Abstract
Background & objectives:
The enzyme paraoxonase (PON), an antioxidant enzyme that has both arylesterase and thiolactonase activity, is well studied in cardiovascular diseases. Although a few studies have shown altered PON activity in ocular diseases such as age-related macular degeneration and diabetic retinopathy, but the tissue-wise expression of PON in its three gene forms has not been studied. This study was conducted to see the ocular distribution of PON for any altered expression in ocular pathologies such as in cataract and diabetes mellitus.
Methods:
Immunohistochemistry (IHC) of the ocular tissues was done for localizing all three forms of the PON in the human donor eyeballs. The PON arylesterase (PON-AREase) and thiolactonase (PON-HCTLase) activities were determined by spectrophotometry in kinetic mode, and the mRNA expression of the PON genes (PON1-3) was determined by reverse transcription-polymerase chain reaction.
Results:
IHC showed the presence of both PON1 and 2 in all the ocular tissues and PON3 was seen only in retina. The mRNA expression analysis showed that PON2 and PON3 were present in all the tissues, whereas PON1 was seen only in ciliary and retina. Both the PON-AREase and PON-HCTLase activities were detected in all ocular tissues and was in the order of lens>retina>choroid>ciliary body>iris. The expression and activity were studied in cataractous lens and in diabetic retina of the donor eyes. A significant decrease in PON-AREase activity was seen in cataractous lens (P<0.05) but not in diabetic retina, and there was an increase in PON- HCTLase activity (P<0.05) only in diabetic retina. Bioinformatic studies and in vitro experiments indicated that advanced glycation end products (AGE) such as carboxymethyl -lysine might decrease the PON- AREase activity of the PON.
Interpretation & conclusions:
Distribution of PON enzyme and its activity in ocular tissues is reported here. The study revealed maximal PON activity in lens and retina, which are prone to higher oxidative stress. Differential activities of PON were observed in the lens and retinal tissues from cataractous and diabetic patients, respectively.
Keywords: Antioxidant, cataract, diabetic retinopathy, ocular tissue, paraoxonase, PON1
The paraoxonase (PON) gene family contains three members, namely, PON1, PON2 and PON3, all of which are clustered in the same region on chromosome 7q21.3-22.1. The sequence identity between the PON genes is 70 per cent and the protein identity is around 60 per cent1. PON2 enzyme is ubiquitously present in all tissues, whereas PON3 is seen both in serum and in tissues. PON1 is synthesized in the liver and secreted into serum, wherein it is associated with the high-density lipoprotein (HDL). The secreted protein retains its hydrophobic leader sequence, which is a structural requirement for PON1 association with HDL. PON1 is widely distributed in liver, kidney, intestine, testis and colon as well as in foetal liver2.
It has been found that all hydrolytic activities of PON1 (lactonase, esterase and phosphotriesterase) are mediated by amino residues with a pKa of 6.3-7.4 that is active in the deprotonated form. Site-directed mutagenesis studies have shown that distinct amino acids at the active site are involved in the catalysis that mediates the lactonase and arylesterase activities compared to the phosphotriesterase activity3,4,5. PON2 and PON3 have been shown to prevent cell-mediated oxidative modification of low-density lipoprotein6,7. Esters of oestrogens have been shown to be one of the natural substrates for PON8.
A significant decrease in serum PON1 activity has been shown in patients with liver cirrhosis9. There is increasing evidence from both animal and human studies linking low PON1 activity in cardiovascular diseases and atherosclerosis10. PON activity is also reported to be decreased in ocular diseases such as Behcet's disease11, age-related macular degeneration12, central retinal vein occlusion (CRVO)13, diabetic retinopathy (DR)14 and cataract15. However, the ocular tissue distribution of the PON enzyme is not yet studied. Therefore, this study was undertaken to localize the various forms of PON in the ocular tissues and also examine their expression and activity in ocular pathology such as in cataract and diabetes.
Material & Methods
This retrospective study was conducted in Vision Research Foundation, Chennai, India, for the period between 2009-2014. Human donor eyeballs obtained from in and around Chennai (n=6: control) through the CU Shah Eye Bank (Sankara Nethralaya, Chennai, India) were used. These were used to determine the tissue distribution of PON, in terms PON activity and mRNA expression by reverse transcription-polymerase chain reaction (RT-PCR) (n=3, mean age: 79±7 yr) and PON protein expression by immunohistochemistry (IHC) (n=3, mean age: 75±10 yr). Lenses with cataract were identified using Pirie classification16 (n=4, mean age 79±12 yr) and compared to non-cataractous control lens (n=4, mean age 28±14 yr).
