Synopsis
Functional reconstruction of craniofacial defects is a major clinical challenge in craniofacial sciences, especially in complex situations involving traumatic injury, cranioplasty and oncologic surgery. The advent of biomaterials has been viewed as a potential alternative to standard autologous/allogenic grafting procedures to achieve clinically successful bone regeneration. Over the years, the field of biomaterials for bone augmentation has swiftly advanced to create novel instructive materials and engineering technologies, emerging as an important therapeutic modality for craniofacial regeneration. This chapter discusses various classes of biomaterials, ranging from bioceramics to biopolymers that are currently employed in craniofacial reconstruction. Further, here we review the clinical applications of biomaterials as delivery agents for the sustained release of stem cells, genes and growth factors. Additionally, we cover recent advancements in 3D printing and bioprinting techniques that appear to be promising for future clinical treatments for craniofacial reconstruction. In summary, the present review highlights relevant topics in the bone regeneration literature exemplifying the potential of biomaterials to repair bone defects.
Keywords: Tissue engineering, stem cells, 3D bioprinting, gene delivery, growth factor delivery, bone regeneration, calcium phosphate
Introduction
The field of craniofacial bone regeneration has experienced tremendous expansion since the inception of the concept of tissue engineering [1], more than 2 decades ago. Arguably, research and development in the area of bone augmentation have contributed significantly to the establishment of tissue engineering as a viable treatment option in medicine and dentistry [2]. Biomaterials represent a fundamental aspect of bone regeneration. It is widely recognized that biomaterials can be tailored to regulate the microenvironment in which cells reside during the process of new bone formation. This essentially means that the ability to manipulate the composition, architecture, and properties of different biomaterials allows one to control the rate of regeneration, and ideally enhance the process of new bone formation [2, 3].
Biomaterials are generally used as biocompatible scaffold systems that allow for the migration, proliferation and differentiation of either resident or externally delivered cells, which are utilized to promote new bone formation. A wide variety of biomaterials have been utilized for craniofacial bone augmentation. These are typically divided into either organic or inorganic materials, where calcium phosphate (CaP) bioceramics represent the majority of inorganic scaffolds, and natural or synthetic biopolymers form the majority of organic scaffolds. In brief, the basic rational behind such materials choice is based on a reductionist attempt to mimic the organic-inorganic composition of native bone, where collagen fibrils (a natural organic polymer) are reinforced with hydroxyapatite crystallites (a natural bioceramic) to form a strong and durable natural biomaterial.
In this article, we review recent developments in the translation of biomaterials design and fabrication for clinical strategies of craniofacial bone augmentation. We describe recently reported aspects of CaP bioceramic regenerative materials, recent work on the synthesis and applications of natural and synthetic polymeric hydrogels, as well as protein delivery in the form of plasma rich fibrin, and hybrids of organic/inorganic scaffolds. Furthermore, we discuss the use of biomaterials as tools to enable the effective delivery of growth factors, stem cells and gene therapy. Lastly, we review recent developments in the 3D printing of regenerative scaffold materials for craniofacial bone augmentation.
Bioceramics
CaP scaffolds and cements
Bioceramics such as calcium phosphates (CaP), calcium carbonates, calcium sulfates, bioactive glasses, and composite materials combining bioactive inorganic materials with biodegradable polymers are some of the most promising biomaterials for application in bone regeneration [4]. Research concerning the ability of CaP bioceramics to stimulate bone growth date back to the 1920s, when an aqueous slurry of ‘triple calcium phosphate’ was used in an attempt to enhance bone formation [5]. Especially since the establishment of tissue engineering as a viable treatment alternative in the late 90s [1], research around CaP materials for bone regeneration has expanded tremendously. These materials have received great attention in the bone regeneration community due to their ability to promote rapid bone formation on their surface (Table 1). Several reasons have been proposed to explain these advantageous properties, including the great compositional similarities of CaP materials to the main constituent of human bone, hydroxyapatite; the ability of osteoprogenitor cells to process and resorb CaP materials; and the complex yet highly effective intracellular signaling of osteogenesis that is triggered by the presence of soluble calcium and inorganic phosphates [6] resulting from byproducts of CaP crystal dissolution (Figure 1).
Table 1.
Calcium phosphate compounds and their solubility/degradation properties
| Name | Chemical formula | Symbol | Ca/P ratio | −log(Ksp) at 298 K |
|---|---|---|---|---|
| Monocalcium phosphate monohydrate | Ca(H2PO4)2·H2O | MCPM | 0.5 | 1.14 |
| Dicalcium phosphate anhydrous | CaHPO4 | DCPA | 1.0 | 6.90 |
| Dicalcium phosphate dihydrate | CaHPO4·2H2O | DCPD | 1.0 | 6.59 |
| Octocalcium phosphate | Ca8H2(PO4)6·5H2O | OCP | 1.33 | 96.6 |
| Hydroxyapatite | Ca10(PO4)6(OH)2 | HA | 1.67 | 116.8 |
| Fluorapatite | Ca10(PO4)6F2 | FA | 1.67 | 120.0 |
| Monocalcium phosphate anhydrous | Ca(H2PO4)2 | MCPA | 1.67 | 1.14 |
| α-Tricalcium phosphate | α-Ca3(PO4)2 | α-TCP | 1.5 | 25.5 |
| β-Tricalcium phosphate | β-Ca3(PO4)2 | β-TCP | 1.5 | 28.9 |
| Tetracalcium phosphate | Ca4(PO4)2O | TTCP | 2.0 | 38.0 |
The parameter −log(Ksp) denotes the solubility product. The lower the −log(Ksp) value, the higher is the solubility. Similarly, it can also be noted that acidic products (MCPM) with lower Ca/P ratio have higher solubility compared to basic compounds, such as hydroxyapatite with higher Ca/P ratio.
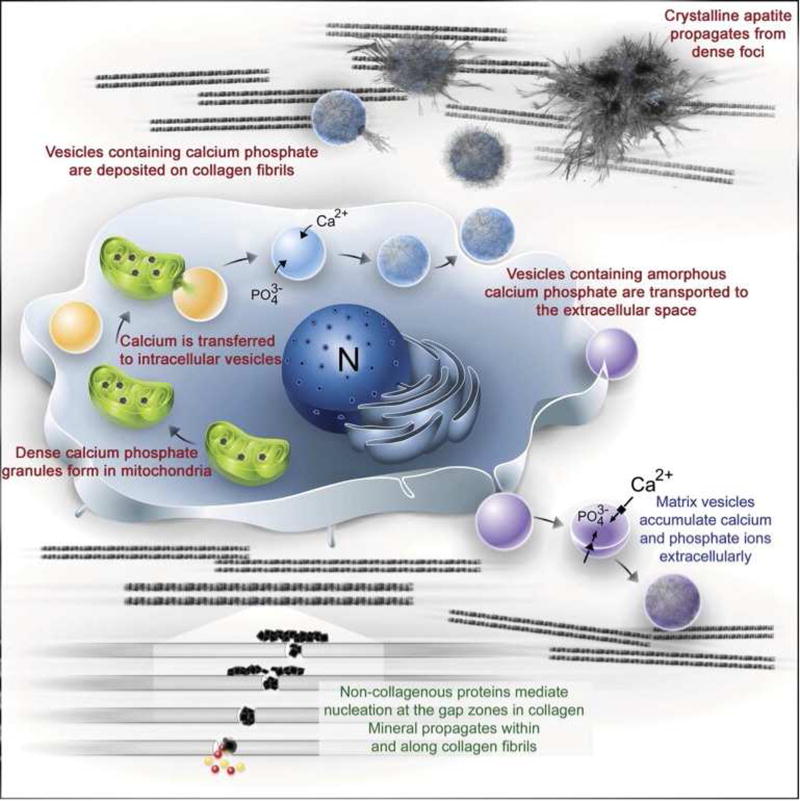
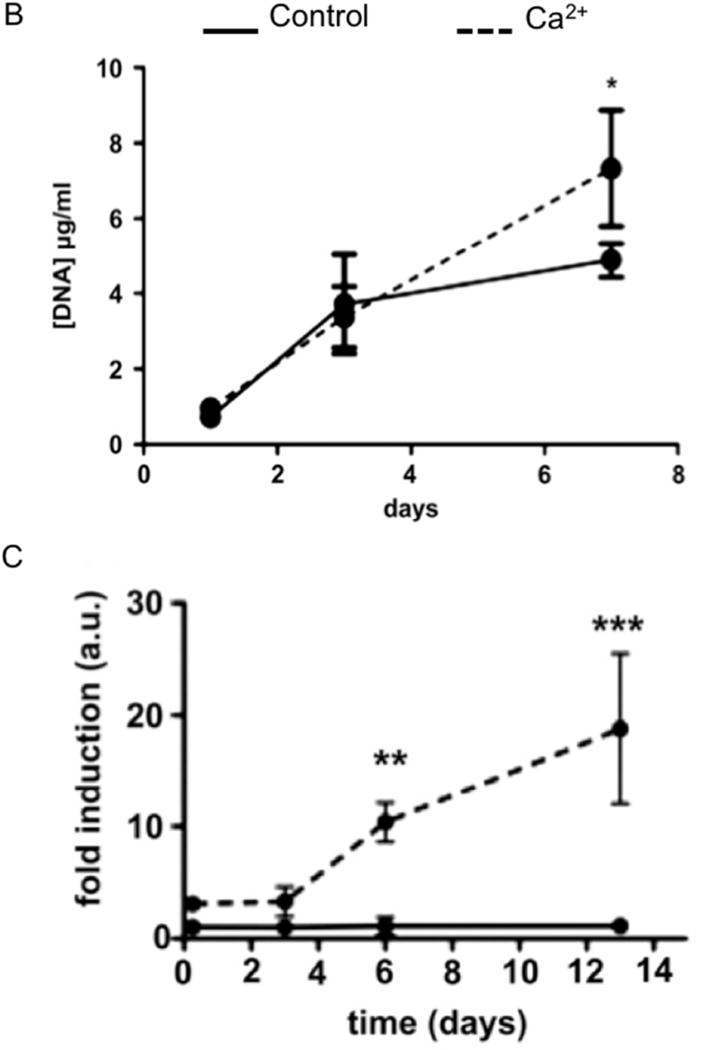
Figure 1.
