Abstract
Background
Microbial degradation of phenoxy acid (PA) herbicides in agricultural soils is important to minimize herbicide leaching to groundwater reservoirs. Degradation may, however, be hampered by exposure of the degrader bacteria to toxic metals as copper (Cu) in the soil environment. Exposure to Cu leads to accumulation of intracellular reactive oxygen species (ROS) in some bacteria, but it is not known how Cu-derived ROS and an ensuing oxidative stress affect the degradation of PA herbicides. Based on the previously proposed paradigm that bacteria deal with environmental stress before they engage in biodegradation, we studied how the degradation of the PA herbicide 2-methyl-4-chlorophenoxyacetic acid (MCPA) by the model PA degrader Cupriavidus pinatubonensis AEO106 was affected by Cu exposure.
Results
Exposure of C. pinatubonensis in batch culture to sublethal concentrations of Cu increased accumulation of ROS measured by the oxidant sensing probe 2,7-dichlorodihydrofluorescein diacetate and flow cytometry, and resulted in upregulation of a gene encoding a protein belong to the Ohr/OsmC protein family. The ohr/osmC gene was also highly induced by H2O2 exposure suggesting that it is involved in the oxidative stress response in C. pinatubonensis. The increased ROS accumulation and increased expression of the oxidative stress defense coincided with a delay in the catabolic performance, since both expression of the catabolic tfdA gene and MCPA mineralization were delayed compared to unexposed control cells.
Conclusions
The current study suggests that Cu-induced ROS accumulation in C. pinatubonensis activates a stress response involving the product of the ohr/osmC gene. Further, the stress response is launched before induction of the catabolic tfdA gene and mineralization occurs.
Keywords: Oxidative stress, ROS, ohr/osmC, Copper, PA degradation, Cupriavidus pinatubonensis
Background
Degradation of the phenoxy acid (PA) herbicides 2,4-dichlorophenoxyacetic acid (2,4-D) and 2-methyl-4-chlorophenoxyacetic acid (MCPA) by soil microorganisms is normally a rapid process, and in experimental soil systems the pesticides are completely degraded within 30 days [1]. Nevertheless, PA herbicides often persist in the soil environment under natural conditions leading to leaching to groundwater reservoirs. The herbicides reach the groundwater even though PA degraders are commonly found in natural soil environments [2]. This suggests that the full mineralization potential of degrader organisms is not as efficiently expressed under in situ soil conditions as seen in laboratory setups. One reason could be the harsh conditions encountered in a soil environment. In soil, the bacteria are confronted with suboptimal conditions caused by for instance fluctuations in substrate availability, pH, temperature, water availability, as well as exposure to toxic, anthropogenic compounds [3–5]. These environmental factors might impose stress that compromise physiological processes in the bacteria and thereby hamper the catabolic potential present in the microbial community.
Co-contamination with metals has previously been shown to reduce degradation of PAs during remediation of contaminated soil [6]. In agricultural soil, the metal copper (Cu) accumulates as Cu2+ ions or Cu-complexes, often as a consequence of the application of Cu-containing manure from pig productions, where Cu is extensively used as a growth promoter [7]. Although Cu is essential for many biological functions, it becomes a stressor and may even become toxic when available in higher concentrations [8, 9]. Several studies indicate that free Cu ion activities correlate well with observed toxicological effects, suggesting that free Cu2+ constitutes the main toxic species responsible for inhibitory effects towards soil microorganisms [10]. Understanding the link between Cu accumulation and impact on PA degradation in agricultural systems is central in order to protect groundwater resources.
Cu is a redox-active metal that may induce oxidative stress in bacteria [11, 12]. Excess of Cu leads to damage on cell components as lipids, proteins and DNA [13, 14]. One type of Cu damage is caused by a Fenton reaction leading to formation of highly reactive hydroxyl radicals [11]. Further, free Cu+ can destabilize iron-sulfur clusters in important dehydratase enzymes [15]. Oxidative stress arises as a result of increased cellular production of reactive oxygen species (ROS) e.g. hydrogen peroxide (H2O2), hydroxyl (OH·)- and superoxide (O2·−) radicals, followed by a subsequent intracellular accumulation of these to levels that exceed the defense capacity of the cell [16]. The involvement of ROS and oxidative stress in response to Cu accumulation in PA degrading strains has however not previously been investigated.
Bacteria have evolved numerous defense mechanisms to keep the intracellular ROS level low, including enzymatic scavenging by superoxide dismutases and catalases [17]. Launching a defense response nevertheless comes with a cost. As expression of the defense system uses elements of the same transcriptional machinery as the catabolic pathways, induction of the defense system may interfere with the expression of catabolic genes. Hence, even subinhibitory levels of ROS potentially impede biodegradation despite the presence of cognate pollutant substrates. For example, the toluene- and xylene-degrading model organism Pseudomonas putida mt-2 downregulates the catabolic xyl genes in response to oxidative- and other stress inducing conditions in pure culture [18, 19]. Based on these findings, the authors proposed the paradigm that this organism responds to stressful conditions by transferring its transcriptional machinery to adapt to a given stressor before it turns on its catabolic machinery for pollutant degradation [11]; hence, stress endurance prevails over degradation of potential carbon substrates. Whether this response is a general trait for degrader organisms is currently unknown, but it could be one of the explainations why biodegradation of pollutants does not always take place at the expected rates in the environment.
The model PA degrader Cupriavidus pinatubonensis JMP134 carries the full set of tfd genes encoding the enzymatic pathway for complete mineralization of the PA herbicides 2,4-D and MCPA, and is able to utilize these as sole carbon- and energy sources [20]. This bacterium, as well as the isogenic tetracyclin-resistant derivate C. pinatubonensis AEO106 [21], has been extensively studied in relation to understanding xenobiotic degradation. Indeed several studies on expression of the tfdA gene, i.e. the gene catalyzing the first step in the sequential degradation of MCPA, have revealed a close link between catabolic gene expression and active mineralization both in controlled pure cultures and in soil microcosms [1, 20, 22]. Yet, its functional performance in relation to specific environmental stressors, e.g. elevated Cu concentrations, has neither been investigated at the functional nor at the genetic level.
