Abstract
Ronald Ma and Barry Popkin discuss the urgent need and challenges to reduce intergenerational transmission of obesity and diabetes.
The diabetes apocalypse?
Globally, efforts to prevent diabetes and obesity have attained mixed success.
Although prevalence of adult-onset diabetes is declining in some countries, the prevalence of childhood obesity and young-onset diabetes, with risk of associated complications, continues to increase in many high- and lower-income countries [1]. More than 1. 9 billion children and adults are overweight and obese, and prevalence is rapidly rising, with the greatest increase found among children [2][3,4].
Ongoing efforts to promote lifestyle intervention have had limited impact on the obesity and diabetes epidemic because of several critical factors. One factor comprises early life effects, a new field that has emerged surrounding developmental origins of many noncommunicable diseases (NCDs). This is the impact of nutritional and other insults during pregnancy and the first 2 years of life on the long-term risk of diabetes. Beginning with pathbreaking work by Barker on the relationship between low birth weight infants and subsequent risk of many adverse cardiometabolic outcomes [5,6], this field expanded to include multigenerational studies in animals and humans to highlight some of the potential mechanisms that may mediate the effect of fetal and infant undernutrition and related insults, including, for example, epigenetic changes affecting pancreatic islets, adipose tissue, and vasculature [7]. A large number of human cohort studies have followed those exposed to nutritional and infection insults in the fetus and infancy through early adulthood and are even exploring intergenerational factors, all showing that early exposures, when interacting with obesogenic dietary and related factors, lead to much greater risks of NCDs [8–10]. Most importantly, the increased risk of obesity and diabetes in individuals exposed in utero to undernutrition appears to be most exacerbated in those whose metabolic capacities are “mismatched” when exposed to metabolic challenges posed by overnutrition later in life [11,12]. This pathway may be particularly relevant to low- and middle-income countries, including India and China, which have undergone rapid socioeconomic and nutritional transition, with both countries witnessing a dramatic increase in the prevalence of diabetes over recent decades [13].
A second critical factor involves risks affecting populations of non-European origin, including Hispanic, African, Middle Eastern, or Asian groups [14–18]. These populations appear to have greater adiposity in the visceral area; yet, at the same body mass index (BMI) as European origin adults, they have a greater likelihood of being susceptible to diabetes, which even led to many Asian countries proposing lower cutoffs for overweight and obesity [19]. There is no clear explanation of the causes for these differences, although they are the subject of much exploratory research [12]. However, more explorations do not explain the explosion of diabetes in China, a country with a relatively low prevalence of adults with BMIs over 30.
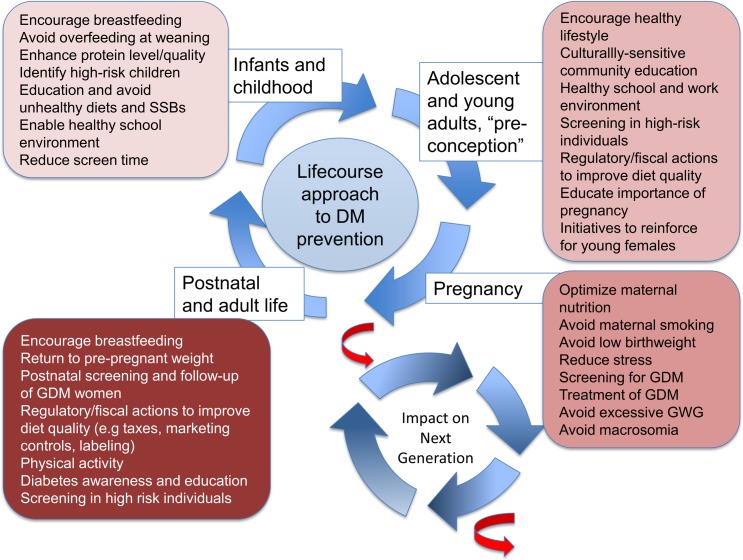
Another aspect of developmental influences relates to maternal hyperglycemia, gestational diabetes mellitus (GDM), maternal obesity, and the resulting in utero overnutrition. Maternal obesity, GDM, and excessive gestational weight gain lead to macrosomia and excess adiposity in offspring and increase the risk of childhood obesity [20,21]. Furthermore, offspring to mothers with maternal hyperglycemia or maternal obesity are likely to develop diabetes at a younger age and may further propagate this intergenerational risk (Fig 1).
