Abstract
Background
Patients with obstructive (≥50% stenosis) left main (LM) coronary artery disease (CAD) are at high risk for adverse events; prior studies have also documented worse outcomes among women than men with severe multivessel/LM CAD. However, the prognostic significance of nonobstructive (1-49% stenosis) LM CAD, including sex-specific differences, have not been previously examined.
Methods and Results
In the long-term CONFIRM registry, patients underwent elective coronary computed tomographic angiography (CCTA) for suspected CAD and were followed for 5 years. After excluding those with obstructive LM CAD, 5,166 patients were categorized as having normal LM or nonobstructive LM (18% of cohort). Cumulative 5-year incidence of death, myocardial infarction, or revascularization was higher among patients with nonobstructive LM than normal LM in both women and men: women (34.3% versus 15.4%, p<0.0001); men (24.6% versus 18.2%, p<0.0001). A significant interaction existed between sex and LM status for the composite outcome (p=0.001). In multivariable Cox regression, the presence of nonobstructive LM plaque increased the risk for the composite outcome in women (HRadj 1.48, p=0.005), but not in men (HRadj 0.98, p=0.806). In subgroup analysis, women with nonobstructive LM CAD had a nearly 80% higher risk for events than men with nonobstructive LM CAD (HRadj 1.78, p=0.017); sex-specific interactions were not observed across other patterns (e.g. location or extent) of nonobstructive plaque.
Conclusion
Nonobstructive LM CAD was frequently detected on CCTA and strongly associated with adverse events among women. Recognizing the sex-specific prognostic significance of nonobstructive LM plaque may augment risk stratification efforts.
Keywords: Left main, Nonobstructive coronary artery disease, Coronary computed tomographic angiography, Sex disparities in coronary heart disease
Obstructive left main (LM) coronary artery disease (CAD), defined as ≥50% luminal stenosis, is associated with significant morbidity and mortality.1 Although the prevalence and burden of obstructive CAD is higher among men, prior studies have described worse outcomes among women than men with severe multivessel or LM CAD, including after revascularization.2-5 Despite abundant prognostic evidence regarding patients with obstructive LM CAD, clinical outcomes of patients with nonobstructive (1-49% luminal stenosis) LM CAD, including sex-specific differences, have not been previously evaluated.
Importantly, nonobstructive CAD is frequently identified on coronary angiography among patients with stable ischemic heart disease (SIHD) and is more prevalent in symptomatic women (∼60%) than men (∼30%).4, 6, 7 Furthermore, recent investigations have described a strong association between nonobstructive CAD and adverse cardiovascular events in both invasive and noninvasive angiographic cohorts, however, comparative prognostic data of women versus men with nonobstructive CAD are limited.8-14 These findings have prompted increased efforts to examine the importance of nonobstructive CAD, including characterizing sex-related differences in outcomes, as a means to improve prognostic models and more precisely identify at-risk patients to target preventive care.7 Notably, nonobstructive plaque within the LM has not been an emphasis within any of these studies to date.
Accordingly, we sought to determine the prognostic significance of nonobstructive LM CAD in a large, ‘real-world’ cohort of patients who underwent elective coronary computed tomographic angiography (CCTA) for the evaluation of suspected CAD. Our objectives were to (1) assess the association between nonobstructive LM CAD and clinical outcomes including all-cause mortality, nonfatal myocardial infarction (MI), and coronary revascularization; and (2) determine whether sex-specific differences in outcomes exist among patients with nonobstructive LM CAD and compared to other subgroups of nonobstructive CAD.
Methods
Study Cohort
In the long-term phase of the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) registry, a total of 12,086 stable outpatients underwent elective CCTA for evaluation of clinically suspected CAD and had prospective follow-up extended to 5 years. A total of 17 participating sites from 9 countries enrolled patients between 2002 and 2009. All sites received institutional review board approval and oversight, and all patients provided informed consent. Patient and site identifiers were not entered into the CONFIRM database. Additional details regarding the CONFIRM registry's design, rationale, site eligibility, and patient recruitment have been previously described.15
The inclusion criteria for our analysis reflected the enrollment indications of the CONFIRM registry, including: (1) adults ≥18 years of age, (2) referral for CCTA to evaluate for suspected CAD given presenting symptoms or for risk stratification using a ≥64-detector row scanner, (3) prospective data collection of CAD risk factors and CCTA data, and (4) standardized reporting of segmental coronary stenosis, as per Society of Cardiovascular Computer Tomography (SCCT) guidelines.16, 17 Excluded from our study were patients with obstructive LM CAD or missing LM stenosis severity (n=1,147), history of known CAD, previous percutaneous coronary intervention, or previous coronary artery bypass surgery (n=1,416), and incomplete adjudication of clinical events (n=4,357) for a final cohort size of 5,166 patients. All CONFIRM investigators have reviewed and approved our study.
Clinical Descriptive Data
All patients enrolled in CONFIRM underwent evaluation by a physician or nurse prior to CCTA. Each participating site uniformly collected self-reported baseline clinical data including age, gender, history of hypertension (HTN), diabetes mellitus (DM), hyperlipidemia (HLD), smoking status, early family history of early CAD (father <55 or mother <65 years of age), left ventricular ejection fraction (LVEF), and presenting symptom characteristics categorized as no chest pain, non-anginal chest pain, atypical angina, or typical angina.
