Abstract
Background
Gonorrhoea is one of the most common bacterial sexually transmitted infections in England. Over 41,000 cases were recorded in 2015, more than half of which occurred in men who have sex with men (MSM). As the bacterium has developed resistance to each first-line antibiotic in turn, we need an improved understanding of fitness benefits and costs of antibiotic resistance to inform control policy and planning. Cefixime was recommended as a single-dose treatment for gonorrhoea from 2005 to 2010, during which time resistance increased, and subsequently declined.
Methods and findings
We developed a stochastic compartmental model representing the natural history and transmission of cefixime-sensitive and cefixime-resistant strains of Neisseria gonorrhoeae in MSM in England, which was applied to data on diagnoses and prescriptions between 2008 and 2015. We estimated that asymptomatic carriers play a crucial role in overall transmission dynamics, with 37% (95% credible interval CrI 24%–52%) of infections remaining asymptomatic and untreated, accounting for 89% (95% CrI 82%–93%) of onward transmission. The fitness cost of cefixime resistance in the absence of cefixime usage was estimated to be such that the number of secondary infections caused by resistant strains is only about half as much as for the susceptible strains, which is insufficient to maintain persistence. However, we estimated that treatment of cefixime-resistant strains with cefixime was unsuccessful in 83% (95% CrI 53%–99%) of cases, representing a fitness benefit of resistance. This benefit was large enough to counterbalance the fitness cost when 31% (95% CrI 26%–36%) of cases were treated with cefixime, and when more than 55% (95% CrI 44%–66%) of cases were treated with cefixime, the resistant strain had a net fitness advantage over the susceptible strain. Limitations include sparse data leading to large intervals on key model parameters and necessary assumptions in the modelling of a complex epidemiological process.
Conclusions
Our study provides, to our knowledge, the first estimates of the fitness cost and benefit associated with resistance of the gonococcus to a clinically relevant antibiotic. Our findings have important implications for antibiotic stewardship and public health policies and, in particular, suggest that a previously abandoned antibiotic could be used again to treat a minority of gonorrhoea cases without raising resistance levels.
In a modeling study, Xavier Didelot and colleagues address the implications of cefixime resistance for treatment of gonorrhea.
Author summary
Why was this study done?
Gonorrhoea is an increasingly common sexually transmitted infection in England.
Gonorrhoea has become resistant to many antibiotics in recent years, to the extent that clinicians are running out of treatment options.
The current recommended treatment is a combination of 2 antibiotics, which is effective, but there are worrying signs that it may not always be so.
Informed decisions need to be made to fight antibiotic-resistant gonorrhoea, but this is currently hampered by a lack of understanding of the dynamics of how resistance emerges and spreads.
What did the researchers do and find?
We developed a mathematical model that captures the dynamics of cefixime resistance observed between 2008 and 2015.
The model includes a fitness benefit of resistance, to explain why cefixime resistance increased when cefixime was widely used up until 2010.
The model also includes a fitness cost of resistance, to explain why cefixime resistance decreased from 2010.
Having inferred the fitness cost and benefit of cefixime resistance, we were able to predict what would happen if cefixime was reintroduced and found that it could be used to treat up to 25% of gonorrhoea cases without risk.
What do these findings mean?
Cefixime had previously been abandoned because of a high level of resistance, but the fitness cost of resistance is such that resistance levels have since dropped, and reintroduction could be considered to treat a minority of cases.
This policy would reduce the risk of resistance developing to the other antibiotics currently used.
Similar modelling studies could be carried out to investigate the fitness costs and benefits of resistance to other antibiotics in gonorrhoea and other bacterial pathogens, providing a strong evidence basis for antibiotic deployment policies.
In the future, there could be a policy of treating patients with gonorrhoea with a range of antibiotic regimens that would be systematically randomized at the individual patient level. The frequency of use of each regimen would be determined by the fitness costs and benefits of resistance to each regimen, once these have been measured.
Introduction
Gonorrhoea, caused by the bacterial pathogen N. gonorrhoeae, is one of the most common sexually transmitted infections in England. Incidence has increased year on year since 2008, culminating in over 41,000 cases in 2015 [1]. Around 22,000 of these cases were found in men who have sex with men (MSM), constituting a 20% annual increase. The greatest cause for concern, however, is the rapid growth in antimicrobial resistance. The bacterium has quickly developed resistance to each first-line antibiotic in turn, from penicillin to cephalosporins, such as cefixime and ceftriaxone [2, 3]. Treatment with ceftriaxone is the last remaining single-drug option in most settings worldwide; however, diminishing susceptibility led England and many other countries to recommend treatment of gonorrhoea with a dual therapy of ceftriaxone and azithromycin [4–6]. Ceftriaxone resistance has been detected only sporadically in England; however, azithromycin resistance is easily selected for and was prevalent in a recent outbreak [7]. Resistance to azithromycin effectively reduces the current treatment to a monotherapy, making resistance trends increasingly important to monitor against the threat of potentially untreatable gonorrhoea.
Public Heath England (PHE) runs the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) [8], which has produced a report annually since 2000 [9–24]. GRASP monitors trends in resistance and susceptibility to a panel of antibiotics used to treat gonorrhoea in England and Wales and thus informs national treatment guidelines and strategy. In 2004, GRASP began testing for cefixime resistance, defined as having a minimum inhibitory concentration (MIC) of ≥0.125 mg/l [13]. A retrospective study of 133 patients returning for test-of-cure in Canada found a cefixime MIC threshold of ≥0.12 mg/l to be associated with a treatment-failure rate of 25% (95% CrI 11%–45%) [25]. In 2005, following worrying increases in resistance to the previous therapy, ciprofloxacin, a new recommendation advising that uncomplicated gonorrhoea should be treated with a single dose of cefixime was introduced [26].
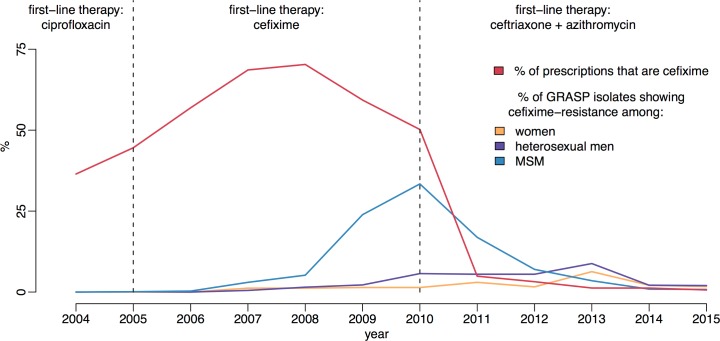
Fig 1 shows the trends in cefixime prescription and resistance in England. Very little resistance was detected until 2007; however, by 2009 the total level of resistance had passed the 5% threshold at which the WHO recommends that first-line treatment guidelines should be changed [16, 27]. At this time, almost 60% of individuals with gonorrhoea diagnoses were being treated with cefixime [18]. The majority of the resistance was concentrated in the MSM population, where it reached a peak of 33% in 2010 [19]. This evidence, combined with increasingly common reports of cefixime treatment failure, formed the basis for the decision in May 2011 for another update to the treatment guidelines for uncomplicated gonorrhoea [28, 29]. Cefixime was no longer recommended as a first-line treatment and was replaced with a combination of 500 mg ceftriaxone and 1 g azithromycin [4]. Since 2011, cefixime prescribing has fallen drastically, in line with the updated guidelines. Over the same period, the proportion of cefixime-resistant isolates has declined steadily in MSM, falling to less than 1% in 2014 [23].
Fig 1. Usage and resistance of cefixime in England and Wales.
The proportion of gonococcal isolates in Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) that are resistant to cefixime over time is compared with the proportion of gonorrhoea diagnoses treated with cefixime. Dashed lines show the dates of treatment guideline changes. MSM, men who have sex with men.