(A) Diagram outlining current models proposed for bone mineral formation. Bone apatite formation likely proceeds via a number of cooperative/redundant mechanisms. (B–C) Calcium induces (B) cell proliferation and (C) BMP-2 expression in hMSCs treated with Ca2+ enriched medium as compared to control medium. Statistical analysis was done with two-Way ANOVA and Bonferroni’s post-hoc tests.
A: From Boonrungsiman S, Gentleman E, Carzaniga R, et al. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc Natl Acad Sci U S A 2012; 109(35): 14174; with permission.
B and C: Adapted from Barradas AM, Fernandes HA, Groen N, et al. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 2012. 33(11): 3205–3215; with permission.
Adapted from Dorozhkin SV. Calcium orthophosphates: Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 2011; 1(2): 122; and Habraken W, Habibovic P, Epple M, et al. Calcium phosphates in biomedical applications: materials for the future? Materials Today 2016; 19(2): 70; and Chow LC. Solubility of calcium phosphates. In: Chow LC, Eanes ED, editors. Octocalcium Phosphate, vol 18. Karger Publishers; 2001, p. 98; with permission.
Currently there exists a myriad of CaP materials that are commercially available for bone regeneration, and typically these include one or more phases of CaP in different mineral phases, crystal structures and processing conditions (Table 2). Research has shown that the specific mineral phase constituting a CaP biomaterial plays a major role in determining the efficacy of the material for osteogenesis. Importantly, it has been demonstrated that the solubility of the CaP mineral phase is a key factor regulating osteoinduction [10]. Initial efforts to develop CaP scaffold materials focused on the synthesis of materials with a similar composition as the mineral found in natural bone, while ensuring high mechanical properties [11]. This was typically achieved by sintering CaP grafts to form sintered hydroxyapatite (HA) or sintered β-tricalcium phosphate (β-TCP), or combinations thereof. However, the sintering process yields scaffold materials that are too brittle for load bearing applications, have little injectability in bulk, and very low solubility, which hinders osteoclast-driven biodegradation and scaffold remodeling. The discovery of bone cements constituted of CaP phases that can be formed at room temperature (calcium deficient HA, brushite, octacalcium phosphate and monetite) opened up a wide range of possibilities in the manufacture of new CaP bone scaffold materials [12–15]. Importantly, these materials have much lower solubility rates and have been shown to transform into a more stable HA phase upon implantation [16–18]. In fact it has been suggested that these less crystalline phases with lower solubility than sintered CaPs have superior biological properties [19].
Table 2.
List of representative commercially available Calcium Orthophosphate Cements
| Commercial name |
Formulations | End product |
Company name |
Applications |
|---|---|---|---|---|
| Biopex® | 75 wt% α-TCP, 18 wt% TTCP, 5 wt% DCPD and 2 wt% HA | Apatite | Mitsubishi Materials | Bone defect repair, Reinforcement of orthopedic screws and implants, Filling gaps between cement-less artificial joints and bone |
| Norian SRS® | MCPM + α-TCP + CaCO3 | Apatite | DePuy Synthes | Skeletal distal radius fractures, craniofacial |
| BoneSource™ | TTCP (73%), DCPD (27%) | Apatite | Stryker Leibinger | Craniofacial |
| ChronOS™ | β-TCP (73%), MCPM (21%), MgHPO4·3H2O (5%) | Brushite | DePuy Synthes | Metaphyseal bone defects, cranioplasty, Onlay Augmentations in the craniomaxillofacial area |
| α-BSM® | ACP (50%), DCPD (50%) | Apatite | ETEX | Filling of bone defects and voids, dental, craniofacial |
| Cementek® | α-TCP, TTCP | Apatite | Teknimed | Filling of bone defects |
| Biocement D® | 58% α-TCP, 24% DCPA, 8.5% CaCO3, 8.5% calcium-deficient HA | Apatite | Merck (GER) Biomet | Filling of bone defects in maxillary surgery |
| Mimix™ | TTCP, α-TCP | Apatite | Walter Lorenz Surgical | Bony contouring of craniofacial skeleton, craniotomy cuts |
| Calcibon® | α-TCP, DCPA, CaCO3, HA | Carbonated apatite | Biomet Inc. | Filling of noninfected, metaphyseal, cancellous bone defects |
Historically, CaP materials have commonly been found in particulate form, but have also been processed as blocks or porous blocks. These have posed relevant limitations on their clinical use, especially in dentistry, since the injectable materials have improved handling and less invasive characteristics. Moreover, controlling shape and architecture using particulate materials is often difficult, in comparison to more user-friendly soft/moldable sponges and polymers, which has likely hindered their clinical use for craniofacial applications where larger reconstruction is necessary.
Although the mechanisms of CaP-induced bone formation are still incompletely understood, there currently exists a breadth of pre-clinical evidence demonstrating the efficacy of these materials for translational applications. A noteworthy study by Yuan et al, for instance, demonstrated that an osteoinductive TCP ceramic material yielded comparable results to an autograft scaffold and scaffolds loaded with a potent osteoinductive growth factor (recombinant human bone morphogenetic protein, or rhBMP-2), where bridging of an ovine critical sized defect was obtained with comparable results regardless of the material utilized [21]. A series of clinical trials on various commercially available products also point to the promising use of CaP materials for orthopedic and craniofacial bone regeneration. Currently, according to clinicaltrials.gov there are over 300 clinical trials being conducted in the world testing the effectiveness of calcium phosphate materials for bone applications, with 60 already completed in the U.S. alone, and an additional 58 in Europe.
In summary, although many phases and compositions of CaP have shown the ability to induce bone formation, studies comparing HA against TCP, or of HA against biphasic calcium phosphate (consisting of HA with TCP), have generally demonstrated that the presence of a more soluble phase enhances bone formation [22–24], although some higher degree of stability in the mineral phase is generally required [25]. Moreover, it has been generally accepted that the surface structural properties of the CaP material, such as microporosity, grain size and specific surface area for adsorption, play an important role in osteoinduction, perhaps even more so than internal porosity. Internal porosity has long been suggested to be more effective in the range of at least 100 µm [26, 27], although other literature has claimed that not only macro-structural porosity, but rather surface micropores in the range of 100 nm to 10 µm are as important to ensure fast scaffold resorption [28].
An emerging area in the synthesis of CaP is the ability of ceramic materials to elicit not only osteogenic, but also vasculogenic properties, and it is relevant to highlight that it is unlikely that any regenerative material for bone formation will be successful without considering the challenges associated with bone vascularization. Novel methods of scaffold fabrication combining CaP materials with pre-fabricated blood vessels and capillaries present an interesting approach moving forward [29, 30] (Figure 2).
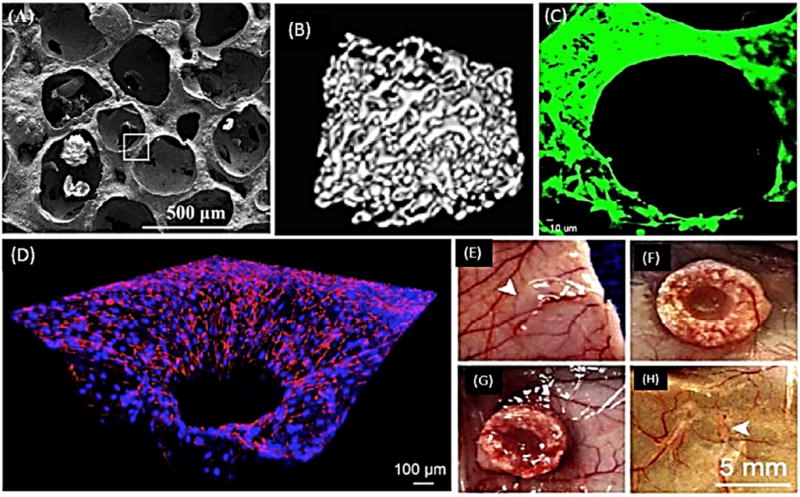
Figure 2.
Porous β-TCP scaffolds with angiogenic and osteogenic potentials. (A) Representative SEM image illustrating the 3D porous architecture of β-TCP scaffold. (B) Micro-CT images showing interconnected pores of scaffold in 3-D. (C) Fluorescent image showing the robust proliferation of HUVEC cells after 7 days of culture. (D) Immunofluorescent image depicting the endothelium lined microchannels of collagen infiltrated, macroporous β-TCP scaffold. (E–H) Photographs showing the subcutaneous implantation of four types of implants, namely (E) Collagen/HUVEC, (F) Collagen/HUVEC/β-TCP, (G) Collagen/Channel/β-TCP and (H) Collagen/Channel β-TCP-based grafts in nude mice.