The details of the donor eyes in terms of cause and time of death as well as the presence and absence of major morbidities were documented. Accordingly, diabetic retina was collected from donor eyes of diabetic patients with no other documented complications and used for the study (n=6 mean age 75±8 yr). Age-matched control retina was collected from donors’ eyes with no other complications (n=6, mean age 75±2 yr).
The study protocol was approved by the institutional ethics committee of Vision Research Foundation, Chennai, India.
Immunohistochemistry (IHC) for the detection of paraoxonase (PON) 1, 2 and 3 in ocular sections: Paraffin sections (5 μm) of donor eyeballs were incubated with 0.2 per cent trypsin/CaCl2 for 30 min and washed in pre-warmed water and tris-buffered saline. Further steps were performed with a polymer detection system (Novolink Min; Novocastra Laboratories Ltd., Newcastle-upon-Tyne, UK). The slides were blocked for peroxidase to avoid non-specific binding. The slides were incubated with a 1:100 diluted rabbit polyclonal antibody directed against human PON1 (Abcam, USA), mouse monoclonal antibody raised against PON2 (Abcam, USA) and goat polyclonal antibody raised against PON3 (Abcam, USA) followed by a polylink, as described by the suppliers. The sections were stained with 0.8 per cent amino ethylcarbazole in acetate buffer (pH 5.0; Sigma-Aldrich, USA) or 3 per cent diaminobenzidine. The sections were counterstained with haematoxylin. A negative control was run along with the experiment, in which the entire procedure was followed with omission of primary antibody.
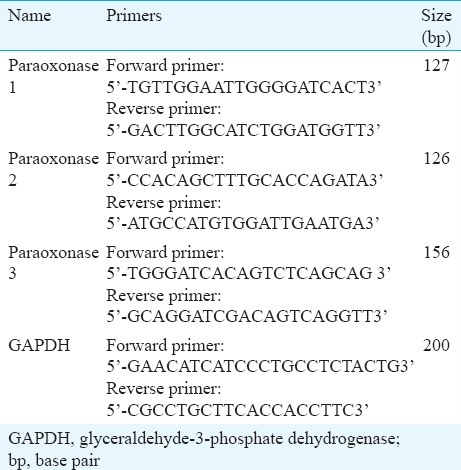
RNA extraction and reverse transcription for paraoxonase (PON) genes: The eyeball was dissected to separate the iris, ciliary body, retina, choroid along with retinal pigment epithelium (RPE) and RNA was extracted within two hours of collection of tissue. Total RNA was extracted from different ocular tissues by the guanidine isothiocyanate and chloroform method (TRI Reagent; Sigma-Aldrich, St. Louis, USA). For all samples, 1 μg of total RNA was used to synthesize first-strand cDNA (BioRad, CA, USA). The primers used for the experiment (Table) were designed using the Primer3 software and commercially procured (ILS, Imperial Life Science, India).
Table.
List of primers used for mRNA expression studies
Assay for paraoxonase (PON) enzyme activity
Tissue extracts preparation: The stored tissues, namely, iris, ciliary body, retina and choroid/RPE were homogenized in lysis buffer containing 125 mM tris hydrochloride, 100 mM sodium chloride and 0.1 per cent Triton X-100 with pH 7.0, at 4°C and centrifuged at 10,000 g for five minutes at 4°C. The supernatants were analyzed immediately for PON assay.
Determination of paraoxonase arylesterase (PON-AREase) activity: The PON-AREase activity was measured using phenyl acetate (PA) (Sigma-Aldrich, USA) as substrate. The activity was measured spectrophotometrically in the kinetic mode by detecting the increase in phenol concentration at 270 nm. Supernatant of the ocular lysates (10 μl) and undiluted vitreous (10 μl) was used for the assay in a buffer, consisting of 10 mmol/l tris and 1 mmol/l CaCl2 was used at pH 8.0. Enzyme activity was expressed as micromoles of PA hydrolyzed per millilitre per minute.
Determination of paraoxonase thiolactonase (PON-HCTLase) activity: The PON-HCTLase activity assay was done using γ-thiobutyrolactone (Sigma-Aldrich, USA) as substrate, and the rate of hydrolysis was measured spectrophotometrically in the kinetic mode at 450 nm. Five millilitres of the undiluted supernatants and 5 μl undiluted vitreous samples were used for the assay, with 5,5’-dithiobis-nitrobenzoic acid as chromogen at pH 7.2, using 100 mmol/l of phosphate buffer. Enzyme activity was expressed in U/l using a formula based on the molecular extinction coefficient of the product17.
In vitro experiment: The bovine retinal endothelial cells were cultured in-house using the protocol which has been reported earlier18, and the cells were lysed using lysis buffer containing 0.06 g tris-hydrochloride, 0.02 g sodium dodecyl sulphate (SDS), 0.1 g sodium deoxycholate and 0.086 g sodium chloride with p H 7.4. The cell lysates were treated with varying concentrations of carboxymethyl Lysine (CML), followed by the measurement of PON-AREase and PON-HCTLase activities. Influence of AGE accumulation on PON activity was assessed by in vitro study using bovine retinal endothelial cells, in which the cell lysates were treated with varying concentration of exogenously added AGE and activity of HCTLase and PON-AREase being measured.
