Abstract
Background
Although incidence rates are well documented for traumatic brain injury, lifetime prevalence in a demographically diverse sample is unknown. We examined the prevalence of self-reported traumatic brain injury (TBI) in a demographically diverse sample.
Methods
History of TBI was examined in 2881 African-Americans and Whites in the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study—a community-based, epidemiological investigation of urban-dwelling adults. Logistic regression analyses examined the odds of TBI as a function of sex, race, poverty status, age quintile and their interactions.
Results
A significant 3-way interaction was noted amongst race, poverty status and age (odds ratio (OR) = 1.57, 95% confidence interval (CI) 1.07–2.31, p = 0.021). Amongst Whites living in poverty, younger (30–36 years of age) individuals had greater odds of TBI than older (58–64 years of age) individuals, whereas older African-Americans living in poverty had greater odds of TBI. Additionally, a main effect of sex (OR = 2.36, 95% CI 1.85–3.03, p < 0.001) indicated that men had greater odds of TBI.
Conclusions
History of TBI is most prevalent in men, older African-Americans in poverty, and younger Whites in poverty. Preventive measures targeting relevant TBI risk factors in these populations are warranted.
Keywords: Traumatic brain injury, prevalence, demographics, sex, race: poverty, age
Introduction
Traumatic brain injury (TBI), defined as an injury from an external force that disrupts the normal function of the brain, is one of the leading causes of death and disability in the world, especially amongst the young [1]. TBI affects about 1.5 million people in the USA every year, with 275 000 hospitalizations and 52 000 deaths [2]. Additionally, substance use is considered one of the most critical risk factors in TBI [3,4]. TBI incidence rates vary greatly based on various demographic characteristics. The literature well documents the disproportionate risk of TBI as a function of age and sex, with more frequent occurrence amongst young and elderly men [5]. Additionally, those who are from a lower socioeconomic status (SES) are more likely to sustain a TBI than higher SES persons [6]. Although African-Americans and Whites have similar incidence, African-Americans are more likely to die from their TBI [5]. However, no studies have examined the interaction of these demographic characteristics with respect to TBI prevalence. While several studies have captured the overall prevalence rates of TBI and sex differences [7], to our knowledge, no studies have examined the lifetime prevalence of TBI as a function of other demographic factors or across demographically diverse subgroups. Accordingly, the present investigation examined the prevalence of TBI in a demographically diverse, urban-dwelling sample of adults.
Methods
Participants
Participants were 2881 African-Americans and Whites in Wave 1 of the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. Full details of the HANDLS study have already been documented [8]. Briefly, HANDLS is an ongoing longitudinal investigation conducted by the National Institute on Aging’s Intramural Research Program focused on race and SES-related health disparities amongst urban-dwelling adults. The study targeted work-aged adults, restricting the age range to 30–64. Participants, who self-identified as African-American and White, were recruited between the years 2004 and 2009 from 13 Baltimore, MD neighbourhoods that reflected socioeconomic and racial diversity [8]. For HANDLS, approximately equal numbers of participants were recruited from separate clusters of contiguous census tracts—neighbourhoods—containing sufficient numbers of residents to fill a factorial cross of sex, race, 5-year age groups, and poverty status (above or below 125% of the Federal poverty guidelines based on household size). Neighbourhoods were selected based on data from the 2000 census. Households were selected randomly for potential participation as were individuals within households. Participants were eligible if they self-identified as either Black/African-American or White/Caucasian. Recruiters visited 32 959 dwellings in which they found 14 799 potentially eligible individuals in 9904 households amongst whom 8150 individuals actually met initial screening criteria [8]. A total of 3720 participants met all inclusion criteria and were enrolled in the entire first wave of the HANDLS study; 2802 participants completed both phases of the protocol. Phase I consisted of screening, recruitment and a household interview. Following inclusion, Phase II was then carried out on mobile medical research vehicles parked in the participant’s neighbourhood, where a medical history, physical examination, cognitive testing, and other diagnostic procedures were performed by licensed physicians, nurses, medical staff and psychometrists. Participants were excluded from the HANDLS study if they were pregnant, were within 6 months of active treatment for cancer (chemotherapy, radiation or biological treatments), were unable to provide informed consent or could not provide a valid picture identification. Participants were excluded from the present analyses if they were missing self-reported history of head TBI or relevant demographic variables. Sample characteristics are displayed in Table I. Participants provided written informed consent according to the guidelines of the National Institute of Environmental Health Science’s Institutional Review Boards.
