Abstract
Purpose of review
Most adolescents begin exploring cannabis in peer contexts, but the neural mechanisms that underlie peer influence on adolescent cannabis use are still unknown. This theoretical overview elucidates the intersecting roles of neural function and peer factors in cannabis use in adolescents.
Recent findings
Novel paradigms using functional magnetic resonance imaging (fMRI) in adolescents have identified distinct neural mechanisms of risk decision-making and incentive processing in peer contexts, centered on reward-motivation and affect regulatory neural networks; these findings inform a theoretical model of peer-driven cannabis use decisions in adolescents.
Summary
We propose four “mechanistic profiles” of social facilitation of cannabis use in adolescents: (1) peer influence as the primary driver of use; (2) cannabis exploration as the primary driver, which may be enhanced in peer contexts; (3) social anxiety; and (4) negative peer experiences. Identification of “neural targets” involved in motivating cannabis use may inform clinicians about which treatment strategies work best in adolescents with cannabis use problems, and via which social and neurocognitive processes.
Keywords: cannabis use, adolescence, peers, reward sensitivity, social neuroscience, fMRI
Introduction
Adolescence, which begins with the onset of puberty and continues into the mid-20s [1], is the developmental period during which adolescents begin to explore what are commonly seen as “adult behaviors”. Many novel behaviors are initially explored within peer-based contexts. Further, these emergent behavioral expressions coincide with a critical time of neurodevelopmental change [2], which cause adolescents to experience an elevated propensity toward exploration and sensation seeking [1,3], as well as a surge in social exploration [4]. Unique to this developmental period, adolescents dramatically increase time spent with peers and decrease time with parents. In addition, they exhibit heightened emotional investment in their peer social relationships [5]. Neuroimaging studies suggest that activation of the brain’s reward-motivation network, notably the ventral striatum (VS), is heightened in adolescents relative to children and adults [3,6], particularly during risky decision-making [7,8]; notably, the very same region is also markedly enhanced during social processing within adolescent peer contexts, such as when anticipating and receiving social evaluation from peers [4,9]. While this same region is central both to risk-taking and to adolescent social development, we have limited insight into the role of these highly salient peer influences on real-world health-relevant behaviors that carry potential long-term health risk for adolescents.
Cannabis use is one such health risk behavior that begins during adolescence, and has high potential for negative health impact. Adolescent cannabis use can pose a threat to psychosocial adjustment [10]; it may also be neurotoxic to the developing brain [11], subsequently leading to sustained cannabis use [12]. In the U.S., rates of cannabis use increase from 15.5% to 44.7% between 8th grade (around age 13–14) and 12th grade (around age 17–18) [13]. About 7.5% of U.S. adolescents have tried cannabis by age 13; furthermore, nationwide average age of initiation has been decreasing in recent years to age 16 (closer to age 12 in high-risk adolescents) [14,15]. At the same time, availability of cannabis has been increasing, with eight states and the District of Columbia recently legalizing recreational cannabis use for ages 21 and older in the U.S. As legalization continues to expand throughout the U.S., adolescents’ estimates of potential harm from cannabis use have been decreasing [16].
Adolescent cannabis use most often begins within peer-based contexts. This is relevant, as many adolescents learn about cannabis use, including about potential harms of cannabis, from the context in which they use [17]. However, while behavioral studies strongly implicate the role of peers in risk for adolescent cannabis use [18–20], we still have not identified how peers influence decisions to use cannabis on a neural level. Doing so will lend specific insights for improving evidence-based treatments (EBTs) targeted toward adolescents with or at high risk for developing cannabis use disorder, or CUD. There has been a growing interest in the integration of developmental cognitive neuroscience with addiction science as a pathway to uncovering the active ingredients in addiction treatments [21–23]. Identification of “neural targets” involved both in motivating substance use and in guiding treatment response has the potential to inform clinicians about which treatment strategies may work best in which populations, and via which social and neurocognitive processes. Nevertheless, critical to understanding the “active ingredients” that guide peer-based treatment efficacy among adolescent cannabis users will be an empirical assessment of how peers influence decision making around cannabis use.
While many recent studies have addressed the neuroscience of adolescent addiction more broadly (e.g.,[10]), given the pre-eminence and impact of peers during this highly socially-sensitive developmental period [4], and given the nature of cannabis use (e.g., primarily in peer contexts; [24,25]), it was our goal to explicitly evaluate the social neuroscience behind the role of peer processes influencing emergent and sustained cannabis use during this period. We begin by reviewing key neurodevelopmental changes that typically occur in adolescence, with a specific focus on two categories: (1) changes in the brain’s reward-motivation network that facilitate risk exploration; and (2) changes in social information processing that lead to social facilitation of cannabis use. Next, we propose a mechanistic model of peer-related motivations toward cannabis use in adolescents. The model has potential to lend valuable and concrete insight into the neural systems – and corresponding psychological processes – that clinicians can target in peer-based and group-based interventions; this is relevant as many treatments for adolescents are delivered in group-based contexts [26,27]. To develop this model, we review behavioral evidence of peer factors associated with adolescent cannabis use. We then review exciting neuroimaging findings that link adolescent cannabis use with the social neuroscience of peer processing, with a focus on translatable models derived from studies on peers and risk/reward processing. With regard to neural mechanisms, we limit the scope of our review to functional magnetic resonance imaging (fMRI) studies, which have been highly useful for the identification of brain-behavior relationships in social and developmental neuroscience. We also discuss the utility of integrating metrics of social neuroscience toward the development of prevention-intervention strategies in adolescents with or at risk for CUD.
Adolescent Brain Development
Over the past several years, structural MRI and fMRI have been critical tools for guiding models of typical neurodevelopmental processes in adolescence. In terms of typical neurodevelopment, MRI/fMRI studies have indicated that synaptic pruning, white matter volume, and myelination proliferate linearly, starting around puberty and continuing well into adulthood, enabling more efficient organization of the cortex and improved cognitive functioning [28–31]. Furthermore, grey matter volume and cortical thickness change in an inverted “U” curve trajectory between childhood and adulthood, with grey matter volume in cortical systems peaking around ages 9–16 and declining thereafter [32]. Notably, some of the largest structural changes during this same age range also occur in subcortical areas, such as the VS [33], particularly in boys [34]. While there is still some contention around the nature of these changes in cortical and subcortical regions [35,36], some have called these structural changes during adolescence a developmental “mismatch” whereby reward-motivational processes are heightened relative to cognitive and behavioral control processes [1,6]. Throughout time, these developing neural changes also allow for a more complex representation of rewards, motivating adolescents to respond to incentives that are more distal and abstract, including social incentives [4].
