Abstract
Background
Bacterial lung infection is a leading cause of death for those 65 years or older, often requiring intensive care unit admission and mechanical ventilation, which consumes considerable health care resources. While administration of antibiotics is the standard of care for bacterial pneumonia, its overuse has led to the emergence of multidrug resistant organisms. Therefore, alternative strategies to help minimize the effects of bacterial pneumonia in the elderly are necessary. As studies have shown that sphingosine has inherent bacterial killing properties, our goal was to assess whether it could act as a prophylactic treatment to protect aged mice from pulmonary infection by Pseudomonas aeruginosa.
Methods
Aged mice (51 weeks) and young (8 weeks) mice C57Bl/6 mice were used in this study. Pulmonary sphingosine levels were determined by histology. Sphingosine content of microparticles was quantified using a sphingosine kinase assay. Pneumonia was induced by intranasally treating mice with 106 CFU Pseudomonas aeruginosa. Microparticles were isolated from young mice while some were further incubated with sphingosine.
Results
We observed that sphingosine levels are reduced in the bronchial epithelial cells as well as the bronchoalveolar lavage microparticles isolated from aged mice, which correlates with a susceptibility to infection. We demonstrate that sphingosine or microparticle treatment can protect aged mice from pulmonary P. aeruginosa infection. Finally, we observed that enriching microparticles with sphingosine prior to treatment eliminated the bacterial load in P. aeruginosa-infected aged mice.
Conclusion
These data suggest that prophylactic treatment with sphingosine could reduce lung bacterial infections for the at-risk elderly population.
Keywords: sphingosine, microparticles, aging, lung infection, anti-microbial
Introduction
Elderly individuals suffering from pneumonia make up almost half of the intensive care unit admissions 1, 2. Pneumonia caused by Pseudomonas aeruginosa (PA), in particular, continues to be a major cause of morbidity and mortality in all hospitalized patients 3–5. Mechanically ventilated patients are particularly susceptible to PA, which is thought to be caused by the endotracheal tube bypassing the innate immune responses of the upper respiratory tract 6, 7. Given that antibiotic resistance to PA is increasingly problematic, it is essential that alternative mechanisms to treat PA infection are identified.
The enzyme acid sphingomyelinase (Asm) catalyzes the hydrolysis of sphingomyelin to ceramide. Peak activity of sphingomyelinases depends on pH and subtypes acid, neutral, and alkaline have been classified. In vitro, the optimum pH for acid sphingomyelinase activity is 4.5–5.0 8. Originally, this indicated that the enzyme was solely lysosomal. However, more recent data indicate that the lipid composition of the membrane alters the Km of the enzyme, thus permitting Asm activity at higher pH 9, 10. Further, recent studies demonstrate that acidic microenvironments exist not only in lysosomes but also in domains on the outer leaflet of plasma membrane, where acid sphingomyelinase localizes after certain stimuli 11. Ceramide is further metabolized to sphingosine by acid ceramidase (Ac). Sphingosine has been reported to have the ability to enhance killing of mycobacteria 12 as well as to kill a variety of Gram-positive and Gram-negative bacteria 13.
Microparticles (MPs) are products of cell membranes which are released in response to cellular stress 14–16. These MPs can contain a mixture of lipids, RNA and proteins. Specifically, it has been shown that microvesicles do contain ceramide 17, but nothing is currently reported concerning SPHcontent. Thus, it is unclear whether MP can curb lung bacterial infections and if the SPH content could be of influence.
The role of SPH and MPs in the response to PA pneumonia in the elderly has yet to be investigated. Given our previous data on cystic fibrosis and after burn 4, we hypothesize that SPH expression is reduced in the lungs of aged mice and this decreases susceptibility to bacterial infection. Restoration of the SPH in the elderly may therefore have a protective mechanism towards infection. Furthermore, utilization of MPs to deliver SPH may be a novel therapy for management of pulmonary infection in the elderly.
Material and Methods
Mice
Eight and 51-week-old male C57Bl/6 mice were purchased from Taconic Farms (Cambridge City, IN) and allowed to acclimate one week prior to conducting experiments. Mice were fed a standard pellet diet and water ad libitum. All mouse experiments were performed using protocols approved by the Institution Animal Care and Use Committee (Protocol number: 08-09-19-01) of the University of Cincinnati.
