Abstract
Background & objectives:
Concomitant feeding and anti-tuberculosis (TB) drug administration are likely to reduce nausea and enhance compliance to treatment. However, food could lower plasma drug concentrations. This study was undertaken to examine the effect of food on two-hour plasma concentrations of rifampicin (RMP), isoniazid (INH) and pyrazinamide (PZA), and pharmacokinetics of these drugs in adult TB patients.
Methods:
Newly diagnosed adult TB patients were recruited from the Revised National Tuberculosis Control Programme (RNTCP) treatment centres in Chennai Corporation, Chennai, India. Two-hour post-dosing plasma concentrations were determined in 25 patients, and a semi-intensive pharmacokinetic study was undertaken in six patients. RMP, INH and PZA concentrations were determined by high-performance liquid chromatography.
Results:
The geometric mean two-hour concentrations with food and under fasting conditions were 2.2 and 5.5 μg/ml for RMP (P<0.001), 3.9 and 11.3 μg/ml for INH (P<0.001), and 18.0 and 28.2 μg/ml for PZA (P<0.001), respectively. Drug administration with food caused the plasma concentration to decrease by 50, 45 and 34 per cent for RMP, INH and PZA, respectively. Significant decreases in peak concentrations and exposures of drugs and delay in time to attain peak concentrations of drugs when taken with food were also observed.
Interpretation & conclusions:
Our findings showed that food lowered anti-TB drug concentrations significantly and delayed absorption. Patients may be explained the beneficial effects of taking anti-TB drugs in a fasting state and advised to do so. There is a need for more research on optimization of dosing to maximize efficacy and safety of currently used drugs.
Keywords: Anti-tuberculosis drug concentrations, food, pharmacokinetics, tuberculosis
Food intake exerts a complex influence on the bioavailability of drugs. It may interfere not only with tablet disintegration, drug dissolution and drug transit through the gastrointestinal tract but may also affect the metabolic transformation of drugs in the gastrointestinal wall and liver1. It has been reported that food intake before administration of anti-tuberculosis (TB) drugs may lead to reduction in blood concentrations. Most of these studies were undertaken in healthy individuals2,3,4,5,6. Some studies undertaken in TB patients have shown food to reduce anti-TB drug levels7,8. A systematic review and meta-analysis by Lin et al9 suggested that food reduced the peak concentration of rifampicin (RMP), isoniazid (INH) and ethambutol (EMB) but not pyrazinamide (PZA). However, there is a paucity of data regarding effect of food on RMP, INH and PZA concentrations in TB patients from India.
In the Revised National Tuberculosis Control Programme (RNTCP) in India10, there is no clear guidance on whether or not to take anti-TB medications with food. It is believed that drugs are not well tolerated on an empty stomach and many patients prefer to have food before taking their medicines. In the RNTCP, all drugs are administered together as directly observed treatment (DOT) in the intensive phase10. This study was undertaken to evaluate the effect of food on plasma concentrations of RMP, INH and PZA and the pharmacokinetics of these drugs in patients with TB.
Material & Methods
The study was planned as a sub-study of a larger pharmacokinetic study and was conducted during June 2014 to November 201411. Adult TB patients with pulmonary or extrapulmonary TB were recruited. They were receiving thrice weekly anti-TB treatment (ATT) under the RNTCP in Chennai Corporation, Chennai, India, with either Category I or Category II ATT. Category I treatment consisted of RMP, INH, PZA and EMB thrice weekly for two months followed by RMP and INH thrice weekly for four months. Category II treatment consisted of RMP, INH, PZA, EMB and streptomycin (S) thrice weekly for two months, RMP, INH, PZA and EMB thrice weekly for one month and RMP, INH and EMB thrice weekly for five months. Diagnosis and treatment were according to the RNTCP guidelines10. The drug doses were RMP 450 mg (600 mg for those >60 kg body weight), INH 600 mg, EMB 1200 mg and PZA 1500 mg. All patients received single drugs (and not fixed-dose combinations). Patients were eligible to take part in this study if they met the following criteria: (i) aged 18 yr or above, (ii) body weight not less than 30 kg, (iii) received at least two weeks of ATT regularly, and (iv) agreed to come to the same DOT centre until completion of the study. All consecutive patients who fulfilled the study criteria were included in the study. The study commenced after obtaining approval from the Institutional Ethics Committee of the ICMR- National Institute for Research in Tuberculosis, Chennai. All patients gave informed written consent.
