Abstract
Background
Pneumococcal disease causes substantial morbidity and mortality, including among adults. Adult pneumococcal vaccines help to prevent these burdens, but they are underused. Accounting for the full benefits of adult pneumococcal vaccination may promote more rational resource allocation decisions with respect to adult pneumococcal vaccines.
Objectives
Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we conducted a systematic review to assess the extent to which the literature has empirically captured (e.g., through measurement or modeling) the full benefits of adult pneumococcal vaccination.
Methods
We systematically searched PubMed and Embase to identify studies published between January 1, 2010 and April 10, 2016 that examine adult pneumococcal vaccination. We included articles if they captured any health or economic benefit of an adult pneumococcal vaccine administered to adults age ≥ 50 or ≥ 18 in risk groups. Finally, we summarized the literature by categorizing the types of benefits captured, the perspective taken, and the strength of the evidence presented. Our protocol is number 42016038335 in the PROSPERO International prospective register of systematic reviews.
Results
We identified 5,857 papers and included 150 studies for analysis. While most capture health gains and healthcare cost savings, far fewer studies consider additional benefit categories, such as productivity gains. However, the studies with a broader approach still exhibit significant limitations; for example, many present only abstracts, while others offer no new measurements. Studies that examine the 13-valent pneumococcal conjugate vaccine focus more on broad economic benefits, but still have limitations.
Conclusions
This review highlights the need for more robust empirical accounting of the full benefits of adult pneumococcal vaccination. Literature outside this realm indicates that these broad benefits may be substantial. Failing to investigate the full benefits may lead society to undervalue vaccines' contributions and therefore underinvest in their development and adoption.
Introduction
Pneumococcal disease causes significant morbidity and mortality in both developing and developed countries, causing 1.6 million deaths annually [1]—more than seasonal influenza [2], malaria [3], or HIV/AIDS [4]. Pneumococcal disease comprises several clinical syndromes caused by the Streptococcus pneumoniae (pneumococcus) bacterium. Case fatality rates vary depending on the manifestation of the disease and can range from approximately 5% for pneumococcal pneumonia to 22% for adult pneumococcal meningitis. Certain pneumococcal infections, especially meningitis, can cause significant long-term sequelae [5].
The elderly and other adults at increased risk of contracting pneumococcal disease—including those with comorbidities or a compromised immune system—bear much of the burden of pneumococcal disease. Because of the potential for interpersonal disease transmission, older adults living in long-term care facilities are at a higher risk for contracting pneumococcal disease [6]. The incidence rate of pneumococcal disease increases with advancing age, and because the number of people age 60 years or older worldwide is expected to double between 2015 and 2050 (from 900 million to 2.1 billion) [7], pneumococcal disease in older adults will continue to be an important public health concern.
Two vaccines offer adults protection from pneumococcal disease: a 23-valent pneumococcal polysaccharide vaccine (PPV23) first introduced in 1983 [8] and a 13-valent pneumococcal conjugate vaccine (PCV13) first introduced in 2009 [9]. In Europe, PCV13 was initially approved for children from six weeks to five years of age in 2009 and then for adults age 50 years and older in 2011. The United States followed a similar pattern of introduction, approving the vaccine for infants and young children ages six weeks through five years in 2010, followed by approval for adults 50 and older at the end of 2011 [10].
While great strides have been made in pneumococcal vaccine distribution since their introduction, significant gaps in adult pneumococcal vaccine coverage persist. For example, in the United States in 2014, only 61.3% of adults 65 and older received their recommended pneumococcal vaccines, and coverage among high-risk adults age 19–64 (such as smokers or those with chronic conditions such as diabetes or chronic obstructive pulmonary disease) was only 20.3% [11]. Across Europe, recommendations and funding for adult pneumococcal vaccinations vary greatly in terms of age and risk groups [12, 13], making implementation and coverage less than optimal. Articulating and empirically measuring the full benefits of adult pneumococcal vaccination can help stimulate efforts to close coverage gaps and ensure adults receive appropriate protection from a vaccine-preventable disease.
The value of vaccination
The finding that health promotes economic well-being—both individually and collectively—is a significant advance in the field of economic development [14–16]. It suggests that health interventions like vaccination programs have benefits that extend beyond the intrinsic value of mortality and morbidity reductions. Along these lines, recent research highlights how economists and policymakers have failed to account for vaccine programs’ full benefits [17–34]. This shortcoming suggests that these programs are substantially undervalued and that many decisions regarding vaccination adoption, scale-up, and investment in vaccine discovery and development were poorly founded.
Given global population aging [35] and the burden of pneumococcal disease among the elderly, adult pneumococcal vaccination in particular merits detailed analysis of the nature and economic magnitude of its potential benefits. This includes a critical review of existing research on the benefits of pneumococcal vaccines in adults. However, our approach differs from other such published systematic reviews in that we aim to determine the extent to which the literature captures broad benefits. Other reviews are typically restricted to a subset of benefits, such as economic benefits [36] or vaccine effectiveness [37–39].
We grounded this review in previous work that describes and attempts to understand the full benefits of vaccination [17, 19, 28, 30, 40–42]. As in other studies focusing on the broad benefits of particular vaccines [17, 28], we devised a taxonomy that identifies a comprehensive set of benefits of adult pneumococcal vaccination. The taxonomy distinguishes the narrow perspective, which includes benefits policymakers commonly think about, from a broader perspective, which includes additional benefits that policymakers rarely consider.
The following benefits constitute the narrow perspective and coincide with similar taxonomies for other diseases [17, 28]:
Healthcare cost savings: The reduction in visits to medical practitioners, inpatient stays, and prescription drugs associated with pneumococcal disease treatment; and
Health gains: The intrinsic value of reduced morbidity, mortality, pain, and suffering from pneumococcal disease.
Similarly, these additional benefits derive from existing benefit taxonomies for other diseases and reflect the broader perspective [17, 28]:
Outcome-related productivity gains: The gains in productivity and income that accrue when immunized adults who are protected from pneumococcal disease are able to work and earn more;
Care-related productivity gains: The value of caretakers’ productive time that is saved when they are released from the care and supervision of adults who are now healthier due to pneumococcal vaccination;
Health-based community externalities: The value of improved health outcomes among nonvaccinated community members. These improved outcomes may be due to herd effects (to the extent that such effects are realized after adult pneumococcal vaccination) or due to a slowing of antibiotics’ loss of effectiveness, which imposes health and economic burdens; and
Risk reduction gains: The value of pneumococcal vaccination’s role in reducing uncertainty and any concomitant anxiety, which would otherwise impose a cost on risk-averse people (e.g., value of peace of mind).
Other broad benefits are particularly relevant to pneumococcal vaccination, including:
Voluntary contributions to family and community: The value of the human capital that accumulates in children and grandchildren through the greater time and education investments their healthier parents and grandparents make in them and the additional contributions adults make to their communities through participation in various activities, which they are better able to do when healthier;
Prevention and amelioration of comorbidities: The inherent health benefits and further economic value of reduced incidence of comorbid health conditions (such as myocardial infarctions) and typical complications that follow from pneumococcal disease;
Reduction in nosocomial infections: The value of avoiding nosocomial infections that could have followed from hospitalizations for pneumococcal disease had an adult not been vaccinated; and
Promotion of social equity: The value of pneumococcal vaccination in diminishing social and economic inequalities, insofar as adult pneumococcal disease disproportionately affects the poor.
Using this taxonomy of benefits of adult pneumococcal vaccination as a guide, we systematically reviewed the literature to identify the breadth and depth of the evidence base on the benefits of adult pneumococcal vaccination.
Methods
Objectives
Our principal research objective was to survey the extent to which the literature empirically captures the full benefits of adult pneumococcal vaccination, through either direct measurement or modeling. Our secondary research objective, which fed into this central goal, was to survey the literature in terms of the breadth (i.e., kinds) of benefits captured and the strength and nature of evidence surrounding these benefits.
Protocol and registration
The study protocol is registered on PROSPERO: International Prospective Register of Systematic Reviews and can be accessed at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016038335 or viewed as supporting information S1 File. We conducted the review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [43, 44].
To identify articles capturing the full benefits of adult pneumococcal vaccination, we performed a search on April 10, 2016 for the timeframe January 1, 2010 through April 10, 2016. We chose this timeframe because it captures a time during which both major adult pneumococcal vaccines were on the market. Although PCV13 had not been approved for older adults in Europe and the United States prior to 2011, we wanted to capture studies conducted to generate evidence on the potential benefits of adult pneumococcal vaccination, including modeling studies. We searched in two databases: PubMed and Embase. We used database terminology (Medical Subject Headings [MeSH] and Emtree, respectively) and keywords to capture more recent articles that had not yet been indexed in the database as of the search date. The search algorithm was based on two main topics: adult pneumococcal vaccination and benefits, which were subdivided into health benefits and economic benefits. S2 File presents the full search string, including the database terminology and keywords used. We exported the identified references into EndNote X7.5 and eliminated duplicate results. To check the sensitivity and quality of the search protocol, we identified 10 key articles that we felt the search should yield and confirmed that the search string returned all 10 articles. We also included articles from expert consultations to be as comprehensive as possible in the search.
Two reviewers screened the identified articles, first including or eliminating articles based on their titles and abstracts according to the inclusion and exclusion criteria that follow. Articles with ambiguous titles and abstracts underwent full text review by both reviewers. In the case the two reviewers disagreed, a third reviewer broke the tie. All reviewers were able to see the authors, institutions, journals of publication, and results when they applied the eligibility criteria. We recorded the reason for exclusion for studies excluded from full text review. To confirm that the reviewers applied the inclusion and exclusion criteria consistently, the two initial reviewers sorted 10 articles using these criteria. We reviewed discrepancies in the two reviewers’ judgments regarding these articles and discussed them to clarify the meaning of the criteria and to facilitate their consistent application.
We obtained most articles from Harvard, Tufts, and University of Massachusetts Boston libraries, or Google Scholar. We attempted to contact the authors of any articles unavailable from these sources and purchased articles that were still inaccessible when possible.
Eligibility criteria
Inclusion criteria
According to our participants, interventions, comparators, outcomes, and study design (PICOS) criteria [43] (Table 1), we included articles in our review if they reported experimental, observational, or model-based studies that capture health or economic benefits of adult pneumococcal vaccination. These could include
Table 1. PICOS criteria for eligibility of studies.
| PICOS Category | Description |
|---|---|
| Population | Vaccinated adults age 50 or above, or adults 18 and older in “risk groups” (as defined by the authors of the paper) |
| Interventions | Vaccination with PCV13 or PPV23 |
| Comparator | Comparators as defined by the authors of the study, can include: • No vaccination; • Placebo; • Vaccination with another product (e.g., PCV13 rather than PPV23); • Co-administration of pneumococcal vaccine with influenza vaccine compared with vaccination with only one, or compared with no vaccination; and • Pneumococcal vaccination of a subgroup compared with another subgroup (e.g., immunocompromised adults compared with healthy adults) |
| Outcomes | Health or economic benefits of adult pneumococcal vaccination, including: • Healthcare cost savings; • Health gains; • Outcome-related productivity gains; • Care-related productivity gains; • Health-based community externalities; • Risk reduction gains; • Voluntary contributions to family and community; • Prevention and amelioration of comorbidities; • Reduction in nosocomial infections; and • Promotion of social equity |
| Study Design | Experimental, observational, or model-based studies that capture health or economic benefits of adult pneumococcal vaccination |
Economic evaluations of adult pneumococcal disease vaccination (including any method of analysis, such as cost-effectiveness analysis, cost-utility analysis, cost-minimization analysis, budget impact analysis, or benefit-cost analysis);
Studies that calculate the benefit of any adult pneumococcal disease vaccination in health terms (e.g., quality-adjusted life years or QALYs, cases averted); or
Studies that capture other benefits of adult pneumococcal vaccination that accrue to individuals or populations (e.g., reduction of hospitalizations due to pneumococcal pneumonia, reduction of nosocomial infections resulting from hospitalization due to pneumococcal disease).