Fig 1. The intergenerational cycle of diabetes and obesity.
Early life undernutrition, maternal diabetes, gestational diabetes, and maternal obesity are associated with increased risk of diabetes and obesity in the offspring, as well as younger age of onset of diabetes. This risk can therefore be transmitted to subsequent generations. Some potential interventions targeting different stages of the reproductive cycle and life course are highlighted.
There is 1 last set of changes we are seeing across many countries. As overweight and obesity has increased globally [4], researchers find increased waist circumferences at the same BMI level over the last decade in many countries, which portends another unknown risk to diabetes [22].
An informed approach to obesity and diabetes prevention
This intergenerational transmission of risk poses particular challenges to our current approach to obesity and diabetes prevention. Recent trials have highlighted that interventions initiated in pregnancy, such as increasing physical activity in overweight mothers, are likely to be too late to have an impact on pregnancy outcome [23–25]. Hence, there is a need to initiate interventions and dietary and physical activity changes in young women (and men) of reproductive age, who traditionally are least likely to come into contact with health services, placing particular challenges on outreach work. This need to engage these new at-risk groups, however, should not distract from ongoing efforts to reduce the pervasive obesogenic environment, including, but not limited to, reducing the intake of sugar-sweetened beverages (SSBs) and other energy-dense snacks and reducing sedentary lifestyle and screen time, especially among children [26]. A recent family-based study utilizing metabolomics has highlighted the important role of shared genetics and familial lifestyle between both parents and offspring in the pathogenesis of obesity [27]. Of note, prenatal and postnatal factors such as maternal smoking during pregnancy, gestational weight gain, duration of breastfeeding, and sleep duration during infancy can be associated with childhood obesity rates, which vary from 6% to 29%, suggesting that interventions that address these early life exposures may impact substantially on childhood obesity [28,29].
Several strategies have been explored over recent years, including restricting advertisement of unhealthy foods to children, front-of-pack labelling, improving school meals, using taxation to reduce the consumption of unhealthy food items, and providing different incentives to encourage intake of healthy foods or to encourage the production of healthier foods (Fig 1). However, the effectiveness and implementation of these remain challenging, and demonstration of impact on a national level remains lacking in most instances. Limited success stories are beginning to emerge, including the experience with taxation on SSBs in Mexico [30–32] and California, United States [33], in which, in both cases, the taxes reduced SSB intake and increased water purchases.
A keen sense of urgency is therefore needed in approaching the current conundrum. Not only is the global burden of diabetes giving rise to escalating healthcare expenditure that is threatening economic development of emerging economies, but the worsening epidemic of childhood obesity and young-onset diabetes and the increasing prevalence of GDM and diabetes affecting women of reproductive age are fuelling an evolving cycle of intergenerational risk transmission. Emerging data linking GDM with adverse neurocognitive development in offspring of mothers with GDM is particularly alarming [34], with maternal GDM potentially impacting on cognition and thereby further exacerbating the social disparity associated with NCDs [35]. Multisectoral efforts and intervention will be required to interrupt the current intergenerational transmission of risk [36] (Fig 1), but it is imperative that societies make this a top priority as the health and related developmental risks facing many countries are immense.
Abbreviations
- BMI
body mass index
- GDM
gestational diabetes mellitus
- NCD
noncommunicable disease
- SSB
sugar-sweetened beverage
Funding Statement
RCWM acknowledges support from the Research Grants Council Theme-based Research Scheme (T12-402/13N), RGC General Research Fund (14110415), and the Health and Medical Research Fund from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region (13140761). BMP acknowledges support from NIH R01DK108148, NIH P2C HD050924, NIH R01DK098072, and Bloomberg Philanthropies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Provenance: Commissioned; not externally peer reviewed.