Study procedure: An open-label, cross-over study design was adopted; each patient was tested on two occasions with an interval of one week between occasions. Patients continued with their regular ATT during this time interval.
On the first occasion, all patients took breakfast followed by anti-TB drugs, and on the second occasion, all patients took the anti-TB drugs after an overnight fast of 12 h and took breakfast after two hours of drug administration. Drug administrations were completely supervised. All patients were provided with a uniform breakfast, which consisted of four idlis with coconut chutney and sambar and a cup of coffee. This comprised 115 g carbohydrate, 25 g protein and 15 g fat and provided 695 calories.
The study was conducted in two phases. During the first phase, a single blood sample was collected two hours after drug administration, on both occasions. During the second phase in a different set of patients, a semi-intensive pharmacokinetic study adopting limited sampling strategy was conducted on both occasions. Blood samples were drawn at pre-dosing and at one, two, three, four, six and eight hours after anti-TB drug administration in heparinized vacutainer tubes. Plasma was separated, ascorbic acid solution was added to plasma to prevent oxidation of RMP. The plasma samples were stored at -20°C until analysis.
Drug estimations: All drug estimations were undertaken within a week of sample collection. Plasma INH, PZA and RMP concentrations were determined by high-performance liquid chromatography (HPLC) using validated methods12,13. Plasma INH and PZA were estimated simultaneously by extraction using para-hydroxybenzaldehyde and trifluoroacetic acid. Analysis was performed using a C8 column at 267 nm. The retention times of PZA and INH were 3 and 5.5 min, respectively. Plasma RMP was extracted using acetonitrile and analysis was performed using C18 column at 254 nm. The retention time of RMP was 1.7 min. The methods were specific with no interfering peaks at the retention times of the drugs. The between-run and within-run variations for all the drugs were below 10 per cent. The lower limits of quantification for RMP, INH and PZA were 0.25, 0.25 and 1.25μg/ml, respectively.
Calculation of pharmacokinetic variables: Pharmacokinetic variables were calculated for the six patients, in whom a semi-intensive pharmacokinetic study was conducted. Peak concentration (Cmax) and time to attain Cmax (Tmax) were determined by visual inspection of data. The linear trapezoidal rule was used to compute exposure or area under the time-concentration curve (zero-eight hours) (AUC0-8).
Statistical evaluation: Analysis of data was performed using SPSS, version 19.0 (IBM, USA). Data were expressed as geometric mean and range. Wilcoxon signed-rank test was performed to study differences in two-hour RMP, INH and PZA concentrations and Cmax, Tmax and AUC0-8 of the drugs between the two occasions (with and without food).
Results
A total of 25 and six patients, respectively, took part in the first and second phases of the study. The patient details are given in Table I. None was HIV-infected. The number of patients with diabetes in the first and second phases was five and one, respectively. Extrapulmonary forms of TB included TB lymphadenitis, miliary TB, TB spine, and TB pleuritis. The two-hour concentrations of RMP, INH and PZA were significantly lower when the drugs were taken immediately after food intake as compared to drug administration under a fasting condition (P<0.001 for all the three drugs). The regression lines drawn in the scatter plots for the drugs were observed to be significant in the case of RMP (P=0.002) and PZA (P<0.001) (Figure). The geometric mean two-hour concentrations with food and under fasting conditions for 25 patients were, respectively, 2.2 and 5.5 μg/ml for RMP (P<0.001), 3.9 and 11.3 μg/ml for INH (P<0.001) and 18.0 and 28.2 μg/ml for PZA (P<0.001). Drug administration with food caused the plasma concentration to decrease by 49.6 per cent (6.4-94.2%) for RMP, 44.8 per cent (4.9-93.4%) for INH and 33.8 per cent (2.0-76.2%) for PZA.