Table I.
Demographic characteristics.
| Demographic variable | With history of head injury (n = 302) |
Without history of head injury (n = 2499) |
|---|---|---|
| Age (M, SD) | 48.25 (8.52) | 48.03 (9.33) |
| Sex (% male) | 62 | 41 |
| Race (% Caucasian) | 57 | 58 |
| Poverty status (% below 125% poverty line) | 51 | 40 |
Biomedical assessment
Basic demographic information including age, sex, race and annual household income were self-reported during an initial household survey. Poverty status was dichotomized at baseline based on reported annual household income below or above 125% of the 2004 US Department of Health and Human Services poverty guidelines [9]. Quintile age groups (30–36, 37–43, 44–50, 51–57, 58–64) were created for the present analyses. Participants underwent a comprehensive medical evaluation that included a structured medical history. Head injury was assessed by asking the participant if they had ever had a head injury that had resulted in a loss of consciousness in a semistructure interview by physician or nurse practitioner. If the participants responded yes, they were then asked to report the number of head injuries they had sustained and what year they had sustained the injury. Wording was altered to the participant to maximize understanding. While self-reported loss of consciousness has relatively low reliability and validity, there remains no standardized measure of head injury, and therefore this remains the standard method of inquiry in the literature.
Statistical analyses
The prevalence of TBI history was examined by logistic regression analysis. Predictors for the analyses included sex, race, poverty status, age quintile, as well as all 2-, 3-, and 4-way interactions amongst the predictors. Backwards elimination was used to remove non-significant, higher-level interactions such that the non-significant 4-way interaction was eliminated first and the regression model recomputed, followed by the removal of non-significant 3-way interactions, and then non-significant 2-way interactions that were not subsumed under significant 3-way interactions. Analyses were then rerun until only significant interactions, and those included in higher-level significantly interactions were present in the final model.
Results
A final model with a 3-way interaction of race, poverty and age (odds ratio (OR) = 1.57, 95% confidence interval (CI) 1.07–2.31, p = 0.021) as well as the main effect of sex (OR = 2.36, 95% CI 1.85–3.03, p < 0.001) was retained (Table II).
Table II.
Results from logistic regression analyses of the retained model of the interactions of age, sex, race and poverty status on history of traumatic brain injury.
| Demographic variable | Odds ratio | 95% confidence interval | p-value |
|---|---|---|---|
| Age | 1.01 | 0.84–1.21 | 0.916 |
| Sex | 2.36 | 1.85–3.03 | <0.001 |
| Race | 1.07 | 0.44–2.61 | 0.880 |
| Poverty status | 3.59 | 1.39–9.30 | 0.008 |
| Race × poverty status | 0.23 | 0.06–0.83 | 0.025 |
| Race × age | 0.95 | 0.74–1.24 | 0.716 |
| Age × poverty status | 0.80 | 0.60–1.06 | 0.124 |
| Age × race × poverty status | 1.57 | 1.07–2.31 | 0.021 |
Bold is significant at p<.05.