We hypothesise that the resistance trend observed can be explained by a net fitness benefit to cefixime resistance when cefixime is widely prescribed but a net fitness cost when cefixime prescriptions decline. Understanding the relationship between antibiotic use and the emergence of resistance in gonorrhoea has been identified as a key research agenda [30]. Here our main aim is to further the understanding of the evolutionary dynamics of cefixime resistance and to use this newfound knowledge to inform public health practice. There is still much we do not understand about the natural history of gonorrhoea, especially since unobserved asymptomatic infections have long been thought to be an important reservoir of infection. The proportion of incident cases that are asymptomatic at each bodily site of infection is known to vary but has not been definitively measured [31–33]. Furthermore, the expected duration of carriage of asymptomatic gonococcal infection is not well studied. Estimates have been traditionally in the region of 6 months; however, recent work using genomic data on pairs of known sexual contacts has suggested that a longer duration of carriage can occasionally happen [34]. We therefore developed and applied a Bayesian statistical approach to account for these uncertainties in the epidemiology of gonorrhoea. The analysis was restricted to MSM, the population in which the cefixime-resistant outbreak of gonorrhoea was concentrated.
Materials and methods
Epidemiological data
The total number of diagnoses of gonorrhoea in MSM in England between 2008 and 2015 was extracted from the Genitourinary Medicine Clinic Activity Dataset (GUMCAD) [35]. This mandatory reporting system provides data on diagnoses of sexually transmitted infections from sexual health services in England, and the GUMCAD data are published annually by PHE. This yearly number of gonorrhoea diagnoses is denoted Y(t).
The number of cases of gonorrhoea in MSM that were cefixime resistant and reported by GRASP between 2008 and 2015 were extracted from the corresponding GRASP reports [17–24] and denoted Yres(t). The coverage of GRASP was calculated for every year between 2008 and 2015 by taking the ratio between the number of cases included in GRASP (irrespective of resistance) and the number of GUMCAD diagnoses in the same year. This GRASP coverage proportion is denoted q(t). GRASP includes a small number of isolates from non-GUM settings, which are not included in GUMCAD. These isolates constitute <3% of the total GRASP sample and are predominantly from women, so while the GRASP data are not strictly a subset of GUMCAD, the effect of the non-GUMCAD cases on the analysis is minimal. The proportion of gonorrhoea cases that were treated with cefixime, as opposed to other antibiotics, was also extracted from the GRASP reports between 2008 and 2015. This time-dependent proportion is denoted π(t) and illustrated in Fig 1.
A recent estimate based on HIV diagnoses and the European MSM Internet Survey (EMIS) has suggested a United Kingdom MSM population of 3.4% (0.6 million) [36]. This is consistent with the third National Survey of Sexual Attitudes and Lifestyles (Natsal) in which 8.4% of men reported same-sex experience at least once, with 2.6% of men having had a same-sex partner in the last 5 years, putting a plausible range for the MSM population at 0.5 and 1.7 million based on a sexually active male population of 20 million [37, 38]. Therefore, we adopt an estimate of the MSM population size of N = 0.6 million.
Given the low prevalence of gonococcal infection in the population, the total population size is not expected to excessively affect the results.
Transmission model
In order to investigate the fitness cost and benefit of cefixime resistance in gonorrhoea, we created a stochastic compartmental model, illustrated in Fig 2 with notation summarised in Table 1. It was important to use a stochastic model because of the small number of resistant cases detected by GRASP in the early and late stages of the outbreak that would not be captured by a deterministic model. High rates of reinfection with gonorrhoea have been observed, suggesting low levels of acquired immunity [39], and experimental urethral infection in male volunteers found no protection was conferred on repeat infection with an identical strain 6 months after the initial infection [40]. It was therefore assumed that no immunity was conferred upon recovery from infection. A closed population of size N was assumed because of the short time period under consideration. Individuals are initially susceptible (S). They become infected with strain s ∈ {sus,res}, denoting cefixime-susceptible and cefixime-resistant strains, respectively. The model assumes that strains do not vary in transmissibility and that the rate of infection from an infectious individual to a susceptible individual is θ/N. Infected individuals initially pass through an incubation period (Us), which they leave at rate σ. A proportion ψ of those infected then go on to develop symptoms (Es), whereas the remainder enter an asymptomatic stage (As). Gonococcal infection can occur in the rectum, pharynx, and/or urethra, resulting in different rates of onward transmission and probabilities of developing symptoms [41]. We do not explicitly model separate sites of infection; therefore, the rate of transmission, θ, and the likelihood of developing symptoms, ψ, should be seen as an average for any infection site. Recovery from asymptomatic infection happens (either naturally or following unrelated antibiotic treatment) at rate ν for the susceptible strain and at rate αν for the resistant strain. The parameter α therefore represents the fitness cost of cefixime resistance. The infected population for each strain s is denoted Is = Us + Es + As, and the total infected population is denoted I = Isus + Ires. All infected individuals are assumed to be infectious. The symptomatic individuals (Es) seek treatment at rate μ. A time-varying proportion π(t) are treated with cefixime (Ts;cef), whereas the remaining 1−π(t) are treated with other antibiotics (Ts;oth). The treated individuals recover from the infection and become susceptible again at rate ρ, with the exception of a proportion ϕ of the individuals infected with a cefixime-resistant strain who have been treated with cefixime (Tres;cef) but experience treatment failure and become asymptomatically infected (Ares) [42, 43].
Fig 2. Flow diagram of model compartments with rates of transition between infection states.
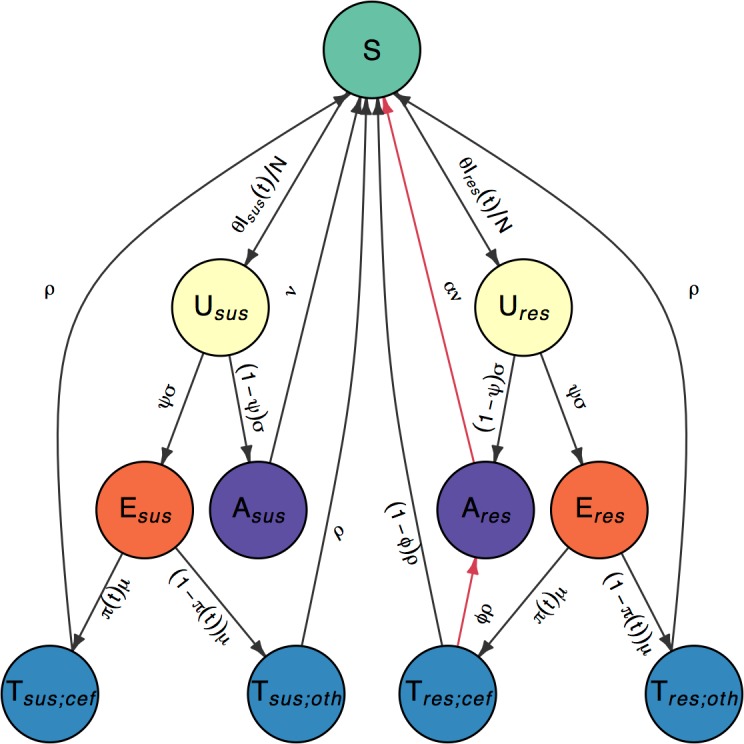
Susceptible individuals (S) become infected with either cefixime-susceptible (s = sus) or cefixime-resistant strains (s = res). Infections initially pass through an incubation period (Us) before the individuals with the infection either develop symptoms (Es) or remain asymptomatic carriers (As). Symptomatic individuals seek treatment (Ts;p) and are prescribed either cefixime (p = cef) or another antibiotic (p = oth). The 2 sides are symmetric with the exception of the 2 arrows highlighted in red, which correspond to the cost and the benefit of resistance.
