Abstract
Wide interest in new hemostatic approaches has stemmed from unmet needs in the hospital and on the battlefield. Many current commercial hemostatic agents fail to fulfill the design requirements of safety, efficacy, cost, and storage. Academic focus has led to the improvement of existing strategies as well as new developments. This review will identify and discuss the three major classes of hemostatic approaches: biologically derived materials, synthetically derived materials, and intravenously administered hemostatic agents. The general class is first discussed, then specific approaches discussed in detail, including the hemostatic mechanisms and the advancement of the method. As hemostatic strategies evolve and synthetic-biologic interactions are more fully understood, current clinical methodologies will be replaced.
Keywords: hemostatic, hemorrhage, hemostasis, trauma, surgery
INTRODUCTION
Uncontrollable bleeding poses significant fatality risks and costs in battlefield, emergency, and hospital settings. In the military, 50% of deaths are the result of exsanguination. Eighty percent of these deaths result from noncompressible injuries making it the leading cause of death in military settings.1,2 It is imperative that hemorrhage is controlled immediately to decrease fatality rates as bleed outs can occur within 5–10 min.3 Improvements to treatment of penetrating and truncal injury is believed to have the greatest potential impact on decreasing killed in action and died of wound rates.4 In emergency civilian settings, hemorrhage accounts for one third of prehospital deaths, a number that has not decreased in the past 30 years.5 In the operating room, surgeries including cardiovascular, hepatic, orthopedic, and spinal procedures have a high incidence of severe blood loss requiring some sort of hemostatic intervention.6
Hemostasis is the body’s multifaceted response to hemorrhage. In primary hemostasis, an initial platelet plug is formed. When vascular tissue is damaged, platelets activate and release chemical signals that induce aggregation and cause adherence to the subendothelial matrix. Activated surface receptors interact and protein bridges are created between the subendothelium and other activated platelets to form a robust initial hemostatic plug.7 Secondary hemostasis, or the coagulation cascade, is divided into two enzymatic pathways, the intrinsic (contact activation) and extrinsic (tissue factor). These pathways converge and result in the formation of a fibrin clot that strengthens the primary platelet plug (Fig. 1). The extrinsic pathway begins when trauma to vasculature exposes tissue factor to blood, activating coagulation factor VII (FVII). The resulting formation of the tissue factor-active FVII complex initiates and amplifies the coagulation cascade. The intrinsic pathway activates factor XII upon surface damage resulting in downstream proteolytic activation of other coagulation factors. The two pathways converge into the common pathway upon the activation of factor X (FX). FX cleaves prothrombin into thrombin, which in turn activates fibrinogen into fibrin, reinforcing the platelet plug.8,9 In cases of severe or uncontrolled blood loss, the body’s natural clotting process is not able to facilitate hemostasis on its own.
FIGURE 1.
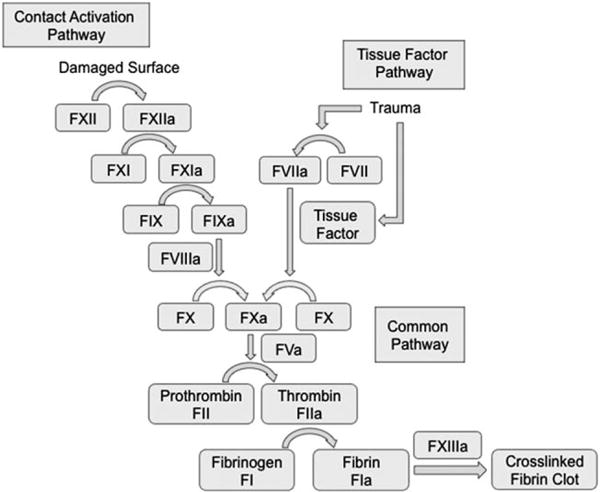
Schematic of the coagulation cascade. Activation of the contact activation and/or the tissue factor pathway causes successive proteolytic steps that result in the formation of a crosslinked fibrin clot. Each coagulation factor is represented with a Roman numeral and active form with the letter a.
The field of hemostatic materials research has lagged behind other medical advances making few major clinical developments. Compression with gauze is still prevalent practice for most injuries and the most common surgical techniques have been in use with minimal modifications for the last decade. The lack of major progress does not reflect a diminishing need. In the most recent comprehensive reviews of topical hemostatic agents,10 wound sealants,11 surgical hemostats,12 and hemostatic agents for military and first response applications,13 the need for future research and development on improved hemostatic agents and devices is emphasized. Their evaluations of hemostatic agents currently available for commercial use in surgery and traumatic hemorrhage control are categorized in Table I. An ideal hemostat is safe, effective, easily stored and used, cheap, and capable of regulatory approval.12,14,15 Many of the materials available at the commercial level fail to meet all of these qualifications.
TABLE I.
Commercially Available Hemostatic Agents
| Categories | Types | Pros | Cons |
|---|---|---|---|
| Physical and Absorbable10,12 | Bone wax, ostene, gelatin foams, sponges, and powders, oxidized cellulose, microfibrillar collagen, bovine and porcine collagen | Tamponades bone surface bleeding, absorbable, and controls small vessel low pressure bleeding | May embolize, prevent bone fusion, reduce structural stability, possible interference with healing process |
| Biologically Active10–12,16,25 | Pooled and recombinant thrombin, thrombin and gelatin, fibrin sealants, platelet gels, albumin and glutaraldehyde | Easily applied, rapid response, effective against mild to moderate bleeding, effective in heparinized patients, and broad applications | Immunological response, viral infection, expensive cost per application, short shelf lives, and adverse distal thrombotic events |
| Synthetic sealants10–12 | Cyanoacrylates, polyethylene glycol hydrogel | Waterproof barrier, replacement for sutures, full strength within minutes, arterial bleeding | Limited topical usages, dangerous if unreacted, and difficult to apply to irregular wounds |
| Hemostatic Dressings10,11,13 | Dry fibrin, chitin, chitosan, alginate, mineral zeolite, kaolin, and smectite | Military and emergency response usage, can stop heavy arterial bleeding, long shelf-life, enhances normal compression treatment, and typically inexpensive | High pressure wounds can expel powders, zeolite causes exothermic reaction, success related to responder training, inconsistent results from animal studies |
While common practice has not changed drastically, there has been significant investigation into new hemostatic approaches at the academic level. These strategies encompass the use of coagulation proteins, in situ forming gels, synthetic polymers, and artificial platelets, among others. This review will focus on material platforms, functionalization strategies, and their targeted hemostatic mechanisms. We have identified three major classes for hemostatic approaches: biologically derived materials, synthetically derived materials, and intravenously administered hemostatic agents. The general class is first discussed, then specific approaches in detail, including the hemostatic mechanisms and the advancement of the method. The categorization of each approach in based on the primary platform; however, many recently developed agents use multiple materials or mechanisms and may tie into several different classes.
BIOLOGICALLY DERIVED MATERIALS
The prevalence of biologically derived hemostatic materials can be attributed to their clear mechanisms of action and efficiency. Naturally occurring proteins and polysaccharides that are commonly used may either have direct roles in hemostasis, such as coagulation factors, or other hemostatic attributes. The latter includes albumin, collagen, gelatin, polypeptides, keratin, chitosan, cellulose, and dextran. Chitosan, cellulose, and dextran are crustacean, plant, and bacteria derived, respectively (Fig. 2). All of these compounds are isolated and manipulated to create effective and easily administered hemostatic agents.
FIGURE 2.
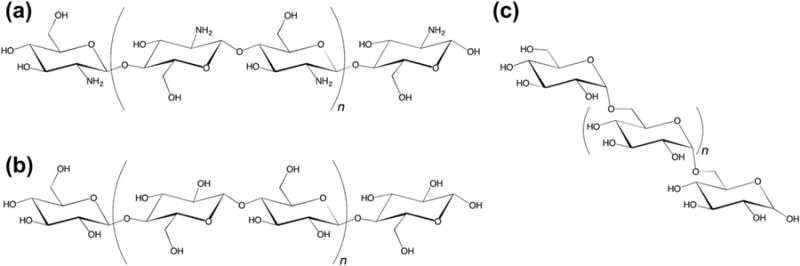
Structures of naturally derived polysaccharides: (a) chitosan, (b) cellulose, and (c) dextran.
Coagulation proteins
Fibrinogen, fibrin, and thrombin have been widely investigated for hemostatic use given their direct role in secondary hemostasis and clot formation. In the final common cascade, thrombin reacts with fibrinogen to create fibrin monomers, which polymerize to form a clot structure. The derivation and concentration techniques for these compounds have changed over time in order to improve efficiency and reduce risk of contamination with pathogens.16 Once isolated, fibrinogen and thrombin can be contained in pressure dressings for arterial hemorrhage control17 and turned into absorbable dry fibrin adhesive bandages.16,18,19 Fibrinogen has also been electrospun into 3D nanofiber structures20 and photochemically crosslinked using ruthenium and persulphate in vivo to produce a potentially noncytotoxic wound sealant.21,22
Recently, novel delivery systems of these coagulation proteins have been investigated. Smeets et al. developed a sponge made of collagen that contains thrombin-loaded biodegradable microspheres for local thrombin release and postsurgical hemorrhage control.23 Shukla et al. coated active coagulation proteins through layer by layer assembly onto a gelatin sponge with water absorbent properties.24 The successes of these types of agents are largely due to the combination of multiple direct hemostatic mechanisms. None of these approaches have fully addressed the issues of short shelf lives, expensive costs per application, potential risk of viral contamination, adverse thrombotic events at distal sites, and significant manufacturing hurdles.16,25
Albumin
Albumin is a water-soluble protein derived from blood plasma, often bovine. It has grown in popularity as a commercially available hemostatic agent in the form of a surgical glue. BioGlue® is commonly used in cardiac surgery and forms a strong tissue sealant through chemical crosslinking of bovine serum albumin and glutaraldehyde.26 While initially effective and relatively affordable, there have been major concerns about glutaraldehyde release causing in vitro and in vivo toxicity.27 Alternatively, Xie et al. demonstrated the use of concentrated albumin as a surgical glue to enhance the effects of argon beam coagulation in a liver injury model.28 The method improved time to hemostasis and overall surgical outcome, but the method required access to an argon beam and does not mitigate the risk of mammalian disease transmission.
