Abstract
Objective
To evaluate if COmbinatie therapie Bij Reumatoïde Artritis (COBRA)-light therapy is cost-effective in treating patients with early rheumatoid arthritis (RA) compared with COBRA therapy.
Methods
This economic evaluation was performed next to the open-label, randomised non-inferiority COBRA-light trial in 164 patients with early RA. Non-responders to COBRA or COBRA-light received etanercept (50 mg/week) for 3–6 months. The societal perspective analysis took medical direct, non-medical direct and indirect costs into account. Costs were measured with patient cost diaries for the follow-up period of 52 weeks. Bootstrapping techniques estimated uncertainty around the cost-effectiveness ratios, presented in cost-effectiveness planes.
Results
164 patients were randomised to either COBRA or COBRA-light strategy. At week 52, COBRA-light proved to be non-inferior to COBRA therapy on all clinical outcome measures. The results of the base-case cost-utility analysis (intention-to-treat analyses) revealed that COBRA-light strategy is more expensive (k€9.3 (SD 0.9) compared with COBRA (k€7.2 (SD 0.8)), but the difference in costs were not significant (k€2.0; 95% CI –0.3 to 4.4). Also, both strategies produced similar quality-adjusted life-years (QALYs). The sensitivity analyses showed robustness of these results. In a per-protocol sensitivity analysis, in which costs of etanercept were assumed to be provided as prescribed according to protocol, both arms had much higher costs: COBRA-light: k€11.5 (8.3) compared with k€8.5 (6.8) for COBRA, and the difference in costs was significant (k€2.9; 0.6 to 5.3).
Conclusions
In the base-case cost-utility analysis, the two strategies produced similar QALYs for similar costs. But it is anticipated that if protocol had been followed correctly, the COBRA-light strategy would have been more costly due to additional etanercept costs, for a limited health gain. Given the limited added benefit and high costs of starting etanercept in the presence of low disease activity in our trial, such a strategy needs better justification than is available now.
Trial registration number
55552928, Results.
Keywords: early rheumatoid arthritis, cost-utility, economic evaluation, etanercept, prednisolone
Key messages.
What is already known about this subject?
Limited information is available on cost-utility studies in which a biological therapy has been added afer 26 to 39 weeks of treatment with intensive combinations of non-biological DMARDs.
This study is the first to assess this in its current form.
What does this study add?
This study adds to the knowledge that adding a biological DMARD after a period of treatment with combination therapies with non-biological DMARDs including prednisolone, seems not cost-effective.
How might this impact on clinical practice?
Cost-effectiveness studies in trials performed using conventional disease-modifying antirheumatic drugs, including prednisolone, as main treatment option are limited. Especially, studies focussing on the cost-effectiveness of adding a biological to the treatment after 52weeks of treatment with intensive combination strategies have not yet been performed. This study provides new information on this matter.
For clinical practice, a better justification of starting a biological after intensive combination strategies, including prednisolone, has to become available as this study concludes that, given the limited added benefit and high costs of starting etanercept in the presence of low disease activity in our trial, such a strategy needs better justification than is available now.
Introduction
Rheumatoid arthritis (RA) is a disabling disease that leads to limitations in daily activities. Prompt treatment in the early stages of the disease has improved the possibility to control the disease activity and limit joint damage, positively influencing the quality of life of patients. Initial intensive treatment with conventional disease-modifying antirheumatic drugs (DMARDs) has a positive effect on long-term outcome. The COBRA strategy (COmbinatie therapie Bij Reumatoïde Artritis) is a strategy that has proven to be very effective in early RA. It comprises a combination of low-dose methotrexate (7.5 mg/day) and sulfasalazine (2 g/day) and initial high-dose prednisolone (60 mg/day, tapered to 7.5 mg/day). COBRA strategy has also been found to be as effective as the tumour necrosis factor (TNF)-alpha agent infliximab.1 2 It is also a cost-effective therapy when compared with sulfasalazine monotherapy, due to identical or lower costs and better effectiveness.3
Recently, the COBRA-light trial was performed as a treat-to-target study, which showed that the COBRA-light strategy is non-inferior to the COBRA strategy. The COBRA-light strategy comprises high-dose methotrexate (25 mg/day), combined with medium-dose prednisolone (30 mg/day, tapered to 7.5 mg/day).4 5 In this trial, etanercept was added at week 26 or 39, if patients still had a disease activity score of 44 joints (DAS44) of 1.6 or higher at these time points. Several studies have shown that etanercept is an effective anti-TNF therapy in reducing disease activity and slowing down joint progression.6–10 However, these drugs are costly, which strongly drives the total direct medical costs of RA, currently estimated at €3.2 billion per year in the Netherlands. Medication, aids and devices count up to 20% of these total direct medical costs.11 Although COBRA-light has shown to be non-inferior to COBRA therapy, more patients in the COBRA-light arm needed etanercept due to remaining high disease activity scores. But patients who received etanercept appeared to have limited added benefit, as the disease activity improved marginally. This study compared the cost-utility of COBRA-light strategy in patients with early RA with COBRA therapy.