References
- 1.Dabelea D, Stafford JM, Mayer-Davis EJ, D'Agostino R Jr., Dolan L, Imperatore G, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317(8):825–35. doi: 10.1001/jama.2017.0686 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Obesity and overweight Factsheet Geneva Switzerland: WHO; 2016. [cited 2017 May 1]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/. [Google Scholar]
- 3.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. doi: 10.1056/NEJMoa1614362 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth J, Qiang X, Marban SL, Redelt H, Lowell BC. The Obesity Pandemic: Where Have We Been and Where Are We Going? Obes Res. 2004;12(suppl_2):88S–101. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker D. Fetal and Infant Origins of Adult Disease: Papers London: British Medical Journal Publishing; 1992. [Google Scholar]
- 7.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–6. doi: 10.1126/science.1095292 . [DOI] [PubMed] [Google Scholar]
- 8.Adair LS, Fall CH, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382(9891):525–34. Epub 2013/04/02. doi: 10.1016/S0140-6736(13)60103-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–57. Epub 2008/01/22. doi: 10.1016/S0140-6736(07)61692-4 ; PubMed Central PMCID: PMC2258311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norris SA, Osmond C, Gigante D, Kuzawa CW, Ramakrishnan L, Lee NR, et al. Size at birth, weight gain in infancy and childhood, and adult diabetes risk in five low- or middle-income country birth cohorts. Diabetes Care. 2012;35(1):72–9. Epub 2011/11/22. doi: 10.2337/dc11-0456 ; PubMed Central PMCID: PMC3241316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, He Y, Qi L, Jaddoe VW, Feskens EJ, Yang X, et al. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes. 2010;59(10):2400–6. doi: 10.2337/db10-0385 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells JC, Pomeroy E, Walimbe SR, Popkin BM, Yajnik CS. The elevated susceptibility to diabetes in India: An evolutionary perspective. Front Public Health. 2016;4:145 doi: 10.3389/fpubh.2016.00145 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanditha A, Ma RC, Ramachandran A, Snehalatha C, Chan JC, Chia KS, et al. Diabetes in Asia and the Pacific: Implications for the global epidemic. Diabetes Care. 2016;39(3):472–85. doi: 10.2337/dc15-1536 . [DOI] [PubMed] [Google Scholar]
- 14.Deurenberg P, Deurenberg-Yap M, Guricci S. Asian are different from Caucasians and from each other in their body mass index/body fat percent relationship. Obes Rev. 2002;3:141–6. [DOI] [PubMed] [Google Scholar]
- 15.Deurenberg-Yap M, Chew SK, Deurenberg P. Elevated body fat percentage and cardiovascular risks at low body mass index levels among Singaporean Chinese, Malays and Indians. Obes Rev. 2002;2:209–15. [DOI] [PubMed] [Google Scholar]
- 16.Stern D, Smith LP, Zhang B, Gordon-Larsen P, Popkin BM. Changes in waist circumference relative to body mass index in Chinese adults, 1993–2009. Int J Obes. 2014;38(12):1503–10. doi: 10.1038/ijo.2014.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albrecht SS, Gordon-Larsen P, Stern D, Popkin BM. Is waist circumference per body mass index rising differentially across the United States, England, China and Mexico[quest]. Eur J Clin Nutr. 2015;69(12):1306–12. doi: 10.1038/ejcn.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim MM, Elamragy AA, Girgis H, Nour MA. Cut off values of waist circumference & associated cardiovascular risk in egyptians. BMC Cardiovascular Disorders. 2011;11(1):53 doi: 10.1186/1471-2261-11-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Obesity Task Force World Health Organization. The Asian-Pacific perspective: redefining obesity and its treatment Caulfield, Victoria: International Diabetes Institute; 2000. [Google Scholar]
- 20.Tam WH, Ma RCW, Ozaki R, Li AM, Chan MHM, Yuen LY, et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care. 2017;40(5):679–86. doi: 10.2337/dc16-2397 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma RC, Chan JC, Tam WH, Hanson MA, Gluckman PD. Gestational diabetes, maternal obesity, and the NCD burden. Clin Obstet Gynecol. 2013;56(3):633–41. doi: 10.1097/GRF.0b013e31829e5bb0 . [DOI] [PubMed] [Google Scholar]
- 22.Albrecht SS, Gordon-Larsen P, Stern D, Popkin BM. Is waist circumference per body mass index rising differentially across the United States, England, China and Mexico? Eur J Clin Nutr. 2015;69(12):1306–12. doi: 10.1038/ejcn.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3(10):767–77. doi: 10.1016/S2213-8587(15)00227-2 . [DOI] [PubMed] [Google Scholar]
- 24.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017;5(1):53–64. doi: 10.1016/S2213-8587(16)30107-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song C, Li J, Leng J, Ma RC, Yang X. Lifestyle intervention can reduce the risk of gestational diabetes: a meta-analysis of randomized controlled trials. Obes Rev. 2016;17(10):960–9. doi: 10.1111/obr.12442 . [DOI] [PubMed] [Google Scholar]
- 26.Popkin BM, Hawkes C. Sweetening of the global diet, particularly beverages: patterns, trends, and policy responses. Lancet Diabetes Endocrinol. 2016;4(2):174–86. doi: 10.1016/S2213-8587(15)00419-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos Ferreira DL, Williams DM, Kangas AJ, Soininen P, Ala-Korpela M, Smith GD, et al. Association of pre-pregnancy body mass index with offspring metabolic profile: Analyses of 3 European prospective birth cohorts. PLoS Med. 2017;14(8):e1002376 doi: 10.1371/journal.pmed.1002376 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillman MW, Rifas-Shiman SL, Kleinman K, Oken E, Rich-Edwards JW, Taveras EM. Developmental origins of childhood overweight: potential public health impact. Obesity (Silver Spring). 2008;16(7):1651–6. doi: 10.1038/oby.2008.260 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gillman MW, Ludwig DS. How early should obesity prevention start? N Engl J Med. 2013;369(23):2173–5. doi: 10.1056/NEJMp1310577 . [DOI] [PubMed] [Google Scholar]
- 30.Colchero MA, Popkin BM, Rivera JA, Ng SW. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ. 2016;352:h6704 doi: 10.1136/bmj.h6704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popkin BM. Preventing type 2 diabetes: Changing the food industry. Best Pract Res Clin Endocrinol Metab. 2016;30(3):373–83. doi: 10.1016/j.beem.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batis C, Rivera JA, Popkin BM, Taillie LS. First-year evaluation of Mexico's tax on nonessential energy-dense foods: An observational study. PLoS Med. 2016;13(7):e1002057 doi: 10.1371/journal.pmed.1002057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silver LD, Ng SW, Ryan-Ibarra S, Taillie LS, Induni M, Miles DR, et al. Changes in prices, sales, consumer spending, and beverage consumption one year after a tax on sugar-sweetened beverages in Berkeley, California, US: A before-and-after study. PLoS Med. 2017;14(4):e1002283 doi: 10.1371/journal.pmed.1002283 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang AH, Wang X, Martinez MP, Walthall JC, Curry ES, Page K, et al. Association of maternal diabetes with autism in offspring. JAMA. 2015;313(14):1425–34. doi: 10.1001/jama.2015.2707 . [DOI] [PubMed] [Google Scholar]
- 35.Daw J. Contribution of four comorbid conditions to racial/ethnic disparities in mortality risk. Am J Prev Med. 2017;52(1S1):S95–S102. doi: 10.1016/j.amepre.2016.07.036 . [DOI] [PubMed] [Google Scholar]
- 36.Hanson MA, Bardsley A, De-Regil LM, Moore SE, Oken E, Poston L, et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: "Think Nutrition First". Int J Gynaecol Obstet. 2015;131(Suppl 4):S213–S53. 10.1016/S0020-7292(15)30034-5 [DOI] [PubMed] [Google Scholar]