Collagen and gelatin
Collagen is a compound present in the extracellular matrix of animal cells. Adherence of platelets to collagen fibrils or insoluble collagen microparticles in a vascular tissue wound is an early step in primary hemostasis.29,30 Several proteins such as platelet glycoproteins and von Willebrand factor molecules have been shown to interact and bind with collagen types I and III.31,32 Recombinant human collagen type III induces platelet aggregation, and in turn, hemostatic activity.33 In lieu of its high cost, collagen’s efficacy has led to its incorporation into commercially available hemostatic sponges, pads, bandages, and foams.10
Gelatin, derived from denatured collage, has been used in a variety of hemostatic materials. Making use of the high porosity and surface roughness of chemically crosslinked gelatin, Hajosch et al. developed a highly blood absorbent sponge.34 The hemostatic activity in an in vitro and in vivo model is attributed to the interaction and adhesion of platelets and coagulation proteins with the rough surface of the sponge. In another study, gelatin, acting in place of a structural protein, was crosslinked in situ with calcium-independent microbial transglutaminase enzyme to form surgical glue mimetic of a natural clot demonstrated in rat liver and femoral artery injuries.35
Ohya et al. investigated a thermoresponsive system that employed an aqueous mixture of poly(N-isopropylacrylamide) (PNIPAM)-grafted hyaluronan and PNIPAM-grafted gelatin macromolecules.36–38 Hyaluronan is a naturally derived cellular component with known biocompatibility and PNIPAM is a synthetic polymer that undergoes sol-to-gel phase transition at physiological temperature. The resulting mixture remained water-soluble at room temperature but gelled in situ at physiological temperature. Tested in needle-prick injury models, the material showed weak adherence and site coverage within a minute and reached complete hemostasis within 1–2 h. While the temperature dependent sol-gel transition could be useful, the extended time to hemostasis on a non-severe injury model brings into question the utility of this specific approach.
Gelatin has also been investigated as an alternative to albumin or fibrin in a crosslinking surgical glue. Initial experiments yielded an increase in bonding strength and resistance to water pressure compared with fibrin glue, and a decrease in cytotoxicity compared with commercial albumin glue.39 Gelatin based materials, although effective, are high in cost, swell excessively, and are often used with coagulation factors such as thrombin to improve hemostatic characteristics.10
Polypeptide
The use of polypeptide hemostatics is attractive because of inherent biocompatibility and the possibility of self-assembly. A poly(L-glutamic acid)-gelatin glue was developed by Otani et al., but it required preheating and a crosslinking step with carbodiimide that may interact with surrounding tissue.40,41 Ruan et al. have used self-assembling complementary amphiphilic peptides that require sonication before application.42 These pre-application steps and the high cost of these approaches limit their potential for future use in a clinical environment.
Self-assembled polypeptides that undergo sol-gel transitions in ionic environments have also been investigated.43,44 These strategies require no pre-application step and are able to immediately induce hemostasis in a variety of rat injury models. Although polypeptide based hemostatics are believed to be biodegradable, no long-term biocompatibility or degradation studies have been investigated for any of the discussed approaches. Additionally, cost and manufacturing issues associated with current commercial hemostatic agents are not addressed.
Keratin
Keratins are proteins that make up protective structures in vertebrates and are generally extracted from hair, but are also present in epidermal and skeletal tissues.45 Van Dyke’s group has investigated the hemostatic potential of keratin-based materials, using human hair derived hydrogels that cause significant red blood cell aggregation, adhere well to tissue, and have similar survivability rates in a lethal liver transection rabbit model as compared with commercial hemostatic agents Hemcon® and Quikclot®.46 Keratin’s ability to foster cell adhesion as a ligand and hemostatic ability in a lethal liver transection swine model were also demonstrated.47,48 Further investigation into keratin hydrogel hemostatic mechanisms revealed direct acceleration of the coagulation cascade, but a specific activation mechanism has not been specified leaving safety concerns.49 Keratin hydrogels are expected to be biocompatible and biodegradable through macrophage phagocytosis.50,51 However, neither has been reported for hemostatic application, and degradation timescales are not well characterized.
Chitosan
Chitosan, a polysaccharide, is the deacetylated form of chitin and is derived from the exoskeletons of crustaceans. Chitosan forms a coagulum in contact with whole blood arising from its polycationic structure and nonspecific binding to cell membranes, leading to wide hemostatic material interest.52 Furthermore, chitosan has been shown to be nontoxic and enzymatically degradable.53 Benesch et al. found that acetylated chitosan was a strong coagulation activator but did not bind fibrinogen or other plasma proteins as deacetylated chitosan did.54 Yang et al. investigated the differences in chitosan molecular weight and degree of deacetylation in addition to comparing the hemostatic mechanism of solid-state chitosan, chitosan in an acetic acid physiological saline solution, and carboxymethyl chitosan in physiological saline solution.55 They concluded that solid-state chitosan aided in hemostasis through platelet adsorption, while chitosan in solution was able to cause erythrocyte aggregation. Additionally, they found that molecular weight and deacetylation had significant effects on hemostatic activity.
Nonmodified chitosan in solution, filament composites, coatings, powder, films, and hydrogels of varying molecular weights, and degrees of deacetylation for hemostatic application have been comprehensively reviewed by Whang et al.56 These material approaches have led to widespread military use in the form of HemCon® and ChitoFlex® dressings.56–58 While they have shown significant advantages over standard gauze, there have been reported limitations with major injury, and bandage variability.59,60 Despite these shortcomings, chitosan is still a very promising candidate for hemostatic material development leading into investigation into its functionalization (Fig. 3) and use in composite materials.
FIGURE 3.
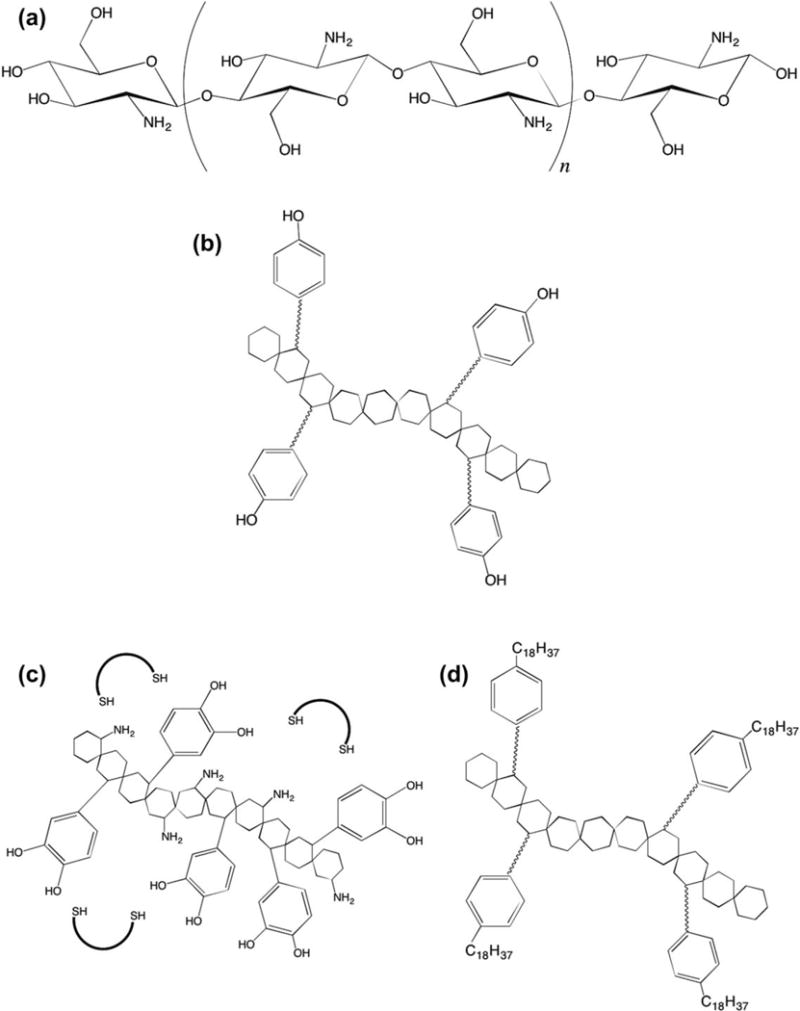
Structure of (a) chitosan, (b) PEG-tyramine functionalized chitosan,68 (c) catechol-conjugated chitosan,67 and (d) hydrophobically modified chitosan.66
Ong et al. took steps to improve chitosan-based hemostatics by including polyphosphate and silver nanoparticles with aims to improve hemostatic and antimicrobial properties. Polyphosphate enhances fibrin clot structure, and acts as a procoagulant.61–63 This approach however resulted in in vitro fibroblast toxicity,64 and imparted substantial costs. Kumar et al. formed microporous chitosan hydrogel zinc oxide composite to improve platelet aggregation and blood absorbance, and to introduce antibacterial elements.65 The material was only tested in wound healing models and increased zinc oxide content led to a decrease in cell viability over 24 h in vitro.
Chitosan modifications have had commendable successes. Dowling et al. hydrophobically modified chitosan to create a “reversible” hemostatic agent with a proposed mechanism of hydrophobic anchoring in red blood cell membranes.66 This mechanism was reversible through the introduction of α-cyclodextrin, which contains a hydrophobic pocket. The addition of aliphatic chains is a simple and cost effective addition that drastically improved femoral artery injury outcomes in both rat and swine femoral artery injury models. Chitosan functionalization was further investigated by Ryu et al. who used catechol and thiolated pluronic to form thermally sensitive composite hydrogels in situ.67 This was accomplished by taking advantage of catechol-chitosan and catechol-thiolated pluronic covalent crosslinking upon oxidation. Lih et al. took a similar approach using PEG-tyramine functionalized chitosan with horseradish peroxidase and hydrogen peroxide.68 This creates a hydrogel through enzymatic crosslinking upon application, as horseradish peroxidase catalyzes the conjugation of phenol and aniline derivatives in situ. These materials have great potential in minimizing cost, thrombotic complications, and disease transmission risk. Further evaluation of long term storage, especially pertaining to approaches that rely on oxidation or enzymatic reactions are needed to fully evaluate their potential.
Cellulose
Oxidized-cellulose, a water-insoluble derivative of cellulose and an important structural component of plant cells, has been widely used and reviewed for clinical applications.69 It is believed to aid in hemostasis through a variety of mechanisms, including calcium and sodium ion interactions, acid induced small vessel contraction, and sealant properties.70–72 To improve on basic cellulose bandages, Wu et al. created a microscale gradient structure that used cellulose of varying hydrophilicties through different degrees of sodium carboxylate functionalization.73 This enabled the material to adopt different hemostatic mechanisms at different stages and led to a two-week degradation profile in vivo. Humphreys et al. took a different approach by using microporous cellulose microparticles that act as molecular sieves to concentrate coagulation proteins and showed efficacy on a spleen injury model.74 Like chitosan, cellulose has great potential in minimizing cost, thrombotic complication, and disease transmission risks. These approaches should also offer the benefit of a long shelf life. Further investigation into using clinically relevant traumatic bleeding models will allow for the full utility of these approached to be assessed.
Dextran
Another polysaccharide, dextran, and its derivatives have been recently incorporated into a wide variety of hemostatic materials. Peng et al. developed in situ forming hydrogels using oxidized dextran and a variety of primary amine containing polymers, including PAA. Utilizing the Schiff-base reaction (Fig. 4), the two mixtures polymerize upon application.75 This material reduced clotting time and improved clot strength as measured using thrombelastography, but hemostatic efficacy was not evaluated in vivo.
