Abstract
Background
Posterior reversible encephalopathy syndrome (PRES) is a variable cerebrovascular syndrome associated with hypertension and autoregulatory failure. Steroids have been reported to both precipitate and treat PRES. We sought to determine the prevalence of steroid therapy at the time of PRES and to assess the relationship between steroid therapy and extent of vasogenic edema.
Methods
We performed a retrospective review of radiology reports between 2008 and 2014 from two academic medical centers to identify cases of PRES. Clinical and radiographic data were collected. Descriptive statistics were used to determine the prevalence of corticosteroid therapy at the time of PRES onset and the latency from steroid initiation to PRES onset. The association between steroid therapy and extent of vasogenic edema was assessed in multiple regression models.
Results
We identified 99 cases of PRES in 96 patients. The median age was 55 years (IQR 30–65) and 74% were women. Steroid therapy at time of PRES onset was identified in 44 of 99 cases. Excluding patients on chronic therapy, the median duration of steroid exposure before PRES onset was 6 (IQR, 3–10) days. Steroid therapy was not associated with extent of vasogenic edema in unadjusted or linear and logistic regression models adjusted for age, sex, and maximum systolic blood pressure on day of onset.
Conclusion
Corticosteroid therapy, often of brief duration, frequently preceded the onset of PRES and was not associated with severity of vasogenic edema.
Keywords: Posterior reversible encephalopathy syndrome, hypertension, corticosteroids, vasogenic edema
INTRODUCTION
Posterior reversible encephalopathy syndrome (PRES) is a cerebrovascular syndrome characterized by acute neurological symptoms accompanied by typically reversible vasogenic edema [11, 17]. Symptoms can include headache, confusion, vision changes, seizures, and focal neurological deficits [27]. At its core, PRES is thought to result from disordered or failed cerebrovascular autoregulation and is often preceded by hypertension [11, 26, 41]. An alternate model posits that PRES results from inflammation- mediated endothelial dysfunction [2].
PRES is associated with autoimmune disorders [10, 21], bone marrow and solid organ transplantation [16, 39, 40], cancer [43], sepsis [3], and the peripartum state [4]. Corticosteroids are frequently used in many of these settings. Steroids are known to increase blood pressure [14, 15] and therefore could precipitate PRES. However, steroids are also used in the treatment of vasogenic edema, such as in patients with intracranial mass lesions [9]. Case reports have implicated steroids as both the precipitant [5, 8, 12, 19, 20, 23, 24, 30, 31, 34, 38, 47, 48] and treatment [1, 6, 8, 12, 13, 18, 25, 28, 30, 33, 36, 37] of PRES. Thus, the role of steroids in PRES remains unclear. We hypothesized that PRES is frequently preceded by initiation of corticosteroid therapy and aimed to assess the relationship between steroid therapy and the extent of vasogenic edema.
METHODS
Study Design
The overall design of our study has been previously described [42]. We conducted a retrospective cohort study of patients diagnosed with PRES at two tertiary-care academic medical centers in New York City: New York Presbyterian Hospital – Weill Cornell Medical Center (NYPH) and Memorial Sloan Kettering Cancer Center (MSK). NYPH is a certified comprehensive stroke center while MSK specializes in oncological care. Patients at MSK typically receive all of their care within the hospital system, and many NYPH patients similarly receive outpatient care in affiliated clinics. Both hospitals have inpatient and outpatient electronic medical records that facilitate detailed, longitudinal data review. Patients transferred to these institutions after presenting elsewhere were included if adequate clinical and radiographic data were available. Our study was approved by the institutional review boards of both institutions. All variables were defined in a data dictionary created by investigator consensus, and data were abstracted in standardized forms [46].
Study Subjects
We performed a text search for “PRES” and “Posterior Reversible Encephalopathy Syndrome” of all brain magnetic resonance imaging (MRI) and computed tomography (CT) reports from 2008 to 2014. The entire reports, including the clinician-entered requisition, were searched. The study cohort was assembled from these screening results by identifying patients with brain parenchymal vasogenic edema on CT or MRI of the brain with associated neurological symptoms (headache, confusion, vision changes, seizures, and/or focal neurological deficits) that could not be attributed to other causes such as infection, malignancy, or stroke [11]. All available imaging studies, including subsequent studies, were available to the radiologist for review when assessing the index study. Patients were included in the cohort by consensus after review by both a neuroradiologist (ADS, RJY, AG) and neurologist (NSP, BBN). Patients under the age of 18 were included as there does not appear to be a distinct pediatric PRES phenotype; however, pediatric patients were excluded in sensitivity analyses [11].
