Abstract
Background & objectives:
Opportunistic virus infections are common in liver transplant (LT) recipients. There is a risk of developing infection with cytomegalovirus (CMV) and herpes-related viruses such as herpes simplex virus-1 and 2 (HSV-1 & 2), Epstein-Barr virus (EBV) and Varicella Zoster virus (VZV), reactivation of infection and recurrent infection. This study was conducted to determine CMV seropositivity in donors and its influence on LT recipients and seropositivity of CMV, HSV-1 and 2, EB viral capsid antigen (EBVCA) and VZV in LT recipients and their reactivation.
Methods:
Pre-transplant data for IgG and IgM for CMV (and donor), HSV-1 and -2, EB viral capsid antigen (VCA) and VZV were available for 153 recipients. All recipients were on ganciclovir or valganciclovir prophylaxis for three months after LT. For reactivation rates, findings of post-transplant CMV quantitative reverse transcription polymerase chain reaction (CMV qRT-PCR) assay were associated with pre-transplant serological profile.
Results:
Of the 153 LT recipients, 131 were men (85.6%). The median age of LT was 46 yr (range 9 months-71 yr). Overall exposure to CMV was 71.8 per cent followed by EB VCA (61.4%) and VZV (49.6%). Susceptibility to both HSV-1 and -2 was high across all decades (P<0.001). Seropositivity of CMV in donor was 90.9 per cent (100 out of 110). Post-transplant CMV qRT- PCR was positive in 17 (26.6%; 3 in recipient negative) of 64 samples tested. qRT-PCR assay was positive in one out of four (25%) tested for HSV-1 and nine out of 19 (47.4%) tested for EBV. Two recipients tested for HSV-2 and one for VZV were negative. There were three deaths in recipients (D+ R+) who were also positive for CMV qRT PCR. There was one death due to HSV-1 pneumonia. One patient with EBV reactivation developed post-transplant lymphoproliferative disorder two years after transplant.
Interpretation & conclusions:
Transplant recipient were at highest risk of acquiring HSV-1 and -2 more so for HSV-2. CMV exposure in transplant recipients and donors were very high and at greatest risk for recipient reactivation rate. Despite this, death related to CMV reactivation was low.
Keywords: Cytomegalovirus, HSV herpes-related viruses, liver transplant, recipient
Liver transplant (LT) recipients are predisposed to several respiratory and gastrointestinal viruses such as cytomegalovirus (CMV) and Herpes-related viruses [herpes simplex virus-1 and -2 (HSV-1 and -2), Epstein–Barr viral capsid antigen and nuclear antigen (EB VCA, EB NA) and Varicella Zoster virus (VZV)]1 and are at constant risk for reactivation and recurrent infection. A typical example is HSV-1 infection which never gets cleared by the immune system and remains dormant in the dorsal root ganglia with reactivation from time to time, especially in an immunosuppressed state. This phenomenon holds true for CMV as well2,3. EB VCA also persists in infected cells and exhibits latency. In children, the virus is associated with significant morbidity and mortality after solid organ transplant. An elevated EBV DNA post-LT is predictive for post-transplant lymphoproliferative disease (PTLD). Serial monitoring of EBV load is mandatory in transplant recipients4,5. For VZV infection, the antibody is protective and serum titre correlates with a history of varicella6.
CMV infection post-LT depends on donor and recipient CMV positivity. The highest risk for infectivity is in D+R- mismatch7 and this can be as high as 44-65 per cent. Infectivity rate in CMV seropositive LT recipient (CMV R+) can be as low as 8-19 per cent8. Post-transplant prophylaxis with ganciclovir or valganciclovir has considerably reduced the risk of CMV infection9. Wadhawan et al10 reported CMV disease in nine of the 306 patients (2.9%) in their study.
Most studies on post-LT viral infection have addressed the influence of donor CMV status in recipients; less information is available on re-infectivity with other herpes-related viruses in the recipient. The aim of the present study was thus, to determine the seropositivity of CMV, HSV-1 and HSV-2, EB VCA and VZV in LT recipients and re-infection rates, CMV seropositivity in donors, and donor-recipient (D-R) CMV mismatch and CMV re-infection in LT recipients.
Material & Methods
This prospective study was done at Gleneagles Global Health City hospital, Chennai, India, among all LT recipients transplanted between August 2009 and December 2013. As per the protocol, all patients listed for LT were screened for CMV, HSV-1, HSV-2, EB VCA, VZV and HIV. Donor screening was done for CMV. The study protocol was approved by the ethics committee of the institution and written informed consent was obtained from all recipients.
The recipients were classified as susceptible (IgG-/IgM-), past-exposure (IgG+/IgM-), acute/recent (IgG-/IgM+) and reactivation (IgG+/IgM+). Equivocal IgG or IgM in recipient was considered as negative. In the donor, equivocal result was considered as positive. Donor-recipient IgG match was done for predicting CMV infection in the recipient7. D+R- implied donor was positive for CMV IgG and the recipient was negative; such recipients were at the greatest risk for CMV infection.
Decision to screen a recipient for post-LT viral infection was individualized. CMV and EBV quantitative reverse transcription polymerase chain reaction (qRT-PCR)11 was indicated when a recipient had unexplained fever, fluctuating transaminitis, diarrhoea, respiratory symptoms, sepsis or seizure. HSV-1, HSV-2 and VZV qRT-PCR assay was indicated if there were skin or mucocutaneous lesions11.
All recipients were on prophylaxis with ganciclovir or valganciclovir for three months. In addition, they were on tapering dose of steroids, tacrolimus and mycophenolate mofetil.
Recipients with serological markers for all the five viruses were included in the study. For donor CMV seropositivity and donor-recipient mismatch, IgG data were mandatory in both donor and recipient.
Statistical analysis: For statistical analysis of data 95 per cent confidence interval, Chi-square for proportions, Kruskal–Wallis for comparison of differences in medians were applied.
Results & Discussion
One hundred and fifty three LT recipients were included in the study. There were 131 (85.6%) males. The median age of presentation was 46 yr (range 9 months to 71 yr). Thirty one recipients were below 17 yr (20.3%) of age. Median follow up of recipients was 334 days (range 4-1601 days). The pre-LT seropositivity for CMV was 71.8 per cent, EBV 61.4 per cent, VZV 49.6 per cent and HSV-1 34.6 per cent (Figure). It was least for HSV-2 (9.8%) (P<0.001) when compared between all five viruses. The overall acute infection rate was 34 per cent. Reactivation of CMV was 21.1 per cent, EBV 24.2 per cent and VZV 24.2 per cent. Susceptibility was high for both HSV-1 (53.3%) and HSV-2 (69.9%), significantly so for HSV-2 (P<0.001). In summary past infection was highest for CMV and EBV, followed by VZV indicating immunity. Acute and reactivation rates of all the virus infections were low (Figure).
Figure.
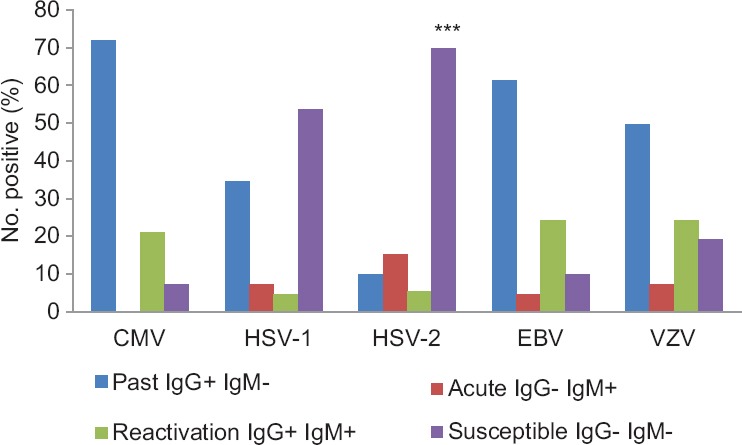
Seropositivity (%) of cytomegalovirus & other herpes-related virus infection in liver transplant recipients. Susceptibility to HSV-2 infection was highly significant. ***P<0.001 compared to HSV-1. Past infection was highest for CMV, EBV, followed by VZV indicating immunity. Acute and reactivation rates for all the virus infections were low. CMV, cytomegalovirus; HSV, herpes simplex virus; EBV, Ebstein–Barr Virus; VZV, Varicella Zoster virus.
Below the age of 17 yr, a substantial number of recipients were exposed to CMV (n=18, 58.1%), EBV (n=9, 29%) and VZV (n=11, 35.3%). By the age of 50, except for HSV-2 (7.9%), there was a significant (P<0.001) exposure to CMV (79.4%), HSV-1 (41.3%), EB VCA (65.1%) and VZV (49.2%). Susceptibility to HSV-2 (69.5%) and HSV-1 (49.2%) was high beyond 50 yr of age. The donor CMV seroprevalence was 90.9 per cent as against the 95 per cent reported among healthy blood donors12.