Bioinformatics: In this study, the homology modelled structure of PON2 based on our previous report19 was utilized for molecular docking studies with CML (Pubchem: 123800) implemented through Autodock 4.0. (http://autodock.scripps.edu/). Further, the docked complex was analyzed for molecular interactions at the active site region of PON2, and these interactions were compared to its native substrates [PA, homocysteine thiolactone (HCTL)] as reported earlier.
Statistical analysis: The Student's t test was used to compare continuous variables between groups.
Results
IHC of PON showed the presence of both PON1 and PON2 in iris, ciliary, retina and choroids. PON2 showed a higher intensity of staining than PON1 in all the tissues indicative of its intracellular nature, while PON3 was detectable only in retina (Fig. 1).
Figure 1.
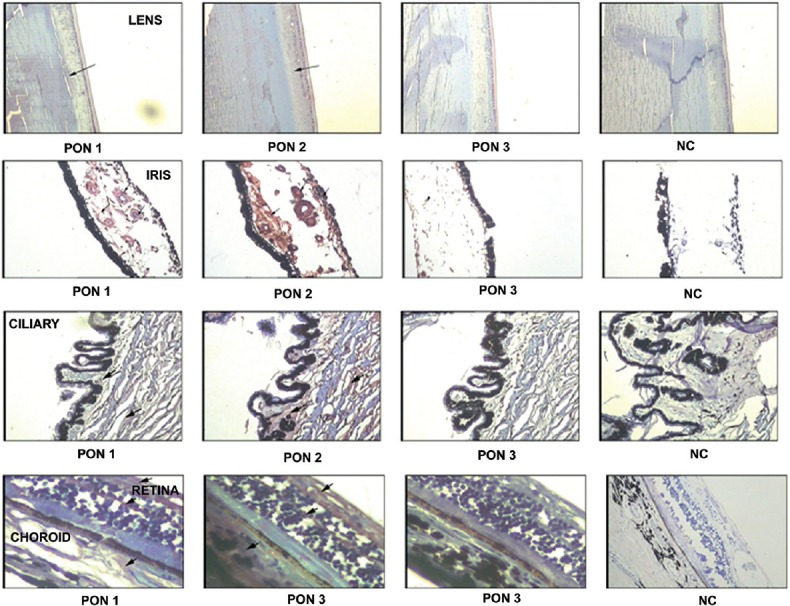
Tissue distribution of paraoxonase 1, 2 and 3. Immunolocalization of paraoxonase in paraffin-embedded sections in normal human donor ocular tissue. Arrow: positive labelling (reddish brown) of the lens, ciliary body, iris, choroid/retinal pigment epithelium and retina, haematoxylin was used as the counterstain. A negative control (NC) was run for all the paraoxonase genes by omitting the primary antibody.
The mRNA expression of PON1, 2 and 3 was done in the ocular tissues (iris, ciliary and choroid) by RT-PCR and PON2 and PON3 were found to be expressed in all these tissues, whereas PON1 was detected only in ciliary tissue (Fig. 2).
Figure 2.
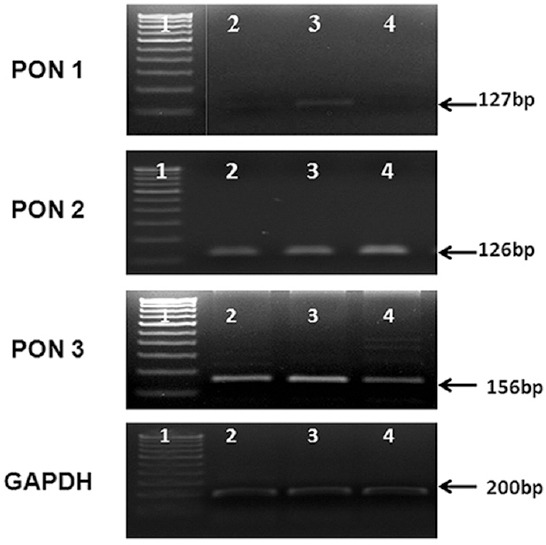
The mRNA expression of paraoxonase genes in ocular tissue. The mRNA expression of paraoxonase 1, 2 and 3 in the ocular tissues, namely, iris, ciliary and choroid was studied. The ciliary tissue alone showed all the paraoxonase genes expression. Lane 1, DNA marker; lane 2, iris; lane 3 ciliary; lane 4, choroid.
Paraoxonase (PON) activity in ocular tissues
Arylesterase (PON-AREase) activity: The AREase activity of PON was highest in the lens tissue (25±2.9 μM/ml/min), followed by retina (15.7±3.8 μM/min/ml), choroid/RPE (CRPE), ciliary body and iris (9.68±1.39, 7.63±1.38, 4.24±0.6 μM/ml/min, respectively) with vitreous showing the least activity as 2.88±0.76 μM/ml/min (Fig. 3A).
Figure 3.
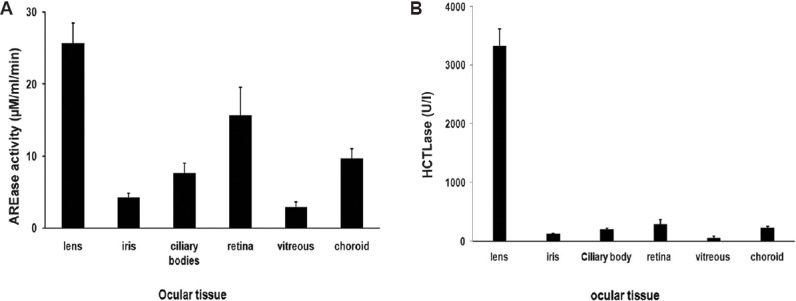
Paraoxonase activity in ocular tissues. (A) Arylesterase (AREase) activity of paraoxonase in ocular tissues: amongst the ocular tissue lysates, the lens had the highest activity, followed by retina, choroid, ciliary, iris and vitreous. (B) Thiolactonase (HCTLase) activity of paraoxonase in ocular tissues: the lens had the highest activity followed by retina, choroid, ciliary, iris and vitreous similar to the arylesterase activity.
Thiolactonase (PON-HCTLase) activity: HCTLase activity of PON was highest in the lens (3328±288 U/l), followed by retina (287±81 U/l), CRPE, ciliary body and iris (228±27; 199±18; 121±10 U/l, respectively), and vitreous had the least (57±30 U/l) (Fig. 3B).
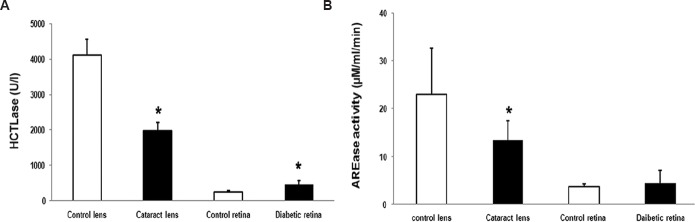
Since lens showed maximal enzyme activity of PON, the mRNA expression and activity were assessed in cataract lens and diabetic retina and compared with control. A significant decrease in HCTLase activity was observed with 1978±227.98 U/l in the cataract lens compared to normal with 4123±447.5 U/L (P<0.05) (Fig. 4A). The AREase activity was also found to be decreased significantly in the cataract lens compared to normal with 13.4±4.1 μM/min/ml in cataract and 23.07±9.7 μM/min/ml in control (P<0.05) (Fig. 4B). In case of diabetic retina, there was no significant change in AREase activity (4.0±2.0 μM/min/ml) compared to normal retina (3.8±3.2 μM/min/ml) (Fig. 4B). However, HCTLase activity was significantly increased in diabetic retina (466±447.5 U/l) compared to normal retina 253±16 U/l (P<0.05) (Fig. 4A).
Figure 4.
Paraoxonase activity in ocular disease condition. (A) There was a significant increase in thiolactonase (HCTLase) activity in the diabetic retina, but in contrast, the cataract lens had a significant drop in the activity. *P<0.05 compared to respective control. (B) There was a significant decrease in arylesterase (AREase) activity in cataract lens but not much change seen in donor retina from patients with diabetes when compared to control.
When the mRNA expression was checked in the control and in cataract lens, there was no PON1 mRNA expression and very minimal PON3 expression observed in the control and cataract lens. However, expression of PON2 was significantly higher (P<0.05) in cataract lens compared to control (Fig. 5), which was also observed in the densitogram (P<0.05). In the donor retina from diabetic patients, both PON2 and PON3 expression was seen, while the PON1 expression was minimal. The mRNA of PON3 was significantly increased (P<0.05) in diabetic retina compared to control, and there was no change in PON2 expression between the diabetic retina and control (Fig. 6A, B).
Figure 5.
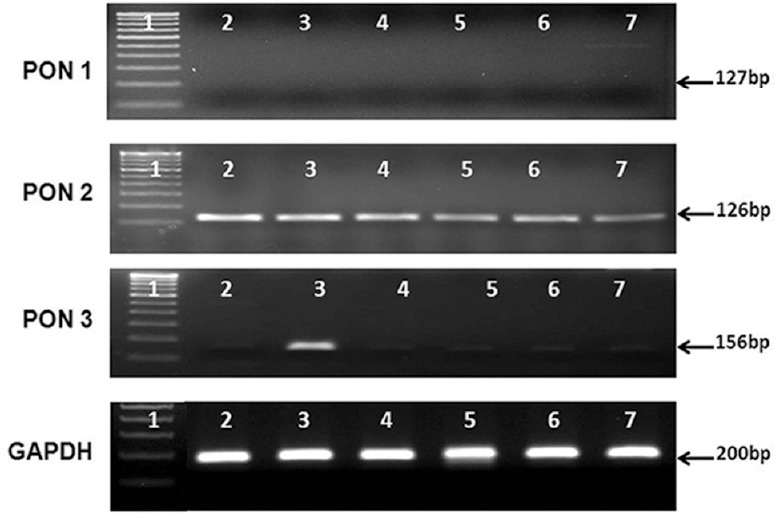
mRNA expression of all PON genes in control and cataract lens. There was no expression of PON1 in both the condition, but there was an significant increase in the PON2 expression in cataract lens compared to control and there was negligible expression of PON3 in both the normal and the cataract lens. All PON mRNA expressions were normalised to GAPDH expression. Lane 1, DNA marker; lanes 2-6, cataract lens, lanes 5-7: control lens.
Figure 6.
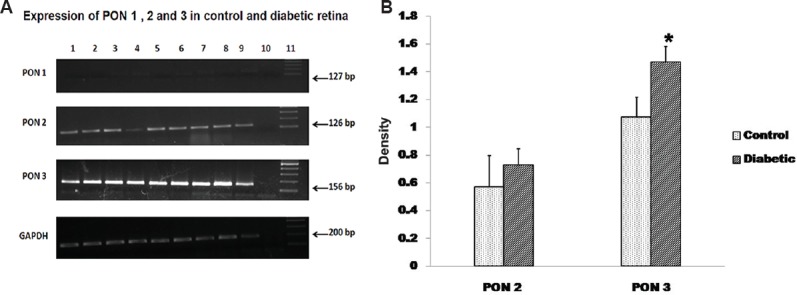
The mRNA expression of all the PON genes were analysed in the control and the donor retina from diabetic patients. (A) There was very negligible expression of PON1 in both the condition, and not much change in the PON2 expression but there was significant increase in the PON3 expression in diabetic retina compared to control retina. Lanes 1-6, control retina; lanes 7-9, diabetic retina; lane 10, NC; lane 11, DNA ladder. (B) Quantification using image J showed a significant (P<0.05) increase in PON3 expression in diabetic retina compared to control.
AGE accumulation is commonly seen in both cataract and diabetes. Assessment of the influence of AGE on PON activity by in vitro study showed a decrease in both HCTLase and AREase activity in response to increasing AGE in dose dependent manner (Fig. 7). Docking studies between PON2 and CML, a common AGE showed that CML binds to the active cavity of PON2, similar to its known substrates by hydrogen boding with Thr170 and localized near the reported putative active site residues (Fig. 8).
Figure 7.
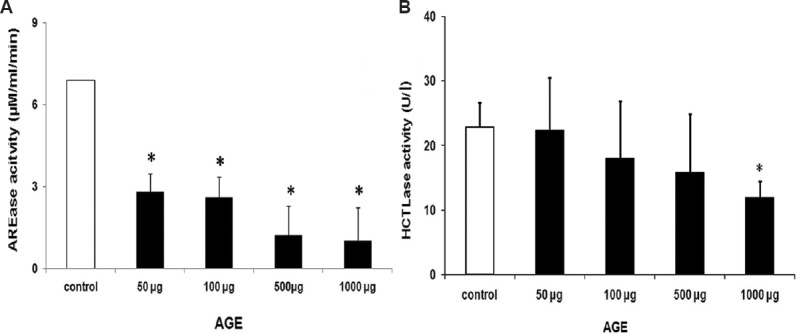
Effect of advanced glycation end products on the arylesterase (AREase) and the thiolactonase (HCTLase) activity. (A) There was a dose-dependent decrease in the arylesterase activity with increasing concentrations of advanced glycation end products. (B) Thiolactonase activity was 50 per cent inhibited at a very high concentration of 1000 μg advanced glycation end products (AGE).
Figure 8.
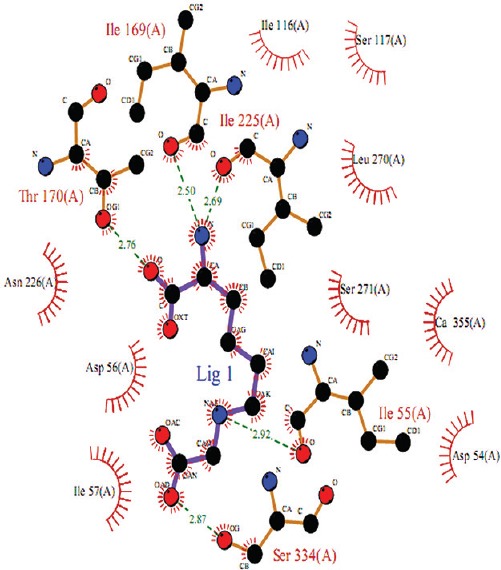
Analysis of carboxymethyl-lysine on paraoxonase 2 model using bioinformatics tools. The paraoxonase 2-carboxymethyllysine molecular interaction as inferred from docking study. Interacting atoms are shown as spheres with respective element colours. Hydrogen bond and hydrophobic interaction are shown as green dashed lines and red spiked arc, respectively.
Discussion
We have reported earlier decreased PON activity associated with increased oxidative stress in the serum of central retinal vein occlusion (CRVO) patients13,20. Decreased PON-AREase activity in the vitreous of proliferative diabetic retinopathy (PDR) cases along with increased HCTLase activity have also been shown21. In this study, the normal distribution of PON enzyme activity in the ocular tissue was looked into. Both PON-AREase and PON-HCTLase activities were observed in all the tissues predominantly in lens and retina. With respect to the mRNA, all ocular tissues showed both PON2 and PON3 expression. Ciliary body and retina showed PON1 expression additionally. Even though PON1 expression has been reported in lens tissue15, it was not observed in our study. PON3 expression was characteristically seen in the retinal tissue.
Lens and retina are highly susceptible to oxidative stress, and therefore, it is likely that PON functions as one of the major antioxidants in these tissues. In cataract lens, the activity of PON-AREase and the PON-HCTLase activities were found to be decreased. This can be attributed to increased oxidative stress induced by ultraviolet, environmental and lifestyle factors such as smoking, drugs, quality of the food and ageing, all of which can be associated with cataractogenesis22. Enzymatic antioxidants, superoxide dismutase and glutathione peroxidase are known to be decreased in the cataract patients associated with an increase in oxidative stress as seen by increased levels of lipid peroxides such as malondialdehyde15,23. Ageing is reported to contribute to the lowering of the PON activity as seen in human plasma. Ageing is also associated with increased oxidative stress and lowered antioxidant that promotes cateractogensis24. PON at mRNA expression level is increased as a defence in response to the increased oxidative stress25. However, the activity is decreased in lens probably in association with accumulation of factors such as advanced glycation end products (AGE). In diabetic retina, PON AREase activity was not found to be altered. However, the HCTLase activity was increased probably owing to accumulating substrate, namely HCTL in the retina. There was also a differential expression of PON with retina showing increase in PON3 unlike PON2 in lens indicating tissue-specific expressions which may also influence the kinetics of the enzyme activity. Drugs such as fenofibrates and statins that are frequently used by patients with diabetes for metabolic dysfunction can augment PON levels26. Our previous observation also supports these data, wherein PDR vitreous showed increased HCTLase activity compared to macular hole (MH) vitreous21. Though PON1 and PON3 are shown to have similar functions, PON3 has been stipulated to have a distinct role from PON1, in the kidney in relation to lipoprotein metabolism7. At the level of eye, there is limited information available. Further studies are needed to relate the PON3 expression with high HCTLase activity, especially in retina.
AGE accumulation is associated with both ageing, as in cataract27 and with hyperglycaemia, as in diabetes28. To understand the decreased AREase activity in cataract and diabetes, a structural bioinformatic approach was applied to derive further insights on the probable inhibitory effect of CML, an AGE found in diabetes on PON2 enzyme. Ferretti et al29 have shown that the glycation of PON results in the modification of the PON1 activity. A bioinformatic study by Saleem et al30 confirmed that structural alterations in the glycated model of PON1 resulted in weak interactions between the docked substrate and the active site cleft. Based on our results of PON2-CML docking studies, it was inferred that CML binding interferes with PON2 function, which falls in line with the observation of Saleem et al30. Our observation was further validated by biochemical studies in which the exogenously added AGE decreased the HCTLase and AREase activities.
In conclusion, our study showed the distribution of PON in terms of its activities as well as the mRNA expression of PON genes in ocular tissue. The PON-AREase activity was found to be decreased in cataract lens indicating lowered antioxidant activity and increased oxidative stress. Although PON2 showed an increase in expression in cataract lens, the other two forms were minimally expressed in this tissue. In donor retina from patients with diabetes, all three PON1, PON2 and PON3 were expressed, and there was a significant increase in PON3 expression compared to control. This might be the reason for the increased thiolactonase activity observed in diabetic retina compared to control. However, in this study we did not assess factors, such as duration of diabetes and glucose control, as well as the drug intake which might influence the enzyme activity and expression. Based on this pilot study, a larger study needs to be done to answer these key questions. Enhancing the activity of PON has been reported to be beneficial31 and therefore, strategies to improve the activity of PON need further studies which may prove to be beneficial in mitigating oxidative stress not only in cataract and diabetic retinopathy but also in other ocular pathologies.
Acknowledgment
Authors acknowledge financial support from Indian Council of Medical Research (ICMR) (No-52/3/2008-BMS) and Department of Biotechnology (DBT) (No-IR/SO/LU/03/2008/1), New Delhi, India.
Footnotes
Conflicts of Interest: None.
References
- 1.Rajkovic MG, Rumora L, Barisic K. The paraoxonase 1, 2 and 3 in humans. Biochem Med (Zagreb) 2011;21:122–30. doi: 10.11613/bm.2011.020. [DOI] [PubMed] [Google Scholar]
- 2.Mackness B, Beltran-Debon R, Aragones G, Joven J, Camps J, Mackness M. Human tissue distribution of paraoxonases 1 and 2 mRNA. IUBMB Life. 2010;62:480–2. doi: 10.1002/iub.347. [DOI] [PubMed] [Google Scholar]
- 3.Khersonsky O, Tawfik DS. The histidine 115-histidine 134 dyad mediates the lactonase activity of mammalian serum paraoxonases. J Biol Chem. 2006;281:7649–56. doi: 10.1074/jbc.M512594200. [DOI] [PubMed] [Google Scholar]
- 4.Mandrich L, Cerreta M, Manco G. An engineered version of human PON2 opens the way to understand the role of its post-translational modifications in modulating catalytic activity. PLoS One. 2015;10:e0144579. doi: 10.1371/journal.pone.0144579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josse D1, Lockridge O, Xie W, Bartels CF, Schopfer LM, Masson P. The active site of human paraoxonase (PON1) J Appl Toxicol. 2001;21(Suppl 1):S7–11. doi: 10.1002/jat.789. [DOI] [PubMed] [Google Scholar]
- 6.Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, et al. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem. 2001;276:44444–9. doi: 10.1074/jbc.M105660200. [DOI] [PubMed] [Google Scholar]
- 7.Reddy ST, Wadleigh DJ, Grijalva V, Ng C, Hama S, Gangopadhyay A, et al. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol. 2001;21:542–7. doi: 10.1161/01.atv.21.4.542. [DOI] [PubMed] [Google Scholar]
- 8.Teiber JF, Billecke SS, La Du BN, Draganov DI. Estrogen esters as substrates for human paraoxonases. Arch Biochem Biophys. 2007;461:24–9. doi: 10.1016/j.abb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Burlina A, Galzigna L. Serum arylesterase isoenzymes in chronic hepatitis. Clin Biochem. 1974;7:202–5. doi: 10.1016/s0009-9120(74)91914-6. [DOI] [PubMed] [Google Scholar]
- 10.Macharia M, Hassan MS, Blackhurst D, Erasmus RT, Matsha TE. The growing importance of PON1 in cardiovascular health: A review. J Cardiovasc Med (Hagerstown) 2012;13:443–53. doi: 10.2459/JCM.0b013e328354e3ac. [DOI] [PubMed] [Google Scholar]
- 11.Isik A, Koca SS, Ustundag B, Selek S. Decreased total antioxidant response and increased oxidative stress in Behcet's disease. Tohoku J Exp Med. 2007;212:133–41. doi: 10.1620/tjem.212.133. [DOI] [PubMed] [Google Scholar]
- 12.Javadzadeh A, Ghorbanihaghjo A, Bahreini E, Rashtchizadeh N, Argani H, Alizadeh S. Serum paraoxonase phenotype distribution in exudative age-related macular degeneration and its relationship to homocysteine and oxidized low-density lipoprotein. Retina. 2012;32:658–66. doi: 10.1097/IAE.0b013e31822529b1. [DOI] [PubMed] [Google Scholar]
- 13.Angayarkanni N, Barathi S, Seethalakshmi T, Punitham R, Sivaramakrishna R, Suganeswari G, et al. Serum PON1 arylesterase activity in relation to hyperhomocysteinaemia and oxidative stress in young adult central retinal venous occlusion patients. Eye (Lond) 2008;22:969–74. doi: 10.1038/sj.eye.6703062. [DOI] [PubMed] [Google Scholar]
- 14.Nowak M, Wielkoszynski T, Marek B, Kos-Kudla B, Swietochowska E, Sieminska L, et al. Antioxidant potential, paraoxonase 1, ceruloplasmin activity and C-reactive protein concentration in diabetic retinopathy. Clin Exp Med. 2010;10:185–92. doi: 10.1007/s10238-009-0084-7. [DOI] [PubMed] [Google Scholar]
- 15.Hashim Z, Ilyas A, Saleem A, Salim A, Zarina S. Expression and activity of paraoxonase 1 in human cataractous lens tissue. Free Radic Biol Med. 2009;46:1089–95. doi: 10.1016/j.freeradbiomed.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Pirie A. Color and solubility of the proteins of human cataracts. Invest Ophthalmol. 1968;7:634–50. [PubMed] [Google Scholar]
