ABSTRACT
Perineural invasion (PNI) has been implicated as a poor prognostic indicator in many cancers. The National Comprehensive Cancer Network recommends consideration of observation or adjuvant therapy in the presence of PNI in early colon cancer. These recommendations are based on single institutional studies that fail to evaluate PNI within the context of adjuvant chemotherapy. The US National Cancer Database (2004–2012) was reviewed for patients with node negative colon cancer, and stratified by PNI and receipt of chemotherapy.
Of 21,488 patients evaluated, 55.2% had T3 disease (n = 11,852), 23.1% had T2 (n = 4,971), 14.4% had T1 (n = 3,088), and 7.3% had T4 disease (n = 1,577); 4.6% (n = 987) had PNI. Most patients (86.8%, n = 18,641) did not have PNI and did not receive chemotherapy; 8.7% (n = 1,860) did not have PNI but received chemotherapy; 3.7% (n = 785) had PNI and did not receive chemotherapy, and 0.9% (n = 202) had PNI and received chemotherapy. Among those with PNI, patients who received chemotherapy tended to be younger (P<0.001), covered by private insurance (P<0.001), with fewer comorbidities (P<0.001), and greater T stage disease (P<0.001). Those with PNI who received chemotherapy had significantly improved survival over those who did not in T3–4 disease (P<0.001), but not in T1–2 disease. On multivariate analysis, those with PNI had a 38% greater hazard of mortality (HR 1.38, P<0.001). Additionally, chemotherapy decreased the hazard of mortality by 43% (HR 0.57, P<0.001). PNI appears to be an independent poor prognostic indicator in stage T3–4 node negative colon cancer. Chemotherapy administered to this patient population is associated with improved survival.
KEYWORDS: Colon cancer, perineural invasion, chemotherapy, negative node colon cancer, National Cancer Data Base, adjuvant chemotherapy, prognostic indicator
Abbreviations
- AJCC
American Joint Committee on Cancer
- CCI
Charlson/Deyo comorbidity index
- NCCN
National Comprehensive Cancer Network
- NCDB
National Cancer Data Base
- PNI
Perineural invasion
Introduction
The role of adjuvant chemotherapy in node positive colon cancer has been established through several randomized trails.1-7 These studies show a 22–32 percent risk reduction in mortality in stage III patients who undergo surgical resection and adjuvant chemotherapy.1-4,5,8 However, the role of chemotherapy in stage II node negative disease remains unclear.6,7,9 Other clinicopathologic features associated with worse prognosis in early stage disease include lymphovascular invasion, bowel obstruction and perforation.10-14 There is conflicting evidence supporting the benefit of adjuvant chemotherapy in these higher-risk subpopulations.2,15-18 Perineural invasion (PNI) has been implicated as a poor prognostic indicator in many cancers, including colon and rectal cancer.19-23 The 5-year overall survival rate has been reported as 72% for PNI-negative tumors versus 25% for PNI-positive tumors.21
To date, guidelines from the National Comprehensive Cancer Network (NCCN) recommend consideration of adjuvant chemotherapy, participation in a clinical trial, or observation in the presence of PNI in early colon cancer.24 These recommendations are based on evidence that PNI is an independent adverse prognostic factor.11,21,22 The majority of existing evidence comes from single institutional studies that fail to evaluate PNI within the context of adjuvant chemotherapy. The objective of this study was to evaluate the impact of PNI on survival in node negative colon cancer in a large Western population.
Results
This study population consisted of 7,960 (37.0%) patients with stage I disease (overall 5-year survival: 69.9%), and 13,528 (63.0%) patients with stage II disease (overall 5-year survival: 58.1%), 51.8% females, with a median age of 72 y (Fig. 1). Of the 21,488 patients evaluated, the majority had T3 disease (n = 11,852, 55.2%), 23.1% had T2 disease (n = 4,971), 14.4% had T1 (n = 3,088), and 7.3% had T4 disease (n = 1,577); 4.6% (n = 987) had PNI. The majority of the study population did not have PNI and did not receive chemotherapy (86.8%, n = 18,641); 8.7% (n = 1,860) did not have PNI but received chemotherapy; 3.7% (n = 785) had PNI and did not receive chemotherapy, and 0.9% (n = 202) had PNI and received chemotherapy.
Figure 1.
