Abstract
Iron deficiency (ID) is the most prevalent single-nutrient deficiency worldwide. There is evidence that ID early in development (preweaning in rat) causes irreversible neurologic, behavioral, and motor development deficits. Many of these effects have been attributed to damage to dopamine systems, including ID-induced changes in transporter and receptor numbers in the striatum and nucleus accumbens. These mesolimbic dopaminergic neurons are, in part, responsible for mediating reward and thus play a key role in addiction. However, there has been relatively little investigation into the behavioral effects of ID on drug addiction. In 2002, we found that rats made ID from weaning (post natal day 21) and throughout the experiment acquired cocaine self-administration significantly more slowly than controls and failed to increase responding when the dose of the drug was decreased. In the present study, we assessed addiction for self-administered cocaine in rats with a history of preweaning ID only during post natal days 4 through 21, and iron replete thereafter. The results showed that while ID did not affect the number of cocaine infusions or the overall addiction-like behavior score, ID rats scored higher on a measure of continued responding for drug than did iron replete controls. This increase in responding, however, was less goal-directed as ID rats also responded more quickly to the non-rewarded manipulandum than did control rats. Thus, while ID early in infancy did not significantly increase addiction-like behaviors for cocaine in this small study, the pattern of data suggests a possible underlying learning or performance impairment. Future studies will be needed to elucidate the exact neuro-behavioral deficits that lead to the increase in indiscriminate responding for drug in rats with a history of perinatal ID.
Keywords: Iron deficiency, Rat, Cocaine, Self-administration, Addiction, Learning
1. Introduction
Iron deficiency (ID) is reported to be the most prevalent single-nutrient deficiency worldwide. As many as two billion people are estimated to be anemic[1] and roughly 50% of anemia is likely the result of dietary ID [2]. There is increasing evidence that ID causes many harmful and probably irreversible neurologic, behavioral, and motor development effects in the fetus, neonates, and infants [3, 4],[5],[6]. Some of these effects of ID have been attributed to damage to dopamine (DA) systems [7]. Moreover, while there is a certain period of time during development when the ID-induced damage to the DA system in the nucleus accumbens (NAc) is reversible [8, 9], gestational ID can result in permanent changes in DA function even when subjects are later iron replete. Thus, there may be a critical window of time, postnatal day 4 through 21 in rats (PND4-21), during which ID causes persistent damage to the DA system in the striatum, including increased dopamine transporters (DAT) and D1 receptors (D1R) and decreased D2 receptors (D2R) [9].
Although there has been ample molecular testing of these ID rats, behavioral testing of the effects of this damage have been mainly limited to assessing locomotor activity, anxiety-like behavior, and hippocampus-mediated learning and memory. Additionally, the exact timing and duration of ID has been varied from study to study. In one, iron replete rats from dams on an ID diet during gestation only displayed locomotor activity, measured by telemetry, that was 25% lower than control rats [10]. Iron deficiency can affect anxiety as well. Eseh and Zimmerberg [11] found that perinatal ID (fetal and neonatal ID, but only through PND10) caused initial anxiety of neonates but was later ameliorated by iron repletion. In another study, Bourque, et. al. [12] found that gestational ID rats exhibited reduced open-field exploratory behavior suggesting greater anxiety, and increased path lengths to reach the hidden platform in a Morris water maze, a common test for learning and memory.
The mesolimbic dopamine system consists of dopamine-producing neurons originating in the ventral tegmental area (VTA) and projecting to the NAc, amygdala, hippocampus, and prefrontal cortex [13]. These pathways are involved in modulating behavioral responses to stimuli related to reward and reinforcement [14]. Dopamine levels within the NAc are elevated following exposure to any number of natural rewards or to drugs of abuse [15], [16], [17], [18], [19]. As such, changes in DA receptor and transporter densities in these areas caused by ID potentially could have profound effects on the functioning of the mesolimbic system and, in turn, on the behavioral response to drugs of abuse.
Previously we found that rats made ID following weaning failed to acquire cocaine (0.33 mg/kg) self-administration when placed on a fixed ratio 10 (FR10) schedule of reinforcement for 1 h/day and failed to work for drug when challenged on a progressive ratio (PR) schedule of reinforcement. This level of ID, however, was viewed as severe and it was ongoing (i.e., it was continued into adulthood). In addition, the 1 h self-administration regimen employed allows for only a limited assessment of addiction-like behavior for drug [20]. In 2004, a different protocol was used by Deroche-Gamonet et al. [21] to generate stable cocaine self-administration and to model addiction-like behaviors as defined by criteria for substance abuse in the Diagnostic and Statistical Manual of Mental Disorders [22] (DSM-IV). In that study, Persistence in drug seeking was measured by persistent responses on the “active” operant during periods of signaled non-availability (SNA) of drug interspersed between daily fixed ratio periods. Continued drug use despite knowledge of physical or psychological harm was evaluated by continued drug seeking in spite of a mild foot shock. Motivation to take drug was assessed using a progressive ratio (PR) schedule of reinforcement where the number of nose pokes required for an iv infusion of drug increased after each infusion. Rats ranking positive on these tests (i.e., in the top tertile) were determined to be exhibiting more ‘addiction-like’ behavior. Here, we used a slight variation of the model developed by Deroche-Gamonet et al. [21] to examine the consequences of ID on addiction-like behavior for cocaine (i.e., on persistence in drug seeking during SNA and motivation to take drug during PR testing). Further, in light of the critical window discussed above, the present study tested addiction in rats with a history of ID limited to PND4-21.
