Abstract
Adjunctive therapies have been proposed for use in at least 5 inflammation pathobiology phenotypes in pediatric sepsis-induced multiple organ failure (MOF). Here, we provide host-pathogen interaction prototypes to facilitate understanding of the rationale for personalized therapy in these phenotypes. Meningococcemic sepsis and Shiga-like toxin-associated atypical Hemolytic Uremic Syndrome sepsis result in thrombocytopenia and MOF due to endothelial dysfunction and formation of small vessel thromboses that can respond to plasma exchange and C5a monoclonal antibody therapy. H1N1 Influenza A sepsis is associated with immune paralysis that can result in opportunistic secondary infection with invasive methicillin resistant Staphylococcus aureus (MRSA) and MOF that can respond to Granulocyte Macrophage Colony Stimulating Factor therapy. Hyperleukocytosis-associated MOF is associated with critical Bordetella Pertussis pulmonary hypertension and cardiovascular collapse which can respond to leukoreduction therapy. Epstein Barr Virus lymphoproliferative disease-associated sequential MOF has high levels of soluble-Fas Ligand that cause liver failure, and can respond to anti-CD20 monoclonal antibody therapy. Viral hemorrhagic fevers such as Ebola or Dengue can lead to macrophage activation syndrome characterized by hyperferritinemia, hepatobiliary dysfunction and disseminated intravascular coagulation (DIC)-related MOF that can theoretically respond to anti-inflammatory therapies. We discuss the literature on adjunctive anti-inflammatory and immune modulation therapies that, in addition to traditional organ support and infection source control, might be part of a personalized precision medicine approach to reverse of each of these inflammatory pathobiology phenotypes.
Keywords: Thrombocytopenia associated MOF, Immune paralysis, Hyperleukocytosis, Sequential MOF, Macrophage Activation Syndrome
Introduction
Adjunctive therapies are considered by clinicians for use in the management of children with sepsis inflammation pathobiology phenotypes and multiple organ failure (MOF). Here, we examine host-pathogen interaction models (or prototypes) that provide the rationale for proven, experimental, or proposed inflammation pathobiology phenotype-targeted therapies in pediatric sepsis-induced MOF. A few general themes for the management of pediatric MOF are always pertinent, including the search for and removal of sources of ongoing infection and inflammation, and support of cardiovascular and other organ functions. In addition to this general approach, one can also use clinical criteria and confirmatory tests to identify one or more of five inflammation phenotypes that can be targeted with pathobiology-based adjunctive therapies (Table 1).
Table 1.
Five inflammation pathobiology phenotypes and putative adjunctive therapies
| Phenotype | Clinical Criteria | Biomarker/Prototype | Adjunctive Therapy |
|---|---|---|---|
| Thrombocytopenia Associated MOF | Platelets < 100,000/mm3 Acute Kidney Injury Elevated LDH |
ADAMTS 13< 57% Discussed Prototypes = Purpura fulminans/Atypical HUS |
a) Plasma Exchange9,,11–15,61 removes ultra large vWF multimers and restores ADAMTS13 activity b) C5a Antibody16–19 Inhibits activated complement (FDA approved for aHUS) |
| Immune paralysis Associated MOF | Persistent or Secondary Infections | Monocyte HLA-DR expression < 30% or 8,000 molecules; Whole blood ex vivo TNF response to LPS < 200 pg/mL; Absolute Lymphocyte Count < 1,000 mm3 Discussed Prototype = H1N1/MRSA | GM-CSF25,29,30 Immune suppressant withdrawal28 Restores TNF response to endotoxin |
| Hyperleukocytosis and pulmonary hypertension associated MOF | Age < 6 months Pulmonary HTN | WBC > 50,000 mm3 Discussed Prototype = Critical Pertussis |
Extracorporeal Leukoreduction36 removes circulating WBC and decreases pulmonary hypertension |
| Sequential MOF with liver failure | Respiratory distress Followed by Hepatobiliary Dysfunction | s-FasL > 200 pg/mL Discussed Prototype = Epstein Barr Virus Lymphoproliferative Disease |
a) Hold immune suppressants b) Give anti CD20 monoclonal antibody44,45 removes EBV reservoir (FDA approved for PTLD) |
| Macrophage Activation Syndrome | Hepatobiliary Dysfunction and Disseminated Intravascular Coagulation | Ferritin > 500 ng/mL Discussed Prototype = Viral Hemorrhagic Fevers |
IVIG + Steroids + Plasma Exchange61 Anakinra48,57 Tocilizumab58,59 decreases macrophage inflammation |
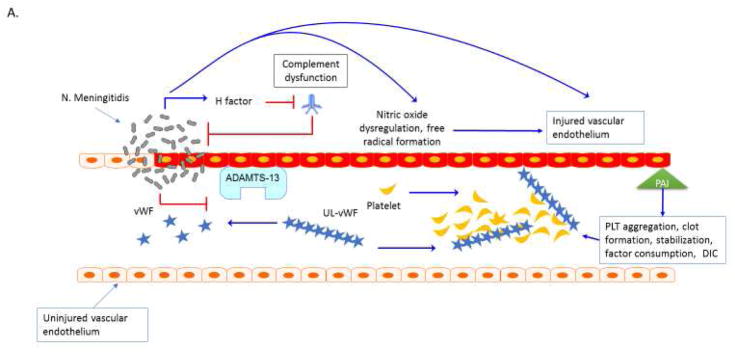
Thrombocytopenia associated MOF (TAMOF) centers on endothelial dysfunction, impairment of metalloproteinase activity of ADAMTS13, and consumptive coagulopathy that results in microvascular impairment and organ injury. We review two host-pathogen interaction models for TAMOF: 1) Neisseria meningitides induced purpura fulminans, associated with complement dysfunction, endothelial injury, production of von Willebrand factor (vWF) ultra large multimers and intravascular coagulation, (Figure 1a); and 2) Atypical hemolytic uremic syndrome (aHUS) as a result of genetic polymorphisms in ADAMTS13 activity or inhibitory complement regulation (Figure 1b). In addition to microbiological source control, both types are amenable to therapeutic plasma exchange, which removes thrombogenic ultra large vWF multimers and restores ADAMTS13 activity. aHUS is also amenable to biologic terminal complement inhibitors such as Eculizumab.
Figure 1. Thrombocytopenia Associated MOF. A. Purpura fulminans.
Neisseria meningitidis can adhere to and form colonies on the vascular endothelium via pilin62. Meningococcal virulence factor such as factor H binding protein can down regulate complement response and allow bacterial proliferation63. Bacterial-endothelial interactions result in endothelial inflammation via NFkB responses and can further promote endothelial infection, capillary leakage and translocation of bacteria across the vessel64–66. Invasive meningococcal disease is associated with decreased activity of the metalloproteinase ADAMST13 and increased activity of von Willebrand factor (vWF) 67, 68. Ultra-large vWF (UL vWF) multimers contribute to platelet (PLT) aggregation and intravascular thrombosis68,69. Endothelial inflammation contributes to platelet dysfunction via N. meningitidis-derived nitric oxide with impaired vascular homeostasis and NO mediated impairment ADP-mediated platelet aggregation70. Inflammatory cytokines change the coagulation profile towards pro-coagulation with a reduction of activated protein C (APC) and anti-thrombin III (ATIII) and upregulation of prothrombin and the anti-fibrinolytic plasminogen activator inhibitor-1 (PAI-1)71. The end result of the interaction of multiple ‘inflammatory’ pathways in the organ is micro-thrombosis, tissue ischemia, oxidative insult, ischemia and cell death. Plasm exchange therapy can reverse this process. B. Atypical Hemolytic Uremic Syndromes. Sterile microangiopathies such as thrombotic thrombocytopenia purpura (TTP) are associated with inhibition of ADAMST13 activity via the presence of auto-inhibitors, whereas atypical HUS or “congenital TTP” has been associated with ADAMST13 and inhibitory complement gene mutations72,73. Upon infection with Shiga toxin (ST)-producing pathogens, ST has direct effects on the vascular endothelium and results in increased release of ultra-large vWF multimers and to directly inhibit ADAMST13 activity level, further promoting thrombosis formation73–74. ST mediated aHUS causes a pro-inflammatory endothelial state by promoting leukocyte adhesion and by producing endothelial derived cytokines such as IL-8, similar to the vascular pathophysiology observed in purpura fulminans. Exposure of human endothelial cells to the pro-inflammatory cytokines TNF-al and IL-1b increase expression of globotriaosylceramide (Gb3) on endothelial cells and result in further susceptibility to ST by a feed-forward mechanism75. Shiga-toxin causes production of Complement 3a (C3a), with loss of thombomodulin (TM), changes in cell surface adhesion molecules and a propensity toward clot production2, 76–78. In aHUS, mutations in the alternative complement regulatory pathway, especially mutations in the complement factor H, impair control of Complement 3b (C3b) on the cell surface by inability to recognize sialic acid and helps to explain the thrombogenic potential in these patients2, 79. Mutations have recently been described in multiple complement regulatory genes in adult patients with aHUS, with 12% of patients having compound mutations80. In addition to plasma exchange therapy, the anti-C5a monoclonal antibody eculizumab is FDA approved for this process.