CCTA Protocol and Anatomic Definitions
Each CONFIRM site was directed by a level III-trained expert in CCTA and followed standardized protocols for performing CCTA as defined by guidelines of the SCCT.16, 17 The percent luminal stenosis in the LM was coded as normal (0% stenosis) or nonobstructive (1-49% stenosis) by visual assessment. Luminal stenosis in non-LM vessels, including the left anterior descending (LAD) artery, left circumflex (LCx) artery, and right coronary artery (RCA) were also gathered and coded as normal, nonobstructive, or obstructive (≥50% stenosis), which were consistent with previous CCTA-derived definitions for obstructive and nonobstructive CAD.2 At all laboratories, intra- and inter-reader reliability were routinely assessed and have been previously described in detail.15
Outcome Data Collection and Follow-up Methods
Our primary outcome was a composite of incident all-cause mortality, nonfatal MI, or late coronary revascularization occurring >90 days from the index CCTA. Each individual endpoint was also evaluated as a secondary outcome. The National Death Index was queried for all-cause death within the United States, or determined through direct interview with the patient's family or physician, telephone call, or review of medical records for events outside of the United States. MI events were confirmed through review of the patient's medical records for hospital documentation of biomarker elevation and electrocardiographic alterations consistent with the Universal Definition of MI.18 Coronary revascularization events were also confirmed through review of medical records, however, target vessel revascularization was not reported. Only late (>90 days from the index CCTA) revascularization events were used as an endpoint; earlier revascularization events represent continued evaluation of the index, but stable CAD course for a patient, while use of late revascularization is a common means to separate elective versus non-elective conditions and consistent with prior literature.15 Additional information on ascertainment and adjudication methods have been previously described.15
Statistical Analysis
Using chi-squared tests for categorical variables and t-tests for normally distributed or Wilcoxon tests for non-normally distributed continuous variables, baseline characteristics were compared between patients with normal LM and nonobstructive LM. We estimated time-to-event using the Kaplan-Meier method and compared differences in cumulative incidence of events between LM groups with log-rank tests.
Next, we tested for an interaction between sex and LM status for the study endpoints and examined sex-specific differences in outcomes according to LM status. After meeting the proportional hazards assumption by graphical assessment, four multivariable Cox proportional hazards models were created using covariables defined a priori based on clinical judgment. Model 1 included age, HTN, DM, HLD, smoking history, and the presence of typical angina to control for baseline demographics, CAD risk factors, and pretest probability for obstructive CAD. Model 2 included the covariables from Model 1 plus the number of non-LM coronary vessels with obstructive plaque in order to adjust for differences in obstructive plaque burden between LM strata. As an alternative method to adjust for co-occurring obstructive plaque, Model 3 included the covariables from Model 1 plus the total number of non-LM coronary artery segments with obstructive plaque. Finally, Model 4 expanded upon the previous models with inclusion of the segment involvement score (SIS, scored 0 to 15 excluding LM), which accounts for overall CAD burden by measuring both nonobstructive and obstructive plaque extent.19
We performed several subgroup and sensitivity analyses to test the robustness of our findings. (1) We assessed time-to-event by LM status in a subset of 3,325 patients without any obstructive CAD to further account for baseline differences in plaque burden. (2) In the same subset, we examined whether sex-specific differences in risk varied based on the location (LM, LAD, LCX, or RCA) or extent (per-segment and per-vessel) of nonobstructive plaque. (3) In order to assess for potential selection bias, the baseline characteristics of patients who were excluded (n=6,920) were compared to those included in the final cohort. We repeated survival analysis on the entire pooled cohort for the endpoint of all-cause mortality, which was the only outcome completely adjudicated in CONFIRM. (4) Since target vessel revascularization was not known and may have been subject to biases by gender or the extent of obstructive CAD, we removed revascularization from the composite endpoint and repeated the survival analysis using death or nonfatal MI as the primary outcome. A two-tailed p-value <0.05 was considered statistically significant for each analysis. All statistical analyses were performed using SAS version 9.4 (Cary, NC).
Results
Clinical and CCTA Characteristics of the Study Cohort
Of 5,166 patients, 82% had normal LM and 18% had nonobstructive LM CAD (Table 1). Patients with nonobstructive LM were older and had higher baseline rates of CAD risk factors including HTN, DM, and HLD (p<0.001). Furthermore, patients with nonobstructive LM CAD had more extensive co-occurring obstructive plaque and higher SIS score (p<0.001). Neither baseline LVEF (p=0.232) nor presenting symptoms (p=0.424) were significantly different by LM status.
Table 1. Baseline Characteristics of Study Cohort by Left Main Status.