2. Material and methods
2.1 Animals
Test rats were produced in the Jones lab at the University Park Campus of the Pennsylvania State University. Pregnant Sprague-Dawley dams were fed either iron sufficient (80 ppm) or ID (3 ppm) diet beginning at gestational day (G) 5 and then throughout pregnancy and lactation. Assignment of the dams to the iron sufficient or ID food group was performed randomly, but balanced for dam weight at G5. This feeding schedule causes iron-sufficient dams to produce iron-sufficient milk and ID dams to produce low-iron milk [23] throughout the postnatal period. At postnatal day (P) 4, pups from iron-sufficient dams were out-fostered to ID dams or to other iron-sufficient dams. This feeding paradigm produced test subjects with a history of being iron deplete (ID, n=9) from postnatal days 4 through 21 (weaning) and iron replete thereafter or always being iron sufficient (Control or CN, n=9). Diets fed ad libitum to dams during gestation and lactation were prepared according to AIN-93 guidelines, with the only difference being iron content. At weaning, all rats were given continuous ad libitum access to Purina Rodent Diet 5001 (240 ppm iron) and to distilled water except where noted. In all cases, rats were housed in a humidity and temperature-controlled (21°C) animal care facility on a standard 12/12 h light/dark cycle (lights on at 7:00am).
After PND21, all male rats were transported 90 miles to the Grigson lab at the Penn State Hershey College of Medicine, Hershey, Pennsylvania. Rats were given a one-week acclimation period before testing. Rats were housed individually in wire bottom cages, in a humidity and temperature-controlled (21°C) animal care facility on a standard 12/12 h light/dark cycle (lights on at 7:00am). Testing was conducted during the light phase of the cycle. All experimental protocols complied with National Institutes of Health Animal Care Guidelines and were approved by the Pennsylvania State University Institutional Animal Care and Use Committee.
2.1.1 Hemoglobin determination
Tail blood was collected at weaning (PND21) and again at surgery (PND39-40) to measure hemoglobin levels to confirm iron sufficiency, deficiency, and repletion. Hemoglobin (Hb) values were determined photometrically using cyanomethemoglobin standard solution and according to the manufacturer’s instructions (Sigma Aldrich, St. Louis, MO).
2.1.2 Surgery
All rats were anesthetized with Ketamine (70 mg/kg) and Xylazine (16 mg/kg) and a jugular catheter was implanted as described [20], [24], [25]. Catheters were implanted into the right external jugular vein with tubing extending subcutaneously past the neck to a capped cannula exiting dorsal to the scapulae. Patency of catheters was maintained by daily flushing with heparinized saline (0.2 ml of 30 IU/ml heparin) and periodically verified by administration through the catheter of 0.2 ml of the short-acting anesthetic 1% Diprivan (propofol). Rats were given one week to recover from surgery before the start of the experiment (habituation) at PND46-47.
2.2 Apparatus
2.2.1 Experimental chambers
Each rat was trained in one of 12 identical operant chambers (Med Associates, St. Albans, VT) measuring 30.5×24.0×29.0 cm and housed in light and sound attenuating cubicles. All chambers have clear Plexiglas tops, fronts, and backs. Sidewalls are aluminum. The floors consist of 19 stainless steel rods (4.8 mm) spaced 1.6 cm apart center to center. Each chamber is equipped with three retractable operant sipper tubes (spouts) that enter the left side of the chamber through 1.3 cm diameter holes spaced at 8.0 cm center to center. A stimulus light is located 6 cm above each spout. A lickometer circuit is used to record spout licks (and contacts such as nose pokes). Each chamber is equipped with a house light (25 W), a tone generator (Sonalert Time Generator, 2900 Hz), and a white noise speaker (75 dB). Self-administration is controlled by an electronic circuit operating a syringe pump (Med Associates). Collection of the data and control of chamber events were performed using a Windows-based computer. Programs were written in Medstate notation language (Med Associates).
2.2.2 Coupling assembly
Prior to each self-administration session, a coupling assembly was attached to the catheter assembly on the back of each rat. The coupling assembly consisted of a metal spring attached to a threaded metal spacer to provide protected passage of the catheter tubing (Tygon) down the center to the animal. The assembly was attached to a counterbalanced swivel (Instech) connected to a syringe pump outside of the cubicle.