FIGURE 4.
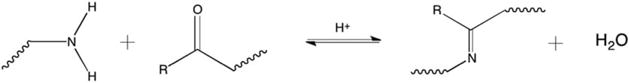
Schematic of the Schiff-base reaction utilized for gel formation or tissue adhesion.
Another adhesive material made of a biocompatible elastomer and modified with oxidized dextran was designed with dense nano-pillared morphology resembling gecko feet. This architecture is more explicitly discussed in the biologically inspired tissue adhesives sections. The oxidized dextran coating provides aldehyde functionality, enabling the material to covalently crosslink with the amine groups of proteins in tissue. Chemical crosslinking and optimization of the pillar array structure contributed to strong adhesive and sealant properties both in vitro and in vivo.76 Dextran based materials are promising because of their low cost, shelf stability, and ease of imparting aldehyde functionality. Further investigation into clinically relevant in vivo injury models is necessary to evaluate the hemostatic potential of dextran-based materials.
SYNTHETICALLY DERIVED MATERIALS
Entirely synthetic materials in various polymeric and mineral forms can be effective hemostatic agents with adhesive, antimicrobial, biocompatible, adsorptive, and biodegradable properties. They can be categorized as biologically mimetic adhesives, in situ forming sealants, direct activators and aggregators, and aluminosilicates. Typically, the synthetic sealants and adhesives are not designed for high-pressure uncontrollable hemorrhage but are applicable in surgery, whereas the mineral-based aluminosilicates are designed for rapid arterial hemorrhage control in the field.
A major advantage to these synthetic polymer systems is the reduced infectious risk or allergic reaction associated with protein-containing products.75,77 In a recent review of hemostatic surgical glues and sealants, Lodi et al. emphasizes the importance of synthetic hemostatic agents in surgery and urges research to focus on better understanding their mechanisms and applications.78
Biologically inspired tissue adhesives
Temporary tissue adhesives often model natural processes observed in biological systems, such as in geckos and marine mussels.76,79,80 Geckos have the ability to climb vertically and upside-down due to unique adhesive properties of their feet.76 A dense array of nanohairs provides high surface area and strong temporary adhesive forces in a dry system. Mussels have a powerful ability to attach to wet surfaces through the secretion of a catechol functional amino acid that cause formation of chemical crosslinks through Michael addition with amine and sulfhydryl groups as well as through the Schiff-base reaction (Fig. 5).81–83 Lee et al. combined these naturally occurring physical and chemical adhesive mechanisms to create a wet/dry biological adhesive with strong potential for medical applications. An organic scaffold composed of nano-pillars was coated with a polymer film based on the mussel adhesive protein.79 The requirement of soft lithography techniques may diminish the advantages of this approach.
FIGURE 5.
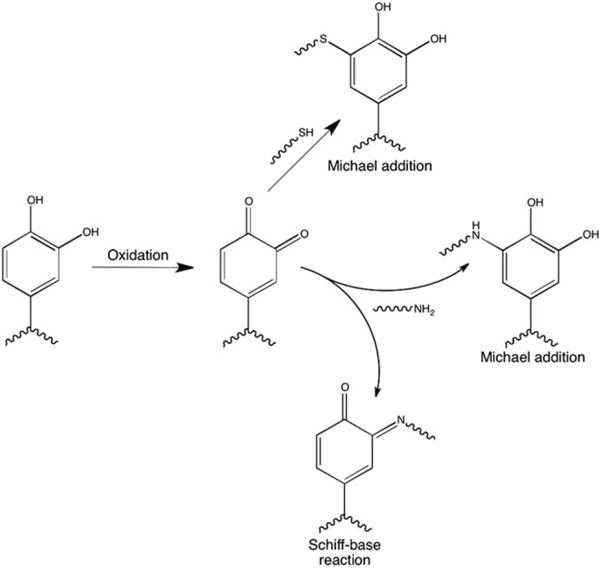
Schematic of catechol reactions with thiol and amine containing compounds.
Mehdizadeh et al. designed and tested injectable citrate-based mussel-inspired bioadhesives (iCMBAs) from citric acid, poly(ethylene glycol) (PEG), and catechol-containing monomers.84 The material promotes strong chemical crosslinking between oxidized catechol and primary amine groups in tissue. This synthetic bioadhesive demonstrated instant hemostasis in a rat dorsum incision wound and has potential as surgical glue. More recently, Barrett et al. demonstrated the general mechanism for chemical crosslinking with controlled swelling and mechanical properties via thermosensitive oxidation of a catechol-modified amphiphilic block copolymer inspired by mussel adhesive proteins.85 For the catechol functionality to be clinically useful, it must be oxidized quickly at the point of injury. This introduces storage issues as well as possible toxicity problems for biologic applications if reducing agents must be present during storage or oxidizing agents must be present for application.
In situ forming
In situ forming synthetic tissue sealants are materials that transition from a liquid to a solid or gel state through physical or chemical crosslinking in a localized site.75 Physical crosslinks may be initiated through temperature-responsive gelation36 or ionic charge interactions.86 Chemical cross-linking seen in hemostatic materials is achieved through chemical reactions such as photoinitiated polymerization,77,87 the Schiff-base reaction between primary amines and aldehyde groups,88,89 and reactions between PAA and PEG derivatives.75
Photoinitiated polymerization can cause gel formation when light irradiation initiates radical activity and subsequent crosslinking between compounds.90 PEG-lactide was used in the formation of a biodegradable occlusive barrier and tissue adherent with promising hemostatic qualities. A primer of eosin-PEG-lactide was brushed on a kidney surgical injury site, PEG-lactide macromer was added, and high intensity xenon light was used to initiate photopolymerization of the two networks in situ.77 Nivasu et al demonstrated another approach in which polyesterpolyols were synthesized from succinic acid and PEGs, acrylated, and photopolymerized using a long-wave UV light.87 The resulting film has variable mechanical strength, swelling, degradation, tensile strength, and elasticity, which can be optimized by varying PEG content and adding reactive diluents. Cytotoxicity is of concern when choosing the photoinitiator but advances with photopolymerization has demonstrated its potential in biomaterials.91 The requirement of a specialized light source increases the difficulty of application for these approaches making them less useful.
The Schiff-base reaction has also been used to chemically crosslink polymers in situ. Crosslinkable aldehyde terminated micelle hydrogels made of poly(ethylene glycol)-poly(DL-lactide) (PEG-PLA) mixed with polyallylamine (PAA) induced local hemostasis and tissue adhesion in mice liver models.89,92 The Schiff-base reaction takes place in seconds as covalent bonds form between terminal aldehyde groups on the micelle surface and amino groups in PAA. PAA was also shown to form a chemically crosslinked hydrogel in situ when mixed with multifunctional PEG.75 Using the Schiff-base reaction to crosslink multiple components typically requires a pre-mixing step or a dual nozzle set-up that diminishes ease of these approaches.
Polymer activators and aggregators
More recently, synthetic materials have been designed to target platelet activation and aggregation in primary hemostasis,93,94 clotting factor activation,95,96 and red blood cell aggregation to promote clot formation.97,98
Platelet activation and aggregation can be indicative of hemostatic potential. Ou et al. synthesized and characterized biodegradable and biocompatible poly(3-hydroxybutyrate-co–4-hydroxybutyrate) block poly(ester-urethane)s (PU3/4HB).94 Using SEM and the lactate dehydrogenase assay, high platelet adhesive and activation properties of PU3/4HB were demonstrated. Several material properties were identified as key factors for influencing platelet adhesion. These include the degree of crystallinity, hydrophobicity, surface free energy, and charge. The presence of urethane linkages increased the degree of negative charge of the polymer, which is associated with increased platelet activation. Another class of polymers with hemostatic potential is polyacrylate-based polymers for their ease of manipulation and functionalization. Using a fluorescence high-throughput polymer microarray technique, several polyacrylate polymers were identified as platelet activators and aggregators.99 Some were shown to selectively bind various proteins including von Willebrand factor and fibrinogen while others bound proteins such as fibronectin, thereby allowing for interaction with platelets. Hemostatic activity of these polymers is attributed to cationic charge content. Higher availability of non-sterically hindered tertiary amines correlated to significant increases in platelet binding. These polymers have not been assessed for hemostatic efficacy in solution, powder, or gel form.
Electrostatic charge and polyelectrolyte complex (PEC) coatings are also common mechanisms by which many of these synthetic hemostats act. Electrospun amphiphilic and PEC-coated polylactic acid and 2-(dimethylamino)-ethyl methacrylate (DMAEMA)-based amine containing materials increased red blood cell content and augmented the intrinsic and extrinsic coagulation pathways shown by a decrease in prothrombin and activated partial thromboplastin time in blood plasma.97,98 Electrostatic charge of the DMAEMA-based functional groups likely dictates the function of these materials. The effect of electrostatic charge on secondary hemostasis was also demonstrated by a primary amine containing synthetic polymer hydrogel that induced FVII activation in vitro.95 Similar cationic hydrogels exhibited high swelling and hemostatic ability in vitro and in vivo.100,101 Synthetic polymers with mechanisms that directly target primary and secondary hemostasis are increasingly prevalent features of hemostatic agents. Concerns with these approaches are long-term immunological response, degradation, and toxicity.
Zeolite, kaolin, and silicates
Mineral zeolite, kaolin, and other related aluminum silicates have been used commercially since the United States military approved the first generation QuikClot® for field treatment in 2003.102 These granular agents are composed of inactive metal oxides, salts, and mineral silicates and are highly porous. Their mechanism of action is thought to be a concentration of clotting factors and platelets in the wound site by rapidly adsorbing water, thereby aiding in clot formation.103 Due to the mechanism of action, zeolite-based products such as first and second generation QuikClot® were shown to cause a severe exothermic reaction upon water adsorption with temperatures ranging from 44 to 95°C and averaging at 67.4°C.104–111 Secondary tissue burns were also recorded in a series of case studies and these products were discontinued by 2008.112 Kaolin, an aluminum silicate similar to zeolite but without notable associated exothermic reactions, replaced zeolite in recent commercial products in the form of an impregnated gauze (Combat Gauze®). Kaolin has been shown to activate the intrinsic clotting pathway and is not able to be left in the injury site.58,103
Biomaterials made with metals oxides have demonstrated hemostatic potential through contact-activated hemostasis due in part to what is known as the glass effect without the damaging exothermic reaction of mineral zeolite. This effect has been attributed to the highly electronegative character of inorganic metal oxides such as silicon dioxide, or silica.113 Inorganic mesoporous bioactive glass microspheres,114 mesoporous silica spheres,115 and mesocellular silicate foams,116 also show hemostatic abilities by nature of their negative charge and highly absorptive pores. These porous biomaterials have been successfully loaded with calcium ions for release in situ to aid in hemostasis and dental or bone reconstruction,114 silver exchanged for antibacterial properties,115 and loaded with thrombin for its direct initiation of fibrin formation upon release.116 Metal oxide based spheres and foams do not induce exothermic reactions and can significantly decrease clotting times, but most of these materials have not yet been shown to be biocompatible or biodegradable.
Gels synthesized from naturally occurring polysaccharides known to be biocompatible can have high porosity and can be combined with other known hemostatic agents to make use of multiple hemostatic mechanisms. Dai et al. combined zeolite with chitosan in a complex synthesis of a xerogel and demonstrated contact activation, high water adsorption, and erythrocyte immobilization.117 In vitro cytotoxicity assays showed proliferation and no cell damage. In vivo efficacy was established in a rabbit lethal artery injury model. Similarly, zeolite composite hollow microspheres made with various biodegradable polymers including gelatin, chitosan, and alginate and loaded antibiotics were shown to have high water absorption and prolonged drug release.118 It is unclear if any of these particles can be left in the wound site or thrombotic complications are a significant risk.
INTRAVENOULSY ADMINISTERED
Intravenously administered hemostatic agents have gained significant interest due to their capacity to treat injury without direct access to the bleeding site. Clinical approaches have focused on the use coagulation factors, antifibrinolytic agents, and lyophilized or frozen platelets. Platelet substitutes have become widely investigated alternatives to these treatment options on an academic level.
Recombinant factor VIIa
Recombinant factor VIIa (rFVIIa), first used as a treatment for bleeding episodes in hemophiliacs, has showed success during clinical investigation for use in surgical and traumatic bleeding.119–122 rFVIIa is a coagulation factor that plays an integral role in the tissue factor (or extrinsic) pathway. Tissue factor is released at an injury site upon damage to the vascular endothelium and complexes with rFVIIa. This complex initiates the common coagulation cascade directly through activation of factor X, or indirectly through activation of factor IX. Since rFVIIa cannot lead to the activation of subsequent steps of the coagulation cascade without the presence of tissue factor, major thrombotic complications are believed to not be a serious threat.123,124 However, complications including myocardial infarction and deep vein thrombosis have still been seen in a significant percentage of patients.125
Antifibrinolytics
Antifibrinolytic approaches that directly inhibit plasmin or the binding of plasmin to fibrin have been used clinically.126,127 Plasmin is a fibrinolytic enzyme that is the active form of plasminogen.128 Aprotinin, aminocaproic acid, and tranexamic acid are pharmaceuticals that act as inhibitors, and have been shown to minimize blood transfusion requirements in surgery.6,129,130 Aprotonin directly inhibits plasmin, kallikrein, and trypsin.131 Aminocaproic acid and tranexamic acid act as lysine analogues, inhibiting binding of plasmin to fibrin.132,133 The use of these therapies has been associated with myocardial infarction, stroke, and renal failure, but some of the reporting clinical studies have undergone scrutiny.6,134,135
Platelets and platlet substitutes
Lyophilized, frozen, and fragments of platelets have all been used intravenously to varying efficacy.136–138 All platelet-derived products require virus inactivation and have variability and storage issues.139,140 To remedy some of these problems platelet substitutes have been widely investigated (Table II). Generally, platelet substitutes aim to augment primary hemostasis using injury-site specific targeting moieties to promote platelet aggregation and fortify platelet plug formation (Fig. 6).
TABLE II.
Intravenous Hemostatic Agents
| Platform | Targeting Moiety | Discussion |
|---|---|---|
| Red Blood Cell | Fibrinogen,141 fibrinogen mimicking peptide142 | Biologically derived, viral transmission risk, autologous red blood cells investigated, high cost, and low stability |
| Albumin | Fibrinogen,144,145 fibrinogen γ-chain dodecapeptide146 | Crosslinked albumin particles, viral transmission risk, and lower cost than red blood cell derived |
| Liposome | Red blood cell and platelet proteins,147 fibrinogen mimicking peptide,148 fibrinogen γ-chain dodecapeptide,149 fibrinogen mimicking peptide, collagen binding peptide, and von Willebrand factor binding peptide150,151 | Earlier approaches still used biologically derived components, later iterations used multiple nonbiologically derived targeting mechanisms, issues with cost and scalability |
| PLGA | Fibrinogen mimicking peptide,152–154 fibrinogen γ-chain dodecapeptide155 | Synthetic and degradable, nonspherical morphologies investigated, issues with cost and scalability |
FIGURE 6.
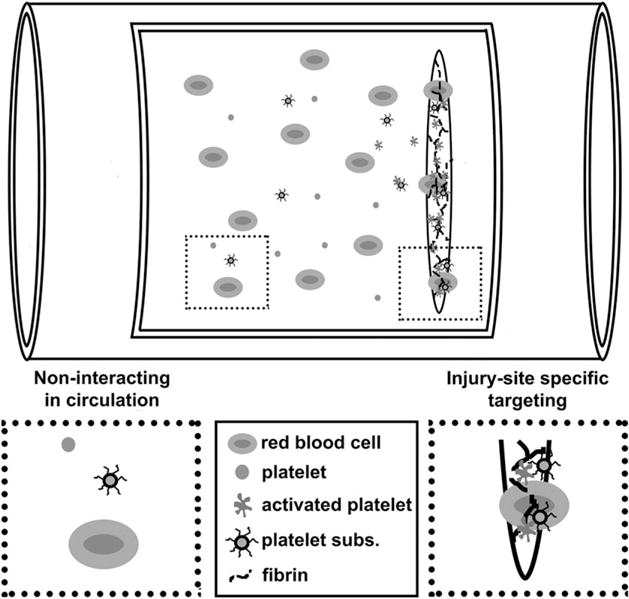
Schematic of platelet substitute hemostatic mechanism in a vascular injury. Platelet substitutes passively circulate until reaching an injury target.
Early platelet substitute approaches that utilized blood derived components were first intended for developing treatments for thrombocytopenia. Fibrinogen has been added to a variety of delivery platforms to serve as a targeting moiety, as it binds to the glycoprotein IIb–IIIa receptors of activated platelets.
Autologous erythrocytes covalently bound to fibrinogen were used with aims of passive participation in platelet aggregation, significantly reducing the bleeding time of thrombocytopenic rats.141 Using a fibrinogen mimicking peptide sequence as the targeting moiety on the surface of autologous red blood cells, Coller et al. removed the risks and difficulties associated with using human derived fibrinogen.142 Beer et al. also investigated the use of immobilized peptide chains to probe the glycoprotein IIb–IIIa receptor.143
Levi et al. and Takeoka et al. used fibrinogen coated albumin microcapsules showing in vitro platelet interaction and administered them to chemotherapy-induced thrombocytopenic rabbits demonstrating significant reduction in bleeding.144,145 Okamura et al. also used albumin particles, but instead used the dodecapeptide (H12) from the fibrinogen γ-chain, replacing fibrinogen as an activated platelet-targeting moiety and showed efficacy in vitro and in thrombocytopenic rats.146
Rybak et al. used liposomes, using a variety of proteins derived from platelets and red blood cells, namely glycoprotein IIb–IIIa and fragmented RBC membrane.147 These proteins were harvested and adsorbed to a heterogeneous liposome. They found that lipid content greatly affected hemostatic efficacy in thrombocytopenic rats. Similarly, surface-conjugated peptides to modulate binding without using human or animal derived components have been used.148 Okamura et al. coupled this approach with the release of adenosine diphosphate, a platelet activator.149 Further iterations of this approach utilized both von Willebrand factor and collagen adhesion promoting peptides motifs in concert with platelet aggregation promoting peptides on the surface of liposomes to increase surface interaction in vitro.150,151
The Lavik group investigated the use of functionalized poly(lactic-co-glycolic acid)-b-poly(L-lysine)-b-PEG nanoparticles that also use glycoprotein IIb–IIIa receptor binding, and confirmed efficacy in surgical and blunt trauma models.152,153 More recently, they have demonstrated precise control of ligand density, showing dramatic improvement in hemostatic ability.154 Okamura et al. have also constructed H12-poly(lactic-co-glycolic acid) nanosheets made of microparticle aggregates, and showed an increased adhesive rate over microparticles alone.155 While these approaches show promise and have the great advantage of not needing direct access to the site of injury, high production cost, and limited scalability may limit clinical utility.
CONCLUSION
The lack of affordable, safe, and effective hemostatic materials has led to wide interest in the development of new approaches. Assessment of new technologies is difficult due to the wide variety of injury models used in hemostatic efficacy studies. Many studies forgo in vivo evaluation all together or use a single small animal injury model. While a rat liver needle-prick injury model could be useful as a proof of concept, the same animal should be used for injuries of varying severity (tail amputation, lung and liver resection). Assessing injury models in large animals such as sheep or swine more accurately predict material performance in humans.
Many material niches exist in the field, but only iterative improvements have been made on existing commercial options. While active systems can achieve impressive hemostatic results and have obvious mechanisms, they carry the risk of thrombotic complications and disease transmission. Passive participation in clot or platelet aggregate formation, as exemplified in the recent advances in intravenous hemostatics, allows for the utilization of natural hemostatic process without this risk. Biologically inspired architectures and chemistries have also led to exciting advances. Only a handful of chemistries behind biocompatible wet-tissue adhesives have been explored. These wet-adhesive approaches deserve further investigation.
Academic strategies must thoughtfully consider the end goal of clinical translation. Complicated delivery methods, high cost, limited scalability, and even safety are often overlooked when developing new solutions. Collaborations with surgeons or other clinicians are encouraged to vet out non-clinically applicable technologies. One-step approaches, topically or intravenously administered, should be the chief focus of future research.
Acknowledgments
The authors would like to thank the Warren Citrin Fellowship and Mr. Steven W. Grant for their support of this work.