Table 1. Parameter notations and prior and posterior distributions.
| Parameter description | Unit | Prior distribution | Prior mean (95% CrI) | Posterior mean (95% CrI) | |
|---|---|---|---|---|---|
| Asus(0) | Initial carriage of cefixime-susceptible infection | # | Uniform(0,∞) | n/a | 618 (411–819) |
| Ares(0) | Initial carriage of cefixime-resistant infection | # | Uniform(0,∞) | n/a | 49 (20–92) |
| θ | Rate of transmission per partner | per year | Gamma(44,10) | 4.4 (3.2–5.8) | 5.2 (4.1–6.5) |
| ψ | Infections that become symptomatic | % | Uniform(0,1) | 50 (2.5–97.5) | 63 (48–76) |
| σ | Rate of departing incubation period | per year | Gamma(17,0.22) | 77 (45–118) | 77 (46–119) |
| ν | Rate of recovery from asymptomatic infection | per year | Gamma(8,3.45) | 2.3 (1.0–4.2) | 1.8 (0.9–3.0) |
| μ | Rate of seeking treatment when symptomatic | per year | Gamma(3,0.02) | 150 (31–361) | 136 (33–350) |
| ρ | Rate of recovery following treatment | per year | Gamma(101,1.9) | 53 (43–64) | 53 (43–63) |
| α | Increased recovery from cefixime-resistant infection | n/a | Uniform(0,∞) | n/a | 1.8 (1.4–2.4) |
| ϕ | Treatment failure rate for cefixime-resistant infections treated with cefixime | % | Uniform(0,1) | 50 (2.5–97.5) | 83 (53–99) |
Calculation of the basic reproduction number
The basic reproduction number, R0, is a measure of the reproductive capacity of an infectious agent and is defined as the average number of secondary cases of gonorrhoea arising from the introduction of a typical infected individual in a completely susceptible population. Where there is direct competition between strains, as in the situation we are modelling, the strain with the highest R0 will outcompete the others.
To calculate R0, we must consider the generation time, defined as the expected time from an individual becoming infected to infecting another individual. By considering the expected time spent in each compartment of the model corresponding to infection with the susceptible strain (i.e., states Usus, Esus, and Asus in Fig 2), we derive an analytical expression of the basic reproduction number for the susceptible strains using the next-generation method, as detailed in S2 Appendix [44]:
| (1) |
Similarly, the basic reproduction number for the resistant strains is given by the following:
| (2) |
Therefore, by equating Eqs 1 and 2, we can obtain the level of cefixime prescriptions above which the resistant strains become fitter than the cefixime-susceptible strains:
| (3) |
Bayesian inference
We considered the data as a partially observed Markov process, with the number of GUMCAD recorded cases, Y(t), and GRASP reported resistant cases, Yres(t), being the observed realisations of the underlying unobserved processes: the total incidence of gonorrhoea infections, Z(t), and the incidence of cefixime-resistant infections, Zres(t). The reporting process assumed that 90% of all gonorrhoea diagnoses are recorded by GUMCAD with a 10% margin of error, consistent with findings that 6%–9% of gonorrhoea is diagnosed in a GP setting [45].
The probability of a cefixime-resistant case of gonorrhoea being sampled by the GRASP study was assumed to be Poisson distributed with a sampling probability denoted q(t) derived from the coverage of the GRASP study over 2008 to 2014:
Based on these observations, we aimed to infer the values of the 10 parameters: Asus(0),Ares(0),θ,ψ,σ,ν,α,μ,ρ, and ϕ.
An analytical expression for the likelihood of the observed data given our model is not available, so we obtained an unbiased estimate of the likelihood using a particle filter [46]. The estimated likelihood was then incorporated into a particle Monte Carlo Markov Chain (pMCMC), which was used to obtain a sample from the posterior distribution of the model parameters [47]. The difficulty of exploring the posterior parameter space with the pMCMC algorithm increases with the number of parameters. The number of symptomatic cases was therefore initialised as Esus(0) = Eres(0) = 0, as after a few days of simulation, these variables reached the stochastic equilibrium values implied by the model and the parameters.
The model fitting was implemented using the R package pomp, which includes a pMCMC algorithm that can be used to perform Bayesian inference [48]. The algorithm was modified to enable parallel computation. The particle filter estimation of the likelihood was based on 1,000 particles, which was sufficiently robust to estimate the likelihood. The pMCMC was run for 1.1 million iterations, with the first 10% discarded as burn-in and the remainder thinned by a factor of 100. Four separate chains were run with dispersed starting points and compared using the R package coda [49]. The chains appeared to have converged to the same posterior distribution based on the multivariate version of the Gelman-Rubin diagnostic, which was less than 1.1 for all inferred parameters [50, 51]. To ensure maximum robustness, the samples from the 4 chains were then combined and found to have an effective sample size of more than 200 for all parameters.
Prior distributions of parameters
Bayesian inference requires setting plausible priors for the model parameters. We used highly uninformative Uniform(0,1) priors for the 2 proportion parameters ϕ and ψ and Uniform(0, ∞) priors for the 3 parameters Asus(0),Ares(0), and α, which is an improper distribution but does not lead to an improper posterior distribution. For the 5 remaining parameters θ,ν,σ,μ, and ρ we assigned informative Gamma priors based on a literature review, as summarised in Table 1.
The transmission rate of infection, represented by the parameter θ, encompasses both the average number of sexual partners per year and the transmission probability per partnership. The Natsal-3 survey observed a mean number of sexual partners per year for MSM of 4.4 [37], and we would therefore expect θ to be slightly lower, to reflect the fact that not all contacts result in transmission. The prior distribution for θ was therefore set such that it was between 2.9 and 6.3 with 99% prior weight.
The expected duration of carriage for asymptomatic gonorrhoea is not well measured. A study of 18 asymptomatic infected men saw no resolution in urethral infection in the 165 days before they received treatment [43]. Estimates of the duration of carriage in modelling studies have been based on calculations that take into account observed prevalence and an assumed proportion of unobserved infection and often assume an average of 6 months [52–54]. This is confirmed by recent work using genomic data, in which the greatest observed time to most recent common ancestor for bacterial genomes from known contact pairs was 8 months [34]. The duration of carriage may depend on the infection site; for pharyngeal gonorrhoea, it has been estimated to be 12 weeks, and for rectal infection, it has been estimated to be 1 year [55, 56]. The parameter ν was therefore assigned a prior that corresponded to a mean duration of carriage between 3 months and 1 year with 99% prior weight.
The duration of the incubation, symptomatic, and treatment stages of infection have been estimated to be short, in the region of days rather than weeks [57–59]. Gamma priors were accordingly assigned to each of the 3 parameters σ,μ, and ρ.
Results
Estimation of model parameters
We fitted our model of gonorrhoea transmission to 2 different time series over the years 2008 to 2014: the total number of gonorrhoea diagnoses in MSM in England [60] and the incidence of cefixime-resistant gonorrhoea [17–23]. The posterior distribution of parameters shown in Fig 3 was obtained through Bayesian inference, implemented using a pMCMC method [47]. For each parameter, we report the posterior mean estimate and 95% credible interval shown in brackets (Table 1). The model suggests that at the end of 2007, the first year that cefixime-resistant cases were detected by GRASP [16], there were 618 cases (95% CrI 411–819) of asymptomatic cefixime-susceptible gonorrhoea (Asus(0)) and 49 cases (95% CrI 20–92) of asymptomatic cefixime-resistant gonorrhoea (Ares(0)).
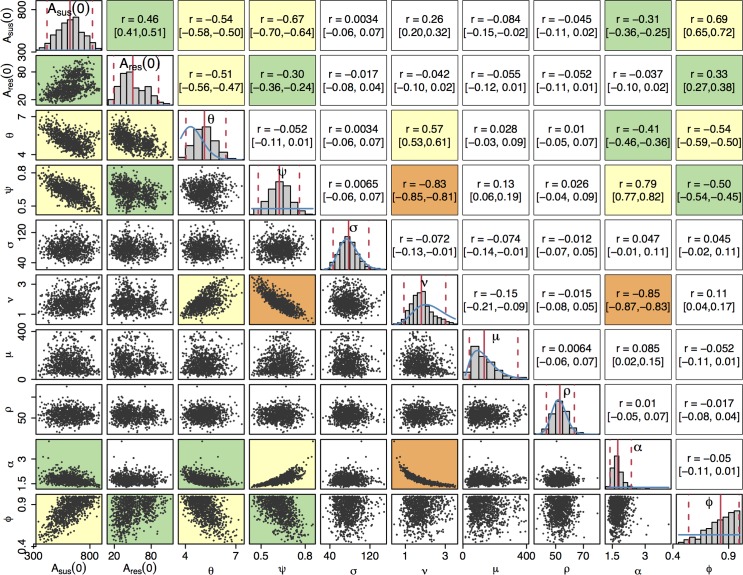
Fig 3. Posterior distributions of parameters.
The diagonal plots show histograms of the posterior distributions for all sampled parameters. The blue lines show prior distributions, and the red lines indicate posterior mean and 95% credible intervals. The plots below the diagonal show scatter plots based on 1,000 samples from the posterior, illustrating the relationships between pairs of estimated parameters. An orange background indicates a correlation higher than 0.8, a yellow background indicates a correlation between 0.5 and 0.8, a green background indicates a correlation between 0.3 and 0.5, and a white background indicates a correlation less than 0.3. The plots above the diagonal show the corresponding correlation coefficients with the 95% credible intervals in parentheses.