2.3 Self-administration
Rats were habituated to sound-attenuating operant chambers as described [24], [25]. The afternoon before habituation, rats were water deprived. During habituation, all rats were given access to water in the operant chambers for 5 min each morning for three days to train them to drink from the available spouts. In each case a cue light was illuminated above the water-containing spout, starting with the left spout on day one, the center spout on day two, and the right spout on day three. No less than 90 min later, rats were given 60 min access to water in the home cage to rehydrate. Following habituation, the rats were returned to ad lib water access and tested in the operant chambers Monday through Friday, with Saturdays and Sundays off to avoid overdose. During this time, all rats were allowed to intravenously (iv) self-administer 0.8 mg/kg of cocaine per infusion. The left spout was not used, the empty center spout was available but “inactive” (no cue light, no reinforcement), the empty right spout was “active” with a cue light and drug reinforcement. Upon achieving the necessary fixed ratio requirement of contacts (spout licks or nose pokes), the light above the active empty spout was extinguished, the active spout retracted (40 s during fixed ratio testing and 20 s during PR testing), and 0.8 mg/kg cocaine was infused iv over a 6 sec period. During this time, no further infusions could be obtained. The spout would then return and the cue light would illuminate. Consistent with the regimen described by Deroche-Gamonet et al. [21], daily testing involved three 40 min drug access periods, separated by two 15 minute signaled non-availability periods (40-15-40-15-40 min). During the SNA periods, the cue light was extinguished, the house light illuminated, and responses on the active spout were not reinforced. Here and elsewhere, responses on the inactive spout were recorded, but led to no consequence. During the first week (Figure 1), rats were trained on a fixed ratio schedule where 5 licks on the active spout (FR5) earned one iv infusion of 0.8 mg/kg of cocaine. The FR requirement was increased to 10 licks during the second week and to 20 licks thereafter. A PR test was conducted each Friday during weeks 3, 4, and 5. During PR testing, the ratio requirement for each successive infusion was increased based on the regimen described by Roberts [26] and reported previously by Deroche-Gamonet et al. [21] (i.e., 10, 20, 30, 45, 65, 85, 115, 145, 185, 225, 275, 325, 385, 445, 515, 585, 665, 745, 835, 925, 1025, 1125, 1235, 1345, 1465, 1585). As described [20], [27], [25], the PR session ended when 30 min elapsed without having earned an infusion or after 5 h.
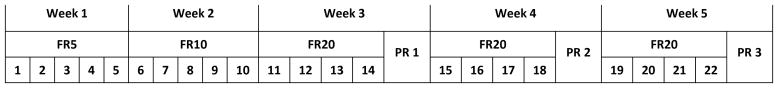
Figure 1.
Rats were tested five days each week, on a FR5 schedule for five trials, then on a FR10 schedule for five trials. Afterwards, rats were tested four days a week on a FR20 schedule for the remaining trials with PR probe tests on Fridays.
2.4 Addiction-like Behavior Score
A useful method to compare groups based on behavior indicative of addiction, as discussed in section 1, is to rank each individual according to criteria related to symptoms of substance abuse described by DSM [28], as reported previously by Deroche-Gamonet et al. [21], our lab [27] and others [29]. Persistence in drug-seeking was defined by the number of active spout responses emitted during SNA, and motivation to take drug was measured by the number of responses required for the last infusion attained (breakpoint) on the PR schedule of reinforcement. A measure of continued drug use despite knowledge of physical or psychological harm was not used in the present study as we chose not to subject rats to footshock. Here, the CN and ID rats were combined and ranked by total responses on the active spout during the two SNA periods during the terminal FR20 session and the breakpoint obtained during the last PR session. One point was given to each rat when ranking in the top tertile for a given criterion. These points were then summed so that each rat could score a 0, 1, or 2, with a score of 2 indicating the strongest addiction-like behavior among the rats tested.
2.5 Statistical analyses
The number of infusions per 2h FR session was analyzed using a 2 x 22 mixed factorial Analysis of Variance (ANOVA) varying diet (ID vs. CN) and trials (1 – 22). The number of active and inactive spout responses emitted during FR drug self-administration and SNA, and the latency(s) to make the first lick on the empty spouts were analyzed using mixed factorial ANOVAs varying diet (ID vs. CN), spout (Active vs. Inactive) and trials (1 – 22). Breakpoint responses emitted during the PR trials were analyzed using a 2 x 3 mixed factorial ANOVA varying diet (ID vs. CN) and test (1 – 3). Post hoc comparisons were conducted, when appropriate, using a Newman-Keuls test or a Student’s t-test. Addiction-like behavior scores were compared using the Mann–Whitney U test. Alpha was set to α = 0.05.
3. Results
3.1 Sample size
One CN rat did not survive catheter implantation surgery. Another CN rat was observed exhibiting extreme perseveration on the active spout during self-administration. After comparison of its data to the other CN rats, it was suspected to be an outlier. The data from this rat failed Grubbs’ test for outliers for the last 12 of the 25 test days. Therefore the data from this rat were discarded. One syringe pump was later discovered to be miscalibrated, thus the data from one ID rat also were discarded. Consequently, we report results for 8 ID and 7 CN rats.
3.2 Iron status and weight
At weaning, the mean hemoglobin of CN rats was 9.87 ± 1.75 g/dl, while that of the ID rats was 4.56 ± 2.61 g/dl. At PND39-40, the hemoglobin of the CN rats was 13.49 ± 0.69 g/dl and that of the ID group was 14.39 ± 0.75 g/dl. Eighteen days of being fed an iron adequate diet, therefore, normalized the Hb of the ID animals [30, 31]. At the beginning of behavioral testing (PND49-50), ID rats weighed 234.2 ± 5.5 g and CN rats weighed 234.0 ± 3.7g, with no significant difference between groups, t < 1.
