Abstract
Malaria causes about half a million deaths annually, with Plasmodium falciparum being responsible for 90% of all the cases. Recent reports on artemisinin resistance in Southeast Asia warrant urgent discovery of novel drugs for the treatment of malaria. However, most bioactive compounds fail to progress to treatments due to safety concerns. Drug repositioning offers an alternative strategy where drugs that have already been approved as safe for other diseases could be used to treat malaria. This study screened approved drugs for antimalarial activity using an in silico chemogenomics approach prior to in vitro verification. All the P. falciparum proteins sequences available in NCBI RefSeq were mined and used to perform a similarity search against DrugBank, TTD and STITCH databases to identify similar putative drug targets. Druggability indices of the potential P. falciparum drug targets were obtained from TDR targets database. Functional amino acid residues of the drug targets were determined using ConSurf server which was used to fine tune the similarity search. This study predicted 133 approved drugs that could target 34 P. falciparum proteins. A literature search done at PubMed and Google Scholar showed 105 out of the 133 drugs to have been previously tested against malaria, with most showing activity. For further validation, drug susceptibility assays using SYBR Green I method were done on a representative group of 10 predicted drugs, eight of which did show activity against P. falciparum 3D7 clone. Seven had IC50 values ranging from 1 μM to 50 μM. This study also suggests drug-target association and hence possible mechanisms of action of drugs that did show antiplasmodial activity. The study results validate the use of proteome-wide target similarity approach in identifying approved drugs with activity against P. falciparum and could be adapted for other pathogens.
Introduction
Malaria is an infectious disease with high morbidity and mortality. Approximately 3.3 billion people are at risk of getting malaria [1]. In 2015 alone, there were an estimated 212 million new cases of malaria worldwide with about 429,000 deaths reported [2]. Out of the total reported malaria cases and deaths, 90% of them occur in Africa, followed by the South-East Asia [1]. This disease burden is aggravated further by rapid development of resistance to antimalarial drugs. Reports of resistance to artemisinin-based combination therapy (ACT), the recommended first-line treatment for Plasmodium falciparum malaria [3–4] in Southeast Asia [5] warrants urgent discovery of new antimalarial drugs.
There are several drug discovery methods that have been used in malaria research [6]. Most approaches involve the use of either target-based or whole cell-based high throughput screens [7–11]. In target-based approaches, extracted proteins that are crucial for the parasite survival are assayed against huge compound libraries, a strategy that was used in the discovery of inhibitors of P. falciparum dihydroorotate dehydrogenase [12]. On the other hand, the whole cell-based approach involves exposing the P. falciparum parasite to test compounds to determine their inhibitory activities. Some antimalarial drugs have been modified from already existing drugs, these include synthetic ozonides which are based on artemisinins [13]. Modifications of drug compounds during drug development is done to either optimize their therapeutic activities, counteract the effect of resistance to the scaffold drug or mitigate the drug’s side effects. Many effective antimalarial drugs have been derived from traditionally used herbal medicines [6], this includes quinine which is extracted from the Cinchona trees and artemisinins are got from the Chinese herb Artemisia annua [14].
Use of Computer Aided Drug Discovery and Development (CADDD) to complement traditional approaches has greatly reduced cost, time and risks in chemotherapy research [15]. CADDD has successfully been used in the discovery of several drugs that have either been approved or are in clinical trials [16]. In silico tools that have been used in drug discovery and development can be broadly classified into bio-chemical databases, chemoinformatics and tools used in structure-based and ligand-based drug design [17].
The effectiveness of an antimalarial drug is dependent on its ability to target a protein or a biological pathway that is essential for the survival of the parasite in the blood stages. The shift of intervention strategies towards pre-elimination in some parts of the world has motivated targeting of other stages of the parasite [9,18–21]. The completion and annotation of the P. falciparum genome [22,23] revealed metabolic pathways that are essential in various stages of the parasite. For instance, heme biosynthesis is essential for P. falciparum in mosquito stage but not in asexual blood stages [24]. Drugs targeting this pathway are unlikely to provide successful antimalarial treatment but may be useful as transmission blockers. Similarly, type II fatty acid biosynthesis is essential for sporozoite development in the mosquito but not in the erythrocytic stages [25]. Tricarboxylic acid (TCA) cycle is nonessential in asexual parasites but has been shown to be indispensable in transmission stages of P. falciparum [26]. Pathways that are crucial in all stages of parasite development include phospholipid biosynthesis [27], coenzyme folate biosynthesis [28], glycolysis [29] and pentose phosphate pathway [30]. Several enzymes and other proteins classes involved in these pathways have been investigated as potential drug targets, with proteases [31–36] and kinases [37–42] being some of the most studied.
Development of new drugs to the point of their introduction into the market is expensive and time-consuming, costing about $100–800 million over a period of 12–15 years [43]. Moreover, most drugs that show activity against malaria fail to get approved due to safety concerns. Consequently, some drug discovery strategies have focused on drug repositioning which entails using already existing drugs for indications different from those they were approved for in order to circumvent approval challenges [44]. This approach has been widely explored in malaria chemotherapy research [45–50].
Through a target-similarity approach, this study sought to predict approved drugs that have undiscovered activity against P. falciparum and hence could be repositioned as antimalarials. This is based on the principle that a drug would have a similar effect on a protein that is similar to its putative target. Two previous studies have used a similar approach in repositioning of approved drugs against P. falciparum apicoplast [51] and Schistosoma mansoni [52]. In this study, each P. falciparum protein sequence in NCBI RefSeq database was used to search for similar to putative drug targets. Functional regions of the successful drugs targets were determined and used to fine tune the similarity search. This study identified approved drugs that have antimalarial activity and possible P. falciparum proteins they could be targeting.
Material and methods
Mining of P. falciparum proteome
A list of all proteins expressed in all stages of P. falciparum was obtained from NCBI Reference Sequence (RefSeq) database release 75 [53]. RefSeq database was preferred over GenBank because of the non-redundant nature of its sequences and the fact that it provides the best available sequence in GenBank (reference sequence) for a each protein. The search at NCBI was made by use of key words “Plasmodium falciparum” and selecting “Protein” database before initiating the search. To further filter the results, “Plasmodium falciparum” was selected in the organisms section and RefSeq as the source database. All the protein sequences were downloaded in a single multi-FASTA file. For easy manipulation, the downloaded sequences were converted into a CSV spreadsheet using R statistical programming software [54].
Identification of putative drug targets using drug databases
Using each P. falciparum protein sequence as a query, a search was done for similar putative drug targets on three publicly available databases; DrugBank [55], Therapeutic Target Database (TTD) [56] and STITCH 4.0 [57]. These databases have information on drugs, their putative targets and other drug-related information. Homologous proteins with output expectation values (E values) lower than 1e-20 [52] were considered for further analysis while the rest were excluded. Here, the E value describes the number of times one can expect to see a match by chance, thus the lower the E value the better. The putative drug targets that met the similarity threshold were retrieved with approved drugs that target them and keyed into a spreadsheet alongside their homologous P. falciparum proteins. For the STITCH database, drugs and other biomolecules that interact with the P. falciparum proteins are already predetermined. Therefore, a search was made in the STITCH database for each of the P. falciparum proteins, drugs that are predicted to interact with the proteins with a confidence score of at least 0.7 were considered for further analysis.
Determination of druggability index
The druggability indices for all the predicted P. falciparum target proteins was obtained from TDR Targets Database v5 [58,59]. Druggability index (D index) describes how druggable a protein is, that is how likely the protein is modulated with a small molecule drug [60]. Druggability indices range from 0 (least druggable) to 1.0 (most druggable). These scores reflect a number of factors such as how similar the protein is to a library of targets at ChEMBL database [61], whether the protein has physiochemical features of known drug targets and empirically determined interactions with drug like compounds. This step was important in resolving viable P. falciparum drug targets. If a drug is predicted to target a protein with low druggability but still exhibits high antimalarial activity then that could imply the drug inhibits another protein with high druggability.
Determination and comparison of functional amino acid residues
All the P. falciparum protein targets that met the inclusion criteria from the drug database search were then analyzed to determine if they share functional amino acid residues with their homologous putative drug targets. Amino acids residues that are conserved by evolution in a protein are believed to perform important structural and/or functional roles in the protein. The previous similarity search in drug databases weighted all amino acids equally while this step only checked for similarity in conserved amino acid residues. This is important in fine tuning the search because two proteins would be more likely to share ligands if they shared functional and structural regions. Before determining the functional residues, a protein-protein pairwise alignment using BLAST [62] was done at NCBI with the drug target as the query sequence and its corresponding P. falciparum homolog as the subject. Only proteins pairs that had more than 80% query coverage were considered for ConSurf server analysis. This step ensured that only proteins that are likely to share a significant number of residues proceeded to analysis using the ConSurf server.
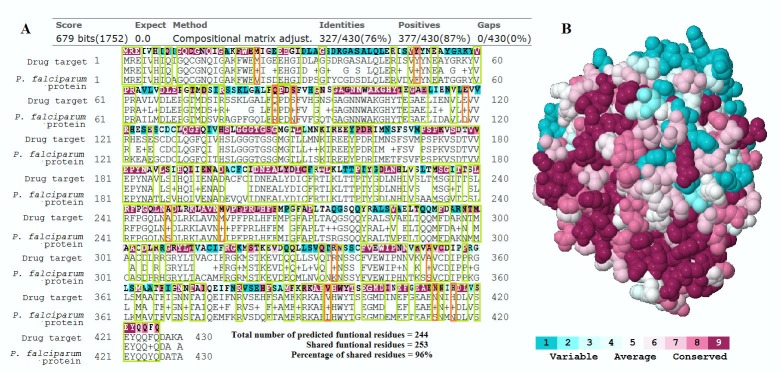
The ConSurf server [63] determines functional amino acid residues by estimating the degree of conservation of amino acids across 150 close sequence homologues obtained from UniProt database [64]. Evolutionary conservation of amino acid positions is estimated based on the phylogenetic relationship between the homologous sequences which is determined by neighbor joining approach with maximum likelihood distance. Conservation scores are calculated using the Bayesian method. The spatial orientation of the amino acids in the 3-D structures of the proteins are also considered in the ConSurf algorithms hence the requirement to have the protein sequence inputs in a PDB format. The 3-D structures were either obtained from Protein Data Bank in Europe [65] or modelled using SWISS-MODEL server [66] if they were not available in the PDB database. The Consurf server result outputs included multiple sequence alignment (MSA) with the amino acid residues color coded according to their conservation scores. The MSA was snipped using Windows® “snipping tool” and overlaid over the BLAST protein-protein pairwise alignment results as shown in Fig 1. This aided in visual comparison and determination of conserved amino acid residues that are shared with the corresponding homologous P. falciparum protein. Amino acid residues with conservation scores of at least six and are shared between the two proteins were counted and the percentage computed. These percentages were categorized according to a criteria adapted from a previous study [52]; high similarity (more than 80%), moderate similarity (50–79%) and low similarity (less than 50%). This was done for 26 protein pairs. Protein pairs with low similarity were excluded from further analysis.
