Abstract
Background:
The relationship between cortisol level and sepsis is not known in Indian patients of severe sepsis/septic shock.
Aims:
The study was done to determine the optimal range of cortisol levels, defining the adrenocortical response, and predicting the mortality, if possible, in the above type of patients.
Settings and Designs:
The study was a single-centered prospective cohort study, conducted in a tertiary referral center, North India.
Materials and Methods:
Sixty patients with severe sepsis (n = 30) and septic shock (n = 30) were recruited. Basal and postcosyntropin (1 μg)-stimulated cortisol levels were measured, and all patients were closely monitored with daily assessments of clinical and laboratory variables. Western diagnostic criteria were followed for defining adrenal insufficiency (AI). The end point was the survival assessed at day 28 or death, whichever came earlier.
Results:
The mean basal (T0) and poststimulation (T30) cortisol levels were 31.77 ± 15.9 μg/dL and 37.58 ± 17.31 μg/dL, respectively. In all sepsis patients, 48.33% qualified as AI at T0 ≤ 24 μg/dL, 61.67% at delta cortisol (Δ = T30-T0) ≤7 μg/dL, and 78.33% at Δ ≤9 μg/dL. Using receiver operating characteristic curve, the area under the curve (AUC) was 0.4954, signifying poor prediction to death.
Conclusions:
Indians have completely different characteristics of cortisol levels in sepsis patients, in comparison to the Western data. They have higher range of basal cortisol levels, higher percentage of AI, and an inability to predict mortality with the cortisol levels. Hence, there is requirement of an international study to confirm the dichotomy of the results.
Keywords: Adrenal insufficiency, corticosteroid, cosyntropin stimulation test, critical illness-related corticosteroid insufficiency
INTRODUCTION
Hemodynamic response in sepsis patients is always co-linked with the functioning of hypothalamic-pituitary-adrenal (HPA) axis.[1,2] Dysfunction of the HPA axis in this contest is known as critical illness-related corticosteroid insufficiency (CIRCI), which is defined as inadequate cellular corticosteroid activity for the severity of the illness.[3] CIRCI occurs either due to decrease in adrenal steroid production (adrenal insufficiency [AI]) or tissue resistance to steroids. The overall incidence of AI in critically ill patients is approximately 20%, with an incidence as high as 60% in patients with severe sepsis and septic shock.[4] Investigators have been using a lack of increment of cortisol in response to corticotropin/cosyntropin adrenocorticotropic hormone (ACTH) or an inappropriately low level or both to diagnose AI.[1,2,5,6] However, as per prevalent guideline, AI is best diagnosed by a delta cortisol (Δ) (after 250 μg cosyntropin) of <9 μg/dL or a random total cortisol of <10 μg/dL.[3] Unfortunately, there is no study from India to support this. This is important because sepsis-activating HPA axis may vary according to the types of infections, chronicity of the illness, and immunity of the individual.
Henceforth, we did a prospective cohort study in a single referral center of North India to answer few hypothesized questions as per our population type: What is the optimal range of cortisol levels in severe sepsis/septic shock patients? How adrenocortical response is defined in these patients? And, can cortisol levels be a predictor of mortality in these patients?
MATERIALS AND METHODS
Study settings
The study was a prospective cohort study, conducted in the Department of Internal Medicine, a referral center at New Delhi, India, from July 2009 to March 2011. It was done to determine the range of basal and poststimulation serum cortisol levels in a group of sepsis patients diagnosed with uniform criteria to define AI and to predict death with the serum cortisol levels.
Participants
Included patients were with severe sepsis and septic shock as defined by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference 1992.[7] Severe sepsis was defined as sepsis with one or more signs of organ dysfunction, which included parameters of cardiovascular system, renal system, respiratory system, hematological system, unexplained metabolic acidosis, or inadequate fluid resuscitation. Septic shock was defined as sepsis with hypotension (arterial blood pressure <90 mmHg systolic, or 40 mmHg less than patients' normal blood pressure) for at least 1 h despite adequate fluid resuscitation or need for vasopressors to maintain systolic blood pressure ≥90 mmHg or mean arterial pressure (MAP) ≥70 mmHg.