Increased Sensitivity of the Brain’s Reward-Motivation System in Adolescence
There is a developmentally-normative interest in risk behavior that peaks during mid- to late-adolescence [1,3,6]. This exploration is believed to be evolutionarily adaptive, and appears to stem from neural changes in the brain’s reward-motivation and cognitive control networks (e.g., [37]). Dual systems models have addressed these aspects of neurodevelopment [1,3,6], stipulating that exploration with risk peaks in adolescence because of the relatively earlier maturation of the reward-motivational system, centered in the brain’s subcortical limbic network that releases dopaminergic signals, thereby heightening the salience of sensational and novel experiences. This system includes the nucleus accumbens (NAc) of the VS, amygdala, hippocampus, and the ventral tegmental area (VTA), the mesolimbic seat of the brain’s dopaminergic reward (DA) system. Simultaneously, the cognitive control system in the brain’s cortical network has not yet completed maturation, placing adolescents at increased risk for engaging in high-risk, high-sensation activities, but with fewer tools to engage in the higher-order cognitive strategies that assess potential impact of risk behaviors [1,3,6]. Indeed, longitudinal evidence points to increased sensitivity to rewarding stimuli from early to late adolescence [38].
Increased Neural Sensitivity to Social Experiences with Peers
Early adolescence marks a time of emergent neurodevelopmental social reorientation, wherein the brain’s social information processing network undergoes significant reorganization [4]. As with changes in reward sensitivity, changes in social behavior during adolescence are linked with the developmental mismatch of cognitive-regulatory and affective systems. In particular, adolescents, relative to other age groups, show increased neurobiological sensitivity to social evaluation [39,40]. Increased sociability and deliberate formation of social relationships emerge, along with increased emotional salience of experiences with peers [41]. As such, the regulatory and motivational systems of the brain become essential for seeking out relationships with peers, appraising oneself among peers, monitoring delayed goals of social affiliation, coping with negative peer experiences, and coordinating social approach/withdrawal behaviors [4,42]. Empirical work using fMRI has implicated the ventrolateral prefrontal cortex (vlPFC) in adolescent response to peer social feedback [43–45]. The VS has also consistently played a role in social evaluation studies, with findings of a linear increase with age in the VS during anticipation of peer acceptance [9], particularly for adolescent girls [46], and enhanced VS responses to peer acceptance compared with peer rejection [9,43]. Overall, the empirical evidence suggests that social experiences represent a specific class of reward for which adolescents experience heightened neural sensitivity [42]. Thus, it stands to reason that social facilitation of cannabis use in adolescence may involve emotional investment in the social rewards (i.e., peer affiliation) inherent in the cannabis use context.
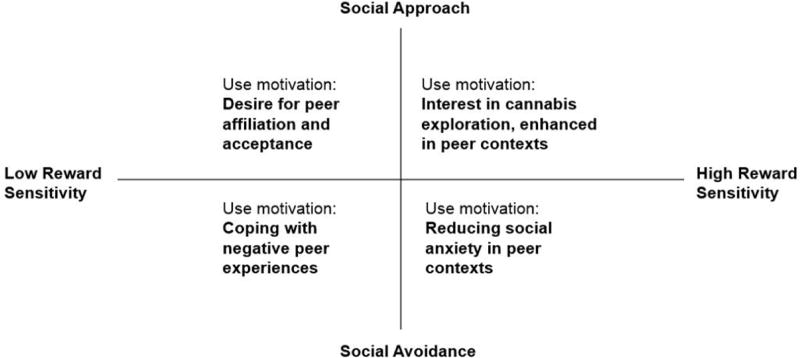
Four Mechanistic Models of Peer Influence on Adolescent Cannabis Use
A large body of work, touched on below, has linked adolescent cannabis use with specific social factors. We focus on a robust subset of those factors that are hypothesized to comprise distinct “neural profiles” related to adolescent cannabis use: peer influence/peer affiliation, adolescent social anxiety, and negative experiences with peers. Compellingly, little is known about what mechanisms underlie the relationships documented in this body of work, or the causal relationships in these peer influence-cannabis use links. This gap in knowledge presents an exciting avenue for future research, and to guide future hypotheses we propose four working models of different peer-related “mechanisms” driving adolescents toward cannabis use (see Figure 1). Our four models synthesize published behavioral work relating peer factors to cannabis use, with social neuroscience studies relating peer factors to risk-taking and reward-seeking behaviors. Specifically, we conjecture that the specific mechanisms motivating use in an individual adolescent depend on two primary dimensions: reward sensitivity (i.e., the rewarding value of the cannabis use behavior) and social orientation (i.e., social avoidance or social approach). To develop our models further, below we discuss known peer-related behavioral correlates of adolescent cannabis use. Then, we integrate these behavioral correlates into the social neuroscience literature to demonstrate how dimensions of reward sensitivity and social orientation might intersect in different ways to facilitate cannabis use.
Figure 1. A Mechanistic Model of Peer Influence on Adolescent Cannabis Use.
Specific peer-related mechanisms motivating cannabis use in an individual adolescent depend on two primary dimensions: reward sensitivity (i.e., the rewarding value of the cannabis use behavior) and social orientation (i.e., social avoidance or social approach).