A–C: From Kang Y, Kim S, Fahrenholtz M, et al. Osteogenic and angiogenic potentials of monocultured and co-cultured hBMSCs and HUVECs on 3D porous β-TCP scaffold. Acta Biomater 2013; 9(1): 4906–4915; with permission.
D–H: From Kang Y, Mochizuki N, Khademhosseini A, et al. Engineering a vascularized collagen-β-tricalcium phosphate graft using an electrochemical approach. Acta Biomater 2015; 11: 449–458; with permission.
Bio-inorganic substitution of CaP materials and bioglasses
Another area of research involving CaP involves doping. Many trace elements are present in the mineral phase of natural bone. Cationic substitution with Mg or Sr on CaP scaffolds can influence the mechanical properties and biological responses due to the changes in the physiochemical properties of CaPs, such as crytallinity, microstructure and solubility [32]. Tarafder investigated the influence of MgO and SrO doping of a β-TCP scaffolds in an animal model and found increased early bone formation in the doped vs. non-doped scaffolds [32]. In addition to the CaPs, bioactive glass and glass-ceramics are materials that have been thoroughly studied for their potential for bone regeneration [33]. Since 1969 when Hench and colleagues discovered that rat bone can bond chemically to certain silicate-based glass compositions, bioactive glass has been investigated and utilized clinically for bone regeneration purposes [4]. Bioactive glass has the ability to chemically react in physiologic body fluids resulting in the formation of a hydroxycarbonate apatite layer to which bone can bind [4]. Although bioactive glass and glass ceramics are still available in particulate form commercially, their limited strength and low fracture toughness have prevented their use for load-bearing implants, and therefore, the repair of larger bony defects at load-bearing anatomical sites remains a challenge [4]. However, due to the potential to improve the osteogenic cell response, bioactive glass is utilized as a filler or coating on polymer based scaffolds. A few examples of clinically available materials for bone regeneration relying on the action of bioactive glass include GlassBone® (Noraker), BonAlive® (BonAlive Biomaterials Ltda), Vitoss® (Stryker), and Perioglass® (NovaBone). Despite the promising results obtained from bioglass based materials, and the claimed advantageous properties of this class of materials over typical CaP sintered ceramics, it is often the long term clinical performance of these systems in-vivo that determine their reliability and efficacy. Therefore, bioglass based systems remain in their infancy as far as their craniofacial and orthopedic applications compared to other existing regenerative materials.
Biopolymers
Natural polymers
Organic scaffolds, such as polymer hydrogels, find use in the delivery of cells and/or growth factors for bone regeneration owing to their cytocompatibility, ability to stimulate an appropriate cellular response, porosity and controlled degradability under physiological conditions. Biopolymers of natural origin, such as collagen, gelatin, chitosan, silk, etc., are employed for this purpose since they mimic the structure, chemical composition and biochemical properties of the natural bone organic matrix, possess low immunogenic properties and are able to stimulate appropriate cell response and function while supporting tissue remodeling [34]. Collagen, for instance, which is the most abundant protein in the extracellular matrix (ECM) of vertebrates, is a logical choice as a biomaterial for tissue regeneration. The main drawback of pure collagen scaffolds remains their poor mechanical properties, which do not approach those of natural bone tissue. Additionally, collagen isolated from animal tissues poses a risk of infection and allergic reactions [41] and cannot be mass produced; although these concerns may be alleviated through the use of recombinant collagen [42]. Natural polysaccharides, such as chitosan, agarose and alginate, are other types of natural polymeric scaffolds. In addition to the desirable properties of all natural polymers, these materials possesses positively charged amino groups on their surface that allow for interactions with anions, such as DNA, lipids, proteins and even cell membranes. Particularly, the cationic nature of chitosan promotes interactions with glycosaminoglycans and proteoglycans which are known to stimulate cytokines and growth factors important for tissue regeneration [54]. This stimulatory effect has been evidenced in multiple studies where chitosan based scaffolds promoted new bone formation in in vivo models. Silk fibroin, another natural polymer, has also demonstrated ability to support cell proliferation [57], induce osteogenesis in vitro and bone formation in in vivo calvarial defect models [58, 59]. Still, despite their biocompatibility and osteoconductive nature, silk based scaffolds have been found to have low compressive strength, thus limiting their application to non-load bearing bone tissue sites [34].
Another category of natural bone substitutes that has been widely applied clinically is demineralized bone matrix (DBM), an allograft obtained by removing the mineral component of bone [43, 44]. DBM is predominantly composed of type I collagen (~90 %) along with various growth factors (bone sialoprotein, Osteopontin, BMPs, IGF1, etc.), in addition to residues of calcium-based particles, inorganic phosphates and some trace cell debris[45, 46]. Though commercially available DBM products are well known for their osteoinductive and osteoconductive nature, each product exhibits large variability in terms of processing conditions, sterilization methods of storage, donor specifications, etc. [47]. Upon comparing 3 different DBM products (Osteofil, Grafton, and Dynagraft) for reliability and efficacy, Wang et al.[48] reported differences in osteoinductive ability due to the inconsistency in processing conditions. It was also reported that different DBM products had variability in handling properties, ultimately resulting in intra-product differences during surgical procedure and after implantation [49]. For optimal surgical handling, DBM is often mixed with binders, such as glycerol (Grafton®), poloxamer carrier (Dynagraft®), hyaluronan (DBX®), gelatin (Regenafil®), calcium sulphate (AlloMatrix™), lecithin (InterGro®), carboxymethyl cellulose (OsteoSelect®), bovine collagen with sodium alginate (PROGENIX® Plus), among other examples. It is also available in the form of freeze-dried powder, granules, gel, putty, or strips [49–51]. Since, DBM possess limited structural support and mechanical strength, it has been commonly utilized as a bone graft extender in well supported, stable skeletal defects [52]. Nonetheless, DBM is considered advantageous over standard autografts as it revascularizes rapidly and facilitates the local endogenous release of growth factors and therapeutic agents to induce new bone formation.
The osteoinductive and vasculogenic potential of scaffolds may be further enhanced by the introduction of appropriate growth factors that induce chemotaxis, proliferation and differentiation of the encapsulated and surrounding cells. Platelet rich plasma (PRP), blood concentrated for thrombocytes, is an autologous source of physiological concentrations of PDGF, TGF-β1, TGF-β2, IGF-I, IGF-II, and VEGF [65] that has been successfully used in bone repair and regeneration [66, 67]. However, the lack of standardization in the method of preparation of PRP has led to discrepancies in the results [68]. Recently, a second generation platelet rich biomaterial, platelet rich fibrin (PRF) has been developed, which is simpler to produce as it does not require the use of the coagulating agents thrombin and calcium chloride [69]. This material has been demonstrably successful both individually and in concert with other scaffold materials in promoting osteogenic differentiation and augmenting bone formation [70, 71]. In addition to the growth factors enumerated above, PRF is rich in leukocytes, cytokines and glycoproteins that participate in wound healing, matrix remodeling, immune activity and stimulation of growth factors [72].
Synthetic polymers
Several synthetic polymer based scaffold materials have been developed, including poly (ε-caprolactone) (PCL), polylactic acid (PLA), polyglycolide (PGA), poly (lactide-co-glycolide) (PLGA), poly(propylene fumarate) (PPF) and polyhydroxyalkanoates (PHA). These have been designed to enable better control over a wider range of mechanical properties of the scaffold through variations in the concentrations and degrees of cross-linking of the polymers, or even through copolymerization of two or more of them. These polymers can be synthesized in large quantities under controlled conditions thus ensuring uniform and reproducible properties while negating risks of infections and immunogenicity. The poly (α hydroxyl) esters - PCL, PLA, PGA and their co-polymer PLGA are the most commonly used synthetic polymers for tissue engineering owing to their mechanical stability, cytocompatibility and resorbability [73]. While PLA and PGA are unsuitable as scaffolds for bone tissue owing to their low osteoconductivity and compressive strength respectively, PLGA co-polymers with varying ratios of PLA and PGA are more soluble, provide a wider range of mechanical properties, enhanced osteoconductivity and controlled rates of degradation [74]. PCL is yet another aliphatic polyester scaffold that is preferred for its flexibility and controlled rate of degradation. In vivo, these scaffolds undergo hydrolytic degradation wherein their monomeric degradation products are removed through natural pathways and are hence approved by the FDA for use in tissue engineering [73]. However, their degradation products are often acidic in nature causing undesirable local changes in pH. Furthermore, their hydrophobic nature is not conducive to cell attachment, and the absence of functional groups results in inferior osteoinduction [73]. These shortcomings may be somewhat diminished in composite scaffolds with hydrophilic polymers, such as polyethylene glycol (PEG), and by coating with natural biomaterials such as collagen [76]. Recent advances in scaffolds for bone regeneration involve the use of hybrid natural and synthetic biomaterials in order to take advantage of the benefits of each. Inclusion of PCL or PCL-PEG-PCL copolymer nanofibers in collagen [82, 83] or chitosan [84] serves to combine the biomimicry and stimulatory effects of natural polymers with the structural and mechanical stability of synthetic polymers, thus offering viable scaffold options with superior osteogenic potential.