Measurements
Patient demographics, comorbidities, and clinical and radiographic PRES characteristics were collected, including granular data regarding oncological history, vascular risk factors, and medication exposures. For incident cases of PRES, we abstracted PRES symptoms, vital signs, and laboratory values. The onset of PRES was defined as the day of symptom onset. The maximum systolic blood pressure on the day of onset was recorded. If symptoms began prior to admission, the maximum blood pressure on the day of admission was recorded. Detailed data regarding steroid therapy were collected including type, dose, duration, and indication for steroid therapy. Chronic steroid therapy was defined as steroid use for over 30 days. All steroid doses were adjusted by glucocorticoid potency relative to methylprednisolone [29].
Outcomes
The primary outcome was extent of vasogenic edema. A neuroradiologist (ADS) independently reviewed the initial imaging study to determine the number of areas with vasogenic edema as reflected either by CT hypoattenuation or MR T2 FLAIR hyperintensity. Diffusion-weighted imaging (DWI) was used to distinguish between vasogenic and cytotoxic edema. The presence or absence of mass effect, configuration (i.e. confluence), and reversibility on follow-up were used to distinguish between vasogenic edema and other causes of parenchymal T2 hyperintensity such as chronic ischemia, post-treatment change, and gliosis of other etiology. The extent of edema was defined as a continuous variable of 1 to 10 pre-defined regions: frontal, parietal, occipital, temporal, basal ganglia, thalamus, brainstem, cerebellum, deep white matter, and corpus callosum. This measure was developed in line with prior studies [22, 35]. We also pre-specified a binary outcome in which edema in 1–4 regions constitutes moderate edema and edema in 5–10 regions constitutes extensive edema.
Statistical Methods
Standard descriptive statistics were used to describe the patient population, PRES characteristics, and prevalence of steroid therapy at time of PRES. We compared patients receiving steroid therapy to patients with no steroid exposure using Chi-square or Fisher’s exact test, as appropriate, for categorical variables and Student’s t-test for numerical variables. Linear and logistic regression models, adjusted for age, sex, and maximum systolic blood pressure on day of onset, were used to assess the effect of steroid exposure on extent of vasogenic edema. Covariates were chosen a priori, and the number of covariates was limited to avoid over-fitting of models. We performed three sensitivity analyses. First, we limited the study cohort to patients 18 and older. Second, we excluded patients who had not undergone MRI. Third, we restricted analyses to patients with at least one repeat MRI available for comparison. Last, in an exploratory analysis, the Spearman correlation between steroid dose, relative to methylprednisolone, and number of areas of vasogenic edema was determined. All p-values were two-sided and evaluated at the 0.05 alpha level. All analyses were performed in SAS v9.3, (SAS Institute, Cary, NC).
RESULTS
We identified 96 patients who met our final inclusion criteria after review of 179 records. Reasons for study exclusion were clinical histories inconsistent with PRES (48 patients), alternate radiological diagnoses (24 patients), and inadequate clinical information (11 patients). Two patients had more than one episode of PRES, such that our study cohort included 96 patients with 99 episodes of PRES; 94 of these 99 cases were diagnosed by MRI. In 64 (65%) cases, a repeat MRI was available for comparison.
Median patient age was 55 years (interquartile range [IQR], 30–65) with 10 patients being under the age of 18. Most patients (74%) were women. Of these 96 patients, 58% had active cancer, 28% had sepsis, 17% had a history of bone marrow transplantation, 1% had solid organ transplantation, 15% had an autoimmune disease, and 8% were peripartum. In 99 cases of PRES, the mean maximum systolic blood pressure on the day of onset was 180 (±32) millimeters mercury.
Corticosteroid therapy was noted at the time of PRES onset in 44 cases. In 10 cases, the patient was on chronic steroid therapy. In the remaining 34 cases, the median duration of steroid use before onset was 6 (IQR, 3–10) days, and 22 (50%) patients had started steroid therapy within 7 days. The most common indications were graft-versus- host disease, chemotherapy-associated indications, and stress-dose steroid dosing for shock. Patients on steroid therapy were more likely to have active cancer (p=0.02) and to be receiving active chemotherapy (p<0.01) or immunosuppressant medication (p<0.01) than patients not receiving steroids (Table 1). However, as the short median duration of steroid therapy prior to PRES suggests, initiation of steroid therapy was a frequent proximate change in medical therapy. Treating clinicians explicitly attributed PRES to recent corticosteroid therapy in 5 of these 44 cases.