Peer-related Behavioral Correlates of Adolescent Cannabis Use
Peer influence and peer affiliation
A well-established correlate of adolescent cannabis use is affiliation with cannabis using peers [19,24,47–50]. The behavioral data offer at least two possible mechanistic explanations for this type of peer influence on adolescent cannabis use. One possibility is that the social relationship with the peer is the primary motivator, wherein adolescents use in order to fit in, obtain social approval and status, or avoid negative peer evaluation (in Figure 1, high social approach, lower reward sensitivity). A second compelling possibility is that interest and exploration itself, this time in the context of cannabis use, is the primary driver, and the rewarding value of the behavior may take place in or be enhanced by the presence of peers, but is not driven by the peer social relationship (high social approach, higher reward sensitivity, Figure 1). Although it can be difficult to establish whether adolescent affiliation with cannabis-using peers is due to one of these mechanisms, it is important to note that there are a handful of studies that suggest adolescents specifically select peers based on cannabis use (i.e., the exploratory behavior itself is the primary use motive) [51–53]. Furthermore, use has been linked to affiliation with both delinquent peers [20,25,53,54], as well as popular peers [25]: these group identity and/or social status motives lend support for the mechanism of social relationship with the peer. Although the majority of these studies have not examined these potential mechanisms as mediators of peer-cannabis use relationships, this is a critical area that merits further empirical investigation.
Social anxiety
While often omitted in examinations of adolescent risk behavior, including cannabis use, there is emerging research highlighting the overlap between social anxiety disorder/social phobia and cannabis use disorder, particularly in late adolescents and young adults [55,56]. In terms of comorbidity, there are positive associations (odds ratios range = 1.24 – 1.68) between anxiety, anxiety/depression, and cannabis use [55,56]. Social anxiety disorder is characterized by an extreme fear of negative evaluation, and in adolescents this fear is often manifested as extreme reticence in peer social situations (e.g., in a school cafeteria surrounded by many unknown peers) [57]. Thus, a third mechanism driving cannabis use, particularly in peer social contexts for adolescents, is effort to reduce social anxiety. Here, teens with elevated social phobia and anxiety may explore cannabis use as a way to mitigate what may feel like imminent and unavoidable negative peer evaluation [58]. Further, adolescents with social anxiety may look to the intoxicating effects of cannabis to help ease the physical and mental experience of social anxiety, and possibly improve what may otherwise be very uncomfortable social interactions for adolescents [59]. Interestingly, studies reflect an inversion in the association between social anxiety and cannabis use, whereby heightened social anxiety relates to lower use in early adolescence [60] and to greater use in late adolescence [59,61–63]. Thus, for the mechanism of adolescent social anxiety, the decisions to use cannabis likely differ from adolescents using for “social relationship with peer” or “interest and exploration” reasons (in Figure 1, high social avoidance, high reward sensitivity). Behavioral studies in late adolescents with social anxiety have associated more frequent cannabis use with greater social concerns [61], greater social avoidance [47], more negative life expectations [63], coping motives [62], and conformity motives [62]. Furthermore, greater belief in use-related impairment predicts more use [59], suggesting that socially anxious adolescents use cannabis, in part, for purposes of perceived escape. Notably, these mechanisms may also drive the association between cannabis use and depression [64].
Negative experiences with peers
Adolescents who report many different classes of negative peer interactions, detailed below, also report greater cannabis use. Cannabis use in this peer context may be driven by the attempt to reduce the affective consequences of negative peer events. Chronic peer rejection in adolescence is associated with suppressed mood and social withdrawal [65,66]. As such, cannabis use in adolescents who have chronic negative experiences with peers might be driven by coping and/or affect regulatory mechanisms (high social avoidance, low reward sensitivity in Figure 1). Published work has linked greater cannabis use in adolescents with peer victimization [67], bullying [68–70], and association with marginalized communities that are often targeted for victimization and harassment (e.g., LGBT and gender-minority adolescents) [71].
Peer-related Neural Mechanisms of Risk and Reward Processing: Implications for Adolescent Cannabis Use
In the absence of experimental fMRI studies relating peer social factors to adolescent cannabis use, one route to understand the interplay between peer behavior and cannabis use among adolescents is to borrow from social neuroscience studies. In this section we review emerging themes from innovative risk decision-making and incentive processing fMRI studies in adolescents. These paradigms have elucidated some useful neural mechanisms of cannabis-use risk among cannabis-using adolescents [72–74], but have historically omitted the potential impact and role of social context. Importantly, the studies in this section integrate peer social factors into risk/reward paradigms and demonstrate high translational potential for illuminating neural mechanisms of peer social influence on adolescent cannabis use.
Peer influence and peer affiliation
To examine parameters of peer influence and peer affiliation on risk behaviors, one group of fMRI studies has utilized a set of paradigms that involve reward incentives and some degree of risk: simulated “go/no go” driving tasks [8,75–78], as well as gambling challenges [79–82]. Across these paradigms, a novel manipulation of peer context has been applied, wherein adolescents complete the task both while being observed by a peer, and while alone. Overall, these studies have reported greater risk-taking behavior in adolescents vs. adults [8,79] in tandem with heightened VS and orbitofrontal cortex (OFC) activity [8], and greater risk-taking in peer-present vs. peer-absent conditions among adolescents [8,75,77,81], also tied to heightened VS/OFC activity for the adolescent age group [8]. Another pair of fMRI studies has simulated different aspects of reward anticipation and reward receipt outside of the risk context, isolating the reward component. In one, adolescents showed greater VS activation when winning a reward in a card guessing task relative to adults, and when a peer was present as compared to when the adolescent was alone [83]. In another, adolescents showed greater behavioral preference for immediate vs. delayed rewards when in the presence of a peer vs. when alone [84]. Taken together, these studies highlight that not only are adolescents neurobiologically “attuned” to the presence of their peers, but that this social dynamic enhances processing of critical reward areas in their brains, which is correlated with greater decisions favoring more risky scenarios, as well as with heightened reward response.
These fMRI studies simulating peer influence and risk/reward processing add a layer of understanding to the question of neurobiological factors driving cannabis use in adolescence. First, they suggest that peers and exploratory behaviors inherent to this developmental period have interactive “reward sensitization” effects, which manifest in the brain’s reward-motivational network (i.e., VS and OFC). Notably, neural response in these regions during incentive processing is shown to be heightened in cannabis-using adolescents relative to non-using controls or to adults [72–74,85,86]; thus, these social neuroscience paradigms suggest that reward incentives may, in fact, be enhanced by social incentives during this age group. Second, in some cases, the social incentive may even act as the primary driver of reward response [76–78]. In other cases, the primary driver appears to be the exploratory behavior, which is enhanced in the presence of peers [8]. Finally, the locus of these studies in the VS and OFC, but not cognitive control regions (e.g., vlPFC), suggest that peers influence risk exploration through incentive salience effects rooted in the brain’s reward-motivation network. This is compelling, as the VS and OFC are part of the cannabinoid network that underlies incentive salience of cannabis [87]. Indeed, distinct neural profiles observed for different substances of abuse (i.e., alcohol, tobacco, cannabis) in adolescents [88,89], particularly in the VS, [89] suggest that cannabis-specific processes may be at play in the developing brain, and also enhanced in peer contexts.