Bacterial preparation
PA 762 strain was grown for 14 hours on a tryptic soy agar plate (BD Biosciences, Franklin Lakes, NJ, USA) and transferred to an Erlenmeyer flask containing 40 ml of tryptic soy broth (BD Biosciences). Bacteria were incubated for 60 min at 37°C and 125 rpm to allow bacteria to be in the logarithmic phase of growth. Bacteria were centrifuged at 2800 rpm for 10 min at 21°C. The bacteria were then resuspended in pre-warmed 40 ml of PBS (Thermo Fischer Scientific; Waltham, MA, USA). An O.D. of 0.04 was obtained using a spectrophotometer and the concentration of the bacteria was determined based on a standard absorption curve. Mice were infected with 106 CFU/20 μl of bacteria. Prior to the inoculation, mice were anesthetized with 3% isoflurane in oxygen. Using a 31-gauge needle on a 1 ml syringe, each mouse was intranasally inoculated with 20 μl of PA.
SPH immunohistochemical analysis
Lungs were harvested from young and aged mice, fixed in 10% neutral buffered formalin (Thermo Fisher Scientific), and stained with Cy3-coupled anti-SPH antibodies (clone NHSPH; Alfresa Pharma Corporation, Osaka, Japan). ImageJ software was used to analyze the immunofluorescence via histology comparison of 10–12 sections in the apical third of tracheal epithelial cells.
MP isolation and sphingosine content analysis
BAL fluid was collected from aged and young mice and centrifuged at 450 × g for 10 minutes to remove cells. The supernatant was collected and centrifuged at 10,000 × g for 10 minutes to remove any residual cells. The supernatant was collected and centrifuged at 25,000 × g for 30 minutes to pellet the MPs. MP pellets were resuspended and Nanoparticle Tracking Analysis (Nanosight, Malvern Instruments Ltd, Worcestershire, UK) used to enumerate MPs. The SPH content of the MPs was quantified using a SPH kinase assay as previously described 6.
SPH and MP administration
Prior to treatment, SPH (D-erythro-sphingosine [d18:1]) was prepared by sonicating for 10 minutes. For SPH treatment, mice inhaled 1 mL of 125 μM SPH (Avanti Polar Lipids, Inc., Alabaster, AL, USA) in normal saline vehicle using a Vios Compressor and Nebulizer (Model 310B0003; PARI Respiratory Equipment, Midlothian, VA, USA) 30 min prior to inoculation with PA. This dose has been shown to be well tolerated and non-toxic to the lungs of mice 18. For SPH-enriched MP treatment, 125 μM SPH was added to MPs isolated from BAL fluid harvested from young mice and incubated for 3 hours at 37°C. MPs were isolated into a pellet by centrifuging at 25,000 × g for 30 minutes and resuspended in normal saline. Mice inhaled 1×109 of these SPH-enriched MPs via nebulizer 30 minutes prior to inoculation with PA.
Determining bacterial load
BAL fluid was harvested 4 hours after bacterial inoculation. Normal saline was instilled through the trachea into the lungs with a 20-gauge catheter, and approximately 1.7 ml of fluid was removed. Samples were serially diluted in sterile saline and cultured on tryptic soy agar plates. After an overnight incubation at 37° C colony enumeration was performed.
Statistical analysis
Using Prism 6.0 (GraphPad Software, La Jolla, CA, USA), statistical comparisons were performed with the Kaplan Meier Log-Rank Test (survival), Student t test (2 groups), or 1-way ANOVA with the Tukey post hoc comparison (>2 groups). Data are reported as means +/− SEM. A p-value of ≤ 0.05 was used for statistical significance.