Biomaterials for controlled delivery
A synergistic combination of cells, proteins, genes and biophysical signals are critical to trigger functional bone regeneration. In the native tissue milieu, the local presentation and spatiotemporal distribution of these combinatorial factors are highly orchestrated by ECM components. This native complex microenvironment has inspired the design and development of biomimetic and biodegradable material carriers possessing ECM-like properties for the controlled delivery and retention of regenerative factors at the injury site over a prolonged period. In light of the tremendous advantage of biomaterials for the targeted and sustained release of therapeutic agents, this section will discuss some of the recent developments in biomaterial-based delivery formulations, from a clinical and translational perspective.
Growth factor delivery
Many growth factors (GFs) have been clinically proven to play a key role in craniofacial growth and development, including TGF-β, FGF, VEGF, PDGF, IGFs and BMPs (BMP-2 and BMP-7) [85],[86],[87]. For example, recombinant human BMPs (rhBMPs), [88] one of the widely investigated FDA approved growth factors, are involved in various developmental processes critical for the formation of soft and hard callus, cranial neural crest, facial primordia, tooth, lip and palate.[89, 90] BMPs act in concert with TGF-β to modulate MSC differentiation during skeletal development, bone formation and bone homeostasis via the activation of the Smad-dependent signaling pathway or MAPK pathway. Likewise, FGF signaling is known to exhibit multiple functions in craniofacial skeletogenesis, [91] whereas PDGFs are potent mediators involved in wound healing, bone repair and remodeling during trauma/infection by inducing proliferation of osteoblastic precursor cells [92]. Similarly, IGFs have an important role in general growth and maintenance of the body skeleton. VEGF, apart from its role in proliferation, vascularization and ossification during bone formation is known to influence calvarial ossification as well as maxillary and palatal mesenchyme [93]. Altogether, an imbalance of these growth factors is associated with severe craniofacial anomalies. Thus, they can be utilized widely in clinical settings as potential therapeutic agents to augment the healing process [94, 95].
Though direct administration of GFs into damaged/degenerated tissues is considered an obvious strategy, often large doses and multiple injections are required to achieve specific biological responses in humans due to its shorter half-life in circulation, slow diffusion, rapid degradation and cleavage [96]. However in vivo, GFs are protected and stabilized by their binding to different extracellular matrix (ECM) molecules. Hence, selecting a suitable biomaterial carrier system has long been considered to be of critical importance to tailor the localized and sustained release of single or multiple GFs [97].
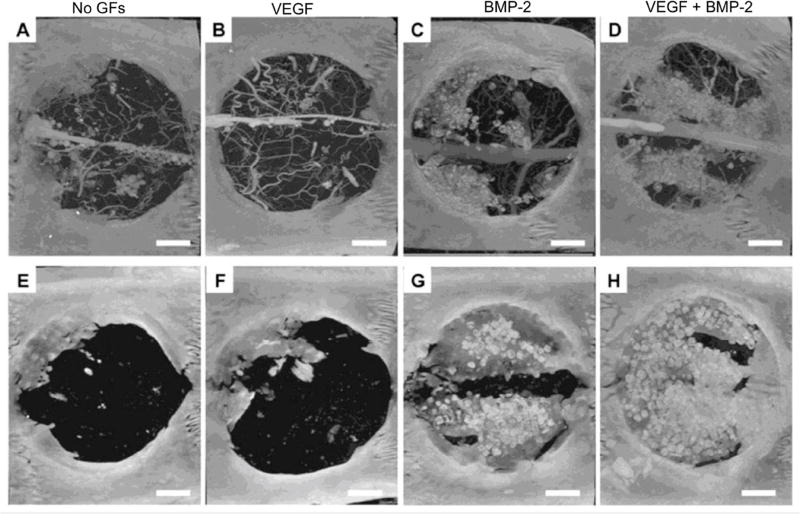
Currently, a plethora of delivery vehicles based on natural and synthetic polymers, inorganic biomaterials, and their composites have been designed in the form of sponges, nanofibrous membranes, micro/nanoparticles, and hydrogels, to either chemically or physically entrap GFs into or onto the substrate [99]. For instance, dual delivery of VEGF and BMP-2 has shown great capacity for nearly complete regeneration of a critical size defect in rats [98] (Figure 3). Depending on the mode of immobilization, the release rate of the GFs may be regulated by processes including diffusion, flow, erosion or degradation, surface charge, charge density, swelling, wettability, dissolution or via an on-demand triggering mechanism including pH, temperature, enzymes, light, electric/magnetic field, ultrasound, etc. [100]. Despite these extensive in vitro studies, the efficacy and safety of these delivery systems are not well established in preclinical and clinical stages.
Figure 3.
Microcomputed tomography images of bone regeneration in rat calvarial critical size defect at 4 (top row) and 12 (bottom row) weeks with no growth factor delivery (A, E), VEGF delivery only (B, F), BMP-2 delivery only (C, G) and VEGF/BMP-2 dual delivery (D, H). Scale bars −200 µm.
From Patel ZS, Young S, Tabata Y, et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008; 43(5): 937; with permission.
Notably, some of the commercially available products that are available for clinical use are based on rhBMPs. For instance, rhBMP-2 infiltrated within absorbable collagen sponge (Infuse® from Medtronic, USA) has FDA approval for various bone defects including sinus lift and localized alveolar ridge augmentation [101]. Similarly, OP-1® Putty (Stryker Biotech), comprised of bovine derived collagen incorporating rhBMP-7, is another commercially available graft material for non-union fractures. Other carrier based grafts recognized for clinical applications in the USA include beta-tricalcium phosphate (β-TCP) porous carrier infused with rhPDGF (GEM 21S® from Osteohealth, USA) to treat periodontally related bone defects and associated gingival recession [102]. Therefore, the inclusion of GFs within biomaterials not only sustains the release kinetics but also provide a porous osteoconductive framework for the bone ingrowth to occur.
Another interesting concept for accelerated bone regeneration is the combined or sequential delivery of multiple GFs. Though this approach seems extremely challenging due to difficulties in choosing the appropriate concentrations of GF cocktails, tailoring the release profiles, controlling the gradients and timings, etc., these dual or multiple delivery vehicles are proven to be effective in stimulating angiogenesis and bone healing [103–105]. For example, a Phase I/II human clinical trial displayed the efficacy and safety of a combination of PDGF/IGF-I in eliciting increased defect fill in periodontal lesions, when co-delivered in a methylcellulose gel vehicle [106].
Stem cell delivery
Another promising application of biomaterials is their use as stem cell-delivery vehicles. Preclinical studies have shown poor engraftment and survival of cells that are directly administered only in saline or media, due to their immediate encounter with harsh conditions such as hypoxia, inflammation, and reactive oxygen species [107]. Biomaterials can function as a substitute to native ECM, conferring a conducive framework for the attachment and growth of encapsulated cells, and thereby preventing anoikis, a form of apoptosis. Moreover, biomaterials can be optimized to offer protection against host immune attack, and they can be manipulated to induce major cellular processes necessary for tissue regeneration [108, 109]. Most of all, the attractive approach of using hydrogels as minimally invasive stem cell delivery vehicles open exciting avenues to reconstruct craniofacial defects resulting from trauma, disease, or congenital abnormalities, without the need for extensive invasive surgeries. For instance, the regenerative potential of injectable composite hydrogels for MSC delivery was demonstrated in a rat critical size cranial defect [110]. However, a drawback of this injectable hydrogel system in craniofacial regeneration is a lack of ability to provide 3D architecture and mechanical stability, especially in cases involving significant bone loss, such as in traumatic injury or oncologic surgery.
The cells that have been predominantly investigated under clinical trials to date include adult stem/progenitor cells from limbus, adipose tissue, bone marrow, placenta, dental pulp and periodontal ligament [111]. Owing to the limitation of the clinical use of pluripotent stem cells (ESCs and iPSCs), the alternate choice for bone and cartilage repair is MSC therapies. This work continues despite a few reports on clinical trial failures in the treatment of ulcerative colitis, ischemic stroke, cardiac repair, acute kidney injury, ischemic stroke, acute respiratory distress syndrome and critical limb ischemia [112]. At this time there are a limited number of commercially available bone graft materials incorporating MSCs for clinical use and these graft materials are all centered around demineralized bone matrix (DBM). The products include Allostem® (AlloSource), Map3™ (rti surgical), Osteocel Plus® (NuVasive) and Trinity Evolution Matrix™ (Orthofix)[113]. It is noteworthy to mention that some of the other commercially available bone grafts are also envisioned as carrier systems for MSC delivery, including collagen sponge (CopiOs sponge, Zimmer), βTCP (Vitoss®; Stryker), Collagen-βTCP composite (Collage™ Putty: Orthofix) and nanoHydroxyapatite – collagen carrier (nanOss® Bioactive (rti surgical) [113, 114]. Another non-invasive source of stem cells is the dental pulp and the inclusion of dental pulp stem/progenitor cells within collagen sponge was clinically established to restore human mandibular bone defects caused by the extraction of third molars [115]. In summary, biomaterial mediated stem cell delivery has significant potency to regenerate oral and maxillofacial structures, though its long term clinical safety and efficacy is yet to be determined.