Social anxiety
Individual differences in social anxiety and related behavioral manifestations, including behavioral inhibition and social phobia, have been associated with distinct neural response in reward tasks, such as monetary incentive delay (MID) [90–92] and gambling tasks [93,94], as well as in peer social evaluation paradigms [95–97]. Across the reward tasks, an emerging theme has been a strong link between anxiety-related behavioral phenotypes and neural responses to reward anticipation or receipt in the VS and amygdala, although the directions of neural response in these studies have varied [90–93]. A second set of studies simulated peer social acceptance and social rejection events. Here, one study evidenced heightened VS response to peer acceptance (vs. rejection) in behaviorally inhibited adolescents [95]. Two more showed, in anxious adolescents, that amygdala activation and amygdala-cortical co-activation were heightened when anticipating peer evaluation [96,97]. In terms of cannabis use implications, the dual roles for the VS and the amygdala in driving behavior in risk-reward and social evaluation paradigms in anxious adolescents suggests that reward seeking behavior in this group might be intimately linked with heightened monitoring of both rewards and threats in the environment. The data from both the non-social reward and the peer evaluation tasks, which pinpoint VS activation in rewarding contexts, suggest reward-related mechanisms may underlie social anxiety in adolescents. This is compelling, as anxiety for a long time has been associated primarily with aberrant threat-related neurobiological function (e.g., amygdala; [98]), while more recent data, on social anxiety in particular, suggests adolescents have competing drives of heightened sensitivity to social reward in tandem with social avoidance [57]. As such, one possible route into cannabis use is heightened neural sensitivity to non-social rewards (i.e., cannabis) in contexts of heightened social concern. Future fMRI studies can test this hypothesis through concurrent assessment of both reward sensitivity and social sensitivity in adolescent cannabis users with and without social anxiety problems.
Negative experiences with peers
To date, only a handful of fMRI studies in adolescents have related negative experiences with peers to neural response in risk-reward scenarios, including MID [99], simulated “go/no go” driving tasks [100,101], and the Balloon Analog Risk Task [102]. An emerging trend across these studies is an intriguing relationship between negative experiences with peers and heightened risky decision-making that is mediated by an affect regulatory network in the brain, including the medial prefrontal cortex (mPFC), temporo-parietal junction (TPJ), dorsal anterior cingulate cortex (dACC), and insula [99–102]. This is in contrast to the peer manipulation contexts (e.g., peer present vs. absent) that pinpointed reward-motivation regions as central to risk and reward processing (e.g., [7,8]). One possible takeaway from this divergence in findings is that the quality of peer experiences plays an important role in guiding neurobehavioral movement toward cannabis use. When peer influences are positive, they may enhance positive affect during incentive processing through activation of the brain’s reward-motivation network. However, when peer influences are negative, adolescents may either reduce negative affect or regulate positive affect during incentive processing through an affect regulatory network that drives cannabis use. The empirical data are in line with work relating chronic negative peer experiences with suppressed reward response and subsequent depression onset in adolescence [66]. As such, the neural mechanisms driving cannabis use behavior in this group of adolescents may be associated with suppressed reward sensitivity and social avoidance, as often occurs in depressed mood in adolescents [66]. Nevertheless, given frequent high associations among peer rejection and both social anxiety [103] and depression outcomes [65] in adolescents, the reward-related mechanisms of cannabis use in adolescents with frequent negative peer experiences are likely to be modulated by comorbid psychopathologies related to withdrawal. Therefore, inclusion of depression and social anxiety measures in adolescent cannabis users, in addition to assessment of negative peer experiences, will be critical in future studies in order to disentangle the neurocognitive profiles related to these often overlapping phenotypes (e.g., whether cannabis use relates to a suppressed or a heightened reward response in frequently rejected adolescents may depend on comorbid depression and/or social anxiety symptoms).
Applications for CUD Prevention and Intervention
The youngest subset of adolescents to initiate cannabis use (typically around age 12 or 13) is at the highest risk for transition into CUD [104]. Development of more effective psychosocial interventions for this high-risk group will necessitate careful consideration of the social environment in which these adolescents begin to engage in substance exploration. Next-level treatments that target neurally-mediated reward-motivational processes, such as motivational interviewing (MI), have already shown promising efficacy for addiction treatment in adolescents [105]. These recent treatment developments can be further refined through an understanding of the different peer social contexts – and their underlying neural mechanisms – that so often drive cannabis initiation in adolescents. For example, in adolescents for whom cannabis use is driven primarily by the desire to enhance “positive affect” through cannabis exploration, but enhanced by peer influence, the most amenable neural targets may be reward-motivational regions, and the most effective therapies may target the cannabis use motivation directly (e.g., cue exposure therapy). Likewise, when cannabis use is driven primarily by the desire for peer affiliation, the neural targets may also be reward-motivational regions, but with peer-focused MI treatment, dyadic therapy sessions with a friend or peer, and group- or peer-led interventions potentially being the more helpful approaches, whereby these therapies harness the rewarding nature of peer acceptance into more health-positive experiences with peers. Alternatively, more data will also help clinicians to determine when peer-based approaches can actually exacerbate cannabis use problems through motivational enhancement, a situation where non-peer based approaches would work better for the patient. Furthermore, in adolescents for whom cannabis use is driven primarily by the desire to decrease “negative affect” in the context of peers (i.e., to reduce social anxiety, or cope with negative peer experiences), more appropriate neural targets may center on affect regulatory regions, as previously implicated in MI treatments [14] and in mindfulness meditation [105]. Finally, future studies will be highly useful for determining how shared and/or distinct underlying neural mechanisms in comorbid psychopathology (i.e., CUD with social anxiety/depression) move adolescents toward use in peer contexts. It is important to note that not all mechanistic profiles in our model are likely to confer the same degree of risk for the development of CUD. Profiles of adolescents that are more strongly associated with adolescent-emergent psychopathology (e.g., social anxiety) or addiction proneness (e.g., incentive salience of cannabis) would likely have more frequent outcomes of CUD than those associated primarily with peer sensitivity, wherein the cannabis use behavior may be more adolescent-limited.