In the current study, we tested the hypotheses that 1) amendment with Cu2+ imposes ROS accumulation in Cupriavidus pinatubonensis and 2) C. pinatubonensis deals with stress release before engaging in catabolic processes. We worked with a polyphasic approach that evaluated the impact of Cu on intracellular ROS accumulation, viability, PA mineralization, and expression of both the catabolic tfdA gene as well as identified a putative oxidative stress response gene in C. pinatubonensis AEO106. The presented data support that the previously proposed paradigm on the trade-off between stress response and catabolic degradation of carbon for growth holds for a wider range of bacteria.
Results
Impact of H2O2-stress on tfdA expression, and establishment of the ohr/osmC gene as a genetic marker for oxidative stress conditions
In initial pure culture experiments C. pinatubonensis was exposed simultaneously to MCPA and hydrogen peroxide (H2O2). H2O2 served as an external ROS source that rapidly enters cells by diffusion. For cells exposed to 1 mM H2O2 and 25 mg L−1 MCPA the average ROS-dependent green fluorescence intensity was 2.3-fold higher than for the control cells after 30 min as measured by the oxidant sensitive probe 2,7-dichlorodihydrofluorescein diacetate (H2DCF-DA) (Fig. 1a). This increase in intracellular ROS coincided with a delayed expression of the tfdA gene i.e. the gene catalyzing the first step in the sequential degradation of MCPA in C. pinatubonensis AEO106. For control cells, tfdA expression peaked 1 h after MCPA addition, whereas a broader peak was observed between 2 and 5 h in H2O2-treated cells (Fig. 2a). No significant differences in viability were detected between the control and the H2O2-treated cells within 8 h after the exposure (t-test, p > 0.05) (Fig. 1b) based on propidium iodide and SYBR Green staining. Hence, the delayed tfdA expression in response to H2O2 could be ascribed to a specific physiological response rather than cell death.
Fig. 1.
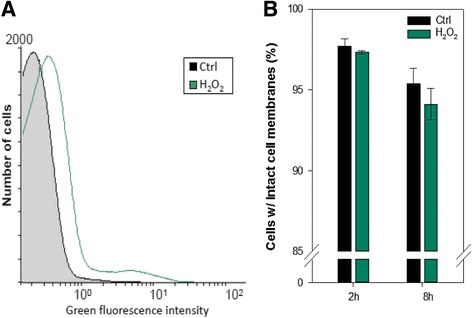
a Determination of intracellular ROS accumulation of cells exposed to 1 mM H2O2. The histogram shows ROS accumulation detected as green fluorescence from ROS-dependent oxidation of the ROS-sensitive probe H2DCF-DA following 30 min of incubation in the presence of H2O2. The experiment was repeated twice in triplicates; the histogram here shows fluorescence values from one representative replica. b Viability of cells following 2 and 8 h of exposure to 1 mM H2O2 measured as cells with intact membranes not stained by propidium iodide. Data are mean values from triplicate cultures from one representative experiment (the experiment was repeated twice). Error bars represents standard deviations
Fig. 2.
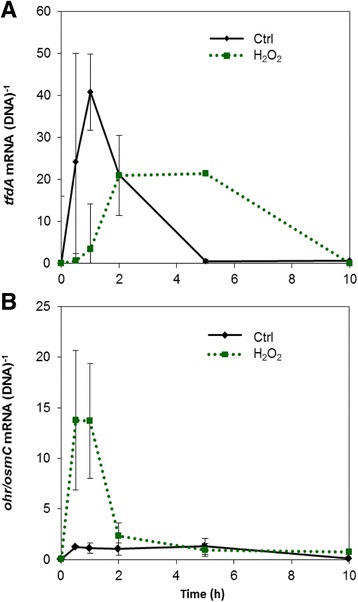
Gene expression by cells exposed to 0 (control) or 1 mM H2O2 measured by qPCR as mRNA normalized to the DNA copy number of the corresponding gene. a Expression of tfdA involved in the first step of MCPA degradation. b Expression of the ohr/osmC-like gene putatively involved in a response to oxidative stress in C. pinatubonensis AEO106. Data are mean values from triplicate cultures from one representative experiment (the experiment was repeated twice). Error bars represent standard errors of means
To investigate if the slower upregulation of tfdA in cells exposed to H2O2 corresponded with a rapid stress release, a putative oxidative stress responsive gene in C. pinatubonensis AEO106 had to be identified for comparison. As no such gene has previously been identified, we performed a search for genes in the C. pinatubonensis AEO106 genome encoding proteins homologous to proteins previously found to be upregulated in response to conditions inducing oxidative stress in C. necator H16 [23]. One of the proteins with the highest expression under the conditions tested by Schwartz and coworkers [23] is the organic hydroperoxide resistance protein Ohr. In C. pinatubonensis AEO16 the product of the gene with locus tag REUT_RS28250 shows 84% amino acid sequence identity to Ohr from H16, and is classified as an osmotically inducible protein, OsmC, by Pfam search. As Ohr/OsmC enzymes belong to a protein family, which is involved in the break-down of hydroperoxides [24, 25], REUT_RS28250 was tested for its response in H2O2-stressed cells. The gene is referred to as ohr/osmC hereafter, as no further attempt to reveal the specific identity of the gene was made in the current study.
Expression of ohr/osmC was induced after 30 min both in the control and the H2O2-treated cells. However, for H2O2-treated cells the up-regulation was approximately 10 fold higher than for control cells (Fig. 2b). The expression was constant throughout the time span of 8 h in the control cells, whereas H2O2-treated cells displayed a clear peak in expression between 30 min and 2 h, i.e. corresponding to the duration of the delay in tfdA expression. Thus, the ohr/osmC gene seemed to be involved in a response against H2O2-induced oxidative stress.
Response to ROS induced by Cu exposure
Cells grown in minimal media amended with MCPA were exposed to three different concentrations of Cu (10, 30 and 50 μM) to examine whether Cu induces ROS accumulation and potential oxidative stress in C. pinatubonensis AEO106, and if so, whether Cu-induced oxidative stress hampers the degradation of MPCA.