Disease endpoints of interest included any form of adult pneumococcal disease, including pneumonia, meningitis, bacteremia, otitis media, sinusitis, bronchitis, ear infection, sinus infection, blood infection, and septicemia, or any comorbidities associated with pneumococcal disease. We included studies from any geographic area. We only included studies examining vaccinated adults age 50 or above, or adults 18 and older in “risk groups” (as defined by the authors of the paper; examples include adults with chronic illness, weakened immune systems, cochlear implants, or cerebrospinal fluid leaks, or adults living in long-term care facilities). We included studies of any pneumococcal vaccine product (as long as the vaccine is given to adults); any vaccination strategy; any length of follow-up; and, for economic studies, any currency.
Exclusion criteria
We excluded economic studies that did not compare costs with outcomes (e.g., price or cost studies, as opposed to, e.g., cost-benefit or cost-effectiveness). We excluded health studies relating solely to internal biological processes (e.g., antibody responses to vaccination) without directly connecting these processes to overall health or economic outcomes (e.g., disability-adjusted life years or DALYs, averted medical costs). We also excluded studies that only explore benefits stemming from vaccinating non-adults. We did not consider studies other than published articles, institutional reports (e.g., World Health Organization reports), and conference proceedings. We therefore excluded reviews, commentaries, editorials, news articles, and policy briefs. We excluded studies reported in any language other than English.
Data extraction
We developed a template for extracting information from the included articles, pilot-tested it on a sample of five studies, and revised the template based on the pilot experience. Two reviewers divided the list of included articles alphabetically by the last name of the first author, extracted data according to the template, and conducted an audit on a subset of the included articles to quality-check the extraction. We extracted the following information from each included study:
Study characteristics (study citation, control and treatment groups, limitations, assumed vaccination coverage, assumed duration of protection, time horizon of analysis, perspective taken, type of study, and follow-up time);
Characteristics of the study population (setting, age group[s], and risk group[s] if applicable);
Characteristics of the vaccination strategy (vaccine product[s] and vaccination strategy[ies]);
Benefits captured in the study (type of outcome[s] measured, units, and results); and
Contextual factors of interest (study sponsor or other comments on the analysis).
See S3 File for the template used to extract study information.
Results
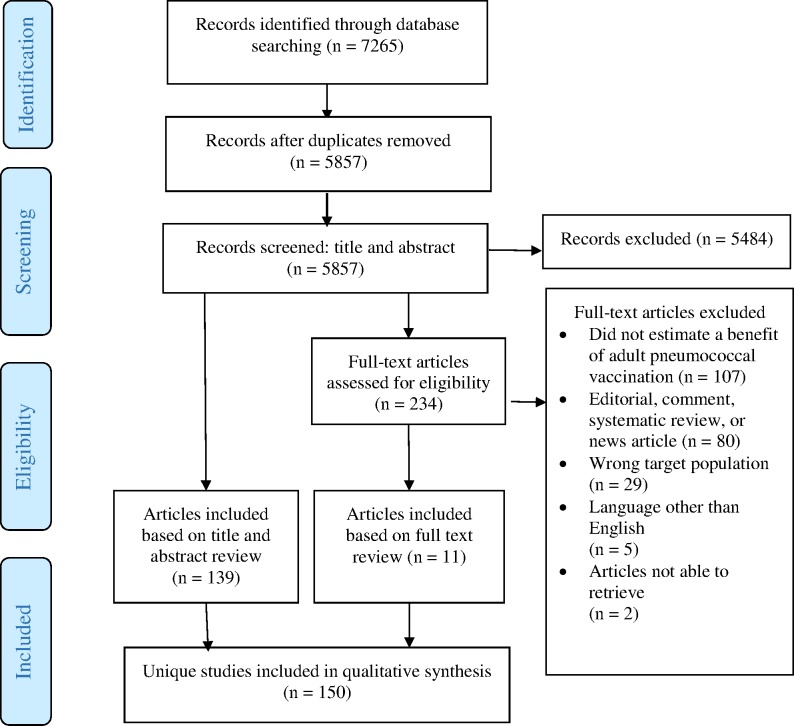
Application of the inclusion and exclusion criteria yielded 150 unique studies for analysis [45–194]. Fig 1 shows the yields at each stage of the review. Table 2 summarizes the reasons for excluding articles that reached the full text review stage. After attempting to access all the articles that reached the full text review stage, we were unable to obtain two articles and therefore excluded them from analysis [195, 196]. While the protocol allowed inclusion of articles recommended by experts during expert consultations and from snowball searching of references in included papers, we did not include any such articles, as our search captured all articles that either experts or reference lists brought to our attention.
Fig 1. Flow diagram of study selection.
Table 2. Summary of primary reasons for study exclusion after full text review.
| Primary reason for exclusion | Number (%) of studies |
|---|---|
| Does not capture a health or economic benefit of pneumococcal vaccination | 107 (47.98%) |
| Is a news article, comment, editorial, or review | 80 (35.87%) |
| Examines a target population that does not meet our criteria (e.g., children) | 29 (13.00%) |
| Is in a language other than English | 5 (2.24%) |
| Could not access article | 2 (0.90%) |
S4 File contains the extracted information for all included articles. We could not to apply criteria to grade the quality of the evidence in the included studies because most studies captured in our review are modeling studies. Major published methods for assessing quality of evidence do not include modeling studies in their hierarchy [197]. In addition, some included articles are not modeling studies, meaning that methods of quality assessment would be difficult to apply in a consistent, meaningful manner. Furthermore, because of the varied nature of included studies, we were not able to assess risk of bias in a meaningful way.
State of the literature
Table 3 summarizes the literature in terms of capturing the full benefits of adult pneumococcal vaccination. We found that, while the literature effectively captures narrow benefits, it rarely (if ever) captures all other benefits.
Table 3. Current state of the literature regarding the full benefits of adult pneumococcal vaccination.
| Perspective | Benefit category | Brief description | Number (%) of studies that capture* | |
|---|---|---|---|---|
| Healthcare cost savings | Averted direct medical costs | 79 studies (52.67%) | ||
| Narrow | Health gains | Inherent value of improved health | 149 studies (99.33%) | |
| Outcome-related productivity gains | Enhanced labor market output | 16 studies (10.67%) | ||
| Care-related productivity gains | Averted costs of formal or informal care | 2 studies (1.33%) | ||
| Voluntary contributions to family and community | Enhanced ability to volunteer and give care | 0 | ||
| Broad | Health-based community externalities | Herd effects or slowed pace of antimicrobial resistance | 1 study (0.67%) | |
| Prevention and amelioration of comorbidities | Value of experiencing fewer or milder comorbidities | 0 | ||
| Reduction in nosocomial infections | Averted hospital-acquired infection costs | 0 | ||
| Risk reduction gains | Value of peace of mind from vaccines | 0 | ||
| Promotion of social equity | Inherent value of narrowing health gaps | 0 | ||
*This represents the number (and percent) of included studies that capture the benefit category in question; categories are not mutually exclusive.
Narrow benefits
Fifty-three percent of studies included in our systematic review included healthcare cost savings, though parameters used to estimate this dimension varied and were often based on estimates. Health gains were included in all but one study included for analysis. Many studies in the review account for this benefit directly. Others account for it indirectly by reporting QALYs or life years (LYs) lost to pneumococcal disease for cost-effectiveness or budget impact calculations.
Other broad benefits
The most commonly captured benefit from the broad perspective was outcome-related productivity gains. Sixteen studies (10.7%) included this benefit [74–76, 81, 85, 87, 98, 108, 115, 117, 127, 132, 142, 144, 157, 165]. These studies estimated how much work individuals miss due to a case of pneumococcal disease and compute the value of that missed work by multiplying missed time by the wage rate. At times, the amount of missed work is weighted by the proportion of the population that is economically active [74]. Two studies (1.3%) accounted for care-related productivity gains [74, 127]. One study (0.7%) accounted for health-based community externalities vis-à-vis the value of pneumococcal vaccination in slowing the rate of antimicrobial resistance [160]. Our included studies did not account for any other benefits in our taxonomy.
Even within the literature that examines benefits other than our narrow ones, study quality and reporting are at times limited. For example, of the 17 unique studies that account for at least one broad benefit, seven (41.2%) present only study abstracts [75, 81, 85, 87, 98, 115, 144]. Of the remaining 10 studies [74, 76, 108, 117, 127, 132, 142, 157, 160, 165], other limitations abound, including, for example, borrowing epidemiological trend data from other countries [108], lack of data necessitating many assumptions and low estimate precision [157], and possible confounding due to a study’s observational nature [160].
Perspectives assumed by economic studies
Examining the perspectives from which economic analyses were performed provides further insights into the state of the literature. Of the 75 included studies that explicitly stated a perspective, 51 (68.0%) examine costs from a health payer’s perspective, meaning they only consider direct costs (i.e., healthcare cost savings). Moreover, 24 studies claim to adopt a societal perspective (either as the only perspective studied or in addition to another perspective, such as health payer’s perspective), implying they consider other broad benefits [198]. However, this likely overstates the number of studies that look beyond narrow benefits. Some studies that were purportedly performed from the societal perspective appear only to consider narrow benefits. We therefore conclude that some studies’ perspectives may have been mislabeled.
Interestingly, three economic modeling studies—two from Brazil and one from Mexico—took an employer’s perspective to analyze the effects of vaccinating employees against pneumococcal disease on outcome-related productivity gains [81, 85, 98]. While the three studies presented only abstracts, and are therefore inconclusive by themselves, their findings unanimously favored the benefits of adult pneumococcal vaccination. This unanimity suggests that perspectives besides health payer might be worth exploring further.
Nature of PCV13 studies
Because PCV13 is the newest adult pneumococcal vaccine, isolating the included studies that analyze PCV13 alone is worthwhile. In general, the 32 (21.3%) studies that present an analysis of PCV13 alone tend to take a more economically focused approach than the other included studies. Twenty-seven (84.4%) of these PCV13-only studies account for healthcare cost savings [55, 63, 64, 80, 82, 87, 91, 98, 110, 117, 123, 127, 128, 130–132, 150, 151, 153, 156–158, 168, 173, 178, 184, 194], compared with 79 (52.7%) of all included studies. Similarly, seven of these studies (21.9%) account for outcome-related productivity gains [81, 87, 98, 117, 127, 132, 157], which represents double the share of all included studies (10.7%).