Excluded patients were with prior pituitary adrenal axis dysfunction, known HIV infection, pregnancy, and already on treatment with corticosteroids or other drugs known to interfere with the HPA axis.
Study variables
Clinical variables measured were as follows:
Demography including age, gender, admission details, and diagnosis at admission
Severity of illness assessed by the Acute Physiology and Chronic Health Evaluation (APACHE) II score and urinary output
Interventions (at physician's discretion) including volume of fluid infusion per 24 h, antibiotics, type and titration of vasopressors, surgical procedures, and the need for mechanical ventilation.
Laboratory variables measured were hematology and biochemical parameters, blood gas analysis, and cultures from the specimen drawn from the site of infection and blood. Random baseline total serum cortisol level was measured (T0). Poststimulation level was collected after 30 min of intravenous injection of 1 μg of tetracosactrin (synthetic ACTH) (T30). The samples thus drawn were sent to the laboratory and centrifuged at 4000 rpm for 5 min and serum separated. The samples would then be stored at 2°C–8°C, and serum cortisol levels were measured using chemiluminescent microparticle immunoassay kits (Architect systems, USA). The cortisol increment levels (Δ, T30-T0) were determined. Although the low-dose (1 μg) ACTH stimulation test was not recommended in defining AI despite being more physiologic and having greater sensitivity than the high dose (250 μg) stimulation test due to limited data, we used the former method considering our different population type and the above characteristics.[3,8,9,10] Similarly, the guideline had supported the diagnosis of AI by a Δ (after 250 μg cosyntropin) of <9 μg/dL or a random total cortisol of <10 μg/dL; however, we took few other cutoff values to define it, which were random total cortisol level of <24 μg/dL and Δ level of < 7 μg/dL and of <9 μg/dL, based on important Western studies.[3,11,12,13]
All patients recruited in the study were closely monitored. The daily assessment was a composite of above clinical conditions, hemodynamics, and laboratory variables. The end point was the survival assessed at day 28 or death, whichever came earlier.
Statistical analysis
The data storage and analysis were performed using Microsoft Excel and STATA® software (version 13, (Version 13, StataCorp, College Station, Texas), respectively. For categorical variables, frequency and percentage were calculated and compared with the help of Chi-square test. Continuous variables were expressed as mean ± standard deviation and compared with unpaired t-test and Mann–Whitney U-test as per the data distribution. The cutoff estimates were found for baseline cortisol and Δ levels, and to predict the mortality, the receiver operating characteristic (ROC) curve was used. Univariate and then multivariate regression analyses were done to determine the predictors of death. Odds ratio (OR, 95% confidence interval) were calculated by logistic regression analysis. P ≤0.05 was considered statistically significant.
Ethical approval and consent
The protocol was approved by the Institutional Ethical Committee, and informed consent was obtained from the patient's next of kin.
RESULTS
Baseline characteristics
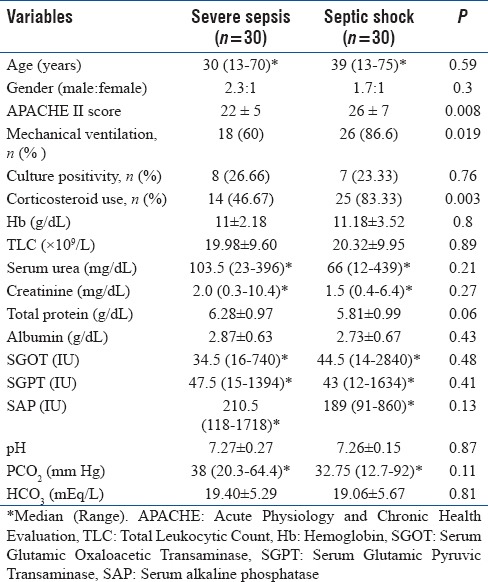
One hundred and thirty-seven patients were screened, out of which sixty patients were found to satisfy criteria proposed for the study. They were in two groups; severe sepsis (n = 30) and septic shock (n = 30). Figure 1 shows the study flowsheet. Both patient groups were comparable in the demographic variables [Table 1]. Culture positivity, hemogram, liver and kidney function tests, and blood gas parameters were even comparable; however, few parameters were significantly different, which includes APACHE II score (22 vs. 26, P = 0.008), mechanical ventilation rate (18 vs. 26, P = 0.019), and corticosteroid uses (14 vs. 25, P = 0.003). These differences were physiological because of very low arterial pressure values, more use of steroids, and more requirements of ventilations in the septic shock group. All patients in the same group had vasopressor support.