Gene delivery
Owing to the limited bioactivity, in vivo instability and high hepatic/renal clearance rates of GFs, gene therapy has been proposed as an alternative to achieve localized and sustained gene expression at the defect site in order to achieve spatiotemporally coordinated protein synthesis.[116, 117] Though a variety of viral vector systems (adenovirus, adeno-associated virus, lentivirus, and retrovirus)[118] have been considered effective due to their high transfection efficiency, biomaterials are often preferred in terms of immunogenicity, safety, ease of manipulation and mutagenesis.[119] To date, several material based systems (lipid-, peptide-, and polymer-based systems) and various gene transfer approaches (microinjection, the “gene gun” and electroporation) have been evaluated for gene delivery [119]. Nevertheless, the rational design of a suitable biomaterial based vector for human clinical trials remain elusive, as the vectors have to bypass a series of systemic, extracellular and sub-cellular barriers including blood serum proteins/enzymes, cell membrane, endosomes and the nuclear membrane. [120–122] All the gene therapy clinical trials reported thus far, for a range of disorders (SCID-X1, cancer, cardiovascular disease, AIDS, cystic fibrosis, muscular dystrophy etc.) were mostly based on viral vectors [123, 124]. Despite encouraging results of non-viral biomaterial based gene delivery in in vitro and in vivo studies, no significant progress has been made toward attaining clinical success. Nevertheless, well-studied biomaterial-mediated gene delivery approaches involves the use of gene activated matrices (GAM), composed of porous collagen sponges for the site specific delivery of plasmid DNA directly to the fracture site.[118] Bonadio et al. established the potency of physically entrapping PTH 1–34/BMP-4 encoding cDNAs in these GAM for new bone formation in a beagle tibia critical defect model.[125, 126] Thus, direct delivery of pure DNA complexes using biomaterial carrier systems offers the flexibility of integrating cells, drugs and groups of other interacting factors to achieve multifunctional therapeutic benefits. Hence, it is believed that biomaterial mediated gene delivery technologies that are currently under development will eventually be used clinically to treat difficult bone loss problems.
3D Printing and Bioprinting
The rapid expansion of the field of 3D printing in the past 5 years has had a considerable and immediate impact in the area of craniofacial bone augmentation. 3D printing addresses a series of significant challenges that up to now have prevented bone tissue engineering from being translated into clinical practice. The benefits of 3D printing include the ability to control the internal and external 3D architecture of scaffold systems, the ease of fabrication of scaffolds that precisely match patient specific needs, the possibility of fabricating scaffolds with multiple materials, and the ability to control cell behavior and mechanical response by pre-defining scaffold architecture[134, 135]. We have addressed the characteristics of 3D printing that make this method especially relevant for craniofacial regeneration in a recent review [134].
While 3D printing and 3D bioprinting are concepts that often have been used interchangeably, in fact they involve different requirements as far as the materials and printing capabilities are concerned. 3D printing is often utilized to describe the fabrication of inert or bioactive scaffold materials without the presence of living cells, whereas 3D bioprinting generally refers to printing of cells and scaffolds together (cell-laden biomaterials) or dense aggregates of cells free from scaffold support [136]. Although there has been immense growth in the number of printing methods available in the past 5 years, the more well established 3D printing modalities for tissue engineering applications are typically categorized as extrusion printing, inkjet printing, laser printing and to a lesser some extent lithography printing (which shares similarities with the laser 3D printing modality). Extrusion 3D printers/bioprinters have been the most relevant for bone augmentation research because, compared to other printers, they allow rapid fabrication of the larger scale constructs required for clinically relevant tissue constructs. Moreover, depending on the materials and hardware characteristics, extrusion 3D printers can be tailored to dispense a wide range of materials that have proven osteoinductive capacity, including CaP injectable pastes, ceramic bases, cell-laden hydrogels, and other types of FDA approved medical grade polymers, such as polycaprolactone (PCL) [137–142].
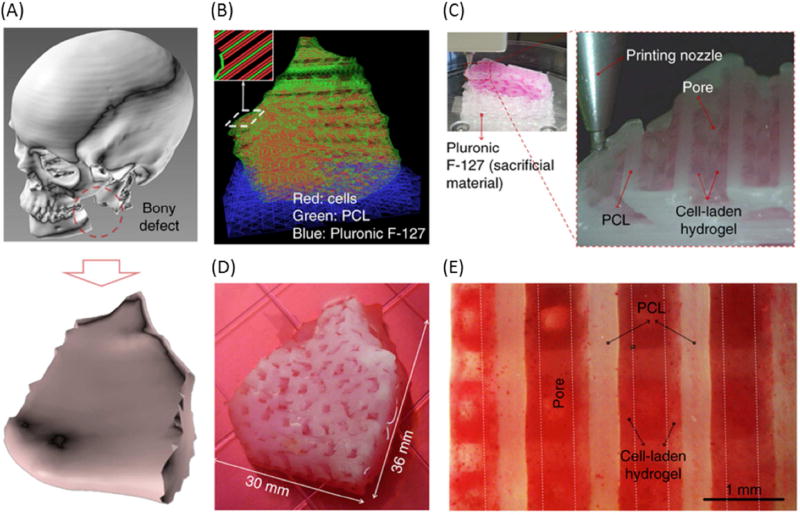
There are noteworthy examples that exemplify how 3D printers can revolutionize the manner in which craniofacial/long bone augmentation is conducted in the clinic. Recent work by Atala’s group demonstrated that a large mandibular bone defect could be regenerated using a sizable multi-typic tissue construct 3D bioprinted with cell-laden hydrogels and a supporting scaffold having controlled porosity to stimulate new vasculature formation (Figure 4) [135]. Another recent example of the successful application of 3D printed scaffolds for bone regeneration was the use of a 3D printed medical-grade PCL with TCP combined with rhBMP-7, which has been reported to enable bridging of a 30 mm long bone defect in a sheep model with superior results compared to the gold standard of autologous bone [143]. Post-surgical biomechanical and microcomputed tomography analyses after 12 months showed significantly greater bone formation and bone quality for the printed scaffolds compared to a bone autograft material.
Figure 4.
3D bioprinted human scale mandible and calvarial bone constructs. (A) 3D CAD model of mandible bony defect obtained by converting the medical CT scan data. (B) Visualized motion program depicting the required dispensing paths of cell-laden hydrogel (red), a mixture of PCL and tricalcium phosphate (green), as a scaffold, and Pluronic F127 (blue), which is used as a temporary support structure. (C) 3D patterning of cell laden hydrogel on PCL platform. (D) Macroscopic image of the 3D printed mandible bone defect construct, grown in osteogenic medium for 28 days. (E) Alizarin Red S staining indicates terminal osteogenic induction and mineral deposition in human amniotic fluid–derived stem cell.
From Kang HW, Lee SJ, Ko IK, et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 2016; 34(3): 312–319; with permission.
Examples of how these 3D printing technologies will be introduced to the market remain uncertain, as the FDA has yet to standardize a series of questions that relate to the process of 3D tissue/scaffold fabrication that go beyond simple validation of the printing material itself. Nevertheless, examples of companies commercializing pre-3D printed scaffold systems for craniofacial regeneration are already commercially available, such as OsteoFlux, a 3D printed CaP osteoinductive material sold by Vivos Dental AG. Despite the uncertainty that is common for the early adoption of a new technology, the current literature presents countless examples of how 3D printing methods can facilitate the fabrication of scaffolds with controlled structure architecture and porosity, of pre-vascularized tissue constructs [144], patient-specific characteristics [145], enhanced mechanical properties [143] and many other advantages. Therefore, one may argue that the future of craniofacial bone augmentation will most certainly cross paths with the next generation of 3D printed materials.[136]
Conclusions
Several decades of intense research have yielded a new generation of biomaterials and novel design strategies with limitless benefits of incorporating cells, drugs and other biochemical signals to promote the formation of engineered bone tissue. With the latest advent of 3D biofabrication technologies, the future of craniofacial reconstruction will witness patient specific surgical implants for large volume bone defects that can fully vascularize and rapidly integrate with the supporting host tissue. Further, the innovative approach of engineering the inherent biomaterial properties to regulate stem cell fate decisions in the host will not only have important implications in fostering bone regeneration but also will nullify the side effects of using biochemical inducers or soluble factors. Apart from the promise of replacing intricate craniofacial deformities with synthetic materials, the field of biomaterials also envisions additional breakthrough approaches involving a combination of physical, chemical, biological and engineering processes to stimulate accelerated bone regeneration.
Key points.
-
-
Calcium phosphate bioceramics remain some the most widely used biomaterials for bone regeneration, particularly owning to their long clinical track-record and well studied mechanisms.
-
-
Both natural and synthetic polymers, despite their comparatively low rigidity, offer a range of physical and biological advantages over bioceramics, such as the possibility of controlling 3D cellular microenvironments for stem cell differentiation and tissue regeneration.
-
-
Biomaterials can be synthesized and/or manipulated to be used for growth factor, gene and stem cell delivery applications with increasingly more successful outcomes.
-
-
3D printing and bioprinting have already revolutionized the field of bone regeneration, and it is likely that the next generation of biomaterials for bone regeneration will take advantage of some method of 3D printing.
Acknowledgments
The authors acknowledge funding from the National Institute of Dental and Craniofacial Research (NIDCR) and the National Institutes of Health (NIH) (R01DE026170 to LEB), and the Medical Research Foundation of Oregon (MRF to LEB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Bertassoni LE, Coelho PG. Engineering mineralized and load bearing tissues. New York, NY: Springer; 2015. [PubMed] [Google Scholar]
- 3.Annabi N, et al. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv Mater. 2014;26(1):85–123. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerhardt L-C, Boccaccini AR. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials. 2010;3(7):3867. doi: 10.3390/ma3073867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albee FH. Studies in Bone Growth: Triple Calcium Phosphate as a Stimulus to Osteogenesis. Ann Surg. 1920;71(1):32–9. doi: 10.1097/00000658-192001000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boonrungsiman S, et al. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc Natl Acad Sci U S A. 2012;109(35):14170–5. doi: 10.1073/pnas.1208916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorozhkin SV. Calcium orthophosphates: Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter. 2011;1(2):121–164. doi: 10.4161/biom.18790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habraken W, et al. Calcium phosphates in biomedical applications: materials for the future? Materials Today. 2016;19(2):69–87. [Google Scholar]
- 9.Chow L. Octacalcium phosphate. Karger Publishers; 2001. Solubility of calcium phosphates; pp. 94–111. [DOI] [PubMed] [Google Scholar]
- 10.Kamakura S, et al. Implanted octacalcium phosphate is more resorbable than beta-tricalcium phosphate and hydroxyapatite. J Biomed Mater Res. 2002;59(1):29–34. doi: 10.1002/jbm.1213. [DOI] [PubMed] [Google Scholar]
- 11.Galea LG, et al. Bone substitute: transforming beta-tricalcium phosphate porous scaffolds into monetite. Biomaterials. 2008;29(24–25):3400–7. doi: 10.1016/j.biomaterials.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 12.Steffen T, et al. Porous tricalcium phosphate and transforming growth factor used for anterior spine surgery. European Spine Journal. 2001;10(2):S132–S140. doi: 10.1007/s005860100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasten P, et al. Comparison of human bone marrow stromal cells seeded on calcium-deficient hydroxyapatite, β-tricalcium phosphate and demineralized bone matrix. Biomaterials. 2003;24(15):2593–2603. doi: 10.1016/s0142-9612(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 14.Munting E, Mirtchi AA, Lemaitre J. Bone repair of defects filled with a phosphocalcic hydraulic cement: an in vivo study. Journal of Materials Science: Materials in Medicine. 1993;4(3):337–344. [Google Scholar]
- 15.Galea LG, et al. Bone substitute: Transforming β-tricalcium phosphate porous scaffolds into monetite. Biomaterials. 2008;29(24–25):3400–3407. doi: 10.1016/j.biomaterials.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 16.Constantz BR, et al. Histological, chemical, and crystallographic analysis of four calcium phosphate cements in different rabbit osseous sites. J Biomed Mater Res. 1998;43(4):451–61. doi: 10.1002/(sici)1097-4636(199824)43:4<451::aid-jbm13>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Bohner M, et al. Compositional changes of a dicalcium phosphate dihydrate cement after implantation in sheep. Biomaterials. 2003;24(20):3463–3474. doi: 10.1016/s0142-9612(03)00234-5. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki O, et al. Bone Formation on Synthetic Precursors of Hydroxyapatite. The Tohoku Journal of Experimental Medicine. 1991;164(1):37–50. doi: 10.1620/tjem.164.37. [DOI] [PubMed] [Google Scholar]
- 19.Kamakura S, et al. Implanted octacalcium phosphate is more resorbable than β-tricalcium phosphate and hydroxyapatite. Journal of Biomedical Materials Research. 2002;59(1):29–34. doi: 10.1002/jbm.1213. [DOI] [PubMed] [Google Scholar]
- 20.Barradas AM, et al. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials. 2012;33(11):3205–15. doi: 10.1016/j.biomaterials.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Yuan H, et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proceedings of the National Academy of Sciences. 2010;107(31):13614–13619. doi: 10.1073/pnas.1003600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan H, et al. A comparison of bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) implanted in muscle and bone of dogs at different time periods. Journal of Biomedical Materials Research Part A. 2006;78A(1):139–147. doi: 10.1002/jbm.a.30707. [DOI] [PubMed] [Google Scholar]
- 23.Yuan H, et al. Cross-species comparison of ectopic bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) scaffolds. Tissue Eng. 2006;12(6):1607–15. doi: 10.1089/ten.2006.12.1607. [DOI] [PubMed] [Google Scholar]
- 24.Habibovic P, et al. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials. 2005;26(17):3565–3575. doi: 10.1016/j.biomaterials.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 25.Habibovic P, et al. Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. Journal of Orthopaedic Research. 2008;26(10):1363–1370. doi: 10.1002/jor.20648. [DOI] [PubMed] [Google Scholar]
- 26.Hulbert SF, et al. Potential of ceramic materials as permanently implantable skeletal prostheses. Journal of Biomedical Materials Research. 1970;4(3):433–456. doi: 10.1002/jbm.820040309. [DOI] [PubMed] [Google Scholar]
- 27.Klawitter JJ, Hulbert SF. Application of porous ceramics for the attachment of load bearing internal orthopedic applications. Journal of Biomedical Materials Research. 1971;5(6):161–229. [Google Scholar]
- 28.Klein CPAT, et al. Interaction of biodegradable β-whitlockite ceramics with bone tissue: An in vivo study. Biomaterials. 1985;6(3):189–192. doi: 10.1016/0142-9612(85)90008-0. [DOI] [PubMed] [Google Scholar]
- 29.Kang Y, et al. Engineering a vascularized collagen-β-tricalcium phosphate graft using an electrochemical approach. Acta Biomaterialia. 2015;11:449–458. doi: 10.1016/j.actbio.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang Y, et al. Osteogenic and angiogenic potentials of monocultured and co-cultured human-bone-marrow-derived mesenchymal stem cells and human-umbilical-vein endothelial cells on three-dimensional porous beta-tricalcium phosphate scaffold. Acta Biomater. 2013;9(1):4906–15. doi: 10.1016/j.actbio.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang Y, et al. Osteogenic and angiogenic potentials of monocultured and co-cultured human-bone-marrow-derived mesenchymal stem cells and human-umbilical-vein endothelial cells on three-dimensional porous beta-tricalcium phosphate scaffold. Acta Biomaterialia. 2013;9(1):4906–4915. doi: 10.1016/j.actbio.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarafder S, et al. 3D printed tricalcium phosphate scaffolds: Effect of SrO and MgO doping on in vivo osteogenesis in a rat distal femoral defect model. Biomater Sci. 2013;1(12):1250–1259. doi: 10.1039/C3BM60132C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaur G, et al. A review of bioactive glasses: Their structure, properties, fabrication and apatite formation. J Biomed Mater Res A. 2014;102(1):254–74. doi: 10.1002/jbm.a.34690. [DOI] [PubMed] [Google Scholar]
- 34.Stoppel WL, et al. Clinical applications of naturally derived biopolymer-based scaffolds for regenerative medicine. Ann Biomed Eng. 2015;43(3):657–80. doi: 10.1007/s10439-014-1206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai K-S, et al. Type I collagen promotes proliferation and osteogenesis of human mesenchymal stem cells via activation of ERK and Akt pathways. Journal of Biomedical Materials Research Part A. 2010;94A(3):673–682. doi: 10.1002/jbm.a.32693. [DOI] [PubMed] [Google Scholar]
- 36.Themistocleous GS, et al. Implants of type I collagen gel containing MG-63 osteoblast-like cells can act as stable scaffolds stimulating the bone healing process at the sites of the surgically-produced segmental diaphyseal defects in male rabbits. In Vivo. 2007;21(1):69–76. [PubMed] [Google Scholar]
- 37.Shih YR, et al. Growth of mesenchymal stem cells on electrospun type I collagen nanofibers. Stem Cells. 2006;24(11):2391–7. doi: 10.1634/stemcells.2006-0253. [DOI] [PubMed] [Google Scholar]
- 38.O'brien FJ. Biomaterials & scaffolds for tissue engineering. Materials today. 2011;14(3):88–95. [Google Scholar]
- 39.Yoshioka SA, Goissis G. Thermal and spectrophotometric studies of new crosslinking method for collagen matrix with glutaraldehyde acetals. J Mater Sci Mater Med. 2008;19(3):1215–23. doi: 10.1007/s10856-007-0151-0. [DOI] [PubMed] [Google Scholar]
- 40.Yunoki S, Matsuda T. Simultaneous processing of fibril formation and cross-linking improves mechanical properties of collagen. Biomacromolecules. 2008;9(3):879–85. doi: 10.1021/bm7012058. [DOI] [PubMed] [Google Scholar]
- 41.Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res B Appl Biomater. 2004;71(2):343–54. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 42.Adachi T, et al. Generation of hybrid transgenic silkworms that express Bombyx mori prolyl-hydroxylase alpha-subunits and human collagens in posterior silk glands: Production of cocoons that contained collagens with hydroxylated proline residues. J Biotechnol. 2006;126(2):205–19. doi: 10.1016/j.jbiotec.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 43.Sawkins MJ, et al. Hydrogels derived from demineralized and decellularized bone extracellular matrix. Acta Biomater. 9(8):7865–7873. doi: 10.1016/j.actbio.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolk A, et al. Current trends and future perspectives of bone substitute materials - from space holders to innovative biomaterials. J Craniomaxillofac Surg. 2012;40(8):706–18. doi: 10.1016/j.jcms.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Gruskin E, et al. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev. 2012;64(12):1063–77. doi: 10.1016/j.addr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holt DJ, Grainger DW. Demineralized bone matrix as a vehicle for delivering endogenous and exogenous therapeutics in bone repair. Adv Drug Deliv Rev. 2012;64(12):1123–8. doi: 10.1016/j.addr.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Kinney RC, et al. Demineralized bone matrix for fracture healing: fact or fiction? J Orthop Trauma. 2010;24(Suppl 1):S52–5. doi: 10.1097/BOT.0b013e3181d07ffa. [DOI] [PubMed] [Google Scholar]