Fig 1. Comparison of conserved amino acid residues.
(A) ConSurf server MSA results (color coded according to conservation scores) of the drug target, the human tubulin beta-1 (NCBI accession number NP_110400.1) is overlaid above its BLAST pair-wise alignment with its P. falciparum homolog (NCBI accession number XP_001347369.1). The percent of the shared conserved residues was then determined; (B) 3D molecular structure of the human tubulin beta-1 chain with residues color coded according to their conservations scores, this was part of the ConSurf server results.
Drug lead list
All approved drugs whose protein targets are similar to P. falciparum proteins were keyed alongside their respective proteins in a spreadsheet. Drugs that are applied topically, nutraceuticals and protein based drugs (e.g. insulin) were excluded from the drug lead list because they are less likely to be used as antimalarial drugs considering their physicochemical properties. Duplicate drug entries were also eliminated. A literature search was carried out at PubMed and Google Scholar to identify which drugs in the lead list had undergone in vitro testing for antimalarial activity. The literature search was done by searching the name of the drug with either “malaria”, “malaria in vitro testing” or “plasmodium falciparum”. Drugs that had been tested but their IC50 not documented were considered as not tested.
In vitro drug susceptibility assays
A representative group of 10 drugs from those predicted to have activity were selected for in vitro testing of antiplasmodial activity. Selection of the drugs was based on their approved uses; two antileukemic drugs (cladribine and clofarabine), three anticancer drugs (oxaliplatin, dasatinib and irinotecan), an antibiotic (levofloxacin), one antidipsotropic (daidzin), an antiasmatic (zafirlukast), an immunosuppressive (tacrolimus) and one used to treat erectile dysfunction (tadafil). Seven of the drugs tested had no documentation of prior in vitro testing and three did. Details of their uses, putative drug targets, predicted P. falciparum drug targets (with druggability indices) and percentage of shared conserved regions between the two proteins are shown in Table 1. Chloroquine, dihydroartemisinin and mefloquine were tested (as reference standards) alongside the candidate drugs. All candidate drugs were bought from Sigma-Aldrich, while reference drugs were provided by World Wide Antimalarial Resistance Network (WWARN) Reference Standards Programme. The P. falciparum 3D7 parasites were obtained from Kenya Medical Research Institute-Walter Reed Project (KEMRI-WRP), Kisumu.
Table 1. Approved uses, putative targets, predicted P. falciparum targets, druggability indices and percentage of shared conserved residues of candidate drugs tested for in vitro antiplasmodial activity.
| Drug | Indication (approved use) | Putative target (UNIPROT ID) |
P. falciparum target (NCBI acc. No.) |
% of shared conserved residues | Druggability index |
|---|---|---|---|---|---|
| Cladribine | Hairy cell leukemia | Adenosine deaminase (P00813) | Adenosine deaminase (XP_001347573.1) | 55% | 1 |
| Daidzin | Anti-dipsotropic | ATP-binding cassette sub-family G member 2 (Q9UNQ0) | ABC transporter (XP_001348418.1) | 51% | 0.5 |
| Zafirlukast | Asthma | ATP-binding cassette sub-family G member 2 (Q9UNQ0) | ABC transporter (XP_001348418.1) | 51% | 0.5 |
| Levofloxacin | Antibacterial | DNA topoisomerase 2-alpha (P11388) | DNA topoisomerase II (XP_001348490.1) | 61% | 0.8 |
| Dasatinib | Anticancer | ATP-binding cassette sub-family G member 2 (Q9UNQ0) | ABC transporter (XP_001348418.1) | 51% | 0.5 |
| Clofarabine | Antileukemia | ATP-binding cassette sub-family G member 2 (Q9UNQ0) | ABC transporter (XP_001348418.1) | 51% | 0.5 |
| Tacrolimus | Organ transplant | NA* | FK506-binding protein (FKBP)-type peptidyl-propyl isomerase (XP_001350859.1) |
NA* | 0.6 |
| Irinotecan | Colorectal cancer | ATP-binding cassette sub-family G member 2 (Q9UNQ0) | ABC transporter (XP_001348418.1) | 51% | 0.5 |
| Oxaliplatin | Colorectal cancer | ATP-binding cassette sub-family G member 2 (Q9UNQ0) | ABC transporter (XP_001348418.1) | 51% | 0.5 |
| Tadafil | Erectile dysfunction | CGMP-specific 3',5'-cyclic phosphodiesterase (O76074) | 3',5'-cyclic nucleotide phosphodiesterase (XP_001349954.1) | > 5% | - |
*Predicted P. falciparum protein targets obtained from STITCH database (e.g. FK506-binding protein) did not have corresponding putative targets for comparison hence were not analyzed by the ConSurf server
The drugs were assayed using a non-radioisotopic assay technique described by Smilkstein and co-workers [8] with modifications [67, 68]. Reference clone chloroquine-sensitive (3D7) were cultured as described by Johnson and colleagues [67]. Drugs and compounds were dissolved in 99.5% dimethylsulfoxide (DMSO) (Sigma-Aldrich) and diluted in complete Roswell Park Memorial Institute 1640 series of Cell Culture Medium (RPMI 1640) prepared as described by Akala et al. [69]. The basic culture medium was prepared from 10.4 g RPMI 1640 powder (Invitrogen, Inc.) augmented with 2 g glucose (Sigma Inc.) and 5.95 g of HEPES (Sigma Inc.) dissolved to homogeneity in one litre of de-ionized water and sterilized with a 0.2 μM filter. Complete RPMI 1640 media (used for all parasite cultures and drug dilutions) consisted of basic RPMI 1640 media with 10% (vol/vol), human ABO pooled plasma, 3.2% (vol/vol) sodium bicarbonate (Thermo Fisher Scientific Inc.) and 4.0 μg/ml hypoxanthine (Sigma Inc.). Complete RPMI 1640 media was stored at 4°C and used within two weeks. Concurrently, two-fold serial dilutions of chloroquine (0.977 to 2,000 ng/ml), mefloquine (0.244 to 500 ng/ml), dihydroartemisinin (0.098 to 200 ng/ml) and drug candidates (24.414 to 50,000 ng/ml) were prepared on a 96-well plate, such that the amount of DMSO was equal to or less than 0.0875%. The 12 doses for each drug were added to wells in a row across the 96-well drug plate. In vitro drug testing was initiated when the culture-adapted P. falciparum at 5% hematocrit with greater than 3% parasitemia were adjusted to 2% hematocrit and 0.5% parasitemia, then added on to the plate containing a dose range of drugs and incubated in gas mixture comprising 5% CO2, 5% O2, and 90% N2 at 37°C. Each drug was tested in three biological replicates. The assay was terminated after 72 hours with SYBR Green dye added in lysis buffer and kept in the dark for 24 hours as described by Cheruiyot et al. [68]. The fluorescence intensity was measured from the bottom of the plate with a GENios Plus plate reader, with excitation wavelengths of 485 nm, emission wavelengths of 535 nm, gain set at 60 and number of flashes set at 10. Parasite growth inhibition was quantified using GraphPad Prism software version 5.02 from GraphPad Software Inc. CA, USA as described by Johnson et al. [67] and presented as mean ± standard deviation.
Results
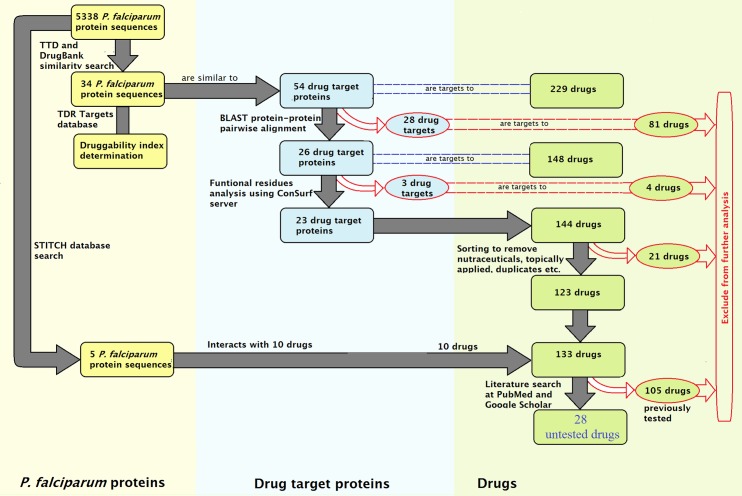
A summary of results for each step in the study is shown in Fig 2 with more details in consecutive sections.
Fig 2. Steps in the chemogenomics repositioning workflow and their corresponding results.
The yellow boxes represent P. falciparum sequences, drug targets are shown in blue boxes and drugs in green. Excluded drugs and proteins target have red box outlines.
P. falciparum proteome
A total of 5,338 protein sequences were obtained from NCBI RefSeq database [53]. This number represents all the P. falciparum protein sequences in RefSeq release 75.
Identification of putative drug targets using drug databases
Each of the 5,338 protein sequences was used to search DrugBank, TTD and STITCH 4.0 databases. Using an E value cutoff of 1e-20 for DrugBank and TTD, 54 approved drug targets were identified to be similar to 34 P. falciparum possible targets. The 54 drug targets were associated with 229 approved drugs, the full list is shown in S1 Table. Using a minimum confidence score 0.7 for STITCH 4.0, 10 drugs were predicted to interact with 5 P. falciparum proteins. It is worth noting that some query results were similar in many of the searches while drugs that had multiple targets appeared more than once in the results.
Druggability index
Druggability indices of 34 predicted P. falciparum drug targets obtained from TDR database are shown in Table 2. The least druggable drug target in the study had a druggability index of 0.3 while five had an index of 1. Eight proteins did not have their druggability indices in TDR database.