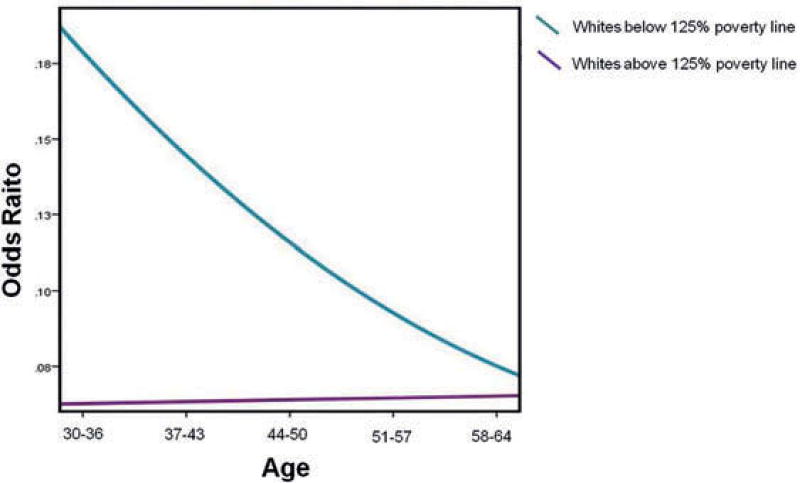
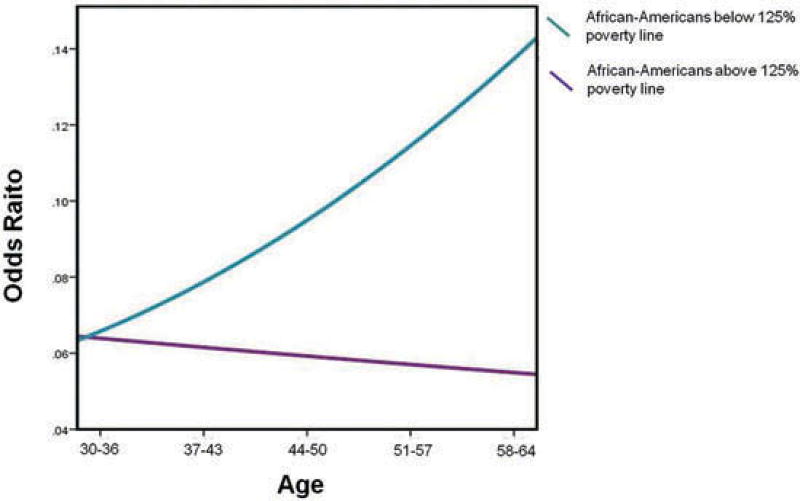
The significant interaction was probed by plotting simple slopes to clarify the nature of the interaction. The effects of age on the odds of prevalence of TBI were stratified by race and poverty status. Amongst Whites below the 125% poverty line, younger adults (age 30–36 years) had greater odds of having had a TBI (Figure 1). There was no significant main effect of age for Whites above the 125% poverty line. Amongst African-Americans below the 125% poverty line, older adults (age 58–64 years) had greater odds of having had a TBI (Figure 2). There was no significant main effect of age for African-Americans above the 125% poverty line.
Figure 1.
Interaction graph of odds of history of head injury by age for Whites above and below the 125% poverty line.
Figure 2.
Interaction graph of odds of history of head injury by age for African-Americans above and below the 125% poverty line.
Discussion
To our knowledge, this is the first investigation to examine interactive relations of age, sex, race and poverty status to TBI prevalence. Results indicated that young Whites and older African-Americans who are living in poverty are more likely to sustain a TBI in their lifetime. Additionally, results indicated that men are 2.4 times more likely to sustain a TBI in their lifetime than women. These results suggest that prevalence of TBI varies across the different demographic characteristics of age, sex, race and poverty status, thus creating a unique picture of lifetime prevalence for individuals.
There are several possible explanations for the present results. The literature shows that individuals with lower SES are more vulnerable to a spectrum of adverse mental and physical health outcomes [10,11]. This vulnerability extends to TBI; lower SES individuals have an elevated risk of sustaining a TBI independent of race [6]. This relative vulnerability may explain why our findings were noted predominantly amongst individuals living in poverty.