| All Patients (N=5,166) | Normal LM (N=4,241) | Nonobstructive LM (N=925) | P- Value* | |
|---|---|---|---|---|
| Age, years | 60±12 | 60±12 | 65±10 | <0.001 |
| Male | 3,255 (63) | 2,592 (61) | 663 (71) | <0.001 |
| Hypertension | 2,769 (54) | 2230 (53) | 539 (59) | 0.001 |
| Diabetes | 865 (17) | 676 (16) | 189 (21) | 0.001 |
| Hyperlipidemia | 2,717 (53) | 2,128 (50) | 589 (64) | <0.001 |
| Smoking History | 1,030 (20) | 827 (20) | 203 (22) | 0.099 |
| Family History of Early CAD | 1,490 (29) | 1,204 (29) | 286 (31) | 0.118 |
| LVEF, % | 60±13 | 60±13 | 61±15 | 0.232 |
| Symptom Characteristics | 0.424 | |||
| Typical angina | 696 (15) | 582 (16) | 114 (14) | |
| Atypical angina | 1,587 (35) | 1,295 (35) | 292 (35) | |
| Non-cardiac | 409 (9) | 341 (9) | 68 (8) | |
| No chest pain | 1,867 (41) | 1,515 (41) | 352 (43) | |
| Extent of Obstructive CAD (per vessel) | <0.001 | |||
| 1-vessel | 986 (19) | 723 (17) | 263 (30) | |
| 2-vessel | 496 (10) | 340 (8) | 156 (18) | |
| 3-vessel | 207 (6) | 205 (5) | 102 (11) | |
| Extent of Obstructive CAD (per segment) | 0.8±1.5; 0 [0-1] |
0.7±1.4; 0 [0-1] |
1.5±1.9; 1 [0-2] |
<0.001 |
| Overall CAD Burden (Segment Involvement Score) | 2.6±2.9; 2 [1-6] |
1.9±2.4; 2 [0-4] |
5.6±2.9; 6 [4-9] |
<0.001 |
Values reported as mean ± standard deviation (median [interquartile range] also reported if non-normally distributed), or N (%)
Comparison of patients with normal LM and nonobstructive LM using chi-squared test for categorical variables and t-test or Wilcoxon tests for continuous variables
LVEF only available in 858 (17%) of patients
CAD: Coronary artery disease; LM: Left main; LVEF: Left ventricular ejection fraction;
Estimating the Risk of Death, Myocardial Infarction, or Revascularization
Through a mean 5.3±1.8 years (median 5.5, interquartile range 5.1-6.2 years) of follow-up, there were 349 deaths, 471 nonfatal MIs, and 364 revascularization events. Cumulative 5-year incidence of the composite outcome was 27.3% for patients with nonobstructive LM CAD compared to 17.2% for patients with normal LM (p<0.0001, Figure 1). Differences in the incidence for the individual endpoints of all-cause mortality, nonfatal MI, and late coronary revascularization by LM status are also displayed.
Figure 1. Cumulative 5-Year Incidence of Events by Left Main Status.
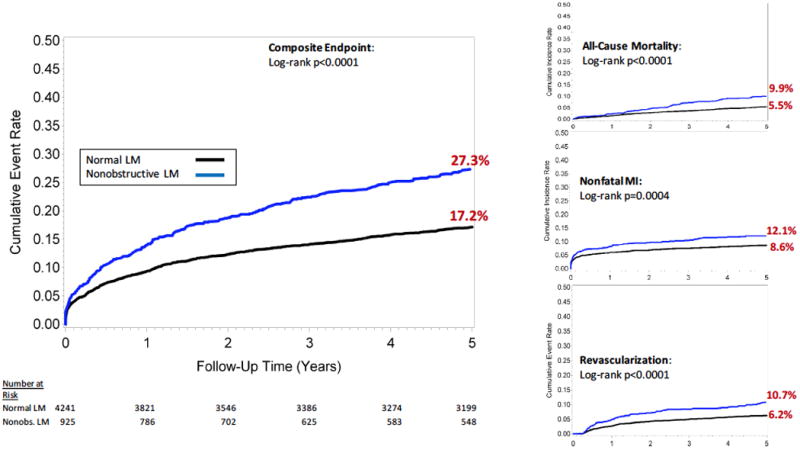
Cumulative incidence of the primary composite outcome of all-cause mortality, nonfatal myocardial infarction or coronary revascularization is displayed using a 90-day landmark time. Cumulative events rates for the secondary endpoints of death, nonfatal myocardial infarction, and revascularization are also shown. Patients are stratified as having normal LM or nonobstructive LM.
LM: Left main; Nonobs: Nonobstructive
Sex-specific Differences in Outcomes
Next, we examined sex-specific differences in outcomes according to LM status. Women had a lower burden of obstructive CAD and SIS scores than men (p<0.001, Supplemental Table 1). In both women and men, those with nonobstructive LM had a higher incidence of composite events than those with normal LM: women (34.3% versus 15.4%, p<0.0001); men (24.6% versus 18.2%, p<0.0001) (Figure 2). Importantly, a significant interaction existed between sex and LM status for the primary outcome (p=0.001). After multivariable adjustment, the association between nonobstructive LM and the composite endpoint remained significant in women (HRadj 1.48 [1.21-1.75], p=0.005), but was not significant in men (HRadj 0.98 [0.81-1.18], p=0.806) (Tables 2 and 3). Similarly, the association between nonobstructive LM and the individual endpoints of death, nonfatal MI, and revascularization also differed by sex and are presented in Tables 2 and 3. The sex-specific HRs for the composite outcome by other measures of CAD extent are displayed in Supplemental Table 2.
Figure 2. Cumulative 5-Year Incidence of Events by Left Main Status in Women and Men.
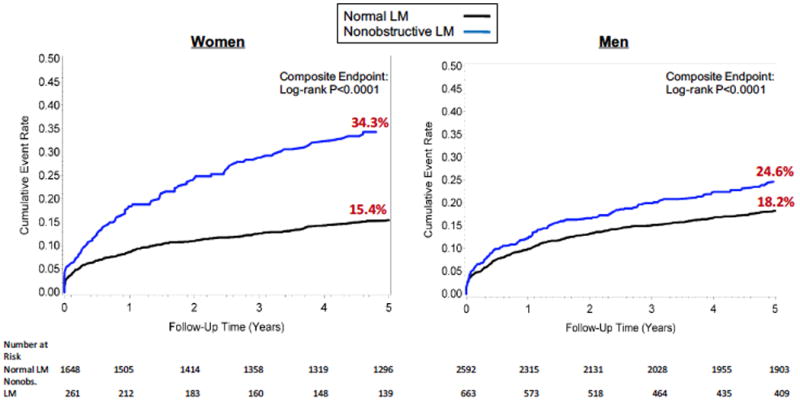
Cumulative incidence of the composite outcome of all-cause mortality, nonfatal myocardial infarction or coronary revascularization are displayed by LM status in women and men.