Table 2. Druggability indices of predicted P. falciparum drug targets.
| P. Falciparum protein name | NCBI accession number | Druggability index |
|---|---|---|
| INOSINE-5'-MONOPHOSPHATE DEHYDROGENASE | XP_001352079.1 | 1 |
| TUBULIN BETA CHAIN | XP_001347369.1 | 1 |
| ADENOSINE DEAMINASE | XP_001347573.1 | 1 |
| ADP/ATP TRANSPORTER ON ADENYLATE TRANSLOCASE | XP_001347650.1 | 1 |
| RIBONUCLEOTIDE REDUCTASE SMALL SUBUNIT | XP_001348226.1 | 1 |
| MO15-RELATED PROTEIN KINASE | XP_001347426.1 | 0.9 |
| DNA TOPOISOMERASE II | XP_001348490.1 | 0.8 |
| HISTONE DEACETYLASE | XP_001352127.1 | 0.8 |
| RIBONUCLEOTIDE REDUCTASE SMALL SUBUNIT | XP_001347439.2 | 0.8 |
| HISTONE DEACETYLASE | XP_001347363.1 | 0.7 |
| CGMP-DEPENDENT PROTEIN KINASE | XP_001348520.1 | 0.7 |
| M1-FAMILY ALANYL AMINOPEPTIDASE | XP_001349846.1 | 0.6 |
| FKBP TYPE PEPTIDYL-PROPYL ISOMERASE | XP_001350859.1 | 0.6 |
| SERINE/THREONINE PROTEIN PHOSPHATASE | XP_001348315.1 | 0.6 |
| PREPROCATHEPSIN C PRECURSOR | XP_001350862.2 | 0.6 |
| ACETYL-COA ACETYLTRANSFERASE | XP_001348658.1 | 0.6 |
| CYCLIC NUCLEOTIDE PHOSPHODIESTERASE | XP_001348846.2 | 0.5 |
| ABC TRANSPORTER | XP_001348418.1 | 0.5 |
| CALCIUM/CALMODULIN-DEPENDENT PROTEIN KINASE | XP_001348401.2 | 0.5 |
| GUANYLYL CYCLASE | XP_001348065.1 | 0.5 |
| TRANSPORTER | XP_001349605.2 | 0.5 |
| STROMAL-PROCESSING PEPTIDASE | XP_001348556.2 | 0.5 |
| ACYL COA:DIACYLGLYCEROL ACYLTRANSFERASE | XP_001351293.1 | 0.4 |
| GUANYLYL CYCLASE BETA | XP_001350316.2 | 0.4 |
| CYSTEINE PROTEINASE FALCIPAIN-1 | XP_001348727.1 | 0.3 |
| FLAVODOXIN-LIKE PROTEIN | XP_002808949.1 | 0.3 |
| CENTRIN-3 | XP_001347555.2 | Not available |
| CGMP-SPECIFIC PHOSPHODIESTERASE | XP_001350504.2 | Not available |
| DELTA-AMINOLEVULINIC ACID DEHYDRATASE | XP_001348555.1 | Not available |
| ORNITHINE AMINOTRANSFERASE | XP_966078.1 | Not available |
| RNA BINDING PROTEIN | XP_001347313.1 | Not available |
| 3',5'-CYCLIC NUCLEOTIDE PHOSPHODIESTERASE | XP_001349954.1 | Not available |
| HEAT SHOCK PROTEIN 110 | XP_001349002.1 | Not available |
| FERROCHELATASE | XP_001350360.2 | Not available |
Determination and comparison of functional regions
A protein-protein pairwise alignment performed between the P. falciparum proteins and their corresponding homologous drug targets revealed 26 out of 54 protein pairs had at least 80% query coverage. The 26 were selected for ConSurf server analysis while the rest were excluded. Comparison of the functional amino acids residues revealed eight protein pairs had high similarity (more than 80%), 11 had a moderate similarity (50–79%) and three low similarity (less than 50%). Those with low similarity were excluded from further analysis.
Drug lead list
The successful 23 putative target proteins were associated with 144 drugs in DrugBank and TTD databases while 5 P. falciparum proteins were predicted to interact with 10 drugs in the STITCH database. This made a total of 154 drugs that were predicted to have antimalarial activity in this study. Out of the 154 drugs, 21 drugs are either applied topically, duplicates, protein based or pure elements, these were eliminated from the drug lead list. A literature search revealed 105 drugs out of the remaining 133 to have been previously tested for antimalarial activity. The IC50 of some of the tested drugs are shown in Table 3 while those that had not been tested previously are summarized in Table 4.
Table 3. IC50 values of some previously tested approved drugs that were predicted to have antiplasmodial activity.
| Predicted P. falciparum target, its druggability index and % of shared conserved residues | Drug | Indication | Antimalarial activity (IC50*) | References |
|---|---|---|---|---|
|
Predicted P. falciparum target: ABC transporter (XP_001348418.1) Putative target: ABC sub-family G member 2 (Q9UNQ0) Druggability index: 0.5 % of shared conserved residues: 51% |
Dactinomycin | Antibiotic | 0.0009 μM | [48] |
| Cisplatin | Anticancer | 0.021 μM | [70] | |
| Cyclosporine | Immuno-suppressant | 0.032 μM | [71] | |
| Docetaxel | Anticancer | 0.01 μM | [72] | |
| Doxorubicin | Antibiotic | 0.21 μM | [48] | |
| Ivermectin | Antiparasitic | 9.1 μM | [73] | |
| Lamivudine | Antiretroviral | > 50 μM | [74] | |
| Saquinavir | Antiretroviral | 5 μM | [74] | |
| Vincristine | Anticancer | 0.0021 μM | [75] | |
|
Predicted P. falciparum target: DNA Topoisomerase II (XP_001348490.1) Putative target: DNA topoisomerase 2-alpha (P11388) Druggability index: 0.8 % of shared conserved residues: 61% |
Amsacrine | Cutaneous T Cell Lymphoma | 0.1 to 2.8 μM | [76] |
| Ciprofloxacin | Antibiotic | 20 μM | [77] | |
| Enoxacin | Antibiotic | 120 μM | [75] | |
| Fleroxacin | Antibiotic | 94 μM | [75] | |
| Lovastatin | Hypolipidemic | >200 μM | [78] | |
| Norfloxacin | Antibiotic | 55 μM | [75] | |
| Ofloxacin | Antibiotic | 180 μM | [75] | |
| Sparfloxacin | Antibiotic | 140 μM | [75] | |
| Trovafloxacin | Antibiotic | 27 μM | [79] | |
| Dactinomycin | Antibiotic | 0.0009 μM | [48] | |
|
Predicted P. falciparum target: Histone deacetylase (XP_001347363.1) Putative target: Histone deacetylase (Q13547) Druggability index: 0.8 % of shared conserved residues: 99% |
Trichostatin A | Antifungal, Antibiotic | — | [80] |
| Valproic Acid | Epilepsy And Seizures Treatment | 210 μM | [75] | |
| Vorinostat | Cutaneous T Cell Lymphoma | 0.12 μM | [49] | |
|
Predicted P. falciparum target: IMP dehydrogenase (XP_001352079.1) Putative target: IMP dehydrogenase 1 (P20839.2) Druggability index: 1 % of shared conserved residues NA |
Azathioprine | Immunosuppressant | ≥ 1 μM | [81] |
|
Predicted P. falciparum target: IMP dehydrogenase (XP_001352079.1) Putative target: IMP dehydrogenase 2 (P12268) Druggability index: 1 % of shared conserved residues: 79% |
Mycophenolic Acid | Immunosuppressant | 5.4 μM | [82] |
|
Predicted P. falciparum target: Serine/threonine protein phosphatase (XP_001348315.1) Putative target: Serine/threonine-protein phosphatase PP1-alpha catalytic subunit (P08129) Druggability index: 0.6 % of shared conserved residues: 99% |
Cantharidin | Warts | 3 μM | [83] |
|
Predicted P. falciparum target: Tubulin beta chain (XP_001347369.1) Putative target: Tubulin beta-4B chain (P68371) Druggability index: 1 % of shared conserved residues: 99% |
Albendazole | Anthelmintic | 2 μM | [48] |
| Vinblastine | Anticancer | 0.0072 μM | [75] | |
| Vindesine | Anticancer | 0.006 μM | [75] | |
| Vincristine | Anticancer | 0.0021 μM | [75] | |
|
Predicted P. falciparum target: Cyclic nucleotide phosphodiesterase (XP_001348846.2) Putative target: cAMP-specific 3',5'-cyclic phosphodiesterase 4A (P27815) Druggability index: 0.5 |
Dipyridamole | Anticoagulants | 0.03 μM | [84] |
|
Predicted P. falciparum target: Adenosine deaminase (XP_001347573.1) Putative target: Adenosine deaminase (P00813) Druggability index: 1 % of shared conserved residues: 55% |
Dipyridamole | Anticoagulants | 0.03 μM | [84] |
|
Predicted P. falciparum target: Centrin-3 (XP_001347555.2) Putative target: Calmodulin (P62158) Druggability index: NA % of shared conserved residues: 76% |
Trifluoperazine | Antipsychotic, Antiemetic | 0.47 μM | [85] |
|
Predicted P. falciparum target: Calcium/calmodulin-dependent protein kinase (XP_001348401.2) Putative target: CaM kinase II subunit gamma (Q13555) Druggability index: 0.5 % of shared conserved residues: 47% |
Bosutinib | Chronic Myelogenous Leukemia (CML) | 0.22 μM | [48] |
*All IC50 values are converted to μM
Table 4. Details of approved drugs predicted to target P. falciparum proteins that have not been tested.
| Drug | UNIPROT ID of putative target | NCBI ACCESSION NUMBER OF P. FALCIPARUM TARGET | CONSURF RESULTS | DRUGGABILITY OF P. FALCIPARUM TARGET |
|---|---|---|---|---|
| Cladribine | P00813 | XP_001347573.1 | 55% | 1 |
| Fludarabine | P00813 | XP_001347573.1 | 55% | 1 |
| Epirubicin | P11388 | XP_001348490.1 | 61% | 0.8 |
| Finafloxacin | P11388 | XP_001348490.1 | 61% | 0.8 |
| Palbociclib | P11802 | XP_001347426.1 | 54% | 0.9 |
| Capridine-beta | P24941 | XP_001347426.1 | 70% | 0.9 |
| Motexafin gadolinium | P31350 | XP_001347439.2 | 60% | 0.8 |
| Aprindine | P62158 | XP_001347555.2 | 76% | - |
| Venlafaxine | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Oxaliplatin | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Zafirlukast | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Clofarabine | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Sumatriptan | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Irinotecan | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Buprenorphine | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Idelalisib | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Cobicistat | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Lenvatinib | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Daclatasvir | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Osimertinib | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Pitavastatin | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Rilpivirine | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Apixaban | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Vandetanib | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Biricodar dicitrate | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Daidzin | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Cabazitaxel | Q9UNQ0 | XP_001348418.1 | 51% | 0.5 |
| Tacrolimus | STITCH | XP_001350859.1 | - | 0.6 |
In vitro drug susceptibility assays
In vitro drug susceptibility tests carried out on ten drugs showed eight with activity within the concentration ranges used. Their mean IC50 are shown in Table 5 while the three biological replicate readings are displayed in S3 Table.