The posterior distribution of the rate of transmission, θ, suggests a higher mean rate of infection than the prior expectation: 5.2 (95% CrI 4.1–6.5), but the prior and posterior credible intervals overlap to a large extent, suggesting that the results are consistent with our prior knowledge. Our model predicts that the proportion ψ of infections that become symptomatic is 63% (95% CrI 48%–76%). The 3 parameters σ, μ, and ρ, corresponding respectively to the durations of the incubation period, the symptomatic infection before seeking treatment, and the treatment phase, had posterior distributions similar to their prior distributions, indicating that the prior distributions were appropriate but that there is little additional information on these parameters in the data set. The posterior distribution of ν has a slightly lower mean than the prior distribution, implying a longer mean duration of carriage of 203 days (95% CrI 122–397). The prior and posterior credible intervals still intersect to a large extent so there is not significant evidence of a departure from the prior based on the data.
The last 2 parameters, α and ϕ, capture the difference between the susceptible and resistant strains in our model. The model predicts that in order to replicate observed incidence patterns, recovery from asymptomatic cefixime-resistant gonorrhoea occurs α = 1.8 (95% CrI 1.4–2.4) times faster than recovery from asymptomatic cefixime-susceptible gonorrhoea, giving rise to a fitness cost. The model suggests a treatment failure proportion of ϕ = 83% (95% CrI 53%–99%) for resistant gonorrhoea treated with cefixime such that resistance confers a fitness benefit in an environment in which cefixime is highly prescribed.
Beyond the marginal posterior distributions of the parameters described above, it is informative to study their posterior correlations. The pairwise posterior relationships between the 10 parameters are depicted in Fig 3. Parameters σ, μ, and ρ did not show a strong correlation with any parameters; as expected, the short duration of the incubation, symptomatic, and treatment stages of infection led to these parameters contributing relatively little to the dynamics of infection. The strongest correlation was found between ν and α, r = −0.85 (95% CrI −0.87 to −0.83), corresponding to the trade-off required to maintain the duration of carriage of resistant infection, which is equal to 1/(αν) and accounts for the nonlinearity of the relationship. Parameters ν and ψ were also highly negatively correlated, r = −0.83 (95% CrI −0.85 to −0.81), which corresponds to the trade-off between duration of carriage, 1/ν, and the proportion of infections entering the carriage state, (1−ψ). The 2 negative correlations of both α and ψ with ν lead to a positive correlation between α and ψ.
Posterior predictive analysis
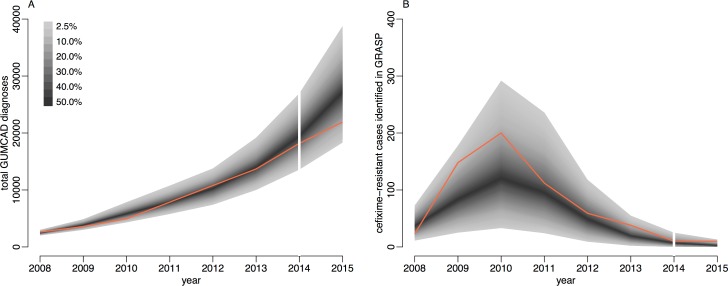
The total number of gonorrhoea cases in MSM observed by GUMCAD and the number of cefixime-resistant infections isolated in MSM by GRASP were compared with simulated data sets using parameters sampled from their posterior distributions to assess the goodness of fit of our model to the data. One thousand parameter sets were sampled, and 1 simulation was performed using each set. Fig 4 demonstrates that the simulated data closely emulate the real data. The real data are within the 95% predictive intervals at all time points, indicating a good fit of the model to the data [61].
Fig 4. Comparison of simulated and observed cases of gonorrhoea.
Panel A shows the total number of cases, and panel B shows only the cefixime-resistant cases. Observed data are shown in orange, with the shaded area showing the 95% posterior predictive interval (based on 1,000 simulations using samples from posterior distribution). Note different scales. GUMCAD, Genitourinary Medicine Clinic Activity Dataset.
The total number of cases of gonorrhoea observed by GUMCAD and the number of cefixime-resistant cases observed by GRASP in 2015 [24] were not used in the model-fitting process and were used to provide an independent check of the model fit. Both data points are within the 95% probability intervals predicted by our model: 21,915 gonorrhoea diagnoses were recorded by GUMCAD, compared to 27,475 (95% CrI 18,307–38,810) diagnoses predicted by the model; 10 cefixime-resistant cases were recorded by GRASP, compared to 4 (95% CrI 0–13) cases predicted by the model. Our modelling suggests that in 2015, 1.7% (95% CrI 1.0%–2.6%) of MSM in England may have been carriers of asymptomatic gonorrhoea, and 0.2% (95% CrI 0.1%–0.4%) may have had symptomatic gonorrhoea, with an overall prevalence of 2.0% (95% CrI 1.1%–2.9%). Our results suggest that asymptomatic gonorrhoea accounted for 89% (95% CrI 82%–93%) of onward transmission in MSM.
Comparative analysis of basic reproduction numbers
A key threshold in epidemic theory associates the persistence of disease in a population with a basic reproduction number greater than 1 [62]. Using Eq 1, we obtain a posterior estimate for the basic reproduction number for cefixime-susceptible infection of = 1.19 (95% CrI 1.10–1.36), which suggests that the cefixime-susceptible strain of gonorrhoea is expected to persist in the population without further intervention (Fig 5A). Under our hypothesis, the basic reproduction number for cefixime-resistant gonorrhoea depends on the frequency of cefixime prescription (Eq 2) and thus can be considered as a function where π is the proportion of gonorrhoea diagnoses being treated by cefixime. In the 2 extreme cases when no cefixime is prescribed (π = 0, meaning that the treatment is always effective) and only cefixime is prescribed (π = 1, meaning that only a proportion 1−ϕ of the treatment is effective), we estimate a basic reproduction number for resistant gonorrhoea of = 0.73 (95% CrI 0.63–0.83) and = 1.61 (95% CrI 1.38–1.90), respectively. At its height in 2008, the frequency of cefixime prescriptions was 70%, and we estimate that at this time the basic reproduction number for resistant gonorrhoea was = 1.35 (95% CrI 1.21–1.52). The former estimate is ≤1, whereas the latter 2 are ≥1; this is consistent with the fact that between 2005 and 2010, when cefixime was often used to treat gonorrhoea, resistance to cefixime increased, whereas with the discontinuation of cefixime usage from 2011, resistance has decreased.
Fig 5.
(A) Histogram of the posterior estimate of . (B) The 95% credible interval of against π with dashed lines showing the 95% credible interval for . (C) Histogram of the posterior estimate of : the threshold of cefixime prescriptions above which .
We can estimate the frequency of cefixime prescriptions above which we expect the resistant strain to persist, corresponding to when the fitness benefit of cefixime resistance is greater that its fitness cost, by setting and solving for π in Eq 2. We denote this threshold and thus obtain a posterior estimate of = 0.31 (95% CrI 0.26–0.36) (Fig 5B). This result suggests that up to a quarter of gonorrhoea treatments could be with cefixime monotherapy without causing a cefixime-resistant epidemic. Another important threshold is the level of cefixime prescriptions above which the resistant strain of gonorrhoea is fitter than the susceptible strain. We denote this threshold . By setting and equating Eqs 1 and 2, we obtain a posterior estimate of = 0.55 (95% CrI 0.44–0.66) (Fig 5C).