But PCV13-only studies have limitations. For example, a common limitation is that vaccine efficacy in adults is merely assumed for the purposes of economic modeling. This limitation was particularly common before the results of the CAPITA trial, a major randomized control trial of PCV13 in adults 65 and older, were published [57].
Discussion
This systematic review shows that the literature does effectively capture some benefits of adult pneumococcal vaccination—notably, health gains and healthcare cost savings. To a limited extent, the literature takes a broader approach to estimating the benefits of adult pneumococcal vaccination. However, most benefits beyond the narrow approach are rarely, if ever, captured.
But this is not to say that these additional benefits are negligible. In fact, existing literature outside the scope of adult pneumococcal vaccines suggests that some benefits are potentially sizeable and are therefore worth investigating further.
The first of these potentially important benefits surrounds the notion that pneumococcal vaccines slow the rate of antimicrobial resistance (AMR) in at least two ways: by slowing the spread of especially resistant serotypes (such as serotype 19A, which is targeted by both commercially available adult vaccines) and by preventing illnesses and thus precluding the need for antibiotics. Although the literature on adult vaccination largely overlooks this effect, pneumococcal conjugate vaccines have been singled out as potentially significant players in the fight to reduce the rate of antibiotic resistance [199, 200]. This effect has clear community health benefits.
Second, recent evidence from the epidemiological literature suggests that some serotypes have low incidence among the young but high incidence among older adults [201]. Therefore, herd effects from vaccinating adults seem possible, which would spread the protective effects of these vaccines to nonvaccinated community members. This could be particularly important in settings with high concentrations of older adults, such as nursing homes. The strength of such herd effects would depend in large part on contextual factors such as childhood vaccine uptake rates [202]. Such factors should be accounted for when estimating the value of adult vaccination strategies.
Third, vaccinating adults attending mass gatherings may be an effective approach to limiting adult transmission [203]. Recognizing this, the Saudi Arabian government requires some vaccines for Hajj and Umrah pilgrims [204], including vaccines against meningitis and poliomyelitis. Including pneumococcal vaccination in the requirements for participants at mass gatherings could carry benefits for many parties: the vaccine recipient, other attendees who come into contact with vaccine recipients, travel companies that could offer the vaccine to appeal to risk-averse attendees, and government health ministries that would save money on treatment costs.
Fourth, parents and grandparents who are healthier are measurably better able to care for children and grandchildren [205, 206] and should in principle make greater voluntary contributions to their communities. Pneumococcal vaccines could contribute to these valuable ends.
Fifth, adults are demonstrably willing to pay to reduce risks to income [207] and to health [208]—both of which pneumococcal vaccines can help ameliorate.
Sixth, insofar as adult pneumococcal disease disproportionately affects the poor, vaccination can diminish social and economic inequalities [209, 210], an outcome that many consider inherently valuable.
Failing to investigate these and other sources of benefit further potentially leads to undervaluing adult pneumococcal vaccines' contributions and therefore underinvesting in their development and adoption. Additional work in this area should create an evidence base aimed at remedying these shortcomings.
Limitations of this study
One limitation of our review is that we only included English-language studies. While we recognize that studies written in other languages likely contribute to the evidence base on the benefits of adult pneumococcal vaccination, our team’s shared language ability is limited to English. Another limitation of our study is the inability to apply a standard bias assessment to the included studies, such as the Cochrane Risk of Bias tool [197], which assesses risk of bias in randomized controlled trials, or ROBIS [211], a tool for assessing the risk of bias in systematic reviews. In lieu of a standard bias assessment, we recognize and comment on many of the limitations in the included studies (see S4 File). A third limitation of our analysis is that while many studies indicated a societal perspective, this was not clearly defined. Thus, we may have miscategorized some studies due to lack of information.
Finally, other vaccinations delivered in the same context, including influenza vaccination among target groups or pediatric vaccination programs, can affect the potential benefits of adult pneumococcal vaccination. This may make generalizing results to a different country context difficult. Several of our included studies demonstrate that co-administration of influenza and pneumococcal vaccination can have positive benefits [61, 62, 88, 164]. A recent systematic review and meta-analysis showed that high childhood vaccination coverage can lead to substantial herd effects, thus protecting unvaccinated adults from disease and reducing the magnitude of the potential benefits of adult vaccination [202]. The studies included in our review inconsistently account for herd effects from childhood vaccination (see S4 File for more detail). Therefore, any analysis of the full benefits of adult pneumococcal vaccination must consider this context.
Conclusions
We conducted this systematic review to identify the breadth and depth of the benefits of adult pneumococcal vaccination captured in the literature. The review followed PRISMA guidelines (See S5 File). We included 150 unique studies for analysis. The literature effectively captures narrow benefits but rarely captures the full range of benefits in our framework. Further research is needed to quantify the broad benefits of adult pneumococcal vaccination.
Supporting information
The Full Benefits of Adult Pneumococcal Vaccination: A Systematic Review.
(PDF)
Search Terms. The Full Benefits of Adult Pneumococcal Vaccination: A Systematic Review.
(PDF)
Extraction Template. The Full Benefits of Adult Pneumococcal Vaccination: A Systematic Review.
(PDF)
Extraction Table. The Full Benefits of Adult Pneumococcal Vaccination: A Systematic Review.
(XLSX)
PRISMA Checklist.
(PDF)
Acknowledgments
Carol Mita of Harvard University’s Countway Library guided the process of generating our search string and contributed useful advice regarding the systematic review process. Mathew McKenna of Data for Decisions, LLC, provided valuable assistance in extracting relevant information from included studies. Many experts in the field of pneumococcal vaccination, including Lakshmi Ganapathi, Peifeng “Perry” Hu, Allison McGeer, Mark Steinhoff, and Ramnath Subbaraman, helped form the foundational understanding of pneumococcal disease and pneumococcal vaccines that has guided this work. Lakshmi Reddy Bloom of Data for Decisions, LLC made valuable contributions to the development of the taxonomy used in this systematic review. JP Sevilla and Daria Burnes of Data for Decisions, LLC, and Kristine Husøy Onarheim of the University of Bergen provided insightful feedback on previous drafts of this manuscript. Finally, we are grateful for helpful comments from colleagues at Pfizer, Inc.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding for this systematic review was provided by Pfizer Inc. (http://www.pfizer.com/) to Data for Decisions, LLC. RS, an employee of Pfizer Inc., is a co-author and played a role in study design, analysis, and preparation of the manuscript. Data for Decisions, LLC provided support in the form of compensation for DEB, ECF, AS, and STJ, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the "author contributions" section.
References
- 1.World Health Organization. Pneumococcal Disease [cited 2016 Nov 3]. Available from: http://www.who.int/ith/diseases/pneumococcal/en/.
- 2.World Health Organization. Inflenza (Seasonal); Fact Sheet N°211 [2014 Mar; cited 2016 Nov 3]. Available from: http://www.who.int/mediacentre/factsheets/fs211/en/.
- 3.World Health Organization. Number of Malaria Deaths [cited 2016 Nov 3]. Available from: http://www.who.int/gho/malaria/epidemic/deaths/en/.
- 4.World Health Organization. Number of Deaths Due to HIV/AIDS [cited 2016 Nov 3]. Available from: http://www.who.int/gho/hiv/epidemic_status/deaths_text/en/.
- 5.Centers for Disease Control and Prevention. Pneumococcal disease In: Hamborsky J, Kroger A, Wolfe S, editors. Epidemiology and prevention of vaccine-preventable diseases. 13th ed. Washington, DC: Public Health Foundation; 2015. pp. 279–296. [Google Scholar]
- 6.Kupronis BA, Richards CL, Whitney CG, the Active Bacterial Core Surveillance Team. Invasive pneumococcal disease in older adults residing in long-term care facilities and in the community. Journal of the American Geriatrics Society. 2003;51(11):1520–1525. [DOI] [PubMed] [Google Scholar]
- 7.United Nations Department of Economic and Social Affairs Population Division. World population prospects: the 2015 revision, key findings & advance tables. [2015; cited 2016 Nov 3]. Working paper no. ESA/P/WP.241. Available from: https://esa.un.org/unpd/wpp/publications/files/key_findings_wpp_2015.pdf
- 8.Merck. PNEUMOVAX 23 patient product information [cited 2016 Nov 3]. Available from: https://www.merck.com/product/usa/pi_circulars/p/pneumovax_23/pneumovax_ppi.pdf.
- 9.European Medicines Agency. Annex 1: summary of product characteristics (Prevenar 13) [cited 2016 Nov 3]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001104/WC500057247.pdf
- 10.Barra M. Prevenar 13/ Prevnar 13 approval history in Europe and in the U.S: PharmUpdates; 2013 [cited 2017 August 2]. Available from: https://pharmupdates.wordpress.com/2013/07/22/prevenar-13-prevnar-13-approval-history-in-europe-and-in-the-u-s/.