LM: Left main; Nonobs: Nonobstructive
Table 2. Risk of Death, Myocardial Infarction, and Revascularization by Left Main Status in Women.
| Unadjusted | Model 1 | Model 2 | Model 3 | Model 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-Value | HR (95% CI) | P-Value | HR (95% CI) | P-Value | HR (95% CI) | P-Value | HR (95% CI) | P-Value | |
| Composite Endpoint | ||||||||||
| Normal LM | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Nonobstructive LM | 2.37 (1.87-3.01) | <0.001 | 2.02 (1.57-2.58) | <0.001 | 1.63 (1.26-2.10) | <0.001 | 1.67 (1.30-2.15) | <0.001 | 1.48 (1.21-1.75) | 0.005 |
| All-Cause Mortality | ||||||||||
| Normal LM | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Nonobstructive LM | 2.52 (2.31-2.90) | <0.001 | 1.96 (1.55-2.36) | 0.001 | 1.87 (1.45-2.29) | 0.003 | 1.92 (1.51-2.32) | 0.002 | 1.58 (1.15-2.2.01) | 0.036 |
| Nonfatal MI | ||||||||||
| Normal LM | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Nonobstructive LM | 1.75 (1.21-2.52) | 0.003 | 1.60 (1.10-2.33) | 0.015 | 1.25 (0.85-1.83) | 0.254 | 1.29 (0.88-1.89) | 0.195 | 1.22 (0.81-1.83) | 0.334 |
| Revascularization | ||||||||||
| Normal LM | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Nonobstructive LM | 2.62 (2.18-3.06) | <0.001 | 2.33 (1.87-2.80) | <0.001 | 1.86 (1.38-2.34) | 0.011 | 1.94 (1.47-2.44) | 0.006 | 1.69 (1.19-2.19) | 0.039 |
Model 1: covariables include age, hypertension, diabetes, hyperlipidemia, smoking, and angina
Model 2: covariables include those in Model 1 plus the number of non-LM vessels with obstructive CAD
Model 3: covariables include those in Model 1 plus the total number of non-LM segments with obstructive CAD
Model 4: covariables include those in Model 1 plus the segment involvement score
CI: Confidence Interval; HR: Hazard ratio; LM: Left main; MI: Myocardial infarction
Table 3. Risk of Death, Myocardial Infarction, and Revascularization by Left Main Status in Men.
| Unadjusted | Model 1 | Model 2 | Model 3 | Model 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-Value | HR (95% CI) | P-Value | HR (95% CI) | P-Value | HR (95% CI) | P-Value | HR (95% CI) | P-Value | |
| Composite Endpoint | ||||||||||
| Normal LM | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Nonobstructive LM | 1.44 (1.21-1.71) | <0.001 | 1.18 (0.99-1.42) | 0.065 | 0.99 (0.82-1.19) | 0.879 | 1.07 (0.90-1.29) | 0.438 | 0.98 (0.81-1.18) | 0.806 |
| All-Cause Mortality | ||||||||||
| Normal LM | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Nonobstructive LM | 1.57 (1.16-2.12) | 0.004 | 1.14 (0.84-1.55) | 0.412 | 1.04 (0.75-1.44) | 0.817 | 1.13 (0.83-1.54) | 0.448 | 0.98 (0.70-1.36) | 0.894 |
| Nonfatal MI | ||||||||||
| Normal LM | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Nonobstructive LM | 1.32 (1.01-1.72) | 0.041 | 1.20 (0.91-1.58) | 0.203 | 0.98 (0.74-1.30) | 0.887 | 1.06 (0.80-1.41) | 0.677 | 1.02 (0.76-1.37) | 0.909 |
| Revascularization | ||||||||||
| Normal LM | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Nonobstructive LM | 1.46 (1.11-1.91) | 0.007 | 1.18 (0.89-1.57) | 0.232 | 0.98 (0.73-1.31) | 0.888 | 1.05 (0.79-1.40) | 0.743 | 0.94 (0.69-1.27) | 0.682 |
Model 1: covariables include age, hypertension, diabetes, hyperlipidemia, smoking, and angina
Model 2: covariables include those in Model 1 plus the number of non-LM vessels with obstructive CAD
Model 3: covariables include those in Model 1 plus the total number of non-LM segments with obstructive CAD
Model 4: covariables include those in Model 1 plus the segment involvement score
CI: Confidence Interval; HR: Hazard ratio; LM: Left main; MI: Myocardial infarction
Subgroup and Sensitivity Analyses
In a subgroup of 3,325 patients without any obstructive CAD, the cumulative 5-year incidence of the composite outcome remained significantly higher among those with nonobstructive LM than those with normal LM (18.6% versus 10.7%, p<0.0001, Figure 3), and was also consistent in sex-stratified Kaplan-Meier analysis: women (28.3% versus 10.5%, p<0.0001); men (14.0% versus 10.8%, p=0.036).
Figure 3. Cumulative 5-Year Incidence of Events among Patients without any Obstructive CAD.