3.3 Drug intake and addiction-like behavior
3.3.1 FR testing
3.3.1.1 Infusions
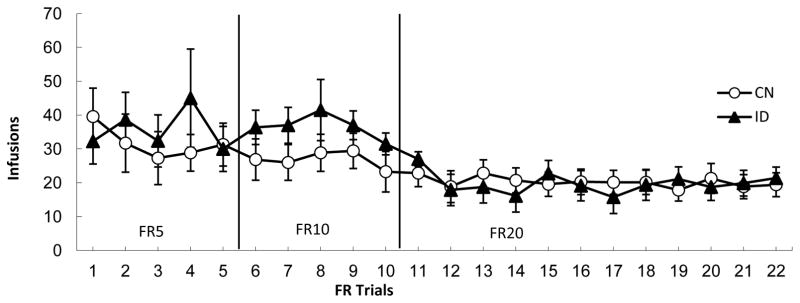
When tested on an FR schedule of reinforcement (Figure 2), there was neither a significant main effect of diet, nor a significant diet x trials interaction, Fs < 1. Additionally, when data from each of the three different FR rates were assessed individually, the results also were not significant, Fs < 1. Thus, regardless of history of ID, all rats self-administered the same number of infusions of cocaine during each phase of FR testing.
Figure 2.
Mean (+/− SEM) number of infusions of cocaine/2h in control (CN) and iron deficient (ID) rats across 5 days of testing on the Fixed Ratio (FR) 5 schedule, 5 days on the FR10 schedule, and 12 days on the FR20 schedule of reinforcement.
3.2.1.2 Signaled non-availability (SNA)
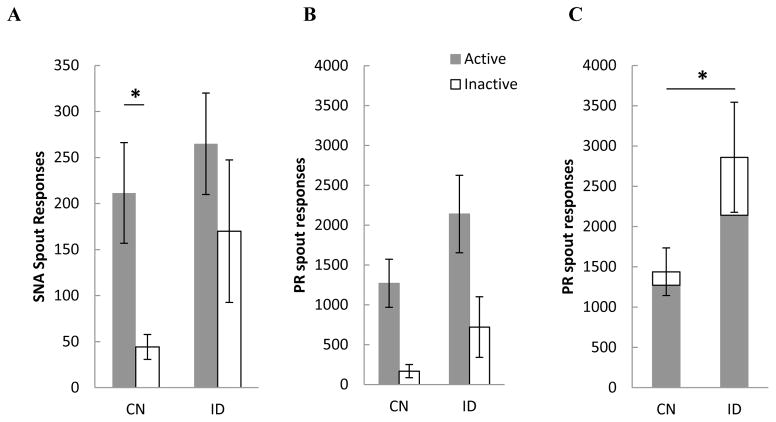
Regarding SNA responding (Figure 3A), neither the main effect of diet, F (1,26) = 2.44, p > 0.13, spout x diet interaction, nor spout x diet x trials interaction reached significance, Fs < 1. On the other hand, there was a significant main effect of spout, F (1,26) = 5.23, p < 0.03, indicating that all rats, regardless of diet history, made more contacts on the active vs. the inactive spout during the SNA period. That said, inspection of the data suggests that the main effect of spout was carried primarily by the control subjects. Accordingly, follow up Student’s t-tests verified that CN, p < 0.05, but not ID rats, p > 0.33 (Figure 3A), made significantly more responses on the active vs. the inactive spout during signaled non-availability.
Figure 3.
A) Mean (+/− SEM) SNA responses on the active and inactive empty spouts in control (CN) and iron deficient (ID) rats. B) Mean (+/− SEM) number of responses on the active and inactive spout for CN and ID rats averaged across progressive ratio (PR) test 1 – 3. C) Average (+/− SEM) number of responses on the active plus the inactive spout in CN and ID rats averaged across PR test 1–3.
3.2.2 PR testing
Breakpoint responses in three progressive ratio probe tests were used as a measure of motivation to take drug. While ID rats tended to take more infusions (have a higher breakpoint) than CN rats, neither an assessment of individual PR trials, nor averaging across PR trials 1, 2, and 3, found significant differences, Fs < 1 in breakpoints. Thus, the total number of responses made on the active and the inactive spout were averaged across the three PR test trials (Figure 3B) and analyzed using a 2 x 2 ANOVA varying diet and spout. The results found a significant main effect of spout, F (1,26) = 14.78, p < 0.001, whereby all rats made more responses on the active vs. the inactive spout during PR testing. The main effect of diet also was significant, F (1,26) = 4.96, p = 0.03, indicating that rats with a history of ID emitted more responses, overall, than did control subjects when working for cocaine (Figure 3C). There were, however, no significant differences between diet groups on active or inactive spout responding, F < 1.