Table 5. In vitro activities of drugs tested against P. falciparum 3D7 strain.
| Drugs | Mean IC50 ± SD (μM) |
|---|---|
| Tadafil | 23.29 ± 2.41 |
| Irinotecan | 14.35 ± 1.70 |
| Levofloxacin | 40.10 ± 5.28 |
| Oxaliplatin | 1.16 ± 0.10 |
| Clofarabine | 48.95 ± 2.03 |
| Tacrolimus | 4.52 ± 0.08 |
| Cladribine | 96.02 ± 16.43 |
| Dasatinib | 8.60 ± 2.22 |
| Reference drugs | |
| Chloroquine | 0.0116 ± 0.0004 |
| Dihydroartemisinin | 0.0026 ± 0.0001 |
| Mefloquine | 0.0395 ± 0.0063 |
The table shows the mean IC50 values and the standard deviation for the drugs in μM as tested in this study. Each drug was tested in three replicates.
Discussion
This study was based on the principle that if a P. falciparum protein is similar to a confirmed drug target, by inference the drug in question would have a similar effect on the P. falciparum protein. Using the full proteome of P. falciparum to do a target-similarity search in drug databases, the study predicted 133 approved drugs could target 34 P. falciparum proteins. A literature search showed 105 of the 133 drugs to have been previously tested against P. falciparum, showing a strong research interest in repositioning approved drugs. Most of the drugs that were previously tested did show activity, validating the use of this approach in drug repositioning. In vitro drug susceptibility tests were done on 10 drugs that were predicted to have antiplasmodial activity. Seven drugs out of the 10 tested did show significant activity with IC50 ranging from 1 μM to 50 μM, these include levofloxacin, dasatinib, clofarabine, tacrolimus, irinotecan, oxaliplatin and tadafil. The drugs that did show activity should be considered for further evaluation and development. The drug-target associations predicted in this study could also explain possible mechanisms of action of drugs that were active, this information could be used to develop more potent antimalarial drugs.
Target-similarity search
This study was based on the assumption that two proteins that similar are by inference likely to share ligands. However, it is important to note that a drug could target a protein/pathway other than the one it is predicted to inhibit. These “off targets” are not uncommon since many drugs have been documented to have multiple targets. In addition, high sequence similarity between two proteins does not necessarily mean they would have similar biological roles. Homologs could have different biological functions but still meet the similarity thresholds used in this study. Nevertheless, the similarity of prospective protein targets to known drug targets has been used as the basis for past repositioning attempts [51,52,86]. This could also explain why most of the drugs predicted to have antimalarial activity in this study are already tested (Table 3).
The whole proteome of P. falciparum was used to perform the similarity search, therefore drugs that could target all stages of the parasite’s life cycle were considered in this study. Most drug development efforts focus on erythrocytic stages because they cause symptoms of the disease and are easier to manipulate in the laboratory. In fact, current antimalarial drugs were discovered on the basis of their activity against the red blood cell stage parasite. Developing drugs targeting the exo-erythrocytic and sporogonic cycles are increasingly drawing interest [9,18–20] because all stages of the parasite need to be targeted if malaria is to be eliminated [21]. The effect of the current antimalarials on all the life cycle stages of Plasmodium has also been studied [87].
The sequence similarity search in both TTD and DrugBank databases found duplication of some results with several P. falciparum proteins picking similar targets. For instance, many of the drug target searches yielded same kinases with low E values. It is interesting to note that kinases are one of the most common classes of proteins investigated as drugs targets in P. falciparum [37–42]. The similarity in the BLAST output could be attributed to the paralogous nature of many P. falciparum proteins and their orthology to many putative drug targets. Among the drug targets that showed up frequently in the output results, Plasmodium merozoite surface protein 1 (PMSP1) was the most common. Being a Plasmodium protein itself, it is expected to be similar to many paralogous P. falciparum proteins we used as queries. Notwithstanding, the similarity of PMSP1 protein to several of P. falciparum proteins is worth investigating further. It is also worth noting that PMSP1 antigen is not a target of any approved or experimental drug, rather it is being investigated for vaccines in clinical trials [88]. Other examples of protein targets that showed up frequently include troponin C, heat shock protein 40, calmodulin, centromeric protein E and Rho-associated protein kinase 1.
ATP-binding cassette transporters
The P. falciparum ATP-binding cassette (ABC) transporter (NCBI accession number Q9UNQ0) was predicted in this study to be a potential target to five drugs that were tested in vitro; dasatinib, clofarabine, irinotecan, daidzin and zafirlukast. The putative target of these drugs is the human ABC sub-family G member 2 (NCBI accession number Q9UNQ0), also known as breast cancer resistance protein (BRCP) and multidrug resistant protein 1 (MRP1). The BRCP is classified among multidrug resistant proteins (MRPs) because of its role in drug resistance and treatment failures in trypanosomatid, apicomplexan and amitochondriate parasites of clinical significance [89,90]. It is believed to cause treatment failures by actively translocating a wide range of structurally and functionally diverse amphipathic compounds across cellular membranes [91]. The ABC transporters have been implicated with high IC50 values in response to chloroquine and quinine in P. falciparum field isolates [92]. Members the MRP family of proteins should also be considered as potential targets for antimalarial drugs because of the vital role they have been shown to play in blood stage multiplication of the Plasmodium species [93]. ABC transporters also have been considered as targets for antibacterial vaccines and chemotherapies because of the part they play in transporting molecules across membranes [94]. The P. falciparum ABC transporter has moderate druggability (D index of 0.5). It also shares 51% of conserved amino acid residues and an E value of 2e-61 when aligned with the human homolog drug target (BRCP) using protein-protein BLAST, suggesting a strong similarity.
Dasatinib has been approved for treatment of chronic myelogenous leukemia (CML) and is currently being evaluated for use in treating other cancers [95–97]. It is widely documented that dasatinib acts by inhibiting a range of tyrosine-protein kinases [98–102], these include Bcr-Abl, Lck and Src family of tyrosine kinases. Dasatinib also has other targets including the human MRP 1 [103,104]. Previous in vitro drug susceptibility assays on P. falciparum have shown dasatinib to have an IC50 of >10 μM [105] compared to 8.599 ± 2.222 μM determined in this study. Other drugs that were tested that could target the P. falciparum ABC transporter are antileukemia clofarabine (48.95 ± 2.032μM) and anticancer drug irinotecan (14.35 ± 1.7 μM). Daidzin (an anti-dipsotropic) and zafirlukast (an antiasthmatic) did not show any activity at the concentration ranges used.
Adenosine deaminase
The target similarity approach used by this study also predicted P. falciparum adenosine deaminase, ADA (XP_001347573.1) to be a target of cladribine, dipyridamole, fludarabine and pentostatin. (S1 Table). This protein has an E value of 2e-29 when aligned with its homologous drug target (P00813.3) and they share 55% of conserved residues. Additionally, the high druggability of P. falciparum ADA (D index of 1) makes it a strong drug target candidate. P. falciparum ADA is essential to the survival of the parasite since the parasite is unable to synthesize purine bases and hence relies on purine salvage and purine recycling to meet its purine needs. P. falciparum ADA is unique because it catalyzes the deamination of both adenosine and 5‘-methylthioadenosine while the human form cannot deaminate the latter [106]. Sriram et al. [107] used a bioinformatics approach to show how quinine and primaquine could bind to the ADA protein. 5‘-methylthio coformycins have also been shown to inhibit the P. falciparum ADA without inhibition of its human homolog [106]. Examples of 5‘-methylthio coformycins that have been tested against 3D7 clone of P. falciparum include 5'-Methylthio-immucillin-H (MT-ImmH) and immucillin-H (ImmH) which have IC50 values of 63 nM and 50 nM respectively [108]. This is comparable to dipyridamole’s IC50 of 30 nM [84] which is also predicted by this study to target the P. falciparum ADA. In vitro tests carried out in this study also showed cladribine to have an IC50 of 96.02 μM which is much higher than that of MT-ImmH, ImmH and dipyridamole. This could probably be attributed cladribine having weaker inhibition of ADA (assuming it is the only target) or other experimental factors. Nevertheless, an antimalarial that could effectively block the parasite’s purine salvage pathway would be efficient in inhibiting the parasite’s growth.
DNA topoisomerases
Levofloxacin, like most broad-spectrum fluoroquinolones, acts by inhibiting two type II DNA topoisomerase enzymes in bacteria; DNA gyrase and topoisomerase IV [109,110]. Levofloxacin was predicted in this study to inhibit the activity of P. falciparum’s DNA topoisomerase II (XP_001348490.1). The P. falciparum DNA topoisomerase II has an E value of 0.0 when aligned to two distinct homologous drug targets; DNA topoisomerase 2-alpha (P11388) and DNA topoisomerase 2-beta (Q02880) suggesting a high similarity. Besides, the P. falciparum DNA topoisomerase II has a D index of 0.8 and shares 61% functional amino acid residues with the drug target DNA topoisomerase 2-alpha. DNA topoisomerases enzymes are involved in overwinding or underwinding of DNA during DNA replication and transcription, hence are considered essential to the survival of many organisms including P. falciparum. Several types of DNA topoisomerases have been characterized and are classified into two major classes depending on how they change the topology of DNA: topoisomerase I and topoisomerase II [111]. Garcia-Estrada et al. [112] proposed DNA topoisomerases as attractive drug targets because of structural differences between host and apicomplexan isoforms, differential expression patterns as well as lack of orthologous topoisomerases in mammals since there are no apicoplast DNA gyrases in mammals. Levofloxacin showed activity against P. falciparum 3D7 with an IC50 of 14.17 μg/ml in this study. A total of 30 approved drugs were predicted to target the P. falciparum DNA topoisomerase II (S1 Table). Examples include moxifloxacin, ciprofloxacin, lucanthone and epirubicin. Camptothecin, a potent DNA topoisomerase I inhibitor has been shown to inhibit nucleic acid biosynthesis in P. falciparum suggesting that it could also be targeting the Plasmodium homolog [113]. Nevertheless, camptothecin was predicted in this study to inhibit the Plasmodium’s ABC transporter (S1 Table) because of its similarity to the MRP1 [114]. Unfortunately, camptothecin cannot be used as an antimalarial considering its toxicity.