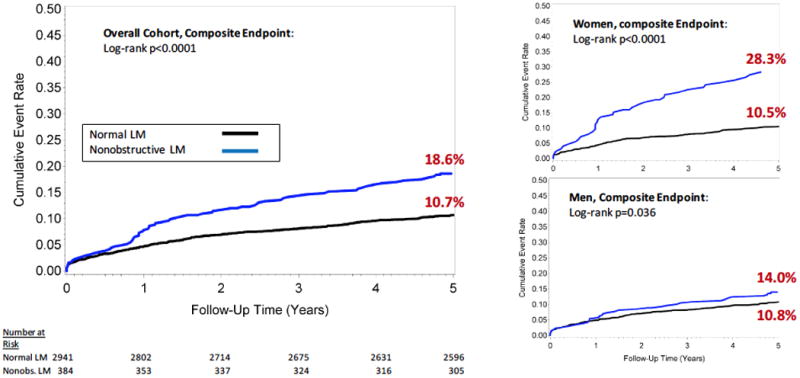
Cumulative incident event rates for the composite outcome of all-cause mortality, nonfatal myocardial infarction or coronary revascularization are displayed among patients without any obstructive CAD. Patients are stratified as having normal LM or nonobstructive LM. Cumulative incidence curves are also displayed in women and men, separately.
CAD: Coronary artery disease; LM: Left main; Nonobs: Nonobstructive
Next, we examined whether sex-related differences in outcomes varied by nonobstructive plaque pattern. As shown in Figure 4, in subgroups of patients with nonobstructive LM CAD, women had a significantly higher risk for adverse events than men (HRadj 1.78 [1.31-2.25], p=0.017). In contrast, outcomes were not significantly different between women and men in other subgroups of nonobstructive plaque.
Figure 4. Sex-specific Differences in Risk by Nonobstructive Plaque Location and Extent.
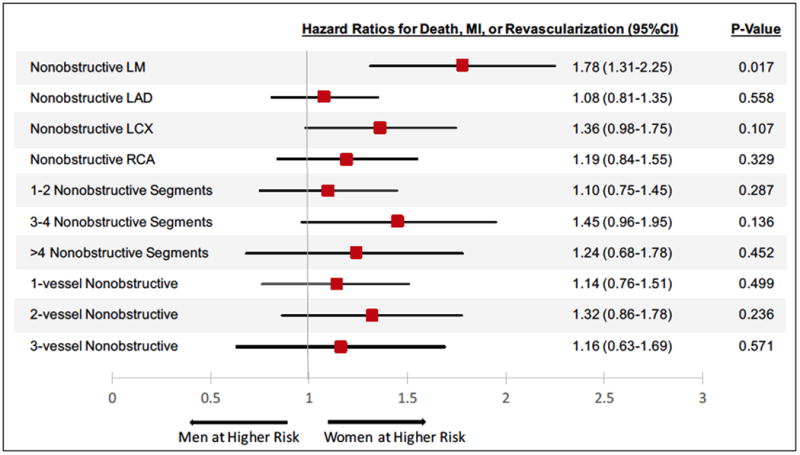
Risk-adjusted hazard ratios comparing women to men for the composite outcome of all-cause mortality, nonfatal myocardial infarction, or revascularization are shown in different subgroups of nonobstructive CAD. All models adjusted for age, hypertension, hyperlipidemia, diabetes, smoking, and angina.
CAD: Coronary artery disease; LM: Left main; LAD: left anterior descending artery; LCX: left circumflex artery; RCA: right coronary artery
In addition, we examined the baseline characteristics of patients excluded (n=6,920) from our analysis (Supplemental Table 3). In a pooled Kaplan-Meier analysis, those with nonobstructive LM CAD had a consistent and elevated incidence of death compared to those with normal LM (13.9% versus 7.9%, p<0.0001). Similar sex-specific differences were also observed: women (18.7% versus 8.1%, p<0.0001), men (11.8% versus 7.7%, p<0.0001, Supplemental Figure 1).
Finally, we removed revascularization events from the composite endpoint. Consistently, patients with nonobstructive LM had higher cumulative incidence of death or MI than patients with normal LM (19.8% versus 13.2%, p<0.0001, Supplemental Figure 2) and when separated by sex: women (26.1% versus 13.1%, p<0.0001), men (17.4%, 13.3%, p=0.001).
Discussion
Although prognosis is well established in the setting of obstructive LM CAD, our findings were the first to reveal sex-specific differences in long-term outcomes for nonobstructive LM CAD. Notably, nonobstructive LM plaque was associated with a nearly 50% higher risk for adverse events among women independent of CAD burden in other vessels, whereas the association between nonobstructive LM CAD and future events was not significant among men after risk adjustment. Furthermore, women with nonobstructive LM plaque had a ∼1.8-fold higher risk for future events than men with nonobstructive LM plaque; sex-specific differences in outcomes were not observed across other patterns of nonobstructive CAD. These findings provide evidence that nonobstructive LM plaque may represent an important risk marker in women that should be considered during risk stratification efforts.
Surprisingly, there has been a paucity of data regarding the prognostic implication of nonobstructive LM plaque within the published literature. One reason may be that previous studies have frequently represented nonobstructive CAD as having a uniform level of risk. For instance, in the Women's Ischemia Syndrome Evaluation (WISE) study9, 5-year event rates for MI were estimated to be 3.9% for patients with any nonobstructive CAD. However, the extent and lesion-specific distribution of nonobstructive CAD were not further delineated.