- 48.Wang JC, et al. A comparison of commercially available demineralized bone matrix for spinal fusion. Eur Spine J. 2007;16(8):1233–40. doi: 10.1007/s00586-006-0282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Acarturk TO, Hollinger JO. Commercially available demineralized bone matrix compositions to regenerate calvarial critical-sized bone defects. Plast Reconstr Surg. 2006;118(4):862–73. doi: 10.1097/01.prs.0000232385.81219.87. [DOI] [PubMed] [Google Scholar]
- 50.Matassi F, et al. New biomaterials for bone regeneration. Clinical Cases in Mineral and Bone Metabolism. 2011;8(1):21–24. [PMC free article] [PubMed] [Google Scholar]
- 51.Drosos GI, et al. Use of demineralized bone matrix in the extremities. World Journal of Orthopedics. 2015;6(2):269–277. doi: 10.5312/wjo.v6.i2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lieberman JR, Friedlaender GE. Bone Regeneration and Repair: Biology and Clinical Applications. Humana Press; 2007. [Google Scholar]
- 53.Croisier F, Jérôme C. Chitosan-based biomaterials for tissue engineering. European Polymer Journal. 2013;49(4):780–792. [Google Scholar]
- 54.Costa-Pinto AR, Reis RL, Neves NM. Scaffolds based bone tissue engineering: the role of chitosan. Tissue Eng Part B Rev. 2011;17(5):331–47. doi: 10.1089/ten.teb.2010.0704. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Stegemann JP. Thermogelling chitosan and collagen composite hydrogels initiated with beta-glycerophosphate for bone tissue engineering. Biomaterials. 2010;31(14):3976–85. doi: 10.1016/j.biomaterials.2010.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Costa-Pinto AR, et al. Chitosan-poly(butylene succinate) scaffolds and human bone marrow stromal cells induce bone repair in a mouse calvaria model. J Tissue Eng Regen Med. 2012;6(1):21–8. doi: 10.1002/term.391. [DOI] [PubMed] [Google Scholar]
- 57.Meechaisue C, et al. Preparation of electrospun silk fibroin fiber mats as bone scaffolds: a preliminary study. Biomed Mater. 2007;2(3):181–8. doi: 10.1088/1748-6041/2/3/003. [DOI] [PubMed] [Google Scholar]
- 58.Uebersax L, et al. Biocompatibility and osteoconduction of macroporous silk fibroin implants in cortical defects in sheep. Eur J Pharm Biopharm. 2013;85(1):107–18. doi: 10.1016/j.ejpb.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, et al. The osteogenic properties of CaP/silk composite scaffolds. Biomaterials. 2010;31(10):2848–56. doi: 10.1016/j.biomaterials.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 60.Mata A, et al. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials. 2010;31(23):6004–12. doi: 10.1016/j.biomaterials.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holmes TC. Novel peptide-based biomaterial scaffolds for tissue engineering. Trends Biotechnol. 2002;20(1):16–21. doi: 10.1016/s0167-7799(01)01840-6. [DOI] [PubMed] [Google Scholar]
- 62.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–8. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 63.Kantlehner M, et al. Surface coating with cyclic RGD peptides stimulates osteoblast adhesion and proliferation as well as bone formation. Chembiochem. 2000;1(2):107–14. doi: 10.1002/1439-7633(20000818)1:2<107::AID-CBIC107>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 64.Gandavarapu NR, et al. Extracellular matrix protein adsorption to phosphate-functionalized gels from serum promotes osteogenic differentiation of human mesenchymal stem cells. Acta Biomater. 2013;9(1):4525–34. doi: 10.1016/j.actbio.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17(2):184–90. [PubMed] [Google Scholar]
- 66.Nash TJ, et al. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994;15(2):203–8. doi: 10.1016/8756-3282(94)90709-9. [DOI] [PubMed] [Google Scholar]
- 67.Aghaloo TL, Moy PK, Freymiller EG. Investigation of platelet-rich plasma in rabbit cranial defects: A pilot study. J Oral Maxillofac Surg. 2002;60(10):1176–81. doi: 10.1053/joms.2002.34994. [DOI] [PubMed] [Google Scholar]
- 68.Sanchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93–103. [PubMed] [Google Scholar]
- 69.Choukroun J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 70.Lee EH, et al. A combination graft of low-molecular-weight silk fibroin with Choukroun platelet-rich fibrin for rabbit calvarial defect. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(5):e33–8. doi: 10.1016/j.tripleo.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, et al. Osteoblastic mesenchymal stem cell sheet combined with Choukroun platelet-rich fibrin induces bone formation at an ectopic site. J Biomed Mater Res B Appl Biomater. 2015;103(6):1204–16. doi: 10.1002/jbm.b.33288. [DOI] [PubMed] [Google Scholar]
- 72.Anitua E, et al. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24(5):227–34. doi: 10.1016/j.tibtech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 73.Gunatillake PA, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;5:1–16. doi: 10.22203/ecm.v005a01. discussion 16. [DOI] [PubMed] [Google Scholar]
- 74.Gentile P, et al. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15(3):3640–59. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Remya KR, et al. Nanohydroxyapatite incorporated electrospun polycaprolactone/polycaprolactone-polyethyleneglycol-polycaprolactone blend scaffold for bone tissue engineering applications. J Biomed Nanotechnol. 2013;9(9):1483–94. doi: 10.1166/jbn.2013.1640. [DOI] [PubMed] [Google Scholar]
- 76.Wang T, et al. Osteoinduction and proliferation of bone-marrow stromal cells in three-dimensional poly (epsilon-caprolactone)/hydroxyapatite/collagen scaffolds. J Transl Med. 2015;13:152. doi: 10.1186/s12967-015-0499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen GQ, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 2005;26(33):6565–78. doi: 10.1016/j.biomaterials.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 78.Cool SM, et al. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) composite biomaterials for bone tissue regeneration: in vitro performance assessed by osteoblast proliferation, osteoclast adhesion and resorption, and macrophage proinflammatory response. J Biomed Mater Res A. 2007;82(3):599–610. doi: 10.1002/jbm.a.31174. [DOI] [PubMed] [Google Scholar]
- 79.Doyle C, Tanner ET, Bonfield W. In vitro and in vivo evaluation of polyhydroxybutyrate and of polyhydroxybutyrate reinforced with hydroxyapatite. Biomaterials. 1991;12(9):841–7. doi: 10.1016/0142-9612(91)90072-i. [DOI] [PubMed] [Google Scholar]
- 80.Timmer MD, et al. Fabrication of poly(propylene fumarate)-based orthopaedic implants by photo-crosslinking through transparent silicone molds. Biomaterials. 2003;24(25):4707–14. doi: 10.1016/s0142-9612(03)00364-8. [DOI] [PubMed] [Google Scholar]
- 81.Fisher JP, et al. Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model. J Biomed Mater Res. 2002;59(3):547–56. doi: 10.1002/jbm.1268. [DOI] [PubMed] [Google Scholar]
- 82.Baylan N, et al. Polycaprolactone nanofiber interspersed collagen type-I scaffold for bone regeneration: a unique injectable osteogenic scaffold. Biomed Mater. 2013;8(4):045011. doi: 10.1088/1748-6041/8/4/045011. [DOI] [PubMed] [Google Scholar]
- 83.Fu S, et al. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials. 2012;33(19):4801–9. doi: 10.1016/j.biomaterials.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 84.Yang X, Chen X, Wang H. Acceleration of osteogenic differentiation of preosteoblastic cells by chitosan containing nanofibrous scaffolds. Biomacromolecules. 2009;10(10):2772–8. doi: 10.1021/bm900623j. [DOI] [PubMed] [Google Scholar]
- 85.Tollemar V, et al. Stem cells, growth factors and scaffolds in craniofacial regenerative medicine. Genes Dis. 2016;3(1):56–71. doi: 10.1016/j.gendis.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Hout WM, et al. Reconstruction of the alveolar cleft: can growth factor-aided tissue engineering replace autologous bone grafting? A literature review and systematic review of results obtained with bone morphogenetic protein-2. Clin Oral Investig. 2011;15(3):297–303. doi: 10.1007/s00784-011-0547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sukul M, et al. Effect of Local Sustainable Release of BMP2-VEGF from Nano-Cellulose Loaded in Sponge Biphasic Calcium Phosphate on Bone Regeneration. Tissue Eng Part A. 2015;21(11–12):1822–36. doi: 10.1089/ten.tea.2014.0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007;77(8):626–31. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- 89.Oryan A, et al. Bone morphogenetic proteins: a powerful osteoinductive compound with non-negligible side effects and limitations. Biofactors. 2014;40(5):459–81. doi: 10.1002/biof.1177. [DOI] [PubMed] [Google Scholar]
- 90.Nie X, Luukko K, Kettunen P. BMP signalling in craniofacial development. Int J Dev Biol. 2006;50(6):511–21. doi: 10.1387/ijdb.052101xn. [DOI] [PubMed] [Google Scholar]
- 91.Nie X, Luukko K, Kettunen P. FGF signalling in craniofacial development and developmental disorders. Oral Dis. 2006;12(2):102–11. doi: 10.1111/j.1601-0825.2005.01176.x. [DOI] [PubMed] [Google Scholar]