Table 1.
Clinical Characteristics in Cases of Posterior Reversible Encephalopathy Syndrome, Stratified by Steroid Exposure
| Characteristica | No Steroids (N=55) | Current Steroids (N=44) | P |
|---|---|---|---|
| Age, mean (SD), y | 50.4 (21.6) | 45.1 (22.0) | 0.23 |
| Female | 43 (78%) | 31 (71%) | 0.38 |
| Hypertension | 24 (44%) | 11 (32%) | 0.79 |
| Chronic kidney disease | 12 (22%) | 6 (14%) | 0.29 |
| Active cancer | 27 (49%) | 32 (73%) | 0.02 |
| Active chemotherapyb | 25 (46%) | 33 (75%) | <0.01 |
| Autoimmune disease | 4 (7%) | 10 (23%) | 0.04 |
| Active immunosuppressionb | 3 (6%) | 18 (41%) | <0.01 |
| History of brain radiation | 2 (4%) | (16%) | 0.07 |
| Status post bone marrow transplantation | 3 (6%) | 16 (36%) | <0.01 |
| Peripartum | 8 (15%) | 0 (0) | 0.01 |
| Active sepsis | 11 (20%) | 16 (36%) | 0.11 |
| Peak systolic blood pressure, mean (SD), mm Hg | 184.5 (35.1) | 174.5 (27.0) | 0.12 |
| Serum creatinine, mean (SD), mg/dL | 1.7 (2.0) | 1.6 (2.0) | 0.89 |
| Serum sodium, mean (SD), mmol/L | 138.1 (4.4) | 137.7 (4.2) | 0.65 |
| Serum albuminc, mean (SD), g/dL | 3.1 (0.8) | 2.8 (0.7) | 0.15 |
Abbreviations: SD, standard deviation; mm Hg, millimeters mercury; mg/dL; milligrams per deciliter; mmol/L, millimoles per liter; g/dL, grams per deciliter.
Data are presented as number (%) unless otherwise specified.
Current or within the last 30 days.
These data were only available for 81 patients.
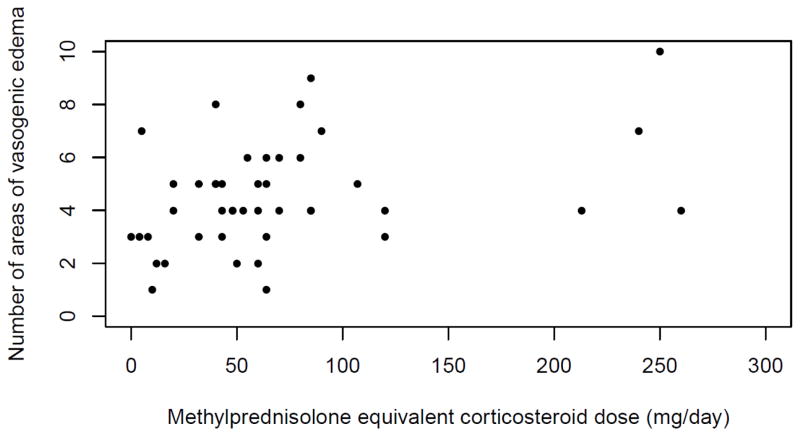
Patients with PRES had a mean of 4.4 (±2.2) brain areas of vasogenic edema with 39 cases (39%) having 5 or more areas with edema. In unadjusted analyses, there was no association between steroid therapy and extent of vasogenic edema when treated as a continuous or binary variable (4.5 [±2.0] areas in patients with steroid use versus 4.2 [±2.4] areas in patients without steroid use, p=0.50) (Table 2). The results remained unchanged in multivariable linear and logistic models adjusting for age, sex, and blood pressure (data not shown). The results were also unchanged in sensitivity analyses limited to patients 18 years and older, cases diagnosed by MRI, and cases with at least one repeat MRI available for comparison (data not shown). Last, in an exploratory analysis of cases in which the patient was receiving steroid therapy, we found a weak, positive association between steroid dose and number of areas of edema (Spearman correlation coefficient, 0.35; p=0.02) (Figure 1).