Histone deacytalase
P. falciparum histone deacytalase, HDAC (XP_001352127.1) shares 99% of functional amino acid residues with drug target histone deacetylase 2 (Q92769) and 85% with histone deacetylase 1 (Q13547). The Plasmodium homologue has an E value 0.0 when aligned with both the histone deacetylase proteins, suggesting a strong similarity. Therefore, the Plasmodium HDAC could be targeted by drugs such as vorinostat (recommended treatment for T cell lymphoma), valproic acid (used for epilepsy treatment) and trichostatin A (an antifungal and antibacterial). All the three drugs have been shown to target the histone deacetylase 2 (Q92769). Plasmodium HDAC has high druggability (a D index of 0.8), making it an attractive antimalarial drug target. A recent study assessed the role of HDAC inhibitors in impeding the growth of P. falciparum both in vivo and in vitro [115]. Vorinostat has displayed high in vitro antimalarial activity with an IC50 of 0.12 μM [49] while valproic acid had 209.34 μM [75]. HDAC inhibitors have also been investigated as drugs for a range of other diseases such as trypanosomiasis, schistosomiasis, leishmaniasis, toxoplasmosis, HIV/AIDS and even cancer [116]. Apicidin, a novel fungal metabolite, has been documented as an inhibitor of HDAC in apicomplexan parasites including malaria [117]. The main challenge about the in vivo use of many HDAC inhibitors is that their zinc-binding hydroxamate group is broken down resulting in lose activity [115].
Inosine 5'-monophosphate dehydrogenase
Inosine 5'-monophosphate dehydrogenase (IMPDH) plays a key role in catalyzing the first committed step of guanosine 5'-monophosphate biosynthesis, an essential pathway in P. falciparum. The P. falciparum IMPDH (XP_001352079.1) has high druggability (D index of 0.8), an E value of 8e-169 when aligned with its putative drug target homologue IMPDH 2 (P12268.2) with which it also shares 79% of conserved amino acid residues. It also has an E value of 4e-173 when aligned with another homologous drug target, the IMPDH 1 (P20839.2). Both these drugs targets are human isoforms. The IMPDH is an attractive target for many therapeutic interventions since most parasites depend on the salvage pathway due to their inability to synthesize purine nucleotides de novo. Inhibitors of IMPDH, ribavirin and mycophenolic acid (both target IMPDH 1 and IMPDH 2) have been used as immunosuppressives, antivirals and anticancer drugs with few side effects to host cells [118–120]. Nevertheless, little has been done concerning their application in treating microorganisms [121]. This study predicted mycophenic acid to inhibit the P. falciparum IMPDH and it has been shown to be active against P. falciparum with an IC50 of 5.4 μM [82].
FK506-binding protein-12
Tacrolimus (FK506) is used to lower the risk of organ rejection after an allogenic organ transplant. It brings about its immunosuppressive activity by binding to FK506-binding protein-12 (FKBP-12) to form a complex that inhibits calcineurin, consequently preventing both T-lymphocyte activation and interleukin-2 transcription [122]. Tacrolimus has been shown by Bell and colleagues [71] to inhibit the growth of P. falciparum in vitro, with an IC50 of 1.9 μM compared to 4.521 ± 0.083 μM established in this study. The study by Bell et al. could not ascertain the mechanism of action considering they could not detect FKBPs in P. falciparum extracts at the time of the study. However, the genome sequence of P. falciparum [22] revealed that it does have a 35-kDa FKBP (PfFKBP35). Though the function of PfFKBP35 is still unknown, the presence of tetratricopeptide repeat motifs suggests it may be involved in transporting and modulating the function of other proteins in the parasite [123]. Bao and colleagues [124] showed tacrolimus could prevent the development of cerebral malaria in Plasmodium berghei ANKA-infected mice though it failed to clear the parasites at the concentrations used. This could mean PfFKBP35 and any other protein tacrolimus targets don’t play a critical role in the survival of the parasite.
Challenges and limitations
The validation approach used in this study assumes that the drugs that have shown activity against Plasmodium would be inhibiting the predicted P. falciparum protein targets. This might not be necessarily true because drugs have been shown to inhibit parasite growth by acting on targets other than the proteins they were expected to considering many drugs interact with several targets. An example of such a drug is dasatinib which targets several tyrosine-protein kinases [98–102], platelet-derived growth factor receptor beta [125], dimethylaniline monooxygenase 3 [126], signal transducer and activator of transcription 5B [127], ABC transporters [103] and a number of cytochrome P450 proteins [128]. Such drugs could bring their inhibitory activity either through concerted efforts of multiple targets or through a few targets that are involved in crucial pathways. This was not factored in the validation process. Nonetheless, multi-target drugs have been documented to be more effective than single-target ones [129] and less prone to drug resistance [130]. Furthermore, targeting different proteins/pathways is the basis for drug combination therapies [4,131].
Based on the low number of similar drug targets detected during the similarity search, it is probable that the parameters used in this study to filter results may have been too stringent. These parameters include an E value of 1e-20 in sequence similarity, a query coverage of 80% in protein-protein pairwise alignment and a minimum of 50% of shared conserved amino residues. For instance, bosutinib has been documented to have high antimalarial activity with an IC50 of 0.22 μM [48]. On the other hand, bosutinib’s predicted P. falciparum target, the calcium/calmodulin-dependent protein kinase (XP_001348401.2) shares 47% of functional amino acids residues with its homologous putative target, the calcium/calmodulin-dependent protein kinase type II subunit gamma (Q13555). This is below the threshold of 50% used in this study hence was eliminated at ConSurf server analysis stage. Out of the 5,338 P. falciparum protein sequences, only 34 possible drug targets met the inclusion criteria used in the DrugBank and TTD databases search and five in the STITCH database. Nevertheless, this had the benefit of increasing the likelihood of finding drug targets that were similar to the P. falciparum proteins hence increase the odds of discovering drugs with antimalarial effects. This also reduced the number of protein targets and drugs that would be analyzed in downstream processes. However, bosutinib represents many drugs (and their possible targets) that could otherwise have been identified by this approach but were not due to the rigorous inclusion criteria used.
Conclusion
With the urgent need to develop new antimalarial drugs to counteract the increasing resistance to current ones, novel P. falciparum pathways should be targeted in the search for the next generation of antimalarial drugs. Repositioning of approved drugs offers such a strategy since most of these drugs have their putative targets documented. This information could be used to identifying approved drugs with antimalarial activity and reveal possible proteins and pathways that could be targeted in the search for new antimalarials. Furthermore, this approach can also be implemented in the search for drugs that are active against pathogens other than P. falciparum. The predicted drugs that did show significant in vitro activity against P. falciparum need be investigated further for antimalarial treatment.
Supporting information
Those obtained from STITCH don’t have putative drug targets since they were associated directly with their corresponding P. falciparum proteins.
(XLSX)
The table details the residue variety in% (across 150 homologs used in Consurf server analysis) for each position in the query sequence (drug target NP_001277159.1). Each column shows the% for that amino-acid, position it is found in the MSA. Non-standard amino acid residues are shown under column 'OTHER'.
(CSV)
(DOCX)
Acknowledgments
We thank members of staff and students at Malaria Drug Resistance, Kenya Medical Research Institute/US Army Medical Research Directorate–Kenya, Kisumu for their input in the design of this work and assistance in vitro assays. We also wish to appreciate Elvira Lukelesia for her invaluable support in this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Japan International Cooperation Agency (AFRICA-ai-JAPAN Research Fund), grant no; JKU/ADM/10B, (url: https://www.jica.go.jp/kenya/english/). The grant number for financial support I received from JICA to facilitate part of this study is iCMOB/05/16 to RMM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Burchard GD. Malaria [Internet]. Der Internist. Springer; Berlin Heidelberg; 2014. February doi: 10.1007/s00108-013-3390-9 [Google Scholar]
- 2.World Health Organisation. World malaria report. World Health Organiization. 2016. ISBN 9789241564106
- 3.World Health Organization, Others. WHO briefing on Malaria Treatment Guidelines and artemisinin monotherapies. Geneva WHO. 2006; 1–28. http://apps.who.int/malaria/docs/Meeting_briefing19April.pdf
- 4.White NJ. Antimalarial drug resistance. JClinInvest. American Society for Clinical Investigation; 2004;113: 1084–1092. doi: 10.1172/JCI200421682.1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herlekar I. The resistance gene in malaria parasite identified. Curr Sci. Nature Publishing Group; 2014;106: 345 doi: 10.1038/nature12876 [Google Scholar]
- 6.Flannery EL, Chatterjee AK, Winzeler EA. Antimalarial drug discovery—approaches and progress towards new medicines. Nat Rev Microbiol. 2013;11: 849–62. doi: 10.1038/nrmicro3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baniecki ML, Wirth DF, Clardy J. High-throughput Plasmodium falciparum growth assay for malaria drug discovery. Antimicrob Agents Chemother. American Society for Microbiology; 2007;51: 716–723. doi: 10.1128/AAC.01144-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and Inexpensive Fluorescence-Based Technique for High-Throughput Antimalarial Drug Screening. Antimicrob Agents Chemother. American Society for Microbiology; 2004;48: 1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchholz K, Burke TA, Williamson KC, Wiegand RC, Wirth DF, Marti M. A high-throughput screen targeting malaria transmission stages opens new avenues for drug development. J Infect Dis. Oxford University Press; 2011;203: 1445–1453. doi: 10.1093/infdis/jir037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gego A, Silvie O, Franetich JF, Farhati K, Hannoun L, Luty AJF, et al. New approach for high-throughput screening of drug activity on Plasmodium liver stages. Antimicrob Agents Chemother. American Society for Microbiology; 2006;50: 1586–1589. doi: 10.1128/AAC.50.4.1586-1589.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macarron R, Banks MN, Bojanic D, Burns DJ, Cirovic DA, Garyantes T, et al. Impact of high-throughput screening. Nature. 2011;10: 188–195. doi: 10.1038/nrd3368 [DOI] [PubMed] [Google Scholar]
