione in cellular redox homeostasis: association with the excitatory amino acid carrier 1 (EAAC1) Molecules. 2015;20:8742–8758. doi: 10.3390/molecules20058742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang A, Lin L, Wang Y. Traditional Chinese herbal medicine Penthorum chinense Pursh: a phytochemical and pharmacological review. Am J Chin Med. 2015;43:601–620. doi: 10.1142/S0192415X15500378. [DOI] [PubMed] [Google Scholar]
- 26.Roslan J, Giribabu N, Karim K, Salleh N. Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed Pharmacother. 2017;86:570–582. doi: 10.1016/j.biopha.2016.12.044. [DOI] [PubMed] [Google Scholar]
- 27.Mattera R, Benvenuto M, Giganti MG, Tresoldi I, Pluchinotta FR, Bergante S, Tettamanti G, et al. Effects of polyphenols on oxidative stress-mediated injury in cardiomyocytes. Nutrients. 2017;9:523. doi: 10.3390/nu9050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruebele A, Zawaski K, Kaplan D, Novak RF. Cytochrome P4502E1-and cytochrome P4502B1/2B2-catalyzed carbon tetrachloride metabolism: effects on signal transduction as demonstrated by altered immediate-early (c-Fos and c-Jun) gene expression and nuclear AP-1 and NF-kappa B transcription factor levels. Drug Metab Dispos. 1996;24:15–22. [PubMed] [Google Scholar]
- 29.Wong FWY, Chan WY, Lee SST. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharm. 1998;153:109–118. doi: 10.1006/taap.1998.8547. [DOI] [PubMed] [Google Scholar]
- 30.Brady JF, Xiao F, Wang MH, Li Y, Ning SM, Gapac JM, Yang CS. Effects of disulfiram on hepatic P450IIE1, other microsomal enzymes, and hepatotoxicity in rats. Toxicol Appl Pharm. 1991;108:366–373. doi: 10.1016/0041-008X(91)90125-X. [DOI] [PubMed] [Google Scholar]
- 31.Ray SD, Mehendale HM. Potentiation of CCl4 and CHCl3 hepatotoxicity and lethality by various alcohols. Fundam Appl Toxicol. 1990;15:429–440. doi: 10.1016/0272-0590(90)90029-J. [DOI] [PubMed] [Google Scholar]
- 32.Wunjuntuk K, Kettawan A, Charoenkiatkul S, Rungruang T. Parboiled germinated brown rice protects against CCl4-induced oxidative stress and liver injury in rats. J Med Food. 2016;19:15–23. doi: 10.1089/jmf.2015.3460. [DOI] [PubMed] [Google Scholar]
- 33.Al-Rasheed N, Faddah L, Al-Rasheed N, Bassiouni YA, Hasan IH, Mahmoud AM, Mohamad RA, et al. Protective effects of silymarin, alone or in combination with chlorogenic acid and/or melatonin, against carbon tetrachloride-induced hepatotoxicity. Pharmacogn Mag. 2016;12:S337–S345. doi: 10.4103/0973-1296.185765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai Y, Cederbaum AI. Inactivation and degradation of human cytochrome P4502E1 by CCl4 in a transfected HepG2 cell line. J Pharmacol Exp Ther. 1995;275:1614–1622. [PubMed] [Google Scholar]
- 35.Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol. 2010;244:57–65. doi: 10.1016/j.taap.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki T, Motohashi H, Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Shertzer HG, Schneider SN, Nebert DW, Dalton TP. Glutamate cysteine ligase catalysis: dependence on ATP and modifier subunit for regulation of tissue glutathione levels. J Biol Chem. 2005;280:33766–33774. doi: 10.1074/jbc.M504604200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