Results
Aged mice demonstrate increased mortality to lung bacterial infection
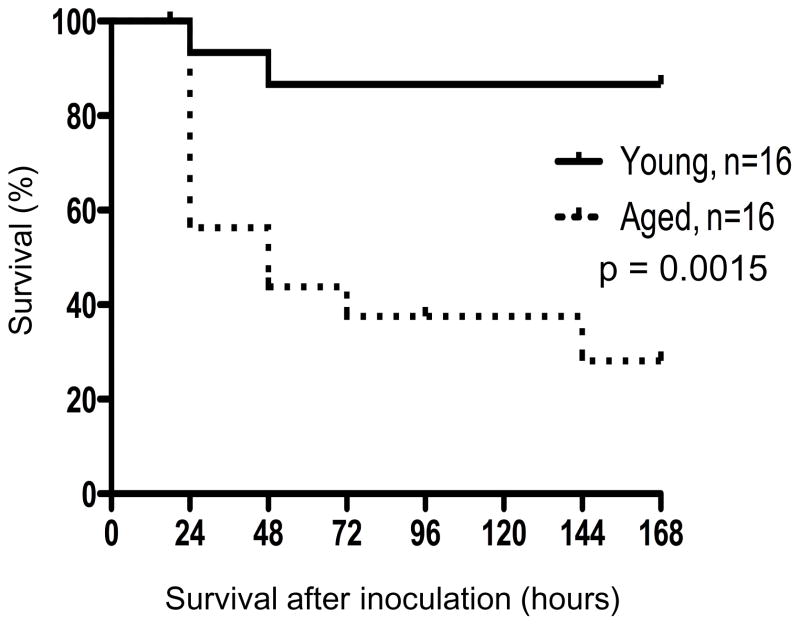
While bacterial pneumonia has low morbidity and mortality in the young, aged individuals tend to be more susceptible with a 10–20% mortality rate 19. To determine the susceptibility of aged mice to pulmonary infection with PA, young and aged (52-week-old) mice were inoculated with 106 CFU of PA and survival was monitored over 7 days (Figure 1). In the first 24 hours, approximately 40% of the aged mice died, which was significantly more than the 6% of the young mice that died. In aged mice, there was 70% mortality after 7 days, whereas only 10% of the young mice succumbed to the infection (p<0.05).
Figure 1. Aged mice demonstrate increased mortality to lung bacterial infection.
Aged and young mice were inoculated with 106 PA 30 minutes after treatment with SPH. Survival was monitored for 7 days after inoculation. Experiments were conducted twice to ensure reproducibility, each with a sample size of 8 young and 8 aged mice. *, p<0.05 as determined by Log-Rank test.
Decreased SPH in bronchial epithelium and BAL MPs in aged mice
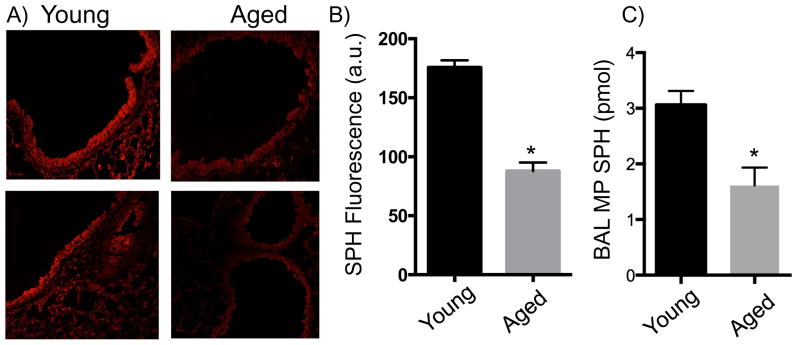
Given that reduction in tracheal epithelial SPH increases susceptibility to pulmonary PA in a cystic fibrosis and burn injury model 6, 20, we hypothesized that this molecule influences the susceptibility of aged mice to infection 21. The SPH expression on the bronchial epithelial cells of young versus aged mice was determined by immunofluorescence (Figures 2a and b). In the absence of injury or infection, the surfaces of bronchial epithelial cells from aged mice express significantly less SPH compared to young mice (p<0.05). As BAL MPs could potentially be a source of SPH, extracellular oriented SPH content was determined as previously described 6. We observed that there is an approximate 50% decrease in extracellular oriented SPH levels on MPs isolated from the BAL fluid from aged mice compared to young mice (Figure 2c).
Figure 2. Decreased SPH in bronchial epithelium and BAL MPs in aged mice.
Fluorescent staining with anti-SPH antibodies of tracheal epithelial cells from A) young and B) aged mice. The top panels represent maximally observed SPH while bottom panels represent minimally observed SPH. C) Quantification of SPH in tracheal epithelial cells based on fluorescence. (n= 4–5 mice). D) Quantification of SPH in BAL MPs based on SPH kinase assay. (n=9–10 mice). Experiments were conducted twice to ensure reproducibility. *, p<0.05 as compared to young mice by Student’s t-test.