Table I.
Details of patients participated in phase I and II study
Figure.
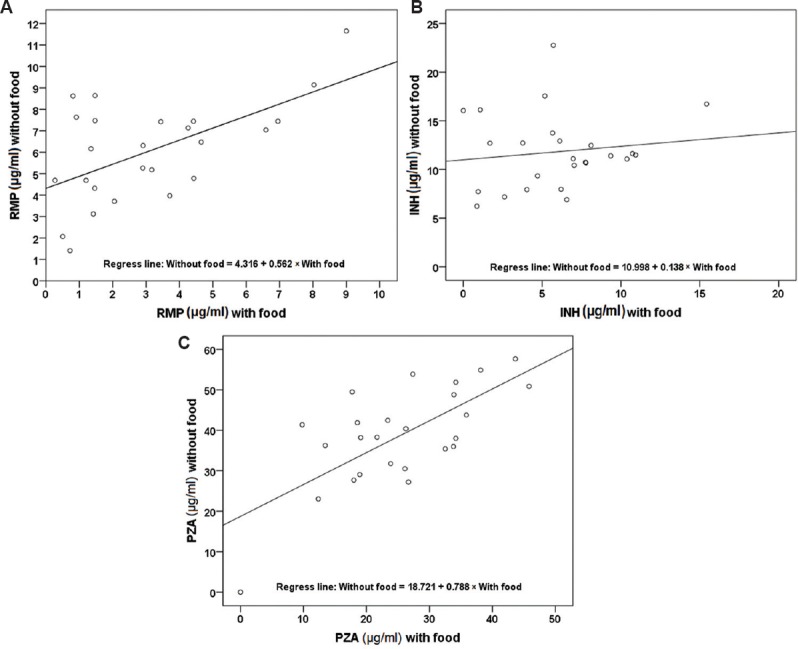
Distribution of two-hour concentrations of rifampicin (RMP) (A), isoniazid (INH) (B) and pyrazinamide (PZA) (C) with and without food (n=25). The diagonal lines indicate the correlation between the points.
For all three drugs (RMP, INH and PZA), the Cmax and AUC0-8 were substantially smaller with food than under fasting conditions, most of the differences being significant (Table II). Furthermore, the corresponding Tmax was significantly higher with food. These findings demonstrated that the consumption of even a limited breakfast (four idlis plus coffee) delayed the absorption of RMP, INH and PZA and thereby resulted in substantial lowering of the drug concentrations.
Table II.
Pharmacokinetics of rifampicin, isoniazid and pyrazinamide with food and under fasting condition (n=6)

Discussion
We examined the effect of prior food intake on plasma concentrations of key first-line anti-TB drugs, namely, RMP, INH and PZA, in adult TB patients. Two-hour plasma concentrations of RMP, INH and PZA were significantly lower when the drugs were taken immediately after food than under fasting conditions. Since two-hour drug concentrations may not always represent peak concentrations (food could delay the absorption of drugs), we undertook a semi-intensive pharmacokinetic study in a smaller group of patients. The findings were similar; Cmax and AUC0-8 of RMP, INH and PZA were lower when the drugs were consumed after food than in a fasting state. Further, a delay in the absorption of drugs was observed as indicated by a delayed Tmax. Thus, drugs taken after food caused not only a delay in the absorption of drugs but also adversely impacted drug concentrations.
It may not be always possible to collect multiple blood samples in the clinical/field setting for logistical and financial reasons; one is typically limited to one or two time points. When only one sample can be obtained, the two-hour post-dose concentrations of RMP, INH and PZA are usually most informative14. Hence, in this study, drug concentrations were estimated at two-hour post-dosing. In a separate study, we observed that RMP, INH and PZA peaked at two hours in more than 95 per cent of patients in a fasting state (unpublished findings). The bactericidal activity of both RMP and INH are related to peak concentrations - higher the concentrations, more effective the bacterial killing15. Further, using a hollow fibre system model of TB, it was observed that low RMP and INH peak concentrations preceded the acquisition of drug resistance and that low drug exposures were predictive of clinical outcomes in TB patients16. It was also observed that drug exposure and peak concentration [indexed to minimum inhibitory concentration (MIC)] were associated with both efficacy and suppression of acquired drug resistance16. Pharmacokinetic data and MIC values can serve as useful predictors of treatment efficacy. The two most relevant pharmacodynamic parameters are peak concentration to MIC and exposure to MIC ratios17. The drug is most effective when these ratios are maximized17.