More recently, both invasive angiographic and CCTA series have characterized gradations of risk based on the extent of nonobstructive CAD. Maddox et al described 1-year MI event rates of 0.24%, 0.56% and 0.59%, respectively, among patients with 1-vessel, 2-vessel, and 3-vessel nonobstructive CAD (defined as 20%-49% stenosis on invasive angiography).12 Similar proportional increases in mortality rates were reported with increasing nonobstructive vessel involvement in CCTA cohorts.20 In contrast to our study, these prior investigations had shorter follow-up times, and nonobstructive LM plaque was classified as ‘1-vessel’ nonobstructive CAD, as a lesion within the LAD territory, or incorporated within the segment involvement score.2, 3, 8, 10, 20 One exception was a small, single-center CCTA study of 76 patients with nonobstructive LM CAD, of whom, none experienced an event after 20 months of follow-up.21 Thus, our investigation expands upon previous findings with longer, 5-year follow-up, and to our knowledge, is the first study sufficiently powered to assess the prognostic significance of nonobstructive LM CAD.
Specifically, our study revealed that nonobstructive LM plaque was strongly associated with adverse events in women but not in men, independent of CAD burden in other vessels. These sex-specific differences in prognosis were not observed for other subgroups of patients with multi-segment or multi-vessel nonobstructive CAD. Our results are in concordance with prior studies by Leipsic and others, who did not find that outcomes in women versus men differed based on the extent of nonobstructive CAD; however, disparities in prognosis based upon the location of nonobstructive plaque (e.g. LM versus other vessel) were not previously explored.11, 14 Shaw et al described both higher in-hospital and 4-year mortality among women with significant atherosclerotic burden or high-risk lesions such as obstructive multivessel or LM CAD as compared to men;2-4 we now extend these observations of sex-based differences in outcomes of LM disease to patients with nonobstructive plaque.
Although elucidating possible mechanisms for the association between nonobstructive LM plaque and adverse outcomes in women requires additional investigation, several potential explanations exist. Independent of body surface area, women are known to have significantly smaller coronary arterial sizes than men, including the luminal area of the LM, which has been associated with worse outcomes in women than men following coronary revascularization and may also increase susceptibility to thrombotic occlusion.22 Numerous pathology examinations and intravascular ultrasound (IVUS) studies have also characterized differences in coronary atherosclerotic composition and progression between women and men.23, 24 Although women have been noted to have less severe and extensive CAD than men, positive coronary artery remodeling was detected in the majority (73%) of women without obstructive CAD in an IVUS sub-study of WISE.24 These nonobstructive, but positively remodeled plaques, have been proposed to serve as precursor lesions vulnerable to future erosion or rupture.25 Given that the LM subtends a large proportion of the myocardium, any sex-related differences in plaque vulnerability in the LM could conceivably place women at a significant and higher risk for downstream coronary events; additional characterization of LM plaque morphology is needed to improve discrimination of at-risk lesions. Moreover, previous studies from the National Cardiovascular Data Registry have shown that patients with nonobstructive CAD are less than half as likely to receive aspirin, statins, or beta-blockers as compared to patients with obstructive CAD.26-28 The preponderance of ‘hidden’ or positively remodeled plaque in women may also lead to an underestimation of true atherosclerotic burden and further therapeutic delay, potentially contributing to disparities in outcomes between women and men with nonobstructive CAD.
Our findings add to the growing body of evidence that depict a heterogeneous distribution of risk among patients with nonobstructive CAD; both the extent and location of nonobstructive plaque appear to confer varying prognostic value. Importantly, estimated rates of obstructive CAD on elective cardiac catheterization have been as low as ∼40% with a disproportionately lower yield for obstructive CAD among women compared to men.6, 29 Guideline recommendations on the management of this large cohort of patients with nonobstructive CAD have remained poorly defined, however, simple reassurance and complacency in clinical management are not likely appropriate given the prognostic implications of nonobstructive plaque. In this context, elucidating and recognizing high-risk patterns of nonobstructive CAD, such as the sex-specific significance of nonobstructive LM plaque, may not only help provide a more granular estimation of cardiovascular risk, but may also identify promising targets to focus preventive care as higher risk patients may derive a greater benefit from risk-modifying therapies. Future prospective randomized trials are still needed to determine optimal strategies for therapeutic risk reduction among patients with nonobstructive CAD.
Study Limitations
Inherently, we were unable to account for all confounders given our retrospective study design, however, we utilized several multivariable regression models that incorporated all available and pertinent clinical characteristics. Selection bias may have also impacted our analysis as we excluded many higher risk patients with obstructive LM or a history of CAD, however, after pooling all patients, those with nonobstructive LM remained at higher risk of death than those with normal LM and similar sex-specific differences in outcomes were observed. Nonetheless, both external and prospective validation of our results remain to be performed, but we chose the CONFIRM registry for our initial analysis as it represents the largest CCTA cohort and with the longest duration of patient follow-up. Moreover, we did not have further details on plaque vulnerability, which may have yielded additional sex-specific predictive information and should be included in future prognostic models derived from invasive (e.g., IVUS or optical coherence tomography) or noninvasive imaging cohorts. Similarly, both LVEF and myocardial ischemia carry important prognostic value and may have influenced downstream clinical outcomes; however, neither were core variables defined by CONFIRM, and were thus not consistently documented by all enrolling sites. Finally, the CONFIRM registry did not collect information regarding medication use or post-CCTA changes in clinical management, which may have differed by sex and impacted patient outcomes.