- 11.Williams WW, Lu PJ, O'Halloran A, Kim DK, Grohskopf LA, Pilishvili T, et al. Surveillance of vaccination coverage among adult populations—United States, 2014. MMWR Surveill Summ. 2016;65(1):1–36. doi: 10.15585/mmwr.ss6501a1 [DOI] [PubMed] [Google Scholar]
- 12.Castiglia P. Recommendations for pneumococcal immunization outside routine childhood immunization programs in Western Europe. Advances in therapy. 2014;31(10):1011–1044. doi: 10.1007/s12325-014-0157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito S, Bonanni P, Maggi S, Tan L, Ansaldi F, Lopalco P, et al. Recommended immunization schedules for adults: Clinical practice guidelines by the Escmid Vaccine Study Group (EVASG), European Geriatric Medicine Society (EUGMS) and the World Association for Infectious Diseases and Immunological Disorders (WAidid). Human Vaccines and Immunotherapeutics. 2016;12(7):1777–1194. doi: 10.1080/21645515.2016.1150396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloom DE, Canning D, Fink G. Disease and development revisited. Journal of Political Economy. 2014;122(6):1355–1366. [Google Scholar]
- 15.Bloom DE, Fink G. The economic case for devoting public resources to health In: Farrar J, Hotez PJ, Junghanss T, Kang H, Lalloo D, White NJ, editors. Manson’s tropical diseases. 23rd ed. Elsevier Health Sciences; 2013. pp. 23–30. [Google Scholar]
- 16.Bloom DE, Canning D. The health and wealth of nations. Science. 2000;287(5456):1207–1209. [DOI] [PubMed] [Google Scholar]
- 17.Bärnighausen T, Bloom DE, Cafiero-Fonseca ET, O'Brien JC. Valuing the broader benefits of dengue vaccination, with a preliminary application to Brazil. Semin Immunol. 2013;25(2):104–113. doi: 10.1016/j.smim.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 18.Bärnighausen T, Bloom DE, Cafiero-Fonseca ET, O'Brien JC. New thinking on the value of vaccination–globally and in India In: Vashinshtha V, Agarwal R, Sukmaran T, editors. Indian Academy of Pediatrics Textbook of Vaccines. New Delhi: Jaypee Brothers; 2014. pp. 563–571. [Google Scholar]
- 19.Bärnighausen T, Bloom DE, Cafiero-Fonseca ET, O'Brien JC. Valuing vaccination. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(34):12313–12319. doi: 10.1073/pnas.1400475111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloom DE, Madhavan G. Vaccines: From valuation to resource allocation. Vaccine. 2015;33, Supplement 2:B52–B54. [DOI] [PubMed] [Google Scholar]
- 21.Bloom DE, Canning D, Weston M. The value of vaccination. World Economics. 2005;6(3):15–39. [Google Scholar]
- 22.Bloom DE. The value of vaccination In: Curtis N, Finn A, Pollard AJ, editors. Hot topics in infection and immunity in children VII. Advances in Experimental Medicine and Biology. 697: Springer New York; 2011. pp. 1–8. [Google Scholar]
- 23.Bärnighausen T, Bloom DE, Canning D, O'Brien J. Accounting for the full benefits of childhood vaccination in South Africa. South African Medical Journal. 2008;98:842–846. [PubMed] [Google Scholar]
- 24.Bärnighausen T, Bloom DE, Canning D, Friedman A, Levine OS, O’Brien J, et al. Rethinking the benefits and costs of childhood vaccination: The example of the Haemophilus influenzae type b vaccine. Vaccine. 2011;29(13):2371–2380. doi: 10.1016/j.vaccine.2010.11.090 [DOI] [PubMed] [Google Scholar]
- 25.Bloom DE, Canning D, Shenoy ES. The effect of vaccination on children's physical and cognitive development in the Philippines. Applied Economics. 2012;44(21):2777–2783. [Google Scholar]
- 26.Bärnighausen T, Berkley S, Bhutta ZA, Bishai DM, Black MM, Bloom DE, et al. Reassessing the value of vaccines. The Lancet Global Health. 2014;2(5):e251–e252. doi: 10.1016/S2214-109X(13)70170-0 [DOI] [PubMed] [Google Scholar]
- 27.van der Putten IM, Evers SM, Deogaonkar R, Jit M, Hutubessy RC. Stakeholders’ perception on including broader economic impact of vaccines in economic evaluations in low and middle income countries: A mixed methods study. BMC Public Health. 2015;15(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bärnighausen T, Bloom DE, Cafiero-Fonseca ET, O'Brien JC. Economic evaluation of vaccination: Capturing the full benefits, with an application to human papillomavirus. Clinical Microbiology and Infection. 2012;18(Suppl. 5):70–76. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Report on the Immunization and Vaccines Related Implementation Research (IVIR): Advisory Committee meeting, Geneva, 25–26 September 2012 [cited 2017 Jan 10]. Available from: http://www.who.int/immunization/research/committees/QUIVER_meeting_report_2012_WHO_IVB_12.12_eng.pdf?ua=1.
- 30.Jit M, Hutubessy R, Png ME, Sundaram N, Audimulam J, Salim S, et al. The broader economic impact of vaccination: reviewing and appraising the strength of evidence. BMC Medicine. 2015;13(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Constenla D, Garcia C, Lefcourt N. Assessing the economics of dengue: results from a systematic review of the literature and expert survey. Pharmacoeconomics. 2015;33(11):1107–1135. doi: 10.1007/s40273-015-0294-7 [DOI] [PubMed] [Google Scholar]
- 32.Timmis JK, Rigat F, Rappuoli R. Core values for vaccine evaluation. Vaccine. 2017;35(Supplement 1):A57–A62. [DOI] [PubMed] [Google Scholar]
- 33.Bloom DE, Brenzel L, Cadarette D, Sullivan J. Moving beyond traditional valuation of vaccination: Needs and opportunities. Vaccine. 2017;35(Suppl 1):A29–A35. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz JL, Mahmoud A. When not all that counts can be counted: economic evaluations and the value of vaccination. Health Affairs. 2016;35(2):208–211. doi: 10.1377/hlthaff.2015.1438 [DOI] [PubMed] [Google Scholar]
- 35.Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451(7179):716–719. doi: 10.1038/nature06516 [DOI] [PubMed] [Google Scholar]
- 36.Porchia BR, Bonanni P, Bechini A, Bonaccorsi G, Boccalini S. Evaluating the costs and benefits of pneumococcal vaccination in adults. Expert Review of Vaccines. 2017;16(2):93–107. [DOI] [PubMed] [Google Scholar]
- 37.Tin Tin Htar M, Stuurman AL, Ferreira G, Alicino C, Bollaerts K, Paganino C, et al. Effectiveness of pneumococcal vaccines in preventing pneumonia in adults, a systematic review and meta-analyses of observational studies. PLOS ONE. 2017;12(5):e0177985 doi: 10.1371/journal.pone.0177985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraicer-Melamed H, O'Donnell S, Quach C. The effectiveness of pneumococcal polysaccharide vaccine 23 (PPV23) in the general population of 50 years of age and older: A systematic review and meta-analysis. Vaccine. 2016;34(13):1540–1550. doi: 10.1016/j.vaccine.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 39.Falkenhorst G, Remschmidt C, Harder T, Hummers-Pradier E, Wichmann O, Bogdan C. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal disease in the elderly: Systematic review and meta-analysis PLOS ONE. 2017;12(1):e0169368 doi: 10.1371/journal.pone.0169368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deogaonkar R, Hutubessy R, van der Putten I, Evers S, Jit M. Systematic review of studies evaluating the broader economic impact of vaccination in low and middle income countries. BMC Public Health. 2012;12(1):878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirelman AJ, Ozawa S, Grewal S. The economic and social benefits of childhood vaccinations in BRICS. Bulletin of the World Health Organization. 2014;92(6):454–456. doi: 10.2471/BLT.13.132597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozawa S, Clark S, Portnoy A, Grewal S, Brenzel L, Walker DG. Return on investment from childhood immunization in low- and middle-income countries, 2011–20. Health Affairs. 2016;35(2):2199–2207. [DOI] [PubMed] [Google Scholar]
- 43.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Annals of Internal Medicine. 2009;151(4):W-65–W-94. [DOI] [PubMed] [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Annals of Internal Medicine. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 45.Abdallah J, Anna K, Hassan T, Jain AK. Vaccination outcomes in inflammatory bowel disease. Gastroenterology. 2014;146(5):S-170. [Google Scholar]
- 46.Akin L, Kaya M, Altinel S, Durand L. Cost of pneumococcal infections and cost-effectiveness analysis of pneumococcal vaccination at risk adults and elderly in Turkey. Human Vaccines. 2011;7(4):441–450. doi: 10.4161/hv.7.4.14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albuja Riofrio MF, Vivero Altamirano RL, Mould Quevedo JF, Roberts CS. Cost-effectiveness analysis of anti-pneumococcal vaccination in high risk and adult patients in Ecuador. Value in Health. 2012;15(4):A194. [Google Scholar]
- 48.Alves M, Lundgren F, Caribé C, Secundo I, Marrocos S, Accioly J, et al. Pneumococcal vaccination: Effects on exacerbation of COPD. European Respiratory Journal. 2013;42(Suppl 57):P3688. [Google Scholar]
- 49.Alvis N, De La Hoz F, Castañeda C, Paternina A. Cost-effectiveness of pneumococcal vaccine polyvalent (PPSV23) in adults older than 60 years old in Colombia. Value in Health. 2010;13(3):A190. [Google Scholar]
- 50.Andrews NJ, Waight PA, George RC, Slack MPE, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30(48):6802–6808. doi: 10.1016/j.vaccine.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 51.Arencibia Jiménez M, Navarro Gracia JF, Delgado de los Reyes JA, Pérez Torregrosa G, López Parra D, López García P. Missed opportunities in antipneumococcal vaccination. Can something more be done for prevention? Archivos de Bronconeumologia. 2014;50(3):93–98. doi: 10.1016/j.arbres.2013.09.016 [DOI] [PubMed] [Google Scholar]
- 52.Becker-Dreps S, Amaya E, Liu L, Rocha J, Briceño R, Moreno G, et al. Impact of a combined pediatric and adult pneumococcal immunization program on adult pneumonia incidence and mortality in Nicaragua. Vaccine. 2015;33(1):222–227. doi: 10.1016/j.vaccine.2014.10.073 [DOI] [PubMed] [Google Scholar]
- 53.Biagini Leandro L, Rojas Ruben R, Fuentealba Francisca F, Pezzani Marcela M. Cost/utility analysis of pneumococcal vaccinees PCV13 versus PPSV23 in adults over 18 years old in Chile. Value in Health. 2015;18(3):A240–A241. [Google Scholar]
- 54.Blommaert A, Bilcke J, Willem L, Verhaegen J, Goossens H, Beutels P. The cost-effectiveness of pneumococcal vaccination in healthy adults over 50: An exploration of influential factors for Belgium. Vaccine. 2016;34(18):2106–2112. doi: 10.1016/j.vaccine.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 55.Boccalini S, Bechini A, Levi M, Tiscione E, Gasparini R, Bonanni P. Cost-effectiveness of new adult pneumococcal vaccination strategies in Italy. Human Vaccines & Immunotherapeutics. 2013;9(3):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bond TC, Spaulding AC, Krisher J, McClellan W. Mortality of dialysis patients according to influenza and pneumococcal vaccination status. Am J Kidney Dis. 2012;60(6):959–965. doi: 10.1053/j.ajkd.2012.04.018 [DOI] [PubMed] [Google Scholar]