Studies conducted in healthy individuals using single doses of individual drugs have shown that food reduced peak concentration, exposure and delayed absorption of RMP2,5, INH3, PZA4 and EMB6. Polasa and Krishnaswamy5 examined the impact of a wheat-based diet on anti-TB drug levels in six healthy individuals, and also examined RMP pharmacokinetics. They also observed a decrease in the total AUC0-8 and prolongation in the time to reach peak concentration (two vs. four hours).
A study in 27 patients with TB showed that concomitant food (carbohydrate and lipid-based) administration decreased the bioavailability of INH and RMP in about half (33-56%) of patients7. A study done in Taiwanese TB patients using a fixed-dose combination formulation observed significantly lower serum concentrations of INH, RMP and PZA under post-prandial conditions than fasting conditions, the percentage decreased ranging from 24 to 40 per cent for Cmax and 12-26 per cent for AUC0-10 and percentage increase of Tmax ranging from 78 to 151 per cent8. In our study also reductions in the range of 29-39 per cent for Cmax and 21-37 per cent for AUC0-8 were observed. However, a study in Peruvian TB patients did not observe significant differences in peak concentration and exposure of INH between fasting and non-fasting conditions although these values were higher under fasting conditions18. Our study findings were similar to those reported in healthy individuals and also those in TB patients2,3,4,5,6,7,8. A systematic review and meta-analysis involving 12 trials and 157 patients suggested that from a pharmacokinetic point of view, it would be ideal for patients to take anti-TB medications under fasting condition9. In vitro studies that were carried out to understand the effect of food on the bioavailability of RMP showed that this was a function of dosage characteristics, such as disintegration time and dissolution rate, which could be affected by the presence of food19.
The strength of this study was that each patient served as his/her own control; thus, patient-related factors (age, sex, comorbidities, drug dosages, disease severity and genetic factors) did not affect the study findings. The meal given was a standard one and all drugs were administered under observation. The study was done under programmatic real-life conditions.
Some of the study limitations were the small number of patients (although each patient served as his/her own control), non-randomization of sequence of occasions (fasting or food administration) and EMB not being estimated. Further, treatment outcomes and development of adverse drug reactions were not related to drug levels in this sub-study.
In conclusion, the results showed that anti-TB drug levels were decreased and absorption delayed when taken after food. Patients can be explained the beneficial effects of taking ATT in a fasting state and encouraged to do so. Our findings highlight the need for more research on optimization of dosing of anti-TB drugs in relation to food, to maximize efficacy and safety of currently used drugs.
Acknowledgment
Authors thank all the patients for participation in the study.
Footnotes
Conflicts of Interest: None.