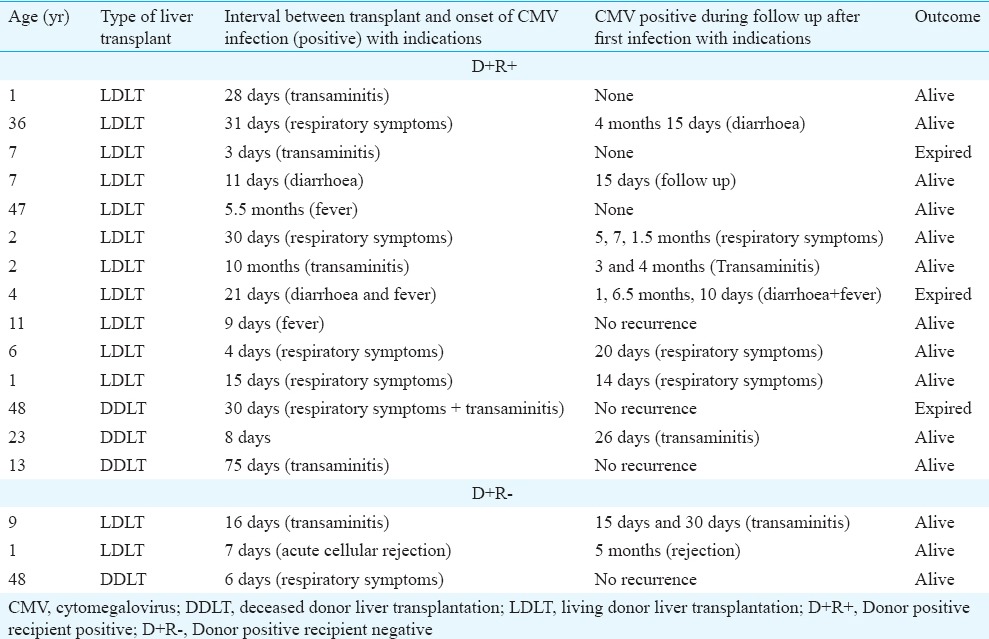
Of the 110 patients, seropositivity of CMV in donor was 90.9 per cent (n=100). One hundred and four (94.5%) recipients received a matched CMV donor liver with low risk for infection [D+R+ 94 (85.5%); D-R- 3 (2.9%); D-R+ 7 (6.4%)]. Donor-recipient mismatch was present in six recipients (5.4%). Of the 64 recipients screened for CMV reactivation, 17 were positive (26.6%). Three (2.7%) of the six D+R- mismatch developed transaminitis, acute cellular rejection and respiratory symtoms, 16, 7 and 6 days after liver transplant; all survived (Table). There were three (17.6%) deaths in D+R+ recipients who were also CMV qRT PCR positive. Cause of death is shown in Table. Reactivation of CMV can occur when high dose of steroid is introduced for acute cellular reaction (ACR). Wadhawan et al10 reported a significant correlation between steroid use for ACR and CMV reactivation (P=0.003) and disease (P=0.002). In our study, only one child (D+R-) with ACR required a high dose.
Table.
Clinical and biochemical presentation of CMV qRT-PCR positive recipients (D+R+: 14; D+R-: 3)
The high CMV infectivity in LT recipient despite a close donor-recipient match has also been reported in renal transplant recipients13,14,15. A study from southern India15 reported the presence of CMV DNA among donors. Sampathkumar and Paya16 reported a low CMV disease (10.5%) and CMV infection (9.5%) despite high CMV-positive antigenemia (77.2%). This study concluded that CMV infection was of little clinical significance as only a subset developed CMV disease.
In our study there were three deaths in recipients who were also CMV qRT-PCR positive (Table). The cause of death may not be directly related to CMV disease. The low infectivity rate (26.6%) may be related to pre-emptive antiviral prophylaxis. Of the 26 symptomatic LT recipients screened for EB VCA, HSV-1 and VZV, HSV-1 was positive in one (expired due to HSV pneumonia) and EBV in nine (47.4%). A child with an elevated level of EBV DNA during follow up developed PTLD two years after LT, an important cause of morbidity and mortality after solid organ transplant in children. Long-term serial monitoring of asymptomatic recipients with chronic high EBV has been recommended for an early detection of PTLD17,18.
In conclusion, past infection in a liver transplant recipient is highest for CMV and EBV, followed by VZV, and peaks around 50 yr of age. Susceptibility is highest for HSV2 infection and tends to increase beyond 50 years. Mismatch rates of CMV positive and CMV negative recipient rates is low at 5.4 per cent. CMV qRT-PCR is indicated in selected situations such as donor recipient mismatch (CMV+ donor, CMV- recipient) or clinical CMV disease (26.6%). EBV requires close monitoring for PTLD for several years post transplant.
Footnotes
Conflicts of Interest: None.
References
- 1.Razonable RR, Paya CV. Herpesvirus infections in transplant recipients: Current challenges in the clinical management of cytomegalovirus and Epstein-Barr virus infections. Herpes. 2003;10:60–5. [PubMed] [Google Scholar]
- 2.Razonable RR, Emery VC. 11th Annual Meeting of the IHMF (International Herpes Management Forum). Management of CMV infection and disease in transplant patients 27-29 February 2004. Herpes. 2004;11:77–86. [PubMed] [Google Scholar]
- 3.Lee SO, Razonable RR. Current concepts on cytomegalovirus infection after liver transplantation. World J Hepatol. 2010;2:325–36. doi: 10.4254/wjh.v2.i9.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens SJ, Verschuuren EA, Pronk I, van Der Bij W, Harmsen MC, The TH, et al. Frequent monitoring of Epstein-Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood. 2001;97:1165–71. doi: 10.1182/blood.v97.5.1165. [DOI] [PubMed] [Google Scholar]
- 5.Kimura H, Ito Y, Suzuki R, Nishiyama Y. Measuring Epstein-Barr virus (EBV) load: The significance and application for each EBV-associated disease. Rev Med Virol. 2008;18:305–19. doi: 10.1002/rmv.582. [DOI] [PubMed] [Google Scholar]
- 6.Hambleton S, Gershon AA. Preventing Varicella–Zoster disease. Clin Microbiol Rev. 2005;18:70–80. doi: 10.1128/CMR.18.1.70-80.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy MS, Varghese J, Venkataraman J, Rela M. Matching donor to recipient in liver transplantation: Relevance in clinical practice. World J Hepatol. 2013;5:603–11. doi: 10.4254/wjh.v5.i11.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh N, Wannstedt C, Keyes L, Wagener MM, Cacciarelli TV. Who among cytomegalovirus-seropositive liver transplant recipients is at risk for cytomegalovirus infection? Liver Transpl. 2005;11:700–4. doi: 10.1002/lt.20417. [DOI] [PubMed] [Google Scholar]
- 9.Azevedo LS, Pierrotti LC, Abdala E, Costa SF, Strabelli TM, Campos SV, et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo) 2015;70:515–23. doi: 10.6061/clinics/2015(07)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadhawan M, Gupta S, Goyal N, Vasudevan KR, Makki K, Dawar R, et al. Cytomegalovirus infection: Its incidence and management in cytomegalovirus-seropositive living related liver transplant recipients: A single-center experience. Liver Transpl. 2012;18:1448–55. doi: 10.1002/lt.23540. [DOI] [PubMed] [Google Scholar]
- 11.Mackay I. Real-time PCR in microbiology: From diagnosis to characterization. Norfolk, England: Caister Academic Press; 2007. p. 440. [Google Scholar]
- 12.Kothari A, Ramachandran VG, Gupta P, Singh B, Talwar V. Seroprevalence of cytomegalovirus among voluntary blood donors in Delhi, India. J Health Popul Nutr. 2002;20:348–51. [PubMed] [Google Scholar]
- 13.Madhavan HN, Samson MY, Ishwarya M, Vijayakumar R, Jambulingam M. pp65 antigenemia and real time polymerase chain reaction (PCR) based-study to determine the prevalence of human cytomegalovirus (HCMV) in kidney donors and recipients with follow-up studies. Virol J. 2010;7:322. doi: 10.1186/1743-422X-7-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson RJ, Clatworthy MR, Birch R, Hammad A, Bradley JA. CMV mismatch does not affect patient and graft survival in UK renal transplant recipients. Transplantation. 2009;88:77–82. doi: 10.1097/TP.0b013e3181aa8d36. [DOI] [PubMed] [Google Scholar]
- 15.Rao M, Finny GJ, Abraham P, Juneja R, Thomas PP, Jacob CK, et al. Cytomegalovirus infection in a seroendemic renal transplant population: A longitudinal study of virological markers. Nephron. 2000;84:367–73. doi: 10.1159/000045613. [DOI] [PubMed] [Google Scholar]
- 16.Sampathkumar P, Paya CV. Management of cytomegalovirus infection after liver transplantation. Liver Transpl. 2000;6:144–56. doi: 10.1002/lt.500060220. [DOI] [PubMed] [Google Scholar]
- 17.D’Antiga L, Del Rizzo M, Mengoli C, Cillo U, Guariso G, Zancan L. Sustained Epstein-Barr virus detection in paediatric liver transplantation. Insights into the occurrence of late PTLD. Liver Transpl. 2007;13:343–8. doi: 10.1002/lt.20958. [DOI] [PubMed] [Google Scholar]
- 18.Bingler MA, Feingold B, Miller SA, Quivers E, Michaels MG, Green M, et al. Chronic high Epstein-Barr viral load state and risk for late-onset posttransplant lymphoproliferative disease/lymphoma in children. Am J Transplant. 2008;8:442–5. doi: 10.1111/j.1600-6143.2007.02080.x. [DOI] [PubMed] [Google Scholar]