- 57.Bonten MJM, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. New England Journal of Medicine. 2015;372(12):1114–1125. doi: 10.1056/NEJMoa1408544 [DOI] [PubMed] [Google Scholar]
- 58.Bussell SA. A role for the pneumococcal vaccine during admission for stroke? Observed protective effect against death in the Medicare population. Chest. 2010;138(4):735A. [Google Scholar]
- 59.Cai J, Shito A, Imai K, Bahadursingh K. An ecological analysis on national trends and correlation between public funding for pneumococcal vaccination and pneumonia disease burden in the Japanese elderly population, 2005–2012. Value in Health. 2014;17(7):A755. [DOI] [PubMed] [Google Scholar]
- 60.Castañeda-Orjuela C, Alvis-Guzmán N, Paternina ÁJ, De la Hoz-Restrepo F. Cost-effectiveness of the introduction of the pneumococcal polysaccharide vaccine in elderly Colombian population. Vaccine. 2011;29(44):7644–7650. doi: 10.1016/j.vaccine.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 61.Chan TC, Hung IFN, Luk JKH, Shea YF, Chan FHW, Woo PCY, et al. Prevention of mortality and pneumonia among nursing home older adults by dual pneumococcal and seasonal influenza vaccination during a pandemic caused by novel pandemic influenza A (H1N1). Journal of the American Medical Directors Association. 2012;13(8):698–703. doi: 10.1016/j.jamda.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 62.Chang YC, Chou YJ, Liu JY, Yeh TF, Huang N. Additive benefits of pneumococcal and influenza vaccines among elderly persons aged 75 years or older in Taiwan—A representative population-based comparative study. Journal of Infection. 2012;65(3):231–238. doi: 10.1016/j.jinf.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 63.Charos A, Balmer P. Cost-effectiveness of adult vaccination of HIV-positive individuals with the 13-Valent Pneumococcal Conjugate Vaccine in the United Kingdom. HIV Medicine. 2012;13(S1):42. [Google Scholar]
- 64.Charos A, Barzey V, Lloyd A, Balmer P. Cost-effectiveness of adult vaccination with 13-valent pneumococcal conjugate vaccine in the United Kingdom. Clinical Microbiology and Infection. 2012;18(S3):20. [Google Scholar]
- 65.Chen J, O’Brien M, Yang HK, Grabenstein J, Dasbach E. Cost-effectiveness of pneumococcal vaccines for adults in the United States. Advances in Therapy. 2014;31(4):392–409. doi: 10.1007/s12325-014-0115-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiou WY, Hung SK, Lai CL, Lin HY, Su YC, Chen YC, et al. Effect of 23-valent pneumococcal polysaccharide vaccine inoculated during anti-cancer treatment period in elderly lung cancer patients on community-acquired pneumonia hospitalization: A nationwide population-based cohort study. Medicine (Baltimore). 2015;94(26):e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cho B-H, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011–6021. doi: 10.1016/j.vaccine.2013.10.024 [DOI] [PubMed] [Google Scholar]
- 68.Chuck A, Waye A, Zanotti G, Jacobs P, Kellner J, Tyrrell G. Pharmacoeconomic evaluation of 13-valent pneumococcal conjugate and 23-valent pneumococcal polysaccharide vaccine in Canadian adults. Value in Health. 2012;15(4):A243. [Google Scholar]
- 69.Cimen P, Unlu M, Kirakli C, Katgi N, Ucsular FD, Ayranci A, et al. Should Patients With COPD Be Vaccinated? Respiratory Care. 2015;60(2):239–243. doi: 10.4187/respcare.03350 [DOI] [PubMed] [Google Scholar]
- 70.Claes C, Pletz MW, Van Der Linden M, Welte T, Von Der Schulenburg Graf JM. Pharmacoeconomic modeling using the 13-valent pneumococcal conjugate vaccine in German adults. Value in Health. 2010;13(7):A435–A436. [Google Scholar]
- 71.Collamati A, Landi F, Poscia A, Topinkova E, Bernabei R, Onder G. Vaccination and survival in a population of older adult living in nursing home. European Geriatric Medicine. 2015;6:S18–S19. [Google Scholar]
- 72.Coulson E, Saravanan V, Hamilton J, Long KS, Morgan L, Heycock C, et al. Pneumococcal antibody levels after pneumovax in patients with rheumatoid arthritis on methotrexate. Annals of the Rheumatic Diseases. 2011;70(7):1289–1291. doi: 10.1136/ard.2010.144451 [DOI] [PubMed] [Google Scholar]
- 73.Cruz RB, Fernandes RA, Takemoto M, Cukier FN, Fujii RK, Roberts CS, et al. Economic evaluation of 13-valent pneumococcal vaccine in high risk elderly population, from the public payer perspective in Brazil. Value in Health. 2012;15(7):A394. [Google Scholar]
- 74.de Soárez PC, Sartori AMC, Freitas AC, Nishikawa ÁM, Novaes HMD. Cost-effectiveness analysis of universal vaccination of adults aged 60 years with 23-valent pneumococcal polysaccharide vaccine versus current practice in Brazil. PLOS ONE. 2015;10(6):e0130217 doi: 10.1371/journal.pone.0130217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dhankhar P, Grabenstein J, O'Brien M, Dasbach EJ. Cost effectiveness of Pneumovax®23 stockpile to prevent secondary pneumococcal infections among a high-risk population in the United States during an influenza pandemic. International Journal of Infectious Diseases. 2010;14:e449. [DOI] [PubMed] [Google Scholar]
- 76.Dhankhar P, Grabenstein JD, O'Brien MA, Dasbach EJ. Cost-effectiveness of stockpiling 23-valent pneumococcal polysaccharide vaccine to prevent secondary pneumococcal infections among a high-risk population in the United States during an influenza pandemic. Clin Ther. 2010;32(8):1501–1516. doi: 10.1016/j.clinthera.2010.07.019 [DOI] [PubMed] [Google Scholar]
- 77.Domínguez A, Castilla J, Godoy P, Delgado-Rodríguez M, Saez M, Soldevila N, et al. Effectiveness of vaccination with 23-valent pneumococcal polysaccharide vaccine in preventing hospitalization with laboratory confirmed influenza during the 2009–2010 and 2010–2011 seasons. Human Vaccines and Immunotherapeutics. 2013;9(4):865–873. doi: 10.4161/hv.23090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Domínguez A, Izquierdo C, Salleras L, Ruiz L, Sousa D, Bayas JM, et al. Effectiveness of the pneumococcal polysaccharide vaccine in preventing pneumonia in the elderly. European Respiratory Journal. 2010;36(3):608–614. doi: 10.1183/09031936.00171309 [DOI] [PubMed] [Google Scholar]
- 79.Fariñas-Alvarez C, Nuñez-Viejo MA, Ansorena-Pool L, Rojo F, Sanz-Salanova JA, Llorca J, et al. Impact of pneumococcal vaccination in preventing invasive pneumococcal disease in Cantabria (SPAIN). Clinical Microbiology and Infection. 2011;17:S243–S244. [Google Scholar]
- 80.Ferreira CN, Manfrin DF, Rufino CS, Gea Y, Haas LC, Fernandes RA. Budget impact analysis of 13-valent pneumococcal vaccine in adult population with comorbidities or immunocompromised from the public payer perspective in Brazil. Value in Health. 2014;17(7):A667–A668. [DOI] [PubMed] [Google Scholar]
- 81.Ferreira CN, Manfrin DF, Rufino CS. Financial analysis of a vaccination campaign using 13 valent pneumococcal conjugated vaccine (PCV13) with employers. Value in Health. 2014;17(3):A270. [DOI] [PubMed] [Google Scholar]
- 82.Ferreira CN, Santana CF, Rufino C. Public health and economic impact of 13-valent pneumococcal conjugate vaccine (PCV13) in public and private system versus PPSV23 and no vaccination in older adults. Value in Health. 2015;18(7):A839. [Google Scholar]
- 83.French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. New England Journal of Medicine. 2010;362(9):812–822. doi: 10.1056/NEJMoa0903029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fujii RK, Roberts CS, Mould J, Presa J, Jardim E, Manfrin DF. Cost effectiveness analysis of vaccination with 13-valent (PCV13) and 23-valent (PPV23) pneumococcal vaccines for senior adults in Sao Paulo state, Brazil—Public perspective. Value in Health. 2012;15(7):A397. [Google Scholar]
- 85.Fujii RK, Presa J, Roberts CS, Gea Y, Manfrin DF, Mould J. Budget impact evaluation of a vaccination with campaign program for corporations using pneumococcal conjugated vaccine (PCV) 13 valent versus pneumococcal polisaccharide vaccine (PPV) 23 with paid campaign for older adults. Value in Health. 2013;16(3):A84–A85. [Google Scholar]
- 86.Fujii RK, Mould JF, Presa J, Jardim E, Sato R, Strutton DR. Cost effectiveness analysis of vaccination with 13-valent (PCV13) and 23-valent (PPV23) pneumococcal vaccines for senior adults in Brazil. Value in Health. 2011;14(7):A279. [Google Scholar]
- 87.Fujii RK, Presa J, Roberts CS, Gea Y, Manfrin DF, Mould J. Budget impact evaluation of a pneumococcal conjugated vaccine (PCV) 13 valent vaccination with free campaign program for corporations vesus no vaccination for older adults. Value in Health. 2013;16(3):A84. [Google Scholar]
- 88.Gilbertson DT, Guo H, Arneson TJ, Collins AJ. The association of pneumococcal vaccination with hospitalization and mortality in hemodialysis patients. Nephrol Dial Transplant. 2011;26(9):2934–2939. doi: 10.1093/ndt/gfq853 [DOI] [PubMed] [Google Scholar]
- 89.Gomez-Junyent J, Garcia-Vidal C, Viasus D, Millat-Martinez P, Simonetti A, Santos MS, et al. Clinical features, etiology and outcomes of community-acquired pneumonia in patients with chronic obstructive pulmonary disease. PLOS ONE. 2014;9(8):e105854 doi: 10.1371/journal.pone.0105854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grzesiowski P, Aguiar-Ibáñez R, Kobryń A, Durand L, Puig P-E. Cost-effectiveness of polysaccharide pneumococcal vaccination in people aged 65 and above in Poland. Human Vaccines & Immunotherapeutics. 2012;8(10):1382–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guijarro P, Echave M. Clinical and economic evaluation of an adult pneumococcal vaccination programme aimed at the Spanish HIV population. Value in Health. 2012;15(7):A389. [Google Scholar]
- 92.Gutiérrez Rodríguez MA, Rosales Statkus ME, Córdoba Deorador E, Taveira Jiménez JA, Arce Arnáez A, Sanz Moreno JC, et al. Invasive pneumococcal disease in adults older than 59 years in the autonomous region of Madrid Spain, 2008–2010. Clinical Microbiology and Infection. 2012;18:149. [Google Scholar]
- 93.Gutiérrez Rodríguez MA, Ordobás Gavín M, García-Comas L, Sanz Moreno JC, Córdoba Deorador E, Lasheras Carbajo MD, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine in adults aged 60 years and over in the Region of Madrid, Spain, 2008–2011. Euro Surveill. 2014;19(40):20922 [DOI] [PubMed] [Google Scholar]