3.2.3 Addiction-like behavior score
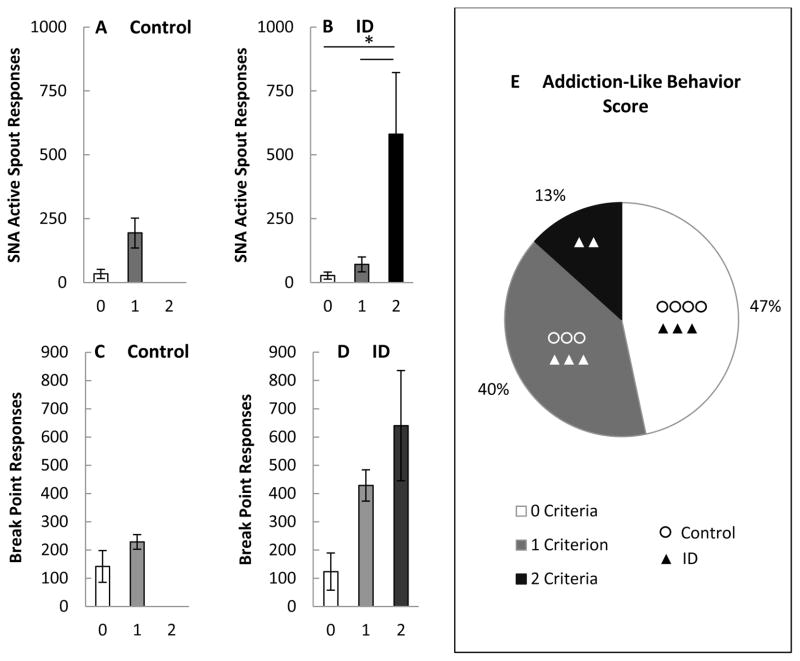
As in the original model [21], there was a trend for the intensity of each of the addiction-like behaviors to be proportional to the number of criteria met (Figure 4A to 4D). SNA. Results from a 2 x 3 mixed factorial ANOVA varying diet (ID, CN) x ALB score (0 – 2) showed no significant main effect of diet, F (1,11) = 1.04, p = 0.33, but did indicate a significant main effect of ALB score, F (2,11) = 17.20, p < 0.001 (Figure 4A and 4B). Post hoc analysis revealed that rats with an ALB score of 2 (all ID rats) responded significantly more on the operant spout during SNA than rats scoring either a 0 or a 1, ps < 0.001 (Figure 4B). PR. Consistent with Deroche-Gamonet et al. [21] and Tacelosky et al. [32], addiction like behavior for PR responding was represented by the number of break point responses made on the active spout on the third and final PR test. Varying diet (ID, CN) x ALB score (0 – 2) x PR breakpoint responses showed no significant main effect of diet, F (1,11) = 1.62, p = 0.23, and no significant main effect of ALB score, F < 1 (Figure 4C and 4D). Finally, while more of the ID rats scored a 2 than did the CN rats (Figure 4E), when analyzed using a Mann–Whitney U test (Wilcoxon rank-sum test), these differences did not attain statistical significance (U = 19.5, p = 0.33).
Figure 4.
Figure 4A, 4B. Mean (+/− SEM) number of responses made on the active spout during signaled non-availability as a function of the number of criterion positive for addiction-like behavior for rats maintained on a control (CN) diet (panel A) or for rats with a history of iron deficiency (ID) (panel B). Figure 4C, 4D. Mean (+/− SEM) number of responses made on the active spout during the final progressive ratio (PR) test as a function of the number of criterion positive for addiction-like behavior for rats maintained on a control (CN) diet (panel C) or for rats with a history of iron deficiency (ID) (panel D). Figure 4E. Pie chart showing the percent of rats positive for 0, 1, or 2 criteria for addiction-like behavior: The empty circle represents rats maintained on a control (CN) diet and the closed triangle rats with a history of iron deficiency (ID).
Overall, then, the ID rats failed to exhibit significantly greater addiction-like behavior. That said, the ID rats did demonstrate an increased response rate on the manipulanda (Figure 3C) and, unlike the CN rats, the ID rats failed to discriminate between the two (Figure 3A). Thus, we chose to revisit the acquisition data (i.e., the FR data) to examine whether ID rats had simply failed to learn the task and were indiscriminately responding in a blind attempt to gain cocaine infusions.
3.3 Reward conditioning/learning
3.3.1 Latency to first response
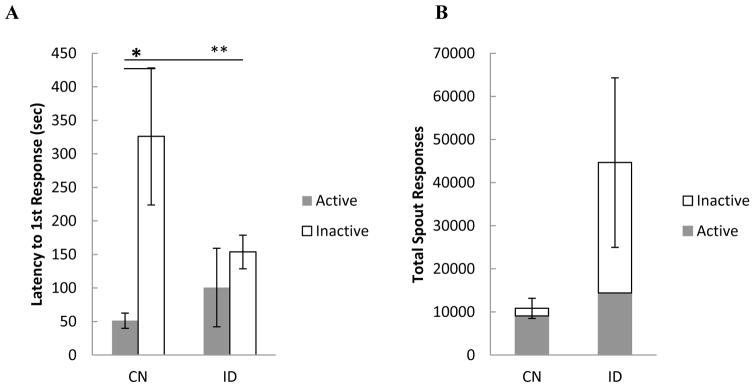
Latency to make the first response on the active vs. the inactive spout can be used as another index of whether the rats have learned to distinguish between the active and inactive spouts. When latency was analyzed by diet (CN, ID), spout (active, inactive), and FR trials (1–22), the main effect of diet was not significant, F (1,26) = 1.09, p < 0.31, nor was the diet x spout x trials interaction, F (21,546) = 1.28, p < 0.18. The main effect of spout was significant, F (1,26) = 7.77, p < 0.01, indicating that rats, as a whole, were faster to initiate licking on the active vs. the inactive spout. Finally, the diet x spout interaction approached statistical significance, F (1,26) = 3.55, p < 0.08. Given this trend, Figure 5A shows the latency to make the first response on the active vs. inactive empty spouts as a function of diet history collapsed across all 22 FR trials. Follow up analysis with Students t-tests confirmed that control rats (p < 0.05), but not rats with a history of ID (p = 0.43), were in fact faster to initiate licking on their respective active vs. inactive spout. Rats with a history of ID, on the other hand, were relatively quick to initiate licking on both spouts.