Conclusions
We elucidated the intersecting roles of neural function and peer factors in cannabis use in adolescents, in order to understand why and how they decide to use. Novel social neuroscience paradigms using fMRI in adolescents have identified distinct neural mechanisms of risk decision-making and incentive processing in peer contexts, centered on reward-motivation and affect regulatory neural networks. These findings have translatable potential for peer-driven cannabis use decisions in adolescents. Specifically, neural mechanisms underlying adolescent cannabis use may depend on the peer context. Integration of social neuroscience and behavioral addiction literature has prompted us to propose four “mechanistic profiles” of social facilitation in adolescent cannabis users: (1) peer affiliation desire; (2) cannabis exploration desire, which is enhanced in peer contexts; (3) social anxiety within peer contexts; and (4) negative peer experiences. Studies that integrate peer social context and neuroscience to examine adolescent cannabis use are merely on the horizon, though we are encouraged by a growing number of such studies conducted in adult cannabis users thus far [106,107]. Though limited in scope, our mechanistic model can begin to disaggregate the complex neurodevelopmental and psychosocial factors that drive adolescent cannabis use. For example, fMRI methods can be used to test whether the mere presence of a real-world cannabis-using friend, or possibly the decision making behaviors of a cannabis-using friend, impact the neural architecture of an adolescent cannabis user’s brain. Furthermore, individual difference factors (e.g., social anxiety, resistance to peer influence, peer victimization history) can be added to these fMRI models to help identify who is maximally sensitive to peer processes in the brain (and may be most responsive to peer-based interventions). Overall, with the legal landscape for cannabis use changing rapidly in the U.S., and the long-term consequences of these changes for adolescents yet unknown, these theoretical models of adolescent use decisions that integrate peer factors and neuroscience are very timely and potentially highly useful for CUD interventions.
Acknowledgments
Source of Funding:
This article is funded by 3R01AA023658-02S1 (PI: Feldstein Ewing)
Footnotes
Conflicts of Interest:
Dr. Justin D. Caouette and Dr. Sarah W. Feldstein Ewing have no conflicts of interest to disclose.
Human and Animal Rights:
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wetherill R, Tapert SF. Adolescent brain development, substance use, and psychotherapeutic change. Psychol Addict Behav. 2013;27:393–402. doi: 10.1037/a0029111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52:216–24. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- 4••.Nelson EE, Jarcho JM, Guyer AE. Social re-orientation and brain development: An expanded and updated view. Dev Cogn Neurosci. 2016;17:118–27. doi: 10.1016/j.dcn.2015.12.008. This review provides evidence from behavioral-cognitive neuroscience for how adolescent neurodevelopment shapes peer experiences, including rendering them more vulnerable to peer influence (e.g., cannabis use). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin KH, Bukowski WM, Bowker JC. Children in peer groups. In: Lerner RM, Bornstein MH, Leventhal T, editors. Handb Child Psychol Dev Sci. 7th. Vol. 4. 2015. Ecol Settings Process. [Google Scholar]
- 6.Luna B, Wright C. Adolescent brain development: Implications to the juvenile criminal justice system. In: Heilbrun K, DeMatteo D, Goldstein NES, editors. APA Handbooks Psychol APA Handb Psychol Juv Justice. Washington, DC: American Psychological Association; 2015. [Google Scholar]
- 7••.Albert D, Chein J, Steinberg L. The teenage brain: Peer influences on adolescent decision making. Curr Dir Psychol Sci. 2013;22:114–20. doi: 10.1177/0963721412471347. This review provides evidence from social neuroscience experimental paradigms for how peers influence risky decision-making in adolescents. The current manuscript applies this evidence to peer-motivated adolescent cannabis use decisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev Sci. 2011;14:F1–10. doi: 10.1111/j.1467-7687.2010.01035.x. This empirical paper provides evidence from an experimental social neuroscience paradigm for how peer observation influences risky decision-making in adolescents. The current manuscript applies this evidence to peer-motivated adolescent cannabis use decisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunther Moor B, van Leijenhorst L, Rombouts SARB, Crone EA, Van der Molen MW, Moor GB, et al. Do you like me? Neural correlates of social evaluation and developmental trajectories. Soc Neurosci. 2010;5:461–82. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- 10.Conrod PJ, Nikolaou K. Annual Research Review: On the developmental neuropsychology of substance use disorders. J Child Psychol Psychiatry. 2016;57:371–94. doi: 10.1111/jcpp.12516. [DOI] [PubMed] [Google Scholar]
- 11.Jacobus J, Tapert SF. Effects of cannabis on the adolescent brain. Curr Pharm Des. 2014;20:2186–93. doi: 10.2174/13816128113199990426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2015: Overview, key findings on adolescent drug use. Ann Arbor, MI: Institute for Social Research, The University of Michigan; 2016. [Google Scholar]
- 14.Feldstein Ewing SW, McEachern AD, Yezhuvath U, Bryan AD, Hutchison KE, Filbey FM. Integrating brain and behavior: Evaluating adolescents’ response to a cannabis intervention. Psychol Addict Behav. 2012;27:510–25. doi: 10.1037/a0029767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kann L, McManus T, Harris WA, Shanklin SL, Flint KH, Hawkins J, et al. Youth Risk Behavior Surveillance – United States, 2015. 2016. [DOI] [PubMed] [Google Scholar]
- 16.Hughes A, Lipari RN, Williams MR. The CBHSQ Report: Marijuana use and perceived risk of harm from marijuana use varies within and across states. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2016. [PubMed] [Google Scholar]
- 17.Kuntsche E, Jordan MD. Adolescent alcohol and cannabis use in relation to peer and school factors. Results of multilevel analyses. Drug Alcohol Depend. 2006;84:167–74. doi: 10.1016/j.drugalcdep.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Creemers HE, Dijkstra JK, Vollebergh WA, Ormel J, Verhulst FC, Huizink AC. Predicting life-time and regular cannabis use during adolescence; the roles of temperament and peer substance use: The TRAILS study. Addiction. 2010;105:699–708. doi: 10.1111/j.1360-0443.2009.02819.x. [DOI] [PubMed] [Google Scholar]