Figure 3a shows that a 30-min exposure to Cu led to an increase in ROS-dependent green fluorescence for all tested Cu concentrations. Longer exposure times did not lead to changes in ROS accumulation (data not shown). For the 10 μM Cu treatment the average fluorescence intensity was 13% higher than for the control cells, which is visualized by the thickened tail and a right-shifted population profile in the histogram. No differences were found between the 30 μM and 50 μM incubations where the average fluorescence intensity increased by 30% compared to the control cultures without Cu amendment. Exposure to 10 μM Cu did not cause a significant reduction in the amount of cells with intact cell membranes within the first 8 h (t-test, p > 0.05); however, over 24 h a significant (t-test, p < 0.05), but small decrease in cells with intact membranes were observed for the cells exposed to 10 μM Cu. The highest concentration of Cu (50 μM) caused significant cell death (t-test, p < 0.05) already after 8 h, although viable cells with intact cell membranes accounted for >80% of the total cells still after 24 h (Fig. 3b).
Fig. 3.
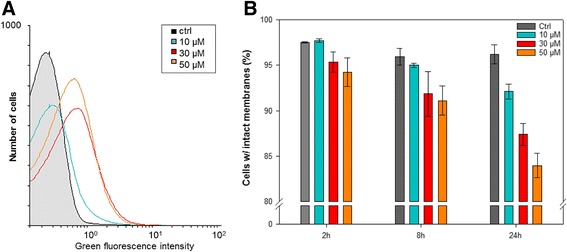
Determination of intracellular ROS accumulation and viability of cells exposed to 0–50 μM CuSO4. a Histogram showing ROS accumulation detected as green fluorescence from ROS-dependent oxidation of the ROS-sensitive probe H2DCF-DA following 30 min of incubation in the presence of Cu. The histogram shows data from a representative experiment. b Viability of cells following 2–24 h of exposure to CuSO4 measured as cells with intact membranes not stained by propidium iodide. Data are mean values from triplicate cultures from one representative experiment (the experiment was repeated twice). Error bars represents standard deviations
The increased amount of intracellular ROS was concurrent with delayed or even impaired growth. Figure 4a shows that non-treated cells started growing immediately, while cells exposed to 10 μM Cu started growing after a lag phase of between 8 and 11 h. Higher concentrations of Cu inhibited cell growth completely during the time course of the experiment.
Fig. 4.
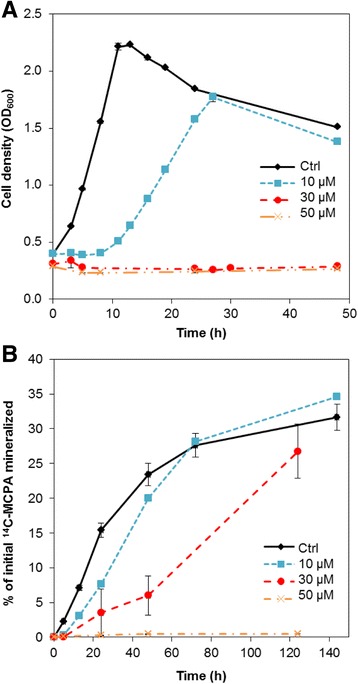
Growth and MCPA mineralization by C. pinatubonensis AEO106 under exposure to 0–50 μM CuSO4. a Growth measured as optical density at 600 nm. b Mineralization of 14C–MCPA measured as accumulation of 14C–CO2 compared to total amount of 14C–MCPA added to the cultures. Data are mean values from triplicate cultures from one representative experiment (the experiment was repeated twice). Error bars represents standard error of means
Mineralization of MCPA was affected by Cu in a concentration-dependent manner. Cells exposed to 10 μM were slower at initiating mineralization than control cells, which corresponded to the delayed growth (compare Fig. 4a and b), but after 72 h the level of mineralization was similar to that observed for the control cells. Though cells exposed to 30 μM Cu did not grow, they were still able to mineralize MCPA after a longer lag phase.
Expression of tfdA was determined within the first 13 h for the control cells and for cells exposed to 10 μM Cu. Cells from both treatments upregulated tfdA within the first hour (Fig. 5a), but the expression level in the control cells was ~3.5 times higher than in the Cu-exposed cells. After 5 h tfdA expression was again downregulated in the control cells; in Cu-treated cells a downregulation appeared already after 1 h. Interestingly, a second tfdA expression peak in Cu-treated cells appeared after 9 h, matching with the onset of mineralization (Fig. 4b). Hence Cu delayed and decreased tfdA expression and only a second expression peak lead to efficient MCPA mineralization.
Fig. 5.
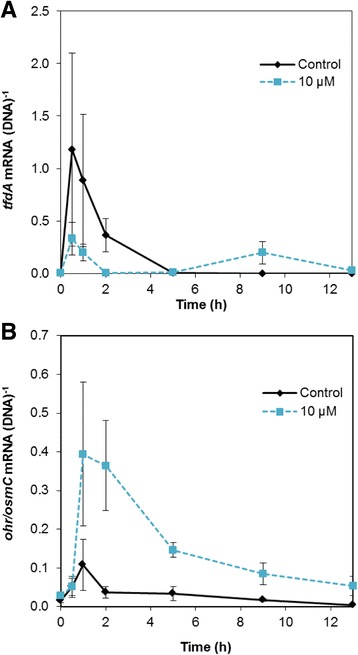
Gene expression by cells in liquid DMM + MCPA exposed to 0 (control) or 10 μM CuSO4 measured by RT-qPCR as mRNA normalized to the DNA copy number of the corresponding gene. a Expression of the catabolic tfdA gene. b Expression of the putatively oxidative stress responsive ohr/osmC-like gene. Data are mean values from triplicate cultures from one representative experiment (the experiment was repeated twice). Error bars represent standard error of means
Subsequently, the expression of ohr/osmC was analyzed to examine if a putative ROS protective mechanism is in play under Cu stress, as observed for cells under H2O2 stress. The expression of ohr/osmC was upregulated ~7 times in control cells after 1 h, but the upregulation was transient and expression quickly went back to a low and steady level. In the Cu-exposed cells the upregulation was at least 30-fold and lasted for at least 5 h (Fig. 5b). After 5 h the expression level in the Cu exposed cells was not statistically significant different from the expression level in the control cells (t-test, p > 0.05).