Patients and methods
This cost-utility analysis was performed alongside the randomised controlled COBRA-light trial to assess non-inferiority of the COBRA-light strategy compared with COBRA strategy in patients with early RA in three medical centres in the Netherlands. Treatment goal of this trial was to reach minimal disease activity (at that time defined as clinical remission: DAS44 <1.6). Intensification of the treatment with methotrexate and/or addition of etanercept (50 mg/week) was protocolised up to 52 weeks, in cases where minimal disease activity was not reached at week 26 or 39. Patient eligibility criteria, randomisation process and study design have been reported previously.4
Study population
This study was a multicentre, randomised, open-label trial (www.controlled–trials.com; ISRCTN55552928). In total, 246 patients with recently diagnosed RA were eligible during March 2008 to April 2011. Eventually, 164 DMARD-naïve patients fulfilled the patient selection criteria,4 and were included in the COBRA-light trial. Patients who were not included either did not meet the medical eligibility criteria (n=29); found the study to intense to participate (n=21); had fear of treatment (n=17) or had other reasons not to participate (n=9). After randomisation, two patients withdrew their consent (both in the COBRA-light arm); their data were discarded for the analyses. It is unlikely that this has influenced the results. In total, 162 patients were treated with one of the treatment arms.
Outcome measures
Cost outcome
Costs and clinical outcome measures were collected at baseline and 3, 6, 9 and 12 months after baseline. Patients were asked to complete cost dairies every 3 months during 1 year. In these diaries, all actual costs due to RA were collected. Patients were asked to record resource use in detail to allow multiplication with unit prices. For direct medical costs, all costs related to visits to the rheumatologists, other specialists, the general practice, paramedic care, days of hospitalisation, medication use, outpatient and inpatient care and alternative therapies were recorded. Direct non-medical costs comprised all costs related to over-the-counter medication, costs of paid and unpaid household help and costs of transportation. To calculate indirect costs, all costs related to sick leave (absenteeism) were collected. Only paid work was included. In addition to the diaries, two other sources were consulted: (1) patient medical records to enrich the dataset on actual medication use and (2) the electronic hospital administration system for radiology costs.
Clinical outcome
Several clinical outcomes of this trial have been described earlier.4 5 In short, in both strategies a decrease of approximately 0.77 points on the Health Assessment Questionnaire (HAQ) was seen, and consistent patterns in single disease activity measures (such as general well-being assessment) over time were seen. Small differences were present in proportions of patients reaching European League against Rheumatism (EULAR) response and American College of Rheumatology (ACR)70 improvement: this did not favour one treatment group.5 Not yet published is the health-related quality of life (utility) of patients, as assessed with the EuroQol-5D (EQ-5D).12 The EQ-5D is a reliable and valid measurement instrument in patients with RA.13
Etanercept
Per protocol, if patients did not reach minimal disease activity (DAS44 <1.6) at week 26 or 39, they were to receive etanercept (50 mg/week) for either 13 or 26 weeks, depending on start at week 26 or 39. But, participating rheumatologists and patients often did not adhere to the protocol, that is, not prescribing etanercept in the face of low (but not minimal) disease activity.5 Significantly more patients in COBRA-light required intensification with etanercept per protocol: 61 patients vs 47 for COBRA. Due to protocol violations, only 40 out of 61 patients in COBRA-light (mean DAS44 at start 2.45 (SD 0.87)) actually received etanercept, and 27 out of 47 patients in the COBRA group (mean DAS44 at start 2.36 (0.65)). Patients who actually received etanercept had limited added benefit as only an improvement in DAS of 0.2 points was seen.5
Economic evaluation
The economic evaluation was performed from a societal perspective: all direct medical, direct non-medical and indirect costs were combined. Prices of 2009 were used, the year most patients were included. Where necessary prices were updated with the Dutch consumer price index (www.cbs.nl). Standard prices for most resources were used (Table 1).14 Because this study had a short follow-up period (1 year), no discounting was used. Prices of medication were obtained through a national website.15 Cost for laboratory tests were obtained through the Dutch website for costs of care,16 and costs for radiology were computed by the Department of Planning and Control of the VU University Medical Center.
Table 1.
Results of the base-case cost-utility analysis, the analysis on complete cases and the different sensitivity analyses
| Costs | Difference in costs | QALYs | Difference in QALYs | |||||||||
| COBRA-light | COBRA | COBRA-light | COBRA | |||||||||
| Main findings | Mean k€ | (SEM) | Mean k€ | (SEM) | k€ | (95% CI) | Mean | (SEM) | Mean | (SEM) | Mean | (95% CI) |
| Base case* | 9.293 | (0.920) | 7.222 | (0.780) | 1.980 | (−0.347 to 4.390) | 0.68 | (0.02) | 0.69 | (0.02) | −0.01 | (−0.06 to 0.04) |
| Analyses on complete cases† | 9.886 | (8.866) | 6.864 | (1.072) | 3.022 | (−0.764 to 6.682) | 0.69 | (0.02) | 0.70 | (0.02) | −0.01 | (−0.08 to 0.06) |
| Sensitivity analyses | ||||||||||||
| 1. Base case with correction for baseline factors* | 9.293 | (0.920) | 7.222 | (0.780) | 1.836 | (−0.347 to 4.189) | 0.68 | (0.02) | 0.69 | (0.02) | −0.01 | (−0.06 to 0.04) |
| 2. Analysis on patients with four or more returned diaries (per-protocol imputation)‡ | 9.897 | (1.139) | 7.343 | (0.910) | 2.554 | (−0.567 to 5.676) | 0.69 | (0.02) | 0.70 | (0.02) | −0.01 | (−0.06 to 0.04) |
| 3. Base case with etanercept use according to protocol* | 11.493 | (8.313) | 8.499 | (6.805) | 2.925 | (0.564 to 5.335) | 0.68 | (0.02) | 0.69 | (0.02) | −0.006 | (−0.06 to 0.04) |
*COBRA-light: n=79 and COBRA: n=77.