- 19.Ali MM, Amialchuk A, Dwyer DS. The social contagion effect of marijuana use among adolescents. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldstein Ewing SW, Filbey FM, Loughran TA, Chassin L, Piquero AR. Which matters most? Demographic, neuropsychological, personality, and situational factors in long-term marijuana and alcohol trajectories for justice-involved male youth. Psychol Addict Behav. 2015;29:603–12. doi: 10.1037/adb0000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldstein Ewing SW, Chung T. Neuroimaging mechanisms of change in psychotherapy for addictive behaviors: Emerging translational approaches that bridge biology and behavior. Psychol Addict Behav. 2013;27:329–35. doi: 10.1037/a0031491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Feldstein Ewing SW, Tapert SF, Molina BS. Uniting adolescent neuroimaging and treatment research: Recommendations in pursuit of improved integration. Neurosci Biobehav Rev. 2016;62:109–14. doi: 10.1016/j.neubiorev.2015.12.011. This review discusses the utility of adolescent neuroimaging toward guiding treatment and intervention for cannabis and other substance use. The current manuscript further develops this idea by considering peer context in uniting neuroimaing with treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tucker JS, de la Haye K, Kennedy DP, Green HD, Jr, Pollard MS. Peer influence on marijuana use in different types of friendships. J Adolesc Heal. 2014;54:67–73. doi: 10.1016/j.jadohealth.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de la Haye K, Green HD, Pollard MS, Kennedy DP, Tucker JS. Befriending risky peers: Factors driving adolescents’ selection of friends with similar marijuana use. J Youth Adolesc. 2015;44:1914–28. doi: 10.1007/s10964-014-0210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DʼAmico EJ, Houck JM, Hunter SB, Miles JN, V, Osilla KC, Ewing BA, et al. Group motivational interviewing for adolescents: Change talk and alcohol and marijuana outcomes. J Consult Clin Psycho. 2015;83:68. doi: 10.1037/a0038155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldstein Ewing SW, Walters S, Baer J. Gr Motiv Interviewing. New York, NY: The Guilford Press; 2012. Group motivational interviewing with adolescents and young adults; pp. 387–406. [Google Scholar]
- 28.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci. 2004:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 29.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 31.Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–92. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- 32.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3585–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–61. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 34.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 35.Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ. The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci. 2014;36:147–60. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- 36.Giedd JN. The amazing teen brain. Sci Am. 2015;312:32–7. doi: 10.1038/scientificamerican0615-32. [DOI] [PubMed] [Google Scholar]
- 37.Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, et al. The dual systems model: Review, reappraisal, and reaffirmation. Dev Cogn Neurosci. 2016;17:103–17. doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urošević S, Collins P, Muetzel R, Lim K, Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Dev Psychol. 2012;48:1488–500. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burnett S, Sebastian C, Cohen Kadosh K, Blakemore SJ. The social brain in adolescence: Evidence from functional magnetic resonance imaging and behavioural studies. Neurosci Biobehav Rev. 2011;35:1654–64. doi: 10.1016/j.neubiorev.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somerville LH. The teenage brain: Sensitivity to social evaluation. Curr Dir Psychol Sci. 2013;22:121–27. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinberg L, Morris AS. Adolescent development. Annu Rev Psychol. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- 42.Foulkes L, Blakemore SJ. Is there heightened sensitivity to social reward in adolescence? Curr Opin Neurobiol. 2016;40:81–5. doi: 10.1016/j.conb.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Soc Cog Affect Neurosci. 2012;7:81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson EE, Guyer AE. The development of the ventral prefrontal cortex and social flexibility. Dev Cogn Neurosci. 2011;1:233–45. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 2010;72:134–45. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009;80:1000–15. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckner JD, Crosby RD, Silgado J, Wonderlich SA, Schmidt NB. Immediate antecedents of marijuana use: An analysis from ecological momentary assessment. J Behav Ther Exp Psychiatry. 2012;43:647–55. doi: 10.1016/j.jbtep.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elkington KS, Bauermeister JA, Zimmerman MA. Do parents and peers matter? A prospective socio-ecological examination of substance use and sexual risk among African American youth. J Adolesc. 2011;34:1035–47. doi: 10.1016/j.adolescence.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fergusson DM, Boden JM, Horwood LJ. The developmental antecedents of illicit drug use: Evidence from a 25-year longitudinal study. Drug Alcohol Depend. 2008;96:165–77. doi: 10.1016/j.drugalcdep.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 50.de Looze M, Harakeh Z, van Dorsselaer SAFM, Raaijmakers QAW, Vollebergh WAM, ter Bogt TFM. Explaining educational differences in adolescent substance use and early sexual debut: The role of parents and peers. J Adolesc. 2012;35:1035–44. doi: 10.1016/j.adolescence.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Becker SJ, Curry JF. Testing the effects of peer socialization versus selection on alcohol and marijuana use among treated adolescents. Subst Use Misuse. 2014;49:234–42. doi: 10.3109/10826084.2013.824479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De La Haye K, Green HD, Kennedy DP, Pollard MS, Tucker JS. Selection and influence mechanisms associated with marijuana initiation and use in adolescent friendship networks. J Res Adolesc. 2013;23:474–86. doi: 10.1111/jora.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillespie NA, Neale MC, Jacobson K, Kendler KS. Modeling the genetic and environmental association between peer group deviance and cannabis use in male twins. Addiction. 2009;104:420–9. doi: 10.1111/j.1360-0443.2008.02457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hampson SE, Andrews JA, Barckley M. Childhood predictors of adolescent marijuana use: Early sensation-seeking, deviant peer affiliation, and social images. Addict Behav. 