References
- 1.Melander A. Influence of food on the bioavailability of drugs. Clin Pharmacokinet. 1978;3:337–51. doi: 10.2165/00003088-197803050-00001. [DOI] [PubMed] [Google Scholar]
- 2.Peloquin CA, Namdar R, Singleton MD, Nix DE. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest. 1999;115:12–8. doi: 10.1378/chest.115.1.12. [DOI] [PubMed] [Google Scholar]
- 3.Peloquin CA, Namdar R, Dodge AA, Nix DE. Pharmacokinetics of isoniazid under fasting conditions, with food, and with antacids. Int J Tuberc Lung Dis. 1999;3:703–10. [PubMed] [Google Scholar]
- 4.Peloquin CA, Bulpitt AE, Jaresko GS, Jelliffe RW, James GT, Nix DE. Pharmacokinetics of pyrazinamide under fasting conditions, with food, and with antacids. Pharmacotherapy. 1998;18:1205–11. [PubMed] [Google Scholar]
- 5.Polasa K, Krishnaswamy K. Effect of food on bioavailability of rifampicin. J Clin Pharmacol. 1983;23:433–7. doi: 10.1002/j.1552-4604.1983.tb01787.x. [DOI] [PubMed] [Google Scholar]
- 6.Peloquin CA, Bulpitt AE, Jaresko GS, Jelliffe RW, Childs JM, Nix DE. Pharmacokinetics of ethambutol under fasting conditions, with food, and with antacids. Antimicrob Agents Chemother. 1999;43:568–72. doi: 10.1128/aac.43.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zent C, Smith P. Study of the effect of concomitant food on the bioavailability of rifampicin, isoniazid and pyrazinamide. Tuber Lung Dis. 1995;76:109–13. doi: 10.1016/0962-8479(95)90551-0. [DOI] [PubMed] [Google Scholar]
- 8.Lin HC, Yu MC, Liu HJ, Bai KJ. Impact of food intake on the pharmacokinetics of first-line antituberculosis drugs in Taiwanese tuberculosis patients. J Formos Med Assoc. 2014;113:291–7. doi: 10.1016/j.jfma.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Lin MY, Lin SJ, Chan LC, Lu YC. Impact of food and antacids on the pharmacokinetics of anti-tuberculosis drugs: Systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010;14:806–18. [PubMed] [Google Scholar]
- 10.TB India 2011. Revised National TB Control Programme. Annual Status Report. New Delhi: Central TB Division Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India; 2011. pp. 98–101. [Google Scholar]
- 11.Ramachandran G, Agibothu Kupparam HK, Vedhachalam C, Thiruvengadam K, Rajagandhi V, Dusthackeer A, et al. Factors influencing tuberculosis treatment outcome in adult patients treated with thrice-weekly regimens in India. Antimicrob Agents Chemother. 2017;61:pii:e02464-16. doi: 10.1128/AAC.02464-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemanth Kumar AK, Chandra I, Ramachandran G, Chelvi SK, Lalitha V, Gurumoorthy P. A validated high performance liquid chromatography method for the determination of rifampicin and desacetyl rifampicin in plasma an d urine. Indian J Pharmacol. 2004;36:231–3. [Google Scholar]
- 13.Hemanth Kumar AK, Sudha V, Ramachandran G. Simple and rapid liquid chromatography method for simultaneous determination of isoniazid and pyrazinamide in plasma. SAARC J Tuberc Lung Dis HIV AIDS. 2012;9:13–8. [Google Scholar]
- 14.Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002;62:2169–83. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Mitchison DA. Pharmacokinetic/pharmacodynamic parameters and the choice of high-dosage rifamycins. Int J Tuberc Lung Dis. 2012;16:1186–9. doi: 10.5588/ijtld.11.0818. [DOI] [PubMed] [Google Scholar]
- 16.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis. 2013;208:1464–73. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stambaugh JJ, Berning SE, Bulpitt AE, Hollender ES, Narita M, Ashkin D, et al. Ofloxacin population pharmacokinetics in patients with tuberculosis. Int J Tuberc Lung Dis. 2002;6:503–9. doi: 10.5588/09640569513011. [DOI] [PubMed] [Google Scholar]
- 18.Requena-Méndez A, Davies G, Waterhouse D, Ardrey A, Jave O, López-Romero SL, et al. Effects of dosage, comorbidities, and food on isoniazid pharmacokinetics in Peruvian tuberculosis patients. Antimicrob Agents Chemother. 2014;58:7164–70. doi: 10.1128/AAC.03258-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panchagnula R, Rungta S, Sancheti P, Agrawal S, Kaul CL. In vitro evaluation of food effect on the bioavailability of rifampicin from antituberculosis fixed dose combination formulations. Farmaco. 2003;58:1099–103. doi: 10.1016/S0014-827X(03)00161-7. [DOI] [PubMed] [Google Scholar]