Conclusion
Although abundant prognostic data have documented poor clinical outcomes among patients with obstructive LM CAD, our findings were the first to reveal an elevated 5-year risk for death, MI, or revascularization associated with nonobstructive LM CAD. Specifically, nonobstructive LM plaque may represent an important risk marker in women, potentially contributing to disparities in outcomes between women and men with nonobstructive CAD. Recognizing the sex-specific prognostic significance of nonobstructive LM plaque may improve future risk stratification efforts in patients undergoing evaluation for CAD.
Supplementary Material
Clinical Perspective.
Although it is well established that obstructive left main (LM) coronary artery disease (CAD) has major prognostic implications, our study reveals that nonobstructive LM plaque is also associated with adverse cardiovascular events. Importantly, the presence of nonobstructive LM CAD was associated with a nearly 50% increase in risk for future events in women, independent of CAD burden or extent in other vessels; however, the association between nonobstructive LM plaque and clinical outcomes was not significant in men. These findings show the heterogeneous profile of risk that exists for nonobstructive CAD as both plaque extent and location carry different prognostic value in women and men. Future risk stratification efforts should recognize nonobstructive LM plaque in women as an important marker of risk.
Acknowledgments
Funding: This work was supported by the National Institutes of Health-National Heart, Lung, and Blood Institute (5T32HL007745-20).
Dr. Berman: consultant with Molecular Dynamics, employed and receives royalties from Cedars-Sinai Medical Center, grant with Bayer Pharmaceutical; Dr. Chow: holds the Saul and Edna Goldfarb Chair in Cardiac Imaging Research, receives research support from GE Healthcare and educational support from TeraRecon Inc; Dr. Andreini: personal fees from GE Healthcare. Dr. Pontone: grants and fees from GE Healthcare, fees from Medtronic, fees from Bracco, grants from Heartflow; Dr. Leipsic: personal fees from Circl CVI, personal fees from GE Healthcare, personal fees from Samsung; Dr. Budoff: grants from NIH, grants from general Electric; Dr. Al-Mallah: fees from Speaker Bureau: GE Healthcare and Phillips; Dr. Min: fees from MDDX, fees from Heartflow, Inc, fees from Arineta.
Footnotes
Disclosures: No other authors have relationships with industry to disclose.
References
- 1.Conley MJ, Ely RL, Kisslo J, Lee KL, McNeer JF, Rosati RA. The prognostic spectrum of left main stenosis. Circulation. 1978;57:947–52. doi: 10.1161/01.cir.57.5.947. [DOI] [PubMed] [Google Scholar]
- 2.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Maffei E, Raff G, Shaw LJ, Villines T, Berman DS, Investigators C. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–60. doi: 10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 3.Shaw LJ, Min JK, Narula J, Lin F, Bairey-Merz CN, Callister TQ, Berman DS. Sex differences in mortality associated with computed tomographic angiographic measurements of obstructive and nonobstructive coronary artery disease: an exploratory analysis. Circ Cardiovasc Imaging. 2010;3:473–81. doi: 10.1161/CIRCIMAGING.109.860981. [DOI] [PubMed] [Google Scholar]
- 4.Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, Block PC, Hewitt K, Weintraub WS, Peterson ED American College of Cardiology-National Cardiovascular Data Registry I. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008;117:1787–801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 5.Oconnor GT, Morton JR, Diehl MJ, Olmstead EM, Coffin LH, Levy DG, Maloney CT, Plume SK, Nugent W, Malenka DJ, Hernandez F, Clough R, Birkmeyer J, Marrin CAS, Leavitt BJ. Differences between Men and Women in-Hospital Mortality Associated with Coronary-Artery Bypass Graft-Surgery. Circulation. 1993;88:2104–2110. doi: 10.1161/01.cir.88.5.2104. [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, Duvernoy C, Walsh MN, Bairey Merz CN Committee ACiW. Emergence of Nonobstructive Coronary Artery Disease: A Woman's Problem and Need for Change in Definition on Angiography. J Am Coll Cardiol. 2015;66:1918–33. doi: 10.1016/j.jacc.2015.08.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bittencourt MS, Hulten E, Ghoshhajra B, O'Leary D, Christman MP, Montana P, Truong QA, Steigner M, Murthy VL, Rybicki FJ, Nasir K, Gowdak LH, Hainer J, Brady TJ, Di Carli MF, Hoffmann U, Abbara S, Blankstein R. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7:282–91. doi: 10.1161/CIRCIMAGING.113.001047. [DOI] [PubMed] [Google Scholar]
- 9.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–50. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hulten E, Villines TC, Cheezum MK, Berman DS, Dunning A, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng VY, Chinnaiyan K, Chow BJ, Cury RC, Delago A, Feuchtner G, Hadamitzky M, Hausleiter J, Kaufmann PA, Karlsberg RP, Kim YJ, Leipsic J, Lin FY, Maffei E, Plank F, Raff GL, Labounty TM, Shaw LJ, Min JK, Investigators C. Usefulness of coronary computed tomography angiography to predict mortality and myocardial infarction among Caucasian, African and East Asian ethnicities (from the CONFIRM [Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter] Registry) Am J Cardiol. 2013;111:479–85. doi: 10.1016/j.amjcard.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Leipsic J, Taylor CM, Gransar H, Shaw LJ, Ahmadi A, Thompson A, Humphries K, Berman DS, Hausleiter J, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chow BJ, Cury RC, Delago AJ, Dunning AL, Feuchtner GM, Hadamitzky M, Kaufmann PA, Lin FY, Chinnaiyan KM, Maffei E, Raff GL, Villines TC, Gomez MJ, Min JK. Sex-based prognostic implications of nonobstructive coronary artery disease: results from the international multicenter CONFIRM study. Radiology. 2014;273:393–400. doi: 10.1148/radiol.14140269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, Leon B, Bhatt DL, Fihn SD, Rumsfeld JS. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–63. doi: 10.1001/jama.2014.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sedlak TL, Lee M, Izadnegahdar M, Merz CN, Gao M, Humphries KH. Sex differences in clinical outcomes in patients with stable angina and no obstructive coronary artery disease. Am Heart J. 2013;166:38–44. doi: 10.1016/j.ahj.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Schulman-Marcus J, o Hartaigh B, Gransar H, Lin F, Valenti V, Cho I, Berman D, Callister T, DeLago A, Hadamitzky M, Hausleiter J, Al-Mallah M, Budoff M, Kaufmann P, Achenbach S, Raff G, Chinnaiyan K, Cademartiri F, Maffei E, Villines T, Kim YJ, Leipsic J, Feuchtner G, Rubinshtein R, Pontone G, Andreini D, Marques H, Shaw L, Min JK. Sex-Specific Associations Between Coronary Artery Plaque Extent and Risk of Major Adverse Cardiovascular Events: The CONFIRM Long-Term Registry. JACC Cardiovasc Imaging. 2016;9:364–72. doi: 10.1016/j.jcmg.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah MH, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan KM, Chow B, Delago A, Hadamitzky M, Hausleiter J, Karlsberg RP, Kaufmann P, Maffei E, Nasir K, Pencina MJ, Raff GL, Shaw LJ, Villines TC. Rationale and design of the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter) Registry. J Cardiovasc Comput Tomogr. 2011;5:84–92. doi: 10.1016/j.jcct.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L, Weigold WG. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2009;3:190–204. doi: 10.1016/j.jcct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Leipsic J, Abbara S, Achenbach S, Cury R, Earls JP, Mancini GJ, Nieman K, Pontone G, Raff GL. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:342–58. doi: 10.1016/j.jcct.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESCAAHAWHFTFfUDoMI, Authors/Task Force Members C. Thygesen K, Alpert JS, White HD, Biomarker S, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Subcommittee ECG, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Imaging S, Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ, Classification S, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Intervention S, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Trials, Registries S, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Trials, Registries S, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Trials, Registries S, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Trials, Registries S, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S, Guidelines ESCCfP. Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Document R, Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–98. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, Lippolis NJ, Berman DS, Callister TQ. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–70. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 20.Lin FY, Shaw LJ, Dunning AM, Labounty TM, Choi JH, Weinsaft JW, Koduru S, Gomez MJ, Delago AJ, Callister TQ, Berman DS, Min JK. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol. 2011;58:510–9. doi: 10.1016/j.jacc.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 21.Maffei E, Seitun S, Martini C, Aldrovandi A, Arcadi T, Clemente A, Messalli G, Malago R, Weustink A, Mollet N, Nieman K, Ardissino D, de Feyter P, Krestin G, Cademartiri F. Prognostic value of CT coronary angiography: focus on obstructive vs. nonobstructive disease and on the presence of left main disease. Radiol Med. 2011;116:15–31. doi: 10.1007/s11547-010-0592-2. [DOI] [PubMed] [Google Scholar]
- 22.Sheifer SE, Canos MR, Weinfurt KP, Arora UK, Mendelsohn FO, Gersh BJ, Weissman NJ. Sex differences in coronary artery size assessed by intravascular ultrasound. Am Heart J. 2000;139:649–53. doi: 10.1016/s0002-8703(00)90043-7. [DOI] [PubMed] [Google Scholar]
- 23.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G, Investigators W. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47:S21–9. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 24.Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, Denardo SJ, McGorray SP, Smith KM, Sharaf BL, Nicholls SJ, Nissen SE, Anderson RD. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) J Interv Cardiol. 2010;23:511–9. doi: 10.1111/j.1540-8183.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmadi A, Leipsic J, Blankstein R, Taylor C, Hecht H, Stone GW, Narula J. Do plaques rapidly progress prior to myocardial infarction? The interplay between plaque vulnerability and progression. Circ Res. 2015;117:99–104. doi: 10.1161/CIRCRESAHA.117.305637. [DOI] [PubMed] [Google Scholar]
- 26.De Ferrari GM, Leonardi S, Baduena L, Chieffo E, Lesce A, Repetto A, Previtali M. Patients with acute coronary syndrome and nonobstructive coronary artery disease in the real world are markedly undertreated. J Cardiovasc Med (Hagerstown) 2011;12:700–8. doi: 10.2459/JCM.0b013e328348e575. [DOI] [PubMed] [Google Scholar]
- 27.Maddox TM, Ho PM, Roe M, Dai D, Tsai TT, Rumsfeld JS. Utilization of secondary prevention therapies in patients with nonobstructive coronary artery disease identified during cardiac catheterization: insights from the National Cardiovascular Data Registry Cath-PCI Registry. Circ Cardiovasc Qual Outcomes. 2010;3:632–41. doi: 10.1161/CIRCOUTCOMES.109.906214. [DOI] [PubMed] [Google Scholar]
- 28.Ramanath VS, Armstrong DF, Grzybowski M, Rahnama-Mohagdam S, Tamhane UU, Gordon K, Froehlich JB, Eagle KA, Jackson EA. Receipt of cardiac medications upon discharge among men and women with acute coronary syndrome and nonobstructive coronary artery disease. Clin Cardiol. 2010;33:36–41. doi: 10.1002/clc.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–44. doi: 10.1093/eurheartj/ehr331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