Hence, exposure to a sublethal concentration (10 μM) of Cu did not prevent induction of gene expression in C. pinatubonensis. Rather, the reduced induction of tfdA coincided with a marked upregulation of ohr/osmC, a gene likely to be involved in an antioxidative response in this bacterium.
Discussion
Little, if anything is known about if and how oxidative stress induced by physicochemical conditions that are relevant for agricultural soil influences degradation of PA herbicides. Oxidative stress hampers expression of catabolic genes in pure cultures of hydrocarbon-degrading pseudomonads [19]; hence, an oxidative stress scenario might influence pesticide-degrading bacteria and their ability to express catabolic genes in a comparable way. In the current work we therefore studied the oxidative stress response caused by Cu exposure at genetic and physiological levels in the model PA degrader strain C. pinatubonensis AEO106 in order to address the question whether stress is dealt with before biodegradation is initiated.
We exposed C. pinatubonensis in liquid culture to concentrations of Cu2+ from 10 to 50 μM. These concentrations are comparable to the concentrations of water extractable Cu that can be found in contaminated agricultural soils [26]. Further, these concentrations are considerably lower than those used to select for Cu-resistant bacteria from soil [26, 27].
Production of antioxidant enzymes e.g. catalases and superoxide dismutases (SODs) are among the major defense lines used by bacteria to exacerbate accumulation of intracellular ROS [8], and detection of their activity is frequently used as evidence for an oxidative stress response [23, 28, 29]. Nevertheless, since changes in gene expression (most often) is the first and most direct response to an environmental change, the expression of a wide array of oxidative stress responsive genes has been studied under various conditions [30–32]. For C. pinatubonensis AEO106/JMP134 no stress responsive genes have been described thus far. We previously identified a gene annotated as a catalase in these strains, but remarkably the gene did not show any response to H2O2 (unpublished data). Interestingly, we on the other hand determined a strong induction of an ohr/osmC gene in response to H2O2. Ohr (organic hydroperoxide resistance protein) and OsmC (osmotically inducible protein C) are homologous proteins belonging to a family of enzymes that are involved in breaking down organic hydroperoxides [33, 34]. Although the proteins belonging to the OsmC subfamily initially were recognized for their role in protection against osmotic stress [34], they are also reported to be involved in the defense against oxidative stress [25, 32, 35, 36]. A study by Saikolappan et al. [37] showed that osmC homologues in Mycobacterium sp. were upregulated as a response to temperature variation and H2O2 exposure, and recently Svenningsen et al. [32] detected increased expression of osmC in Pseudomonas putida exposed to H2O2. In Mycoplasma genitalium, which has the smallest genome among self-replicating bacteria, no genes encoding catalases or SODs have been found [38]. Instead Zhang and coworkers [38] have localized an osmC homologue, which is crucial in the oxidative stress defense of this bacterium. The conservation of osmC in a genome-streamlined bacterium suggests that the gene possesses an important biological function. In contrast to OsmC, the Ohr proteins seem to function specifically against organic hydroperoxides and not against inorganic hydroperoxides [24, 39].
In the current study we observed induction of the ohr/osmC gene under Cu-induced intracellular ROS accumulation. This supports a role of this gene in the response to oxidative stress. However, as the gene is even slightly induced under control growth conditions, we cannot rule out that it may be involved in protection against other stress conditions in AEO106. In contrast to H2O2 that passively diffuses across the cell membrane and accumulates in the exposed cells, accumulation of ROS upon exposure to Cu results from an indirect mechanism possibly involving a Fenton-like reaction, where Cu replaces iron [11, 40]. Another suggestion is that Cu disrupts iron-sulfur complexes leading to elevated iron concentrations, which then drive the Fenton reaction that is responsible for the Cu-mediated ROS accumulation [11]. Hence, it makes sense that the upregulation of the ohr/osmC gene was slower in the Cu exposed cells compared to the H2O2 treated cells, as it takes longer time for the cells to accumulate ROS generated by a physiological process than by diffusion.
Concomitant analysis of transcription dynamics of tfdA and the ohr/osmC gene for C. pinatubonensis AEO106 exposed to H2O2 and Cu, respectively, allowed us to address the question of whether this bacterium copes with stress before initiating biodegradation. Remarkably, the upregulation of the ohr/osmC gene in response to H2O2 coincided very accurately with a delay in expression of the catabolic tfdA gene (encoding the first enzyme involved in MCPA degradation). For cells exposed to Cu, the tfdA expression was reduced and delayed, and expression appeared in two peaks. The downregulation of tfdA after the first peak coincided with upregulation of the ohr/osmC gene. Hence both results for H2O2- and Cu exposure support that stress endurance prevails over biodegradation. Therefore, our current data for C. pinatubonensis strongly indicate that this bacterium cope with stress before initiating biodegradation in agreement with the notion developed by Velázquez and coworkers [19] for another important model strain for biodegradation, Pseudomonas putida mt-2.
A striking observation was that cells exposed to the higher concentrations of Cu (30 μM) were not able to increase their biomass as measured by OD, but they were still able to mineralize MCPA, albeit with a longer lag phase than the cells treated with 10 μM. Increased respiration without growth due to stress is a known phenomenon for bacteria exposed to hydrocarbons that induce stress by disrupting the cell membrane and thereby interfere with energy generation [41]. Cu can inactivate iron-sulfur clusters primarily of enzymes belonging to the dehydratase family [11]. These enzymes are involved in central catabolic pathways e.g. the citric acid cycle [42]. We therefore speculate that the higher doses of Cu impair energy generation, as well as regeneration of reducing powers in the form of NAD(P)H. Depletion of energy and reducing powers might in turn lead to increased activity of catabolic pathways, but at the cost of anabolic pathway activity. For Pseudomonas in particular, there seems to be a tight link between stress endurance and metabolic pathways that are involved in regeneration of the reducing powers from NAD(P)H [43, 44], and Obruca et al. [45] have reported a related link for Cupriavidus necator H16. An alternative explanation for investing in the complete mineralization of MPCA without incorporation of carbon into biomass could be that MCPA, or its degradation products, might be toxic. Hence, the removal of MCPA and degradation products by complete mineralization could be interpreted as part of the stress defense. Nevertheless, we have not been able to detect increased ROS accumulation in AEO106 in response to MCPA exposure (data not shown). In agreement with this observation, neither Delftia acidovorans MC1 nor Pseudomonas putida KT2440 experienced oxidative stress when exposed to PA herbicides in previous studies [46, 47]. However, other stress scenarios upon exposure to such chlorinated aromatic compounds than oxidative stress are also possible, as they are known for instance to impact the cell membrane and uncouple oxidative phosphorylation [47, 48].