†COBRA-light: n=37 and COBRA: n=40.
‡COBRA-light: n=61 and COBRA: n=63.
COBRA, COmbinatie therapie Bij Reumatoïde Artritis; QALY, quality-adjusted life-years.
The friction cost method was used to value sick leave (absenteeism) from paid work: only sick leave during a friction period (23 weeks) needed to replace a person is taken into account.17 18 Assuming a friction period of 23 weeks, which equals 0.4423 years, and a total number of working hours per year of (when excluding holidays), at most 0.4423×1540=681.2 working hours may be accounted as costs of productivity loss per person. Sex-dependent and age-dependent wages were used to value lost productivity. The shadow price of informal care (eg, care by family) was assumed to be equal to the tariff for cleaning work.
Statistical analyses
All patients who received at least one medication dose were included in the modified intention-to-treat protocol (ITT), and using actual costs as mentioned by patients in the patient diary, as well as the medical record and the electronic hospital administration. For the analyses, all paramedical therapies such as physiotherapy and occupational therapy were combined into one result. The same procedure was applied to types of help in the housekeeping (eg, paid, help from families).
Missing data were imputed as total costs or utility score per time point per treatment arm separately with multiple imputation (predictive mean matching) by chained equations. Linear and logistic regression analyses were performed to investigate which variables (ie, clinical data) were associated with missingness, with observed costs (total costs at each time point, gender, CCP positivity) or utility scores (ie, EULAR good responder). Variables found to be associated with missingness, with observed costs or utility scores were included in the multiple imputation model. Ten imputed data sets were created which were all analysed separately. Results of the 10 analyses were pooled using Rubin’s rules.19
For the main findings, at first a base-case cost-utility analysis was performed including all patients included in the modified ITT protocol. Second, analysis on complete cases were performed to provide information on type of costs; their relative importance (ie, its contribution to the mean total costs per group), and the mean utility scores per group. The complete case analysis comprised those patients who completed all cost diaries until week 52.
In order to perform incremental cost-utility analyses, the cumulative costs and number of QALYs per patient per treatment group were calculated. For all patients cumulative costs between baseline and week 52 were calculated by summing all costs at every visit. The number of QALYs per patient was calculated by multiplying the EQ-5D utility score by the appropriate time period, using linear interpolation between measurement times.
Bias-corrected accelerated bootstrapping techniques were used to estimate CIs for costs, given their skewed distribution. With the bootstrapping technique, 1000 datasets of the same sample size of the original dataset were sampled with replacement from the original data.20 21 All analyses were performed with the IBM Statistical Package for the Social Science V.20 and STATA V.12.1.
Sensitivity analyses
To assess the robustness of the main findings, several additional analyses were performed: (1) an analysis in which the base-case analysis was adjusted using multivariate regression analyses for total costs at baseline; (2) an analysis on patients who returned four or more diaries in which missing data were imputed with per-protocol costs. The per-protocol costs are the sum of the mean costs of a visit to a nurse and rheumatologist, medication use according to protocol, laboratory diagnostics and radiographs and DEXA scans every 6 months and (3) an analysis in which costs of etanercept were assumed to be as prescribed per protocol.
Cost-utility
The uncertainty around costs and effects were assessed by bootstrapping the 10 imputed datasets in STATA with 5000 replications. The results were projected on a cost-utility plane. In a cost-utility plane, the cost difference between the intervention and control group is presented on the y-axis, while the difference in QALYs is presented on the x-axis, resulting in four different quadrants. When COBRA-light is more effective but at additional costs (north-east quadrant), a trade-off has to be made between gained QALYs and additional costs (ie, do gained QALYs justify the additional costs). A cost-utility acceptability curve was therefore plotted, which presents the probability that COBRA-light is cost-effective compared with COBRA for different willingness to pay values for an additional QALY.
Incremental cost-effectiveness ratios were not calculated due to small differences in health effects between both strategies.
Results
In total, 162 patients were included in this trial and treated according to the modified ITT protocol with either COBRA-light (intervention) or COBRA strategy (control). During the 52 weeks of treatment, seven patients dropped out due to serious adverse events: four in COBRA-light and three in COBRA. COBRA-light as well as COBRA therapy both showed major improvements in DAS44, HAQ and VAS scores (Table 2). Analyses showed that COBRA-light is non-inferior to COBRA therapy. No difference in improvement of the EQ-5D score between baseline and week 52 was found between both groups (2.5 points; 95% CI −5.3 to 10.4).
Table 2.