Abbreviations: Thombocytopenia Associated Multiple Organ Failure (TA-MOF); Nuclear factor kappa b (NFkb); A disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMST13); von Willebrand factor (vWF), Platelet (PLT); Nitric Oxide (NO); Activated protein C (APC); Anti-thrombin III (ATIII); plasminogen activator inhibitor-1 (PAI-1); thrombotic thrombocytopenia purpura (TTP); hemolytic uremic syndrome (HUS); atypical HUS (aHUS); shiga toxin (ST); tumor necrosis factor alpha (TNF-a); interleukin 1 beta (IL-1b); globotriaosylceramide (Gb3); complement 3a (C3a); thrombomodulin (TM), complement 3b (C3b).
There are also host genetic risk factors for the development of TAMOF including ADAMTS13 deficiency syndrome (Upshaw-Schulman Syndrome, also known as congenital thrombotic thrombocytopenic purpura), and deficiency in complement H1,2. Environmental risk factors include elaboration of inflammatory cytokines resulting in direct endothelial activation or injury, liver failure, and inhibitory ADAMTS13 antibodies as seen in acquired thrombotic microangiopathy (TMA)3–5. Free hemoglobin resulting from red blood cell hemolysis in TAMOF is a driver for further endothelial and other organ injury. Other sources of pathologic free hemoglobin include any aged blood cells prone to hemolysis upon transfusion, cardiopulmonary bypass, extracorporeal membrane oxygenation (ECMO), or continuous renal replacement therapy (CRRT)6–8.
There are a number of TAMOF-directed adjunctive therapeutic options. In a small, single center prospective randomized clinical study, plasma exchange therapy (1.5 volumes on day 1 followed by 1 volume on days number 2 through end, with end being determined by return of organ function and platelet count) was associated with removal of ultra large vWF multimers, restoration of ADAMTS13 functional activity, and improvement in end-organ functional markers9,10. Meta-analyses indicate a mortality benefit with use of plasma exchange therapy in adults11–13. Although the data for use of plasma exchange therapy in critically adult and pediatric ICU populations for the indication of sepsis and septic shock is mixed, there is potential benefit in the TAMOF syndrome, based upon biologic plausibility and a track record of efficacy of its use in microangiopathies14,15. The decision to provide plasma exchange should be made based on the clinical condition, including degree of coagulopathy and platelet count. In addition, plasma exchange should be considered in the setting of severe neurologic disease. Evidence for activation of the complement system should also prompt a consideration for plasma exchange therapy. Eculizumab, a C5 terminal complement cleavage inhibitory monoclonal antibody, can be considered in the setting of TAMOF. This therapy has been most extensively studied in the setting of aHUS associated with an ineffective inhibitory complement response, where eculizumab has been shown to improve renal function, need for renal support, and improve quality of life among adult patients16. Eculizumab has been approved for use in pediatric aHUS17. Moreover, earlier administration of the antibody in an aHUS disease course is associated with improved renal recovery18,19.
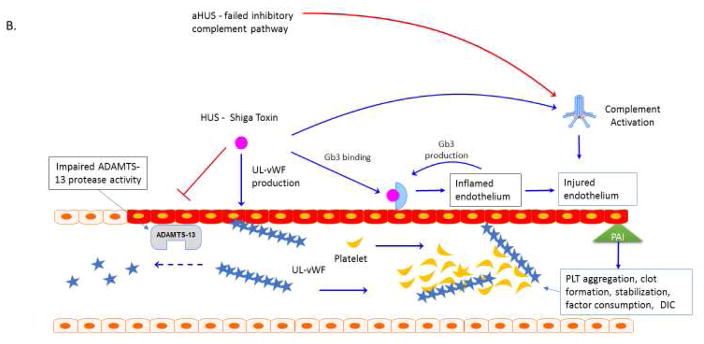
Immunoparalysis associated MOF centers on impairment of both innate and adaptive immune function with resulting inability for the host to contain a primary or secondary infection. The host pathogen example we use for this phenotype of MOF is H1N1 Influenza A infection with impairment of monocyte function and contraction of adaptive immune populations (Figure 2). In addition to antiviral and antibacterial therapy to control pathogen burden and removal (when appropriate) of pharmacologic sources of immune suppression, providing granulocyte macrophage colony stimulating factor (GM CSF) has been successfully used to reverse innate immune paralysis. Programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) blockade as well as provision of recombinant lymphocyte survival factors such as IL-7 are being evaluated as methods to restore adaptive immune function.
Figure 2. Immune Paralysis MOF.
H1N1 influenza has adaptive and innate immune suppressive effects and is associated with the development of MRSA superinfection and death27 in children, and altered bone marrow microenvironment and with reduced leukocyte output in an animal model, with reduced human leukocyte antigen (HLA)-DR expression and reduction in proinflammatory tumor necrosis factor alpha (TNF-a) and interferon gamma (IFN-g) responses22, 80–85. Alterations in innate immune cell function impact polarization and homeostasis of T cell populations, as T cells interacting with anergic monocytes have increases in CTLA-4 mediated negative co-stimulation and anti-inflammatory IL-10 responses86. Altered adaptive immune homeostasis favors contraction of immune populations and lymphopenia. Combined adaptive and innate leukopenia and decreased immune function predisposes to secondary infection with methicillin-resistant Staphylococcus aureus (MRSA). MRSA elaboration of Panton-Valentine Leukocidin (PVL) further reduces innate immune cell number and leads to cytotoxic lung and soft tissue damage87, 88. S. aureus collagen adhesin (CNA) is a virulence factor in invasive pulmonary disease, and may contribute to septic embolization of the pathogen88. Low dose GM-CSF administration can be given to reverse immune paralysis.
Abbreviations: Human Leukocyte Antigen DR (HLA-DR); tumor necrosis factor alpha (TNF-a); interferon gamma (IFN-g); Cytotoxic T lymphocyte antigen 4 (CTLA4); Interleukin 10 (IL-10); Methicillin Resistant Stapholococcus aureus (MRSA); Panton-Valentine Leukocidin (PVL); collagen adhesin (CNA).
Immunoparalysis associated MOF is associated with decreased ex vivo TNF-alpha response < 200 pg/mL and decreased expression of the MHC-II molecule HLA-DR <8000 molecules or <30% of control level beyond 3 days of illness20–22. Among patients with severe sepsis, lymphopenia is an independent predictor of mortality23. A phenotype of prolonged lymphocyte depletion was described among spleen and other lymphoid tissue samples from pediatric and adult patients who died of sepsis associated MOF20,24. Poor outcomes associated with the immunoparalysis phenotype include increased association of ventilator associated pneumonia, reactivation of latent herpesvirus family infections, and secondary opportunistic infections, upon which multi-organ failure may be superimposed25,26.