Impact of cefixime usage on simulated resistance trends
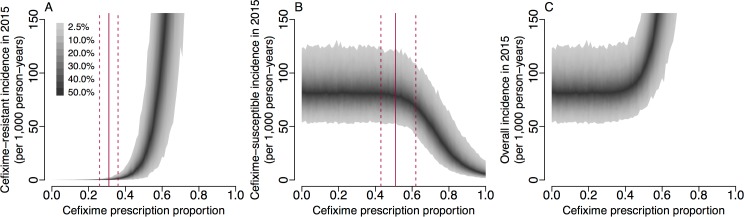
The basic reproduction numbers derived above are informative but do not capture completely the complex dynamics of infection transmission that occur when accounting for stochasticity, competition between susceptible and resistant strains, and non-negligible fractions of the population becoming infected. To further study the impact of cefixime prescribing on the cefixime-resistant and cefixime-susceptible epidemics, we performed stochastic model simulations over 8 years from 2008 to 2015 using parameters drawn from their posterior distributions and examining scenarios with a frequency of cefixime prescriptions ranging from no use of cefixime (π = 0) to all gonorrhoea cases being treated by cefixime (π = 1). The prescription frequency in each case was kept constant throughout the entire simulation period. Fig 6A shows that, when fewer than 27% of gonorrhoea cases were treated with cefixime, there was a 95% probability that the resistant outbreak no longer persisted in 2015. This is comparable to our estimate of = 0.31 (95% CrI 0.26–0.36) (Fig 5B), the level of prescriptions above which the fitness benefit of cefixime resistance is greater than the fitness cost.
Fig 6. Incidence of gonorrhoea in 2015 based on simulations from 2004 to 2015 with varying levels of cefixime prescribing.
(A) Incidence of the cefixime-resistant strain. The red lines show the 95% credible interval for . (B) Incidence of the cefixime-susceptible strain. The red lines show the 95% credible interval for . (C) The overall incidence from both cefixime-resistant and cefixime-susceptible strains. The shaded areas show the 95% posterior predictive intervals (based on 1,000 simulations using samples from posterior distribution).
Fig 6B shows that, when more than 50% of gonorrhoea cases were treated with cefixime, the simulated incidence of cefixime-susceptible infection began to fall, with the cefixime-resistant strain becoming more common. This supports our analytical estimate of = 0.55 (95% CrI 0.44–0.66) (Fig 5C), the level of cefixime prescriptions above which the resistant strain becomes fitter than the susceptible strain. If cefixime were used to treat more than 58% of cases, then the level of cefixime resistance would become greater than 50% at the end of the 8-year simulation period, and if cefixime were used to treat all cases, resistance would be close to 100%.
Discussion
Main findings
We have used mathematical modelling and Bayesian inference methods to uncover insights into the dynamics of cefixime resistance in gonorrhoea. We quantified both the fitness cost and fitness benefit of resistant strains, which allowed us to make predictions about the future prevalence of resistance as a function of how often cefixime is prescribed. Our results indicate that cefixime could be used to treat uncomplicated cases of gonorrhoea without incurring the risk of causing a resistant epidemic like the one that happened in 2007–2012, provided its frequency of use were controlled, enabling continued use of an ‘abandoned’ antibiotic. Our analysis uses the complementary approaches of analysing the basic reproduction number and posterior predictive analysis to suggest that cefixime could be used to treat up to 25% of cases. This threshold should still be used cautiously, however, for reasons described below. N. gonorrhoeae has developed resistance to each first-line antibiotic in turn, from penicillin to third-generation cephalosporins. Our modelling work lays the foundation for a rational scientific approach to extending and ultimately preserving the usefulness of existing antibiotics.
Study strengths and limitations
The strengths of our methodology reside in the explicit stochastic model of gonorrhoea infection we developed and the state-of-the-art approach to Bayesian inference employed to estimate model parameters. We were able to explain and reproduce the observed boom-and-bust trend of cefixime resistance by modelling both the fitness cost and the fitness benefit of resistant strains relative to susceptible strains. These 2 fitness properties had not been estimated before, and yet, we demonstrate that they are the key to making predictions about future resistance and incidence levels and therefore to making rational decisions about antibiotic usage policies. We deliberately kept our model as simple as possible, firstly to avoid the computational challenges that arise with a complex model and secondly to minimise the number of parameters for which the data analysed here would not be informative. However, it is important and interesting to consider the validity of our model assumptions and the effect they could have on our results.
Our model ignores the fact that gonorrhoea can infect different body sites, including in MSM the rectum, pharynx, or urethra, which results in different rates of onward transmission and probability of developing symptoms [41]. Reliable statistics are lacking for the relative prevalence, transmissibility, and pathogenicity of gonorrhoea by site of infection; consequently, this aspect would be difficult to add to our model. The parameters we estimate should be seen as averages for any infection site. Unless sexual practices were to change over time, we would expect the relative contributions to prevalence, symptomatic infections, asymptomatic infections, and transmission of each anatomical site to remain the same over time. We have no evidence that sexual practices did change over the time period considered; therefore, we expect that this averaging should have a minimal effect on the overall results. Likewise, we did not model heterogeneity in sexual behaviours or the underlying sexual network within the population, both of which can affect gonorrhoea spread within a population [63, 64], but these would be expected to impact both susceptible and resistant strains in the same fashion and therefore not affect our results on the dynamics of cefixime resistance. Indeed, a recent modelling study showed that differences of behaviour do not explain differences in resistance levels [65]. Our model assumes that treating a cefixime-resistant infection with cefixime results in treatment failure with probability ϕ, whereupon individuals become asymptomatic carriers. This is necessary to provide a significant benefit to the resistant strain, allowing it to spread when cefixime was used frequently. Some treatment failures would in fact have been detected and led to successful re-treatment, which we do not model explicitly but can be thought of as being part of the probability 1−ϕ of treatment success in spite of using cefixime for a resistant case. Control measures such as repeated testing of high-risk individuals or partner notifications are also not modelled explicitly but would likely affect the prevalence of both susceptible and resistant cases in a similar fashion and therefore not alter significantly our conclusions about the fitness differences caused by resistance. Furthermore, partner notification often occurs at low rates: only circa 10% of gonorrhoea that is diagnosed in England and reported through GUMCAD is found through partner notification, with the proportion likely to be lower in MSM than among heterosexuals [60].
It was assumed that all cefixime-susceptible infections were cured, regardless of which antibiotic was prescribed. The prescription data show that between 2008 and 2015 the vast majority of non-cefixime prescriptions were for ceftriaxone, either alone or in combination with azithromycin, so the assumption of cure is reasonable given that ceftriaxone resistance reports remain sporadic in England. The GRASP data we used in this study reflect a definition of cefixime resistance (MIC ≥ 0.125 mg/l) that is less stringent than the European Committee on Antimicrobial Susceptibility Testing (EUCAST) definition (MIC > 0.125 mg/l) and do not include information about concurrent prescription of azithromycin against any concomitant Chlamydia trachomatis infection [66], even though this is also an effective treatment against gonorrhoea [67]. Both these factors should contribute to a relatively low probability ϕ of treatment failure. Our estimate of ϕ was 83% with a wide 95% credible interval from 53% to 99%. A study on treatment failure in Toronto, Canada, estimated that only 25% (95% CrI 11%–45%) of patients with a cefixime MIC of >0.12 mg/l experienced treatment failure when treated with cefixime [25]. This figure is based on 7 out of 28 patients who returned for test of cure. A further 31 patients with a cefixime MIC of >0.12 mg/l detected at first treatment did not return for test of cure, resulting in the low sample size. The study reports that, initially, 13 patients with a cefixime MIC of >0.12mg/l failed the test of cure; however, 4 of these did not have an explicit denial of sexual re-exposure in their medical record. The study makes the strong assumption that all of these cases were in fact reinfections, which may have artificially reduced the treatment failure rate observed.
Our model implicitly assumes that there is no coinfection with both strains and no evolution of resistance happening within host. Ignoring within-host competition between resistant and susceptible strains following coinfection is justified here by the fact that both strains have low prevalence, making coinfection very unlikely. In a genomic study in the United States, only 2 clades of cefixime resistance were detected, suggesting that de novo acquisition of resistance is rare [3]. This simplification has been used in a number of other studies on the epidemiology of antimicrobial resistance [68, 69]. Within-host evolution of resistance was included in a recent gonorrhoea modelling study [65], but clearly, this is a rare event that only increases by 1 the number of resistant infections, which we estimated to be 49 at the start of the epidemic simulation on 1 January 2008.