Treatment with SPH protects susceptible aged mice from bacterial lung infection
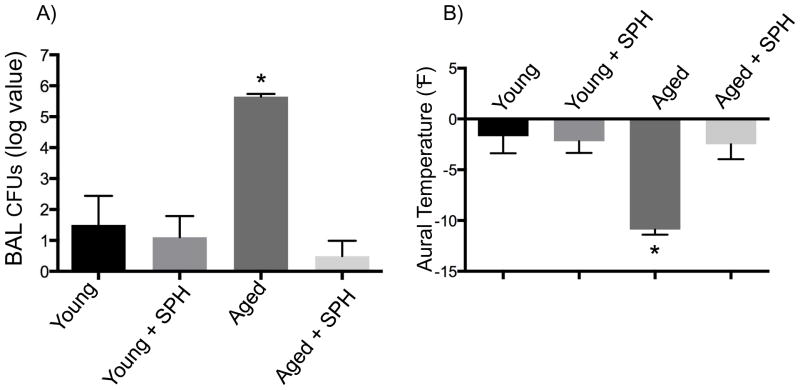
To determine whether SPH treatment in aged mice protects against pulmonary PA infection, mice were pre-treated with inhaled SPH 30 minutes prior to bacterial inoculation. As demonstrated in Figure 3a, young mice were able to clear most of the PA without pre-treatment. Administration of SPH did not provide additional reduction in the already low level of bacteria. Aged animals, without treatment, retained approximately 1 million bacterial CFU. Exogenous administration of SPH led to an approximate 5-log reduction in bacterial burden in the lungs of aged mice and allowed for clearance similar to that of a young mouse.
Figure 3. Treatment with SPH protects susceptible aged mice from bacterial lung infection.
Aged and young mice were inoculated with 106 PA 30 minutes after treatment with SPH. A) 4 hours after inoculation, BAL fluid was collected and CFU enumerated. B) Change in aural temperature of mice 4 hours after inoculation with PA (n=5 mice). Experiments were conducted twice to ensure reproducibility and data representative of one is shown. *, p>0.05 compared to young mice, young mice treated with SPH, and aged mice treated with SPH by ANOVA.
To determine whether this bacterial burden was clinically significant in the aged mice, the aural temperature was therefore recorded 4 hours after inoculation. We observed that there were minimal temperature fluctuations in the young mice in the presence or absence of SPH in response to pulmonary PA infection (Figure 3b). In contrast, PA inoculation caused a significant hypothermic reaction in the aged mice. This reaction was mitigated when aged mice were pre-treated with SPH.
Treatment with MP and SPH-enriched MP reduce lung bacterial loads
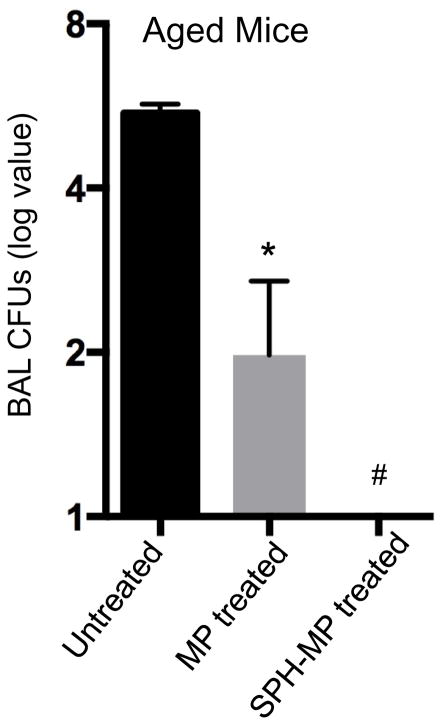
Our previous data demonstrated a decrease in BAL MP SPH levels (Figure 2c). We hypothesized that decreased SPH in MPs contribute to infection susceptibility. Upon testing, we observed that aged mice without treatment have an approximately 6 log of bacterial burden 4 hours after infection (Figure 4). With administration of MPs alone, the bacterial burden after only 4 hours, is reduced by approximately 4 logs. Notably, with administration of SPH-enriched MPs, however, we were unable to detect any bacteria from the BAL fluid within the same 4 hour time period. After 4 hours, young mice demonstrated 10 CFU of bacterial load and MP treatment did not significantly change this already low level (data not shown).
Figure 4. Treatment with MP and SPH-enriched MP reduce lung bacterial loads.
Aged mice were treated with MPs or SPH-incubated MPs and inoculated with 106 PA 30 minutes later. BAL fluid was collected after 4 hours and CFUs enumerated. (n=4 mice). Experiments were conducted twice to ensure reproducibility and data representative of one is shown. *p<0.05 compared to untreated mice and #p<0.05 compared to MP treated mice by ANOVA.