An examination of the clinical features associated with mortality during the 2009–2010 H1N1 influenza pandemic highlighted the relationship between viral immune suppression and the development of opportunistic methicillin resistant staphylococcus aureus infection (MRSA). In a retrospective observational cohort of 838 patients <21 years admitted to pediatric ICUs across the United States, overall mortality was 8.9%27. Leukopenia and neutropenia were associated with mortality (RR 1.8, 95% CI 1.2–2.9; RR 2.8, 95% CI 1.5–5.5, respectively). Interestingly, bacterial pneumonia (co-infection) with MRSA, but not methicillin sensitive staphylococcus aureus (MSSA), was associated with death (RR 3.2, 95% CI 1.8–5.9). In a multivariate model, among the 251 previously healthy children enrolled in the study, only presumed MRSA infection was significantly associated with mortality (RR 8, 95% CI 3.9–20.6). Whole blood TNF alpha hyporeponsiveness to endotoxin was highly and independently associated with both mortality and length of ICU stay.
The potential treatments for immune paralysis depend on the background immune status of the host. For chronically immune suppressed patients such as transplant recipients, withholding or reducing immune suppression is indicated in the setting of severe sepsis associated MODS.28 Among patients with immunoparalysis associated MOF who had a pre-intervention RR of death of 11 [95% CI 1.4–89], Hall et al showed that treatment with low-dose GM-CSF at 125 micrograms/kg per day for seven days given over a minimum of 12 hours as an infusion prevented secondary infection, death, and restored ex vivo TNF response to LPS29. Similarly, in randomized double-blinded study of adults undergoing general surgical procedures with immunosuppression defined by HLA-DR expression <10,000 molecules on the surface of monocytes, administration of a single dose of GM-CSF, but not influenza vaccine, was associated with restored HLA-DR expression, improved WBC count and less severe delirium compared to placebo30. Therefore, the immune paralysis phenotype of MODS can be considered a reversible condition with appropriate modulation of the host immune response.25
Recent mouse model data has shown that PD-1 and PD-L1 blockade results in improved survival in a model of fungal sepsis31 and ex-vivo data has implicated PD-1 blockade as a potential therapy in patients with severe sepsis and the immune paralysis phenotype.32 Clinical trials are currently enrolling adult patients with severe sepsis to test the role of PD-1-PD-L1 axis blockade using BMS-936559 (anti-PD-L1) in sepsis survival and organ function (https://clinicaltrials.gov/ct2/show/NCT02576457).
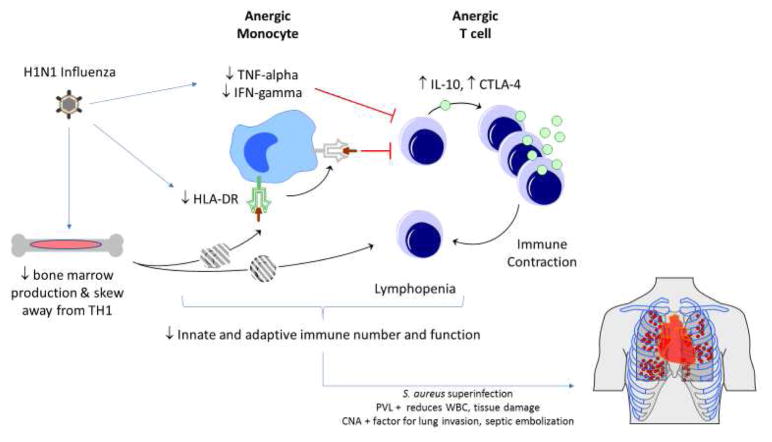
Critical pertussis associated MOF (Figure 3). results in impairment of the innate immune response, and a poor adaptive immune response. In addition, both epithelial targeting and direct effects of pertussis toxin on leukocyte populations causes robust margination of neutrophils and lymphocytes from the tissues to the circulation and the infected epithelial surfaces. The result is a unique pathophysiology of hyperleukocytosis in very young infants. The high viscosity and endothelial dysregulation with cellular plugging of pulmonary arterioles and venules results in pulmonary hypertension and cardiopulmonary collapse. The critical pertussis syndrome is associated with bacterial superinfection, and broad spectrum antimicrobial source control should be initiated in patients with this MOF phenotype. In addition, leukoreduction therapy by therapeutic apheresis, organ support (potentially with the use of ECMO), and a trial of inhaled nitric oxide therapy are reasonable but unproven approaches to treatment.
Figure 3. Hyperleukocytosis – Critical Pertussis MOF.
Bordetella pertussis infects the airway epithelium inducing inflammation and altered mucosal immune response. Bacterial production of the virulence factor CyaA has several deleterious effects on CD11b+ dendritic cells, including decreased ability to perform phagocytosis, decreased capacity to present antigen to T cells, and decreased expression of co-stimulatory molecules. CyaA leads to dendritic cell apoptosis89. The virulence factor filamentous hemagglutinin (FHA) induces NF-kB mediated up-regulation of epithelial ICAM-1 at sites of bacterial invasion89,93. This, in part, results in the histopathological findings typical of pertussis infection including leukocyte activation and infiltration90. Leukocytosis and lymphocytosis and subsequent plugging of pulmonary arterioles and venules is specific to early infancy, predominantly due to margination of cells from tissues90–92. Pertussis toxin (PT) decreases the ability of leukocytes to exit the blood vessel via impairment of CD62L expression and LFA-192. Pertussis toxin has a further role in impairing macrophage and neutrophil activation and function via alterations in toll like receptor (TLR)-4 signaling, whereas prolonged exposure of innate cells to FHA modulates NFkB responses downward93, 94. Complement mediated opsonization and phagocytosis and intracellular killing of B. pertussis is impaired. Another virulence factor, ACT, leads to innate immune cell apoptosis94. In addition to leukocyte plugging and high blood viscosity, perturbations in nitric oxide mediated regulation of pulmonary vascular tone contribute to pulmonary hypertension90–92. Infants have responded to extracorporeal leukocyte reduction therapies as well as inhaled nitric oxide and ECMO for reversal of cardiovascular collapse.
Abbreviations: filamentous hemagglutinin (FHA); intercellular adhesion molecule 1 (ICAM-1); pertussis toxin (PT); toll-like receptor 4 (TLR-4).
The risk factors associated with mortality among <120 day old infants include lower birth weight, younger gestational age, younger age at time of onset, higher peak WBC and lymphocyte count >30,000 cells per microliter. In multivariate analysis, WBC count and birth weight were the only pre-ICU admission factors that were associated with death33. Among patients admitted to the ICU, 43% go on to require endotracheal intubation and mechanical ventilation34. A median WBC count > 27,000 mm3 was significantly associated with need for mechanical ventilation, the presence of pulmonary arterial hypertension and death. Despite the brisk leukocytosis observed among infants, there is evidence that pertussis toxin has direct immune inhibitory effects on macrophage and neutrophil activation putting the infants at risk for co-infection (Figure 3).
Therapies for critical pertussis include treatment of primary infection, prevention of secondary infection, ventilatory support, and leukoreduction to minimize the pathologic effects of hyperleukocytosis. Unfortunately, limited data exists regarding whether the timing of Bordetella pertussis-directed antimicrobial therapy impacts the important clinical outcomes of hospitalization days, need for mechanical ventilation, or death35. In addition to macrolide therapy, broad spectrum antimicrobial therapy is employed for critically ill pertussis patients with severe sepsis or septic shock with special consideration for the possibility of bacterial respiratory superinfection. Rowlands and colleagues proposed an algorithm for use of leukoreduction therapy in critical pertussis based on white blood cell counts > 50,000 mm3 and presence of cardiopulmonary compromise36,37. Additional therapies specific to pertussis pathophysiology as a toxin-driven disease include anti-Pertussis toxin-enriched intravenous immune globulin (P-IVIG). Although standard IVIG therapy has been shown to be ineffective in changing the severity of pertussis disease or in decreasing symptoms, preliminary trials have indicated that targeting the toxin has symptomatic benefit as well as a reduction in the degree of lymphocytosis without significant adverse events38,39. Further study is needed to determine the efficacy of P-IVIG or a combination of leukoreduction and anti-toxin therapy in the critical pertussis cohort, specifically.