Figure 5.
A) Mean (+/− SEM) latency (sec) to make the 1st response on the active and the inactive spout by control (CN) rats and by rats with a history of iron deficiency (ID) averaged across 22 fixed ratio trials. B) Mean (+/− SEM) total responses on the active plus the inactive spout for CN and ID rats averaged across 22 fixed ratio trials.
3.3.2 Spout responding during drug availability
Although the ID rats took the same amount of drug as the CN rats during FR responding (Figure 2), upon re-inspection it became clear that ID rats exhibited a lot of behavior in gaining infusions (e.g., Figure 3C). When examining the total number of spout responses emitted during FR trials, however, the results of a 2 x 2 x 22 mixed factorial ANOVA did not attain a significant main effect of diet, F(1,26) = 2.37, p =0.14, or any interaction thereof, ps > 0.05 (see Figure 5B). Thus, while ID rats exhibited a fourfold increase in active plus inactive spout responding compared to the CN controls, this was only a trend.
4. Discussion
Rats with a history of ID self-administered the same amount of drug as did the CN controls during FR testing. While these data point toward the ID rats being neither more nor less motivated to take drug than CN rats, the absence of a group effect is expected here. Specifically, Piazza’s regimen generally leads to stable drug-taking across trials, with little or no group differences being evident during the FR phase of testing [21, 27]. When challenged during SNA or PR, there was some evidence for greater addiction-like behavior in the rats with a history of having been ID. The ID rats tended to make more spout responses during both SNA and PR than the CN rats and only the ID rats scored a 2 (i.e., fell in the top tertile for both SNA and PR responding). However, these effects were not significantly different between groups. Of course, inclusion of the third criterion (punished responding) as used by Piazza and colleagues, may have resulted in a more sensitive test. That said, closer analysis of the data revealed that, while ID rats may have tended to exhibit greater responding for cocaine than the CN rats, the nature of responding was largely indiscriminate. During FR testing, CN rats exhibited a shorter latency to make contact with the active vs. the inactive spout, while the ID rats were quick to make a first contact on both spouts. Because of a tendency to generate more inactive spout responses, ID rats also tended to make more overall responses during FR testing than the CN rats. During SNA testing, CN rats demonstrated goal-directed behavior by making more responses on the active vs. the inactive spout [33]. ID rats, on the other hand, emitted a high number of responses on both the active and the inactive spout. Finally, during PR testing, the ID rats, like the CN rats, made more licks on the active vs. the inactive spout, but the ID rats again tended to make more licks on the inactive spout – leading to a non-significant, yet large number of overall responses generated.
Initially, these differences in responding may be attributed to unconditioned effects of the drug such as increased locomotor activity (hyperactivity), impulsivity, or anxiety. However, this protocol of combining self-administration with measures of addiction-like behavior is a robust model. That is, Piazza and colleagues have shown that rats under these conditions did not differ on sensitivity to the effects of cocaine, measured by locomotion during self-administration. Additionally, the group has shown that these addiction measures are independent of impulsivity. Using factor analysis they showed that continued responding under extinction conditions (a measure of impulsivity) loaded as an independent factor from the SNA and PR responding. The addiction-like behaviors, then, are measures of a single factor, likely indicating compulsive drug use. Theirs and other studies (see Deroche-Gamonet, et al, 2004 Supporting Online Material[21]) have shown that characteristics such as spontaneous motor activity [34–36] and anxiety-like behaviors [37, 38] are not explained by differences in these addiction-like behavior measures. Further, there is direct evidence that, as stated in section 1, gestational ID instead may reduce locomotor activity.
Abundant responding on both the active and inactive spouts by the ID rats (Figure 3A and 5B) may indicate a failure to distinguish between the two spouts, that is, to learn which spout is the operant and thus linked to obtaining drug. As mentioned in section 1, perinatal ID has been shown to negatively affect learning and memory. If indeed this is a learning deficit by ID rats, it would not be fully unexpected as the dopaminergic system is known to contribute to reward learning and memory [39]. Deficiency of iron could contribute to deficits in any number of systems, the dopaminergic system being one of many. Nonetheless, dopamine D1 and D2 receptors are altered by ID [9], and have been shown to participate in learning and memory [40, 41]. Dysfunction of this system by ID could conceivably alter an animal’s ability to learn the proper sequence of events in order to efficiently gain access to drug, resulting in greater responding on both spouts in a blind effort to solve the problem. In fact, we saw evidence of this in our previous study [20] where rats fed an ID diet beginning at weaning and continuing throughout the experiment were much slower to acquire responding on an empty spout operant for cocaine self-administration than iron replete controls. At the time, this outcome was interpreted as a learning and/or a motivational deficit, but not a motor deficit, as these rats readily licked for sucrose. Here, we see evidence that the greater activity could indicate a failure to discriminate between spouts, that is, a failure of reward learning. This is but one plausible explanation. ID may very well have negatively affected intracellular signaling or other neurotransmitter systems besides what has been shown. This possibility must be given due consideration. Nonetheless, our early work showed that the mesolimbic and nigrostriatal pathways are affected by early ID and may mimic what has been shown by treating animals with DA antagonists and partial agonists [42]. Thus, we hypothesize that these behavioral deficits, at least in part, may be due to the irreversible molecular changes in the dopaminergic reward system caused by acute ID during a critical period of development.