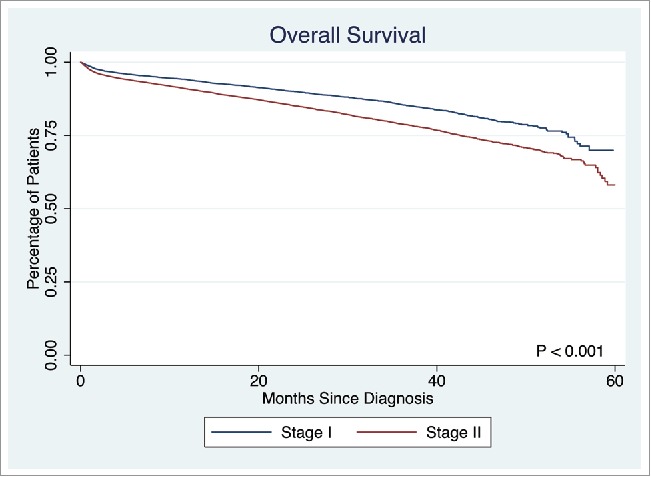
Overall survival in Stage I and Stage II disease.
Patient, disease and treatment characteristics, stratified by presence of PNI and receipt of chemotherapy, are displayed in Table 1. Patients with PNI who received chemotherapy tended to be younger (p < 0.0001), covered by private insurance (p < 0.0001), with fewer comorbidities (p < 0.0001). They tended to have more advanced T stage (p < 0.0001), and pathological stage disease. There was no difference in type of surgery received (p = 0.939) or number of lymph nodes resected (p = 0.084). Patients without PNI who received chemotherapy also tended to be younger (p < 0.0001), covered by private insurance (p < 0.0001), with fewer comorbidities (p < 0.0001). They too, had more advanced T stage (p < 0.0001), and pathological stage disease (p < 0.0001), and greater number of lymph nodes resected (p < 0.0001).
Table 1.
Patient, disease and surgery demographics.
| Neural invasion |
No neural invasion |
||||||
|---|---|---|---|---|---|---|---|
| Chemotherapy | None | Chemotherapy | None | ||||
| (n = 202) | (n = 785) | p-value | (n = 1,860) | (n = 18,641) | p-value | p-value | |
| Age | 60.4 | 72.2 | <0.0001 | 60.7 | 71.2 | <0.0001 | <0.0001 |
| 18–59 | 46.0% | 17.8% | 44.7% | 18.5% | |||
| 60–69 | 31.2% | 19.9% | 30.3% | 22.3% | |||
| 70–79 | 18.3% | 27.3% | 20.3% | 29.7% | |||
| 80–90 | 4.5% | 35.0% | 4.8% | 29.4% | |||
| Sex | 0.400 | 0.027 | 0.043 | ||||
| Male | 48.0% | 51.3% | 50.5% | 47.8% | |||
| Female | 52.0% | 48.7% | 49.5% | 52.2% | |||
| Race | 0.455 | <0.0001 | <0.0001 | ||||
| White (non-Hispanic) | 70.3% | 75.4% | 70.5% | 77.2% | |||
| Black (non-Hispanic) | 15.3% | 11.7% | 13.4% | 10.5% | |||
| Other (non-Hispanic) | 3.5% | 3.4% | 3.7% | 3.0% | |||
| Hispanic | 10.9% | 9.4% | 12.4% | 9.3% | |||
| Insurance | <0.0001 | <0.0001 | <0.0001 | ||||
| Private | 48.0% | 23.3% | 45.4% | 27.2% | |||
| Medicare | 36.6% | 67.0% | 38.0% | 64.9% | |||
| Medicaid and other government | 6.9% | 3.7% | 8.4% | 3.8% | |||
| Unknown | 0.5% | 1.9% | 1.2% | 1.3% | |||
| Not ensured | 7.9% | 4.1% | 7.0% | 2.7% | |||
| Median income | 0.269 | 0.05 | 0.166 | ||||
| <58,000 | 16.3% | 19.9% | 20.4% | 18.0% | |||
| 58,000 -74,000 | 21.3% | 25.4% | 25.6% | 25.2% | |||
| 74,000 - 93,000 | 30.7% | 26.0% | 25.3% | 26.6% | |||
| >93,000 | 30.7% | 27.8% | 28.3% | 29.6% | |||