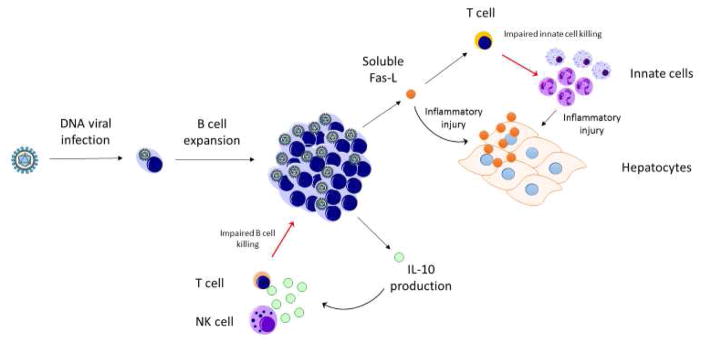
Sequential MOF (SMOF) is a sequential respiratory and then hepato-renal failure syndrome that is driven by perturbation in the immune system’s ability to perform activation-induced cell death (AICD) that leads to lymphoproliferation, and to perform DNA viral infection killing that leads to uncontrolled viremia (Figure 4). This phenotype is most commonly found among patients with organ transplantation who are maintained on T cell immunosuppressant therapy and have Epstein Barr Virus (EBV) or other DNA viral infection. In this condition, EBV infection of B cells provides a pool for viral proliferation, resulting in post-transplant lymphoproliferative disorder (PTLD), and impairment of the normal Fas-Fas ligand (Fas-FasL) mediated apoptotic pathways. Soluble FasL interferes with the T and NK cells ability to cull infected B cells, and also leads to direct hepatocyte injury through FasL-Fas interaction. Expanded innate populations further damage organs by inflammatory cytokine and direct effects. The treatment of sequential MODS includes withdrawal of immune suppression as able and B cell reduction therapy with rituximab (anti-CD20 antibody) in addition to antiviral therapy.
Figure 4. Sequential MOF.
The host-pathogen relationship model in sequential MOF is Epstein Barr Virus (EBV) infection of host B cells, such as occurs in the transplant recipients. Virulence factors such as viral-encoded IL-10 modulate the host immune antigen presenting, NK cell, and T cell antiviral response and contribute to viral survival and lymphoproliferation95, 96. B cells, among other cell types, have been hypothesized to be a source for soluble Fas ligand (s-FasL)40. Membrane bound FasL on T and NK cells interacts with Fas on target cells and results in cellular apoptosis required to cull immune populations in activation induced cell death (AICD)97. The production of large amounts of s-FasL by B cells may interfere with this pathway, impairing cytotoxic culling of B cells that are the cellular pool required for EBV infection. Production of s-FasL impacts T cells ability to kill innate immune cells, which leads to inflammatory injury at end organs including hepatocytes. Furthermore, engagement of Fas on hepatocytes by anti-Fas antibody results in direct hepatic injury and sFasL >200 pg/mL in patients was associated with hepatocyte destruction and mortality40, 98–100. Immune suppressant withdrawal allows recovery of NK function and FDA approved use of the anti CD20 monoclonal antibody Rituximab removes the reservoir of infection.
Abbreviations: Epstein Barr Virus (EBV); Fas ligand (FasL); soluble Fas ligand (sFasL); activation induced cell death (AICD).
The mortality has been approximately 50% once the diagnosis is made. The clinical SMOF phenotype was correlated with both high serum IL-10 concentrations and soluble Fas (s-Fas) and soluble Fas-Ligand (s-FasL), suggesting perturbation of Fas-FasL mediated activation induced cell death pro-apoptotic pathway (Figure 4)40,41. The at-risk host population for SMOF includes recipients of transplanted organs or tissues requiring immunosuppression, similar to the risk factor profile for PTLD. Conversely, SMOF has also been observed in viral disease without transplantation in certain host genetic backgrounds, such as X-linked lymphoproliferative disease (XLP-1). XLP1 patients with mutations in the SLAM-associated protein (SAP) are susceptible to EBV infection due to a signal transduction defect in T cells and NK cells, rendering ineffective cytotoxic control of EBV-infected cell proliferation42. This differs from X-linked inhibitor of apoptosis (XIAP) mutations in BIRC4 (XLP-2), in which patients are susceptible to EBV infection, but present with splenomegaly and recurrent episodes of hemophagocytic syndrome, and inflammatory bowel disease43. In addition, mutations in this apoptotic signaling pathway have been found in approximately 75% of individuals with the autoimmune lymphoproliferative syndrome (ALPS), in which immune contraction due to AICD is impaired.
Similar to other MOF phenotypes, therapy for SMOF depends on the ascertained etiology. For cases involving clinical SMOF, the magnitude of DNA viremia is important to consider. For PTLD-associated SMOF, the mainstay of therapy has been reduction of immune suppression to allow for cytotoxic T and NK cell control of the causative DNA virus. Reduction in immune suppression is balanced by risk of rejection in the setting of solid or hematopoietic organ transplantation. The use of rituximab has emerged as an additional method that reduces the B cell, and in turn, the DNA viral reservoir44. The therapeutic principle is that CD20 on mature B cells is engaged by the monoclonal antibody, which in turn has direct signaling effects on the B cell (apoptosis, B cell receptor down regulation) and activation of cytotoxic response (NK cell and T cells via direct engagement and antibody dependent cytotoxicity)45. Based on soluble Fas levels in adult patients with diffuse large B cell lymphomas, the measured Fas-FasL axis is related to rituximab treatment response46. Antiviral therapy remains critical to controlling DNA viremia and expansion of the infected pool. The observation that NK cell cytotoxic activity is impaired in the setting of pediatric PTLD, and is associated with increased PD-1 expression may result in new attention to monitoring NK cell numbers and functional capacity in the host response47.
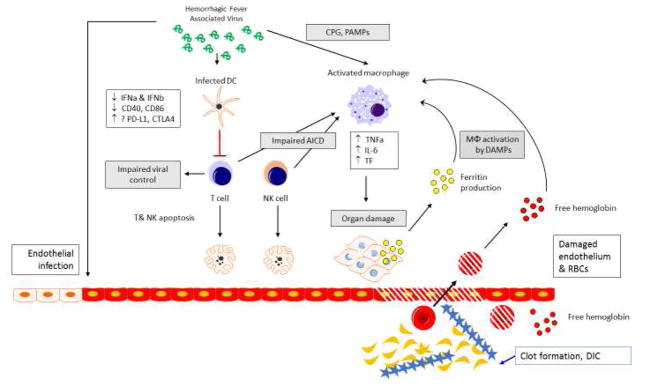
Severe sepsis associated Macrophage Activation Syndrome (MAS) is exemplified by the host pathogen interaction of filoviruses (i.e. Ebola virus) or other viral families associated with hemorrhagic fevers (Figure 5). The hallmark of severe sepsis associated MAS is high ferritin, hepatobiliary dysfunction and DIC. In Ebola hemorrhagic fever (EHF), viral infection activates innate immune cells, impairs innate cells ability to present antigen and co-stimulate T cells, and results in adaptive and NK cell immune paralysis. The infection of vascular endothelium and macrophage production of pro-coagulant inflammatory mediators leads to DIC. Pathogen associated molecular patterns and damage associated molecular patterns (such as hemoglobin – haptoglobin complexes, and putatively, ferritin) act to damage tissues and activate macrophages as well as lead to direct cellular injury. The treatments for EHF specifically may include immune serum in the form of chimeric monoclonal antibodies (i.e. ZMapp) to control viremia. In general terms, inflammation reduction therapy, such as steroids, IVIG and plasma exchange, is useful in severe sepsis associated MAS. Anti-cytokine therapy such as anakinra has also been shown to be beneficial as treatment of hyperinflammation in severe sepsis associated MAS, and other cytokine blocking therapies are being evaluated48. The pathophysiology of hemorrhagic fever driven MAS is based on unchecked and unabated inflammation and hemolysis driven myeloid innate immune cell activation (Figure 5).
Figure 5. Severe Sepsis Associated Macrophage Activation Syndrome.