Discussion
These data demonstrate that aged mice are significantly more susceptible to pulmonary infection with PA (Figure 1). We observed that aged mice had reduced SPH expressed both on the bronchial epithelial surface and on MPs located in BAL fluid (Figure 2). When aged mice are pre-treated with SPH, their airway bacterial burden is significantly decreased and this is associated with overall clinical improvement (Figure 3). Finally, treatment of aged mice with BAL MPs isolated from young mice also robustly decreased the bacterial burden in aged mice, while SPH augmentation on these BAL MPs eliminated the bacteria (Figure 4).
The exact mechanism of SPH-mediated bacterial killing is currently unknown. However, it is clear that SPH has the ability to directly kill a number of bacteria and fungi, to include E. coli, F. nucleatum, S. aureus, S. sanguinis, C. bovis, C. striatum, P. aeruginosa, S. pneumonia,, M. catarrhalis, B. burgdorferi, H. influenza, mycobacteria and fungi 12, 13, 22. Reconstitution experiments applying sphingosine and sphingosine containing microparticles to the lung strongly suggest that sphingosine mediates the anti-bacterial effects. We have shown that SPH expression is also significantly decreased in the lungs after burn injury 20, but we also observed a corresponding increase in expression of its precursor lipid, ceramide. When ceramide accumulates in ceramide-rich domains, this allows for clustering of other receptors and amplification of their downstream signaling to promote various cellular activities, including initiation of apoptotic pathways. Since we have shown that SPH can directly kill bacteria, perhaps it is both the absence of SPH along with the increased ceramide that allows for the inability for aged mice to ineffectively clear bacteria.
Altogether, our data provide a proof of principle that lung epithelial cell sphingosine levels are diminished in aged mice and restoration can ameliorate lung infection. However, studies have not addressed the protective duration of inhaled sphingosine. Further, we did not demonstrate that it could be given as a supplement to treat severe pulmonary infections in the elderly. Ongoing and future studies will examine the duration of the prophylactic effect and whether SPH can act as an alternative therapeutic regimen to minimize the need for antibiotics.
Conclusion
These data suggest a novel mechanism for increased susceptibility to bacterial pulmonary infection in the elderly and offers a potential strategy for protecting these individuals from pneumonia. Exploiting the methods of measuring SPH levels in BAL fluid in the current study will eventually allow us to translate these results into humans. Future studies are needed, however, to determine the exact mechanism of decreased SPH in the lungs of aged mice. In addition, it is unclear whether SPH contributes to direct bacterial killing via alteration of the lipid membrane either through direct changes in membrane fluidity or through pore formation or whether there is a direct drug toxicity to the bacteria. Regardless of the mechanism, there is a potential role for sphingolipids in the response to infection. Further exploitation of this pathway is therefore imperative to improve outcomes for elderly individuals in response to pulmonary infection.
Acknowledgments
Funding Source: The project described was supported by Award Number R01 GM100913 (CCC) and T32 GM08478 (TCR and AMP) from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
Presentation: Portions of this work were presented at the 12th Annual Academic Surgical Congress in Las Vegas, NV, on February 7–9, 2017.
Author contributions: TCR, AMP and APS: Design of the work, Data collection, Data analysis and interpretation, Drafting the article, Critical revision of the article, and Final approval of the version to be published. EG, VN and CCC: Conception of the work, Data analysis and interpretation, Drafting the article, Critical revision of the article, and Final approval of the version to be published.