- 12.Baldwin J, Michnoff CH, Malmquist NA, White J, Roth MG, Rathod PK, et al. High-throughput screening for potent and selective inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase. J Biol Chem. American Society for Biochemistry and Molecular Biology; 2005;280: 21847–21853. doi: 10.1074/jbc.M501100200 [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Dong Y, Vennerstrom JL. Synthetic peroxides as antimalarizals. Med Res Rev. 2004;24: 425–448. doi: 10.1002/med.10066 [DOI] [PubMed] [Google Scholar]
- 14.Meshnick SR, Dobson MJ. The History of Antimalarial Drugs. Antimalar Chemother Mech Action, Resist New Dir Drug Discov. Humana Press; 2001; 15–25. doi: 10.1007/978-1-59259-111-4_2 [Google Scholar]
- 15.Boruah L, Das A, Nainwal L, Agarwal N, Shankar B. In-Silico Drug Design: A revolutionary approach to change the concept of current Drug Discovery Process. Ijpbr. 2013;1: 60–73. Available: http://ijpbr.in/wp-content/uploads/2012/06/11 Neha.pdf [Google Scholar]
- 16.Talele TT, Khedkar S a, Rigby AC. Successful applications of computer aided drug discovery: moving drugs from concept to the clinic. Curr Top Med Chem. 2010;10: 127–141. doi: 10.2174/156802610790232251 [DOI] [PubMed] [Google Scholar]
- 17.Liao C, Sitzmann M, Pugliese A, Nicklaus MC. Software and resources for computational medicinal chemistry. Future Med Chem. 2011;3: 1057–1085. doi: 10.4155/fmc.11.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derbyshire ER, Mota MM, Clardy J. The next opportunity in anti-malaria drug discovery: The liver stage. Rall GF, editor. PLoS Pathog. Public Library of Science; 2011;7: e1002178 doi: 10.1371/journal.ppat.1002178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandhi V, Kantarjian H, Faderl S, Bonate P, Du M, Ayres M, et al. Pharmacokinetics and Pharmacodynamics of Plasma Clofarabine and Cellular Clofarabine Triphosphate in Patients with Acute Leukemias. Clin Cancer Res. Clinical Cancer Research; 2003;9: 6335–6342. doi: 10.1200/jco.20.3.665 [PubMed] [Google Scholar]
- 20.Graewe S, Rankin KE, Lehmann C, Deschermeier C, Hecht L, Froehlke U, et al. Hostile takeover by plasmodium: Reorganization of parasite and host cell membranes during liver stage egress. Striepen B, editor. PLoS Pathog. Public Library of Science; 2011;7: e1002224 doi: 10.1371/journal.ppat.1002224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White NJ. The role of anti-malarial drugs in eliminating malaria. Malar J. 2008;7: S8 doi: 10.1186/1475-2875-7-S1-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardner MJ, Hall N, Fung E, White O, Berriman M, Richard W, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. 2013;419: 3–9. doi: 10.1038/nature01097.Genome [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh I, Altman RB. Drug Targets for Plasmodium falciparum: a post-genomic review/survey. Mini Rev Med Chem. 2006;6: 177–202. Available: http://www.ncbi.nlm.nih.gov/pubmed/16472186 [DOI] [PubMed] [Google Scholar]
- 24.Ke H, Sigala PA, Miura K, Morrisey JM, Mather MW, Crowley JR, et al. The Heme Biosynthesis Pathway Is Essential for Plasmodium falciparum Development in Mosquito Stage but Not in Blood Stages. J Biol Chem. 2014;289: 34827–34837. doi: 10.1074/jbc.M114.615831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Schaijk BCL, Kumar TRS, Vos MW, Richman A, van Gemert G- J, Li T, et al. Type II fatty acid biosynthesis is essential for Plasmodium falciparum sporozoite development in the midgut of Anopheles mosquitoes. Eukaryot Cell. American Society for Microbiology (ASM); 2014;13: 550–9. doi: 10.1128/EC.00264-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ke H, Lewis IA, Morrisey JM, McLean KJ, Ganesan SM, Painter HJ, et al. Genetic investigation of tricarboxylic acid metabolism during the Plasmodium falciparum life cycle. Cell Rep. NIH Public Access; 2015;11: 164–74. doi: 10.1016/j.celrep.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Déchamps S, Wengelnik K, Berry-Sterkers L, Cerdan R, Vial HJ, Gannoun-Zaki L. The Kennedy phospholipid biosynthesis pathways are refractory to genetic disruption in Plasmodium berghei and therefore appear essential in blood stages. Mol Biochem Parasitol. 2010;173: 69–80. doi: 10.1016/j.molbiopara.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 28.Metz J. Folic Acid Metabolism and Malaria. Food Nutr Bull. 2007;28: S540–S549. doi: 10.1177/15648265070284S407 [DOI] [PubMed] [Google Scholar]
- 29.van Niekerk DD, Penkler GP, du Toit F, Snoep JL. Targeting glycolysis in the malaria parasite Plasmodium falciparum. FEBS J. 2016;283: 634–646. doi: 10.1111/febs.13615 [DOI] [PubMed] [Google Scholar]
- 30.Preuss J, Jortzik E, Becker K. Glucose-6-phosphate metabolism in Plasmodium falciparum. IUBMB Life. 2012;64: 603–611. doi: 10.1002/iub.1047 [DOI] [PubMed] [Google Scholar]
- 31.Azin Nezami ‡, Tooru Kimura §, Koushi Hidaka §, Aiko Kiso §, Jun Liu ‖, Yoshiaki Kiso §, et al. High-Affinity Inhibition of a Family of Plasmodium falciparum Proteases by a Designed Adaptive Inhibitor†. American Chemical Society; 2003; 10.1021/BI034131Z [DOI] [PubMed]
- 32.Andrews KT, Fairlie DP, Madala PK, Ray J, Wyatt DM, Hilton PM, et al. Potencies of human immunodeficiency virus protease inhibitors in vitro against Plasmodium falciparum and in vivo against murine malaria. Antimicrob Agents Chemother. American Society for Microbiology; 2006;50: 639–48. doi: 10.1128/AAC.50.2.639-648.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alam A, Asrar. Serine Proteases of Malaria Parasite Plasmodium falciparum: Potential as Antimalarial Drug Targets. Interdiscip Perspect Infect Dis. Hindawi Publishing Corporation; 2014;2014: 453186 doi: 10.1155/2014/453186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wegscheid-Gerlach C, Gerber H-D, Diederich W. Proteases of Plasmodium falciparum as Potential Drug Targets and Inhibitors Thereof. Curr Top Med Chem. 2010;10: 346–367. doi: 10.2174/156802610790725461 [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Wu Y. Computer Assisted Searches for Drug Targets with Emphasis on Malarial Proteases and their Inhibitors. Curr Drug Target -Infectious Disord. 2004;4: 25–40. doi: 10.2174/1568005043480952 [DOI] [PubMed] [Google Scholar]
- 36.Lee BJ, Singh A, Chiang P, Kemp SJ, Goldman EA, Weinhouse MI, et al. Antimalarial activities of novel synthetic cysteine protease inhibitors. Antimicrob Agents Chemother. American Society for Microbiology; 2003;47: 3810–4. doi: 10.1128/AAC.47.12.3810-3814.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doerig C, Meijer L, Mottram JC. Protein kinases as drug targets in parasitic protozoa. Trends Parasitol. 2002;18: 366–371. doi: 10.1016/S1471-4922(02)02321-8 [DOI] [PubMed] [Google Scholar]
- 38.Knockaert M, Greengard P, Meijer L. Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol Sci. 2002;23: 417–425. doi: 10.1016/S0165-6147(02)02071-0 [DOI] [PubMed] [Google Scholar]
- 39.Doerig C, Billker O, Haystead T, Sharma P, Tobin AB, Waters NC. Protein kinases of malaria parasites: an update. Trends Parasitol. 2008;24: 570–577. doi: 10.1016/j.pt.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 40.Lucet IS, Tobin A, Drewry D, Wilks AF, Doerig C. Plasmodium kinases as targets for new-generation antimalarials. Future Med Chem. Future Science Ltd London, UK; 2012;4: 2295–2310. doi: 10.4155/fmc.12.183 [DOI] [PubMed] [Google Scholar]
- 41.Doerig C. Protein kinases as targets for anti-parasitic chemotherapy. Biochim Biophys Acta—Proteins Proteomics. 2004;1697: 155–168. doi: 10.1016/j.bbapap.2003.11.021 [DOI] [PubMed] [Google Scholar]
- 42.Jirage D, M. Keenan S, C. Waters N. Exploring Novel Targets for Antimalarial Drug Discovery: Plasmodial Protein Kinases. Infect Disord—Drug Targets. 2010;10: 134–146. doi: 10.2174/187152610791163381 [DOI] [PubMed] [Google Scholar]
- 43.Morgan S, Grootendorst P, Lexchin J, Cunningham C, Greyson D. The cost of drug development: A systematic review. Health Policy (New York). 2011;100: 4–17. doi: 10.1016/j.healthpol.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 44.Sekhon BS. Repositioning drugs and biologics: retargeting old/ existing drugs for potential new therapeutic applications. J Pharm Educ Res. 2013;4: 1–15. [Google Scholar]
- 45.Ekins S, Williams AJ. Finding promiscuous old drugs for new uses. Pharm Res. 2011;28: 1785–1791. doi: 10.1007/s11095-011-0486-6 [DOI] [PubMed] [Google Scholar]
- 46.Matthews H. Accelerating antimalarial drug discovery through repositioning. 2013;
- 47.Wu Zikai, Wang Yong, Chen L. Network-based drug repositioning. Mol Biosyst. 2013;9: 1268–1281. doi: 10.1039/c3mb25382a [DOI] [PubMed] [Google Scholar]
- 48.Lotharius J, Gamo-Benito FJ, Angulo-Barturen I, Clark J, Connelly M, Ferrer-Bazaga S, et al. Repositioning: the fast track to new anti-malarial medicines? Malar J. BioMed Central; 2014;13: 143 doi: 10.1186/1475-2875-13-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engel JA, Jones AJ, Avery VM, Sumanadasa SDM, Ng SS, Fairlie DP, et al. Profiling the anti-protozoal activity of anti-cancer HDAC inhibitors against Plasmodium and Trypanosoma parasites. Int J Parasitol Drugs Drug Resist. Elsevier; 2015;5: 117–126. doi: 10.1016/j.ijpddr.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chong CR, Chen X, Shi L, Liu JO, Sullivan DJ. A clinical drug library screen identifies astemizole as an antimalarial agent (supplemental file: figure 1). Nat Chem Biol. Nature Publishing Group; 2006;2: 415–6. doi: 10.1038/nchembio806 [DOI] [PubMed] [Google Scholar]