Contract grant sponsor: National Science Foundation; contract grant number: DMR-1041535
References
- 1.Kelly JF, Ritenour AE, McLaughlin DF, Bagg KA, Apodaca AN, Mallak CT, Pearse L, Lawnick MM, Champion HR, Wade CE, Holcomb JB. Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003–2004 versus 2006. J Trauma. 2008;64(2 Suppl):S21–6. doi: 10.1097/TA.0b013e318160b9fb. discussion S26–27. [DOI] [PubMed] [Google Scholar]
- 2.Katzenell U, Ash N, Tapia AL, Campino GA, Glassberg E. Analysis of the causes of death of casualties in field military setting. Mil Med. 2012;177:1065–1068. doi: 10.7205/milmed-d-12-00161. [DOI] [PubMed] [Google Scholar]
- 3.Champion HR, Bellamy RF, Roberts CP, Leppaniemi A. A profile of combat injury. J Trauma. 2003;54(5 Suppl):S13–19. doi: 10.1097/01.TA.0000057151.02906.27. [DOI] [PubMed] [Google Scholar]
- 4.Blackbourne LH, Czarnik J, Mabry R, Eastridge B, Baer D, Butler F, Pruitt B. Decreasing killed in action and died of wounds rates in combat wounded. J Trauma. 2010;69:S1–S4. doi: 10.1097/TA.0b013e3181e4206f. [DOI] [PubMed] [Google Scholar]
- 5.Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: Comprehensive population-based assessment. World J Surg. 2010;34:158–163. doi: 10.1007/s00268-009-0266-1. [DOI] [PubMed] [Google Scholar]
- 6.Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356:2301–2311. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 7.Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, Deckmyn H. Platelets at work in primary hemostasis. Blood Rev. 2011;25:155–167. doi: 10.1016/j.blre.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 9.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 10.Achneck HE, Sileshi B, Jamiolkowski RM, Albala DM, Shapiro ML, Lawson JH. A comprehensive review of topical hemostatic agents efficacy and recommendations for use. Ann Surg. 2010;251:217–228. doi: 10.1097/SLA.0b013e3181c3bcca. [DOI] [PubMed] [Google Scholar]
- 11.Peng HT, Shek PN. Novel wound sealants: Biomaterials and applications. Expert Rev Med Devices. 2010;7:639–659. doi: 10.1586/erd.10.40. [DOI] [PubMed] [Google Scholar]
- 12.Spotnitz WD. Hemostats, sealants, and adhesives: A practical guide for the surgeon. Am Surg. 2012;78:1305–1321. [PubMed] [Google Scholar]
- 13.Gordy SD, Rhee P, Schreiber MA. Military applications of novel hemostatic devices. Expert Rev Med Devices. 2011;8:41–47. doi: 10.1586/erd.10.69. [DOI] [PubMed] [Google Scholar]
- 14.Spotnitz WD, Burks S. Hemostats, sealants, and adhesives: Components of the surgical toolbox. Transfusion. 2008;48:1502–1516. doi: 10.1111/j.1537-2995.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 15.Spotnitz WD. Fibrin sealant: past, present, and future: A brief review. World J Surg. 2010;34:632–634. doi: 10.1007/s00268-009-0252-7. [DOI] [PubMed] [Google Scholar]
- 16.Alston SM, Solen KA, Sukavaneshvar S, Mohammad SF. In Vivo efficacy of a new autologous fibrin sealant. J Surg Res. 2008;146:143–148. doi: 10.1016/j.jss.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Larson MJ, Bowersox JC, Lim RC, Hess JR. Efficacy of a fibrin hemostatic bandage in controlling hemorrhage from experimental arterial injuries. Thromb Haemost. 1995;73:1465–1465. doi: 10.1001/archsurg.1995.01430040082018. [DOI] [PubMed] [Google Scholar]
- 18.Anema JG, Morey AF, Harris R, MacPhee M, Cornum RL. Potential uses of absorbable fibrin adhesive bandage for genitourinary trauma. World J Surg. 2001;25:1573–1577. doi: 10.1007/s00268-001-0152-y. [DOI] [PubMed] [Google Scholar]
- 19.Pusateri AE, Kheirabadi BS, Delgado AV, Doyle JW, Kanellos J, Uscilowicz JM, Martinez RS, Holcomb JB, Modrow HE. Structural design of the dry fibrin sealant dressing and its impact on the hemostatic efficacy of the product. J Biomed Mater Res B Appl Biomater. 2004;70B:114–121. doi: 10.1002/jbm.b.30031. [DOI] [PubMed] [Google Scholar]
- 20.Wnek GE, Carr ME, Simpson DG, Bowlin GL. Electrospinning of nanofiber fibrinogen structures. Nano Lett. 2003;3:213–216. [Google Scholar]
- 21.Elvin CM, Brownlee AG, Huson MG, Tebb TA, Kim M, Lyons RE, Vuocolo T, Liyou NE, Hughes TC, Ramshaw JAM, Werkmeister JA. The development of photochemically crosslinked native fibrinogen as a rapidly formed and mechanically strong surgical tissue sealant. Biomaterials. 2009;30:2059–2065. doi: 10.1016/j.biomaterials.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 22.Elvin CM, Danon SJ, Brownlee AG, White JF, Hickey M, Liyou NE, Edwards GA, Ramshaw JAM, Werkmeister JA. Evaluation of photo-crosslinked fibrinogen as a rapid and strong tissue adhesive. J Biomed Mater Res A. 2010;93A:687–695. doi: 10.1002/jbm.a.32572. [DOI] [PubMed] [Google Scholar]
- 23.Smeets R, Gerhards F, Stein JM, Paz RM, Vogt S, Pautke C, Weitz J, Kolk A. A novel hemostatic delivery device for thrombin: Biodegradable poly(D,L-lactide-co-glycolide) 50:50 microspheres. J Biomed Mater Res A. 2011;96:177–185. doi: 10.1002/jbm.a.32970. [DOI] [PubMed] [Google Scholar]
- 24.Shukla A, Fang JC, Puranam S, Jensen FR, Hammond PT. Hemostatic multilayer coatings. Adv Mater. 2012;24:492–496. doi: 10.1002/adma.201103794. [DOI] [PubMed] [Google Scholar]
- 25.Joch C. The safety of fibrin sealants. Cardiovasc Surg. 2003;11:23–28. doi: 10.1016/S0967-2109(03)00068-1. [DOI] [PubMed] [Google Scholar]
- 26.Bhamidipati CM, Coselli JS, LeMaire SA. BioGlue in 2011: What is its role in cardiac surgery? J Extra Corpor Technol. 2012;44:P6–12. [PMC free article] [PubMed] [Google Scholar]
- 27.Furst W, Banerjee A. Release of glutaraldehyde from an albumin-glutaraldehyde tissue adhesive causes significant in vitro and in vivo toxicity. Ann Thorac Surg. 2005;79:1522–1529. doi: 10.1016/j.athoracsur.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 28.Xie H, Wolf RF, Burke AP, Gustafson SB, Gregory KW, Prahl SA. Concentrated albumin as a biological glue for hemorrhage control on hepatic resection with argon beam coagulation. J Biomed Mater Res B Appl Biomater. 2004;71:84–89. doi: 10.1002/jbm.b.30077. [DOI] [PubMed] [Google Scholar]
- 29.Moroi M, Jung SM, Nomura S, Ordinas A, DiazRicart M. Analysis of the involvement of the von Willebrand factor-GPIb interaction in platelet adhesion to a collagen-coated surface under flow conditions. Thromb Haemost. 1997:P2627–P2627. [PubMed] [Google Scholar]
- 30.Moroi M, Jung SM, Nomura S, Sekiguchi S, Ordinas A, DiazRicart M. Analysis of the involvement of the von Willebrand factor glycoprotein Ib interaction in platelet adhesion to a collagen-coated surface under flow conditions. Blood. 1997;90:4413–4424. [PubMed] [Google Scholar]
- 31.Moroi M, Jung SM. Platelet receptors for collagen. Thromb Haemost. 1997;78:439–444. [PubMed] [Google Scholar]
- 32.Chiang TM. Collagen-platelet interaction: platelet non-integrin receptors. Histol Histopathol. 1999;14:579–585. doi: 10.14670/HH-14.579. [DOI] [PubMed] [Google Scholar]
- 33.Yang C, Hillas P, Tang J, Balan J, Notbohm H, Polarek J. Development of a recombinant human collagen-type III based hemostat. J Biomed Mater Res B Appl Biomater. 2004;69B:18–24. doi: 10.1002/jbm.b.20030. [DOI] [PubMed] [Google Scholar]
- 34.Hajosch R, Suckfuell M, Oesser S, Ahlers M, Flechsenhar K, Schlosshauer B. A novel gelatin sponge for accelerated hemostasis. J Biomed Mater Res B Appl Biomater. 2010;94:372–379. doi: 10.1002/jbm.b.31663. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Kopelman D, Wu LQ, Hijji K, Attar I, Preiss-Bloom O, Payne GF. Biomimetic sealant based on gelatin and microbial transglutaminase: an initial in vivo investigation. J Biomed Mater Res B Appl Biomater. 2009;91:5–16. doi: 10.1002/jbm.b.31368. [DOI] [PubMed] [Google Scholar]
- 36.Ohya S, Sonoda H, Nakayama Y, Matsuda T. The potential of poly(N-isopropylacrylamide) (PNIPAM)-grafted hyaluronan and PNIPAM-grafted gelatin in the control of post-surgical tissue adhesions. Biomaterials. 2005;26:655–659. doi: 10.1016/j.biomaterials.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Ohya S, Kidoaki S, Matsuda T. Poly(N-isopropylacrylamide) (PNI-PAM)-grafted gelatin hydrogel surfaces: Interrelationship between microscopic structure and mechanical property of surface regions and cell adhesiveness. Biomaterials. 2005;26:3105–3111. doi: 10.1016/j.biomaterials.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Ohya S, Matsuda T. Poly(N-isopropylacrylamide) (PNIPAM)-grafted gelatin as thermoresponsive three-dimensional artificial extracellular matrix: Molecular and formulation parameters vs. cell proliferation potential. J Biomater Sci Polym Ed. 2005;16:809–827. doi: 10.1163/1568562054255736. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki S, Ikada Y. Sealing effects of cross-linked gelatin. J Biomater Appl. 2013;27:801–810. doi: 10.1177/0885328211426491. [DOI] [PubMed] [Google Scholar]
- 40.Otani Y, Tabata Y, Ikada Y. Effect of additives on gelation and tissue adhesion of gelatin-poly(L-glutamic acid) mixture. Biomaterials. 1998;19:2167–2173. doi: 10.1016/s0142-9612(98)00123-9. [DOI] [PubMed] [Google Scholar]
- 41.Otani Y, Tabata Y, Ikada Y. Hemostatic capability of rapidly curable glues from gelatin, poly(L-glutamic acid), and carbodiimide. Biomaterials. 1998;19:2091–2098. doi: 10.1016/s0142-9612(98)00121-5. [DOI] [PubMed] [Google Scholar]
- 42.Ruan L, Zhang H, Luo H, Liu J, Tang F, Shi YK, Zhao X. Designed amphiphilic peptide forms stable nanoweb, slowly releases encapsulated hydrophobic drug, and accelerates animal hemostasis. Proc Natl Acad Sci USA. 2009;106:5105–5110. doi: 10.1073/pnas.0900026106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellis-Behnke RG, Liang YX, Tay DK, Kau PW, Schneider GE, Zhang S, Wu W, So KF. Nano hemostat solution: Immediate hemostasis at the nanoscale. Nanomedicine. 2006;2:207–215. doi: 10.1016/j.nano.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Song H, Zhang L, Zhao X. Hemostatic efficacy of biological self-assembling peptide nanofibers in a rat kidney model. Macromol Biosci. 2010;10:33–39. doi: 10.1002/mabi.200900129. [DOI] [PubMed] [Google Scholar]
- 45.Goddard DR, Michaelis L. A Study on Keratin. J Biol Chem. 1934;106:605–614. [Google Scholar]
- 46.Aboushwareb T, Eberli D, Ward C, Broda C, Holcomb J, Atala A, Van Dyke M. A keratin biomaterial gel hemostat derived from human hair: Evaluation in a rabbit model of lethal liver injury. J Biomed Mater Res B Appl Biomater. 2009;90:45–54. doi: 10.1002/jbm.b.31251. [DOI] [PubMed] [Google Scholar]
- 47.Burnett LR, Richter JG, Rahmany MB, Soler R, Steen JA, Orlando G, Abouswareb T, Van Dyke ME. Novel keratin (KeraStatTM) and polyurethane (Nanosan(R)-Sorb) biomaterials are hemostatic in a porcine lethal extremity hemorrhage model. J Biomater Appl Forthcoming. doi: 10.1177/0885328213484975. [DOI] [PubMed] [Google Scholar]
- 48.Burnett LR, Rahmany MB, Richter JR, Aboushwareb TA, Eberli D, Ward CL, Orlando G, Hantgan RR, Van Dyke ME. Hemostatic properties and the role of cell receptor recognition in human hair keratin protein hydrogels. Biomaterials. 2013;34:2632–2640. doi: 10.1016/j.biomaterials.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 49.Rahmany MB, Hantgan RR, Van Dyke M. A mechanistic investigation of the effect of keratin-based hemostatic agents on coagulation. Biomaterials. 2013;34:2492–2500. doi: 10.1016/j.biomaterials.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Dias GJ, Mahoney P, Swain M, Kelly RJ, Smith RA, Ali MA. Keratin-hydroxyapatite composites: biocompatibility, osseointegration, and physical properties in an ovine model. J Biomed Mater Res A. 2010;95:1084–1095. doi: 10.1002/jbm.a.32908. [DOI] [PubMed] [Google Scholar]
- 51.Dias GJ, Peplow PV, McLaughlin A, Teixeira F, Kelly RJ. Biocompatibility and osseointegration of reconstituted keratin in an ovine model. J Biomed Mater Res A. 2010;92:513–520. doi: 10.1002/jbm.a.32394. [DOI] [PubMed] [Google Scholar]
- 52.Malette WG, Quigley HJ, Gaines RD, Johnson ND, Rainer WG. Chitosan: A new hemostatic. Ann Thorac Surg. 1983;36:55–58. doi: 10.1016/s0003-4975(10)60649-2. [DOI] [PubMed] [Google Scholar]
- 53.Rao SB, Sharma CP. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J Biomed Mater Res. 1997;34:21–28. doi: 10.1002/(sici)1097-4636(199701)34:1<21::aid-jbm4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 54.Benesch J, Tengvall P. Blood protein adsorption onto chitosan. Biomaterials. 2002;23:2561–2568. doi: 10.1016/s0142-9612(01)00391-x. [DOI] [PubMed] [Google Scholar]
- 55.Yang J, Tian F, Wang Z, Wang Q, Zeng YJ, Chen SQ. Effect of chitosan molecular weight and deacetylation degree on Hemostasis. J Biomed Mater Res B Appl Biomater. 2008;84B:131–137. doi: 10.1002/jbm.b.30853. [DOI] [PubMed] [Google Scholar]
- 56.Whang HS, Kirsch W, Zhu YH, Yang CZ, Hudson SM. Hemostatic agents derived from chitin and chitosan. J Macromol Sci Polym Rev. 2005;C45:309–323. [Google Scholar]
- 57.Cox ED, Schreiber MA, McManus J, Wade CE, Holcomb JB. New hemostatic agents in the combat setting. Transfusion. 2009;49:248S–255S. doi: 10.1111/j.1537-2995.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- 58.Littlejohn LF, Devlin JJ, Kircher SS, Lueken R, Melia MR, Johnson AS. Comparison of Celox-A, ChitoFlex, WoundStat, and combat gauze hemostatic agents versus standard gauze dressing in control of hemorrhage in a swine model of penetrating trauma. Acad Emerg Med. 2011;18:340–350. doi: 10.1111/j.1553-2712.2011.01036.x. [DOI] [PubMed] [Google Scholar]
- 59.Kheirabadi BS, Acheson EM, Deguzman R, Sondeen JL, Ryan KL, Delgado A, Dick EJ, Holcomb JB. Hemostatic efficacy of two advanced dressings in an aortic hemorrhage model in swine. J Trauma. 2005;59:25–34. doi: 10.1097/01.ta.0000171458.72037.ee. [DOI] [PubMed] [Google Scholar]
- 60.Neuffer MC, McDivitt J, Rose D, King K, Cloonan CC, Vayer JS. Hemostatic dressings for the first responder: A review. Mil Med. 2004;169:716–720. doi: 10.7205/milmed.169.9.716. [DOI] [PubMed] [Google Scholar]
- 61.Smith SA, Morrissey JH. Polyphosphate as a general procoagulant agent. J Thromb Haemost. 2008;6:1750–1756. doi: 10.1111/j.1538-7836.2008.03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith SA, Morrissey JH. Polyphosphate enhances fibrin clot structure. Blood. 2008;112:2810–2816. doi: 10.1182/blood-2008-03-145755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith SA, Choi SH, Davis-Harrison R, Huyck J, Boettcher J, Reinstra CM, Morrissey JH. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 2010;116:4353–4359. doi: 10.1182/blood-2010-01-266791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ong SY, Wu J, Moochhala SM, Tan MH, Lu J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29:4323–4332. doi: 10.1016/j.biomaterials.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 65.Kumar PTS, Lakshmanan VK, Anilkumar TV, Ramya C, Reshmi P, Unnikrishnan AG, Nair SV, Jayakumar R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. Acs Appl Mater Interfaces. 2012;4:2618–2629. doi: 10.1021/am300292v. [DOI] [PubMed] [Google Scholar]
- 66.Dowling MB, Kumar R, Keibler MA, Hess JR, Bochicchio GV, Raghavan SR. A self-assembling hydrophobically modified chitosan capable of reversible hemostatic action. Biomaterials. 2011;32:3351–3357. doi: 10.1016/j.biomaterials.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 67.Ryu JH, Lee Y, Kong WH, Kim TG, Park TG, Lee H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules. 2011;12:2653–2659. doi: 10.1021/bm200464x. [DOI] [PubMed] [Google Scholar]
- 68.Lih E, Lee JS, Park KM, Park KD. Rapidly curable chitosan-PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta Biomater. 2012;8:3261–3269. doi: 10.1016/j.actbio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Tomizawa Y. Clinical benefits and risk analysis of topical hemostats: A review. J Artif Organs. 2005;8:137–142. doi: 10.1007/s10047-005-0296-x. [DOI] [PubMed] [Google Scholar]
- 70.Krizova P, Masova L, Suttnar J, Salaj P, Dyr JE, Homola J, Pecka M. The influence of intrinsic coagulation pathway on blood platelets activation by oxidized cellulose. J Biomed Mater Res A. 2007;82A:274–280. doi: 10.1002/jbm.a.31060. [DOI] [PubMed] [Google Scholar]
- 71.Larson PO. Topical hemostatic agents for dermatologic surgery. J Dermatol Surg Oncol. 1988;14:623–632. doi: 10.1111/j.1524-4725.1988.tb03390.x. [DOI] [PubMed] [Google Scholar]
- 72.Schonauer C, Tessitore E, Barbagallo G, Albanese V, Moraci A. The use of local agents: Bone wax, gelatin, collagen, oxidized cellulose. Eur Spine J. 2004;13:S89–S96. doi: 10.1007/s00586-004-0727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu YD, He JM, Cheng WL, Gu HB, Guo ZH, Gao S, Huang YD. Oxidized regenerated cellulose-based hemostat with microscopically gradient structure. Carbohydr Polym. 2012;88:1023–1032. [Google Scholar]
- 74.Humphreys MR, Castle EP, Andrews PE, Gettman MT, Ereth MH. Microporous polysaccharide hemospheres for management of laparoscopic trocar injury to the spleen. Am J Surg. 2008;195:99–103. doi: 10.1016/j.amjsurg.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 75.Peng HT, Blostein MD, Shek PN. Experimental optimization of an in situ forming hydrogel for hemorrhage control. J Biomed Mater Res B Appl Biomater. 2009;89:199–209. doi: 10.1002/jbm.b.31206. [DOI] [PubMed] [Google Scholar]
- 76.Mahdavi A, Ferreira L, Sundback C, Nichol JW, Chan EP, Carter DJ, Bettinger CJ, Patanavanich S, Chignozha L, Ben-Joseph E, Galakatos A, Pryor H, Pomerantseva I, Masiakos PT, Faquin W, Zumbuehl A, Hong S, Borenstein J, Vacanti J, Langer R, Karp JM. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc Natl Acad Sci USA. 2008;105:2307–2312. doi: 10.1073/pnas.0712117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramakumar S, Roberts WW, Fugita OE, Colegrove P, Nicol TM, Jarrett TW, Kavoussi LR, Slepian MJ. Local hemostasis during laparoscopic partial nephrectomy using biodegradable hydrogels: initial porcine results. J Endourol. 2002;16:489–494. doi: 10.1089/089277902760367458. [DOI] [PubMed] [Google Scholar]
- 78.Lodi D, Iannitti T, Palmieri B. Management of haemostasis in surgery: Sealant and glue applications. Blood Coagul Fibrinolysis. 2012;23:465–472. doi: 10.1097/MBC.0b013e32835496d8. [DOI] [PubMed] [Google Scholar]
- 79.Lee H, Lee BP, Messersmith PB. A reversible wet/dry adhesive inspired by mussels and geckos. Nature. 2007;448:338–341. doi: 10.1038/nature05968. [DOI] [PubMed] [Google Scholar]
- 80.Xue J, Wang T, Nie J, Yang D. Preparation and characterization of a photocrosslinkable bioadhesive inspired by marine mussel. J Photochem Photobiol B. 2013;119:31–36. doi: 10.1016/j.jphotobiol.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 81.Schweigert N, Zehnder AJB, Eggen RIL. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ Microbiol. 2001;3:81–91. doi: 10.1046/j.1462-2920.2001.00176.x. [DOI] [PubMed] [Google Scholar]
- 82.Land EJ, Ramsden CA, Riley PA. Quinone chemistry and melanogenesis. Methods Enzymol. 2004;378:88–109. doi: 10.1016/S0076-6879(04)78005-2. [DOI] [PubMed] [Google Scholar]