Conflicts of interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feldman C. Pneumonia in the elderly. Clin Chest Med. 1999;20(3):563–73. doi: 10.1016/s0272-5231(05)70236-7. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan V, Angus DC, Griffin MF, et al. Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165(6):766–72. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 3.Cook DJ, Kollef MH. Risk factors for ICU-acquired pneumonia. JAMA. 1998;279(20):1605–6. doi: 10.1001/jama.279.20.1605. [DOI] [PubMed] [Google Scholar]
- 4.National Nosocomial Infections Surveillance (NNIS) System Report, Data Summary from January 1992–June 2001, issued August 2001. Am J Infect Control. 2001;29(6):404–21. doi: 10.1067/mic.2001.119952. [DOI] [PubMed] [Google Scholar]
- 5.Morrison AJ, Jr, Wenzel RP. Epidemiology of infections due to Pseudomonas aeruginosa. Rev Infect Dis. 1984;6(Suppl 3):S627–42. doi: 10.1093/clinids/6.supplement_3.s627. [DOI] [PubMed] [Google Scholar]
- 6.Pewzner-Jung Y, Tavakoli Tabazavareh S, Grassme H, et al. Sphingoid long chain bases prevent lung infection by Pseudomonas aeruginosa. EMBO Mol Med. 2014;6(9):1205–14. doi: 10.15252/emmm.201404075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies. A consensus statement, American Thoracic Society, November 1995. Am J Respir Crit Care Med. 1996;153(5):1711–25. doi: 10.1164/ajrccm.153.5.8630626. [DOI] [PubMed] [Google Scholar]
- 8.Teichgraber V, Ulrich M, Endlich N, et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008;14(4):382–91. [Google Scholar]
- 9.Schissel SL, Jiang X, Tweedie-Hardman J, et al. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J Biol Chem. 1998;273(5):2738–46. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- 10.Schissel SL, Keesler GA, Schuchman EH, et al. The cellular trafficking and zinc dependence of secretory and lysosomal sphingomyelinase, two products of the acid sphingomyelinase gene. J Biol Chem. 1998;273(29):18250–9. doi: 10.1074/jbc.273.29.18250. [DOI] [PubMed] [Google Scholar]
- 11.Xu M, Xia M, Li XX, et al. Requirement of translocated lysosomal V1 H(+)-ATPase for activation of membrane acid sphingomyelinase and raft clustering in coronary endothelial cells. Mol Biol Cell. 2012;23(8):1546–57. doi: 10.1091/mbc.E11-09-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutierrez MG, Gonzalez AP, Anes E, et al. Role of lipids in killing mycobacteria by macrophages: evidence for NF-kappaB-dependent and -independent killing induced by different lipids. Cell Microbiol. 2009;11(3):406–20. doi: 10.1111/j.1462-5822.2008.01263.x. [DOI] [PubMed] [Google Scholar]
- 13.Fischer CL, Drake DR, Dawson DV, et al. Antibacterial activity of sphingoid bases and fatty acids against Gram-positive and Gram-negative bacteria. Antimicrob Agents Chemother. 2012;56(3):1157–61. doi: 10.1128/AAC.05151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 15.Morel O, Jesel L, Freyssinet JM, et al. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31(1):15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 16.Kuethe JW, Mintz-Cole R, Johnson BL, 3rd, et al. Assessing the immune status of critically ill trauma patients by flow cytometry. Nurs Res. 2014;63(6):426–34. doi: 10.1097/NNR.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulbins A, Grassme H, Hoehn R, et al. Regulation of Neuronal Stem Cell Proliferation in the Hippocampus by Endothelial Ceramide. Cell Physiol Biochem. 2016;39(2):790–801. doi: 10.1159/000447789. [DOI] [PubMed] [Google Scholar]
- 18.Martin GE, Boudreau RM, Couch C, et al. Sphingosine’s role in epithelial host defense: A natural antimicrobial and novel therapeutic. Biochimie. 2017 doi: 10.1016/j.biochi.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Meehan TP, Chua-Reyes JM, Tate J, et al. Process of care performance, patient characteristics, and outcomes in elderly patients hospitalized with community-acquired or nursing home-acquired pneumonia. Chest. 2000;117(5):1378–85. doi: 10.1378/chest.117.5.1378. [DOI] [PubMed] [Google Scholar]
- 20.Rice TC, Seitz AP, Edwards MJ, et al. Frontline Science: Sphingosine rescues burn-injured mice from pulmonary Pseudomonas aeruginosa infection. J Leukoc Biol. 2016;100(6):1233–1237. doi: 10.1189/jlb.3HI0416-197R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker KA, Riethmuller J, Luth A, et al. Acid sphingomyelinase inhibitors normalize pulmonary ceramide and inflammation in cystic fibrosis. Am J Respir Cell Mol Biol. 2010;42(6):716–24. doi: 10.1165/rcmb.2009-0174OC. [DOI] [PubMed] [Google Scholar]
- 22.Henry B, Ziobro R, Becker KA, et al. Acid sphingomyelinase. Handb Exp Pharmacol. 2013;(215):77–88. doi: 10.1007/978-3-7091-1368-4_4. [DOI] [PubMed] [Google Scholar]