While it appears that the ID rats may have a learning impairment, they do not appear to have a general motivation deficit. There were no significant differences in breakpoint between the groups during PR testing, a test for motivation. However, the increase in responding on both the active and the inactive spout by the ID rats, if not due to increased locomotor activity (see section 1), could therefore be interpreted as evidence of motivation. The argument then, would be that if the ID rats failed to distinguish between the active and the inactive spout (a measure of goal-directed behavior) due to a learning deficit, but were still motivated, they would need to generate a great deal of random responses to obtain drug. In fact, they were just as fast to make contact with both the active and the inactive spouts, and when considering the combined number of active and inactive spout responses, generated up to four fold more responses than CN rats during drug and SNA periods of FR testing, and during PR testing. Are the ID rats, then, exhibiting greater motivation than iron normal rats? Given the relatively small sample in the present study (7–8 subjects per group), statistical significance was not reached in many measures, and there is a considerable amount of variation within groups. However, taken together, these data suggest that while ID early in infancy impairs the ability to acquire efficient goal-directed behavior for cocaine, the motivation for drug could possibly be left intact, as these ID rats were willing to generate a great amount of extraneous effort to seek and to take drug.
5. Conclusions
Although the CN and ID rats took the same amount of drug during FR trials, the ID rats tended to score higher on the addiction-like behavior scale than CN rats. Upon closer inspection of the data, however, it was evident that responding by rats made ID during PN days 4–21, while high, was not goal-directed, i.e., the ID rats exhibited high indiscriminant responding on both the active and inactive spouts and were just as quick to respond on both manipulanda. This finding is not likely due to locomotor activity. It has been shown that responding in the Piazza model is not linked to differences in locomotor activity, and that ID may, if anything, reduce locomotion. Additionally, these ID rats scored equally high on the PR test. Thus, they appear to be as motivated for drug, but incapable of selectively responding on the active spout operant. Future work should parse out behavioral deficits in the ID rats that are displayed during cocaine self-administration testing, measure the motivation to take drug, test for deficits in learning and memory, and eliminate, if any, possible locomotor and impulsivity changes caused by preweaning ID. Nevertheless, perinatal ID in rat is known to cause irreversible damage to the nervous system and the present work has indicated that this damage perhaps has detrimental effects on reward learning.
Highlights.
Iron deficiency during postnatal days 4–21 results in greater non-reinforced responding for cocaine.
Iron deficient rats failed to discriminate between the cocaine-contingent and non-contingent manipulanda.
Responding by iron deficient rats is less goal-directed and may be evidence of a learning disability.
Acknowledgments
This work was supported by the National Institutes of Health DA009815 and the Social Sciences Research Institute at Penn State.
Abbreviations
- ID
iron deficiency, iron deficient
- DA
dopamine
- DAT
dopamine transporter
- PND
postnatal day
- D1R
dopamine type-1 receptor
- D2R
dopamine type-2 receptor
- NAc
nucleus accumbens
- VTA
ventral tegmental area
- iv
intravenous
- FR
fixed ratio
- PR
progressive ratio
- CN
control
- SNA
signaled non-availability
Footnotes
The authors do not have any actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization, C. f. D. C. a. P. Assessing the Iron Status of Populations. 2. Geneva: World Health Organization; 2007. [Google Scholar]
- 2.World Health Organization, C. f. D. C. a. P. The World Health Report 2002: Reducing Risks, Promoting Healthy Life. Geneva: World Health Organization; 2002. [Google Scholar]
- 3.Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med. 1991;325:687–94. doi: 10.1056/NEJM199109053251004. [DOI] [PubMed] [Google Scholar]
- 4.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–43. doi: 10.1301/nr.2006.may.S34-S43. discussion S72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beard JL. Why iron deficiency is important in infant development. The Journal of nutrition. 2008;138:2534–6. doi: 10.1093/jn/138.12.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collard KJ. Iron homeostasis in the neonate. Pediatrics. 2009;123:1208–16. doi: 10.1542/peds.2008-1047. [DOI] [PubMed] [Google Scholar]
- 7.Beard JL, Erikson KM, Jones BC. Neurobehavioral analysis of developmental iron deficiency in rats. Behavioural brain research. 2002;134:517–24. doi: 10.1016/s0166-4328(02)00092-x. [DOI] [PubMed] [Google Scholar]
- 8.Beard JL, Unger EL, Bianco LE, Paul T, Rundle SE, Jones BC. Early postnatal iron repletion overcomes lasting effects of gestational iron deficiency in rats. The Journal of nutrition. 2007;137:1176–82. doi: 10.1093/jn/137.5.1176. [DOI] [PubMed] [Google Scholar]
- 9.Beard J, Erikson KM, Jones BC. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J Nutr. 2003;133:1174–9. doi: 10.1093/jn/133.4.1174. [DOI] [PubMed] [Google Scholar]
- 10.Komolova M, Bourque SL, Nakatsu K, Adams MA. Sedentariness and increased visceral adiposity in adult perinatally iron-deficient rats. Int J Obes (Lond) 2008;32:1441–4. doi: 10.1038/ijo.2008.97. [DOI] [PubMed] [Google Scholar]