Amongst the young, the most common stated causes of TBI include substance use and vehicular accidents [5]. It is possible that, in the present sample, young Whites below the poverty line are more likely to engage in these behaviours than their young African-American and their more affluent counterparts, ultimately leading to higher TBI prevalence. In the general population, Whites are indeed more likely than African-Americans to be involved in motor vehicle crashes, with higher percentages of youth being involved in alcoholimpaired motor vehicle crashes [12]. Additionally, whereas young African-Americans are more likely to be non-drinkers, their White counterparts are more likely to be moderateheavy drinkers [13]. There is also a growing risk of morbidity associated with opioids drug use in young to middle age Whites [14], which may also result in a higher prevalence of TBI in this group. In the elderly, TBI has typically been associated with falls associated with frailty [5]. In the elderly, African-Americans are more likely than Whites to be dependent on their activities of daily living [15,16], perhaps as a result of increased frailty. Older African-Americans therefore may be more likely to fall and sustain a TBI than older Whites, which would also explain our findings. In the HANDLS study, it has been noted that low SES African-American men have higher levels of frailty beginning in middle age than White men (Personal Communication Felicia Griffin 2016).
The current findings regarding sex differences are consistent with the TBI literature. Sex differences in TBI have been well documented, with incidence rates for men being more than double that of women [17]. Similarly, a recent meta-analysis of 12 prevalence studies showed that rates are twice as high in men (16.7%) than in women (8.6%) [7]. Importantly, because no other study has investigated age, race and poverty status as they relate to sex difference in lifetime prevalence of TBI, our study further expands upon the literature by clarifying that sex differences are independent of other demographic variables.
Our results have several potential implications. Prevention of TBI can be targeted towards individual demographic groups based on their relative risk, especially amongst those who are living in poverty. Further research should focus on identifying the exact mechanisms of vulnerability for these demographic groups, e.g., evaluating potential influences of frailty in older African-Americans and risky behaviours associated with substance use in younger Whites. The present investigation study, in conjunction with future studies, may help suggest targeted prevention strategies for older African-Americans (such as limiting frailty-related head injuries), as well as interventions for younger White adults living in poverty (such as substance use education and screening). Additionally, it is possible that a history of TBI increases subsequent vulnerability of the brain. In that regard, prior research has shown that TBI may increase the risk of dementia [18]. Thus, the increased prevalence of TBI in African-Americans living in poverty may, in part, explain their increased dementia risk [19,20]. This issue warrants further research.
The current study had several limitations. First, the measure of TBI was based on self-report, which could mislabel an individual if inaccurate. There was also no information collected on the nature, location or severity of the injury. Additionally, the data obtained were cross-sectional, thus precluding any temporal inferences. Also nearly 25% of the initial sample did not complete both phases of the baseline assessment. Finally, our cohort was limited to individuals over the age of 30 living in Baltimore, which may limit generalizability.
In conclusion, the present study is the first to demonstrate interactive relations amongst demographic variables on the lifetime prevalence of TBI. Men as well as young Whites and older African-Americans below the 125% poverty line showed higher lifetime prevalence than their counterparts. Further investigation is warranted to understand the implications of these findings, and whether each group recovers differently from a TBI. Longitudinal data can help clarify what role TBI might play in progressive dementias in this demographically diverse sample.
Acknowledgments
The National Institute on Aging’s Intramural Research Program and National Institutes of Health Grant 1RO1AG034161 supported this research.
Footnotes
Declaration of Interest
The authors report no conflicts of interests.