- 51.Bispo NA, Culleton R, Silva LA, Cravo P. A Systematic In Silico Search for Target Similarity Identifies Several Approved Drugs with Potential Activity against the Plasmodium falciparum Apicoplast. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neves BJ, Braga RC, Bezerra JCB, Cravo PVL, Andrade CH. In Silico Repositioning-Chemogenomics Strategy Identifies New Drugs with Potential Activity against Multiple Life Stages of Schistosoma mansoni. PLoS Negl Trop Dis. 2015;9: e3435 doi: 10.1371/journal.pntd.0003435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pruitt KD, Tatusova T, Maglott DR. NCBI reference sequences (RefSeq): A curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. Oxford University Press; 2007;35: D61–5. doi: 10.1093/nar/gkl842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Team RDC. R: A language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing. Vienna, Austria; 2004. citeulike-article-id:708579
- 55.Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, et al. DrugBank 3.0: A comprehensive resource for “Omics” research on drugs. Nucleic Acids Res. 2011;39: D1035–41. doi: 10.1093/nar/gkq1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu F, Shi Z, Qin C, Tao L, Liu X, Xu F, et al. Therapeutic target database update 2012: A resource for facilitating target-oriented drug discovery. Nucleic Acids Res. 2012;40: D1128–36. doi: 10.1093/nar/gkr797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuhn M, Szklarczyk D, Franceschini A, Von Mering C, Jensen LJ, Bork P. STITCH 3: Zooming in on protein-chemical interactions. Nucleic Acids Res. 2012;40: D876–80. doi: 10.1093/nar/gkr1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.T D R Targets. The TDR Targets Database v5 [Internet]. 2015 [cited 14 Dec 2015] pp. 1–5. Available: http://www.tdrtargets.org
- 59.Magariños MP, Carmona SJ, Crowther GJ, Ralph SA, Roos DS, Shanmugam D, et al. TDR targets: A chemogenomics resource for neglected diseases. Nucleic Acids Res. Oxford University Press; 2012;40: D1118–27. doi: 10.1093/nar/gkr1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Owens J. Determining druggability. Nat Rev Drug Discov. 2007;6: 187–187. doi: 10.1038/nrd2275 [Google Scholar]
- 61.Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, et al. ChEMBL: A large-scale bioactivity database for drug discovery. Nucleic Acids Res. Oxford University Press; 2012;40: D1100–7. doi: 10.1093/nar/gkr777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Altschul SF, Gish W, Miller WT, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215: 403–410. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 63.Glaser F, Pupko T, Paz I, Bell RE, Bechor-Shental D, Martz E, et al. ConSurf: Identification of functional regions in proteins by surface-mapping of phylogenetic information. Bioinformatics. 2003;19: 163–164. doi: 10.1093/bioinformatics/19.1.163 [DOI] [PubMed] [Google Scholar]
- 64.Bateman A, Martin MJ, O’Donovan C, Magrane M, Apweiler R, Alpi E, et al. UniProt: A hub for protein information. Nucleic Acids Res. Oxford University Press; 2015;43: D204–D212. doi: 10.1093/nar/gku989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Velankar S, Alhroub Y, Best C, Caboche S, Conroy MJ, Dana JM, et al. PDBe: Protein Data Bank in Europe. Nucleic Acids Res. Oxford University Press; 2012;40: D445–D452. doi: 10.1093/nar/gkr998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modelling. Bioinformatics. Oxford University Press; 2006;22: 195–201. doi: 10.1093/bioinformatics/bti770 [DOI] [PubMed] [Google Scholar]
- 67.Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. Assessment and continued validation of the malaria SYBR Green I-based fluorescence assay for use in malaria drug screening. Antimicrob Agents Chemother. American Society for Microbiology (ASM); 2007;51: 1926–1933. doi: 10.1128/AAC.01607-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheruiyot AC, Auschwitz JM, Lee PJ, Yeda RA, Okello CO, Leed SE, et al. Assessment of the Worldwide antimalarial resistance network standardized procedure for in vitro malaria drug sensitivity testing using SYBR green assay for field samples with various initial parasitemia levels. Antimicrob Agents Chemother. American Society for Microbiology; 2016;60: 2417–2424. doi: 10.1128/AAC.00527-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akala HM, Eyase FL, Cheruiyot AC, Omondi AA, Ogutu BR, Waters NC, et al. Antimalarial drug sensitivity profile of western Kenya Plasmodium falciparum field isolates determined by a SYBR green I in vitro assay and molecular analysis. Am J Trop Med Hyg. 2011;85: 34–41. doi: 10.4269/ajtmh.2011.10-0674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nair L, Bhasin VK. Cure With Cisplatin (Ii) of Murine Malaria Infection and in Vitro Inhibition of a Chloroquine-Resistant Plasmodium Falciparum Isolate. Japanese J Med Sci Biol. National Institute of Infectious Diseases, Japanese Journal of Infectious Diseases Editorial Committee; 1994;47: 241–252. doi: 10.7883/yoken1952.47.241 [DOI] [PubMed] [Google Scholar]
- 71.Bell A, Wernli B, Franklin RM. Roles of peptidyl-prolyl CIS-trans isomerase and calcineurin in the mechanisms of antimalarial action of cyclosporin a, FK506, and rapamycin. Biochem Pharmacol. 1994;48: 495–503. doi: 10.1016/0006-2952(94)90279-8 [DOI] [PubMed] [Google Scholar]
- 72.Sinou V, Grellier P, Schrevel J. In vitro and in vivo inhibition of erythrocytic development of malarial parasites by docetaxel. Antimicrob Agents Chemother. American Society for Microbiology (ASM); 1996;40: 358–361. Available: http://www.ncbi.nlm.nih.gov/pubmed/8834880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nasveld P, Russell B, Kotecka B, Rieckmann K. Lack of in vitro effect of ivermectin on Plasmodium falciparum. Southeast Asian J Trop Med Public Health. 2003;34: 552–553. Available: http://www.ncbi.nlm.nih.gov/pubmed/15115126 [PubMed] [Google Scholar]
- 74.Nsanzabana C, Rosenthal PJ. In vitro activity of antiretroviral drugs against Plasmodium falciparum. Antimicrob Agents Chemother. American Society for Microbiology (ASM); 2011;55: 5073–5077. doi: 10.1128/AAC.05130-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mahmoudi N, Ciceron L, Franetich JF, Farhati K, Silvie O, Eling W, et al. In vitro activities of 25 quinolones and fluoroquinolones against liver and blood stage Plasmodium spp. Antimicrob Agents Chemother. 2003;47: 2636–2639. doi: 10.1128/AAC.47.8.2636-2639.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Figgitt D, Denny W, Chavalitshewinkoon P, Wilairat P, Ralph R. In vitro study of anticancer acridines as potential antitrypanosomal and antimalarial agents. Antimicrob Agents Chemother. American Society for Microbiology; 1992;36: 1644–1647. doi: 10.1128/AAC.36.8.1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dennis Shanks G, Edstein MD, Pavanand K, Kyle Webster H, Wechgritaya S. Ciprofloxacin treatment of drug-resistant falciparum malaria. J Infect Dis. Oxford University Press; 1991;164: 602–604. doi: 10.1093/infdis/164.3.602 [DOI] [PubMed] [Google Scholar]
- 78.Pradines B, Torrentino-Madamet M, Fontaine A, Henry M, Baret E, Mosnier J, et al. Atorvastatin is 10-fold more active in vitro than other statins against Plasmodium falciparum [3]. Antimicrob Agents Chemother. American Society for Microbiology (ASM); 2007;51: 2654–2655. doi: 10.1128/AAC.01330-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamzah J, Skinner-Adams T, Davis TME. In vitro antimalarial activity of trovafloxacin, a fourth-generation fluoroquinolone. Acta Trop. 2000;74: 39–42. doi: 10.1016/S0001-706X(99)00051-0 [DOI] [PubMed] [Google Scholar]
- 80.Andrews KT, Walduck A, Kelso MJ, Fairlie DP, Saul A, Parsons PG. Anti-malarial effect of histone deacetylation inhibitors and mammalian tumour cytodifferentiating agents. Int J Parasitol. 2000;30: 761–768. doi: 10.1016/S0020-7519(00)00043-6 [DOI] [PubMed] [Google Scholar]
- 81.Bobbala D, Koka S, Geiger C, Föller M, Huber SM, Lang F. Azathioprine favourably influences the course of malaria. Malar J. BioMed Central; 2009;8: 102 doi: 10.1186/1475-2875-8-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veletzky L, Rehman K, Lingscheid T, Poeppl W, Loetsch F, Burgmann H, et al. In vitro activity of immunosuppressive drugs against Plasmodium falciparum. Malar J. BioMed Central; 2014;13: 476 doi: 10.1186/1475-2875-13-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bajsa J, McCluskey A, Gordon CP, Stewart SG, Hill TA, Sahu R, et al. The antiplasmodial activity of norcantharidin analogs. Bioorganic and Medicinal Chemistry Letters. 2010. doi: 10.1016/j.bmcl.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 84.Akaki M, Nakano Y, Ito Y, Nagayasu E, Aikawa M. Effects of dipyridamole on Plasmodium falciparum-infected erythrocytes. Parasitol Res. Springer-Verlag; 2002;88: 1044–1050. doi: 10.1007/s00436-002-0690-8 [DOI] [PubMed] [Google Scholar]
- 85.Menezes CMS, Kirchgatter K, Di Santi SM, Savalli C, Monteiro FG, Paula GA, et al. In vitro chloroquine resistance modulation study on fresh isolates of Brazilian Plasmodium falciparum: Intrinsic antimalarial activity of phenothiazine drugs. Mem Inst Oswaldo Cruz. Fundação Oswaldo Cruz; 2002;97: 1033–1039. S0074-02762002000700018 [pii] [DOI] [PubMed] [Google Scholar]
- 86.Li Q, Lai L. Prediction of potential drug targets based on simple sequence properties. BMC Bioinformatics. BioMed Central; 2007;8: 353 doi: 10.1186/1471-2105-8-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, et al. The activities of current antimalarial drugs on the life cycle stages of plasmodium: A comparative study with human and rodent parasites. PLoS Med. Public Library of Science; 2012;9: e1001169 doi: 10.1371/journal.pmed.1001169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stowers AW, Cioce V, Shimp RL, Lawson M, Hui G, Muratova O, et al. Efficacy of two alternate vaccines based on Plasmodium falciparum merozoite surface protein 1 in an Aotus challenge trial. Infect Immun. American Society for Microbiology; 2001;69: 1536–1546. doi: 10.1128/IAI.69.3.1536-1546.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leslie EM, Deeley RG, Cole SPC. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense [Internet]. Toxicology and Applied Pharmacology. 2005. pp. 216–237. doi: 10.1016/j.taap.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 90.Leprohon P, Legare D, Ouellette M. ABC transporters involved in drug resistance in human parasites. Essays Biochem. 2011;50: 121–144. doi: 10.1042/bse0500121 [DOI] [PubMed] [Google Scholar]
- 91.Koenderink JB, Kavishe RA, Rijpma SR, Russel FGM. The ABCs of multidrug resistance in malaria. Trends Parasitol. Elsevier; 2010;26: 440–6. doi: 10.1016/j.pt.2010.05.002 [DOI] [PubMed] [Google Scholar]