Finally, our model also makes assumptions concerning the cost of cefixime resistance. The fitness cost of the mutation conferring resistance is assumed to be constant over time; however, compensatory mutations that reduce the initially high fitness cost of antibiotic resistance have been observed in other bacterial pathogens [70, 71]. It is clear from our analysis that there was a substantial fitness cost to cefixime resistance when the prescription protocol was changed in 2010, which is the reason why the resistance level subsequently fell. We cannot rule out that compensatory mutations took place after resistance initially emerged, but this would mean that the initial cost was even higher, and in these conditions, resistance would have been unlikely to emerge at all. Our formulation of the dynamics of the fitness cost of resistance was via a reduction in the duration of asymptomatic carriage. In the absence of evidence of the resistance mechanism, the fitness cost could plausibly be modelled through reduced transmissibility of the resistant strain [65]; we have confirmed with analysis that this would not affect our overall conclusions, in particular regarding the basic reproduction number analysis and predictions of the impact of cefixime usage on future resistance trends.
Implications for policy and research
The ceftriaxone-azithromycin dual therapy is currently effective, but it represents a last resort; thus, we urgently need a strategy for what would be done if it stopped working. It is likely to be just a matter of time before this happens, with the first reported failure of the dual therapy having occurred in 2015 [72]. Resistance to azithromycin was detected in a recent outbreak that started in the north of England [7] and is now reported in almost 10% of tested isolates [24]. Resistance to ceftriaxone remains rare, but MIC levels have been steadily increasing [73]. If alternative treatment options could be used, even for a minority of cases, then it would delay and maybe even prevent the emergence of resistance to the dual-therapy antibiotics by reducing the fitness benefit it would confer.
For some previously used antibiotics, such as penicillin or ciprofloxacin, significant levels of resistance remain in the gonococcal population (24% and 39% in 2015, respectively [24]) such that they cannot be recommended even for a small fraction of cases. These antibiotics could be prescribed only if drug sensitivity could be quickly established, for example, using real-time PCR assays [74, 75], or whole genome sequencing [76, 77], which both remain experimental. In contrast, the fact that resistance to cefixime has become very low in England (around 1% in 2015, [24]) makes it a prime candidate for return into action without the need for case-by-case susceptibility testing. Using 2 different methods—namely, a basic reproduction number analysis and posterior predictive simulations—we estimated that a quarter of gonorrhoea cases could be treated with cefixime. This model prediction is based on a randomised treatment strategy at the patient level whereby cefixime is allocated to a subset of uncomplicated cases for which resistance profiles are unknown. Combining this treatment strategy with point-of-care susceptibility testing could bring further benefits to avoid prescribing antibiotics for which resistance is detected. When coinfection with chlamydia is demonstrated or suspected, cefixime could be combined with azithromycin; this or other dual therapies could be useful more generally as well, and future modelling work should consider the evolutionary dynamics of multiple antibiotics concurrently. Perhaps the greatest threat posed by this proposed strategy would be the evolution of compensatory mutations that could reduce the fitness cost of cefixime resistance. As previously mentioned, compensatory mutations do not seem to have emerged during the 2007–2012 cefixime-resistant epidemic, but if they did occur, then the acceptable prescribing proportion would be lowered, and the probability of persistence of cefixime resistance increased. Therefore, a redeployment of cefixime would require the continuation, and perhaps reinforcement, of monitoring of resistance trends in England [8] and beyond [78, 79]. Ideally, surveillance systems would routinely record the prescription and treatment outcome for each individual case, as part of antimicrobial stewardship and monitoring of antibiotic resistance.
Conclusion
Fighting antibiotic-resistant gonorrhoea requires initially understanding the dynamics of how resistance emerges and spreads, in order to make informed decisions about treatment. Here we focused on an antibiotic, cefixime, which was previously used but had to be abandoned due to rising resistance levels. We estimated that resistance to cefixime comes at a significant cost for the pathogen; thus, when cefixime is not used, resistance tends to disappear. We also quantified the benefit of cefixime resistance for gonorrhoea, which is an increasing function of how often cefixime is used to treat gonorrhoea. Our results suggest that cefixime could be used again to treat a minority of gonorrhoea cases without risk of worsening the resistance problem.
Supporting information
(PDF)
(PDF)
Acknowledgments
We wish to thank David Eyre, John Paul, and Brian Spratt for stimulating discussions and constructive comments on an earlier version of this manuscript.
Disclaimer: The views expressed are those of the authors and not necessarily those of the Department of Health, MRC, NIHR, NHS, or Public Health England.
Abbreviations
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- GRASP
Gonococcal Resistance to Antimicrobials Surveillance Programme
- GUMCAD
Genitourinary Medicine Clinic Activity Dataset
- MIC
minimum inhibitory concentration
- MSM
men who have sex with men
- PHE
Public Heath England
- pMCMC
particle Monte Carlo Markov Chain
Data Availability
The number of cases of gonorrhea in MSM that were cefixime-resistant and reported by GRASP between 2008 and 2015 were extracted from the GRASP reports, which are available at https://www.gov.uk/government/publications/gonococcal-resistance-to-antimicrobials-surveillance-programme-grasp-report. The GUMCAD annual diagnosis figures are available at http://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables.
Funding Statement
This work was funded by the UK National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Modelling Methodology at Imperial College London in partnership with Public Health England (PHE) (grant HPRU-2012-10080), the MRC Centre for Outbreak Analysis and Modelling (grant MR/K010174/1), and the Medical Research Council (grant MR/N010760/1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Public Health England. Infection Report: HIV—STIs. Public Health England; 2015. https://www.gov.uk/government/publications/health-protection-report-volume-8-2014
- 2.Lewis DA. The Gonococcus fights back: is this time a knock out? Sexually Transmitted Infections. 2010;86(6):415–421. doi: 10.1136/sti.2010.042648 [DOI] [PubMed] [Google Scholar]
- 3.Grad YH, Kirkcaldy RD, Trees D, Dordel J, Harris SR, Goldstein E, et al. Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the USA: A retrospective observational study. The Lancet Infectious Diseases. 2014;14(3):220–226. doi: 10.1016/S1473-3099(13)70693-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bignell C, FitzGerald M. UK national guideline for the management of gonorrhoea in adults, 2011. International Journal of STD & AIDS. 2011;22(10):541–547. doi: 10.1258/ijsa.2011.011267 [DOI] [PubMed] [Google Scholar]
- 5.Bignell C, Unemo M. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. International Journal of STD & AIDS. 2013;24(2):85–92. doi: 10.1177/0956462412472837 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Gonococcal Infections—2015 STD Treatment Guidelines; 2015.
- 7.Chisholm SA, Wilson J, Alexander S, Tripodo F, Al-Shahib A, Schaefer U, et al. An outbreak of high-level azithromycin resistant Neisseria gonorrhoeae in England. Sexually Transmitted Infections. 2015; doi: 10.1136/sextrans-2015-052312 [DOI] [PubMed] [Google Scholar]
- 8.Ison CA, Town K, Obi C, Chisholm S, Hughes G, Livermore DM, et al. Decreased susceptibility to cephalosporins among gonococci: Data from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) in England and Wales, 2007–2011. The Lancet Infectious Diseases. 2013;13(9):762–768. doi: 10.1016/S1473-3099(13)70143-9 [DOI] [PubMed] [Google Scholar]
- 9.GRASP Steering Group. Annual Report 2000. London: Health Protection Agency; 2001. [Google Scholar]
- 10.GRASP Steering Group. The Gonococcal Resistance to Antimicrobials Surveillance Programme 2001. London: Health Protection Agency; 2002. [Google Scholar]
- 11.GRASP Steering Group. The Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) 2002. London: Health Protection Agency; 2003. [Google Scholar]
- 12.GRASP Steering Group. Annual Report Year 2003. London: Health Protection Agency; 2004. [Google Scholar]
- 13.GRASP Steering Group. The Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) Year 2004 report. London: Health Protection Agency; 2005. [Google Scholar]
- 14.GRASP Steering Group. The Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) 2005 report. London: Health Protection Agency; 2006. [Google Scholar]
- 15.GRASP Steering Group. Annual Report 2006. London: Health Protection Agency; 2007. [Google Scholar]
- 16.GRASP Steering Group. Annual Report 2007. London: Health Protection Agency; 2008. [Google Scholar]
- 17.GRASP Steering Group. The Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) Year 2008 report. London: Health Protection Agency; 2009. [Google Scholar]
- 18.GRASP Steering Group. Health Protection Report: Gonococcal Resistance to Antimicrobials Surveillance Programme in England and Wales (GRASP) report of 2009 data London: Health Protection Agency; 2010. 34. [Google Scholar]
- 19.GRASP Steering Group. GRASP 2010 Report. London: Health Protection Agency; 2011. [Google Scholar]
- 20.GRASP Steering Group. GRASP 2011 Report: The Gonococcal Resistance to Antimicrobials Surveillance Programme London: Health Protection Agency; 2012. [Google Scholar]
- 21.GRASP Steering Group. GRASP 2012 Report: The Gonococcal Resistance to Antimicrobials Surveillance Programme London: Public Health England; 2013. [Google Scholar]
- 22.GRASP Steering Group. GRASP 2013 Report: The Gonococcal Resistance to Antimicrobials Surveillance Programme (England and Wales); 2014.