Demographic and disease activity measures at baseline and at 52 weeks
| COBRA-light (n=81) |
COBRA (n=81) |
p Value | |
| Demographics at baseline | |||
| Female, n (%) | 58 (69) | 54 (66) | 0.66 |
| Age, years | 51 (12) | 53 (13) | 0.44 |
| Disease duration, months | 24 (22) | 21 (17) | 0.36 |
| EQ-5D | 54.2 (20.0) | 53.8 (20.0) | 0.92 |
| HAQ | 1.4 (0.7) | 1.4 (0.7) | 0.87 |
| Patient global assessment, by VAS, mm | 58.3 (25.3) | 60.5 (21.9) | 0.55 |
| Disease activity and response at week 52 | |||
| EQ-5D | 71.9 (19.3) | 74.8 (15.0) | 0.31 |
| HAQ | 0.61 (0.6) | 0.57 (0.5) | 0.71 |
| Patient global assessment, by VAS, mm | 28.8 (26.2) | 31.2 (26.2) | 0.57 |
| ACR/Boolean remission | 14 (17) | 12 (15) | 0.72 |
| EULAR response, n (%) | |||
| Non-responders | 5 (6) | 5 (6) | 0.97 |
| Good responders | 49 (60) | 56 (69) | 0.18 |
| ACR response, n (%) | |||
| ACR non-responders | 20 (25) | 19 (23) | 0.89 |
| ACR70 | 28 (35) | 25 (31) | 0.65 |
Data are expressed as mean (SD) unless otherwise stated.
ACR, American College of Rheumatology; anti-CCP, anticyclic citrullinated peptide; COBRA, COmbinatie therapie Bij Reumatoïde Artritis; EULAR, European League against Rheumatism; EQ-5D, EuroQol 5 Dimensions; HAQ, Health Assessment Questionnaire; VAS, visual analogue scale.
In total, 87 patients (60%) returned all five cost diaries. The number of patients who returned two, three or four diaries were 5 (3%), 17 (11%) and 41 (25%), respectively. Twelve patients (7%) returned one or zero diaries and were excluded from all analyses. Of these 12 patients, 9 received COBRA therapy; they resembled the other patients on clinical outcome.
Direct and indirect costs (main findings)
The results of the base-case analysis revealed that COBRA-light strategy is more expensive than COBRA (k€9.3 (SD 0.9) compared with k€7.2 (SD 0.8)), but the difference in costs was not significant (k€2.0, 95% CI −0.3 to 4.4; Table 1).
Second, analysis on those patients who completed all cost diaries until week 52 (complete cases) were performed. Based on QALY as outcome measure, total costs were higher for COBRA-light compared with COBRA as can be seen in Table 1 (cost difference: k€3.0, 95% CI −0.8 to 6.7), but this difference was not statistically significant. The differences in costs are mainly driven by higher direct non-medical costs after 1 year of treatment (k€1.0 (SD 1.8) for COBRA-light and k€0.3 (SD 0.6) for COBRA). Also, indirect costs were roughly 50% higher in the COBRA-light group compared with the COBRA group (non-significant): k€3.0 (SD 6.4) and k€1.6 (SD 3.9) for COBRA-light and COBRA, respectively. At baseline, indirect costs account for 45.6% for COBRA-light strategy and 51.4% for COBRA strategy. After 1 year of treatment, the indirect costs decreased to 31.3% and 22.7% for COBRA-light and COBRA strategy, respectively.
Analyses in patients with data on the other effect measures, showed similar trends, but did result in significantly differences in costs between COBRA-light and COBRA. For example, in patients with data on ACR70 response and HAQ, results were k€3.0 (95% CI 0.5 to 5.3) and k€2.9 (95% CI 0.6 to 5.3), respectively, in favour of COBRA (data not shown). The effect differences on almost all outcome measures were close to 0, and all did not significantly differ (data not shown).
Sensitivity analyses
Results of the sensitivity analysis with correction for baseline costs and the analysis in the group of patients who returned four or more diaries were similar to the base-case analysis, although differences in costs and effects were somewhat lower (Table 1). Differences in costs and effects remained non-significant.
As can be seen in Table 3, based on actual costs as reported by the patients, costs for etanercept were lower in the COBRA-light group, despite higher number of patients needing intensification with etanercept compared with COBRA. Due to protocol violations, less patients actually received etanercept in the COBRA-light group. Therefore, for the last sensitivity analysis, cost of etanercept use was assumed to be as indicated in the protocol. In this analysis, the costs for COBRA-light were higher compared with COBRA: €11.493 (SD 8.313) vs €8.499 (SD 6.805), respectively. This difference in costs was significant: €2.925 (564 to 5.335).
Table 3.