- 92.Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29(12):1795–803. doi: 10.1002/jor.21462. [DOI] [PubMed] [Google Scholar]
- 93.Duan X, et al. VEGF stimulates intramembranous bone formation during craniofacial skeletal development. Matrix Biol. 2016;52–54:127–40. doi: 10.1016/j.matbio.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schilephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg. 2002;31(5):469–84. doi: 10.1054/ijom.2002.0244. [DOI] [PubMed] [Google Scholar]
- 95.Francis CS, et al. rhBMP-2 with a demineralized bone matrix scaffold versus autologous iliac crest bone graft for alveolar cleft reconstruction. Plast Reconstr Surg. 2013;131(5):1107–15. doi: 10.1097/PRS.0b013e3182865dfb. [DOI] [PubMed] [Google Scholar]
- 96.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8(55):153–70. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Azevedo HS, Pashkuleva I. Biomimetic supramolecular designs for the controlled release of growth factors in bone regeneration. Adv Drug Deliv Rev. 2015;94:63–76. doi: 10.1016/j.addr.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 98.Patel ZS, et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931–40. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee SH, Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 2007;59(4–5):339–59. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 100.King WJ, Krebsbach PH. Growth factor delivery: how surface interactions modulate release in vitro and in vivo. Adv Drug Deliv Rev. 2012;64(12):1239–56. doi: 10.1016/j.addr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Triplett RG, et al. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2009;67(9):1947–60. doi: 10.1016/j.joms.2009.04.085. [DOI] [PubMed] [Google Scholar]
- 102.Kretlow JD, et al. Injectable biomaterials for regenerating complex craniofacial tissues. Adv Mater. 2009;21(32–33):3368–93. doi: 10.1002/adma.200802009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Richardson TP, et al. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 104.Strobel C, et al. Sequential release kinetics of two (gentamicin and BMP-2) or three (gentamicin, IGF-I and BMP-2) substances from a one-component polymeric coating on implants. J Control Release. 2011;156(1):37–45. doi: 10.1016/j.jconrel.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 105.Mehta M, et al. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Deliv Rev. 2012;64(12):1257–76. doi: 10.1016/j.addr.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Howell TH, et al. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol. 1997;68(12):1186–93. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- 107.O'Neill HS, et al. Biomaterial-Enhanced Cell and Drug Delivery: Lessons Learned in the Cardiac Field and Future Perspectives. Adv Mater. 2016;28(27):5648–61. doi: 10.1002/adma.201505349. [DOI] [PubMed] [Google Scholar]
- 108.Qi C, et al. Biomaterials as carrier, barrier and reactor for cell-based regenerative medicine. Protein Cell. 2015;6(9):638–53. doi: 10.1007/s13238-015-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell. 2008;2(3):205–13. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 110.Vo TN, et al. Injectable dual-gelling cell-laden composite hydrogels for bone tissue engineering. Biomaterials. 2016;83:1–11. doi: 10.1016/j.biomaterials.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17(1):11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 112.Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 17(1):11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 113.Temple HT, Malinin TI. Orthobiologics in the Foot and Ankle. Foot and Ankle Clinics. 2016;21(4):809–823. doi: 10.1016/j.fcl.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 114.Nandi S, et al. Orthopaedic applications of bone graft & graft substitutes: a review. 2010 [PubMed] [Google Scholar]
- 115.d'Aquino R, et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- 116.Ito H, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11(3):291–7. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scheller EL, Villa-Diaz LG, Krebsbach PH. Gene therapy: implications for craniofacial regeneration. J Craniofac Surg. 2012;23(1):333–7. doi: 10.1097/SCS.0b013e318241dc11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Winn SR, et al. Gene therapy approaches for modulating bone regeneration. Adv Drug Deliv Rev. 2000;42(1–2):121–38. doi: 10.1016/s0169-409x(00)00057-0. [DOI] [PubMed] [Google Scholar]
- 119.Han S, et al. Development of biomaterials for gene therapy. Mol Ther. 2000;2(4):302–17. doi: 10.1006/mthe.2000.0142. [DOI] [PubMed] [Google Scholar]
- 120.Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58(4):467–86. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 121.Dang JM, Leong KW. Natural polymers for gene delivery and tissue engineering. Adv Drug Deliv Rev. 2006;58(4):487–99. doi: 10.1016/j.addr.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 122.Franceschi RT. Biological approaches to bone regeneration by gene therapy. J Dent Res. 2005;84(12):1093–103. doi: 10.1177/154405910508401204. [DOI] [PubMed] [Google Scholar]
- 123.Kohn DB. Gene therapy for XSCID: the first success of gene therapy. Pediatr Res. 2000;48(5):578. doi: 10.1203/00006450-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 124.Kumar SRP, et al. Clinical development of gene therapy: results and lessons from recent successes. Molecular Therapy - Methods & Clinical Development. 2016;3 doi: 10.1038/mtm.2016.34. Article 16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baltzer AW, Lieberman JR. Regional gene therapy to enhance bone repair. Gene Ther. 2004;11(4):344–50. doi: 10.1038/sj.gt.3302195. [DOI] [PubMed] [Google Scholar]
- 126.Bonadio J, et al. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5(7):753–9. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 127.Breitbart AS, et al. Gene-enhanced tissue engineering: applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg. 1999;42(5):488–95. [PubMed] [Google Scholar]
- 128.Schek RM, et al. Tissue engineering osteochondral implants for temporomandibular joint repair. Orthod Craniofac Res. 2005;8(4):313–9. doi: 10.1111/j.1601-6343.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 129.Chang SC, et al. Ex vivo gene therapy in autologous critical-size craniofacial bone regeneration. Plast Reconstr Surg. 2003;112(7):1841–50. doi: 10.1097/01.PRS.0000091168.73462.1A. [DOI] [PubMed] [Google Scholar]
- 130.D'Mello SR, et al. A Pilot Study Evaluating Combinatorial and Simultaneous Delivery of Polyethylenimine-Plasmid DNA Complexes Encoding for VEGF and PDGF for Bone Regeneration in Calvarial Bone Defects. Curr Pharm Biotechnol. 2015;16(7):655–60. doi: 10.2174/138920101607150427112753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kushibiki T, Tabata Y. Future Direction of Gene Therapy in Tissue Engineering. Topics in Tis sue Engineering. 2005:1–33. [Google Scholar]
- 132.L Santos J, et al. Non-viral gene delivery to mesenchymal stem cells: methods, strategies and application in bone tissue engineering and regeneration. Current gene therapy. 2011;11(1):46–57. doi: 10.2174/156652311794520102. [DOI] [PubMed] [Google Scholar]
- 133.Tokatlian T, et al. Design and characterization of microporous hyaluronic acid hydrogels for in vitro gene transfer to mMSCs. Acta Biomater. 2012;8(11):3921–31. doi: 10.1016/j.actbio.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Obregon F, et al. Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial Tissue Engineering. Journal of Dental Research. 2015;94(9 suppl):143S–152S. doi: 10.1177/0022034515588885. [DOI] [PubMed] [Google Scholar]
- 135.Kang H-W, et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotech. 2016;34(3):312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 136.Obregon F, et al. Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial Tissue Engineering. J Dent Res. 2015;94(9 Suppl):143S–52S. doi: 10.1177/0022034515588885. [DOI] [PubMed] [Google Scholar]
- 137.Trombetta R, et al. 3D Printing of Calcium Phosphate Ceramics for Bone Tissue Engineering and Drug Delivery. Ann Biomed Eng. 2017;45(1):23–44. doi: 10.1007/s10439-016-1678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Inzana JA, et al. 3D Printing of Composite Calcium Phosphate and Collagen Scaffolds for Bone Regeneration. Biomaterials. 2014;35(13):4026–4034. doi: 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Do A-V, et al. 3D Printing of Scaffolds for Tissue Regeneration Applications. Advanced healthcare materials. 2015;4(12):1742–1762. doi: 10.1002/adhm.201500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shim J-H, et al. Comparative Efficacies of a 3D-Printed PCL/PLGA/β-TCP Membrane and a Titanium Membrane for Guided Bone Regeneration in Beagle Dogs. Polymers. 2015;7(10):1500. [Google Scholar]
- 141.Wu Z, et al. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Scientific Reports. 2016;6:24474. doi: 10.1038/srep24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 143.Reichert JC, et al. A Tissue Engineering Solution for Segmental Defect Regeneration in Load-Bearing Long Bones. Science Translational Medicine. 2012;4(141):141ra93–141ra93. doi: 10.1126/scitranslmed.3003720. [DOI] [PubMed] [Google Scholar]
- 144.Bertassoni LE, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab on a Chip. 2014;14(13):2202–2211. doi: 10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morrison RJ, et al. Mitigation of tracheobronchomalacia with 3D-printed personalized medical devices in pediatric patients. Science Translational Medicine. 2015;7(285):285ra64–285ra64. doi: 10.1126/scitranslmed.3010825. [DOI] [PMC free article] [PubMed] [Google Scholar]