Figure 1.
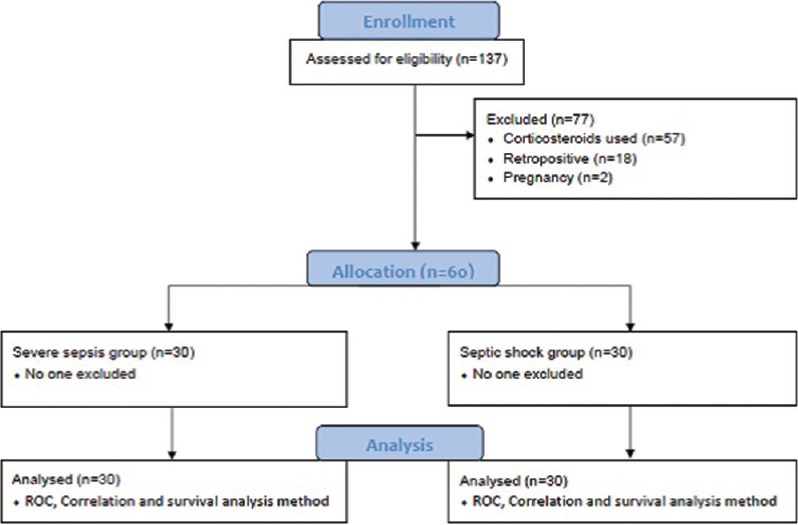
Study flowsheet. ROC: Receiver operating characteristics
Table 1.
Baseline characteristics between severe sepsis and septic shock patients
Primary outcome analysis
The overall mean of total serum cortisol levels at T0 and T30 were 31.77 ± 15.9 μg/dL and 37.58 ± 17.31 μg/dL, respectively, and the difference between the two groups was insignificantly minimal [Figure 2, Box plot graph]. The overall mean Δ level was 5.82 ± 3.29 μg/dL. However, among the two groups of severe sepsis and septic shock, there was significant increments in cortisol levels (Δ), with 5.73 ± 3.55 μg/dL (P = 0.0001) and 5.89 ± 3.09 μg/dL (P = 0.0001), respectively.
Figure 2.
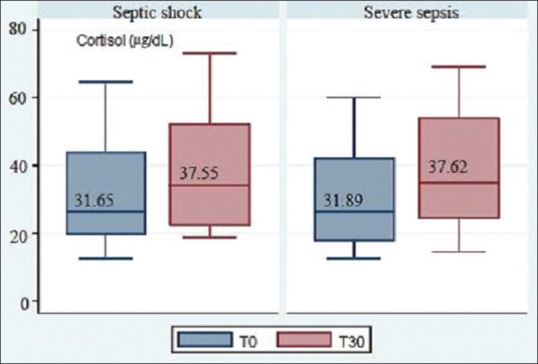
Box plot showing the rise in cortisol levels following adrenocorticotropic hormone stimulation test in both groups
Using the different cutoffs prescribed by the Western studies, discussed above, to define AI, the following results were obtained. When total cortisol level <24 μg/dL was used to define, 48.33% of the patients qualified as AI. When a Δ <7 μg/dL was used to define, 61.67% of the patients categorized as being AI. Using the cutoff value of Δ <9 μg/dL, the incidence was increased to 78.33%.