Severe sepsis associated MAS can be modeled after the host pathogen interaction of the hemorrhagic fever viruses, including the filoviral family member Ebola. The unchecked inflammatory response is driven by myeloid lineage cells including monocytes and macrophages, and functionally impaired T and NK cell responses. T cells are skewed away from robust antiviral responses and towards regulatory T cell responses, in part due to lack of co-stimulation from professional antigen presenting cells and potentially due to negative costimulation and induction of apoptosis101–103. Macrophages are activated toward production of IL-6, TNF-a, and tissue factor (TF). IL-6 and TNF-a contribute to the cytokine storm phenotype, and TF promotes endothelial dysfunction and consumptive coagulopathy104. Danger associated molecular pattern (DAMP) and pathogen associated molecular pattern (PAMP) pattern recognition receptor (PRR) engagement through production of ferritin from either cellular damage or by the reticuloendothelial system, elaboration of free hemoglobin (via formation of hemoglobin-haptoglobin complexes and stimulation of CD163 receptors on monocytes and macrophages), or by toll-like receptor (TLR)-9 stimulation via CpG, may provide a feed forward mechanism by which monocytes and macrophages are further stimulated toward a pro-inflammatory phenotype. The pro-inflammatory cytokines IL-1 and TNF-alpha, which are produced at high levels by monocytes and macrophages, have been shown to have direct injurious effects on end organs and tissues (heart, liver, kidney, etc.)105, 106. High ferritin levels were recently associated with mortality among patients with epidemic Ebola hemorrhagic fever50. Anti-inflammatory therapies effective in reversing MAS have not been evaluated in Ebola infected patients.
Abbreviations: Interleukin 6 (IL-6); tissue factor (TF); Danger associated molecular pattern (DAMP); pathogen associated molecular pattern (PAMP); pattern recognition receptor (PRR); toll-like receptor 9 (TLR-9).
Viral hemorrhagic fever syndromes, such as those associated with the filoviruses (Ebola, Marburg), some flaviviruses (Dengue) and some arenaviruses (Lassa), have been reported as examples of viral disease associated MAS. Ebola has the clinical features of macrophage activation syndrome with associated cytopenias, disseminated intravascular coagulation, ongoing capillary leak syndrome, and hepatic dysfunction49,50. Of note, a recent examination of 55 biomarkers in Ebola hemorrhagic fever (EHF) patients indicated that elevations in serum thrombomodulin and serum ferritin were both associated with hemorrhage and mortality51,52. Elevation of serum ferritin has been shown to correlate with mortality among pediatric patients with MAS in multiple settings53,54.
Treatment for severe sepsis associated MAS are varied but fall into two broad categories: 1) control of source of inflammation and 2) modulation of immune functional and inflammation pathways. With regard to EHF, treatment with multiple chimeric monoclonal antibodies directed against viral components (ZMapp) has been evaluated in nonhuman primates and in a clinical trial during the 2014 Sierra Leone outbreak, with inconclusive results. While there may have been potential clinical benefit, there was an absence of statistical significance, requiring further study55,56. With regard to anti-inflammatory and immune modulatory therapies for MAS, there are several potential approaches. One of the most studied anti-cytokine therapies has been IL-1 blockade with recombinant IL-1RA (anakinra), which has been associated with a two-fold decrease in mortality in adults with severe sepsis associated MAS.48,57. Anti-IL-6 monoclonal antibody tocilizumab has shown efficacy in the treatment of systemic onset juvenile idiopathic arthritis induced MAS, and in cytokine release syndrome after chimeric antigen receptor (CAR) T cell treatment for blood cancers58,59. Anti-IL-18 therapies, which may be promising for MAS, are currently under development. Unrelated to biologic therapies, a study of Turkish children with severe sepsis and features of MAS compared the hemophagocytic lymphohistiocytosis (HLH)-94 chemotherapy protocol with a regimen of plasma exchange and IVIG or methylprednisolone and found the HLH-94 protocol had an associated survival of only 50% whereas the IVIG and steroid regimens had a survival of 100%, p=0.002 when combined with plasma exchange therapy, supporting an anti-inflammatory approach for MAS patients60,61.
Conclusions
We have described 5 inflammation pathobiology driven phenotypes of pediatric sepsis induced MOF, using specific host-pathogen interaction models to illustrate therapeutic opportunity for personalized precision medicine approaches that have the potential to improve outcomes in selected children. Further evaluation is necessary.
Key Points.
Adjunctive therapies are considered by clinicians for use in the management of children with sepsis inflammation pathobiology phenotypes and multiple organ failure (MOF).
A few general themes for the management of pediatric MOF are always pertinent, including the search for and removal of sources of ongoing infection and inflammation, and support of cardiovascular and other organ functions.
One can also use clinical criteria and confirmatory tests to identify one or more of five inflammation phenotypes that can be targeted with pathobiology-based adjunctive therapies
Acknowledgments
The authors would like to acknowledge Dr. Carol Vetterly for her ideas and expertise.
Funding Support: Funded in part by R01GM108616 and 5UG1HD049983 (JAC)
Text Abbreviations
- MOF
Multiple Organ Failure
- MRSA
methicillin resistant Staphylococcus aureus
- DIC
disseminated intravascular coagulation
- ADAMTS13
thrombospondin type 1 motif, member 13
- TAMOF
Thrombocytopenia associated MOF
- vWF
von Willebrand factor
- ADAMTS13
a thrombospondin type 1 motif, member 13
- aHUS
Atypical hemolytic uremic syndrome
- TTP
thrombotic thrombocytopenic purpura
- TMA
thrombotic microangiopathy
- ECMO
extracorporeal membrane oxygenation
- CRRT
continuous renal replacement therapy
- GM-CSF
granulocyte macrophage colony stimulating factor
- PD-1
Programmed cell death protein 1
- PD-L1
programmed cell death ligand 1
- P-IVIG
anti-Pertussis toxin-enriched intravenous immune globulin
- SMOF
Sequential MOF
- AICD
activation induced cell death
- EBV
Epstein Barr Virus
- Fas-L
Fas ligand
- PTLD
post-transplant lymphoproliferative disorder
- s-Fas
soluble Fas
- s-FasL
soluble Fas-Ligand
- XLP-1
X-linked lymphoproliferative disease
- SAP
SLAM-associated protein
- XIAP
X-linked inhibitor of apoptosis
- XLP-2
BIRC4
- ALPS
autoimmune lymphoproliferative syndrome
- MAS
Macrophage Activation Syndrome
- EHF
Ebola hemorrhagic fever
- CAR
chimeric antigen receptor
- HLH
hemophagocytic lymphohistiocytosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sadler JE, Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112(1):11–18. doi: 10.1182/blood-2008-02-078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noris M. Complement Factor H Mutation in Familial Thrombotic Thrombocytopenic Purpura with ADAMTS13 Deficiency and Renal Involvement. J Am Soc Nephrol. 2005;16(5):1177–1183. doi: 10.1681/ASN.2005010086. [DOI] [PubMed] [Google Scholar]
- 3.Gando S. Microvascular thrombosis and multiple organ dysfunction syndrome. Crit Care Med. 2010;38(2 Suppl):S35–42. doi: 10.1097/CCM.0b013e3181c9e31d. [DOI] [PubMed] [Google Scholar]
- 4.Okano E, Ko S, Kanehiro H, Matsumoto M, Fujimura Y, Nakajima Y. ADAMTS13 activity decreases after hepatectomy, reflecting a postoperative liver dysfunction. [Accessed March 28, 2016];Hepatogastroenterology. 57(98):316–320. http://www.ncbi.nlm.nih.gov/pubmed/20583434. [PubMed] [Google Scholar]
- 5.Reuken PA, Kussmann A, Kiehntopf M, et al. Imbalance of von Willebrand factor and its cleaving protease ADAMTS 13 during systemic inflammation superimposed on advanced cirrhosis. Liver Int. 2015;35(1):37–45. doi: 10.1111/liv.12657. [DOI] [PubMed] [Google Scholar]