Mean costs at baseline and over 52 weeks of treatment of complete cases (n=87)
| COBRA-light (n=44) | COBRA (n=43) | ||||||||
| Reference price per unit (€) | Baseline (€) | (SD) | 1 year period (€) | (SD) | Baseline (€) | (SD) | 1 year period (€) | (SD) | |
| Direct medical costs | 1073 | (986) | 5598 | (3450) | 918 | (581) | 5069 | (3853) | |
| General practitioner | 28 | 65 | (408) | 101 | (83) | 71 | (52) | 66 | (92) |
| Occupational health specialist | 23 | 9 | (25) | 32 | (70) | 3 | (11) | 28 | (67) |
| Paramedical therapies* | 22–65 | 70 | (168) | 415 | (775) | 100 | (193) | 257 | (465) |
| Alternative therapies | 45 | 20 | (85) | 36 | (121) | 6 | (39) | 7 | (25) |
| Specialist care† | 72; 136 | 339 | (266) | 908 | (631) | 238 | (200) | 710 | (487) |
| Polyclinic care (nurses)† | 64; 129 | 19 | (34) | 96 | (130) | 23 | (34) | 76 | (94) |
| Patient day care (day) | 251 | 11 | (76) | 6 | (38) | 0 | (0) | 0 | (0) |
| Hospital admissions* | 435; 575 | 131 | (867) | 340 | (1185) | 974 | (531) | 107 | (701) |
| Laboratory diagnostics | 8 | 382 | (141) | 251 | (129) | 361 | (124) | 256 | (77) |
| X-ray | 43 | 300 | (0) | 300 | (0) | ||||
| Dexa scan | 193 | 886 | (158) | 876 | (165) | ||||
| Other radiological diagnostics* | 108–280 | 69 | (0) | 69 | (0) | ||||
| DMARD use* | 0.13–0.39 | 2 | (6) | 98 | (93) | 4 | (9) | 130 | (112) |
| Etanercept use | 280.1 | 0 | (0) | 1979 | (2436) | 0 | (0) | 2091 | (3066) |
| Other drug use* | 0.02–0.28 | 26 | (45) | 79 | (67) | 19 | (31) | 95 | (66) |
| Direct non-medical costs | 333 | (916) | 1044 | (1800) | 68 | (148) | 343 | (608) | |
| Professional home care | 68 | 0 | (0) | 57 | (348) | 0 | (0) | 15 | (72) |
| Informal home care | ‡ | 0 | (0) | 180 | (722) | 0 | (0) | 29 | (193) |
| Housekeeping | 25 | 22 | (104) | 744 | (1554) | 19 | (84) | 209 | (471) |
| Aids | § | 23 | (86) | 34 | (132) | 1 | (6) | 60 | (283) |
| Reimbursement for swimming | § | 1 | (5) | 10 | (30) | 3 | (13) | 7 | (24) |
| Travel expenses | 0.19 per km | 6 | (18) | 20 | (43) | 8 | (26) | 23 | (60) |
| Total indirect costs¶ | ** | 1177 | (2947) | 3025 | (6393) | 1042 | (2805) | 1591 | (3922) |
| Total costs | 2583 | (3543) | 9667 | (8234) | 2028 | (2918) | 7003 | (6749) | |
*Dependent on type of assessment or drug.
†Dependent whether the hospital is academic or private.
‡Shadow price, being equal to the hour price for professional home care.
§Average cost per hour according to the friction cost method. In the analyses, sex-dependent and age-dependent costs are used.
¶Based on absenteeism of paid labour.
**Prices were provided by patients.
COBRA, COmbinatie therapie Bij Reumatoïde Artritis; DEXA, dual-energy X-ray absorptiometry.
Cost-effectiveness analyses
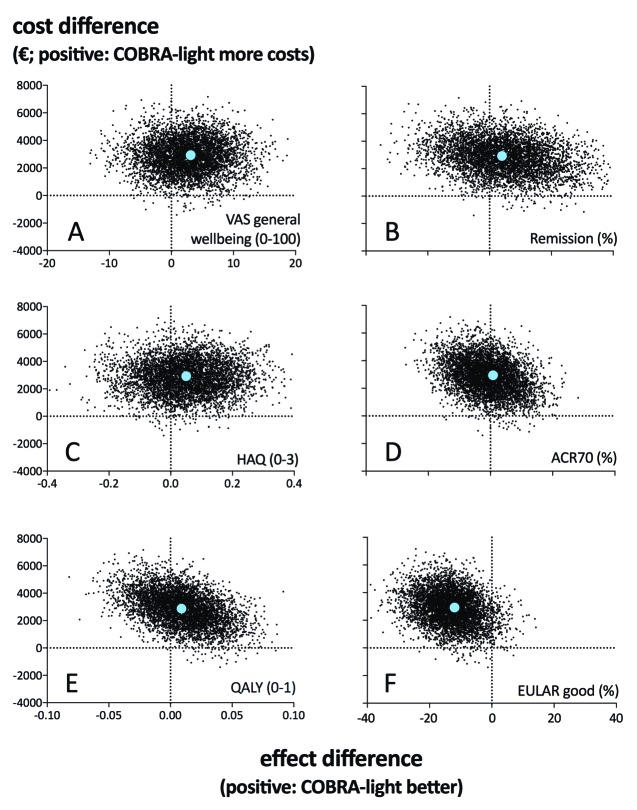
For the base-case analysis, figure 1 shows the cost-effectiveness planes of COBRA-light strategy of the base-case analyses depicting the difference in costs against the difference in health effect for the different health measures. With respect to the outcome measure QALY (figure 1e), in total 59% of the bootstrapped cost-utility pairs fell in the northwest quadrant, representing the probability that COBRA-light is more expensive and less effective compared with COBRA. Also 40% of the pairs fell in the northeast quadrant representing the probability that COBRA-light is more expensive but more effective compared with COBRA. For analyses with patient global assessment (figure 1a), ACR/Boolean remission (figure 1b), HAQ (figure 1c) and ACR70 response (figure 1d) as outcome measures, percentage of cost-utility pairs fell mainly in the northeast quadrants (75%, 53%, 69% and 63%, respectively). Strikingly, nearly all cost-utility pairs for EULAR good response fell in the northwest quadrant as only 6% ended up in the northeast quadrant (figure 1f). Note that for the dichotomous outcome variables, the difference in health effect corresponds to a difference in the percentage of patients with a score of 1 (ie, the difference in the percentage of patients reaching ACR70).
Figure 1.
Cost-effectiveness planes of the base-case analyses. ACR, American College of Rheumatology; COBRA, COmbinatie therapie Bij Reumatoïde Artritis; EULAR, European League against Rheumatism; HAQ, Health Assessment Questionnaire; QALY, quality-adjusted life-years; remission, Boolean remission; VAS, visual analogue scale.