- 17.Koubaa N, Hammami S, Nakbi A, Ben Hamda K, Mahjoub S, Kosaka T, et al. Relationship between thiolactonase activity and hyperhomocysteinemia according to MTHFR gene polymorphism in Tunisian Behçet's disease patients. Clin Chem Lab Med. 2008;46:187–92. doi: 10.1515/CCLM.2008.046. [DOI] [PubMed] [Google Scholar]
- 18.Barathi S, Angayarkanni N, Sumantran VN. GLUT-1 expression in bovine retinal capillary endothelial cells and pericytes exposed to advanced glycation end products. Invest Ophthalmol Vis Sci. 2010;51:6810–4. doi: 10.1167/iovs.10-5312. [DOI] [PubMed] [Google Scholar]
- 19.Barathi S, Charanya M, Muthukumaran S, Angayarkanni N, Umashankar V. Comparative modeling of PON2 and analysis of its substrate binding interactions using computational methods. J Ocul Biol Dis Infor. 2010;3:64–72. doi: 10.1007/s12177-011-9057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharathi Devi SR, Suganeswari G, Sharma T, Thennarasu M, Angayarkanni N. Homocysteine induces oxidative stress in young adult central retinal vein occlusion. Br J Ophthalmol. 2012;96:1122–6. doi: 10.1136/bjophthalmol-2011-301370. [DOI] [PubMed] [Google Scholar]
- 21.Barathi S, Angayarkanni N, Pasupathi A, Natarajan SK, Pukraj R, Dhupper M, et al. Homocysteinethiolactone and paraoxonase: Novel markers of diabetic retinopathy. Diabetes Care. 2010;33:2031–7. doi: 10.2337/dc10-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtul N, Söylemez S, Celik M. Plasma paraoxonase and arylesterase activities in smokers and smokeless tobacco users as maras powder. Inhal Toxicol. 2014;26:235–9. doi: 10.3109/08958378.2013.878007. [DOI] [PubMed] [Google Scholar]
- 23.Hashim Z, Zarina S. Assessment of paraoxonase activity and lipid peroxidation levels in diabetic and senile subjects suffering from cataract. Clin Biochem. 2007;40:705–9. doi: 10.1016/j.clinbiochem.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Mehdi MM, Rizvi SI. Human plasma paraoxonase 1 (PON1) arylesterase activity during aging: Correlation with susceptibility of LDL oxidation. Arch Med Res. 2012;43:438–43. doi: 10.1016/j.arcmed.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Jasna JM, Anandbabu K, Bharathi SR, Angayarkanni N. Paraoxonase enzyme protects retinal pigment epithelium from chlorpyrifos insult. PLoS One. 2014;9:e101380. doi: 10.1371/journal.pone.0101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phuntuwate W, Suthisisang C, Koanantakul B, Chaloeiphap P, Mackness B, Mackness M. Effect of fenofibrate therapy on paraoxonase1 status in patients with low HDL-C levels. Atherosclerosis. 2008;196:122–8. doi: 10.1016/j.atherosclerosis.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Franke S, Dawczynski J, Strobel J, Niwa T, Stahl P, Stein G. Increased levels of advanced glycation end products in human cataractous lenses. J Cataract Refract Surg. 2003;29:998–1004. doi: 10.1016/s0886-3350(02)01841-2. [DOI] [PubMed] [Google Scholar]
- 28.Yamagishi S. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol. 2011;46:217–24. doi: 10.1016/j.exger.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Ferretti G, Bacchetti T, Marchionni C, Caldarelli L, Curatola G. Effect of glycation of high density lipoproteins on their physicochemical properties and on paraoxonase activity. Acta Diabetol. 2001;38:163–9. doi: 10.1007/s592-001-8074-z. [DOI] [PubMed] [Google Scholar]
- 30.Saleem A, Azam SS, Zarina S. Docking and molecular dynamics simulation studies on glycation-induced conformational changes of human paraoxonase 1. Eur Biophys J. 2012;41:241–8. doi: 10.1007/s00249-011-0779-z. [DOI] [PubMed] [Google Scholar]
- 31.Schrader C, Rimbach G. Determinants of paraoxonase 1 status: Genes, drugs and nutrition. Curr Med Chem. 2011;18:5624–43. doi: 10.2174/092986711798347216. [DOI] [PubMed] [Google Scholar]