- 6.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: Exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121(8):1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janz DR, Ware LB. The role of red blood cells and cell-free hemoglobin in the pathogenesis of ARDS. J Intensive Care. 2015;3(1):1–7. doi: 10.1186/s40560-015-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gladwin MT, Kanias T, Kim-Shapiro DB. Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease. J Clin Invest. 2012;122(4):1205–1208. doi: 10.1172/JCI62972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen TC, Han YY, Kiss JE, et al. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med. 2008;36(10):2878–2887. doi: 10.1097/CCM.0b013e318186aa49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen TC, Liu A, Liu L, et al. Acquired ADAMTS-13 deficiency in pediatric patients with severe sepsis. [Accessed March 23, 2016];Haematologica. 2007 92(1):121–124. doi: 10.3324/haematol.10262. http://www.ncbi.nlm.nih.gov/pubmed/17229645. [DOI] [PubMed] [Google Scholar]
- 11.Rimmer E, Houston BL, Kumar A, et al. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: a systematic review and meta-analysis. Crit Care. 2014;18(6):699. doi: 10.1186/s13054-014-0699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F, Peng Z, Murugan R, Kellum JA. Blood purification and mortality in sepsis: a meta-analysis of randomized trials. Crit Care Med. 2013 Sep;41(9):2209–20. doi: 10.1097/CCM.0b013e31828cf412. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darmon M, Azoulay E, Thiery G, Ciroldi M, Galicier L, Parquet N, Veyradier A, Le Gall JR, Oksenhendler E, Schlemmer B. Time course of organ dysfunction in thrombotic microangiopathy patients receiving either plasma perfusion or plasma exchange. Crit Care Med. 2006 Aug;34(8):2127–33. doi: 10.1097/01.CCM.0000227659.14644.3E. [DOI] [PubMed] [Google Scholar]
- 14.Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325(6):393–397. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 15.Thejeel B, Garg AX, Clark WF, Liu AR, Iansavichus AV, Hildebrand AM. Long-Term Outcomes of Thrombotic Microangiopathy Treated with Plasma Exchange: A Systematic Review. Am J Hematol. 2016 Feb; doi: 10.1002/ajh.24339. [DOI] [PubMed] [Google Scholar]
- 16.Fakhouri F, Hourmant M, Campistol JM, et al. Terminal Complement Inhibitor Eculizumab in Adult Patients With Atypical Hemolytic Uremic Syndrome: A Single-Arm, Open-Label Trial. Am J Kidney Dis. 2016:1–10. doi: 10.1053/j.ajkd.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Greenbaum LA, Fila M, Ardissino G, et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016:701–711. doi: 10.1016/j.kint.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 18.Vande Walle J, Delmas Y, Ardissino G, Wang J, Kincaid JF, Haller H. Improved renal recovery in patients with atypical hemolytic uremic syndrome following rapid initiation of eculizumab treatment. J Nephrol. 2016 Mar; doi: 10.1007/s40620-016-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenbaum LA, Fila M, Ardissino G, Al-Akash SI, Evans J, Henning P, Lieberman KV, Maringhini S, Pape L, Rees L, van de Kar NC, Vande Walle J, Ogawa M, Bedrosian CL, Licht C. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016 Mar;89(3):701–11. doi: 10.1016/j.kint.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Felmet KA, Hall MW, Clark RSB, Jaffe R, Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174(6):3765–3772. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall MW, Geyer SM, Guo C-Y, et al. Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med. 2013;41(1):224–236. doi: 10.1097/CCM.0b013e318267633c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42(5):383–391. doi: 10.1097/SHK.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boomer JSJ, To K, Chang KKC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. Jama. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathias B, Szpila BE, Moore FA, Efron PA, Moldawer LL. A Review of GM-CSF Therapy in Sepsis. Medicine (Baltimore) 2015;94(50):1–10. doi: 10.1097/MD.0000000000002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kollef KE, Schramm GE, Wills AR, Reichley RM, Micek ST, Kollef MH. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest. 2008;134(2):281–287. doi: 10.1378/chest.08-1116. [DOI] [PubMed] [Google Scholar]
- 27.Randolph AG, Vaughn F, Sullivan R, et al. Critically ill children during the 2009–2010 influenza pandemic in the United States. Pediatrics. 2011;128(6):e1450–8. doi: 10.1542/peds.2011-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manez R, Kusne S, Linden P, et al. Temporary withdrawal of immunosuppression for life-threatening infections after liver transplantation. [Accessed May 19, 2016];Transplantation. 1994 57(1):149–151. doi: 10.1097/00007890-199401000-00023. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2977934&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37(3):525–532. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spies C, Luetz A, Lachmann G, et al. Influence of Granulocyte-Macrophage Colony-Stimulating Factor or Influenza Vaccination on HLA-DR, Infection and Delirium Days in Immunosuppressed Surgical Patients: Double Blind, Randomised Controlled Trial. PLoS One. 2015;10(12):e0144003. doi: 10.1371/journal.pone.0144003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang KC, Burnham C-A, Compton SM, et al. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17(3):R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang K, Svabek C, Vazquez-Guillamet C, et al. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit Care. 2014;18(1):R3. doi: 10.1186/cc13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winter K, Zipprich J, Harriman K, et al. Risk Factors Associated with Infant Deaths from Pertussis: A Case-Control Study. Clin Infect Dis. 2015;61(7):1099–1106. doi: 10.1093/cid/civ472. [DOI] [PubMed] [Google Scholar]
- 34.Burr JS, Jenkins TL, Harrison R, et al. The Collaborative Pediatric Critical Care Research Network Critical Pertussis Study: collaborative research in pediatric critical care medicine. Pediatr Crit Care Med. 2011;12(4):387–392. doi: 10.1097/PCC.0b013e3181fe4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singhi S, Benkatti G. Critical Pertussis. Pediatr Crit Care Med. 2013;14(4):434–436. doi: 10.1097/PCC.0b013e31828a82f2. [DOI] [PubMed] [Google Scholar]
- 36.Rowlands HE, Goldman AP, Harrington K, Karimova A, Brierley J, Cross N, Skellett S, Peters MJ. Impact of rapid leukodepletion on the outcome of severe clinical pertussis in young infants. Pediatrics. 2010 Oct;126(4):e816–27. doi: 10.1542/peds.2009-2860. [DOI] [PubMed] [Google Scholar]
- 37.Sawal M1, Cohen M, Irazuzta JE, Kumar R, Kirton C, Brundler MA, Evans CA, Wilson JA, Raffeeq P, Azaz A, Rotta AT, Vora A, Vohra A, Abboud P, Mirkin LD, Cooper M, Dishop MK, Graf JM, Petros A, Klonin H. Fulminant pertussis: a multi-center study with new insights into the clinico-pathological mechanisms. Pediatr Pulmonol. 2009 Oct;44(10):970–80. doi: 10.1002/ppul.21082. [DOI] [PubMed] [Google Scholar]
- 38.Granstrom M, Olinder-Nielsen AM, Holmblad P, Mark A, Hanngren K. Specific immunoglobulin for treatment of whooping cough. [Accessed October 7, 2016];Lancet (London, England) 1991 338(8777):1230–1233. doi: 10.1016/0140-6736(91)92101-7. http://www.ncbi.nlm.nih.gov/pubmed/1682643. [DOI] [PubMed] [Google Scholar]
- 39.Bruss JB, Malley R, Halperin S, et al. Treatment of severe pertussis: a study of the safety and pharmacology of intravenous pertussis immunoglobulin. [Accessed October 7, 2016];Pediatr Infect Dis J. 1999 18(6):505–511. doi: 10.1097/00006454-199906000-00006. http://www.ncbi.nlm.nih.gov/pubmed/10391179. [DOI] [PubMed] [Google Scholar]
- 40.Doughty L, Clark RSB, Kaplan SS, Sasser H, Carcillo J. sFas and sFas ligand and pediatric sepsis-induced multiple organ failure syndrome. Pediatr Res. 2002;52(6):922–927. doi: 10.1203/01.PDR.0000036279.41965.F7. [DOI] [PubMed] [Google Scholar]