2008;33:1140–7. doi: 10.1016/j.addbeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kedzior K, Laeber L. Cannabis use and disorder: Epidemiology, comorbidity, health consequences, and medico-legal status. BMC Psychiatry. 2014;14 doi: 10.1186/1471-244X-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kedzior K, Laeber L. A positive association between anxiety disorders and cannabis use disorders in the general population – a meta-analysis of 31 studies. BMC Psychiatry. 2014;14 doi: 10.1186/1471-244X-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caouette JD, Guyer AE. Gaining insight into adolescent vulnerability for social anxiety from developmental cognitive neuroscience. Dev Cogn Neurosci. 2014;8:65–76. doi: 10.1016/j.dcn.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buckner JD, Heimberg RG, Matthews RA, Silgado J. Marijuana-related problems and social anxiety: The role of marijuana behaviors in social situations. Psychol Addict Behav. 2012;26:151–6. doi: 10.1037/a0025822. [DOI] [PubMed] [Google Scholar]
- 59.Buckner JD, Schmidt NB. Marijuana effect expectancies: Relations to social anxiety and marijuana use problems. Addict Behav. 2008;33:1477–83. doi: 10.1016/j.addbeh.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmits E, Mathys C, Quertemont E. A longitudinal study of cannabis use initiation among high school students: Effects of social anxiety, expectancies, peers and alcohol. J Adolesc. 2015;41:43–52. doi: 10.1016/j.adolescence.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Buckner JD, Heimberg RG, Schmidt NB. Social anxiety and marijuana-related problems: The role of social avoidance. Addict Behav. 2011;36:129–32. doi: 10.1016/j.addbeh.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buckner JD, Bonn-Miller MO, Zvolensky MJ, Schmidt NB. Marijuana use motives and social anxiety among marijuana-using young adults. Addict Behav. 2007;32:2238–52. doi: 10.1016/j.addbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buckner JD, Schmidt NB. Social anxiety disorder and marijuana use problems: The mediating role of marijuana effect expectancies. Depress Anxiety. 2009;26:864–70. doi: 10.1002/da.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med [Internet] 2014;44:797–810. doi: 10.1017/S0033291713001438. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23795762. [DOI] [PubMed] [Google Scholar]
- 65.Platt B, Kadosh KC, Lau JYF. The role of peer rejection in adolescent depression. Depress Anxiety. 2013:809–21. doi: 10.1002/da.22120. [DOI] [PubMed] [Google Scholar]
- 66.Davey CG, Yücel M, Allen NB. The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev. 2008:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 67.Wormington SV, Anderson KG, Tomlinson KL, Brown SA. Alcohol and Other Drug Use in Middle School: The Interplay of Gender, Peer Victimization, and Supportive Social Relationships. J Early Adolesc. 2013;33:610–34. doi: 10.1177/0272431612453650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kelly EV, Newton NC, Stapinski LA, Slade T, Barrett EL, Conrod PJ, et al. Concurrent and prospective associations between bullying victimization and substance use among Australian adolescents. Drug Alcohol Depend. 2015;154:63–8. doi: 10.1016/j.drugalcdep.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 69.Kim MJ, Catalano RF, Haggerty KP, Abbott RD. Bullying at elementary school and problem behaviour in young adulthood: A study of bullying, violence and substance use from age 11 to age 21. Crim Behav Ment Heal. 2011;21:136–44. doi: 10.1002/cbm.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tharp-Taylor S, Haviland A, D’Amico EJ. Victimization from mental and physical bullying and substance use in early adolescence. Addict Behav. 2009;34:561–7. doi: 10.1016/j.addbeh.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldbach JT, Schrager SM, Dunlap SL, Holloway IW. The application of minority stress theory to marijuana use among sexual minority adolescents. Subst Use Misuse. 2015;6084:1–10. doi: 10.3109/10826084.2014.980958. [DOI] [PubMed] [Google Scholar]
- 72.Cousijn J, Wiers RW, Ridderinkhof KR, Van Den Brink W, Veltman DJ, Porrino LJ, et al. Individual differences in decision making and reward processing predict changes in cannabis use: A prospective functional magnetic resonance imaging study. Addict Biol. 2013;18:1013–23. doi: 10.1111/j.1369-1600.2012.00498.x. [DOI] [PubMed] [Google Scholar]
- 73.Jager G, Block RI, Luijten M, Ramsey NF. Tentative evidence for striatal hyperactivity in adolescent cannabis-using boys: A cross-sectional multicenter fMRI study. J Psychoactive Drugs. 2013;45:156–67. doi: 10.1080/02791072.2013.785837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Filbey FM, Dunlop J. Differential reward network functional connectivity in cannabis dependent and non-dependent users. Drug Alcohol Depend. 2014;140:101–11. doi: 10.1016/j.drugalcdep.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Segalowitz SJ, Santesso DL, Willoughby T, Reker DL, Campbell K, Chalmers H, et al. Adolescent peer interaction and trait surgency weaken medial prefrontal cortex responses to failure. Soc Cogn Affect Neurosci. 2012;7:115–24. doi: 10.1093/scan/nsq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simons-Morton BG, Bingham CR, Falk EB, Li K, Pradhan AK, Ouimet MC, et al. Experimental effects of injunctive norms on simulated risky driving among teenage males. Health Psychol. 2014;33:616–27. doi: 10.1037/a0034837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cascio CN, Carp J, O’Donnell MB, Tinney FJ, Bingham CR, Shope JT, et al. Buffering social influence: Neural correlates of response inhibition predict driving safety in the presence of a peer. J Cogn Neurosci. 2015;27:83–95. doi: 10.1162/jocn_a_00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vorobyev V, Kwon MS, Moe D, Parkkola R, Hämäläinen H. Risk-taking behavior in a computerized driving task: Brain activation correlates of decision-making, outcome, and peer influence in male adolescents. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haddad ADM, Harrison F, Norman T, Lau JYF. Adolescent and adult risk-taking in virtual social contexts. Front Psychol. 2014;5 doi: 10.3389/fpsyg.2014.01476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silva K, Shulman EP, Chein J, Steinberg L. Peers increase late adolescents’ exploratory behavior and sensitivity to positive and negative feedback. J Res Adolesc. 2015;26:696–705. doi: 10.1111/jora.12219. [DOI] [PubMed] [Google Scholar]