Our current results indicate that an oxidative stress protection program is launched prior to PA herbicide mineralization in C. pinatubonensis AEO106. Hence, the ability of this bacterium to degrade PA herbicides under environmental conditions, where it is likely to be continuously confronted with oxidative stress inducing conditions may not primarily depend on induction of the catabolic pathways, but rather on a rapid launching of a stress response.
Conclusion
The effect of ROS on PA herbicide degradation by C. pintubonensis has not been evaluated in previous studies. Here we showed that Cu, which is a relevant stress factor in many agricultural soils, leads to increased accumulation of ROS in C. pinatubonensis AEO106 that in turn launces a protective response against oxidative stress, including a gene homologous to ohr and osmC. The Cu-induced stress results in delayed cell growth, delayed MCPA mineralization and delayed induction of the catabolic tfdA gene. Hence, the data suggest that C. pinatubonensis, like other degraders of xenobiotic compounds, cope with environmental stress before engaging in biodegradation. The novel insight into the stress physiology of PA degrader cells adds valuable input to understanding the soil filter function and highlights the need for including agricultural management practices such as manure application in models predicting leaching of pesticides to groundwater reservoirs.
Methods
Strain and standard medium
Cupriavidus pinatubonensis AEO106 (pRO101) is a tetracyclin resistant derivative of the naturally occurring 2,4-D and MCPA-degrading soil bacterium C. pinatubonensis JMP134 (pJP4), which carries the genes for complete mineralization of 2,4-D and MCPA [49].
C. pinatubonensis AEO106 was routinely grown in Davis Minimal Medium (DMM) (Difco, USA) supplemented with 1 ml L−1 of trace element solution, containing 20 mg CoCl2·6H2O, 30 mg H3BO3, 10 mg ZnSO4·7H2O, 1 mg of CuCl2·2H2O, 2 mg NiCl2·6H2O, 3 mg NaMoO4·2H2O, 10 mg FeSO4·7H2O, and 2.6 mg MnSO4·H2O per liter. The medium was amended with tetracycline to a final concentration of 10 μg mL−1 and incubation was carried out in 500 ml flasks containing 100 ml medium at 28 °C with continuous shaking at 150 rpm.
Growth and mineralization of MCPA under exposure to H2O2 or CuSO4 in pure culture
Overnight cultures of C. pinatubonensis AEO106 were diluted 100× in fresh DMM and incubated until an optical density at 600 nm (OD600nm) of approximately 0.3 was reached; then 20 ml culture was transferred to 100 ml infusion bottles. Sterile-filtered MCPA dissolved in MilliQ water (pH 7), was added to a final concentration of 25 mg L−1. At the same time specific stressors were added: H2O2 (final concentration 1 mM) or Cu (CuSO4; final concentrations 10 μM, 30 μM and 50 μM). Ring-U-14C labelled MCPA (specific activity 5.975 MBq mg−1; radiochemical purity 99.26%; IZOTOP, Budapest, Hungary) was added to a final activity of 10,000 dpm mL−1. A glass vial containing 1 mL 1 M NaOH (basetrap) was placed inside the bottle to trap CO2 produced during incubation. Upon sampling, the content of the traps was transferred to polyethylene vials and mixed with 4 mL scintillation liquid (Optiphase ‘Hisafe’3, Perkin Elmer) while fresh NaOH was added to the CO2 traps. Samples were analyzed in a scintillation counter (Tri-Carp 2910TR, Perkin Elmer), and total mineralization at a given time was calculated as accumulated 14C–CO2 compared to total amount of 14C added to the cultures.
At given time points 100 μL culture was sampled for DNA/RNA extraction (including a sampling point before addition of MCPA and H2O2 or CuSO4), flash frozen in liquid N2 and stored at −70 °C until further processing. Cell growth was measured simultaneously as OD600nm.
Parallel experiments were run for detection of intracellular ROS and for viability staining, but without addition of radiolabeled MCPA. All experiments were performed in triplicates and repeated twice.
Detection of intracellular ROS and viability staining
Detection of intracellular ROS was performed using the oxidant-sensing probe, 2′,7′-dichlorodihydrofluorescein (H2DCF-DA; Sigma Aldrich Co.). After 30 min of incubation in the presence of either H2O2, CuSO4 or without any additional stressor (control), samples of 500 μL culture were incubated for 30 min at room temperature in the dark with 10 μL of 1 mg mL−1 H2DCF-DA prepared in DMSO. The samples were then analyzed by flow cytometry on a BD FACSCalibur flow cytometer (Becton Dickinson, CA) equipped with an argon-ion laser of 15 mW with excitation at 488 nm. Fluorescence from intracellular ROS-dependent oxidation of H2DCF-DA was recorded in the FL1 channel (515–545 nm) after gating of cells in a side scatter (SSC) vs. forward scatter (FSC) plot.
Viability staining and quantification of cells with intact or injured membranes following exposure to H2O2 or CuSO4 by flow cytometry was performed as in DeRoy et al. [50]. Culture samples of 500 μl were incubated in the dark for 30 min with 10 μL mL−1 EDTA (500 mM, pH 8) and 10 μL mL−1 staining solution (400 μM propidium iodide in DMSO from the BacLight Kit; Invitrogen, and 100× SYBR Green I in DMSO; Invitrogen) before flow cytometry analysis. After SSC vs. FSC gating, cells with intact membranes were distinguished from cells with injured membranes in a plot of fluorescence detected in the FL1 vs. FL3 channel (675–715 nm).