- 41.Doughty L, Carcillo JA, Kaplan S, Janosky J. The compensatory anti-inflammatory cytokine interleukin 10 response in pediatric sepsis-induced multiple organ failure. Chest. 1998;113(6):1625–1631. doi: 10.1378/chest.113.6.1625. [DOI] [PubMed] [Google Scholar]
- 42.Marsh RA, Madden L, Kitchen BJ, et al. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood. 2010;116(7) doi: 10.1182/blood-2010-01-256099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelsen JR, Dawany N, Martinez A, Martinez A, Grochowski CM, Maurer K, … Devoto M. A de novo whole gene deletion of XIAP detected by exome sequencing analysis in very early onset inflammatory bowel disease: a case report. BMC Gastroenterology. 2015;15:160. doi: 10.1186/s12876-015-0394-z. http://doi.org/10.1186/s12876-015-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen JI, Bollard CM, Khanna R, Pittaluga S. Current understanding of the role of Epstein-Barr virus in lymphomagenesis and therapeutic approaches to EBV-associated lymphomas. Leukemia & Lymphoma. 2008;49(Suppl 1):27–34. doi: 10.1080/10428190802311417. http://doi.org/10.1080/10428190802311417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47(2):115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hara T, Tsurumi H, Goto N, et al. Serum soluble Fas level determines clinical outcome of patients with diffuse large B-cell lymphoma treated with CHOP and R-CHOP. J Cancer Res Clin Oncol. 2009;135(10):1421–1428. doi: 10.1007/s00432-009-0586-4. [DOI] [PubMed] [Google Scholar]
- 47.Wiesmayr S, Webber SA, Macedo C, et al. Decreased NKp46 and NKG2D and elevated PD-1 are associated with altered NK-cell function in pediatric transplant patients with PTLD. Eur J Immunol. 2012;42(2):541–550. doi: 10.1002/eji.201141832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, Cron RQ, Opal SM. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med. 2016 Feb;44(2):275–81. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Ven AJAM, Netea MG, van der Meer JWM, de Mast Q. Ebola Virus Disease has Features of Hemophagocytic Lymphohistiocytosis Syndrome. Front Med. 2015;2:4. doi: 10.3389/fmed.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McElroy AK, Erickson BR, Flietstra TD, et al. Ebola Hemorrhagic Fever: Novel Biomarker Correlates of Clinical Outcome. J Infect Dis. 2014;210(4):558–566. doi: 10.1093/infdis/jiu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cron RQ, Behrens EM, Shakoory B, Ramanan AV, Chatham WW. Does Viral Hemorrhagic Fever Represent Reactive Hemophagocytic Syndrome? J Rheumatol. 2015 Jul;42(7):1078–80. doi: 10.3899/jrheum.150108. [DOI] [PubMed] [Google Scholar]
- 52.Rollin PE, Bausch DG, Sanchez A. Blood Chemistry Measurements and d -Dimer Levels Associated with Fatal and Nonfatal Outcomes in Humans Infected with Sudan Ebola Virus. J Infect Dis. 2007;196(s2):S364–S371. doi: 10.1086/520613. [DOI] [PubMed] [Google Scholar]
- 53.Garcia PCR, Longhi F, Branco RG, Piva JP, Lacks D, Tasker RC. Ferritin levels in children with severe sepsis and septic shock. Acta Paediatr. 2007;96(12):1829–1831. doi: 10.1111/j.1651-2227.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 54.Bennett TD, Hayward KN, Farris RWD, Ringold S, Wallace CA, Brogan TV. Very high serum ferritin levels are associated with increased mortality and critical care in pediatric patients. Pediatr Crit Care Med. 2011;12(6):e233–e236. doi: 10.1097/PCC.0b013e31820abca8. [DOI] [PubMed] [Google Scholar]
- 55.Group TPIW. A Randomized, Controlled Trial of ZMapp for Ebola Virus Infection. N Engl J Med. 2016;375(15):1448–1456. doi: 10.1056/NEJMoa1604330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014 Aug; doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic Role of Anakinra, an Interleukin-1 Receptor Antagonist, in the Management of Secondary Hemophagocytic Lymphohistiocytosis/Sepsis/Multiple Organ Dysfunction/Macrophage Activating Syndrome in Critically Ill Children. Pediatr Crit Care Med. 2014:1–8. doi: 10.1097/PCC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 58.Yokota S, Imagawa T, Mori M, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371(9617):998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 59.Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121(26) doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Demirkol D, Yildizdas D, Bayrakci B, et al. Hyperferritinemia in the critically ill child with secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome: what is the treatment? Crit Care. 2012;16(2):R52. doi: 10.1186/cc11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qu L, Kiss JE, Dargo G, Carcillo JA. Outcomes of previously healthy pediatric patients with fulminant sepsis-induced multisystem organ failure receiving therapeutic plasma exchange. J Clin Apher. 2011;26(4):208–13. doi: 10.1002/jca.20296. Epub 2011 Jul. [DOI] [PubMed] [Google Scholar]
- 62.Pron B, Taha MK, Rambaud C, et al. Interaction of Neisseria maningitidis with the components of the blood-brain barrier correlates with an increased expression of PilC. J Infect Dis. 1997;176:1285–1292. doi: 10.1086/514124. [DOI] [PubMed] [Google Scholar]
- 63.Madico G, Welsch JA, Lewis LA, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177(1):501–510. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller F, Lecuyer H, Join-Lambert O, et al. Neisseria meningitidis colonization of the brain endothelium and cerebrospinal fluid invasion. Cell Microbiol. 2013;15(4):512–519. doi: 10.1111/cmi.12082. [DOI] [PubMed] [Google Scholar]
- 65.Dixon GL, Heyderman RS, Kotovicz K, et al. Endothelial adhesion molecule expression and its inhibition by recombinant bactericidal/permeability-increasing protein are influenced by the capsulation and lipooligosaccharide structure of Neisseria meningitidis. [Accessed March 29, 2016];Infect Immun. 1999 67(11):5626–5633. doi: 10.1128/iai.67.11.5626-5633.1999. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=96935&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dixon GLJ, Heyderman RS, van der Ley P, Klein NJ. High-level endothelial E-selectin (CD62E) cell adhesion molecule expression by a lipopolysaccharide-deficient strain of Neisseria meningitidis despite poor activation of NF-kappaB transcription factor. [Accessed March 29, 2016];Clin Exp Immunol. 2004 135(1):85–93. doi: 10.1111/j.1365-2249.2004.02335.x. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1808929&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hollestelle MJ, Sprong T, Bovenschen N, et al. Von Willebrand factor activation, granzyme-B and thrombocytopenia in meningococcal disease. J Thromb Haemost. 2010;8(5):1098–1106. doi: 10.1111/j.1538-7836.2010.03811.x. [DOI] [PubMed] [Google Scholar]
- 68.Bongers TN, Emonts M, De Maat MPM, et al. Reduced ADAMTS13 in children with severe meningococcal sepsis is associated with severity and outcome. Thromb Haemost. 2010;103(6):1181–1187. doi: 10.1160/TH09-06-0376. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen TC, Carcillo JA. Bench-to-bedside review: thrombocytopenia-associated multiple organ failure--a newly appreciated syndrome in the critically ill. Crit Care. 2006;10(6):235. doi: 10.1186/cc5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kobsar A, Siauw C, Gambaryan S, et al. Neisseria meningitidis induces platelet inhibition and increases vascular endothelial permeability via nitric oxide regulated pathways. Thromb Haemost. 2011;106(6):1127–1138. doi: 10.1160/TH11-07-0491. [DOI] [PubMed] [Google Scholar]