Table 2.
Association Between Steroid Exposure and Vasogenic Edema
| Vasogenic edema | No steroids (N=55) | Current steroids (N=44) | P |
|---|---|---|---|
| Areasa of vasogenic edema, mean (SD) | 4.2 (2.4) | 4.5 (2.0) | 0.50 |
| Number with extensiveb vasogenic edema | 20 (36%) | 19 (43%) | 0.49 |
Areas of vasogenic edema were quantified by assessing 10 pre-defined regions for FLAIR hyperintensity or CT hypoattenuation: frontal, parietal, occipital, temporal, basal ganglia, thalamus, brainstem, cerebellum, deep white matter, and corpus callosum.
Extensive vasogenic edema was defined as having at least 5 areas of vasogenic edema.
Fig. 1.
Scatter plot of methylprednisolone equivalent corticosteroid dose and number of areas of vasogenic edema in posterior reversible encephalopathy syndrome (n = 44). Spearman coefficient, 0.35; p=0.02
DISCUSSION
In our multicenter, heterogeneous cohort of patients with PRES, we found a high prevalence of corticosteroid therapy, frequently of brief duration, at the time of PRES onset. In multiple models, active steroid therapy was not associated with decreased severity of vasogenic edema. Instead, a weak positive correlation was observed between steroid dose and extent of vasogenic edema.
Our finding of frequent steroid exposure prior to PRES is consistent with numerous case reports demonstrating a temporal association between steroid exposure and PRES [5, 8, 12, 19, 20, 23, 24, 30, 31, 34, 38, 47, 48]. In our cohort and in the literature, steroid therapy is often co-administered with other potential culprit drugs or administered in the context of nephritis, which causes hypertension. However, the brief median duration of steroid exposure prior to PRES in our cohort raises the possibility that steroids may also contribute to the development of PRES. This is consistent with reports of PRES occurring after isolated exposure to steroids in non-autoimmune disorders, such as in asthma and brain metastasis [20, 24]. Similarly, PRES has been reported in patients receiving steroid therapy for autoimmune conditions that do not cause nephritis, such as neuromyelitis optica and multiple sclerosis [30, 34]. Among patients with lupus, PRES has been described after high-dose steroids in the absence of nephritis [5]. Additionally, two studies of lupus patients demonstrated univariate associations between steroid dose and incident PRES, [21, 32] and our findings suggest that there may be a weak association between steroid dose and extent of vasogenic edema.
The high prevalence of steroid therapy at the time of PRES onset has a number of potential explanations. First, steroids may play a role in the development of PRES by increasing blood pressure or through other effects on vascular tone.[45] This possibility has also been raised for a related disorder – reversible cerebral vasoconstriction syndrome.[44] Alternatively, it is plausible that steroid administration simply reflects the presence of an underlying inflammatory or oncological disease, which may itself cause PRES through endothelial dysfunction [2]. However, were this the case, we would expect to find less vasogenic edema in patients receiving steroids. Steroids have potent anti-inflammatory effects that would be expected to ameliorate PRES if it were caused by inflammation [7]. To our knowledge, our analysis is the first to examine the association between steroid therapy and extent of vasogenic edema, and we did not find such a relationship. In fact, we observed a weak correlation between steroid dose and extent of edema.
Our findings should be considered in light of several potential limitations. First, the study design was retrospective, which limits our ability to draw conclusions regarding causal relationships. Additionally, retrospective design may lead to under-ascertainment of PRES, especially in mild cases that may not have undergone neuroimaging. Second, the cohort was assembled from two academic tertiary care centers; the results therefore may not be generalizable to all healthcare settings. Third, steroid exposure was heterogeneous and included a variety of corticosteroids. Particular corticosteroids may have idiosyncratic effects on vasogenic edema; however, our sample size did not permit more granular analyses. Last, the degree of vasogenic edema was assessed by visual inspection; this method may have limited precision and accuracy. However, vasogenic edema was measured without consideration of clinical information, and the alternative approach of automated quantitative segmentation methods may be prone to misclassifying areas of T2 hyperintensity from other causes as vasogenic edema. Additionally, a similar manual approach has been used previously to evaluate disease extent in PRES [35].