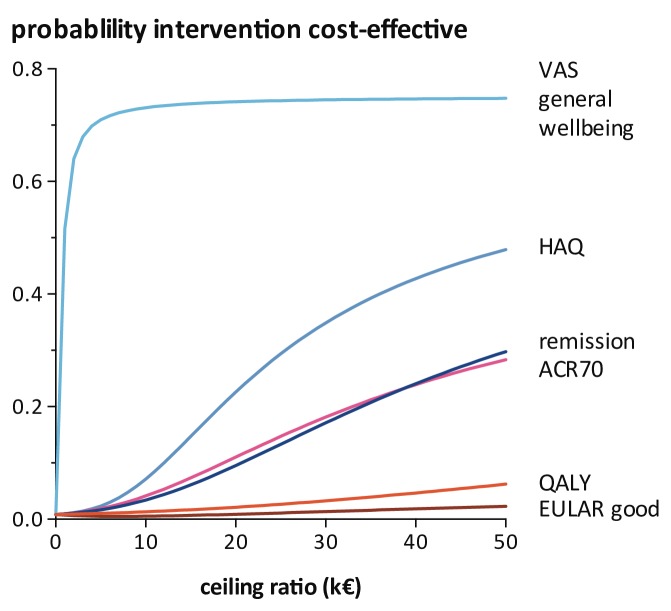
The cost-utility acceptability curves (figure 2) show that COBRA-light has a probability of close to zero to be cost-effective compared with COBRA at a willingness to pay value of €0, per QALY. Moreover, even at a willingness to pay of €50 000, per QALY, the probability that COBRA-light is cost-effective compared with COBRA does not exceed 10%. When looking at the other effect measures, the cost-effectiveness results are more favourable for the COBRA-light strategy. For example, with VAS general well-being as outcome, the probability that COBRA-light is cost-effective compared with COBRA is approximately 70% at a willingness to pay value of k€5.0.
Figure 2.
Cost-utility acceptability curves of the base-case analyses. The (cost-effectivenes acceptability curves) CEAC lines are all for COmbinatie therapie Bij Reumatoïde Artritis (COBRA)-light strategy compared with COBRA strategy. VAS general well-being, HAQ and QALY are continuous outcome. Remission, ACR70 and EULAR good responder are binary outcome. ACR, American College of Rheumatology; EULAR, European League against Rheumatism; HAQ, Health Assessment Questionnaire; QALY, quality-adjusted life-years; remission, Boolean remission; VAS, visual analogue scale.
Etanercept
The cost-effectiveness analyses were also performed on the sensitivity analyses with etanercept use according to protocol. Based on the etanercept costs, nearly all cost-utility pairs of the COBRA-light strategy fell in the northeast quadrants for all outcome measures except for ACR70 response and EULAR good responder (see online supplementary appendix 1). In other words, COBRA-light is more effective, and more expensive when compared with COBRA strategy. With ACR70, costs were higher for COBRA-light with comparable effectiveness between both strategies. And with EULAR good response, COBRA-light is more expensive and less effective than COBRA strategy. The cost-utility acceptability curves (see online supplementary appendix 2) show the same results as for the base-case analyses (figure 2).
rmdopen-2017-000502supp001.jpg (373.2KB, jpg)
rmdopen-2017-000502supp002.jpg (172.5KB, jpg)
Discussion
The aim of this study was to assess the cost-effectiveness of COBRA-light compared with COBRA strategy in the treatment of patients with early onset RA. Based on the ITT analyses, no significant differences in effects and costs were found between COBRA-light and COBRA strategies.
Cost-effectiveness studies in trials performed using conventional DMARDs as main treatment option are limited.22–24 In the BehandelStrategieën bij reumatoïde artritis (BeSt) study, COBRA therapy was compared with three other arms: (1) sequential monotherapy with methotrexate and thereafter monotherapy with other DMARDs, (2) step-up combination therapy and (3) methotrexate with infliximab.24 For the economic evaluation, the COBRA strategy (n=78) was compared with initial sulfasalazine monotherapy (n=78). Mean total costs per patient per year were k€9.2 (calculated from US dollars; exchange rate of 1:0.904 on 9 June 2016) for COBRA and k€11.6 for the sulfasalazine monotherapy group. In our study, costs for COBRA therapy were much lower (k€7.0) and mainly driven by total direct medical costs (k€5.1 compared with k€5.0 in the BeSt trial), and much lower productivity costs (k€1.6 compared with k€4.3 in the BeSt trial).
The SWEFOT (Swedish Pharmacotherapy) trial found that addition of infliximab after failure on 4 months of methotrexate monotherapy treatment resulted in similar health effects but higher costs, when compared with the arm receiving sulfasalazine and hydroxychloroquine (intensive combination treatment).22 This is similar to our trial, but patients in the SWEFOT trial did not receive prednisolone and infliximab was added after 4 months of monotherapy, making comparison with our results difficult.
In the recently published Treatment of Early Aggressive Rheumatoid Arthritis (TEAR) study, four arms were compared: initial triple therapy, initial etanercept, step-up triple therapy and step-up etanercept.23 The triple therapy consisted of methotrexate (20 mg/week), sulfasalazine (1000 g/day after 6 weeks) and hydrochloroquine (400 mg/day). All arms had similar clinical effects after 1 year therapy. The triple therapy was the optimal strategy, as it was most efficacious and least expensive. Costs of the triple therapy were k€9.6 per patient per year. In the step-up etanercept group, patients received etanercept at week 24 if DAS28-ESR ≥3.2, which is similar to our trial. Costs for this arm were k€16.1, which is much higher than the total costs of COBRA-light therapy in our trial. Therapy and patient inclusion criteria differed from our study, making comparison with our results difficult.