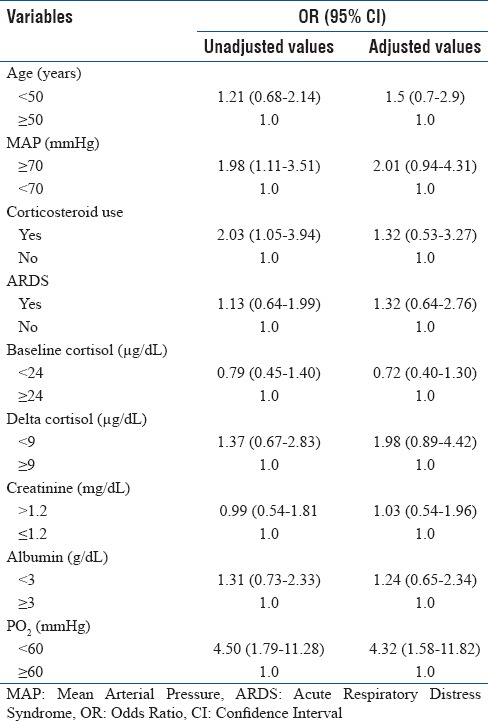
Using ROC analysis, area under the curve (AUC) was 0.4954 [Figure 3]. When Δ ≥4.6 μg/dL was used as a predictor of mortality, the study showed poor sensitivity (61.22%) and poor specificity (54.55%) and hence, it could not predict the mortality (AUC, near 1 is considered to be good). The positive likelihood ratio was also low (1.34). Apart from cortisol levels, various clinical, biological, and laboratory parameters were analyzed for possible prediction toward mortality. On univariate analysis, hypoxia (PO2<60 mmHg) was found to be a significant predictor of the mortality (OR, 4.50) along with MAP <70 mmHg (OR, 1.98) and lack of corticosteroid use (OR, 2.03). On multivariate analysis too, patients with hypoxia had 4.32 times more odd of dying than nonhypoxic group of patients. Other variables were not significantly associated with the mortality [Table 2].
Figure 3.
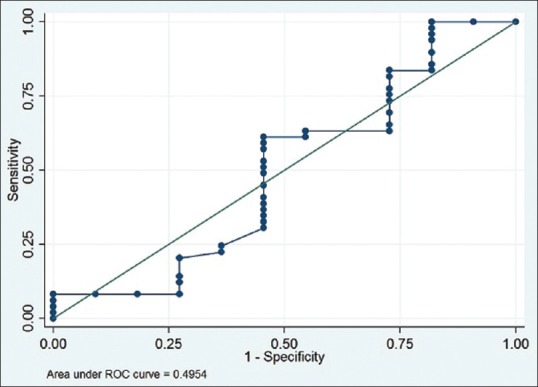
Receiver operating characteristic curve analysis of delta cortisol (T30-T0) values with reference to 28-day mortality
Table 2.
Multivariate logistic regression analysis of the clinically important variables of the study population
DISCUSSION
This is the first Indian study on adult sepsis patients, characteristically describes basal, peak after low-dose ACTH stimulation, and delta serum cortisol levels, defining AI with respect to different criteria, and in predicting the death. In comparison to previous studies, it has major differences with respect to primary objectives. However, at this moment of time, it is difficult to say that these are characteristics of our population type (Indian) without direct comparison in an international study using the same study protocol.
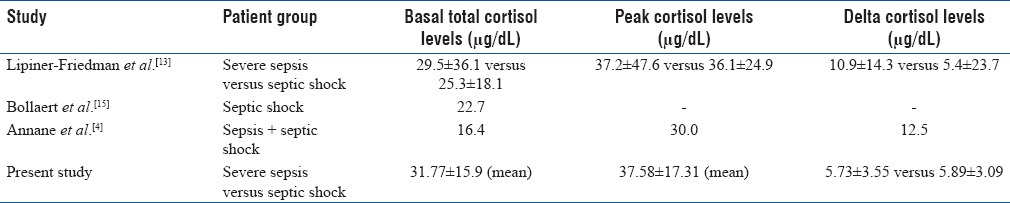
To answer the first hypothesis of “cortisol levels in Indian sepsis patients,” the present study shows higher ranges of basal (>30 μg/dL), peak (>35 μg/dL), and Δ (>5 μg/dL) levels. There is only one Indian study, done in the children, which shows characteristically higher cortisol levels (>70 μg/dL).[14] Although both studies including the present one show higher ranges of cortisol levels in sepsis patients, it will be premature to infer anything from these Indian studies. If we see the Western data of adult patients, lower values (<30 μg/dL) of total cortisol levels have been found compared to our study [Table 3].[4,13,15] We may speculate the reasons for this major difference among Indian and Western data based on different population characteristics and the study protocol, but we need evidences for the same with the help of international studies, which are lacking at the moment. A high mean basal cortisol value would mean that, prior to the ACTH stimulation itself, the adrenal is already in a stimulated state and its ability to respond to continuous stress is limited. If we compare in-between the two sepsis groups, the difference in mean Δ levels is insignificantly very less. However, a study by Lipiner-Friedman et al. shows it to be significantly greater in severe sepsis than in septic shock patients.[13]
Table 3.