- 94.Hanna JN, Humphreys JL, Murphy DM, Smith HV. Invasive pneumococcal disease in non-Indigenous people in north Queensland, 2001–2009. Med J Aust. 2010;193(7):392–396. [DOI] [PubMed] [Google Scholar]
- 95.Hechter RC, Chao C, Jacobsen SJ, Slezak JM, Quinn VP, Van Den Eeden SK, et al. Clinical effectiveness of pneumococcal polysaccharide vaccine in men: California Men's Health Study. Vaccine. 2012;30(38):5625–5630. doi: 10.1016/j.vaccine.2012.06.085 [DOI] [PubMed] [Google Scholar]
- 96.Hoshi SL, Kondo M, Okubo I. Economic evaluation of immunisation programme of 23-valent pneumococcal polysaccharide vaccine and the inclusion of 13-valent pneumococcal conjugate vaccine in the list for single-dose subsidy to the elderly in Japan. PLOS ONE. 2015;10(10):e0139140 doi: 10.1371/journal.pone.0139140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hsieh MJ, Tsai YH, Chang CJ, Wen YW, Hu HC, Chao YN, et al. The efficacy of 23-valent pneumococcal polysaccharide vaccine in preventing pneumonia and invasive pneumococcal disease in the elderly aged 75 years and older in Taiwan. Chest. 2013;144(4):254A. [Google Scholar]
- 98.Huicochea-Bartelt JL, Muciño-Ortega E, Vargas-Valencia JJ. Economic impact of a 13-valent pneumococcal conjugate vaccination programme in a Mexican corporate setting. Value in Health. 2014;17(3):A277. [Google Scholar]
- 99.Hung IF, Leung AY, Chu DW, Leung D, Cheung T, Chan CK, et al. Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clin Infect Dis. 2010;51(9):1007–1016. doi: 10.1086/656587 [DOI] [PubMed] [Google Scholar]
- 100.Ignatova G, Antonov V, Rodionova O. Effectiveness of vaccination of patients with combination of COPD and coronary heart disease (CHD). European Respiratory Journal. 2015;46(S59):PA3673. [Google Scholar]
- 101.Inoue S, Watanuki Y, Kaneko T, Sato T, Miyazawa N, Kaneko T, et al. Heterogeneity of the efficacy of the 23-valent pneumococcal polysaccharide vaccine caused by various underlying conditions of chronic pulmonary disease in older patients: prospective cohort study. BMJ Open. 2011;1(1):e000105 doi: 10.1136/bmjopen-2011-000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang Y, Gauthier A, Annemans L, Van Der Linden M, Nicolas-Spony L, Bresse X. A cost-effectiveness analysis of vaccinating the elderly with 23-valent pneumococcal polysaccharide vaccine (PPV23) in Germany. Value in Health. 2011;14(7):A279. [DOI] [PubMed] [Google Scholar]
- 103.Jiang Y, Gauthier A, Annemans L, Van Der Linden M, Nicolas-Spony L, Bresse X. A public health and budget impact analysis of vaccinating at risks and elderly adults with polysaccharide pneumococcal vaccine (PPV23) compared to pneumococcal conjugate vaccine (PCV13) in Germany. European Geriatric Medicine. 2011;2(S1):S85. [Google Scholar]
- 104.Jiang Y, Gauthier A, Annemans L, Van Der Linden M, Nicolas-Spony L, Bresse X. Modelling budget impact (BI) of vaccinating at-risk adults and the elderly with 23-valent pneumococcal polysaccharide vaccine (PPV23) compared to 13-valent pneumococcal conjugate vaccine (PCV13) in Germany. Value in Health. 2011;14(7):A268. [Google Scholar]
- 105.Jiang Y, Gauthier A, Keeping ST, Carroll SM. A budget impact (bi) analysis of vaccinating the elderly and at-risk adults with the 23-valent pneumococcal polysaccharide vaccine (ppv23) compared to no vaccination (novac) or 13-valent pneumococcal conjugate vaccine (pcv13) in the UK. Value in Health. 2012;15(7):A388. [Google Scholar]
- 106.Jiang Y, Gauthier A, Annemans L, van der Linden M, Nicolas-Spony L, Bresse X. A public health and budget impact analysis of vaccinating at-risk adults and the elderly against pneumococcal diseases in Germany. Expert Review of Pharmacoeconomics & Outcomes Research. 2012;12(5):631–643. [DOI] [PubMed] [Google Scholar]
- 107.Jiang Y, Gauthier A, Keeping ST, Carroll SM. Assessing the cost-effectiveness (CE) of vaccinating the elderly and at-risk adults with the 23-valent pneumococcal polysaccharide vaccine (PPV23) compared to the 13-valent pneumococcal conjugate vaccine (PCV13) or no vaccination (NOVAC) in the UK. Value in Health. 2012;15(7):A396–A397. [Google Scholar]
- 108.Jiang Y, Gauthier A, Annemans L, van der Linden M, Nicolas-Spony L, Bresse X. Cost–effectiveness of vaccinating adults with the 23-valent pneumococcal polysaccharide vaccine (PPV23) in Germany. Expert Review of Pharmacoeconomics & Outcomes Research. 2012;12(5):645–660. [DOI] [PubMed] [Google Scholar]
- 109.Jiang Y, Gervais F, Gauthier A, Baptiste C, Martinon P, Bresse X. A comparative public health and budget impact analysis of pneumococcal vaccines. The French case. Value in Health. 2014;17(7):A668. [DOI] [PubMed] [Google Scholar]
- 110.Jiang Y, Gauthier A, Keeping S, Carroll S. A public health and budget impact analysis of vaccinating the elderly and at-risk adults with the 23-valent pneumococcal polysaccharide vaccine or 13-valent pneumococcal conjugate vaccine in the UK. Expert Rev Pharmacoecon Outcomes Res. 2014;14(6):901–911. doi: 10.1586/14737167.2014.953932 [DOI] [PubMed] [Google Scholar]
- 111.Jiang Y, Gauthier A, Keeping S, Carroll S. Cost–effectiveness of vaccinating the elderly and at-risk adults with the 23-valent pneumococcal polysaccharide vaccine or 13-valent pneumococcal conjugate vaccine in the UK. Expert Review of Pharmacoeconomics & Outcomes Research. 2014;14(6):913–927. [DOI] [PubMed] [Google Scholar]
- 112.Jingu D, Yajima T, Ubukata S, Shoji M, Watanabe H, Takahashi H. Impact of the vaccination campaign on pneumococcal pneumonia after the great East Japan earthquake. European Respiratory Journal. 2015;46(S59):PA1854. [Google Scholar]
- 113.Kaseer B, Chahin J, Weyandt R, Zahid M. Lack of association between pneumococcal vaccination and outcomes in patients presenting with ST-elevation myocardial infarction. Journal of Cardiovascular Disease Research. 2014;5(2):20–25. [Google Scholar]
- 114.Kawakami K, Ohkusa Y, Kuroki R, Tanaka T, Koyama K, Harada Y, et al. Effectiveness of pneumococcal polysaccharide vaccine against pneumonia and cost analysis for the elderly who receive seasonal influenza vaccine in Japan. Vaccine. 2010;28(43):7063–7069. doi: 10.1016/j.vaccine.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 115.Kotsopoulos N, Bresse X, Connolly M. A macro economic analysis of 65 year-old 'rendez-vous vaccinal 'in France: What is the return on investment? Value in Health. 2014;17(7):A680. [DOI] [PubMed] [Google Scholar]
- 116.Koutsovasilis AG, Papazafiropoulou A, Sotiropoulos A, Vergidou P, Gougourelas D, Bakomitrou F, et al. Prevalence and impact of influenza and pneumococcal vaccination in type 2 diabetes mellitus patients. Diabetes. 2014;63:A642. [Google Scholar]
- 117.Kuhlmann A, Theidel U, Pletz MW, von der Schulenburg JMG. Potential cost-effectiveness and benefit-cost ratios of adult pneumococcal vaccination in Germany. Health Economics Review. 2012;2(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuhlmann A, Treskova M, Ultsch B, Weidemann F, Wichmann O, Falkenhorst G, et al. Cost-effectiveness of pneumococcal vaccination of elderly in Germany. Value in Health. 2015;18(7):A584. [Google Scholar]
- 119.Le Meur JB, Lefebvre B, Proulx JF, Dery S, Pepin J, De Wals P. Impact of pneumococcal vaccines use on invasive pneumococcal disease in Nunavik (Quebec) from 1997 to 2010. International Journal of Circumpolar Health. 2014;73(1):22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leventer-Roberts M, Feldman BS, Brufman I, Cohen-Stavi CJ, Hoshen M, Balicer RD. Effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive disease and hospital-treated pneumonia among people aged ≥65 years: A retrospective case-control study. Clinical Infectious Diseases. 2015;60(10):1472–1480. doi: 10.1093/cid/civ096 [DOI] [PubMed] [Google Scholar]
- 121.Li C, Gubbins PO, Chen GJ. Prior pneumococcal and influenza vaccinations and in-hospital outcomes for community-acquired pneumonia in elderly veterans. J Hosp Med. 2015;10(5):287–293. doi: 10.1002/jhm.2328 [DOI] [PubMed] [Google Scholar]
- 122.Liao WH, Lin SH, Lai CC, Tan CK, Liao CH, Huang YT, et al. Impact of pneumococcal vaccines on invasive pneumococcal disease in Taiwan. Eur J Clin Microbiol Infect Dis. 2010;29(4):489–492. doi: 10.1007/s10096-010-0873-7 [DOI] [PubMed] [Google Scholar]
- 123.Liguori G, Parlato A, Zamparelli AS, Belfiore P, Galle F, Di Onofrio V, et al. Adult immunization with 13-valent pneumococcal vaccine in Campania region, South Italy: an economic evaluation. Hum Vaccin Immunother. 2014;10(2):492–497. doi: 10.4161/hv.26888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin CJ, Zimmerman RK, Smith KJ. Cost-effectiveness of pneumococcal and influenza vaccination standing order programs. The American Journal of Managed Care. 2013;19(1):e30–37. [PMC free article] [PubMed] [Google Scholar]
- 125.Luk JKH, Chan WK, Ng WC, Chiu PKC, Ho C, Chan TC, et al. Mortality and health services utilisation among older people with advanced cognitive impairment living in residential care homes. Hong Kong Medical Journal. 2013;19(6):518–524. doi: 10.12809/hkmj133951 [DOI] [PubMed] [Google Scholar]
- 126.Mahamat A, Daurès JP, De Wazières B. Additive preventive effect of influenza and pneumococcal vaccines in the elderly Results of a large cohort study. Human Vaccines and Immunotherapeutics. 2013;9(1):128–135. doi: 10.4161/hv.22550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mangen MJ, Rozenbaum MH, Huijts SM, van Werkhoven CH, Postma DF, Atwood M, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015;46(5):1407–1416. doi: 10.1183/13993003.00325-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mankinen PT, Soini EJ, Laine J, Linna M, Åhman H, Martikainen J. Health economic impact of 13-valent pneumococcal conjugate vaccine in Finnish home care customers ≥ 50 years with underlying chronic medical conditions. Value in Health. 2015;18(7):A579. [Google Scholar]
- 129.Manzur A, Izquierdo C, Ruiz L, Sousa D, Bayas JM, Celorrio JM, et al. Influence of prior pneumococcal and influenza vaccination on outcomes of older adults with community-acquired pneumonia. Journal of the American Geriatrics Society. 2011;59(9):1711–1716. doi: 10.1111/j.1532-5415.2011.03541.x [DOI] [PubMed] [Google Scholar]
- 130.Marbaix S, Sato R, Mignon A, Atwood M, Weycker D. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine among patients aged 65–84 years with co-morbidities or immunosuppression in Belgium. Value in Health. 2015;18(7):A584. [Google Scholar]