- 23.GRASP Steering Group. The Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) 2014 report. London: Public Health England; 2015. [Google Scholar]
- 24.GRASP Steering Group. Surveillance of antimicrobial resistance in Neisseria gonorrhoeae Key findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP); 2016. October.
- 25.Allen VG, Mitterni L, Seah C, Rebbapragada A, Martin IE, Lee C, et al. Neisseria gonorrhoeae Treatment Failure and Susceptibility to Cefixime in Toronto, Canada. JAMA. 2013;309(2):163 doi: 10.1001/jama.2012.176575 [DOI] [PubMed] [Google Scholar]
- 26.Bignell C. National Guideline on the Diagnosis and Treatment of Gonorrhoea in Adults 2005; 2005. https://www.bashh.org/documents/116/116.pdf
- 27.World Health Organization. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. World Health Organization; 2012. [Google Scholar]
- 28.Ison C, Hussey J, Sankar K, Evans J, Alexander S. Gonorrhoea treatment failures to cefixime and azithromycin in England, 2010. Eurosurveillance. 2011;16(14):1–4. [PubMed] [Google Scholar]
- 29.Forsyth S, Penney P, Rooney G. Cefixime-resistant Neisseria gonorrhoeae in the UK: a time to reflect on practice and recommendations. International Journal of STD and AIDS. 2011;22(5):296–297. doi: 10.1258/ijsa.2009.009191 [DOI] [PubMed] [Google Scholar]
- 30.Grad YH, Goldstein E, Lipsitch M, White PJ. Improving Control of Antibiotic-Resistant Gonorrhea by Integrating Research Agendas Across Disciplines: Key Questions Arising From Mathematical Modeling. Journal of Infectious Diseases. 2016;213(6):883–890. doi: 10.1093/infdis/jiv517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bissessor M, Tabrizi SN, Fairley CK, Danielewski J, Whitton B, Bird S, et al. Differing Neisseria gonorrhoeae bacterial loads in the pharynx and rectum in men who have sex with men: Implications for gonococcal detection, transmission, and control. Journal of Clinical Microbiology. 2011;49(12):4304–4306. doi: 10.1128/JCM.05341-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kent CK, Chaw JK, Wonq W, Liska S, Gibson S, Hubbard G, et al. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clinical Infectious Diseases. 2005;41(1):67–74. doi: 10.1086/430704 [DOI] [PubMed] [Google Scholar]
- 33.Norris Turner A, Carr Reese P, Ervin M, Davis JA, Fields KS, Bazan JA. HIV, rectal chlamydia and rectal gonorrhea in men who have sex with men attending an STD clinic in a midwestern US city. Sexually Transmitted Diseases. 2013;40(6). doi: 10.1097/OLQ.0b013e31828fd163.HIV [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Didelot X, Dordel J, Whittles L, Collins C, Bilek N, Bishop C, et al. Genomic analysis and comparison of two gonorrhoea outbreaks. mBio. 2016; e00525–16. doi: 10.1128/mBio.00525-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savage EJ, Mohammed H, Leong G, Duffell S, Hughes G. Improving surveillance of sexually transmitted infections using mandatory electronic clinical reporting: the genitourinary medicine clinic activity dataset, England, 2009 to 2013. Eurosurveillance. 2014;19(48):20981 doi: 10.2807/1560-7917.ES2014.19.48.20981 [DOI] [PubMed] [Google Scholar]
- 36.Marcus U, Hickson F, Weatherburn P, Schmidt AJ. Estimating the size of the MSM populations for 38 European countries by calculating the survey-surveillance discrepancies (SSD) between self-reported new HIV diagnoses from the European MSM internet survey (EMIS) and surveillance-reported HIV diagnoses am. BMC Public Health. 2013;13(1):919 doi: 10.1186/1471-2458-13-919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercer CH, Tanton C, Prah P, Erens B, Sonnenberg P, Clifton S, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). The Lancet. 2013;382(9907):1781–1794. doi: 10.1016/S0140-6736(13)62035-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Office for National Statistics. United Kingdom population mid-year estimate; 2016. http://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/timeseries/ukpop/pop
- 39.Hosenfeld CB, Workowski Ka, Berman S, Zaidi A, Dyson J, Mosure D, et al. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sexually Transmitted Diseases. 2009;36(8):478–489. doi: 10.1097/OLQ.0b013e3181a2a933 [DOI] [PubMed] [Google Scholar]
- 40.Schmidt K, Schneider H, Lindstrom J, Boslego J, Warren R, Van de Verg L, et al. Homologous Gonococci in Male Volunteers. Sexually Transmitted Diseases. 2001; 28(10):555–564. [DOI] [PubMed] [Google Scholar]
- 41.Platt R, Rice P, McCormack W. Risk of acquiring gonorrhea and prevalence of abnormal adnexal findings among women recently exposed to gonorrhea. Journal of Acquired Immune Deficiency Syndromes. 1983;250:3205–3209. doi: 10.1001/jama.250.23.3205 [PubMed] [Google Scholar]
- 42.Johnson LF, Dorrington RE, Bradshaw D. The role of immunity in the epidemiology of gonorrhoea, chlamydial infection and trichomoniasis: insights from a mathematical model. Epidemiology and Infection. 2011;139(12):1875–1883. doi: 10.1017/S0950268811000045 [DOI] [PubMed] [Google Scholar]
- 43.Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic Gonorrhea in Men. New England Journal of Medicine. 1974;290(3):117–123. doi: 10.1056/NEJM197401172900301 [DOI] [PubMed] [Google Scholar]
- 44.Diekmann O, Heesterbeek JAP, Metz JAJ. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. Journal of Mathematical Biology. 1990;28(4):365–382. doi: 10.1007/BF00178324 [DOI] [PubMed] [Google Scholar]
- 45.Wetten S, Mohammed H, Yung M, Mercer CH, Cassell JA, Hughes G. Diagnosis and treatment of chlamydia and gonorrhoea in general practice in England 2000–2011: a population-based study using data from the UK Clinical Practice Research Datalink. BMJ Open. 2015; 5:e007776 doi: 10.1136/bmjopen-2015-007776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Moral P, Doucet A, Jasra A. Sequential Monte Carlo samplers. Journal of the Royal Statistical Society Series B: Statistical Methodology. 2006;68(3):411–436. doi: 10.1111/j.1467-9868.2006.00553.x [Google Scholar]
- 47.Andrieu C, Doucet A, Holenstein R. Particle Markov chain Monte Carlo methods. Journal of the Royal Statistical Society Series B-Statistical Methodology. 2010;72(3):269–342. doi: 10.1111/j.1467-9868.2009.00736.x [Google Scholar]
- 48.King AA, Nguyen D, Ionides EL. Statistical Inference for Partially Observed Markov Processes via the R Package pomp. Journal of Statistical Software. 2016;69(12):1–43. doi: 10.18637/jss.v069.i12 [Google Scholar]
- 49.Plummer M, Best N, Cowles K, Vines K. CODA: convergence diagnosis and output analysis for MCMC. R News. 2006;6(March):7–11. doi: 10.1159/000323281 [Google Scholar]
- 50.Gelman A, Rubin DB. Inference from Iterative Simulation Using Multiple Sequences. Statistical Science. 1992;7(4):457–472. [Google Scholar]
- 51.Brooks SP, Gelman AG. General methods for monitoring convergence of iterative simulations. Journal of Computational and Graphical Statistics. 1998;7(4):434–455. doi: 10.2307/1390675 [Google Scholar]
- 52.Yorke J, Hethcote H, Nold A. Dynamics and control of the transmission of gonorrhea. Sexually Transmitted Diseases. 1978;5(2):51–56. doi: 10.1097/00007435-197804000-00003 [DOI] [PubMed] [Google Scholar]