In conclusion, PRES is frequently preceded by initiation of steroid therapy, which is not associated with reduction in vasogenic edema. Our findings may prompt increased vigilance for signs and symptoms of PRES when administering steroids, especially to patients with preexisting risk factors for PRES. Additionally, our findings do not support the use of corticosteroids for treatment of vasogenic edema in PRES.
Acknowledgments
Funding:
Ms. Joanne Chin, from the Department of Radiology, Memorial Sloan Kettering Cancer Center, provided assistance in the editing of this paper.
This research was funded in part through the National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748. Babak B Navi was supported by a grant (K23NS091395) from the National Institute of Neurological Disorders and Stroke and the Florence Gould Endowment for Discovery in Stroke. Ashley E. Giambrone was partially supported by a grant from the Clinical and Translational Science Center at Weill Cornell Medicine (UL1-TR000457-06).
Footnotes
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
This study was approved by the institutional review boards of Weill Cornell Medicine and Memorial Sloan Kettering Cancer Center. Informed consent was not required.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aridon P, Ragonese P, Mazzola MA, Quintini G, Lo Re M, Talamanca S, Terruso V, D’Amelio M, Savettieri G. Reversible posterior leukoencephalopathy syndrome in a patient with thrombotic thrombocytopenic purpura. Neurol Sci. 2011;32:469–472. doi: 10.1007/s10072-010-0465-4. [DOI] [PubMed] [Google Scholar]
- 2.Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29:1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartynski WS, Boardman JF, Zeigler ZR, Shadduck RK, Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006;27:2179–2190. [PMC free article] [PubMed] [Google Scholar]
- 4.Brewer J, Owens MY, Wallace K, Reeves AA, Morris R, Khan M, LaMarca B, Martin JN. Posterior reversible encephalopathy syndrome in 46 of 47 patients with eclampsia. Am J Obstet Gynecol. 2013;208:468e461–466. doi: 10.1016/j.ajog.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Chennareddy S, Adapa R, Kishore BK, Rajasekhar L. Posterior reversible encephalopathy syndrome in systemic lupus erythematosus following methylprednisolone: report of two cases. Int J Rheum Dis. 2013;16:786–788. doi: 10.1111/1756-185X.12148. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhary M, Kabbani AA, Tobey D, Hope TD. Posterior reversible encephalopathy syndrome in a woman with focal segmental glomerulosclerosis. Neuropsychiatr Dis Treat. 2015;11:1111–1114. doi: 10.2147/NDT.S84010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhillon A, Velazquez C, Siva C. Rheumatologic diseases and posterior reversible encephalopathy syndrome: two case reports and review of the literature. Rheumatol Int. 2012;32:3707–3713. doi: 10.1007/s00296-012-2476-3. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol. 2011;4:233–242. doi: 10.1586/ecp.11.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85:427–432. doi: 10.4065/mcp.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914–925. doi: 10.1016/S1474-4422(15)00111-8. [DOI] [PubMed] [Google Scholar]
- 12.Fujita M, Komatsu K, Hatachi S, Yagita M. Reversible posterior leukoencephalopathy syndrome in a patient with Takayasu arteritis. Mod Rheumatol. 2008;18:623–629. doi: 10.1007/s10165-008-0097-1. [DOI] [PubMed] [Google Scholar]
- 13.Fukushima K, Hineno A, Kodaira M, Machida K, Ishii W, Kaneko T, Shimojo H, Uhara H, Yamamoto K, Morita H, Yoshida K, Ikeda SI. Reversible extensive leukoencephalopathy in Sweet disease: a case report. J Neurol Sci. 2008;275:178–180. doi: 10.1016/j.jns.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Grossman A, Messerli FH, Grossman E. Drug induced hypertension--An unappreciated cause of secondary hypertension. Eur J Pharmacol. 2015;763:15–22. doi: 10.1016/j.ejphar.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Grossman E, Messerli FH. Drug-induced hypertension: an unappreciated cause of secondary hypertension. Am J Med. 2012;125:14–22. doi: 10.1016/j.amjmed.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Hammerstrom AE, Howell J, Gulbis A, Rondon G, Champlin RE, Popat U. Tacrolimus-associated posterior reversible encephalopathy syndrome in hematopoietic allogeneic stem cell transplantation. Am J Hematol. 2013;88:301–305. doi: 10.1002/ajh.23402. [DOI] [PubMed] [Google Scholar]