- 11.Eseh R, Zimmerberg B. Age-dependent effects of gestational and lactational iron deficiency on anxiety behavior in rats. Behavioural brain research. 2005;164:214–21. doi: 10.1016/j.bbr.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Bourque SL, Iqbal U, Reynolds JN, Adams MA, Nakatsu K. Perinatal iron deficiency affects locomotor behavior and water maze performance in adult male and female rats. The Journal of nutrition. 2008;138:931–7. doi: 10.1093/jn/138.5.931. [DOI] [PubMed] [Google Scholar]
- 13.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain research bulletin. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 14.Tisch S, Silberstein P, Limousin-Dowsey P, Jahanshahi M. The basal ganglia: anatomy, physiology, and pharmacology. The Psychiatric clinics of North America. 2004;27:757–99. doi: 10.1016/j.psc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiology & behavior. 1988;44:599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- 16.Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain research. 2001;904:76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- 17.Hajnal A, Norgren R. Repeated access to sucrose augments dopamine turnover in the nucleus accumbens. Neuroreport. 2002;13:2213–6. doi: 10.1097/00001756-200212030-00010. [DOI] [PubMed] [Google Scholar]
- 18.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. American journal of physiology Regulatory, integrative and comparative physiology. 2004;286:R31–7. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 19.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones BC, Wheeler DS, Beard JL, Grigson PS. Iron deficiency in rats decreases acquisition of and suppresses responding for cocaine. Pharmacology, biochemistry, and behavior. 2002;73:813–9. doi: 10.1016/s0091-3057(02)00906-1. [DOI] [PubMed] [Google Scholar]
- 21.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association, A. P. A. T. F. o. D.-I. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 23.Rao R, Tkac I, Unger EL, Ennis K, Hurst A, Schallert T, et al. Iron supplementation dose for perinatal iron deficiency differentially alters the neurochemistry of the frontal cortex and hippocampus in adult rats. Pediatric research. 2013;73:31–7. doi: 10.1038/pr.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grigson PS, Twining RC. Cocaine-induced suppression of saccharin intake: a model of drug-induced devaluation of natural rewards. Behavioral neuroscience. 2002;116:321–33. [PubMed] [Google Scholar]
- 25.Twining RC, Bolan M, Grigson PS. Yoked delivery of cocaine is aversive and protects against the motivation for drug in rats. Behavioral neuroscience. 2009;123:913–25. doi: 10.1037/a0016498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of neuroscience methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 27.Puhl MD, Cason AM, Wojnicki FH, Corwin RL, Grigson PS. A history of bingeing on fat enhances cocaine seeking and taking. Behavioral neuroscience. 2011;125:930–42. doi: 10.1037/a0025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Psychiatric Association, A. P. A. D. S. M. T. F. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- 29.Velazquez-Sanchez C, Ferragud A, Moore CF, Everitt BJ, Sabino V, Cottone P. High trait impulsivity predicts food addiction-like behavior in the rat. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2014;39:2463–72. doi: 10.1038/npp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson-Delaney CA, Harrison LR. Exotic companion medicine handbook for veterinarians. Lake Worth, Fla: Wingers Pub; 1996. [Google Scholar]
- 31.Fox JG. Laboratory animal medicine. 2. Amsterdam New York: Academic Press; 2002. American College of Laboratory Animal Medicine. [Google Scholar]
- 32.Tacelosky DM, Alexander DN, Morse M, Hajnal A, Berg A, Levenson R, et al. Low expression of D2R and Wntless correlates with high motivation for heroin. Behavioral neuroscience. 2015;129:744–55. doi: 10.1037/bne0000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology. 1998;135:151–60. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- 34.Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 35.Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:4226–32. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nature neuroscience. 2004;7:389–97. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- 37.Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F, et al. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology. 1995;122:369–73. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- 38.Homberg JR, van den Akker M, Raaso HS, Wardeh G, Binnekade R, Schoffelmeer AN, et al. Enhanced motivation to self-administer cocaine is predicted by self-grooming behaviour and relates to dopamine release in the rat medial prefrontal cortex and amygdala. The European journal of neuroscience. 2002;15:1542–50. doi: 10.1046/j.1460-9568.2002.01976.x. [DOI] [PubMed] [Google Scholar]
- 39.Wise RA. Dopamine, learning and motivation. Nature reviews Neuroscience. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 40.Xu TX, Yao WD. D1 and D2 dopamine receptors in separate circuits cooperate to drive associative long-term potentiation in the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16366–71. doi: 10.1073/pnas.1004108107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujishiro H, Umegaki H, Suzuki Y, Oohara-Kurotani S, Yamaguchi Y, Iguchi A. Dopamine D2 receptor plays a role in memory function: implications of dopamine-acetylcholine interaction in the ventral hippocampus. Psychopharmacology. 2005;182:253–61. doi: 10.1007/s00213-005-0072-x. [DOI] [PubMed] [Google Scholar]
- 42.Koob GF, Le HT, Creese I. The D1 dopamine receptor antagonist SCH 23390 increases cocaine self-administration in the rat. Neuroscience letters. 1987;79:315–20. doi: 10.1016/0304-3940(87)90451-4. [DOI] [PubMed] [Google Scholar]