Data from at least 75,000 cells were collected from each sample and analyzed in the software CyflogicTM 1.2.1 (CyFlo Ltd.) for all flow cytometry analyses.
Nucleic acid extraction and gene expression analyses
DNA and RNA were co-extracted using the AllPrep DNA/RNA Mini kit (Qiagen). Prior to extraction samples were treated with 10 μL of lysozyme (1 mg mL−1 in 10 mM Tris-Cl, pH 8) for 20 min at room temperature; otherwise the manufacturer’s protocol was followed. RNA was subsequently treated with the RQ1 DNase (Promega) followed by reverse transcription with the Omniscript Reverse Transcriptase (Qiagen) as described previously [32].
Quantitative PCR (qPCR) was performed using SYBR Green detection with the Stratagene Brilliant III Ultra-Fast SYBR® Green QRT-PCR Master Mix (Agilent Technologies) with primers targeting the tfdA gene (Bælum et al., 2006), and primers for the ohr/osmC–like gene putatively involved in an oxidative stress response in C. pinatubonensis AEO106 (this study). The gene was identified based on a BLAST search for ORFs homologues to genes induced under oxidative stress inducing conditions in C. necator H16 [15] using TBLASTX (NCBI). Annotation and protein family membership was searched for by Pfam search of translated nucelotides (http://pfam.xfam.org/).
The qPCRs were performed in 20 μL with 0.4 μM of each primer (see Table 1 for primer details) and 1 μg Bovine Serum Albumin. DNA and cDNA was diluted 1:10 prior to analysis. The PCR programs were 95 °C for 30 s, followed by 40 cycles of 95 °C for 20 s, Ta (Table 1) for 30 s, 72 °C for 45 s. Subsequently a melting curve was run to check for specificity of the amplification products, and qPCRs of DNase treated RNA were run in parallel to test for DNA contamination of the RNA. Standard curves were constructed based on 10-fold dilution series of DNA extracted from a culture of C. pinatubonensis AEO106 with a known OD, and sample tfdA or ohr/osmC gene copy numbers were calculated by relating the Ct values to these standards. No standard curves for cDNA were constructed; instead the cDNA was quantified as DNA equivalents. All standard curves had efficiencies above 80%. Gene copy numbers in cDNA samples were then converted to mRNA equivalents by accounting for dilutions in the DNase-treatment and reverse transcription steps. Finally, cell specific gene expression was calculated as mRNA copies per DNA copies.
Table 1.
List of primers used for qPCR analyses
| Target gene | Primer | Sequence (5′-3′) | Product size (bp) | Ta (°C) | Reference |
|---|---|---|---|---|---|
| tfdA | tfdA**F | GAG CAC TAC GCR CTG AAY TCC CG | 260 | 64 | [20] |
| tfdA**R | CTT CGG CCA CCG GAA GGC CT | ||||
| ohr/osmC | B5559 F | GTC GAG CAC ATT GAC GAA GA | 257 | 60 | This study |
| B5559 R | AGA CCG ATG TGG AGT CGT TC |
The asterisk in the tfdA primer name is merely nomenclature, and does not reflect any reference to statistical or other measures
Acknowledgements
We would like to thank Dorthe Ganzhorn for technical assistance.
Funding
The project was supported by the Villum Kann Rasmussen Foundation through the Center for Environmental and Agricultural Microbiology (CREAM). The Villum Kann Rasmussen Foundation did not participate in the design of the study, in collection, analysis and interpretation of data, or in writing the manuscript.
Availability of data and materials
The datasets produced and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Cu
Copper
- H2DCF-DA
2′,7′-dichlorodihydrofluorescein diacetate
- MCPA
2-methyl-4-chlorophenoxyacetic acid
- OD
Optical density
- PA
Phenoxy acid
- ROS
Reactive oxygen species
Authors’ contributions
MD, NBS and MR carried out the experimental work and analyzed the data. NBS, DPP, MD, ON and MN designed the study, and NBS, MD, ON and MN drafted the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nanna Bygvraa Svenningsen, Email: nbsv@plen.ku.dk.
Mette Damgaard, Email: d_mette@hotmail.com.
Maria Rasmussen, Email: maria.kat.ras@gmail.com.
Danilo Pérez-Pantoja, Email: dperezpantoja@gmail.com.
Ole Nybroe, Email: oln@plen.ku.dk.
Mette Haubjerg Nicolaisen, Email: meni@plen.ku.dk.