- 92.Raj DK, Mu J, Jiang H, Kabat J, Singh S, Sullivan M, et al. Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione. J Biol Chem. 2009;284: 7687–7696. doi: 10.1074/jbc.M806944200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rijpma SR, van der Velden M, Annoura T, Matz JM, Kenthirapalan S, Kooij TWA, et al. Vital and dispensable roles of Plasmodium multidrug resistance transporters during blood- and mosquito-stage development. Mol Microbiol. 2016;101: 78–91. doi: 10.1111/mmi.13373 [DOI] [PubMed] [Google Scholar]
- 94.Garmory HS, Titball RW. ATP-binding cassette transporters are targets for the development of antibacterial vaccines and therapies. Infect Immun. American Society for Microbiology; 2004;72: 6757–63. doi: 10.1128/IAI.72.12.6757-6763.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yu EY, Wilding G, Posadas E, Gross M, Culine S, Massard C, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15: 7421–8. doi: 10.1158/1078-0432.CCR-09-1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson FM, Saigal B, Talpaz M, Donato NJ. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11: 6924–6932. doi: 10.1158/1078-0432.CCR-05-0757 [DOI] [PubMed] [Google Scholar]
- 97.Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat. Springer US; 2007;105: 319–326. doi: 10.1007/s10549-006-9463-x [DOI] [PubMed] [Google Scholar]
- 98.Imming P, Sinning C, Meyer A. Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006;5: 821–834. doi: 10.1038/nrd2132 [DOI] [PubMed] [Google Scholar]
- 99.Quintás-Cardama A, Kantarjian H, Cortes J. Targeting ABL and SRC kinases in chronic myeloid leukemia: experience with dasatinib. Futur Oncol. 2006;2: 655–665. doi: 10.2217/14796694.2.6.655 [DOI] [PubMed] [Google Scholar]
- 100.Serrels A, Macpherson IRJ, Evans TRJ, Lee FY, Clark EA, Sansom OJ, et al. Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with dasatinib. Mol Cancer Ther. 2006;5: 3014–3022. doi: 10.1158/1535-7163.MCT-06-0382 [DOI] [PubMed] [Google Scholar]
- 101.Kamath AV., Wang J, Lee FY, Marathe PH. Preclinical pharmacokinetics and in vitro metabolism of dasatinib (BMS-354825): a potent oral multi-targeted kinase inhibitor against SRC and BCR-ABL. Cancer Chemother Pharmacol. 2008;61: 365–376. doi: 10.1007/s00280-007-0478-8 [DOI] [PubMed] [Google Scholar]
- 102.Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin R V., et al. 2-Aminothiazole as a Novel Kinase Inhibitor Template. Structure−Activity Relationship Studies toward the Discovery of N -(2-Chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1- piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide (Dasatinib, BMS-354825) as a Potent pan -Src Kinase Inhibitor. J Med Chem. 2006;49: 6819–6832. doi: 10.1021/jm060727j [DOI] [PubMed] [Google Scholar]
- 103.Dohse M, Scharenberg C, Shukla S, Robey RW, Volkmann T, Deeken JF, et al. Comparison of ATP-Binding Cassette Transporter Interactions with the Tyrosine Kinase Inhibitors Imatinib, Nilotinib, and Dasatinib. Drug Metab Dispos. 2010;38: 1371–1380. doi: 10.1124/dmd.109.031302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hegedűs C, Özvegy-Laczka C, Apáti Á, Magócsi M, Német K, Őrfi L, et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. Br J Pharmacol. 2009;158: 1153–1164. doi: 10.1111/j.1476-5381.2009.00383.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kesely KR, Pantaleo A, Turrini FM, Olupot-Olupot P, Low PS. Inhibition of an erythrocyte tyrosine kinase with imatinib prevents Plasmodium falciparum egress and terminates parasitemia. PLoS One. 2016;11: 1–19. doi: 10.1371/journal.pone.0164895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tyler PC, Taylor EA, Fröhlich RFG, Schramm VL. Synthesis of 5???-methylthio coformycins: Specific inhibitors for malarial adenosine deaminase. J Am Chem Soc. American Chemical Society; 2007;129: 6872–6879. doi: 10.1021/ja0708363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sriram S, Srilakshmi JK, Meenaa V, Sasikumar C. Adenosine deaminase from Plasmodium falciparum as a Potential Drug Target in Anti- Malarial Drug Designing: A Bioinformatic Approach. Africa (Lond). 2009; 13–16. [Google Scholar]
- 108.Ting LM, Shi W, Lewandowicz A, Singh V, Mwakingwe A, Birck MR, et al. Targeting a novel Plasmodium falciparum purine recycling pathway with specific immucillins. J Biol Chem. 2005;280: 9547–9554. doi: 10.1074/jbc.M412693200 [DOI] [PubMed] [Google Scholar]
- 109.Davis R, Bryson HM. Levofloxacin: A Review of its Antibacterial Activity, Pharmacokinetics and Therapeutic Efficacy. Drugs. Springer International Publishing; 1994;47: 677–700. doi: 10.2165/00003495-199447040-00008 [DOI] [PubMed] [Google Scholar]
- 110.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61: 377–392. D—NLM: PMC232616 EDAT- 1997/09/18 MHDA- 1997/09/18 00:01 CRDT- 1997/09/18 00:00 PST—ppublish [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Champoux JJ. DNA Topoisomerases: Structure, Function, and Mechanism. Annu Rev Biochem. 2001;70: 369–413. doi: 10.1146/annurev.biochem.70.1.369 [DOI] [PubMed] [Google Scholar]
- 112.Garcia-Estrada C, Prada CF, Fernandez-Rubio C, Rojo-Vazquez F, Balana-Fouce R. DNA topoisomerases in apicomplexan parasites: promising targets for drug discovery. Proc Biol Sci. 2010;277: 1777–1787. doi: 10.1098/rspb.2009.2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bodley AL, Cumming JN, Shapiro TA. Effects of camptothecin, a topoisomerase I inhibitor, on Plasmodium falciparum. Biochem Pharmacol. 1998;55: 709–711. doi: 10.1016/S0006-2952(97)00556-X [DOI] [PubMed] [Google Scholar]
- 114.Ma Y, Wink M. The beta-carboline alkaloid harmine inhibits BCRP and can reverse resistance to the anticancer drugs mitoxantrone and camptothecin in breast cancer cells. Phyther Res. 2010;24: 146–149. doi: 10.1002/ptr.2860 [DOI] [PubMed] [Google Scholar]
- 115.Andrews K, Tran T, Wheatley N, Fairlie D. Targeting Histone Deacetylase Inhibitors for Anti-Malarial Therapy. Curr Top Med Chem. 2009;9: 292–308. doi: 10.2174/156802609788085313 [DOI] [PubMed] [Google Scholar]
- 116.Andrews KT, Haque A, Jones MK. HDAC inhibitors in parasitic diseases. Immunol Cell Biol. 2012;90: 66–77. doi: 10.1038/icb.2011.97 [DOI] [PubMed] [Google Scholar]
- 117.Darkin-Rattray SJ, Gurnett AM, Myers RW, Dulski PM, Crumley TM, Allocco JJ, et al. Apicidin: A novel antiprotozoal agent that inhibits parasite histone deacetylase (cyclic ). Med Sci. National Academy of Sciences; 1996;93: 13143–13147. doi: 10.1073/pnas.93.23.13143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bentley R. Mycophenolic acid: A one hundred year odyssey from antibiotic to immunosuppressant. Chem Rev. American Chemical Society; 2000;100: 3801–3825. doi: 10.1021/cr990097b [DOI] [PubMed] [Google Scholar]
- 119.Chen L, Pankiewicz KW. Recent development of IMP dehydrogenase inhibitors for the treatment of cancer. Curr Opin Drug Discov Devel. 2007;10: 403–12. Available: http://www.ncbi.nlm.nih.gov/pubmed/17659481 [PubMed] [Google Scholar]
- 120.Tam RC, Lau JYN, Hong Z. Mechanisms of action of ribavirin in antiviral therapies. Antivir Chem Chemother. SAGE Publications; 2001;12: 261–272. doi: 10.1177/095632020101200501 [DOI] [PubMed] [Google Scholar]
- 121.Hedstrom L, Liechti G, B. Goldberg J, R. Gollapalli D. The Antibiotic Potential of Prokaryotic IMP Dehydrogenase Inhibitors. Curr Med Chem. 2011;18: 1909–1918. doi: 10.2174/092986711795590129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thomson AW, Bonham CA, Zeevi A. Mode of action of tacrolimus (FK506): molecular and cellular mechanisms. Ther Drug Monit. 1995;17: 584–91. Available: http://www.ncbi.nlm.nih.gov/pubmed/8588225 [DOI] [PubMed] [Google Scholar]
- 123.Monaghan P, Bell A. A Plasmodium falciparum FK506-binding protein (FKBP) with peptidyl–prolyl cis–trans isomerase and chaperone activities. Mol Biochem Parasitol. 2005;139: 185–195. doi: 10.1016/j.molbiopara.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 124.Bao LQ, Nhi DM, Huy NT, Hamano S, Hirayama K. Tacrolimus prevents murine cerebral malaria. Immunology. 2017;150: 155–161. doi: 10.1111/imm.12661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen Z, Lee FY, Bhalla KN, Wu J. Potent Inhibition of Platelet-Derived Growth Factor-Induced Responses in Vascular Smooth Muscle Cells by BMS-354825 (Dasatinib). Mol Pharmacol. 2006;69: 1527–1533. doi: 10.1124/mol.105.020172 [DOI] [PubMed] [Google Scholar]
- 126.Wang L, Christopher LJ, Cui D, Li W, Iyer R, Humphreys WG, et al. Identification of the Human Enzymes Involved in the Oxidative Metabolism of Dasatinib: An Effective Approach for Determining Metabolite Formation Kinetics. Drug Metab Dispos. 2008;36: 1828–1839. doi: 10.1124/dmd.107.020255 [DOI] [PubMed] [Google Scholar]
- 127.Fiskus W, Pranpat M, Balasis M, Bali P, Estrella V, Kumaraswamy S, et al. Cotreatment with Vorinostat (Suberoylanilide Hydroxamic Acid) Enhances Activity of Dasatinib (BMS-354825) against Imatinib Mesylate-Sensitive or Imatinib Mesylate-Resistant Chronic Myelogenous Leukemia Cells. Clin Cancer Res. 2006;12: 5869–5878. doi: 10.1158/1078-0432.CCR-06-0980 [DOI] [PubMed] [Google Scholar]
- 128.van Erp NP, Gelderblom H, Guchelaar H-J. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev. 2009;35: 692–706. doi: 10.1016/j.ctrv.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 129.Csermely P, Ágoston V, Pongor S. The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharmacol Sci. 2005;26: 178–182. doi: 10.1016/j.tips.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 130.Hampton T. “Promiscuous” Anticancer Drugs That Hit Multiple Targets May Thwart Resistance. Jama. American Medical Association; 2004;292: 419 doi: 10.1001/jama.292.4.419 [DOI] [PubMed] [Google Scholar]
- 131.Zimmermann GR, Lehár J, Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12: 34–42. doi: 10.1016/j.drudis.2006.11.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Those obtained from STITCH don’t have putative drug targets since they were associated directly with their corresponding P. falciparum proteins.
(XLSX)
The table details the residue variety in% (across 150 homologs used in Consurf server analysis) for each position in the query sequence (drug target NP_001277159.1). Each column shows the% for that amino-acid, position it is found in the MSA. Non-standard amino acid residues are shown under column 'OTHER'.
(CSV)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.