- 17.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 18.Honkaniemi J, Kähärä V, Dastidar P, Latvala M, Hietaharju A, Salonen T, Keskinen L, Ollikainen J, Vähämäki L, Kellokumpu-Lehtinen P, Frey H. Reversible posterior leukoencephalopathy after combination chemotherapy. Neuroradiology. 2000;42:895–899. doi: 10.1007/s002340000482. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda M, Ito S, Hataya H, Honda M, Anbo K. Reversible posterior leukoencephalopathy in a patient with minimal-change nephrotic syndrome. Am J Kidney Dis. 2001;37:E30. doi: 10.1016/s0272-6386(01)90016-2. [DOI] [PubMed] [Google Scholar]
- 20.Irvin W, MacDonald G, Smith JK, Kim WY. Dexamethasone- induced posterior reversible encephalopathy syndrome. J Clin Oncol. 2007;25:2484–2486. doi: 10.1200/JCO.2007.10.9991. [DOI] [PubMed] [Google Scholar]
- 21.Jung SM, Moon SJ, Kwok SK, Ju JH, Park KS, Park SH, Kim HY. Posterior reversible encephalopathy syndrome in Korean patients with systemic lupus erythematosus: risk factors and clinical outcome. Lupus. 2013;22:885–891. doi: 10.1177/0961203313496341. [DOI] [PubMed] [Google Scholar]
- 22.Karia SJ, Rykken JB, McKinney ZJ, Zhang L, McKinney AM. Utility and Significance of Gadolinium-Based Contrast Enhancement in Posterior Reversible Encephalopathy Syndrome. AJNR Am J Neuroradiol. 2016;37:415–422. doi: 10.3174/ajnr.A4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Rajam L. Posterior reversible encephalopathy syndrome (PRES/RPLS) during pulse steroid therapy in macrophage activation syndrome. Indian J Pediatr. 2011;78:1002–1004. doi: 10.1007/s12098-011-0368-2. [DOI] [PubMed] [Google Scholar]
- 24.Kurahashi H, Okumura A, Koide T, Ando Y, Hirata H, Magota M, Watabane K. Posterior reversible encephalopathy syndrome in a child with bronchial asthma. Brain Dev. 2006;28:544–546. doi: 10.1016/j.braindev.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Lv F, Wei Y, Yan B, Xie P. Posterior reversible encephalopathy syndrome in a patient with systemic lupus erythematosus after cessation of oral prednisone. Neurol Sci. 2013;34:2241–2242. doi: 10.1007/s10072-013-1479-5. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Gor D, Walicki D, Jenny D, Jones D, Barbour P, Castaldo J. Spectrum and potential pathogenesis of reversible posterior leukoencephalopathy syndrome. J Stroke Cerebrovasc Dis. 2012;21:873–882. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Liman TG, Bohner G, Heuschmann PU, Endres M, Siebert E. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: the retrospective Berlin PRES study. J Neurol. 2012;259:155–164. doi: 10.1007/s00415-011-6152-4. [DOI] [PubMed] [Google Scholar]
- 28.Lioger B, Diot E, Ebbo M, Schleinitz N, Aaron L, Michot JM, Lambotte O, Dhote R, De Boysson H, Ponce E, Maillot F. Posterior reversible encephalopathy syndrome and systemic vasculitis: report of six cases. Clin Exp Rheumatol. 2015;34:S7–11. [PubMed] [Google Scholar]
- 29.Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, Brown JP, Cohen A, Kim H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9:30. doi: 10.1186/1710-1492-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magaña SM, Matiello M, Pittock SJ, McKeon A, Lennon VA, Rabinstein AA, Shuster E, Kantarci OH, Lucchinetti CF, Weinshenker BG. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology. 2009;72:712–717. doi: 10.1212/01.wnl.0000343001.36493.ae. [DOI] [PubMed] [Google Scholar]
- 31.Magnano MD, Bush TM, Herrera I, Altman RD. Reversible posterior leukoencephalopathy in patients with systemic lupus erythematosus. Semin Arthritis Rheum. 2006;35:396–402. doi: 10.1016/j.semarthrit.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Merayo-Chalico J, Apodaca E, Barrera-Vargas A, Alcocer-Varela J, Colunga-Pedraza I, González-Patiño A, Arauz A, Abud-Mendoza C, Martínez-Martínez M, Gómez-Martín D. Clinical outcomes and risk factors for posterior reversible encephalopathy syndrome in systemic lupus erythematosus: a multicentric case-control study. J Neurol Neurosurg Psychiatry. 2015;87:287–94. doi: 10.1136/jnnp-2014-310145. [DOI] [PubMed] [Google Scholar]
- 33.Min L, Zwerling J, Ocava LC, Chen IH, Putterman C. Reversible posterior leukoencephalopathy in connective tissue diseases. Semin Arthritis Rheum. 2006;35:388–395. doi: 10.1016/j.semarthrit.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Morrow SA, Rana R, Lee D, Paul T, Mahon JL. Posterior Reversible Encephalopathy Syndrome due to High Dose Corticosteroids for an MS Relapse. Case Rep Neurol Med. 2015;2015:325657. doi: 10.1155/2015/325657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mueller-Mang C, Mang T, Pirker A, Klein K, Prchla C, Prayer D. Posterior reversible encephalopathy syndrome: do predisposing risk factors make a difference in MRI appearance? Neuroradiology. 2009;51:373–383. doi: 10.1007/s00234-009-0504-0. [DOI] [PubMed] [Google Scholar]
- 36.Murphy T, Al-Sharief K, Sethi V, Ranger GS. Posterior Reversible Encephalopathy Syndrome (PRES) After Acute Pancreatitis. West J Emerg Med. 2015;16:1173–1174. doi: 10.5811/westjem.2015.8.28347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishio M, Yoshioka K, Yamagami K, Morikawa T, Konishi Y, Hayashi N, Himuro K, Imanishi M. Reversible posterior leukoencephalopathy syndrome: a possible manifestation of Wegener’s granulomatosis- mediated endothelial injury. Mod Rheumatol. 2008;18:309–314. doi: 10.1007/s10165-008-0052-1. [DOI] [PubMed] [Google Scholar]
- 38.Ozkok A, Elcioglu OC, Bakan A, Atilgan KG, Alisir S, Odabas AR. Reversible posterior leukoencephalopathy in the course of Goodpasture syndrome. Ren Fail. 2012;34:254–256. doi: 10.3109/0886022X.2011.647211. [DOI] [PubMed] [Google Scholar]
- 39.Pruitt AA, Graus F, Rosenfeld MR. Neurological complications of solid organ transplantation. Neurohospitalist. 2013;3:152–166. doi: 10.1177/1941874412466090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pruitt AA, Graus F, Rosenfeld MR. Neurological complications of transplantation: part I: hematopoietic cell transplantation. Neurohospitalist. 2013;3:24–38. doi: 10.1177/1941874412455338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabinstein AA, Mandrekar J, Merrell R, Kozak OS, Durosaro O, Fugate JE. Blood pressure fluctuations in posterior reversible encephalopathy syndrome. J Stroke Cerebrovasc Dis. 2012;21:254–258. doi: 10.1016/j.jstrokecerebrovasdis.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Schweitzer AD, Parikh NS, Askin G, Nemade A, Lyo J, Karimi S, Knobel A, Navi BB, Young RJ, Gupta A. Imaging characteristics associated with clinical outcomes in posterior reversible encephalopathy syndrome. Neuroradiology. 2017 doi: 10.1007/s00234-017-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singer S, Grommes C, Reiner AS, Rosenblum MK, DeAngelis LM. Posterior Reversible Encephalopathy Syndrome in Patients With Cancer. Oncologist. 2015;20:806–811. doi: 10.1634/theoncologist.2014-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singhal AB, Topcuoglu MA. Glucocorticoid-associated worsening in reversible cerebral vasoconstriction syndrome. Neurology. 2017;88:228–236. doi: 10.1212/WNL.0000000000003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ullian ME. The role of corticosteriods in the regulation of vascular tone. Cardiovasc Res. 1999;41:55–64. doi: 10.1016/s0008-6363(98)00230-2. [DOI] [PubMed] [Google Scholar]
- 46.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 47.Youssef D, Fawzy F. Posterior reversible encephalopathy syndrome in a child with steroid sensitive nephrotic syndrome. Arab J Nephrol Transplant. 2012;5:163–166. [PubMed] [Google Scholar]
- 48.ıncecik F, Hergüner M, Yıldızdaş D, Yılmaz M, Mert G, Horoz Ö, Altunbaşak Ş. Posterior reversible encephalopathy syndrome due to pulse methylprednisolone therapy in a child. Turk J Pediatr. 2013;55:455–457. [PubMed] [Google Scholar]