- 53.Garnett GP, Mertz KJ, Finelli L, Levine WC, St Louis ME. The transmission dynamics of gonorrhoea: modelling the reported behaviour of infected patients from Newark, New Jersey. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 1999;354(1384):787–97. doi: 10.1098/rstb.1999.0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White PJ, Ward H, Cassell JA, Mercer CH, Garnett GP. Vicious and virtuous circles in the dynamics of infectious disease and the provision of health care: gonorrhea in Britain as an example. The Journal of infectious diseases. 2005;192(5):824–836. doi: 10.1086/432004 [DOI] [PubMed] [Google Scholar]
- 55.Fairley CK, Chen MY, Bradshaw CS, Tabrizi SN. Is it time to move to nucleic acid amplification tests screening for pharyngeal and rectal gonorrhoea in men who have sex with men to improve gonorrhoea control? Sexual Health. 2011;8(1):9–11. doi: 10.1071/SH10134 [DOI] [PubMed] [Google Scholar]
- 56.Jin F, Prestage GP, Mao L, Kippax SC, Pell CM, Donovan B, et al. Incidence and risk factors for urethral and anal gonorrhoea and chlamydia in a cohort of HIV-negative homosexual men: the Health in Men Study. Sexually Transmitted Infections. 2007;83(2):113–119. doi: 10.1136/sti.2006.021915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harrison WO, Hooper RR, Wiesner PJ, Campbell AF, Karney WW, Reynolds GH, et al. A trial of minocycline given after exposure to prevent gonorrhea. New England Journal of Medicine. 1979;300(19):1074–1078. doi: 10.1056/NEJM197905103001903 [DOI] [PubMed] [Google Scholar]
- 58.McCutchan JA. Epidemiology of venereal urethritis: comparison of gonorrhea and nongonococcal urethritis. Reviews of Infectious Diseases. 1984;6(5):669–688. [DOI] [PubMed] [Google Scholar]
- 59.Korenromp EL, Sudaryo MK, de Vlas SJ, Gray RH, Sewankambo NK, Serwadda D, et al. What proportion of episodes of gonorrhoea and chlamydia becomes symptomatic? International journal of STD & AIDS. 2002;13(2):91–101. doi: 10.1258/0956462021924712 [DOI] [PubMed] [Google Scholar]
- 60.Public Health England. STI diagnoses & rates in England by gender, 2006–2015; 2015.
- 61.Gelman A, Meng XL, Stern H. Posterior predictive assessment of model fitness via realized discrepancies. Vol.6, No.4. Statistica Sinica. 1996;6(4):733–807. [Google Scholar]
- 62.Dietz K. The estimation of the basic reproduction number for infectious diseases. Statistical Methods in Medical Research. 1993;2(1):23–41. doi: 10.1177/096228029300200103 [DOI] [PubMed] [Google Scholar]
- 63.Garnett G, Anderson R. Contact Tracing and the Estimation of Sexual Mixing Patterns. Sexually Transmitted Diseases. 1993;20(4):181–191. doi: 10.1097/00007435-199307000-00001 [DOI] [PubMed] [Google Scholar]
- 64.Ghani A, Swinton J, Garnett G. The Role of Sexual Partnership Networks in the Epidemology of Gonorrhea. Sexually Transmitted Diseases. 1997;24(1):45–56. doi: 10.1097/00007435-199701000-00009 [DOI] [PubMed] [Google Scholar]
- 65.Fingerhuth SM, Bonhoeffer S, Low N, Althaus CL. Antibiotic-Resistant Neisseria gonorrhoeae Spread Faster with More Treatment, Not More Sexual Partners. PLoS Pathogens. 2016;12(5):e1005611 doi: 10.1371/journal.ppat.1005611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bignell C. 2009 European (IUSTI / WHO) Guideline on the Diagnosis and Treatment of Gonorrhoea in Adults. International Journal of STD & AIDS. 2009;20:453–457. doi: 10.1258/ijsa.2009.009160 [DOI] [PubMed] [Google Scholar]
- 67.Bignell C, Garley J. Azithromycin in the treatment of infection with Neisseria gonorrhoeae. Sexually Transmitted Infections. 2010;86:422–426. doi: 10.1136/sti.2010.044586 [DOI] [PubMed] [Google Scholar]
- 68.Lipsitch M, Bergstrom CT, Levin BR. The epidemiology of antibiotic resistance in hospitals: paradoxes and prescriptions. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(4):1938–1943. doi: 10.1073/pnas.97.4.1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kouyos RD, zur Wiesch PA, Bonhoeffer S. On being the right size: The impact of population size and stochastic effects on the evolution of drug resistance in hospitals and the community. PLoS Pathogens. 2011;7(4): e1001334 doi: 10.1371/journal.ppat.1001334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Melnyk AH, Wong A, Kassen R. The fitness costs of antibiotic resistance mutations. Evolutionary Applications. 2015;8(3):273–283. doi: 10.1111/eva.12196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Didelot X, Walker AS, Peto TE, Crook DW, Wilson DJ. Within-host evolution of bacterial pathogens. Nature Reviews Microbiology. 2016;14(3):150–162. doi: 10.1038/nrmicro.2015.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fifer H, Natarajan U, Jones L, Alexander S, Hughes G, Golparian D, et al. Failure of Dual Antimicrobial Therapy in Treatment of Gonorrhea. New England Journal of Medicine. 2016;374(25):2504–2506. doi: 10.1056/NEJMc1512757 [DOI] [PubMed] [Google Scholar]
- 73.Town K, Obi C, Quaye N, Chisholm S, Hughes G. Drifting towards ceftriaxone treatment failure in gonorrhoea: risk factor analysis of data from the Gonococcal Resistance to Antimicrobials Surveillance Programme in England and Wales. Sexually Transmitted Infections. 2017;93(1):39–45. doi: 10.1136/sextrans-2016-052583 [DOI] [PubMed] [Google Scholar]
- 74.Pond MJ, Hall CL, Miari VF, Cole M, Laing KG, Jagatia H, et al. Accurate detection of Neisseria gonorrhoeae ciprofloxacin susceptibility directly from genital and extragenital clinical samples: Towards genotype-guided antimicrobial therapy. Journal of Antimicrobial Chemotherapy. 2016;71(4):897–902. doi: 10.1093/jac/dkv432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buckley C, Trembizki E, Donovan B, Chen M, Freeman K, Guy R, et al. Real-time PCR detection of Neisseria gonorrhoeae susceptibility to penicillin. Journal of Antimicrobial Chemotherapy. 2016;71:3090–3095. doi: 10.1093/jac/dkw291 [DOI] [PubMed] [Google Scholar]
- 76.Grad YH, Harris SR, Kirkcaldy RD, Green AG, Marks DS, Bentley SD, et al. Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013. Journal of Infectious Diseases. 2016;214(10):1579–87. doi: 10.1093/infdis/jiw420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eyre DW, De Silva D, Cole K, Peters J, Cole MJ, Grad YH, et al. WGS to predict antibiotic MICs for Neisseria gonorrhoeae. Journal of Antimicrobial Chemotherapy. 2017; doi: 10.1093/jac/dkx067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kirkcaldy RD, Hook EW, Olusegun O, del Rio C, Kubin G, Zenilman JM, et al. Trends in Neisseria gonorrhoeae susceptibility to cephalosporins in the United States, 2006–2014. JAMA. 2015;314(17):1869–1871. doi: 10.1001/jama.2015.10347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kirkcaldy RD, Schlanger K, Papp JR, Torrone EA. Considerations for Strengthening Surveillance of Neisseria gonorrhoeae Antimicrobial Resistance and Interpreting Surveillance Data. Sexually Transmitted DIseases. 2017;44(3):154–156. doi: 10.1097/OLQ.0000000000000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
The number of cases of gonorrhea in MSM that were cefixime-resistant and reported by GRASP between 2008 and 2015 were extracted from the GRASP reports, which are available at https://www.gov.uk/government/publications/gonococcal-resistance-to-antimicrobials-surveillance-programme-grasp-report. The GUMCAD annual diagnosis figures are available at http://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables.