Abstract
BACKGROUND
Cognitive-behavioral therapy (CBT) and selective serotonin reuptake inhibitors (SSRIs) are the two first-line treatments for depression, but little is known about their effects on quality of life.
AIMS
To investigate the efficacy of these two interventions for depression on quality of life (QOL).
METHOD
A meta-analysis was conducted to examine changes in QOL in adults with major depressive disorder who received CBT (24 studies examining 1,969 patients) or SSRI treatment (13 studies examining 4,286 patients) for their depression.
RESULTS
Moderate improvements in QOL from pre to post-treatment were observed in both CBT (Hedges’ g = 0.63) and SSRI (Hedges’ g = 0.79) treatments. The effect size remained stable over the course of the follow-up period for CBT. No data were available to examine follow-ups in the SSRI group. QOL effect sizes decreased linearly with publication year, and greater improvements in depression were significantly associated with greater improvement in QOL for CBT, but not for SSRIs.
CONCLUSION
CBT and SSRIs for depression were both associated with moderate improvements in QOL, but are possibly caused by different mechanisms.
Keywords: Quality of Life, Life Satisfaction, Depression, Cognitive Behavioral Therapy, Selective Serotonin Reuptake Inhibitors
Depression is one of the most costly and common disorders worldwide (World Health Organization, 2005). The lifetime prevalence rate of DSM-IV major depressive disorder (MDD) in the United States is 16.2% and the 12-month prevalence rate is 6.6% (Kessler, R. C. et al., 2003). The large economic burden is driven by its high prevalence rate and debilitating nature of the illness (Greenberg, Fournier, Sisitsky, Pike & Kessler, 2015; Marcus & Olfson, 2010). MDD has a significant impact on the patient’s quality of life (QOL). Quality of life refers to subjective well-being, life satisfaction, perceptions of social relationships, physical health, economic status, and functioning in daily activities and work and is typically assessed through subjective views of one’s life circumstances, perceptions of mental and physical health, social and family relationships, and functioning at work and home (Angermeyer & Kilian, 2006).
Effective treatments of this pervasive and chronic disorder can lead to a reduction in depressive symptoms, improvement of psychosocial functioning, and greater QOL (Merikangas, et al., 2007; Angermeyer & Katschnig, 2006). However, the treatment effects on QOL have not received nearly as much attention as clinical measures of depression. It is possible that regulatory agencies have not placed much value on QOL measures because they are not primary outcome measures in clinical trials, including those leading to drug marketing approval.
Although depression severity is correlated with QOL impairment, (Judd, et al., 2000) the changes in QOL are not fully accounted for by changes in depression, (Hirschfeld, et al., 2002) and QOL changes more slowly than symptoms of depression (Trivedi, 2006). Furthermore, treatments that reduce depression symptoms do not necessarily result in improved QOL. A meta-analysis examining adjunctive atypical antipsychotic treatment for depression, for instance, showed that while observer ratings of depression decreased with pharmacotherapy use, there was little evidence of improvement in patients’ QOL (Spielmans, Berman, Linardatos, Rosenlicht, Perry, & Tsai, 2013). Additionally, a meta-analysis investigating the efficacy of antidepressants for depressed youths demonstrated that despite improvement in clinician-rated depression symptoms following the use of antidepressants, patients did not exhibit improvement in overall well-being and QOL (Spielmans & Gerwig, 2014).
It has been suggested that psychotherapy might be more effective for changing QOL because it directly targets general well-being, whereas pharmacotherapy more indirectly targets QOL by focusing on symptoms (Angermeyer & Kilian, 2006; Gladis, Gosch, Dishuk, & Crits-Christoph, 1999) but there is little empirical data to support this argument. For instance, Farabaugh et al. (2015) randomized depressed individuals to 12 weeks of CBT (n = 15) or escitalopram (n = 11). The authors found no statistically significant differences between treatment groups on any of the outcome measures, including QOL. The study by Orjuela-Rojas et al. (2015) randomized patients with temporal lobe epilepsy to receive 12 weeks of CBT (n = 7) or SSRI (n = 8) for the treatment of major depressive disorder. After treatment, both groups showed improved QOL and reduced severity of depression symptoms, with no statistically significant group differences.
Numerous guidelines, including the Practice Guidelines by the American Psychiatric Association (Gelenberg, et al., 2010) recommend cognitive behavioral therapy (CBT) and selective serotonin reuptake inhibitors (SSRIs) as treatment for depression. While there are other effective psychological short-term treatments for depression that are not part of the general CBT family, we chose to limit our analyses to CBT for a number of reasons. First, the number of clinical trials examining the efficacy of CBT for depression is very large (Hofmann et al., 2012), providing a large sample of potential studies. Second, the clinical trials involving CBT typically show a relatively high rigor or methodological quality; and third, restricting the review to CBT reduces the heterogeneity of empirically supported psychological therapies.
The aim of this study was to examine the effects of CBT and SSRIs for depression on quality of life. This is an important aim, because an effective treatment for a common mental disorder, such as depression, should ideally not be limited to only symptom improvement but also include enhancing the person’s QOL. Using a meta-analytic approach, we hypothesized that both treatments would be associated with improvements in quality of life. Due to the small number of available studies directly comparing CBT and SSRIs, we did not predict that one treatment modality would be associated with greater improvements in QOL than the other. Instead, we examined the effects of both treatments on QOL independently.
Methods
Search
A search of PubMed and PsycINFO databases for articles published from 1994 to the present was conducted on June 20, 2014, and then updated October 17, 2016. We used a 20-year time span to focus on the relatively recent literature and to limit the heterogeneity of the studies in terms of treatment approach, diagnostic criteria, and measures used for depression and QOL. The following three sets of search terms were used simultaneously: ((quality of life OR quality-of-life)) AND (((((cognitive therapy OR cognitive behavio* therapy) OR (behavior therapy OR behavio* therapy)) OR (cognitive-behavioral OR cognitive-behavioural)) OR (pharmacological OR pharmacotherapy)) AND (depression OR depressive)). The initial search produced 3,862 results, with 3,665 studies remaining after duplicates were excluded. In accordance with the guidelines set forth in Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (Moher, et al., 2015). the protocol for this meta-analysis was registered in with the International Prospective Register of Systematic Reviews (PROSPERO) on July 10, 2014, and was last updated on January 16, 2015 (registration number CRD42014009831).
Study Selection
Studies were selected by the second through fourth authors and a team of independent trained assessors. Studies were included in the present meta-analysis if: 1) at least one treatment condition consisted of CBT or treatment with an SSRI; 2) they included a sample diagnosed with current major depression; 3) they included a sample of adults at or above the age of 18; 4) they included an adequate measure of QOL at pre- and post-intervention. Following the recommendation of Moons, Budts and De Geest (2006), we conceptualized QOL in terms of life satisfaction. Therefore studies with QOL measures that exclusively focused on mental or physical symptoms or health status without tapping a subjective aspect of satisfaction with one’s life were excluded. QOL measures that do not meet this criteria might be more adequately understood of as assessments of health status, which has been shown to be conceptually distinct from QOL (Smith, Avis, & Assmann, 1999). We also confirmed that all included QOL measures had demonstrated adequate reliability and validity; and 5) they provided sufficient data on the intervention of interest for calculating an effect size to use in our meta-analysis.
Studies were excluded if: 1) major depression was secondary to another psychiatric condition; 2) the data in one study overlapped with data reported in another study considered for inclusion; 3) CBT or SSRI was administered in conjunction with another active treatment. In cases of disagreement, the authors discussed the case until consensus was reached. If the data necessary to calculate an effect size were not reported, we requested these data from the corresponding author.
Data Extraction
For each selected study, we extracted data on QOL and depression symptoms at pre-treatment, post-treatment, and 6-month follow-up (or closest available follow-up date if included in study) for the CBT or SSRI treatment arms, along with data from control conditions if included. In addition, we extracted data on sample and study characteristics, including sample size, length of treatment, SSRI type and dosage, CBT treatment modality (e.g. individual, group, or computer-based) and dosage (i.e. hours of therapy), gender, age, psychiatric medication use (for CBT samples) and medical and psychiatric comorbidity. In studies where more than one active treatment condition was examined, data from the more intensive form of therapy was used (e.g. individual rather than group therapy). If a study used multiple eligible measures of QOL, we extracted data from the measure that was in closer accordance with our operational definition of QOL. Specifically, we selected the Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ; Endicott, Nee, Harrison, & Blumenthal, 1993) over the Short-Form 36 Health Survey (SF-36; Ware & Sherbourne, 1992) and the Spitzer Quality of Life Index, and the Quality of Life Inventory (QOLI; Frisch et al., 2005) over the SF-36. When studies utilized multiple measures of depression, we chose clinician-administered scales over self-report measures. Data were extracted on two separate occasions by independent raters and compared to ensure accuracy, with discrepancies resolved by the second and third authors.
Risk of Bias Assessment
In accordance with the Cochrane guidelines for systematic reviews, (Higgins & Green, 2011) we assessed study quality with the Cochrane Collaboration’s tool for assessing risk of bias (Higgins & Altman, 2008). This tool involves assessing each study as containing a high, low or unclear level of bias risk in a number of domains (sequence generation, allocation concealment, incomplete outcome data, and selective outcome reporting). Although this tool was initially designed for assessment of randomized controlled trials, the Cochrane guidelines specify that it may be adapted for the evaluation of non-randomized trials (Higgins, & Green, 2011). In doing so, we assigned non-randomized trials a high risk rating in the sequence generation category. Following recommendations from the Cochrane guidelines, a total bias assessment was created for each study such that an ‘unclear’ rating in any category meant an ‘unclear risk’ overall rating, a ‘high’ rating in any category lead to a ‘high risk’ overall rating, and ‘low risk’ studies had to be rated as ‘low’ in all four categories. The second and third authors independently rated each study and then met to resolve any discrepancies. Inter-rater reliability for the total bias assessment was strong (Kappa = .80; SE = .09).
Quantitative Data Synthesis
We used a random effects model because of the heterogeneity within the studies. Within-group and controlled effect sizes were calculated using Hedges’ g (Hedges & Olkin, 1985). Specifically, within-group effect sizes reflect pre- to post-treatment changes, and controlled effect sizes represent differences in efficacy between the treatment and control conditions. To compute the within-group effect size, the following formulas were utilized: , such that reflects the pre-treatment mean, reflects the post-treatment mean, Sdifference reflects the standard deviation of the difference, and r reflects the correlation between pre-treatment and post-treatment scores. Hedges’ g was computed by multiplying d with correction factor , such that df represents the degrees of freedom to estimate the within-group standard deviation. The controlled effect sizes were computed using the following formula: , such that is the mean pre- to posttreatment change, SD is the standard deviation of post-treatment scores, n is the sample size, TREAT refers to the active treatment condition (i.e., CBT or SSRI), and CONT refers to the control condition. Following Rosenthal (1984), we estimated the pre-post correlation to be r = .70.
To investigate potential moderator effects on QOL outcome, we employed the between-group heterogeneity statistic (QB) recommended by Hedges and Olkin (Hedges & Olkin, 1985) and meta-regression procedures for categorical and continuous moderators, respectively. Moderators of interest included both treatment characteristics (i.e., study year, treatment dose, risk of bias, assessment type, treatment format, sex distribution, frequency of contact with study physician, concomitant medication, completer percentage) and clinical characteristics (i.e., depression symptom improvement and comorbidity with a medical condition). In addition, for CBT studies we also investigated whether inclusion of patients stable on psychiatric medication predicted QOL outcome, and for SSRI studies, we tested the impact of frequency of visits with study physician.
To examine the presence of publication bias, we inspected the funnel plot. In addition, we used the fail-safe N method to determine the number of additional studies with a null result needed to reduce the overall effect size to non-significance (Rosenthal, 1991). If the fail-safe N exceeds 5 multiplied by K (i.e., the number of studies in the meta-analysis) + 10, then the results may be considered statistically robust. We also examined the funnel plot to evaluate symmetry relative to the mean effect size, with greater symmetry corresponding to decreased likelihood of publication bias. To complement funnel plot inspection, the trim and fill method (Duval, & Tweedie, 2000) was utilized to determine the nature of potential publication bias and compute an imputed effect size that accounts for it. Furthermore, we examined Egger’s regression intercept to determine whether results might be biased as a consequence of study number. Due to space constraints, we limited the funnel plot analysis to only the main analyses. All meta-analytic procedures were conducted in Comprehensive Meta-Analysis, Version 3 (Comprehensive Meta-Analysis, 2016).
Results
Study Flow and Characteristics
The flow diagram in Figure 1 shows the number of studies excluded at each stage of study selection, and the reasons for exclusion. Of the 4,426 unique studies initially identified, 37 (24 CBT, 13 SSRI) were determined to be eligible and included in the final analysis. Together these studies examined 1,969 participants receiving CBT and 4,286 participants receiving SSRI treatment. Of note, only two studies directly examined the effects of both SSRI and CBT for depression on QOL (Farabaugh et al., 2015; Orjuela-Rojas, Martínez-Juárez, Ruiz-Chow & Crail-Melendez, 2015). In order to avoid double counting these studies by using them for analyses of both treatment modalities, we excluded it from our analyses.
Figure 1.
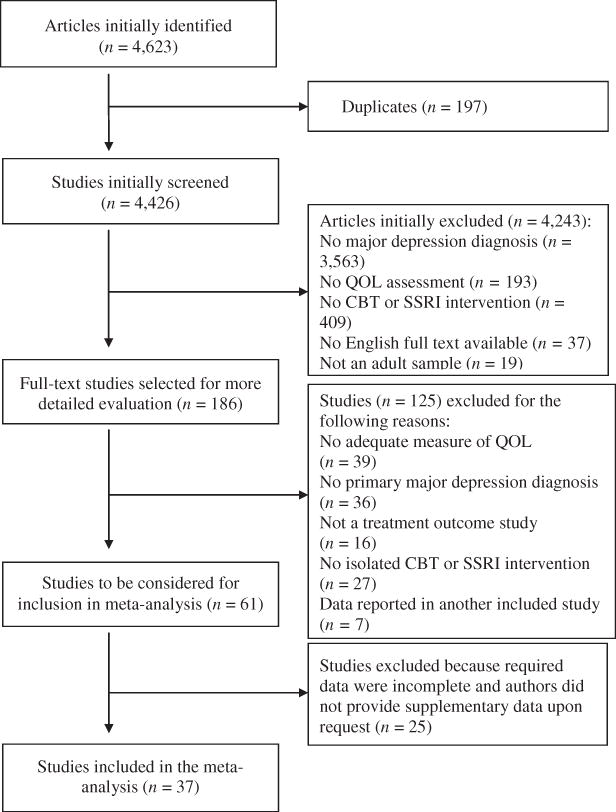
Flow diagram of study selection process
Study characteristics are presented in Table 1. Results from our risk of bias assessment showed that most studies had an unclear (10 CBT, 4 SSRI) or high risk (11 CBT, 8 SSRI) bias, with one SSRI and three CBT studies determined to be low risk in all four of the rated categories. There was no difference in bias ratings between intervention types (Fisher’s Exact Test = 0.85, p = n.s.). In addition, no difference was found in the percentage of patients who completed treatment in CBT (M = 77.39, SD = 10.19) and SSRI studies (M = 82.02, SD = 15.02; t (29) = 0.84, p = n.s.).
Table 1.
Study Characteristics
| SSRI Studies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | SSRI Type | Dosage (mg) |
RCT | Length of Treatment (weeks) |
Sample Size |
% Female |
Mean Age (SD) |
Psychiatric Comorbidity |
Medically Comorbid Sample |
QOL Measure |
Depressi on Measure |
Risk of Bias SG AC ID SR Total |
| Aberg-Wistedt et al, 2000 | Sertraline Paroxetine |
83 27 |
Yes | 24 | 353 | 67.5 | 42.5 (NA) |
Unknown | No | BQOL | MADRS | ? ? ? ✓? |
| Bellino et al, 2006 | Fluoxetine | 20–40 | Yes | 24 | 19 | 62.5 | 26.4 (3.7) |
All Borderline Pers. Disorder | No | SAT-P | Ham-D | ? ? X ✓ X |
| Demyttenaere et al, 2008 | Escitalopram | 10–20 | Yes | 8 | 523 | 56.4 | 40 (11.5) |
Unknown | No | QLESQ | MADRS | ? ? ? ✓ ? |
| Gleason et al, 2002 | Citalopram | 26.7 | No | 8 | 15 | 33.3 | 42.6 (6.4) |
Unknown | Hepatitis C | SF-36 | SCL-90 | X ? X ✓ X |
| Ishak et al, 2013 | Citalopram | NA | No | 12 | 2280 | 62.8 | NA | Unknown | No | QLESQ | QIDS-SR | X ? ? ✓ X |
| Lewis-Fernandez et al, 2013 | Sertraline | NA | No | 12 | 42 | 52 | 39.8 (12.2) |
Unknown | No | QLESQ | Ham-D | X ? X ✓ X |
| Nicolau et al, 2013 | Citalopram | 20 | No | 26 | 38 | 52 | 60.4 (10.7) |
Allowed but unknown | Type 2 diabetes | SF-36 | BDI | X X ? ✓ X |
| Paile-Hyvärinen et al, 2003 | Paroxetine | 20 | Yes | 10 | 7 | 100 | 61.1 (8.6) |
Unknown | Type 2 diabetes | RAND-36 | MADRS | ✓ ✓ ✓ ✓ ✓ |
| Peveler et al, 2005 | Fluoxetine Sertraline Citalopram Paroxetine |
20.7 66.1 19.4 21.6 |
Yes | 12 | 87 | 71 | 44.6 (NA) |
Allowed but unknown | No | SF-36 | HADS-D | X ✓ ✓ ✓ X |
| Rabkin et al, 1994 | Sertraline | 120 | No | 8 | 20 | 5 | 41.0 (6.6) |
No comorbid anxiety allowed | HIV positive | QLESQ | Ham-D | X ? X ✓ X |
| Saveanu et al, 2015 | Escitalopram Sertraline |
12.3 61.1 |
Yes | 8 | 672 | 54.9 | 38.1 (12.45) |
Comorbid anxiety: 41.4% | No | WHOQOL | Ham-D | ✓ ? ✓ ✓ ? |
| Shelton et al, 2006 | Sertraline | 50 | Yes | 8 | 82 | 46 | 41.2 (12.0) |
Allowed but unknown | No | QLESQ | Ham-D | ? ? ? ✓ ? |
| Trivedi et al, 2004 | Paroxetine CR | 25 | Yes | 8 | 148 | 59.5 | 39.4 (10.8) |
Allowed but unknown | No | QLESQ | Ham-D | ✓ ✓ X ✓ X |
| CBT Studies | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | CBT Form at |
Dose of Treat. (hours) |
Length of Treat. (weeks) |
RCT | Sample Size |
% Female |
Mean Age (SD) |
Psychiatric Medication Use |
Psychiatric Comorbidity |
Medically Comorbid Sample |
QOL Measure |
Depression Measure |
Risk of Bias SG AC ID SR Total |
| Almlöv et al, 2009 | Comp | NA | NA | Yes | 103 | 77.7 | 40.8 (13.6) |
Unknown | Unknown | No | QOLI | BDI-II | ? ? ? ✓ ? |
| Brothers et al, 2011 | Ind. | 11 | 26 | No | 36 | 92 | 49.0 (11.0) |
Unspecified: 35.8% | Comorbid anxiety: 53% | Cancer | SF-36 | Ham-D | X ? X ✓ X |
| Craigie & Nathan, 2007 | Group | 20 | 10 | No | 115 | 71.3 | 38.6 (11.8) |
Any: 76.2% | Panic, GAD, SAD, OCD, PTSD | No | QLESQ | BDI-II | ✓ ? ? ✓ X |
| Craigie et al 2009 | Ind. | 8.4 | NA | No | 116 | 60 | 34.0 (11.5) |
Any: 69% | Any: 54%, comorbid anxiety: 47% | No | QLESQ | BDI-II | X ? ✓ ✓ X |
| Dobkin et al, 2011 | Ind. | 12.5 | 10 | Yes | 41 | 39 | 63.7 (9.9) |
Anti-depressants: 54% | Comorbid anxiety: 63% | Parkinson’s | SF-36 | Ham-D | ✓ ? ✓ ✓ ? |
| Freedland et al, 2009 | Ind. | 12 | 12 | Yes | 41 | 56 | 62.0 (11.0) |
Anti-depressants: 54% | Unknown | Coronary artery bypass surgery recoverers | SF-36 | Ham-D | ✓ ✓ ✓✓ ✓ |
| Hopko et al, 2008 | Ind. | 9 | 9 | No | 13 | 84.6 | 52.2 (10.9) |
Anti-depressant or antianxiety medication: 61.5% | GAD 54%; SAD 23%; panic 8%, OCD 8%; specific phobia 8%; anxiety NOS 8% | Cancer | QOLI | Ham-D | X ? ✓ ✓ X |
| Hopko et al, 2011 | Ind. | 8 | 12 | Yes | 42 | 100 | 56.4 (11.1) | Anti-depressant or antianxiety medication: 57% | GAD 50%; SAD 10%; PTSD: 5%, specific phobia 5%, panic 5%, anxiety NOS 3%. | Cancer | QOLI | Ham-D | ✓ ? ✓ ✓ ? |
| Jha et al, 2015 | Ind | 18 | 12 | No | 492 | 68.1 | 42.6 | Unknown | Unknown | No | QLESQ | Ham-D | X ? ✓ ✓ X |
| Johansson et al, 2012 | Comp | NA | 10 | Yes | 36 | 74.4 | 45.7 (10.9) |
Anti-depressants: 23.1% | Any 97%; anxiety disorder 62% | No | QOLI | BDI-II | ✓ ? ✓ ✓ ? |
| Johansson et al, 2013 | Comp | NA | 12 | No | 44 | 68.2 | 45.2 (13.0) |
Any: 20.4% | Any anxiety disorder 50%; SAD 30%; GAD 23%; panic 5%; OCD 2% | No | QOLI | BDI-II | X ? ✓ ✓ X |
| Kanter et al, 2015 | Ind. | NA | 12 | Yes | 21 | 76.2 | 38.7 (11.7) |
Not allowed | Any: 33.3% | No | QLESQ | Ham-D | ✓ ? ✓ ✓ ? |
| Laidlaw et al, 2008 | Ind. | NA | 8 | Yes | 20 | 60 | 74 (8.39) |
Not allowed | Any: 10% | No | WHO-QOL | Ham-D | ✓ ? ? ✓ ? |
| Lemmens et al, 2015 | Ind. | NA | 18 | Yes | 76 | 71.1 | 41.2 (12.4) |
Not allowed | Unknown | No | SF-36 | BDI-II | ✓✓✓✓ ✓ |
| Levin et al, 2011 | Comp | 4.3 | 6 | Yes | 100 | 78 | 44 (13.3) |
Unknown | Comorbid anxiety disorder 45%, substance abuse 13% | No | PES | CES-D | ✓? ✓ ✓ ? |
| McEvoy et al, 2013 | Group | 20 | 10 | No | 142 | 68.1 | 38.6 (13.7) |
Any: 82.5% | Any 66%; GAD 28%; SAD: 22%, dysthymia 19%, panic 10% | No | QLESQ | BDI-II | X ? ✓ ✓ X |
| Oei & McAlinden, 2014 | Group | 28 | 4 | No | 53 | 66 | 44.3 (11.6) |
Any: 71.2% | Unknown | No | QOLI | ZSDS | X ? ✓ ✓ X |
| Parker et al, 2013 | Ind. | 10 | 12 | Yes | 11 | 72.7 | 48.0 (9.5) |
Not allowed | Unknown | No | QLESQ-SF | Ham-D | ✓ ✓ ? ✓ ? |
| Qiu et al, 2013 | Group | 20 | 10 | Yes | 31 | 100 | 51.7 (6.0) |
Not allowed | Unknown | Breast cancer | FACT-B | Ham-D | ✓ ? ✓ ✓ ? |
| Richards et al, 2016 | Ind. | 12.5 | 16 | Yes | 168 | 68 | 43 (14.1) |
Antidepressants: 79% | Unknown | No | SF-36 | PHQ-9 | ✓✓✓✓ ✓ |
| Saulsman et al, 2006 | Group | 20 | 10 | Yes | 109 | 69 | 36.3 (11.0) |
Any: 77% | All: 74% | No | QLESQ | BDI-II | ? ? ? ✓ ? |
| Swan et al, 2009 | Group | 20 | 10 | No | 49 | 65.5 | 38.1 (11.5) |
Any: 76.7% | Any 59%; panic 14%, GAD 25%; OCD 3%; SAD 22%; simple phob. 2%; substance abuse 2% | No | QLESQ | BDI-II | X ? X ✓ X |
| Vilhauer et al, 2013 | Group | 30 | 10 | No | 20 | 65 | 52.4 (13.5) |
Any: 90% | Personality disorder 10%; anxiety disorder 50%; substance abuse 10%; psychosis 5% | No | QLESQ-SF | QIDS | X ? ✓ ✓ X |
| Watson & Nathan 2008 | Ind. | 11 | 11 | No | 90 | 61.5 | 34.5 (11.7) |
Any: 73% | Any: 64%; panic 16%; GAD 14%, SAD: 12% | No | QLESQ | BDI-II | X ? ✓ ✓ X |
Note. Definitions of abbreviations are listed below:
Quality of life measures: BQOL= Battelle Quality of Life Questionnaire (Revicki, Turner, Brown, & Martindale, 1992); FACT-B = Functional Assessment of Cancer Therapy – Breast (Wang, Zhang, & Tang, 2003); PES = Pleasant Events Schedule, Mood-Related subscale (MacPhillamy, & Lewinsohn, 1982); QLESQ = Quality of Life Enjoyment and Satisfaction Questionnaire (Endicott, Nee, Harrison, & Blumenthal, 1993); QLESQ-SF = Quality of Life Enjoyment and Satisfaction Questionnaire – Short Form (Endicott, Nee, Harrison, & Blumenthal, 1993); QOLI = Quality of Life Inventory (Frisch, MClark, Rouse, Rudd, Paweleck, Greenstone, & Kopplin, 2005); RAND-36 = The RAND-36 Measure of Health-Related Quality of Life (Hays, Sherbourne, & Mazel, 1993); SAT-P= Satisfaction Profile for quality of life (Majani, et al., 1999); SF-36 = The Short Form (36) Health Survey (Ware, & Sherbourne, 1992); WHOQOL= The World Health Organization Quality of Life (World Health Organization, 1995).
Depression measures: BDI = Beck Depression Inventory (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961); BDI-II = Beck Depression Inventory – II (Beck, Steer, & Brown,1996); CES-D = Center for Epidemiologic Studies Depression Scale (Radloff, 1977); Ham-D = Hamilton Depression Rating Scale (Hamilton, 1960); HADS-D = Hospital Anxiety and Depression Scale – Depression subscale (Zigmond, & Snaith, 1983); MADRS = Montgomery–Åsberg Depression Rating Scale (Montgomery, & Asberg, 1979); PHQ-9 = Patient Health Questionnaire 9 (Kroencke, Spitzer & Williams, 2001); SCL-90 = Symptom Checklist 90 – depression section (Derogatis, 2000); QIDS–SR = Quick Inventory of Depressive Symptomatology–Self Report (Rush, et al., 2003); ZSDS = Zung Self-Reported Depression Scale (Zung, 1965).
Pscyhiatric Comorbidity: GAD = Generalized Anxiety Disorder; OCD = Obsessive Compulsive Disorder; SAD = Social Anxiety Disorder; Anxiety NOS = Anxiety Not Otherwsie Specified; PTSD = Posttraumatic Stress Disorder.
Risk of Bias: SG = Sequence Generation; AC = Allocation Concealment; ID = Incomplete Outcome Data; SR = Selective Outcome Reporting; X = High Risk of Bias; ? = Unclear Risk of Bias; ✓ = Low Risk of Bias.
Across intervention types, the mean study sample consisted of 65.81% female participants (SD = 18.20), with a greater percentage in CBT studies (M = 71.33, SD = 13.46) than SSRI studies (M = 55.62, SD = 21.73; t(35) = 2.72, p < .05). The mean age of each study sample was 45.39 (SD = 9.73), with no difference between CBT (M = 46.46, SD = 9.90) and SSRI studies (M = 43.25, SD = 9.42; t(34) = 0.93, p = n.s.). Mean treatment duration across intervention type was 11.94 weeks (SD = 5.40), and was equivalent in CBT (M = 11.36 weeks, SD = 4.35) and SSRI studies (M = 12.92 weeks, SD = 6.91; t(17.71) = −0.82, p = n.s.). Only one SSRI study reported data on the frequency of psychiatric comorbidity (Saveanu, et al., 2015). Among the 15 CBT studies reporting such data, the mean percentage of participants with at least one comorbid condition was 55.80% (SD = 20.16). Of the 20 CBT studies that provided information on psychiatric medication use, 15 allowed participants to enter the study if stable on psychiatric medication, and on average 47.00% (SD = 32.56) of participants used medication. Only four of the 13 SSRI trials provided information on psychotherapy use among study participants, with all four trials excluding concomitant psychotherapy treatment during the trial. Ten studies reported follow-up data, with the mean follow-up assessment being 5.0 months post-treatment (SD = 1.81; range = 1–7 months).
To determine whether baseline symptom severity influenced the effect sizes of the two interventions, we examined differences in baseline depression severity across interventions. For interventions using the BDI-II as the primary outcome measure, no differences were found between CBT (M = 28.00, SD = 5.64) and SSRI treatments (M = 22.00, SD = 3.06; t(9) = 1.01, p = n.s.). Likewise, for interventions using the HAM-D as the primary outcome instrument, no differences were found between CBT (M = 18.73, SD = 3.06) and SSRI treatments (M = 20.89, SD = 2.78; t(11) = 1.09, p = n.s.).
Quantitative Data Synthesis
Treatment Effects on Depression
Pre-post within-group effects
For the within-group effects of treatment of depression, one outlier (Farabaugh, et al., 2015) (Hedges’ g = 5.16) was identified and removed. The pre-post within-group random effect size on depression symptoms was Hedges’ g = 1.30 (95% CI: 1.16–1.45, z = 17.81, p < .0001). The fail-safe N for measures of depression was 38,715 (z = 65.22), using an alpha level of .05. The fail-safe N values for SSRIs and CBT were 7,427 (z = 48.79) and 12,210 (z = 45.20), respectively. The Egger's regression intercept was not significant (intercept = 1.87, 2-tailed p = n.s.), suggesting that the parameter estimates were not influenced by the number of studies. The Q-value was significant (Q-value = 454.67, p < 0.01), and the I2 value was 95.16, suggesting considerable heterogeneity.
Pre-follow-up within-group effects
To examine the long-term effects of the treatments on depression, we examined the change in depression from pre-treatment to follow-up. Ten studies, all of which investigated CBT interventions, included follow-up data. One study with an effect size that was more than 2 standard deviations above the mean of the effect sizes of the other studies was identified (Qiu, et al., 2013) and removed from subsequent analyses (Hedges’ g = 3.73). The random effects meta-analysis yielded an effect size of Hedges’ g = 1.33 (95% CI: 0.99–1.67, z = 7.82, p < .0001). The fail-safe N was 1,265 (z = 23.32). The Egger's regression intercept was significant (intercept = 6.66, 2-tailed p < 0.05), suggesting that the parameter estimates could be influenced by the number of studies. The Q-value was significant (Q-value = 83.92, p < 0.01), and the I2 value was 90.47, suggesting considerable heterogeneity.
Pre-post controlled effects
To examine the treatment effect on depression in controlled studies, we examined the controlled effect size and found an overall large effect of Hedges’ g = 1.21 (95% CI: 0.55–1.87, z = 3.59, p < .0001). The fail-safe N was robust with N = 221 (z = 11.41); however, the Egger's regression intercept was significant (intercept = 1.71, 2-tailed p < 0.05). The Q-value was significant (Q-value = 105.96, p < 0.01), and the I2 value was 94.34, suggesting considerable heterogeneity.
Treatment Effects on Quality of Life
Pre-post within-group effects
For the within-group analysis, one outlier with 2 standard deviations above the mean (Hedges’ g = 3.28) was identified (Åberg-Wistedt, Ågren, Ekselius, Bengtson & Åkerblad, 2000). and removed from subsequent analyses. The random effects meta-analysis yielded an overall QOL effect size of Hedges’ g = 0.69 (95% CI: 0.61–0.78, z = 15.52, p < .0001). The fail-safe N analyses for the CBT and SSRI treatments were robust with N = 5,282 (z = 29.14) and 5,233 (z = 39.37), respectively. Likewise, the Egger's regression intercept was not significant for all studies (intercept = −0.63, 2-tailed p = n.s.). The Q-value was significant (Q-value = 381.24, p < 0.01), and the I2 value was 90.56, suggesting considerable heterogeneity.
Using the trim and fill method (Figure 2), 5 studies would need to fall to the left of the mean (i.e., have an effect size smaller than the mean) and 0 studies would need to fall to the right of the mean (i.e., have an effect size larger than the mean) to make the overall random-effects plot symmetrical. The random-effects model for the new imputed mean effect size revealed a Hedges’ g = 0.68 (95% CI: 0.66–0.70).
Figure 2.
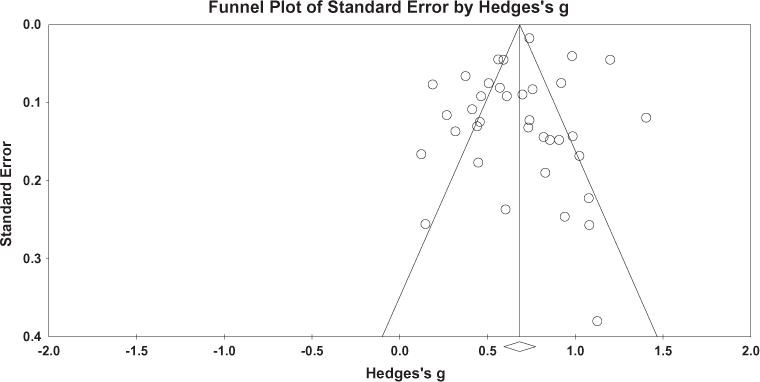
Funnel plot of precision by Hedges’ g for quality of life measures in the pooled meta-analysis.
Note: This funnel plot reflects a random effects model. Horizontal and vertical axes plot the effect size and standard error of the effect size, respectively.
Pre-follow-up within-group effects
Ten studies, all of which investigated CBT interventions, assessed QOL at follow-up. The random effects analysis yielded an effect size of Hedges’ g = 0.72 (95% CI: 0.48–0.97, z = 5.76, p < .0001), which did not significantly differ from the CBT pre-post within-group effect size (QB = 0.09, df = 1, p = n.s.). The fail-safe N was 614 (z = 15.47); however, the Egger's regression intercept was significant (intercept = 6.26, 2-tailed p < 0.05). The Q-value was significant (Q-value = 79.06, p < 0.01), and the I2 value was 88.62, suggesting considerable heterogeneity.
Pre-post controlled effect sizes
Eight of the trials included a non-active control or comparison group, including pill placebo, attention control, clinical monitoring, online discussion groups, treatment as usual, and waitlist. In total, these controlled trials together yielded a QOL controlled effect size of Hedges’ g = 0.29 (95% CI: 0.12–0.47, z = 3.31, p < .01). Using an alpha level of .05, the fail-safe N for measures of QOL was 43 (z = 4.94). Because this N is smaller than 5k + 10, the controlled effect sizes cannot be considered statistically robust. However, the Egger's regression intercept was not significant (intercept = 0.21, 2-tailed p = n.s.), suggesting that the parameter estimates were not influenced by the number of studies. The Q-value was not significant (Q-value = 13.63, p = 0.06), and the I2 value was 48.63, suggesting moderate heterogeneity.
Moderator Analyses
Results revealed no significant relationship between pre-post QOL effect sizes and study quality (QB = 0.37, df = 2, p = n.s.) or percentage of patients who completed treatment (B = −0.01, SE = 0.01, p = n.s). Moreover, comorbidity with a medical disorder did not significantly moderate QOL effect sizes (QB = .25, df = 1, p = n.s.), nor did sex distribution (B = −0.003, SE = 0.00, p = n.s.).
For both SSRI and CBT interventions, treatment efficacy was not moderated by dosage (B = 0.008, SE = 0.01, p = n.s.) or total treatment hours (B = −0.004, SE = 0.01, p = n.s.). The current results suggest that neither delivery format of CBT (i.e., individual, group, computer-based) (QB = 1.63, df = 2, p = n.s.),type of SSRI (QB = 5.19, df = 5, p = n.s.), nor frequency of contact with study physician in SSRI studies (B = 0.001, SE = 0.07, p = n.s.) moderated improvement in QOL. Moreover, the efficacy of CBT on QOL was not moderated by whether a study included patients who were on a stable dose of psychiatric medication (QB = 0.32, df = 1, p = n.s.). We also examined likelihood of being placed in placebo condition as predictor of pre-post effect size in SSRI studies, and found no effect (B = 0.00, SE = 0.00, p = n.s.).
However, quality of life effect sizes were moderated by publication year (B = −0.02, SE = 0.01, p < 0.05), indicating that the effect sizes decreased linearly with time. The decrease in effect size across time was likely not accounted for by diminishing study quality or sample size, Furthermore, of the 6 most recently published studies, all of them still had statistically significant within-group effect sizes. In fact, of the 36 studies, only 2 of them (Paile-Hyvärinen, Wahlbeck, & Eriksson, 2003; Vilhauer, Cortes, Moali, Chung, Mirocha, & Ishak, 2013) had non-significant within-group effect sizes. Improvement in QOL was also significantly moderated by improvement in depression (B = 0.22, SE = 0.05, p < .0001). Depression improvement was positively associated with QOL improvement for those who received CBT (B = 0.21, SE = 0.06, p < .0001), whereas no significant relationship occurred for those receiving SSRIs (B = 0.21, SE = 0.13, p = n.s.). However, there was no significant interaction effect of changes in depression and intervention type on QOL improvement (B = − 0.13, SE = 0.23, p = n.s.).
Based on prior research demonstrating differential effect sizes resulting from the use of clinician-administered versus self-report measures of depression, (Cuijpers, Li, Hofmann, & Andersson, 2010) we investigated type of assessment instrument as a moderator of depression outcome. Consistent with such research, our analyses similarly showed that clinician administered measures yielded larger effect sizes (Hedges’ g = 1.56) than did self-report instruments (Hedges’ g = 1.01) (QB = 12.74, df = 1, p < 0.05.). Furthermore, the type of assessment instrument for depression moderated the relationship between depression effect sizes and QOL effect sizes (B = −0.37, SE = 0.18, p < 0.05.). Specifically, the relationship between depression and QOL improvement was weaker for clinician administered measures (B = 0.13) than for self-report measures (B = 0.51).
Discussion
To examine the effects of the two most common treatments for depression (SSRI and CBT) on QOL, we conducted a meta-analytic review. Our initial search produced 3,665 unique studies. Of those, 37 studies (24 CBT, 13 SSRI) examining 1,969 participants receiving CBT and 4,286 participants receiving SSRI treatment were used for this meta-analytis. Only two studies directly examined the effects of SSRI and CBT for depression on QOL (Farabaugh, et al., 2015; Orjuela-Rojas et al., 2015), and as described above were not included in the analysis. Therefore, this research was not designed to directly compare the effects of CBT vs. SSRIs for depression on QOL. Rather, the present study provides an assessment of the size of the effect that SSRIs and CBT have on QOL in patients treated for depression, as well as the strength of the evidence for such effects.
Results showed that both SSRIs and CBT were associated with large reductions in QOL from pre to post-treatment, and small effects compared to control treatments. Furthermore, computer-based, individual, and group-based CBT for depression similarly improved QOL. A small number of studies (all of them were CBT trials) also included follow-up data and the results showed that this effect was maintained over a follow-up period of 1 to 7 months, supporting the current practice guidelines (Gelenberg, et al., 2010). Together, these results suggest that the effects of SSRIs and CBT for MDD extend beyond symptoms of depression, broadly impacting patients’ quality of life.
The primary analysis showed that QOL significantly improved after SSRIs (Hedges’ g = 0.79) and CBT (Hedges’ g = 0.63) for depression. Similar effects on QOL were observed in other studies with CBT (Hofmann, Wu, & Boettcher, 2014) and pharmacotherapy (Hofmann, Wu, Boettcher, & Sturm, 2014) for anxiety disorders. Of note, a significant portion of participants in most CBT trials (45%) began treatment while also receiving some form of pharmacotherapy. Although patients were stabilized on their medication throughout the CBT treatment, it is possible that the medication already enhanced the patients’ QOL, making it more difficult for CBT to further improve QOL. On the other hand, the majority of SSRI trials (9 of 13) did not provide information on concomitant psychotherapy use, making it difficult to evaluate whether SSRI response could have been influenced by additional treatment.
Interestingly, improvement in depression was positively associated with changes in QOL only for patients who received CBT but not for those who received SSRIs. This is somewhat consistent with other meta-analytic research on psychotherapy for depression showing that changes in mental health-related quality of life over the course of treatment were partially accounted for by changes in depressive symptoms (Kolovos, Kleiboer & Cuijpers, 2016). It is possible that CBT improves QOL primarily by reducing symptoms of depression, whereas SSRIs are more broad-band therapies that target a multitude of psychiatric problems, including anxiety and stress (Gorman, & Kent, 1999). Combining SSRIs and CBT might result in a more complete and long-lasting improvement by not only reducing depression but also improving the patient’s QOL. Future combination studies with mediation analyses that examine the causal pathway would provide valuable data to examine this possible explanation.
Although only a minority of studies clearly demonstrated a low risk of bias, risk of bias was unrelated to the effect size estimates of the interventions on QOL in the present study. The suboptimal risk of bias ratings obtained in the current meta-analysis are also in accordance with those found in other large scale meta-analyses of CBT for depression (Cristea, Huibers, David, Hollon, Andersson, & Cuijpers, 2015), which is not uncommon in systematic reviews using the Cochrane Collaboration’s Risk of Bias tool (Armijo‐Olivo, Stiles, Hagen, Biondo, & Cummings, 2012; Robertson, Ramsay, Gurung, Mowatt, Pickard, & Sharma, 2015; Sinha, Craig, Sureshkumar, Hayen, & Brien, 2015). Further, we observed no difference in the risk of bias between CBT and SSRI trials. Nevertheless, the small number of high quality studies is clearly a limitation; more high quality studies in the depression field are needed. In addition, our analyses showed that treatment intensity, type of SSRI sample size, comorbidity, and sex distribution did not moderate the effects. Later publication year was associated with a smaller increase in QOL. We do not have a convincing explanation for this finding, but note that it is in line with the observation that effect sizes in clinical trials tend to decrease over time (Ioannidis, 2005; Johnsen, & Friborg, 2015).
There are several limitations that should be considered when interpreting these findings. First, despite the relatively large number of clinical trials of one of the most common mental health problems, we identified just two studies that directly examined the effects of SSRI and CBT for depression on QOL, and no study examined the combined effect on QOL. Therefore, we cannot conclude that, despite the lack of significant differences, SSRIs and CBT are as effective for improving QOL. Without a direct comparison of these modalities, it is not possible to control the various potential factors that might mediate and moderate the effects, leaving the results to be tentative. This is clearly an area for future research, and would enable more robust examinations of differential efficacy. Second, a limited number of studies had control conditions to use as a comparison for the effects of CBT or SSRIs, and the pooled effect sizes for such studies was rather small. This makes it difficult to establish whether such effects are specific to the treatments examined in this study. A recent meta-analysis by Kolovos et al. (2016), for instance, found no difference between the effect of CBT and other forms of psychotherapy on quality of life in depression, further underscoring the need for more comparative studies. Moreover, there were not enough studies to conduct a moderator analysis to examine differences in effect size across type of control group.
Third, only CBT studies reported data at follow-up, thus the long-term effect of SSRIs on QOL could not be examined. Fourth, only one SSRI study reported data on psychiatric comorbidity. Again, this confound does not allow for a direct comparison between the effects of CBT and SSRI on QOL. Fifth, in some instances, results of the moderator analyses were based on a small number of studies. Relatedly, the effects of the two treatment modalities on QOL might be different between patients with and without certain other comorbid conditions (e.g., cardiovascular disease, pain, cancer, etc), because the treatments might have different effects of these conditions, thereby differentially improving QOL. Many more large-scale trials would be needed to control for all these potentially confounding factors. Sixth, although the risk of bias was not related to effect sizes, the sample of included studies was relatively small and there is no measure or procedure that can perfectly detect and correct publication bias. Therefore, it is difficult, if not impossible, to accurately detect the number of unpublished studies or their magnitude on the overall estimate of treatment effects. Finally, there was some level of heterogeneity in the measures used to assess QOL. While measures that primarily focused on symptoms or health status without assessing satisfaction with one’s life were excluded in accordance with prior conceptualizations of QOL (Moons, Budts, & De Geest, 2006; Smith, et al., 1999), instruments still varied to some degree (e.g., different emphases on satisfaction with physical vs. psychological functioning).
Despite these limitations, our results add to the current state of knowledge regarding the effect of CBT and SSRIs for depression on QOL. Our review suggests that both treatment modalities improve QOL in patients with depression, with no evidence for differential effects between treatments. Improvements in QOL were more strongly linked to symptom reduction after CBT than SSRIs, possibly pointing to a different mechanism through which the treatments enhance QOL. However, in order to directly compare CBT and SSRIs for depression on QOL, large-scale studies are needed that directly compare these treatment modalities (and their combination).
Table 2.
Hedges’ g for within-group pre-post, within-group pre-follow-up, and controlled pre-post QOL effect sizes in each study
| Intervention | Study | Pre-Post Effect Size | Pre-FU Effect Size | Pre-Post Control. Effect Size | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hedges’ g (95% CI) |
Z | p | Hedges’ g (95% CI) |
Z | p | Hedges’ g (95% CI) |
Z | p | ||
| Individual CBT | Brothers et al, 2011 | .32 (.05–.59) | 2.33 | .02 | – | – | – | – | – | – |
| Craigie et al, 2007 | .51 (.36–.66) | 6.72 | .00 | – | – | – | – | – | – | |
| Dobkin et al, 2011 | .46 (.21–.71) | 3.67 | .00 | .27 (.03–.51) | 2.22 | .02 | .72 (.27–1.17) | 3.15 | .00 | |
| Freedland et al, 2009 | .91 (.62–1.20) | 6.14 | .00 | .73 (.45–1.00) | 5.16 | .00 | – | – | – | |
| Hopko et al, 2008 | .94 (.46–1.43) | 3.82 | .00 | .91 (.43–1.39) | 3.72 | .00 | – | – | – | |
| Hopko et al, 2011 | .99 (.71–1.27) | 6.87 | .00 | 1.25 (.94–1.56) | 7.93 | .00 | – | – | – | |
| Jha et al, 2015 | 1.20 (1.11–1.29) | 26.178 | .00 | – | – | – | – | – | – | |
| Kanter et al, 2015 | .83 (.46–1.21) | 4.37 | .00 | – | – | – | – | – | – | |
| Laidlaw et al, 2008 | .45 (.10–.80) | 2.54 | .01 | .28 (–.05–.62) | 1.65 | .00 | – | – | – | |
| Lemmens et al, 2015 | .47 (.29–.65) | 5.04 | .00 | .52 (.33–.70) | 5.49 | .00 | – | – | – | |
| Parker et al, 2013 | .61 (.14–1.07) | 2.55 | .01 | – | – | – | – | – | – | |
| Richards et al, 2016 | .67 (.61–.78) | 15.52 | .00 | |||||||
| Watson & Nathan, 2008 | .70 (.53–.88) | 7.77 | .00 | – | – | – | – | – | – | |
| Subtotal | .70 (.49–.91) | 6.74 | .00 | .64 (.36–.93) | 4.41 | .00 | .72 (.27–1.17) | 3.15 | .00 | |
|
| ||||||||||
| Group CBT | Craigie & Nathan, 2009 | .82 (.54–1.11) | 5.68 | .00 | – | – | – | – | – | – |
| McEvoy et al, 2013 | .38 (.25–.51) | 5.66 | .00 | – | – | – | – | – | – | |
| Oei & McAlinden, 2014 | .42 (.20–.63) | 3.81 | .00 | – | – | – | – | – | – | |
| Qiu et al, 2013 | 1.03 (.70–1.36) | 6.08 | .00 | 1.55 (1.15–1.95) | 7.59 | .00 | .75 (.24–1.26) | 2.89 | .00 | |
| Saulsman et al, 2006 | .76 (.60–.93) | 9.08 | .00 | – | – | – | – | – | – | |
| Swan et al, 2009 | .74 (.50–.99) | 6.02 | .00 | – | – | – | – | – | – | |
| Vilhauer et al, 2013 | .13 (−.20–.45) | .76 | .45 | – | – | – | – | – | – | |
| Subtotal | .63 (.44–.82) | 6.55 | .00 | 1.55 (1.15–1.95) | 7.59 | .00 | .75 (.24–1.26) | 2.89 | .00 | |
|
| ||||||||||
| Computer-based CBT | Almlöv et al, 2009 | .58 (.41–.74) | 7.02 | .00 | – | – | – | – | – | – |
| Johansson et al, 2012 | .86 (.57–1.15) | 5.78 | .00 | .89 (.59–1.18) | 5.92 | .00 | .03 (–.39–.45) | .15 | .88 | |
| Johansson et al, 2013 | .27 (.04–.50) | 2.33 | .02 | .81 (.55–1.07) | 6.12 | .00 | – | – | – | |
| Levin et al, 2011 | .19 (.04–.34) | 2.47 | .01 | .25 (.09–.40) | 3.15 | .00 | .02 (−.26–.30) | .12 | .91 | |
| Subtotal | .46 (.19–.73) | 3.33 | .01 | .64 (.19–1.09) | 2.78 | .00 | .02 (−.21–.26) | .18 | .86 | |
|
| ||||||||||
| All CBT | .63 (.49–.77) | 8.88 | .00 | .72 (.48–.97) | 5.76 | .00 | .35 (−.04–.75) | 1.72 | .09 | |
|
| ||||||||||
| Citalopram | Gleason et al, 2002 | 1.09 (.58–1.60) | 4.21 | .00 | – | – | – | – | – | – |
| Ishak et al, 2013 | .74 (.71–.78) | 40.56 | .00 | – | – | – | – | – | – | |
| Nicolau et al, 2013 | .45 (.19–.70) | 3.39 | .00 | – | – | – | .55 (−.14–1.25) | 1.56 | .12 | |
| Subtotal | .70 (.47–.96) | 5.37 | .00 | – | – | – | .55 (−.14–1.25) | 1.56 | .12 | |
|
| ||||||||||
| Paroxetine | Paile-Hyvärinen et al, 2003 | .15 (−.35–.65) | .59 | .56 | – | – | – | −.29 (−1.31–.73) | −.56 | .58 |
| Trivedi et al, 2004* | .92 (.78–1.07) | 12.20 | .00 | – | – | – | .26 (.01–.50) | 2.06 | .04 | |
| Subtotal | .58 (−.18–1.33) | 1.50 | .13 | – | – | – | .22 (−.06–.49) | 1.51 | .13 | |
|
| ||||||||||
| Sertraline | Lewis-Fernandez et al, 2013 | .74 (.48–1.00) | 5.55 | .00 | – | – | – | – | – | – |
| Rabkin et al, 1994 | 1.13 (.38–1.88) | 2.97 | .00 | – | – | – | – | – | – | |
| Saveneau et al, 2015 | .60 (.51–.69) | 12.96 | .00 | – | – | – | – | – | – | |
| Shelton et al, 2006 | 1.41 (1.17–1.64) | 11.72 | .00 | – | – | – | – | – | – | |
| Subtotal | .94 (.50–1.39) | 4.18 | .00 | – | – | – | – | – | – | |
|
| ||||||||||
| Escitalopram | Demyttenaere et al, 2008 | .99 (.91–1.07) | 23.91 | .00 | – | – | – | .30 (.17-.43) | 4.60 | .00 |
| Saveneau et al, 2015 | .57 (.48–.66) | 12.42 | .00 | – | – | – | – | – | – | |
| Subtotal | .78 (.36–1.19) | 3.69 | .00 | – | – | – | – | – | – | |
|
| ||||||||||
| Fluoxetine | Bellino et al, 2006 | 1.08 (.64–1.52) | 4.84 | .00 | – | – | – | – | – | – |
|
| ||||||||||
| Multiple | Aberg-Wistedt et al, 2000 | 4.41 (4.03–4.79) | 22.73 | .00 | – | – | – | – | – | – |
| Peveler et al, 2005 | .62 (.43-.80) | 6.64 | .00 | – | – | – | – | – | – | |
|
| ||||||||||
| All SSRI | .79 (.67–.91) | 12.82 | .00 | – | – | – | .29 (.18–.40) | 5.12 | .00 | |
Note: Control conditions for pre-post controlled effect sizes were as follows: Dobkin et al., (2011) – clinical monitoring; Qiu et al., (2013) – waitlist; Johansson et al., (2012) – online discussion group; Levin et al., (2011) – treatment as usual; Nicolau et al. (2013) – attention control; Paile-Hyvärinen et al., (2003) – placebo; Trivedi et al. (2004) – placebo; Demyttenaere et al., (2008) – placebo.
Used Paroxetine CR;
Not included in combined group analysis due to being an outlier.
Acknowledgments
Dr. Hofmann receives support from NIH/NCCIH (R01AT007257), NIH/NIMH (R01MH099021, R34MH099311, R34MH086668, R21MH102646, R21MH101567, K23MH100259), the James S. McDonnell Fundation 21st Century Science Initiative in Understanding Human Cognition – Special Initiative, and the Department of the Army for work unrelated to the studies reported in this article. He receives compensation for his work as an advisor from the Palo Alto Health Sciences and Otsuka Digital Health, Inc., and for his work as a Subject Matter Expert from John Wiley & Sons, Inc. and SilverCloud Health, Inc. He also receives royalties and payments for his editorial work from various publishers for work unrelated to the studies reported in this article.
Funding/Support and Role of the sponsor: This study is not supported by any funding agencies.
Additional Contributions: The authors would like to thank Muyu Lin, Elizabeth Mundy, Almudena Duque Sanches, Fangfang Shangguan, Can Sabaner and Aleksandrina Skvortsova for their help with retrieving articles, entering them into the data base, or cross-checking some of the entries. None of these individuals received any financial compensation.
Footnotes
Author Contributions: All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Stefan G. Hofmann.
Acquisition of data: Joshua Curtiss, Joseph Carpenter, and Shelley Kind
Analysis and interpretation of data: Stefan G. Hofmann, Joshua Curtiss, Joseph Carpenter, and Shelley Kind.
Drafting of the manuscript: Stefan G. Hofmann, Joshua Curtiss, Joseph Carpenter, and Shelley Kind.
Critical revision of the manuscript for important intellectual content: Stefan G. Hofmann, Joshua Curtiss, Joseph Carpenter, and Shelley Kind.
Statistical analysis: Joshua Curtiss and Joseph Carpenter.
Obtained funding: The study is not funded.
Administrative, technical, or material support: Stefan G. Hofmann.
Study supervision: Stefan G. Hofmann
Previous Presentations: Preliminary results from this study were presented as a poster in November 2015 at the 49th Annual Convention of the Association for Behavioral and Cognitive Therapies.
References
Note. All references listed with a * refer to studies included in the meta-analysis.
- *.Åberg-Wistedt A, Ågren H, Ekselius L, Bengtson F, Åkerblad A. Sertraline versus paroxetine in major depression: Clinical outcome after six months of continuous therapy. Journal of Clinical Psychopharmacology. 2000;20:645–652. doi: 10.1097/00004714-200012000-00010. [DOI] [PubMed] [Google Scholar]
- *.Almlöv J, Carlbring P, Berger T, Cuijpers P, Andersson G. Therapist factors in Internet-delivered cognitive behavioural therapy for major depressive disorder. Cognitive Behavioral Therapy. 2009;38:247–254. doi: 10.1080/16506070903116935. [DOI] [PubMed] [Google Scholar]
- Angermeyer MC, Katschnig H. Psychotropic medication and quality of life: A conceptual framework for assessing their relationship. In: Katschnig H, Freeman H, Sartorius N, editors. Quality of life in mental disorders. 2nd. England: Wiley; 2006. pp. 215–25. [Google Scholar]
- Angermeyer MC, Kilian R. Theoretical models of quality of life for mental disorders. In: Katschnig H, Freeman H, Sartorius N, editors. Quality of life in mental disorders. 2nd. Wiley; 2006. pp. 21–31. [Google Scholar]
- Armijo‐Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: Methodological research. Journal of Evaluation in Clinical Practice. 2012;18:12–18. doi: 10.1111/j.1365-2753.2010.01516.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory–II Manual. San Diego, California: Harcourt Brace Jovonovich; 1996. [Google Scholar]
- *.Bellino S, Zizza M, Rinaldi C, Bogetto F. Combined treatment of major depression in patients with borderline personality disorder: A comparison with pharmacotherapy. Canadian Journal of Psychiatry. 2006;51:453–460. doi: 10.1177/070674370605100707. [DOI] [PubMed] [Google Scholar]
- *.Brothers B, Yang H, Strunk D, Andersen B. Cancer patients with major depressive disorder: Testing a biobehavioral/cognitive behavior intervention. Journal of Consulting and Clinical Psychology. 2011;79:253–260. doi: 10.1037/a0022566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comprehensive Meta-Analysis (Version 3) [Computer software] Englewood, NJ: Biostat; 2015. Available from https://www.meta-analysis.com/ [Google Scholar]
- *.Craigie M, Nathan P. A nonrandomized effectiveness comparison of broad-spectrum group CBT to individual CBT for depressed outpatients in a community mental health setting. Behavior Therapy. 2009;40:302–314. doi: 10.1016/j.beth.2008.08.002. [DOI] [PubMed] [Google Scholar]
- *.Craigie M, Saulsman L, Lampard A. MCMI-III personality complexity and depression treatment outcome following group-based cognitive-behavioral therapy. Journal of Clinical Psychology. 2007;63:1153–1170. doi: 10.1002/jclp.20406. [DOI] [PubMed] [Google Scholar]
- Cristea IA, Huibers MJ, David D, Hollon SD, Andersson G, Cuijpers P. The effects of cognitive behavior therapy for adult depression on dysfunctional thinking: A meta-analysis. Clinical Psychological Review. 2015;42:62–71. doi: 10.1016/j.cpr.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Li J, Hofmann SG, Andersson G. Self-reported versus clinician-rated symptoms of depression as outcome measures in psychotherapy research on depression: a meta-analysis. Clinical Psychology Review. 2010;30:768–778. doi: 10.1016/j.cpr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- *.Demyttenaere K, Andersen H, Reines E. Impact of escitalopram treatment on Quality of Life Enjoyment and Satisfaction Questionnaire scores in major depressive disorder and generalized anxiety disorder. International Clinical Psychopharmacology. 2008;23:276–286. doi: 10.1097/YIC.0b013e328303ac5f. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Task Force for the Handbook of Psychiatric Measures Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association; 2000. Symptom Checklist-90-Revised (SCL-90-R). [Self-report Measure] pp. 81–84. [Google Scholar]
- *.Dobkin RD, Menza M, Allen LA, Gara MA, Mark MH, Tiu J, Friedman J. Cognitive-behavioral therapy for depression in Parkinson’s disease: A randomized, controlled trial. American Journal of Psychiatry. 2011;168:1066–1074. doi: 10.1176/appi.ajp.2011.10111669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: A simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R. Quality of life enjoyment and satisfaction questionnaire: A new measure. Psychopharmacological Bulletin. 1993;29:321–326. [PubMed] [Google Scholar]
- Farabaugh A, Fisher L, Nyer M, Holt D, Cohen M, Alpert JE. Similar changes in cognitions following cognitive-behavioral therapy or escitalopram for major depressive disorder: Implications for mechanisms of change. Annals of Clinical Psychiatry. 2015;27:118–25. [PubMed] [Google Scholar]
- *.Freedland KE, Skala JA, Carney RM, Rubin EH, Lustman PJ, Dávila-Román VG, Hogue CR., Jr Treatment of depression after coronary artery bypass surgery: A randomized controlled trial. Archives of General Psychiatry. 2009;66:387–396. doi: 10.1001/archgenpsychiatry.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch MB, Clark MP, Rouse SV, Rudd MD, Paweleck JK, Greenstone A, Kopplin DA. Predictive and treatment validity of life satisfaction and the quality of life inventory. Assessment. 2005;12:66–78. doi: 10.1177/1073191104268006. [DOI] [PubMed] [Google Scholar]
- Gelenberg AJ, Freeman MP, Markowitz JC, Rosenbaum JF, Thase ME, Trivedi MH, Van Rhoads RS. Practice guideline for the treatment of patients with major depressive disorder. 3rd. American Psychiatric Association; 2010. [Google Scholar]
- Gladis MM, Gosch EA, Dishuk NM, Crits-Christoph P. Quality of life: Expanding the scope of clinical significance. Journal of Consulting and Clinical Psychology. 1999;67:320–331. doi: 10.1037//0022-006x.67.3.320. [DOI] [PubMed] [Google Scholar]
- *.Gleason O, Yates W, Isbell M, Philipsen M. An open-label trial of citalopram for major depression in patients with hepatitis C. Journal of Clinical Psychiatry. 2002;63:194–198. doi: 10.4088/jcp.v63n0304. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Kent JM. SSRIs and SNRIs: broad spectrum of efficacy beyond major depression. Journal of Clinical Psychiatry. 1999;60:33–39. [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) Journal of Clinical Psychiatry. 2015;76:155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A Rating scale for depression. Journal of Neurology Neurosurgery & Psychiatry. 1960;23:54–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays R, Sherbourne C, Mazel R. The RAND 36-Item Health Survey 1.0. Health Economics. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- Hedges L, Olkin I. Statistical Methods for Meta-analysis. Amsterdam: The Netherlands: Academic Press; 1985. [Google Scholar]
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011 Retrieved from: http://www.cochrane-handbook.org.
- Higgins J, Altman DG. Chapter 8: Assessing risk of bias in included studies. Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0. 2008 Retrieved from http://www.cochrane-handbook.org.
- Hirschfeld RM, Dunner DL, Keitner G, Klein DN, Koran LM, Kornstein SG, Keller MB. Does psychosocial functioning improve independent of depressive symptoms? A comparison of nefazodone, psychotherapy, and their combination. Biological Psychiatry. 2002;51:123–133. doi: 10.1016/s0006-3223(01)01291-4. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Asnaani A, Vonk JJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cognitive Therapy and Research. 2012;36:427–440. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Wu JQ, Boettcher H. Effect of cognitive-behavioral therapy for anxiety disorders on quality of life: A meta-analysis. Journal of Consulting and Clinical Psychology. 2014;82:375. doi: 10.1037/a0035491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Wu JQ, Boettcher H, Sturm J. Effect of pharmacotherapy for anxiety disorders on quality of life: a meta-analysis. Quality of Life Research. 2014;23:1141–1153. doi: 10.1007/s11136-013-0573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hopko DR, Armento ME, Robertson S, Ryba MM, Carvalho JP, Colman LK, Lejuez CW. Brief behavioral activation and problem-solving therapy for depressed breast cancer patients: Randomized trial. Journal of Consulting and Clinical Psychology. 2011;79:834–849. doi: 10.1037/a0025450. [DOI] [PubMed] [Google Scholar]
- *.Hopko DR, Bell JL, Armento M, Robertson S, Mullane C, Wolf N, Lejuez CW. Cognitive-behavior therapy for depressed cancer patients in a medical care setting. Behavior Therapy. 2008;39:126–136. doi: 10.1016/j.beth.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP. Contradicted and initially stronger effects in highly cited clinical research. JAMA. 2005;294:218–228. doi: 10.1001/jama.294.2.218. [DOI] [PubMed] [Google Scholar]
- *.Ishak WW, Christensen S, Sayer G, Ha K, Li N, Miller J, Cohen RM. Sexual satisfaction and quality of life in major depressive disorder before and after treatment with citalopram in the STAR*D study. Journal of Clinical Psychiatry. 2013;74:256–261. doi: 10.4088/JCP.12m07933. [DOI] [PubMed] [Google Scholar]
- *.Jha MK, Minhajuddin A, Thase ME, Jarrett RB. Improvement in self-reported quality of life with cognitive therapy for recurrent major depressive disorder. Journal of Affective Disorders. 2014;167:37–43. doi: 10.1016/j.jad.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Johansson R, Nyblom A, Carlbring P, Cuijpers P, Andersson G. Choosing between Internet-based psychodynamic versus cognitive behavioral therapy for depression: A pilot preference study. BMC Psychiatry. 2013;13:268. doi: 10.1186/1471-244X-13-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Johansson R, Sjöberg E, Sjögren M, Johnsson E, Carlbring P, Andersson T, Andersson G. Tailored vs. standardized internet-based cognitive behavior therapy for depression and comorbid symptoms: A randomized controlled trial. Plos ONE. 2012;7:e36905. doi: 10.1371/journal.pone.0036905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen T, Friborg O. The effects of cognitive behavioral therapy as an anti-depressive treatment is falling: A meta-analysis. Psychological Bulletin. 2015;141:747–768. doi: 10.1037/bul0000015. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Zeller PJ, Paulus M, Leon AC, Maser JD, Keller MD. Psychosocial disability during the long-term course of unipolar major depressive disorder. Archives of General Psychiatry. 2000;57:375–380. doi: 10.1001/archpsyc.57.4.375. [DOI] [PubMed] [Google Scholar]
- *.Kanter JW, Santiago-Rivera AL, Santos MM, Nagy G, López M, Hurtado GD, West P. A randomized hybrid efficacy and effectiveness trial of behavioral activation for Latinos with depression. Behavior Therapy. 2015;46:177–192. doi: 10.1016/j.beth.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kolovos, Kleiboer, Cuijpers Effect of psychotherapy for depression on quality of life: meta-analysis. British Journal of Psychiatry. 2016;209:460–468. doi: 10.1192/bjp.bp.115.175059. [DOI] [PubMed] [Google Scholar]