In our study, indirect costs accounted for approximately 50% of the total costs at baseline for both strategies, which decreased after 1 year of treatment to 31% and 23% for COBRA-light and COBRA strategy, respectively, based on the complete case analyses. If the indirect costs would be discarded in the analyses, the total costs for COBRA-light would be k€6.6 and k€5.4 for COBRA (Table 3). The cost difference would then be approximately k€1.2, strengthening our conclusion that both strategies produced similar costs.
Our study has strengths and limitations. A first limitation is the fact that cost diaries are a useful tool to collect hard-to-observe data such as cost data.25 However, we noticed that patients frequently forget to track all expenses in the diary and filled out the diary at the end of each 3 months period, possibly leading to recall bias and most likely an underestimation of the true costs. Furthermore, selection bias can occur with diaries, as it is a demanding and skilled activity.25 Therefore, patients who find it difficult to answer all questions may stop completing the diaries, leaving only diaries from ‘willing and able’ patients. We think this did not have a major influence, as results were similar in patients who filled out less diaries.
Another limitation of this study is that we needed to impute missing data on the quality of life scores (EQ-5D) and resource use. This was done using multiple imputation techniques. Rather than imputing individual cost items, total costs per visit and utility scores per visit were imputed for both arms separately. Although this method is generally considered state-of-the-art in economic evaluations, data missingness generally weakens the analysis. Nevertheless, the amount of missingness was similar in both trial arms, and as such we think the data imputation did not influence the comparison between groups.
The main limitation is the fact that actual costs on etanercept use did not properly represent the costs if the protocol had been followed as intended, as shown in the sensitivity analysis. Given the large costs of etanercept, this is a main driver of the analysis. Also, the small sample size of 162 patients, especially with a complete case analysis of only 87 patients provides uncertainty of our findings. To assess the impact of uncertainty, we carried out several sensitivity analyses, with generally robust results, except for the data with etanercept as provided according to protocol. In these last analyses, costs for etanercept use were calculated based on the assumption that all patients who needed intensification with etanercept, had actually received it (61 patients in COBRA-light and 47 in COBRA). The per-protocol sensitivity analyses showed that the costs raised in both strategies, and cost differences between both strategy became significant, in favour of COBRA strategy. We think that if protocol had been followed, it is unlikely that the extra costs of etanercept would have been justified by the improvement in patient health as the benefit of etanercept was low (0.2 DAS points) in patients who actually received it. It would be interesting to perform a study to assess the cost-utility of biologicals after intensive combination strategies as main research question. For example, a trial in which non-responder patients on COBRA-light treatment, would be randomised to receive a biological or a placebo for the following 6 months. Based on the social perspective, a cost-effectiveness analyses could be performed. But, based on the high prices and limited effect, we do think that the cost-effectiveness of adding a biologicals after intensive treatment with combination strategies might be limited. This might change in the era of biosimilars.
Conclusion
Although COBRA-light strategy produces fewer QALYs and was more expensive than COBRA strategy in observed practice, there was no statistical difference found between both therapies. However, it is possible that when the original DAS44-driven treat-to-target protocol had been fully followed, the larger number of etanercept users in the COBRA-light arm would have made this arm significantly more expen’sive than the COBRA arm, and, probably also does not lead to large gain in QALYs. Given the modest efficacy and high costs of starting etanercept in the presence of low disease activity in our trial, such a strategy needs better justification than is available now.
Acknowledgments
The authors would like to thank all patients, as well as all doctors who enrolled patients in this study, and all research nurses who were involved in patient management.
Footnotes
Contributors: All authors have substantially contributed to the conception or design of the work; the acquisition, analysis and interpretation of data for the work. The authors revised the article critically and approved the final version to be published. All agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This research was performed within the framework of project T1–106 of the Dutch Top Institute group, and additionally funded by an unrestricted grant from Pfizer.
Competing interests: WFL has received speaker’s fee from Merck, AbbVie, Roche and Pfizer. MSN has received research grants from AbbVie, BMS, MSD, Pfizer, UCB and Roche. He has also acted as a consultant for AbbVie, BMS, Pfizer and Roche. Furthermore, he has participated in speakers bureau for AbbVie, BMS, Pfizer and Roche. All other authors have nothing to declare.
Patient consent: Obtained.