References
- 1.Bælum J, Nicolaisen MH, Holben WE, Strobel BW, Sørensen J, Jacobsen CS. Direct analysis of tfdA gene expression by indigenous bacteria in phenoxy acid amended agricultural soil. ISME J. 2008;2:677–687. doi: 10.1038/ismej.2008.21. [DOI] [PubMed] [Google Scholar]
- 2.Bælum J, Prestat E, David MM, Strobel BW, Jacobsen CS. Modeling of phenoxy acid herbicide mineralization and growth of microbial degraders in 15 soils monitored by quantitative real-time PCR of the functional tfdA gene. Appl Environ Microbiol. 2012;78:5305–5312. doi: 10.1128/AEM.00990-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Veen JA, Van Overbeek S, Van Elsas JD. Fate and activity of microorganisms introduced into soil. Microbiol Mol Biol R. 1997;61:121–135. doi: 10.1128/mmbr.61.2.121-135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Or D, Smets BF, Wraith JM, Dechesne a, Friedman SP. Physical constraints affecting bacterial habitats and activity in unsaturated porous media – a review. Adv Water Resour. 2007;30:1505–1527. doi: 10.1016/j.advwatres.2006.05.025. [DOI] [Google Scholar]
- 5.Roszak DB, Colwell RR. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987;51:365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roane TM, Josephson KL, Pepper IL. Dual-bioaugmentation strategy to enhance remediation of cocontaminated soil. Appl Environ Microbiol. 2001;67:3208–3215. doi: 10.1128/AEM.67.7.3208-3215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovi P, Bonazzi G, Maestri E, Marmiroli N. Accumulation of copper and zinc from liquid manure in agricultural soils and crop plants. Plant Soil. 2003;250:249–257. doi: 10.1023/A:1022848131043. [DOI] [Google Scholar]
- 8.Bååth E. Effects of heavy metals in soil on microbial processes and populations (a review) Water Air Soil Pollut. 1989;47:335–379. doi: 10.1007/BF00279331. [DOI] [Google Scholar]
- 9.Vulkan R, Zhao F-J, Barbosa-Jefferson V, Preston S, Paton GI, Tipping E, McGrath SP. Copper speciation and impacts on bacterial biosensors in the pore water of copper-contaminated soils. Environ Sci Technol. 2000;34:5115–5121. doi: 10.1021/es0000910. [DOI] [Google Scholar]
- 10.Nybroe O, Brandt KK, Ibrahim YM, Tom-Petersen A, Holm PE. Differential bioavailability of copper complexes to bioluminescent Pseudomonas Fluorescens reporter strains. Environ Toxicol Chem. 2008;27:2246–2252. doi: 10.1897/08-025.1. [DOI] [PubMed] [Google Scholar]
- 11.Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teitzel GM, Parsek MR. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl Environ Microbiol. 2003;69:2313–2320. doi: 10.1128/AEM.69.4.2313-2320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osman D, Cavet JS. Copper homeostasis in bacteria. Adv Appl Microbiol. 2008;65:217–247. doi: 10.1016/S0065-2164(08)00608-4. [DOI] [PubMed] [Google Scholar]
- 14.Solioz M, Abict HK, Mermod M, Mancini S. Response of gram-positive bacteria to copper stress. J Biol Inorg Chem. 2010;15:3–14. doi: 10.1007/s00775-009-0588-3. [DOI] [PubMed] [Google Scholar]
- 15.Chillappagari S, Seubert A, Trip H, Kuipers OP, Marahiel MA, Miethke M. Copper stress affects iron homeostasis by destabilizing iron-sulfur cluster formation in Bacillus subtilis. J Bacteriol. 2010;192:2512–2524. doi: 10.1128/JB.00058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabiscol E, Tamarit J, Ros J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol. 2000;3:3–8. [PubMed] [Google Scholar]
- 17.Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol. 2013;11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domínguez-Cuevas P, González-Pastor JE, Marqués S, Ramos JL, de Lorenzo V. Transcriptional tradeoff between metabolic and stress-response programs in Pseudomonas putida KT2440 cells exposed to toluene. J Biol Chem. 2006;281:11981–11991. doi: 10.1074/jbc.M509848200. [DOI] [PubMed] [Google Scholar]
- 19.Velázquez F, de Lorenzo V, Valls M. The m-xylene biodegradation capacity of Pseudomonas putida mt-2 is submitted to adaptation to abiotic stresses: evidence from expression profiling of xyl genes. Environ Microbiol. 2006;8:591–602. doi: 10.1111/j.1462-2920.2005.00936.x. [DOI] [PubMed] [Google Scholar]
- 20.Leveau JH, van der Meer JR. The tfdR gene product can successfully take over the role of the insertion element-inactivated TfdT protein as a transcriptional activator of the tfdCDEF gene cluster, which encodes chlorocatechol degradation in Ralstonia eutropha JMP134 (pJP4) J Bacteriol. 1996;178:6824–6832. doi: 10.1128/jb.178.23.6824-6832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harker AR, Olsen RH, Seidler RJ. Phenoxyacetic acid degradation by the 2,4-dichlorophenoxyacetic acid (TFD) pathway of plasmid pJP4: mapping and characterization of the TFD regulatory gene, tfdR. J Bacteriol. 1989;171:314–320. doi: 10.1128/jb.171.1.314-320.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolaisen MH, Bælum J, Jacobsen CS, Sørensen J. Transcription dynamics of the functional tfdA gene during MCPA herbicide degradation by Cupriavidus necator AEO106 (pRO101) in agricultural soil. Environ Microbiol. 2008;10:571–579. doi: 10.1111/j.1462-2920.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz E, Voigt B, Zühlke D, Pohlmann A, Lenz O, Albrecht D, et al. A proteomic view of the facultatively chemolithoautotrophic lifestyle of Ralstonia eutropha H16. Proteomics. 2009;9:5132–5142. doi: 10.1002/pmic.200900333. [DOI] [PubMed] [Google Scholar]
- 24.Alegria TGP, Meireles DA, Cussiol JRR, Hugo M, Trujillo M, de Oliveira MA, et al. Ohr plays a central role in bacterial responses against fatty acid hydroperoxides and peroxynitrite. Proc Natl Acad Sci U S A. 2016;114:E132–E141. doi: 10.1073/pnas.1619659114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lesniak J, Nikolov DB, Barton WA. Structural and functional features of the Escherichia coli hydroperoxide resistance protein OsmC. Prot Sci. 2003;12:2838–2843. doi: 10.1110/ps.03375603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg J, Brandt KK, Al-Soud WA, Holm PE, Hansen LH, Sørensen SJ, Nybroe O. Selection for cu-tolerant bacterial communities with altered composition, but unaltered richness, via long-term cu exposure. Appl Environ Microbiol. 2012;78:7438–7446. doi: 10.1128/AEM.01071-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg J, Tom-Petersen A, Nybroe O. Copper amendment of agricultural soil selects for bacterial antibiotic resistance in the field. Lett Appl Microbiol. 2005;40:146–151. doi: 10.1111/j.1472-765X.2004.01650.x. [DOI] [PubMed] [Google Scholar]