- 81.Smith AR, Chein J, Steinberg L. Peers increase adolescent risk taking even when the probabilities of negative outcomes are known. Dev Psychol. 2014;50:1564–8. doi: 10.1037/a0035696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Braams BR, Peters S, Peper JS, Güroǧlu B, Crone EA. Gambling for self, friends, and antagonists: Differential contributions of affective and social brain regions on adolescent reward processing. Neuroimage. 2014;100:281–9. doi: 10.1016/j.neuroimage.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 83.Smith A, Steinberg L, Strang N, Chein J. Age differences in the impact of peers on adolescents’ and adults’ neural response to reward. Dev Cogn Neurosci. 2015;11:75–82. doi: 10.1016/j.dcn.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weigard A, Chein J, Albert D, Smith A, Steinberg L. Effects of anonymous peer observation on adolescents’ preference for immediate rewards. Dev Sci. 2014;17:71–8. doi: 10.1111/desc.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Approach-bias predicts development of cannabis problem severity in heavy cannabis users: results from a prospective FMRI study. PLoS One. 2012;7:e42394. doi: 10.1371/journal.pone.0042394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheetham A, Allen NB, Whittle S, Simmons JG, Yücel M, Lubman DI. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: A 4-year longitudinal and prospective study. Biol Psychiatry. 2012;71:684–92. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 87.Filbey FM, DeWitt SJ. Cannabis cue-elicited craving and the reward neurocircuitry. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:30–5. doi: 10.1016/j.pnpbp.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thayer RE, Montanaro E, Weiland BJ, Callahan TJ, Bryan AD. Exploring the relationship of functional network connectivity to latent trajectories of alcohol use and risky sex. Curr HIV Res. 2014;12 doi: 10.2174/1570162x12666140721124441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karoly HC, Bryan AD, Weiland BJ, Mayer A, Dodd A, Feldstein Ewing SW. Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev Cogn Neurosci. 2015;16:5–15. doi: 10.1016/j.dcn.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, et al. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol Sci. 2009;20:1009–18. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci. 2006;26:6399–405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry. 2012;169:205–12. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93•.Galván A, Peris TS. Neural correlates of risky decision making in anxious youth and healthy controls. Depress Anxiety. 2014;31:591–8. doi: 10.1002/da.22276. This empirical paper identifies neural underpinnings of risky decision-making in adolescents with anxiety. Anxiety disorders, including social anxiety, are highly comorbid with cannabis use. The current manuscript discusses neural mechanisms that underlie social anxiety-related decisions to use cannabis in adolescents. [DOI] [PubMed] [Google Scholar]
- 94.Richards JM, Patel N, Daniele-Zegarelli T, MacPherson L, Lejuez CW, Ernst M. Social anxiety, acute social stress, and reward parameters interact to predict risky decision-making among adolescents. J Anxiety Disord. 2015;29:25–34. doi: 10.1016/j.janxdis.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guyer AE, Benson B, Choate VR, Bar-Haim Y, Perez-Edgar K, Jarcho JM, et al. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Dev Psychopathol. 2014;26:229–43. doi: 10.1017/S0954579413000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guyer AE, Lau JYF, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008;65:1303–12. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spielberg JM, Jarcho JM, Dahl RE, Pine DS, Ernst M, Nelson EE. Anticipation of peer evaluation in anxious adolescents: Divergence in neural activation and maturation. Soc Cogn Affect Neurosci. 2015;10:1084–91. doi: 10.1093/scan/nsu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bishop SJ. Neurocognitive mechanisms of anxiety: An integrative account. Trends Cogn Sci. 2007;11:307–16. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 99•.Casement MD, Guyer AE, Hipwell AE, McAloon RL, Hoffmann AM, Keenan KE, et al. Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Dev Cogn Neurosci. 2014;8:18–27. doi: 10.1016/j.dcn.2013.12.003. This empirical paper identifies neural underpinnings of reward processing in adolescents who have experienced chronic peer victimization. The current manuscript discusses neural mechanisms that underlie negative peer experiences and their documented association with adolescent cannabis use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Falk E, Cascio C, Brook O’Donnell M, Carp J, Tinney F, Jr, Bingham C, et al. Neural responses to exclusion predict susceptibility to social influence. J Adolesc. 2014;54:S22–31. doi: 10.1016/j.jadohealth.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peake SJ, Dishion TJ, Stormshak EA, Moore WE, Pfeifer JH. Risk-taking and social exclusion in adolescence: Neural mechanisms underlying peer influences on decision-making. Neuroimage. 2013;82:23–34. doi: 10.1016/j.neuroimage.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Telzer EH, Fuligni AJ, Lieberman MD, Miernicki ME, Galvan A. The quality of adolescents peer relationships modulates neural sensitivity to risk taking. Soc Cogn Affect Neurosci. 2013;10:389–98. doi: 10.1093/scan/nsu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.La Greca AM, Harrison HM. Adolescent peer relations, friendships, and romantic relationships: Do they predict social anxiety and depression? J Clin Child Adolesc Psychol. 2005;34:49–61. doi: 10.1207/s15374424jccp3401_5. [DOI] [PubMed] [Google Scholar]
- 104.Simpson AK, Magid V. Cannabis use disorder in adolescence. Child Adolesc Psychiatr Clin North Am. 2014;25:431–43. doi: 10.1016/j.chc.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 105.Thayer RE, Feldstein Ewing SW. Adolescent psychotherapy for addiction medicine: From brain development to neurocognitive treatment mechanisms. Prog Brain Res. 2016;224:305–22. doi: 10.1016/bs.pbr.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 106.Gilman JM, Lee S, Kuster JK, Lee MJ, Kim BW, van der Kouwe A, et al. Variable activation in striatal subregions across components of a social influence task in young adult cannabis users. Brain Behav. 2016;6:e00459. doi: 10.1002/brb3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gilman JM, Schuster RM, Curran MT, Calderon V, van der Kouwe A, Evins AE. Neural mechanisms of sensitivity to peer information in young adult cannabis users. Cogn Affect Behav Neurosci. 2016;16:646–61. doi: 10.3758/s13415-016-0421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]