- 83.Jain R, Agarwal A, Kierski PR, Schurr MJ, Murphy CJ, McAnulty JF, Abbott NL. The use of native chemical functional groups presented by wound beds for the covalent attachment of polymeric microcarriers of bioactive factors. Biomaterials. 2013;34:340–352. doi: 10.1016/j.biomaterials.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mehdizadeh M, Weng H, Gyawali D, Tang L, Yang J. Injectable citrate-based mussel-inspired tissue bioadhesives with high wet strength for sutureless wound closure. Biomaterials. 2012;33:7972–7983. doi: 10.1016/j.biomaterials.2012.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barrett DG, Bushnell GG, Messersmith PB. Mechanically robust, negative-swelling, mussel-inspired tissue adhesives. Adv Healthc Mater. 2013;2:745–755. doi: 10.1002/adhm.201200316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Tomme SR, van Steenbergen MJ, De Smedt SC, van Nostrum CF, Hennink WE. Self-gelling hydrogels based on oppositely charged dextran microspheres. Biomaterials. 2005;26:2129–2135. doi: 10.1016/j.biomaterials.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 87.Nivasu VM, Reddy TT, Tammishetti S. In situ polymerizable polyethyleneglycol containing polyesterpolyol acrylates for tissue sealant applications. Biomaterials. 2004;25:3283–3291. doi: 10.1016/j.biomaterials.2003.09.091. [DOI] [PubMed] [Google Scholar]
- 88.Peng HT, Shek PN. Development of in situ-forming hydrogels for hemorrhage control. J Mater Sci Mater Med. 2009;20:1753–1762. doi: 10.1007/s10856-009-3721-5. [DOI] [PubMed] [Google Scholar]
- 89.Murakami Y, Yokoyama M, Okano T, Nishida H, Tomizawa Y, Endo M, Kurosawa H. A novel synthetic tissue-adhesive hydrogel using a crosslinkable polymeric micelle. J Biomed Mater Res A. 2007;80:421–427. doi: 10.1002/jbm.a.30911. [DOI] [PubMed] [Google Scholar]
- 90.Odian GG. Principles of polymerization Hoboken. New Jersey: Wiley-Interscience; 2004. p. xxiv, 812. [Google Scholar]
- 91.Yagci Y, Jockusch S, Turro NJ. Photoinitiated polymerization: Advances, challenges, and opportunities. Macromolecules. 2010;43:6245–6260. [Google Scholar]
- 92.Murakami Y, Yokoyama M, Nishida H, Tomizawa Y, Kurosawa H. In vivo and in vitro evaluation of gelation and hemostatic properties of a novel tissue-adhesive hydrogel containing a cross-linkable polymeric micelle. J Biomed Mater Res B Appl Biomater. 2009;91B:102–108. doi: 10.1002/jbm.b.31378. [DOI] [PubMed] [Google Scholar]
- 93.Hansen A, McMillan L, Morrison A, Petrik J, Bradley M. Polymers for the rapid and effective activation and aggregation of platelets. Biomaterials. 2011;32:7034–7041. doi: 10.1016/j.biomaterials.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 94.Ou W, Qiu H, Chen Z, Xu K. Biodegradable block poly(ester-urethane)s based on poly(3-hydroxybutyrate-co-4-hydroxybutyrate) copolymers. Biomaterials. 2011;32:3178–3188. doi: 10.1016/j.biomaterials.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 95.Casey BJ, Behrens AM, Hess JR, Wu ZJ, Griffith BP, Kofinas P. FVII dependent coagulation activation in citrated plasma by polymer hydrogels. Biomacromolecules. 2010;11:3248–3255. doi: 10.1021/bm101147w. [DOI] [PubMed] [Google Scholar]
- 96.Broekema FI, van Oeveren W, Zuidema J, Visscher SH, Bos RR. In vitro analysis of polyurethane foam as a topical hemostatic agent. J Mater Sci Mater Med. 2011;22:1081–1086. doi: 10.1007/s10856-011-4276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yancheva E, Paneva D, Manolova N, Mincheva R, Danchev D, Dubois P, Rashkov I. Tuning of the surface biological behavior of poly(L-lactide)-based electrospun materials by polyelectrolyte complex formation. Biomacromolecules. 2010;11:521–532. doi: 10.1021/bm901307x. [DOI] [PubMed] [Google Scholar]
- 98.Spasova M, Manolova N, Paneva D, Mincheva R, Dubois P, Rashkov I, Maximova V, Danchev D. Polylactide stereocomplex-based electrospun materials possessing surface with antibacterial and hemostatic properties. Biomacromolecules. 2010;11:151–159. doi: 10.1021/bm901016y. [DOI] [PubMed] [Google Scholar]
- 99.Hansen A, McMillan L, Morrison A, Petrik J, Bradley M. Polymers for the rapid and effective activation and aggregation of platelets. Biomaterials. 2011;32:7034–7041. doi: 10.1016/j.biomaterials.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 100.Casey BJ, Behrens AM, Tsinas ZI, Hess JR, Wu ZJ, Griffith BP, Kofinas P. In vitro and in vivo evaluation of polymer hydrogels for hemorrhage control. J Biomater Sci Polym Ed. 2013;24:1781–1793. doi: 10.1080/09205063.2013.801707. [DOI] [PubMed] [Google Scholar]
- 101.Behrens AM, Sikorski MJ, Li T, Wu ZJ, Griffith BP, Kofinas P. Blood aggregating hydrogel particles for use as a hemostatic agent. Acta Biomater Forthcoming. doi: 10.1016/j.actbio.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 102.Granville-Chapman J, Jacobs N, Midwinter MJ. Pre-hospital haemostatic dressings: A systematic review. Injury. 2011;42:447–459. doi: 10.1016/j.injury.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 103.Gordy SD, Rhee P, Schreiber MA. Military applications of novel hemostatic devices. Expert Rev Med Devices. 2011;8:41–47. doi: 10.1586/erd.10.69. [DOI] [PubMed] [Google Scholar]
- 104.Pusateri AE, Delgado AV, Dick EJ, Martinez RS, Holcomb JB, Ryan KL. Application of a granular mineral-based hemostatic agent (QuikClot) to reduce blood loss after grade V liver injury in swine. J Trauma. 2004;57:555–562. doi: 10.1097/01.ta.0000136155.97758.cd. [DOI] [PubMed] [Google Scholar]
- 105.Acheson EM, Kheirabadi BS, Deguzman R, Dick EJ, Holcomb JB. Comparison of hemorrhage control agents applied to lethal extremity arterial hemorrhages in swine. J Trauma. 2005;59:865–874. doi: 10.1097/01.ta.0000187655.63698.9f. [DOI] [PubMed] [Google Scholar]
- 106.Ward KR, Tiba MH, Holbert WH, Blocher CR, Draucker GT, Proffitt EK, Bowlin GL, Ivatury RR, Diegelmann RF. Comparison of a new hemostatic agent to current combat hemostatic agents in a swine model of lethal extremity arterial hemorrhage. J Trauma. 2007;63:276–283. doi: 10.1097/TA.0b013e3180eea8a5. [DOI] [PubMed] [Google Scholar]
- 107.Alam HB, Uy GB, Miller D, Koustova E, Hancock T, Inocencio R, Anderson D, Llorente O, Rhee P. Comparative analysis of hemostatic agents in a swine model of lethal groin injury. J Trauma. 2003;54:1077–1082. doi: 10.1097/01.TA.0000068258.99048.70. [DOI] [PubMed] [Google Scholar]
- 108.Alam HB, Chen Z, Jaskille A, Querol RI, Koustova E, Inocencio R, Conran R, Seufert A, Ariaban N, Toruno K, Rhee P. Application of a zeolite hemostatic agent achieves 100% survival in a lethal model of complex groin injury in Swine. J Trauma. 2004;56:974–983. doi: 10.1097/01.ta.0000127763.90890.31. [DOI] [PubMed] [Google Scholar]
- 109.Arnaud F, Tomori T, Saito R, McKeague A, Prusaczyk WK, McCarron RM. Comparative efficacy of granular and bagged formulations of the hemostatic agent QuikClot. J Trauma. 2007;63:775–782. doi: 10.1097/TA.0b013e31805f7023. [DOI] [PubMed] [Google Scholar]
- 110.Kozen BG, Kircher SJ, Henao J, Godinez FS, Johnson AS. An alternative hemostatic dressing: Comparison of CELOX, HemCon, and QuikClot. Acad Emerg Med. 2008;15:74–81. doi: 10.1111/j.1553-2712.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- 111.Wright JK, Kalns J, Wolf EA, Traweek F, Schwarz S, Loeffler CK, Snyder W, Yantis LD, Jr, Eggers J. Thermal injury resulting from application of a granular mineral hemostatic agent. J Trauma. 2004;57:224–230. doi: 10.1097/01.ta.0000105916.30158.06. [DOI] [PubMed] [Google Scholar]
- 112.McManus J, Hurtado T, Pusateri A, Knoop KJ. A case series describing thermal injury resulting from zeolite use for hemorrhage control in combat operations. Prehosp Emerg Care. 2007;11:67–71. doi: 10.1080/10903120601021176. [DOI] [PubMed] [Google Scholar]
- 113.Ostomel TA, Shi Q, Stoimenov PK, Stucky GD. Metal oxide surface charge mediated hemostasis. Langmuir. 2007;23:11233–11238. doi: 10.1021/la701281t. [DOI] [PubMed] [Google Scholar]
- 114.Ostomel TA, Shi Q, Tsung CK, Liang H, Stucky GD. Spherical bioactive glass with enhanced rates of hydroxyapatite deposition and hemostatic activity. Small (Weinheim an der Bergstrasse, Germany) 2006;2:1261–1265. doi: 10.1002/smll.200600177. [DOI] [PubMed] [Google Scholar]
- 115.Dai C, Yuan Y, Liu C, Wei J, Hong H, Li X, Pan X. Degradable, antibacterial silver exchanged mesoporous silica spheres for hemorrhage control. Biomaterials. 2009;30:5364–5375. doi: 10.1016/j.biomaterials.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 116.Baker SE, Sawvel AM, Fan J, Shi Q, Strandwitz N, Stucky GD. Blood clot initiation by mesocellular foams: Dependence on nanopore size and enzyme immobilization. Langmuir. 2008;24:14254–14260. doi: 10.1021/la802804z. [DOI] [PubMed] [Google Scholar]
- 117.Dai CL, Liu CS, Wei J, Hong H, Zhao QH. Molecular imprinted macroporous chitosan coated mesoporous silica xerogels for hemorrhage control. Biomaterials. 2010;31:7620–7630. doi: 10.1016/j.biomaterials.2010.06.049. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Y, Xu C, He Y, Wang X, Xing F, Qiu H, Liu Y, Ma D, Lin T, Gao J. Zeolite/polymer composite hollow microspheres containing antibiotics and the drug release. J Biomater Sci Polym Ed. 2011;22:809–822. doi: 10.1163/092050610X496242. [DOI] [PubMed] [Google Scholar]