Ethics approval: Medical Ethics Committees at each participating centre approved the protocol; patients gave written informed consent before inclusion, and the study was conducted in accordance with the Declaration of Helsinki/Good Clinical Practice.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Boers M, Verhoeven AC, Markusse HM, et al. . Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet 1997;350:309–18. 10.1016/S0140-6736(97)01300-7 [DOI] [PubMed] [Google Scholar]
- 2.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, et al. . Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381–90. 10.1002/art.21405 [DOI] [PubMed] [Google Scholar]
- 3.Verhoeven AC, Bibo JC, Boers M, et al. . Cost-effectiveness and cost-utility of combination therapy in early rheumatoid arthritis: randomized comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone. Cobra trial group. Combinatietherapie bij reumatoide artritis. Br J Rheumatol 1998;37:1102–9. 10.1093/rheumatology/37.10.1102 [DOI] [PubMed] [Google Scholar]
- 4.den Uyl D, ter Wee M, Boers M, et al. . A non-inferiority trial of an attenuated combination strategy (‘COBRA-light’) compared to the original COBRA strategy: clinical results after 26 weeks. Ann Rheum Dis 2014;73:1071–8. 10.1136/annrheumdis-2012-202818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ter Wee MM, den Uyl D, Boers M, et al. . Intensive combination treatment regimens, including prednisolone, are effective in treating patients with early rheumatoid arthritis regardless of additional etanercept: 1-year results of the COBRA-light open-label, randomised, non-inferiority trial. Ann Rheum Dis 2015;74:1233–40. 10.1136/annrheumdis-2013-205143 [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner SW, Fleischmann RM, Moreland LW, et al. . Etanercept (Enbrel) in patients with rheumatoid arthritis with recent onset versus established disease: improvement in disability. J Rheumatol 2004;31:1532–7. [PubMed] [Google Scholar]
- 7.Fleischmann RM, Baumgartner SW, Tindall EA, et al. . Response to etanercept (Enbrel) in elderly patients with rheumatoid arthritis: a retrospective analysis of clinical trial results. J Rheumatol 2003;30:691–6. [PubMed] [Google Scholar]
- 8.Geborek P, Crnkic M, Petersson IF, et al. . Etanercept, infliximab, and leflunomide in established rheumatoid arthritis: clinical experience using a structured follow up programme in southern Sweden. Ann Rheum Dis 2002;61:793–8. 10.1136/ard.61.9.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreland LW, Schiff MH, Baumgartner SW, et al. . Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med 1999;130:478–86. [DOI] [PubMed] [Google Scholar]
- 10.Moreland LW, Cohen SB, Baumgartner SW, et al. . Long-term safety and efficacy of etanercept in patients with rheumatoid arthritis. J Rheumatol 2001;28:1238–44. [PubMed] [Google Scholar]
- 11.van den Akker-van Marle ME, Chorus AM, Vliet Vlieland TP, et al. . Cost of rheumatic disorders in The Netherlands. Best Pract Res Clin Rheumatol 2012;26:721–31. 10.1016/j.berh.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 12.Lamers LM, Stalmeier PF, McDonnell J, et al. . [Measuring the quality of life in economic evaluations: the Dutch EQ-5D tariff]. Ned Tijdschr Geneeskd 2005;149:1574–8. [PubMed] [Google Scholar]
- 13.Linde L, Sørensen J, Ostergaard M, et al. . Health-related quality of life: validity, reliability, and responsiveness of SF-36, 15D, EQ-5D [corrected] RAQoL, and HAQ in patients with rheumatoid arthritis. J Rheumatol 2008;35:1528–37. [PubMed] [Google Scholar]
- 14.Tan SS, Bouwmans CA, Rutten FF, et al. . Update of the Dutch manual for costing in economic evaluations. Int J Technol Assess Health Care 2012;28:152–8. 10.1017/S0266462312000062 [DOI] [PubMed] [Google Scholar]
- 15.Medicijnkosten Themasite van het College voor zorgverzekeringen (CVZ). Medication costs. www.medicijnkosten.nl (accessed 26 Jun 2012).
- 16.Nederlanse Zorgautoriteit (NZa). http://dbc-zorgproducten-tarieven.nza.nl/nzaZpTarief/Welkom.aspx (accessed 6 Jun 2012).
- 17.Koopmanschap MA, Rutten FF. A practical guide for calculating indirect costs of disease. Pharmacoeconomics 1996;10:460–6. 10.2165/00019053-199610050-00003 [DOI] [PubMed] [Google Scholar]
- 18.Oostenbrink JB, Koopmanschap MA, Rutten FF. Standardisation of costs: the dutch manual for costing in economic evaluations. Pharmacoeconomics 2002;20:443–54. [DOI] [PubMed] [Google Scholar]
- 19.Rubin DB. Multiple imputation for non response in surveys. New York: Wiley, 1987. [Google Scholar]
- 20.Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219–36. [DOI] [PubMed] [Google Scholar]
- 21.Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ 2000;320:1197–200. 10.1136/bmj.320.7243.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eriksson JK, Karlsson JA, Bratt J, et al. . Cost-effectiveness of infliximab versus conventional combination treatment in methotrexate-refractory early rheumatoid arthritis: 2-year results of the register-enriched randomised controlled SWEFOT trial. Ann Rheum Dis 2015;74:1094–101. 10.1136/annrheumdis-2013-205060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jalal H, O’Dell JR, Bridges SL, et al. . Cost-effectiveness of triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis. Arthritis Care Res 2016;68:1751–7. 10.1002/acr.22895 [DOI] [PubMed] [Google Scholar]
- 24.Korthals-de Bos I, Van Tulder M, Boers M, et al. . Indirect and total costs of early rheumatoid arthritis: a randomized comparison of combined step-down prednisolone, methotrexate, and sulfasalazine with sulfasalazine alone. J Rheumatol 2004;31:1709–16. [PubMed] [Google Scholar]
- 25.Alaszewski A. Conclusion: exploiting the potential of research diaries Using diaries for social research. 1st edn London: SAGE Publications LTD, 2006:112–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2017-000502supp001.jpg (373.2KB, jpg)
rmdopen-2017-000502supp002.jpg (172.5KB, jpg)