- Kroencke K, Spitzer R, Williams J. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Laidlaw K, Davidson K, Toner H, Jackson G, Clark S, Law J, Cross S. A randomised controlled trial of cognitive behaviour therapy vs treatment as usual in the treatment of mild to moderate late life depression. International Journal of Geriatric Psychiatry. 2008;23:843–850. doi: 10.1002/gps.1993. [DOI] [PubMed] [Google Scholar]
- *.Lemmens LH, Arntz A, Peeters F, Hollon SD, Roefs A, Huibers MJ. Clinical effectiveness of cognitive therapy v. interpersonal psychotherapy for depression: results of a randomized controlled trial. Psychological Medicine. 2015;45:2095–2110. doi: 10.1017/S0033291715000033. [DOI] [PubMed] [Google Scholar]
- *.Levin W, Campbell D, McGovern K, Gau J, Kosty D, Seeley JR, Lewinsohn P. A computer-assisted depression intervention in primary care. Psychological Medicine. 2011;41:1373–1383. doi: 10.1017/S0033291710001935. [DOI] [PubMed] [Google Scholar]
- *.Lewis-Fernández R, Balán IC, Patel SR, Sánchez-Lacay JA, Alfonso C, Gorritz M, Moyers TB. Impact of motivational pharmacotherapy on treatment retention among depressed Latinos. Psychiatry. 2013;76:210–222. doi: 10.1521/psyc.2013.76.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhillamy D, Lewinsohn P. The pleasant events schedule: Studies on reliability, validity, and scale intercorrelation. Journal of Consulting and Clinical Psychology. 1982;50:363–380. [Google Scholar]
- Majani G, Callegari S, Pierobon A, Giardini A, Viola L, Baiardini I, Sommaruga M. A new instrument in quality-of-life assessment: The Satisfaction Profile (SAT-P) International Journal of Mental Health. 1999;28:77–82. [Google Scholar]
- Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Archives of General Psychiatry. 2010;67:1265–1273. doi: 10.1001/archgenpsychiatry.2010.151. [DOI] [PubMed] [Google Scholar]
- *.McEvoy P, Burgess M, Nathan P. The relationship between interpersonal problems, negative cognitions, and outcomes from cognitive behavioral group therapy for depression. Journal of Affective Disorders. 2013;150:266–275. doi: 10.1016/j.jad.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Ames M, Cui L, Stang PE, Ustun TB, Von Korff M, Kessler RC. The impact of comorbidity of mental and physical conditions on role disability in the US adult household population. Archives of General Psychiatry. 2007;64:1180–1188. doi: 10.1001/archpsyc.64.10.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart L. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015;349:7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- Moons P, Budts W, De Geest S. Critique on the conceptualisation of quality of life: A review and evaluation of different conceptual approaches. International Journal of Nursing Studies. 2006;43:891–901. doi: 10.1016/j.ijnurstu.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:322–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- *.Nicolau J, Rivera R, Francés C, Chacártegui B, Masmiquel L. Treatment of depression in type 2 diabetic patients: Effects on depressive symptoms, quality of life and metabolic control. Diabetes Research and Clinical Practice. 2013;101:148–152. doi: 10.1016/j.diabres.2013.05.009. [DOI] [PubMed] [Google Scholar]
- *.Oei TP, McAlinden NM. Changes in quality of life following group CBT for anxiety and depression in a psychiatric outpatient clinic. Psychiatry Research. 2014;220:1012–1018. doi: 10.1016/j.psychres.2014.08.036. [DOI] [PubMed] [Google Scholar]
- Orjuela-Rojas JM, Martínez-Juárez IE, Ruiz-Chow A, Crail-Melendez D. Treatment of depression in patients with temporal lobe epilepsy: A pilot study of cognitive behavioral therapy vs. selective serotonin reuptake inhibitors. Epilepsy & Behavior. 2015;51:176–181. doi: 10.1016/j.yebeh.2015.07.033. [DOI] [PubMed] [Google Scholar]
- *.Paile-Hyvärinen M, Wahlbeck K, Eriksson JG. Quality of life and metabolic status in mildly depressed women with type 2 diabetes treated with paroxetine: A single-blind randomised placebo controlled trial. BMC Family Practice. 2003;4:1–6. doi: 10.1186/1471-2296-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Parker G, Blanch B, Paterson A, Hadzi-Pavlovic D, Sheppard E, Manicavasagar V, Perich T. The superiority of antidepressant medication to cognitive behavior therapy in melancholic depressed patients: A 12-week single-blind randomized study. Acta Psychiatry Scandanavica. 2013;128:271–281. doi: 10.1111/acps.12049. [DOI] [PubMed] [Google Scholar]
- *.Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, Thomson C. A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine. Health Technology Assessment. 2005;9:1–134. doi: 10.3310/hta9160. [DOI] [PubMed] [Google Scholar]
- *.Qiu J, Chen W, Gao X, Xu Y, Tong H, Yang M, Yang M. A randomized controlled trial of group cognitive behavioral therapy for Chinese breast cancer patients with major depression. Journal of Psychosomatic Obstetrics & Gynecology. 2013;34:60–67. doi: 10.3109/0167482X.2013.766791. [DOI] [PubMed] [Google Scholar]
- *.Rabkin JG, Wagner G, Rabkin R. Effects of sertraline on mood and immune status in patients with major depression and HIV illness: an open trial. Journal of Clinical Psychiatry. 1994;55:433–339. [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Revicki D, Turner R, Brown R, Martindale J. Reliability and validity of a health-related quality of life battery for evaluating outpatient antidepressant treatment. Quality of Life Research. 1992;1:257–266. doi: 10.1007/BF00435635. [DOI] [PubMed] [Google Scholar]
- *.Richards DA, Ekers D, McMillan D, Taylor RS, Byford S, Warren FC, O’Mahen H. Cost and outcome of behavioural activation versus cognitive behavioural therapy for depression (COBRA): a randomised, controlled, non-inferiority trial. The Lancet. 2016;388:871–880. doi: 10.1016/S0140-6736(16)31140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C, Ramsay C, Gurung T, Mowatt G, Pickard R, Sharma P. Practicalities of using a modified version of the Cochrane Collaboration risk of bias tool for randomised and non‐randomised study designs applied in a health technology assessment setting. Research Synthesis Methods. 2015;5:200–211. doi: 10.1002/jrsm.1102. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic Procedures for Social Research. Thousand Oaks, CA: Sage Publications; 1984. [Google Scholar]
- Rosenthal R. Meta-analytic Procedures for Social Research. Revised. Thousand Oaks, CA: Sage Publications; 1991. [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Keller MD. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- *.Saulsman L, Coall D, Nathan P. The association between depressive personality and treatment outcome for depression following a group cognitive-behavioral intervention. Journal of Clinical Psychology. 2006;62:1181–1196. doi: 10.1002/jclp.20278. [DOI] [PubMed] [Google Scholar]
- *.Saveanu R, Etkin A, Duchemin AM, Goldstein-Piekarski A, Gyurak A, Debattista C, Williams LM. The International Study to Predict Optimized Treatment in Depression (iSPOT-D): Outcomes from the acute phase of antidepressant treatment. Journal of Psychiatric Research. 2015;61:1–12. doi: 10.1016/j.jpsychires.2014.12.018. [DOI] [PubMed] [Google Scholar]
- *.Shelton RC, Haman KL, Rapaport MH, Kiev A, Smith WT, Hirschfeld RM, Dunner DL. A randomized, double-blind, active-control study of sertraline versus venlafaxine XR in major depressive disorder. Journal of Clinical Psychiatry. 2006;67:1674–1681. doi: 10.4088/jcp.v67n1102. [DOI] [PubMed] [Google Scholar]
- Sinha Y, Craig J, Sureshkumar P, Hayen A, Brien J. Risk of bias in randomized trials of pharmacological interventions in children and adults. Journal of Pediatrics. 2015;165:367–371. doi: 10.1016/j.jpeds.2014.03.058. [DOI] [PubMed] [Google Scholar]
- Smith K, Avis N, Assmann S. Distinguishing between quality of life and health status in quality of life research: A meta-analysis. Quality of Life Research. 1999;8:447–59. doi: 10.1023/a:1008928518577. [DOI] [PubMed] [Google Scholar]
- Spielmans, Berman, Linardatos, Rosenlicht, Perry, Tsai Adjunctive atypical antipsychotic treatment for major depressive disorder: A meta-analysis of depression, quality of life, and safety outcomes. PLOS Medicine. 2013 doi: 10.1371/journal.pmed.1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmans, Gerwig The efficacy of antidepressants on overall well-being and self-reported depression symptom severity in youth: a meta-analysis. Psychotherapy and Psychosomatics. 2014;83:158–164. doi: 10.1159/000356191. [DOI] [PubMed] [Google Scholar]
- Spitzer WO, Dobson AJ, Hall J, Chesterman E, Levi J, Shepherd R, Catchlove BR. Measuring the quality of life of cancer patients: a concise QL-index for use by physicians. Journal of Chronic Diseases. 1981;34:585–597. doi: 10.1016/0021-9681(81)90058-8. [DOI] [PubMed] [Google Scholar]
- *.Swan A, Watson H, Nathan P. Quality of life in depression: An important outcome measure in an outpatient cognitive-behavioural therapy group programme? Clinical Psychological Psychotherapy. 2009;16:485–496. doi: 10.1002/cpp.588. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Warden D, McKinney W, Downing M, Sakeim HA. Factors associated with health-related quality of life among outpatients with major depressive disorder: a STAR*D report. Journal of Clinical Psychiatry. 2006;67:185–195. doi: 10.4088/jcp.v67n0203. [DOI] [PubMed] [Google Scholar]
- *.Vilhauer J, Cortes J, Moali N, Chung S, Mirocha J, Ishak W. Improving quality of life for patients with major depressive disorder by increasing hope and positive expectations with future directed therapy (FDT) Innovative Clinical Neuroscience. 2013;10:12–22. [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Zhang DM, Tang XL. The standardization of Chinese version of Functional Assessment of Cancer Therapy – Breast (FACT-B) Chinese Mental Health Journal. 2003;17:298–300. [Google Scholar]
- Ware J, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- *.Watson H, Nathan P. Role of gender in depressive disorder outcome for individual and group cognitive-behavioral treatment. Journal of Clinical Psychology. 2008;64:1323–1337. doi: 10.1002/jclp.20524. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The World Health Organization Quality of Life Assessment (WHOQOL): Position paper from the World Health Organization. Social Science & Medicine. 1995;41:1403–1409. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- World Health Organization, WHO Press. The Global Burden of Disease: 2004 Update. 2005 Retrieved from: http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Rating Scale. Acta Psychiatry Scandanavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Zung W. A self-rating depression scale. Archives Of General Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]