- 131.Martikainen JA, Soini EJ, Laine J, Ahman H, Postila V, Klemets P. Economic impact of 13-valent pneumococcal conjugate vaccine (PCV13) in persons over 50 years of age with underlying chronic medical conditions in Finland. Value in Health. 2012;15(7):A388–A389. [Google Scholar]
- 132.Martikainen JA, Soini EJ, Laine J, Åhman H, Postila V, Klemets P. Economic impact of 13-valent pneumococcal conjugate vaccine in Finnish adults ≥50 years with underlying chronic medical conditions. Journal of Evaluation in Clinical Practice. 2014;20(4):333–341. doi: 10.1111/jep.12131 [DOI] [PubMed] [Google Scholar]
- 133.Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004 doi: 10.1136/bmj.c1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Menendez R, Rodríguez-GonzálezMoro JM, Gros B, Echave M, Oyagüez I, Lwoff N, et al. Cost-effectiveness of a 13-valent conjugate pneumococcal vaccination program in COPD patients aged ≥ 50 years in Spain: Preliminary results. Value in Health. 2013;16(7):A353. [Google Scholar]
- 135.Menzies RI, Jayasinghe SH, Krause VL, Chiu CK, McIntyre PB. Impact of pneumococcal polysaccharide vaccine in people aged 65 years or older. Med J Aust. 2014;200(2):112–115. [DOI] [PubMed] [Google Scholar]
- 136.Michaelidis CI, Zimmerman RK, Nowalk MP, Smith KJ. Cost-effectiveness of a program to eliminate disparities in pneumococcal vaccination rates in elderly minority populations: an exploratory analysis. Value in Health. 2013;16(2):311–317. doi: 10.1016/j.jval.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Michaelidis CI, Zimmerman RK, Nowalk MP, Smith KJ. Cost-effectiveness of single-and multi-component vaccination programs to eliminate disparities in influenza and pneumococcal vaccination rates in elderly minorities. Journal of General Internal Medicine. 2013;28:S55–S56. [Google Scholar]
- 138.Moberley S, Krause V, Cook H, Mulholland K, Carapetis J, Torzillo P, et al. Failure to vaccinate or failure of vaccine? Effectiveness of the 23-valent pneumococcal polysaccharide vaccine program in Indigenous adults in the Northern Territory of Australia. Vaccine. 2010;28(11):2296–2301. doi: 10.1016/j.vaccine.2009.12.066 [DOI] [PubMed] [Google Scholar]
- 139.Montserrat-Capdevila J, Godoy P, Marsal JR, Barbe F, Galvan L. Risk of exacerbation in chronic obstructive pulmonary disease: a primary care retrospective cohort study. BMC Fam Pract. 2015;16(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nagel J, Geborek P, Saxne T, Jönsson G, Englund M, Petersson IF, et al. The risk of pneumococcal infections after immunization with pneumococcal conjugate vaccine compared to non-vaccinated inflammatory arthritis patients. Scandinavian Journal of Rheumatology. 2015;44(4):271–279. doi: 10.3109/03009742.2014.984754 [DOI] [PubMed] [Google Scholar]
- 141.Nagel J, Geborek P, Saxne T, Jönsson G, Englund M, Petersson IF, et al. The association between antibody levels before and after 7-valent pneumococcal conjugate vaccine immunization and subsequent pneumococcal infection in chronic arthritis patients. Arthritis Research and Therapy. 2015;17:124 doi: 10.1186/s13075-015-0636-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neto JT, Tannus Branco de Araujo G, Gagliardi A, Pinho A, Durand L, Fonseca M. Cost-effectiveness analysis of pneumococcal polysaccharide vaccination from age 60 in São Paulo State, Brazil. Human Vaccines. 2011;7(10):1037–1047. doi: 10.4161/hv.7.10.15987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nuñez SM, Mould-Quevedo JF, Gutierrez-Ardila MV, Roberts CS, La Rotta JE. Cost effectiveness analysis of vaccination with 13-valent (PCV13) and 23-valent (PPSV23) pneumococcal vaccines for adults in Colombia. Value in Health. 2012;15(4):A242. [Google Scholar]
- 144.O'Brien M, Dhankhar P, Grabenstein J, Dasbach EJ. Cost-effectiveness of the use 23-valent pneumococcal polysaccharide vaccine to prevent secondary bacterial infections related to pandemic influenza in Brazil. International Journal of Infectious Diseases. 2010;14:e449–e450. [DOI] [PubMed] [Google Scholar]
- 145.Ochoa-Gondar O, Vila-Corcoles A, Rodriguez-Blanco T, Gomez-Bertomeu F, Figuerola-Massana E, Raga-Luria X, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against community-acquired pneumonia in the general population aged ≥60 years: 3 years of follow-up in the CAPAMIS study. Clin Infect Dis. 2014;58(7):909–917. doi: 10.1093/cid/ciu002 [DOI] [PubMed] [Google Scholar]
- 146.Okapuu JM, Chétrit E, Lefebvre B, Quach C. How many individuals with asthma need to be vaccinated to prevent one case of invasive pneumococcal disease? Canadian Journal of Infectious Diseases and Medical Microbiology. 2014;25(3):147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oliveira AKB, Pedreschi M, Barros MT, Cohon A, Kalil J, Kokron CM. Clinical evaluation in patients with common variable immunodeficiency after administration of polysaccharide and protein antigens. Journal of Clinical Immunology. 2011;31:S65–S66. [Google Scholar]
- 148.Ordoñez Molina JE, Gutierrez-Ardila MV, Vargas Zea N. Cost effectiveness analysis of vaccination with 13-valent (PCV13) and 23-valent (PPSV23) pneumococcal vaccines for adults in a private Colombian institution. Value in Health. 2013;16(3):A90. [Google Scholar]
- 149.Ordoñez Molina JE, Gutierrez-Ardila MV, Vargas Zea N. Cost effectiveness analysis of vaccination with 13-valent (PCV13) and 23-valent (PPSV23) pneumococcal vaccines for adults in Bogota, Colombia—public scenario. Value in Health. 2013;16(3):A90. [Google Scholar]
- 150.Ordóñez JE, Orozco JJ. Cost-effectiveness analysis of pneumococcal conjugate vaccine 13-valent in older adults in Colombia. BMC Infectious Diseases. 2014;14(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pradas R, Gil De Miguel A, Alvaro A, Gil-Prieto R, Guijarro P, Lorente R, et al. Clinical and economical impact of pneumococcal vaccination in Spanish adult population measured by a dynamic model. Value in Health. 2011;14(7):A269. [Google Scholar]
- 152.Pradas R, Guijarro P, De Salas-Cansado M, Lorente R, Antoñanzas F. Use of dynamic models to measure health outcomes of pneumococcal vaccination in Spanish adult population. Value in Health. 2011;14(7):A286. [Google Scholar]
- 153.Pradas R, Gil de Miguel A, Alvaro A, Gil-Prieto R, Lorente R, Mendez C, et al. Budget impact analysis of a pneumococcal vaccination programme in the 65-year-old Spanish cohort using a dynamic model. BMC Infectious Diseases. 2013;13:175 doi: 10.1186/1471-2334-13-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rodríguez González-Moro JM, Menéndez R, Campins M, Lwoff N, Oyagüez I, Echave M, et al. Cost effectiveness of the 13-valent pneumococcal conjugate vaccination program in chronic obstructive pulmonary disease patients aged 50+ years in Spain. Clinical Drug Investigation. 2016;36(1):41–53. doi: 10.1007/s40261-015-0345-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rosales Statkus ME, Gutiérrez Rodríguez MA, Martínez Blanco M, Lasheras Carbajo MD, Martín Martínez F, Arce Arnáez A, et al. Invasive pneumococcal disease in the autonomous region of Madrid, Spain: from 2008 to 2010. Clinical Microbiology and Infection. 2012;18:149–150. [Google Scholar]
- 156.Rozenbaum MH, Hak E, Van Der Werf TS, Postma MJ. Is routine immunization of elderly with the 13-valent pneumococcal conjugate vaccine likely to be considered as cost-effective? Value in Health. 2010;13(7):A378. [Google Scholar]
- 157.Rozenbaum MH, Hak E, van der Werf TS, Postma MJ. Results of a cohort model analysis of the cost-effectiveness of routine immunization with 13-valent pneumococcal conjugate vaccine of those aged ≥65 years in the Netherlands. Clinical Therapeutics. 2010;32(8):1517–1532. doi: 10.1016/j.clinthera.2010.06.016 [DOI] [PubMed] [Google Scholar]
- 158.Rozenbaum MH, van Hoek AJ, Fleming D, Trotter CL, Miller E, Edmunds WJ. Vaccination of risk groups in England using the 13 valent pneumococcal conjugate vaccine: economic analysis. BMJ. 2012;345:e6879 doi: 10.1136/bmj.e6879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rudnick W, Liu Z, Shigayeva A, Low DE, Green K, Plevneshi A, et al. Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995–2011. Vaccine. 2013;31(49):5863–5871. doi: 10.1016/j.vaccine.2013.09.049 [DOI] [PubMed] [Google Scholar]
- 160.Sangil A, Xercavins M, Rodríguez-Carballeira M, Andrés M, Riera M, Espejo E, et al. Impact of vaccination on invasive pneumococcal disease in adults with focus on the immunosuppressed. Journal of Infection. 2015;71(4):422–427. doi: 10.1016/j.jinf.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 161.Shigayeva A, Rudnick W, Green K, Chen DK, Demczuk W, Gold WL, et al. Invasive pneumococcal disease among immunocompromised persons: Implications for vaccination programs. Clinical Infectious Diseases. 2016;62(2):139–147. doi: 10.1093/cid/civ803 [DOI] [PubMed] [Google Scholar]
- 162.Siemieniuk RA, Gregson DB, Gill MJ. A decade of experience with invasive pneumococcal disease in HIV patients. Canadian Journal of Infectious Diseases and Medical Microbiology. 2011;22:20B–21B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Siemieniuk RA, Gregson DB, Gill MJ. The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study. BMC Infectious Diseases. 2011;11:314 doi: 10.1186/1471-2334-11-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Smith KJ, Lee BY, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of dual influenza and pneumococcal vaccination in 50-year-olds. Vaccine. 2010;28(48):7620–7625. doi: 10.1016/j.vaccine.2010.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Smith KJ, Raymund M, Nowalk MP, Roberts MS, Zimmerman RK. Cost-effectiveness of pneumococcal polysaccharide vaccine among healthcare workers during an influenza pandemic. The American Journal of Managed Care. 2010;16(3):200–206. [PMC free article] [PubMed] [Google Scholar]
- 166.Smith KJ, Wateska AR, Nowalk M, Raymund M, Nuorti J, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307(8):804–812. doi: 10.1001/jama.2012.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smith KJ, Wateska A, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination strategies in us adults aged 65 and older. Journal of General Internal Medicine. 2012;27:S150. [Google Scholar]