- 71.Pathan N, Faust SN, Levin M. Pathophysiology of meningococcal meningitis and septicaemia. Arch Dis Child. 2003;88(7):601–607. doi: 10.1136/adc.88.7.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng S, Eyler SJ, Zhang Y, et al. Partial ADAMTS13 deficiency in atypical hemolytic uremic syndrome. Blood. 2013;122(8):1487–1494. doi: 10.1182/blood-2013-03-492421.S.F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Furlan M, Robles R, Galbusera M, et al. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339(22):1578–1584. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 74.Louise CB, Obrig TG. Shiga toxin-associated hemolytic-uremic syndrome: combined cytotoxic effects of Shiga toxin, interleukin-1 beta, and tumor necrosis factor alpha on human vascular endothelial cells in vitro. Infect Immun. 1991;59(11):4173–4179. doi: 10.1128/iai.59.11.4173-4179.1991. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=259013&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bauwens A, Betz J, Meisen I, Kemper B, Karch H, Muthing J. Facing glycosphingolipid-Shiga toxin interaction: Dire straits for endothelial cells of the human vasculature. Cell Mol Life Sci. 2013;70(3):425–457. doi: 10.1007/s00018-012-1060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morigi M, Galbusera M, Gastoldi S, et al. Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J Immunol. 2011;187(1):172–180. doi: 10.4049/jimmunol.1100491. [DOI] [PubMed] [Google Scholar]
- 77.Muthing J, Schweppe CH, Karch H, Friedrich AW. Shiga toxins, glycosphingolipid diversity, and endothelial cell injury. Thromb Haemost. 2009;101(2):252–264. doi: 10.1160/TH08-05-0317. [DOI] [PubMed] [Google Scholar]
- 78.Lehtinen MJ, Rops AL, Isenman DE, van der Vlag J, Jokiranta TS. Mutations of factor H impair regulation of surface-bound C3b by three mechanisms in atypical hemolytic uremic syndrome. J Biol Chem. 2009;284(23):15650–15658. doi: 10.1074/jbc.M900814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hyvarinen S, Meri S, Jokiranta TS. Disturbed sialic acid recognition on endothelial cells and platelets in complement attack causes atypical hemolytic uremic syndrome. Blood. 2016 doi: 10.1182/blood-2015-11-680009. [DOI] [PubMed] [Google Scholar]
- 80.Maga TK, Nishimura CJ, Weaver AE, Frees KL, Smith RJH. Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat. 2010;31(6):1445–1460. doi: 10.1002/humu.21256. [DOI] [PubMed] [Google Scholar]
- 81.Noone CM, Lewis EA, Frawely AB, et al. Novel mechanism of immunosuppression by influenza virus haemagglutinin: selective suppression of interleukin 12 p35 transcription in murine bone marrow-derived dendritic cells. J Gen Virol. 2005;86(7):1885–1890. doi: 10.1099/vir.0.80891-0. [DOI] [PubMed] [Google Scholar]
- 82.Erturk M, Jennings R, Oxley KM, Hastings MJ. Effect of influenza A on phagocytic cell function. [Accessed May 19, 2016];Med Microbiol Immunol. 1989 178(4):199–209. doi: 10.1007/BF00202553. http://www.ncbi.nlm.nih.gov/pubmed/2747589. [DOI] [PubMed] [Google Scholar]
- 83.Larson HEE, Blades R. Impairment of human polymorphonuclear leucocyte function by influenza virus. Lancet. 1976;307(7954):283. doi: 10.1016/S0140-6736(76)91407-0. [DOI] [PubMed] [Google Scholar]
- 84.Hoeve MA, Nash AA, Jackson D, Randall RE, Dransfield I. Influenza virus A infection of human monocyte and macrophage subpopulations reveals increased susceptibility associated with cell differentiation. PLoS One. 2012;7(1):e29443. doi: 10.1371/journal.pone.0029443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lina G, Piemont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine Leukocidin--Producing Staphylococcus aureus in Primary Skin Infections and Pneumonia. Clin Infect Dis. 1999;29(5):1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 86.Ayukawa H, Matsubara T, Kaneko M, Hasegawa M, Ichiyama T, Furukawa S. Expression of CTLA-4 (CD152) in peripheral blood T cells of children with influenza virus infection including encephalopathy in comparison with respiratory syncytial virus infection. Clin Exp Immunol. 2004;137(1):151–155. doi: 10.1111/j.1365-2249.2004.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loffler B, Hussain M, Grundmeier M, et al. Staphylococcus aureus Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils. In: Cheung A, editor. PLoS Pathog. 1. Vol. 6. 2010. p. e1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gonzalez BE, Hulten KG, Dishop MK, et al. Pulmonary Manifestations in Children with Invasive Community-Acquired Staphylococcus aureus Infection. Clin Infect Dis. 2005;41(5):583–590. doi: 10.1086/432475. [DOI] [PubMed] [Google Scholar]
- 89.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18(2):326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paddock CD, Sanden GN, Cherry JD, et al. Pathology and Pathogenesis of Fatal Bordetella pertussis Infection in Infants. Clin Infect Dis. 2008;47(3):328–338. doi: 10.1086/589753. [DOI] [PubMed] [Google Scholar]
- 91.Carbonetti NH. Bordetella pertussis. Curr Opin Infect Dis. 2016;29(3):287–294. doi: 10.1097/QCO.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carbonetti NH. Pertussis leukocytosis: mechanisms, clinical relevance and treatment. In: Bavoil P, editor. Pathog Dis. 7. Vol. 74. 2016. p. ftw087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abramson T, Kedem H, Relman DA. Modulation of the NF-κB Pathway by Bordetella pertussis Filamentous Hemagglutinin. In: Ratner AJ, editor. PLoS One. 11. Vol. 3. 2008. p. e3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Gouw D, Diavatopoulos DA, Bootsma HJ, Hermans PWM, Mooi FR. Pertussis: a matter of immune modulation. FEMS Microbiol Rev. 2011;35(3):441–474. doi: 10.1111/j.1574-6976.2010.00257.x. [DOI] [PubMed] [Google Scholar]
- 95.Jochum S, Moosmann A, Lang S, Hammerschmidt W, Zeidler R. The EBV Immunoevasins vIL-10 and BNLF2a Protect Newly Infected B Cells from Immune Recognition and Elimination. In: Stevenson PG, editor. PLoS Pathog. 5. Vol. 8. 2012. p. e1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Salek-Ardakani S, Arrand JR, Mackett M. Epstein–Barr Virus Encoded Interleukin-10 Inhibits HLA-Class I, ICAM-1, and B7 Expression on Human Monocytes: Implications for Immune Evasion by EBV. Virology. 2002;304(2):342–351. doi: 10.1006/viro.2002.1716. [DOI] [PubMed] [Google Scholar]
- 97.Waring P, Mullbacher A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol. 1999;77(4):312–317. doi: 10.1046/j.1440-1711.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- 98.Faouzi S, Burckhardt BE, Hanson JC, et al. Anti-Fas induces hepatic chemokines and promotes inflammation by an NF-kappa B-independent, caspase-3-dependent pathway. J Biol Chem. 2001;276(52):49077–49082. doi: 10.1074/jbc.M109791200. [DOI] [PubMed] [Google Scholar]
- 99.Ogasawara J, Watanabe-Fukunaga R, Adachi M, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364(6440):806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 100.Kakinuma C, Takagaki K, Yatomi T, et al. Acute Toxicity of an Anti-Fas Antibody in Mice. Toxicol Pathol. 1999;27(4):412–420. doi: 10.1177/019262339902700404. [DOI] [PubMed] [Google Scholar]
- 101.Bradfute SB, Braun DR, Shamblin JD, et al. Lymphocyte Death in a Mouse Model of Ebola Virus Infection. J Infect Dis. 2007;196(s2):S296–S304. doi: 10.1086/520602. [DOI] [PubMed] [Google Scholar]
- 102.Gupta M, Spiropoulou C, Rollin PE. Ebola virus infection of human PBMCs causes massive death of macrophages, CD4 and CD8 T cell sub-populations in vitro. Virology. 2007;364(1):45–54. doi: 10.1016/j.virol.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 103.Mohamadzadeh M, Chen L, Schmaljohn AL. How Ebola and Marburg viruses battle the immune system. Nat Rev Immunol. 2007;7(7):556–567. doi: 10.1038/nri2098. [DOI] [PubMed] [Google Scholar]
- 104.Geisbert TW, Hensley LE, Jahrling PB, et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003;362(9400):1953–1958. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 105.Drosatos K, Lymperopoulos A, Kennel PJ, Pollak N, Schulze PC, Goldberg IJ. Pathophysiology of sepsis-related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Curr Heart Fail Rep. 2015;12(2):130–140. doi: 10.1007/s11897-014-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mizuhara H, O’Neill E, Seki N, et al. T cell activation-associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. [Accessed February 14, 2017];J Exp Med. 1994 179(5):1529–1537. doi: 10.1084/jem.179.5.1529. http://www.ncbi.nlm.nih.gov/pubmed/8163936. [DOI] [PMC free article] [PubMed] [Google Scholar]