| Comorbidities | <0.0001 | <0.0001 | <0.0001 | ||||
| CCI score 0 | 78.7% | 64.6% | 75.6% | 65.3% | |||
| CCI score 1 | 15.8% | 23.3% | 19.6% | 24.7% | |||
| CCI score 2 | 5.4% | 12.1% | 4.8% | 10.0% | |||
| Facility type | 0.026 | 0.068 | 0.001 | ||||
| Community | 14.4% | 13.9% | 15.8% | 16.2% | |||
| Comprehensive community | 42.1% | 50.3% | 50.2% | 53.1% | |||
| Academic/research | 27.7% | 29.0% | 24.6% | 24.0% | |||
| Other | 0.0% | 0.1% | 0.3% | 0.2% | |||
| Facility location | 0.236 | 0.001 | 0.001 | ||||
| Northeast | 19.3% | 22.4% | 18.6% | 18.6% | |||
| South | 44.1% | 38.6% | 40.3% | 40.2% | |||
| Midwest | 21.8% | 24.1% | 24.7% | 25.1% | |||
| West | 8.9% | 13.4% | 12.2% | 15.0% | |||
| TNM: T | <0.0001 | <0.0001 | <0.0001 | ||||
| T1 | 0.0% | 4.6% | 0.8% | 16.3% | |||
| T2 | 1.0% | 11.2% | 2.9% | 25.9% | |||
| T3 | 59.4% | 69.9% | 68.5% | 53.2% | |||
| T4 | 39.6% | 14.3% | 27.8% | 4.7% | |||
| Surgery type | 0.998 | 0.001 | <0.0001 | ||||
| Partial or hemicolectomy | 95.5% | 95.5% | 94.1% | 95.8% | |||
| Total colectomy | 4.5% | 4.5% | 5.9% | 4.2% | |||
| Number of regional lymph nodes removed | 19.9 | 19.0 | 0.285 | 20.8 | 19.2 | <0.0001 | 0.007 |
| Pathological stage | <0.0001 | <0.0001 | <0.0001 | ||||
| Stage 1 | 0.5% | 15.5% | 3.4% | 41.7% | |||
| Stage 2 | 99.5% | 84.5% | 96.6% | 58.3% | |||
The presence of PNI was not associated with a difference in survival in T1 or T2 stage disease (p = 0.7839, p = 0.1379, respectively). However, PNI was associated with significantly lower survival rate in T3 and T4 stage disease (5-year survival: 53.7% vs. 58.3%, p < 0.0001, 45.9% vs. 57.6%, p = 0.0033, respectively). Overall survival in T3 disease is depicted in Fig. 2. Patients with PNI who did not receive chemotherapy had significantly worse survival than those without PNI who did not receive chemotherapy, those with PNI who received chemotherapy, and those without PNI who received chemotherapy (5-year survival: 49.1%, 54.1%, 81.1%, and 83.8%, respectively, p < 0.001). A similar statistically significant survival pattern was observed in T4 disease (5-year survival: 21.9%, 53.1%, 74.0%, and 69.9%, respectively, p < 0.001) (Fig. 3).
Figure 2.
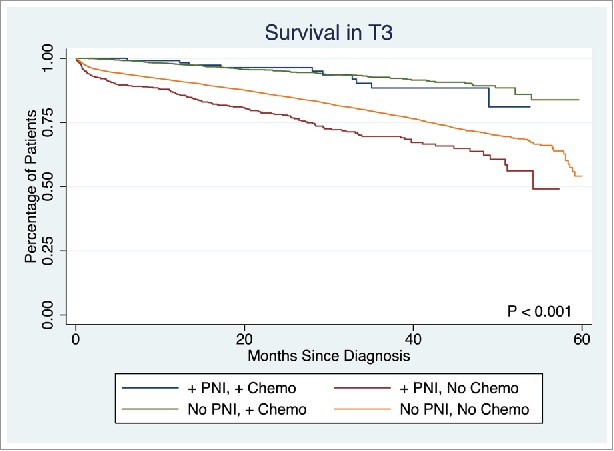
Survival in Stage T3 disease.
Figure 3.
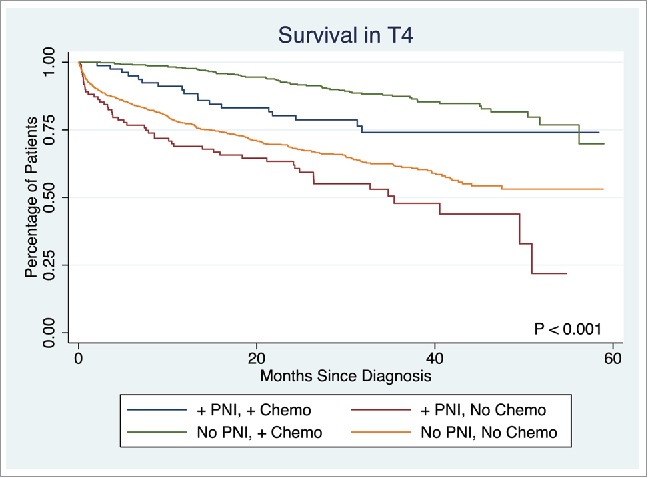
Survival in Stage T4 disease.