- 168.Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31(37):3950–3956. doi: 10.1016/j.vaccine.2013.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smith KJ, Wateska AR, Nowalk MP, Raymund M, Lee BY, Zimmerman RK. Modeling of cost effectiveness of pneumococcal conjugate vaccination strategies in U.S. older adults. American Journal of Preventive Medicine. 2013;44(4):373–381. doi: 10.1016/j.amepre.2012.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Soneji S, Metlay J. Mortality reductions for older adults differ by race/ethnicity and gender since the introduction of adult and pediatric pneumococcal vaccines. Public Health Rep. 2011;126(2):259–269. doi: 10.1177/003335491112600217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sotiropoulos A, Koutsovasilis AG, Vergidou P, Grozou A, Apostolou O, Panagiotou D, et al. Influenza and pneumococcal vaccination rates among Greek diabetes patients between 2003–2013 and its influence on their morbidity and hospitalization. Diabetes. 2015;64:A441. [Google Scholar]
- 172.Steens A, Vestrheim DF, de Blasio BF. Pneumococcal vaccination in older adults in the era of childhood vaccination: Public health insights from a Norwegian statistical prediction study. Epidemics. 2015;11:24–31. doi: 10.1016/j.epidem.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 173.Stoecker C, Kim L, Gierke R, Pilishvili T. Incremental cost-effectiveness of 13-valent pneumococcal conjugate vaccine for adults age 50 years and older in the United States. J Gen Intern Med. 2016;31(8):901–908. doi: 10.1007/s11606-016-3651-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tsai YH, Hsieh MJ, Chang CJ, Wen YW, Hu HC, Chao YN, et al. The 23-valent pneumococcal polysaccharide vaccine is effective in elderly adults over 75 years old—Taiwan's PPV vaccination program. Vaccine. 2015;33(25):2897–2902. doi: 10.1016/j.vaccine.2015.04.068 [DOI] [PubMed] [Google Scholar]
- 175.Tseng H, Slezak JM, Quinn VP, Sy LS, Van Den Eeden SK, Jacobsen SJ. Pneumococcal vaccination and risk of acute myocardial infarction and stroke in men. JAMA. 2010;303(17):1699–1706. doi: 10.1001/jama.2010.529 [DOI] [PubMed] [Google Scholar]
- 176.van Hoek AJ, Miller E. Cost-effectiveness of vaccinating immunocompetent ≥65 year olds with the 13-valent pneumococcal conjugate vaccine in England. PLOS ONE. 2016;11(2):e0149540 doi: 10.1371/journal.pone.0149540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Van Werkhoven CH, Huijts SM, Bolkenbaas M, Grobbee DE, Bonten MJM. The impact of age on the efficacy of 13-valent pneumococcal conjugate vaccine in elderly. Clinical Infectious Diseases. 2015;61(12):1835–1838. doi: 10.1093/cid/civ686 [DOI] [PubMed] [Google Scholar]
- 178.Varona JL, Lorente MR, Antoñanzas F, Rejas J. A dynamic model to estimate the budget impact of a neumococcal vaccination program in Spain. Value in Health. 2015;18(7):A580. [Google Scholar]
- 179.Viasus D, Garcia-Vidal C, Cruzado JM, Adamuz J, Verdaguer R, Manresa F, et al. Epidemiology, clinical features and outcomes of pneumonia in patients with chronic kidney disease. Nephrology Dialysis Transplantation. 2011;26(9):2899–2906. [DOI] [PubMed] [Google Scholar]
- 180.Vila-Corcoles A, Ochoa-Gondar O, Guzmán JA, Rodriguez-Blanco T, Salsench E, Fuentes CM. Effectiveness of the 23-valent polysaccharide pneumococcal vaccine against invasive pneumococcal disease in people 60 years or older. BMC Infectious Diseases. 2010;10:73 doi: 10.1186/1471-2334-10-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, Gutierrez-Perez A, Vila-Rovira A. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in patients with chronic pulmonary diseases: a matched case-control study. Hum Vaccin Immunother. 2012;8(5):639–644. doi: 10.4161/hv.19466 [DOI] [PubMed] [Google Scholar]
- 182.Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, Gutierrez-Perez A, Vila-Rovira A, Gomez F, et al. Clinical effectiveness of pneumococcal vaccination against acute myocardial infarction and stroke in people over 60 years: the CAPAMIS study, one-year follow-up. BMC Public Health. 2012;12:222 doi: 10.1186/1471-2458-12-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wen YW, Chang CJ. Economic benefit of the 23-valent pneumococcal polysaccharide vaccination program for the elder aged over 75 years in Taiwan. Value in Health. 2014;17(3):A271. [Google Scholar]
- 184.Weycker D, Sato R, Strutton D, Edelsberg J, Atwood M, Jackson LA. Public health and economic impact of 13-valent pneumococcal conjugate vaccine in US adults aged ≥50 years. Vaccine. 2012;30(36):5437–5444. doi: 10.1016/j.vaccine.2012.05.076 [DOI] [PubMed] [Google Scholar]
- 185.Wiemken TL, Carrico RM, Klein SL, Jonsson CB, Peyrani P, Kelley RR, et al. The effectiveness of the polysaccharide pneumococcal vaccine for the prevention of hospitalizations due to Streptococcus pneumoniae community-acquired pneumonia in the elderly differs between the sexes: results from the Community-Acquired Pneumonia Organization (CAPO) international cohort study. Vaccine. 2014;32(19):2198–2203. doi: 10.1016/j.vaccine.2014.02.048 [DOI] [PubMed] [Google Scholar]
- 186.Williams E, Nahman S, Colombo R, Kintziger K, Kheda M, Huber L, et al. Cofactors for mortality in HIV-positive dialysis patients. Journal of Investigative Medicine. 2015;63(2):438. [Google Scholar]
- 187.Wright LB, Hughes GJ, Chapman KE, Gorton R, Wilson D. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in people aged 65 years and over in the North East of England, April 2006-July 2012. Trials in Vaccinology. 2013;2(1):45–48. [Google Scholar]
- 188.Wu DBC, Chang CJ, Chien L, Fang HCH, Roberts CS. The cost-effectiveness analysis of 13-valent pneumococcal conjugate vaccine (PCV13) and 23-valent pneumococcal polysaccharide vaccine (PPV23) in Taiwan. Value in Health. 2012;15(7):A602. [Google Scholar]
- 189.Wu WC, Jiang L, Friedmann PD, Trivedi A. Association between process quality measures for heart failure and mortality among US veterans. American Heart Journal. 2014;168(5):713–720. doi: 10.1016/j.ahj.2014.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang HK, Chen J, O'Brien M, Grabenstein J, Dasbach E. Cost-effectiveness of pneumococcal vaccines for adults in the United States. Value in Health. 2013;16(3):A93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang HK, O'Brien M, Chen J, Grabenstein J, Dasbach E. Public health impact and cost effectiveness of pneumococcal vaccination for adults in Puerto Rico. Value in Health. 2013;16(7):A714. [Google Scholar]
- 192.Yang HK, Dasbach EJ, Guarin D, Hernandez G, Lemos E. Assessing the public health impact and cost effectiveness of pneumococcal vaccines for adults 65 years of age in Colombia. Value in Health. 2015;18(7):A871. [Google Scholar]
- 193.Yilmaz D, Uzaslan E, Ege E. Impact of pneumococcal polysaccharide vaccine on acute exacerbation and quality of life in COPD patients. American Journal of Respiratory and Critical Care Medicine. 2013;187:A2182. [Google Scholar]
- 194.Zigmond J, Tichopad A, Kolek V, Roberts CS, Hajek P. Cost-effectiveness of 13-valent versus 23-valent pneumococcal polysaccharide vaccine and no vaccination in the Czech national vaccination program. Value in Health. 2013;16(3):A73–A74. [Google Scholar]
- 195.Zanotti G, Antoun M, Sato R, Strutton D, Weycker D, McNeil S. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine in adults aged 50 years and older in Canada. Canadian Journal of Infectious Diseases and Medical Microbiology. 2012;23:40B. [Google Scholar]
- 196.Pneumococcal immunization in older adults. Clinical Geriatrics. 2010;18(6):13–14. [Google Scholar]
- 197.The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Higgins JPT, Green S, editors [updated 2011 Mar; cited 2016 Nov 3]. Available from: http://handbook.cochrane.org/.
- 198.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- 199.Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, Reingold A, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. New England Journal of Medicine. 2006;354(14):1455–1463. doi: 10.1056/NEJMoa051642 [DOI] [PubMed] [Google Scholar]
- 200.Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen J-A, Klugman K, et al. Access to effective antimicrobials: a worldwide challenge. The Lancet.387(10014):168–175. [DOI] [PubMed] [Google Scholar]
- 201.Slotved H-C, Dalby T, Hoffmann S. The effect of pneumococcal conjugate vaccines on the incidence of invasive pneumococcal disease caused by ten non-vaccine serotypes in Denmark. Vaccine. 2016;34(6):769–774. doi: 10.1016/j.vaccine.2015.12.056 [DOI] [PubMed] [Google Scholar]
- 202.Shiri T, Datta S, Madan J, Tsertsvadze A, Royle P, Keeling MJ, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e51–e59. doi: 10.1016/S2214-109X(16)30306-0 [DOI] [PubMed] [Google Scholar]
- 203.Abubakar I, Gautret P, Brunette GW, Blumberg L, Johnson D, Poumerol G, et al. Global perspectives for prevention of infectious diseases associated with mass gatherings. The Lancet Infectious Diseases. 2012;12(1):66–74. doi: 10.1016/S1473-3099(11)70246-8 [DOI] [PubMed] [Google Scholar]
- 204.Ministry of Health of Saudi Arabia. Health requirements for travellers to Saudi Arabia for pilgrimage to Makkah (2016/1437H Hajj) [2016; cited 2016 Nov 3]. Available from: http://www.moh.gov.sa/en/Hajj/Pages/HealthRegulations.aspx
- 205.Hayslip B, Kaminski PL. Grandparents raising their grandchildren: A review of the literature and suggestions for practice. The Gerontologist. 2005;45(2):262–269. [DOI] [PubMed] [Google Scholar]
- 206.Minkler M, Fuller-Thomson E. The health of grandparents raising grandchildren: Results of a national study. American Journal of Public Health. 1999;89(9):1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Arrow KJ. Aspects of the theory of risk-bearing Helsinki: Yrjö Jahnssonin Säätiö; 1965. [Google Scholar]
- 208.Viscusi WK. The value of risks to life and health. Journal of Economic Literature. 1993;31(4):1912–1946. [Google Scholar]
- 209.Luyten J, Beutels P. The social value of vaccination programs: Beyond cost-effectiveness. Health Affairs. 2016;35(2):212–218. doi: 10.1377/hlthaff.2015.1088 [DOI] [PubMed] [Google Scholar]
- 210.Chapman KE, Wilson D, Gorton R. Invasive pneumococcal disease and socioeconomic deprivation: a population study from the North East of England. J Public Health (Oxf). 2013;35(4):558–569. [DOI] [PubMed] [Google Scholar]
- 211.Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. Journal of Clinical Epidemiology. 2016;69:225–234. doi: 10.1016/j.jclinepi.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Full Benefits of Adult Pneumococcal Vaccination: A Systematic Review.
(PDF)
Search Terms. The Full Benefits of Adult Pneumococcal Vaccination: A Systematic Review.
(PDF)
Extraction Template. The Full Benefits of Adult Pneumococcal Vaccination: A Systematic Review.
(PDF)
Extraction Table. The Full Benefits of Adult Pneumococcal Vaccination: A Systematic Review.
(XLSX)
PRISMA Checklist.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.