Comparison of basal, peak, and delta cortisol levels among various studies
To answer the second hypothesis of “adrenocortical response in Indian sepsis patients,” the present study establishes approximately half of sepsis patients to be AI using criteria of T0 <24 μg/dL, which is lower than the Western study that reported to be 61%.[16] This is due to use of different criteria for defining AI. Whereas, when we use the criteria of cortisol increment (Δ <7 μg/dL and Δ <9 μg/dL), higher percentages of AI are found compared to the Western data (61.67% and 78.33% vs. 34% and 38%).[11,12] This variability among different criteria may be due to a different profile of the patient populations. Indians have higher prevalence of malnutrition, tuberculosis, parasitic infections, and other chronic infectious diseases; these may have contributed to this phenomenon. Persistent stimulations as discussed above lead to exhaustion of adrenal function and on further ACTH stimulation, even physiologic doses (1 μg), it is showing higher rate of AI. Cause of this disparity has to be evaluated in the future clinical studies. Henceforth, the cutoff to define AI in sepsis patients remains highly variable till date if we consider the different population types.
To answer the third hypothesis of “cortisol levels as predictor of mortality in sepsis patients,” we analyze basal and Δ values using ROC analysis with respect to 28-day mortality. However, it fails in predicting the death rate (AUC, 0.4954 and low likelihood ratio). The poor specificity and sensitivity may be due to very small sample size of survived patients. The present study also does not find any predictors of death except hypoxia, which is physiological too, on multivariate analysis. Bollaert et al. in a retrospective study show a basal cortisol level >20 μg/dL and Δ level <9 μg/dL which are independent predictors of 28-day mortality.[17] However, our study confirms that the cortisol levels may not be used as predictor of death or criteria for prognostication in sepsis.
The study has limitations. Not being a randomized control trial, it has its own demerits. Smaller sample size and single-center nature of the study are the major limitations to make it less generalized even in our own population (e.g., South India). Our study is based on total serum cortisol levels and not the free cortisol levels which might negate the effects of low levels of albumin and cortisol-binding globulins in critically ill patients. Although the guideline does not recommend free cortisol levels in replacing total concentration for diagnosing AI, the dissociation between the two is most prominent at serum albumin <2.5 g/dL[3,18] That might be one reason why the study does not find cortisol levels as a useful predictor of mortality contradicting Western literature reviews where free levels are used.