- 119.Kenet G, Walden R, Eldad A, Martinowitz U. Treatment of traumatic bleeding with recombinant factor VIIa. Lancet. 1999;354:1879–1879. doi: 10.1016/S0140-6736(99)05155-7. [DOI] [PubMed] [Google Scholar]
- 120.Aldouri M. The use of recombinant factor VIIa in controlling surgical bleeding in non-haemophiliac patients. Pathophysiol Haemost Thromb. 2002;32(Suppl 1):41–46. doi: 10.1159/000057301. [DOI] [PubMed] [Google Scholar]
- 121.Lusher JM, Arkin S, Abildgaard CF, Schwartz RS. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. Safety, efficacy, and development of inhibitors. Kogenate Previously Untreated Patient Study Group. N Engl J Med. 1993;328:453–459. doi: 10.1056/NEJM199302183280701. [DOI] [PubMed] [Google Scholar]
- 122.Mohr AM, Holcomb JB, Dutton RP, Duranteau J. Recombinant activated factor VIIa and hemostasis in critical care: A focus on trauma. Crit Care. 2005;9(Suppl 5):S37–42. doi: 10.1186/cc3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roberts HR, Monroe DM, III, Hoffman M. Safety profile of recombinant factor VIIa. Semin Hematol. 2004;41(1 Suppl 1):101–108. doi: 10.1053/j.seminhematol.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 124.Nakar C, Cooper DL, DiMichele D. Recombinant activated factor VII safety and efficacy in the treatment of cranial haemorrhage in patients with congenital haemophilia with inhibitors: An analysis of the Hemophilia and Thrombosis Research Society Registry (2004–2008) Haemophilia. 2010;16:625–631. doi: 10.1111/j.1365-2516.2010.02208.x. [DOI] [PubMed] [Google Scholar]
- 125.Howes JL, Smith RS, Helmer SD, Taylor SM. Complications of recombinant activated human coagulation factor VII. Am J Surg. 2009;198:895–899. doi: 10.1016/j.amjsurg.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 126.Royston D, Bidstrup BP, Taylor KM, Sapsford RN. Effect of aprotinin on need for blood-transfusion after repeat open-heart-surgery. Lancet. 1987;2:1289–1291. doi: 10.1016/s0140-6736(87)91190-1. [DOI] [PubMed] [Google Scholar]
- 127.Speekenbrink RG, Wildevuur CR, Sturk A, Eijsman L. Low-dose and high-dose aprotinin improve hemostasis in coronary operations. J Thorac Cardiovasc Surg. 1996;112:523–530. doi: 10.1016/S0022-5223(96)70281-7. [DOI] [PubMed] [Google Scholar]
- 128.Vassalli JD, Sappino AP, Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991;88:1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Attar S, Katsaros D. Tranexamic acid reduces postbypass blood use: A double-blinded, prospective, randomized study of 210 patients-Discussion. Ann Thorac Surg. 1996;61:1135–1135. doi: 10.1016/0003-4975(96)00022-7. [DOI] [PubMed] [Google Scholar]
- 130.Munoz JJ, Birkmeyer NJO, Birkmeyer JD, O’Connor GT, Dacey LJ. Is epsilon-aminocaproic acid as effective as aprotinin in reducing bleeding with cardiac surgery? A meta-analysis Circulation. 1999;99:81–89. doi: 10.1161/01.cir.99.1.81. [DOI] [PubMed] [Google Scholar]
- 131.Longstaff C. Studies on the mechanisms of action of aprotinin and tranexamic acid as plasmin inhibitors and antifibrinolytic agents. Blood Coagul Fibrinolysis. 1994;5:537–542. [PubMed] [Google Scholar]
- 132.Hoylaerts M, Lijnen HR, Collen D. Studies on the mechanism of the anti-fibrinolytic action of tranexamic acid. Biochim Biophys Acta. 1981;673:75–85. [PubMed] [Google Scholar]
- 133.Brockway WJ, Castelli Fj. Mechanism of inhibition of plasmin activity by epsilon-aminocaproic acid. J Biol Chem. 1971;246:4641. [Google Scholar]
- 134.Henry DA, Moxey AJ, Carless PA, O’Connell D, McClelland B, Henderson KM, Sly K, Laupacis A, Fergusson D. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2001:CD00 1886. doi: 10.1002/14651858.CD001886. [DOI] [PubMed] [Google Scholar]
- 135.Levy JH. Aprotinin is useful as a hemostatic agent in cardiopulmonary surgery: Yes. J Thromb Haemost. 2006;4:1875–1878. doi: 10.1111/j.1538-7836.2006.02107.x. [DOI] [PubMed] [Google Scholar]
- 136.Alving BM, Reid TJ, Fratantoni JC, Finlayson JS. Frozen platelets and platelet substitutes in transfusion medicine. Transfusion. 1997;37:866–876. doi: 10.1046/j.1537-2995.1997.37897424413.x. [DOI] [PubMed] [Google Scholar]
- 137.Graham SS, Gonchoroff NJ, Miller JL. Infusible platelet membranes retain partial functionality of the platelet GPIb/IX/V receptor complex. Am J Clin Pathol. 2001;115:144–147. doi: 10.1309/CCDV-3BEP-XXKP-BKDM. [DOI] [PubMed] [Google Scholar]
- 138.Hawksworth JS, Elster EA, Fryer D, Sheppard F, Morthole V, Krishnamurthy G, Tomori T, Brown TS, Tadaki DK. Evaluation of lyophilized platelets as an infusible hemostatic agent in experimental non-compressible hemorrhage in swine. J Thromb Haemost. 2009;7:1663–1671. doi: 10.1111/j.1538-7836.2009.03562.x. [DOI] [PubMed] [Google Scholar]
- 139.Bertolini F, Murphy S. A multicenter inspection of the swirling phenomenon in platelet concentrates prepared in routine practice. Biomedical Excellence for Safer Transfusion (BEST) Working Party of the International Society of Blood Transfusion. Transfusion. 1996;36:128–132. doi: 10.1046/j.1537-2995.1996.36296181924.x. [DOI] [PubMed] [Google Scholar]
- 140.Barrett BB, Andersen JW, Anderson KC. Strategies for the avoidance of bacterial contamination of blood components. Transfusion. 1993;33:228–233. doi: 10.1046/j.1537-2995.1993.33393174449.x. [DOI] [PubMed] [Google Scholar]
- 141.Agam G, Livne AA. Erythrocytes with covalently bound fibrinogen as a cellular replacement for the treatment of thrombocytopenia. Eur J Clin Invest. 1992;22:105–112. doi: 10.1111/j.1365-2362.1992.tb01943.x. [DOI] [PubMed] [Google Scholar]
- 142.Coller BS, Springer KT, Beer JH, Mohandas N, Scudder LE, Norton KJ, West SM. Thromboerythrocytes. In vitro studies of a potential autologous, semi-artificial alternative to platelet transfusions. J Clin Invest. 1992;89:546–555. doi: 10.1172/JCI115619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Beer JH, Springer KT, Coller BS. Immobilized Arg-Gly-Asp (RGD) peptides of varying lengths as structural probes of the platelet glycoprotein IIb/IIIa receptor. Blood. 1992;79:117–128. [PubMed] [Google Scholar]
- 144.Levi M, Friederich PW, Middleton S, de Groot PG, Wu YP, Harris R, Biemond BJ, Heijnen HF, Levin J, ten Cate JW. Fibrinogen-coated albumin microcapsules reduce bleeding in severely thrombocytopenic rabbits. Nat Med. 1999;5:107–111. doi: 10.1038/4795. [DOI] [PubMed] [Google Scholar]
- 145.Takeoka S, Teramura Y, Okamura Y, Handa M, Ikeda Y, Tsuchida E. Fibrinogen-conjugated albumin polymers and their interaction with platelets under flow conditions. Biomacromolecules. 2001;2:1192–1197. doi: 10.1021/bm015554o. [DOI] [PubMed] [Google Scholar]
- 146.Okamura Y, Takeoka S, Teramura Y, Maruyama H, Tsuchida E, Handa M, Ikeda Y. Hemostatic effects of fibrinogen gamma-chain dodecapeptide-conjugated polymerized albumin particles in vitro and in vivo. Transfusion. 2005;45:1221–1228. doi: 10.1111/j.1537-2995.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 147.Rybak MEM, Renzulli LA. A liposome based platelet substitute, the plateletsome, with hemostatic efficacy. Biomater Artif Cells Immobilization Biotechnol. 1993;21:101–118. doi: 10.3109/10731199309117350. [DOI] [PubMed] [Google Scholar]
- 148.Huang G, Zhou Z, Srinivasan R, Penn MS, Kottke-Marchant K, Marchant RE, Gupta AS. Affinity manipulation of surface-conjugated RGD peptide to modulate binding of liposomes to activated platelets. Biomaterials. 2008;29:1676–1685. doi: 10.1016/j.biomaterials.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Okamura Y, Takeoka S, Eto K, Maekawa I, Fujie T, Maruyama H, Ikeda Y, Handa M. Development of fibrinogen gamma-chain peptide-coated, adenosine diphosphate-encapsulated liposomes as a synthetic platelet substitute. J Thromb Haemost. 2009;7:470–477. doi: 10.1111/j.1538-7836.2008.03269.x. [DOI] [PubMed] [Google Scholar]
- 150.Ravikumar M, Modery CL, Wong TL, Dzuricky M, Sen Gupta APD. Mimicking Adhesive Functionalities of Blood Platelets using Ligand-Decorated Liposomes. Bioconjug Chem. 2012;23:1266–1275. doi: 10.1021/bc300086d. [DOI] [PubMed] [Google Scholar]
- 151.Ravikumar M, Modery CL, Wong TL, Gupta AS. Peptide-decorated liposomes promote arrest and aggregation of activated platelets under flow on vascular injury relevant protein surfaces in vitro. Biomacromolecules. 2012;13:1495–1502. doi: 10.1021/bm300192t. [DOI] [PubMed] [Google Scholar]
- 152.Bertram JP, Williams CA, Robinson R, Segal SS, Flynn NT, Lavik EB. Intravenous hemostat: nanotechnology to halt bleeding. Sci Transl Med. 2009;1:11ra22. doi: 10.1126/scitranslmed.3000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shoffstall AJ, Atkins KT, Groynom RE, Varley ME, Everhart LM, Lashof-Sullivan MM, Martyn-Dow B, Butler RS, Ustin JS, Lavik EB. Intravenous hemostatic nanoparticles increase survival following blunt trauma injury. Biomacromolecules. 2012;13:3850–3857. doi: 10.1021/bm3013023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shoffstall AJ, Everhart LM, Varley ME, Soehnlen ES, Shick AM, Ustin JS, Lavik EB. Tuning ligand density on intravenous hemostatic nanoparticles dramatically increases survival following blunt trauma. Biomacromolecules. 2013;14:2790–2797. doi: 10.1021/bm400619v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Okamura Y, Fukui Y, Kabata K, Suzuki H, Handa M, Ikeda Y, Takeoka S. Novel platelet substitutes: disk-shaped biodegradable nanosheets and their enhanced effects on platelet aggregation. Bioconjug Chem. 2009;20:1958–1965. doi: 10.1021/bc900325w. [DOI] [PubMed] [Google Scholar]