- 28.Krayl M, Benndorf D, Loffhagen N, Babel W. Use of proteomics and physiological characteristics to elucidate ecotoxic effects of methyl tert-butyl ether in Pseudomonas putida KT2440. Proteomics. 2003;3:1544–1552. doi: 10.1002/pmic.200300477. [DOI] [PubMed] [Google Scholar]
- 29.Peters LP, Carvalho G, Martins PF, Dourado MN, Vilhena MB, Pileggi M, et al. Differential responses of the antioxidant system of Ametryn and Clomazone tolerant bacteria. PLoS One. 2014;9:e112271. doi: 10.1371/journal.pone.0112271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jangiam W, Loprasert S, Smith DR. Tungpradabkul. Burkholderia pseudomallei RpoS regulates OxyR and the katG-dpsA operon under conditions of oxidative stress. Microbiol Immunol. 2010;54:389–397. doi: 10.1111/j.1348-0421.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- 31.Ochsner UA, Vasil ML, Alsabbagh E, Parvatiyar K, Hassett DJ. Role of the Pseudomonas aeruginosa oxyR-recG operon in oxidative stress defense and DNA repair: OxyR-dependent regulation of katB-ankB, ahpB and ahpC-ahpF. J Bacteriol. 2000;182:4533–4544. doi: 10.1128/JB.182.16.4533-4544.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svenningsen NB, Pérez-Pantoja D, Níkel NMH, de Lorenzo V, Nybroe O. Pseudomonas putida mt-2 tolerates reactive oxygen species generated during matric stress by inducing a major oxidative defense response. BMC Microbiol. 2015;15:202. doi: 10.1186/s12866-015-0542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mongkolsuk S, Praituan W, Loprasert S, Fuangthong M, Chamnongpol S. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestri pv. Phaseoli. J Bacteriol. 1998;180:2636–2643. doi: 10.1128/jb.180.10.2636-2643.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atichartpongkul S, Loprasert S, Vattanaviboon P, Whangsuk W, Helmann JD, Mongkolsuk S. Bacterial Ohr and OsmC paralogues define two protein families with distinct functions and patterns of expression. Microbiology. 2001;147:1775–1782. doi: 10.1099/00221287-147-7-1775. [DOI] [PubMed] [Google Scholar]
- 35.Conter A, Sturny R, Gutierrez C, Cam K. The RcsCB his-asp phosphorelay system is essential to overcome chlorpromazine-induced stress in Escherichia coli. J Bacteriol. 2002;184:2850–2853. doi: 10.1128/JB.184.10.2850-2853.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutierrez C, Devedjian JC. Osmotic induction of gene osmC expression in Escherichia coli K12. J Mol Biol. 1991;220:959–973. doi: 10.1016/0022-2836(91)90366-E. [DOI] [PubMed] [Google Scholar]
- 37.Saikolappan S, Das K, Sasindran SJ, Jagannath C, Dhandayuthapani S. OsmC proteins of Mycobacterium tuberculosis and Mycobacterium smegmatis protect against organic hydroperoxide stress. Tuberculosis. 2011;91:119–127. doi: 10.1016/j.tube.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Baseman JB. Functional characterization of osmotically inducible protein C (MG_427) from Mycoplasma genitalium. J Bacteriol. 2014;196:1012–1019. doi: 10.1128/JB.00954-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lesniak J, Barton W, Nikolov D. Structural and functional characterization of the Pseudomonas hydroperoxide resistance protein Ohr. EMBO J. 2002;21:6649–6659. doi: 10.1093/emboj/cdf670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinto E, Sigaud-kutner TCS, Leitão MAS, Okamoto OK, Morse D, Colepicolo P. Heavy metal-induced oxidative stress in algae. J Phycol. 2003;39:1008–1018. doi: 10.1111/j.0022-3646.2003.02-193.x. [DOI] [Google Scholar]
- 41.Labud V, Garcia C, Hernandez T. Effect of hydrocarbon pollution on the microbial properties of a sandy and clay soil. Chemosphere. 2006;66:1863–1871. doi: 10.1016/j.chemosphere.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 42.Flint DH, Emptage MH, Guest JR, Fumarase A. From Escherichia coli: Putification and characterization as an iron-sulfur cluster containing enzyme. Biochemist. 1992;31:10331–10337. doi: 10.1021/bi00157a022. [DOI] [PubMed] [Google Scholar]
- 43.Chavarría M, Nikel PI, Pérez-Pantoja D, de Lorenzo V. The Entner-Doudoroff pathway empowers Pseudomonas putida KT2440 with a high tolerance to oxidative stress. Environ Microbiol. 2013;15:1772–1785. doi: 10.1111/1462-2920.12069. [DOI] [PubMed] [Google Scholar]
- 44.Mailloux RJ, Lemire J, Appanna VD. Metabolic networks to combat oxidative stress in Pseudomonas fluorescens. Antonie Van Leeuwenhoek. 2011;99:433–442. doi: 10.1007/s10482-010-9538-x. [DOI] [PubMed] [Google Scholar]
- 45.Obruca S, Marova I, Stankova M, Mravcova L, Svoboda Z. Effect of ethanol and hydrogen peroxide on poly(3-hydroxybutyrate) biosynthetic pathway in Cupriavidus necator H16. World J Microbiol Biotechnol. 2010;26:1261–1267. doi: 10.1007/s11274-009-0296-8. [DOI] [PubMed] [Google Scholar]
- 46.Benndorf D, Babel W. Assimilatory detoxification of herbicides by Delftia acidovorans MC1: induction of two chlorocatechol 1,2-dioxygenases as a response to chemostress. Microbiology. 2002;147:2883–2888. doi: 10.1099/00221287-148-9-2883. [DOI] [PubMed] [Google Scholar]
- 47.Benndorf D, Thiersch M, Loffhagen N, Kunath C, Harms H. Pseudomonas putida KT2440 responds specifically to chlorophenoxy herbicides and their initial metabolites. Proteomics. 2006;6:3319–3329. doi: 10.1002/pmic.200500781. [DOI] [PubMed] [Google Scholar]
- 48.García-Cruz U, Celis LB, Poggi H, Meraz M. Inhibitory concentrations of 2,4D and its possible intermediates in sulfate reducing biofilms. J Hazardous Metals. 2010;179:591–595. doi: 10.1016/j.jhazmat.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 49.Pemberton JM, Corney B, Don RH. Evolution and spread of pesticide degrading ability among soil microorganisms. In: Plasmids of medical, environmental and commercial importance. 1979. Timmis, KN, Pühler a (eds). Amsterdam: Elsevier/North-Holland Biomedical Press. p. 287–99.
- 50.De Roy K, Clement L, Thas O, Wang Y, Boon N. Flow cytometry for fast microbial community fingerprinting. Water Res. 2012;46:907–919. doi: 10.1016/j.watres.2011.11.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets produced and analyzed during the current study are available from the corresponding author on reasonable request.