CONCLUSIONS
Baseline total cortisol levels are in higher range in Indian sepsis cohort in contrast to the Western population; the reason is not known. Indian patients have higher percentage of AI and cortisol levels (both basal and postcosyntropin stimulation) which are not true predictors of mortality in patients with sepsis, which is also negating the Western data. This study evokes urgency of a multicenter (both national and international) study to confirm the dichotomy of results and to solve the issue of the diagnostic criteria for AI in sepsis patients, especially Indian patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bouachour G, Tirot P, Gouello JP, Mathieu E, Vincent JF, Alquier P. Adrenocortical function during septic shock. Intensive Care Med. 1995;21:57–62. doi: 10.1007/BF02425155. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM, Udwadia ZF, Lawler PG. Cortisol response to corticotropin and survival in septic shock. Lancet. 1991;337:582–3. doi: 10.1016/0140-6736(91)91641-7. [DOI] [PubMed] [Google Scholar]
- 3.Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: Consensus statements from an international task force by the American college of Critical Care Medicine. Crit Care Med. 2008;36:1937–49. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 4.Annane D, Maxime V, Ibrahim F, Alvarez JC, Abe E, Boudou P. Diagnosis of adrenal insufficiency in severe sepsis and septic shock. Am J Respir Crit Care Med. 2006;174:1319–26. doi: 10.1164/rccm.200509-1369OC. [DOI] [PubMed] [Google Scholar]
- 5.Soni A, Pepper GM, Wyrwinski PM, Ramirez NE, Simon R, Pina T, et al. Adrenal insufficiency occurring during septic shock: Incidence, outcome, and relationship to peripheral cytokine levels. Am J Med. 1995;98:266–71. doi: 10.1016/S0002-9343(99)80373-8. [DOI] [PubMed] [Google Scholar]
- 6.Zaloga GP, Marik P. Hypothalamic-pituitary-adrenal insufficiency. Crit Care Clin. 2001;17:25–41. doi: 10.1016/s0749-0704(05)70150-0. [DOI] [PubMed] [Google Scholar]
- 7.American college of chest physicians/society of critical care medicine consensus conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 8.Mayenknecht J, Diederich S, Bähr V, Plöckinger U, Oelkers W. Comparison of low and high dose corticotropin stimulation tests in patients with pituitary disease. J Clin Endocrinol Metab. 1998;83:1558–62. doi: 10.1210/jcem.83.5.4831. [DOI] [PubMed] [Google Scholar]
- 9.Rasmuson S, Olsson T, Hagg E. A low dose ACTH test to assess the function of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf) 1996;44:151–6. doi: 10.1046/j.1365-2265.1996.600482.x. [DOI] [PubMed] [Google Scholar]
- 10.Abdu TA, Elhadd TA, Neary R, Clayton RN. Comparison of the low dose short synacthen test (1 microg), the conventional dose short synacthen test (250 microg), and the insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in patients with pituitary disease. J Clin Endocrinol Metab. 1999;84:838–43. doi: 10.1210/jcem.84.3.5535. [DOI] [PubMed] [Google Scholar]
- 11.Tordjman K, Jaffe A, Trostanetsky Y, Greenman Y, Limor R, Stern N. Low-dose (1 microgram) adrenocorticotrophin (ACTH) stimulation as a screening test for impaired hypothalamo-pituitary-adrenal axis function: Sensitivity, specificity and accuracy in comparison with the high-dose (250 microgram) test. Clin Endocrinol (Oxf) 2000;52:633–40. doi: 10.1046/j.1365-2265.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- 12.Zarkovic M, Ciric J, Stojanovic M, Penezic Z, Trbojevic B, Drezgic M, et al. Optimizing the diagnostic criteria for standard (250-microg) and low dose (1-microg) adrenocorticotropin tests in the assessment of adrenal function. J Clin Endocrinol Metab. 1999;84:3170–3. doi: 10.1210/jcem.84.9.5947. [DOI] [PubMed] [Google Scholar]
- 13.Lipiner-Friedman D, Sprung CL, Laterre PF, Weiss Y, Goodman SV, Vogeser M, et al. Adrenal function in sepsis: The retrospective Corticus cohort study. Crit Care Med. 2007;35:1012–8. doi: 10.1097/01.CCM.0000259465.92018.6E. [DOI] [PubMed] [Google Scholar]
- 14.Sarthi M, Lodha R, Vivekanandhan S, Arora NK. Adrenal status in children with septic shock using low-dose stimulation test. Pediatr Crit Care Med. 2007;8:23–8. doi: 10.1097/01.pcc.0000256622.63135.90. [DOI] [PubMed] [Google Scholar]
- 15.Bollaert PE, Fieux F, Charpentier C, Lévy B. Baseline cortisol levels, cortisol response to corticotropin, and prognosis in late septic shock. Shock. 2003;19:13–5. doi: 10.1097/00024382-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003;31:141–5. doi: 10.1097/00003246-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26:645–50. doi: 10.1097/00003246-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629–38. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]