References
- 1.Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mount Sinai J Med. 2009;76(2):97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- 2.Sosin DM, Sniezek JE, Thurman DJ. Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj. 1996;10(1):47–54. doi: 10.1080/026990596124719. [DOI] [PubMed] [Google Scholar]
- 3.Harrison DA, Griggs KA, Gomes M, Menon DK, Rowan KM. Prospective external validation of risk prediction models for acute traumatic brain injury in UK critical care units: The rain study. Intensive Care Med. 2012;38:S240. [Google Scholar]
- 4.Ilie G, Adlaf E, Mann R, Ialomiteanu A, Hamilton H, Rehm J, Asbridge M, Cusimano M. Associations between a history of traumatic brain injuries and current cigarette smoking, cannabis use, nonmedical opioid use, elevated psychological distress in a population sample of Canadian adults. J Neurotrauma. 2015;32(14):1130–4. doi: 10.1089/neu.2014.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevent. Traumatic brain injury and concussion: Data and statistics. 2013 Retrieved from https://www.cdc.gov/traumaticbraininjury/data/index.html.
- 6.Kraus J, McArthur D. Epidemiology of brain injury. In: Evans R, editor. Neurology and trauma. New York: Oxford University Press, Inc; 2006. pp. 3–18. [Google Scholar]
- 7.Frost RB, Farrer TJ, Primosch M, Hedges DW. Prevalence of traumatic brain injury in the general adult population: A meta-analysis. Neuroepidemiology. 2013;40:154–9. doi: 10.1159/000343275. [DOI] [PubMed] [Google Scholar]
- 8.Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, Zonderman AB. Healthy aging in neighborhoods of diversity across the life span (HANDLS): Overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethnicity Dis. 2010;20:267–75. [PMC free article] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services. Health and human services poverty guidelines. Federal Registry. 2004;69(30):7336–7338. [Google Scholar]
- 10.Nguyen HT, Evans M, Zonderman AB. Poverty and self-reported mental disorders: Findings from the Healthy Aging in Neighborhoods of Diversity Across the Life Span Study (HANDLS) Ann Epidemiol. 2007;17(9):736. [Google Scholar]
- 11.Smith J. Unraveling the SES: Health connection. Popul Dev Rev. 2004;30(Marmot):108–32. [Google Scholar]
- 12.National Highway Traffic Safety Administration. Traffic Safety Facts 2006. Washington (DC): National Center for Statistics and Analysis; 2009. [Google Scholar]
- 13.Sloan FA, Malone PS, Kertesz SG, Wang Y, Costanzo PR. Racial differences in the relationship between alcohol consumption in early adulthood and occupational attainment at midlife. Am J Public Health. 2009;99(12):2261–7. doi: 10.2105/AJPH.2007.127621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci. 2015;112:201518393. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wee CC, Huskey KW, Ngo LH, Fowler-Brown A, Leveille SG, Mittlemen MA, McCarthy EP. Obesity, race, and risk for death or functional decline among medicare beneficiaries: A cohort study. Ann Internal Med. 2011;154(10):645–55. doi: 10.7326/0003-4819-154-10-201105170-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. J Am Med Assoc. 2003;289(18):2387–92. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 17.Faul M, Xu L, Wald M, Coronado V. Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. 2010:891–904. [Google Scholar]
- 18.Wang H-L, Lin S-H, Sun P-S, Wu M-H, Hung K-W, Wang L-C, Huang C-Y, Lu K, Chen H-J, Tsai K-J. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry. 2012;83:1080–5. doi: 10.1136/jnnp-2012-302633. [DOI] [PubMed] [Google Scholar]
- 19.Guland B, Wilder D, Lantigua R, Mayeux R, Stern Y, Chen J, Killeffer E. Difference in rates of dementia between ethno-racial groups. In: Martin LG, Soldo BJ, editors. National Research Council (US) `Committee on Population. Washington (DC): National Acadmies Press; 1997. pp. 233–69. [Google Scholar]
- 20.Anttila T, Helkala E-L, Kivipelto M, Hallikainen M, Alhainen K, Heinonen H, Mannermaa A, Tuomilehto J, Soininen H, Nissinen A. Midlife income, occupation, APOE status, and dementia: A population-based study. Neurology. 2002;59(6):887–93. doi: 10.1212/wnl.59.6.887. [DOI] [PubMed] [Google Scholar]