Results of a multivariable Cox proportional hazards model, controlling for patient, disease, and treatment characteristics are reported in Table 2. The presence of PNI was associated with a 41% greater hazard of mortality (HR 1.41, p < 0.001). Receipt of adjuvant chemotherapy was associated with a 44% reduction in the hazard of mortality (HR 0.56, p < 0.001).
Table 2.
Multivariate survival analysis.
| 95% Confidence interval |
||||
|---|---|---|---|---|
| Hazard ratio | lower | upper | p-value | |
| Perineural invasion | ||||
| None | Reference | |||
| Present | 1.38 | 1.20 | 1.57 | <0.001 |
| Age | ||||
| 18–59 | Reference | |||
| 60–69 | 1.38 | 1.20 | 1.57 | <0.001 |
| 70–79 | 1.68 | 1.44 | 1.98 | <0.001 |
| 80–90 | 2.72 | 2.32 | 3.19 | <0.001 |
| Sex | ||||
| Male | Reference | |||
| Female | 0.78 | 0.73 | 0.84 | <0.001 |
| Race | ||||
| White (non-Hispanic) | Reference | |||
| Black (non-Hispanic) | 1.03 | 0.92 | 1.16 | 0.575 |
| Other (non-Hispanic) | 1.02 | 0.81 | 1.27 | 0.893 |
| Hispanic | 1.01 | 0.90 | 1.14 | 0.848 |
| Insurance | ||||
| Private | Reference | |||
| Medicare | 1.27 | 1.14 | 1.41 | <0.001 |
| Medicaid and other government | 1.56 | 1.28 | 1.91 | <0.001 |
| Unknown | 1.37 | 1.01 | 1.87 | 0.043 |
| Not ensured | 1.39 | 1.07 | 1.80 | 0.013 |
| Median income | ||||
| <58,000 | Reference | |||
| 58,000 -74,000 | 1.02 | 0.92 | 1.12 | 0.766 |
| 74,000 - 93,000 | 0.94 | 0.85 | 1.04 | 0.241 |
| >93,000 | 0.87 | 0.78 | 0.96 | 0.006 |
| Comorbidities | ||||
| CCI score 0 | Reference | |||
| CCI score 1 | 1.34 | 1.24 | 1.44 | <0.001 |
| CCI score 2 | 2.11 | 1.93 | 2.31 | <0.001 |
| Facility type | ||||
| Community | Reference | |||
| Comprehensive community | 1.00 | 0.92 | 1.09 | 1.000 |
| Academic/research | 0.94 | 0.85 | 1.03 | 0.188 |
| Other | 0.97 | 0.50 | 1.87 | 0.917 |
| Facility location | ||||
| Northeast | Reference | |||
| South | 0.99 | 0.90 | 1.09 | 0.873 |
| Midwest | 0.98 | 0.89 | 1.08 | 0.661 |
| West | 0.99 | 0.88 | 1.11 | 0.884 |
| Surgery type | ||||
| Partial or hemicolectomy | Reference | |||
| Total colectomy | 1.44 | 1.25 | 1.67 | <0.001 |
| TNM: T | ||||
| T1 | Reference | |||
| T2 | 1.15 | 1.01 | 1.31 | 0.031 |
| T3 | 1.49 | 1.32 | 1.67 | <0.001 |
| T4 | 2.91 | 2.51 | 3.37 | <0.001 |
| Adjuvant therapy | ||||
| None | Reference | |||
| Chemotherapy | 0.57 | 0.48 | 0.67 | <0.001 |
| Radiation | 1.22 | 0.58 | 2.56 | 0.608 |
| Chemoradiation | 1.08 | 0.70 | 1.65 | 0.736 |
Discussion
The results of this study suggest the poor prognostic implications of PNI, and the survival benefits adjuvant chemotherapy offers in stage T3 and T4 node negative colon cancer.
Multiple single institutional studies suggest the presence of PNI is a poor prognostic indicator.19-23 The current large, national, study found patients with PNI had a 38% greater hazard of mortality (p < 0.001). A much smaller retrospective study of 2,649 patients from the Swedish Colon Cancer Registry with TNM stage II disease reported increased risk of recurrence in patients with PNI.19 Huh et al. reported a retrospective study of 1,437 patients who underwent surgery for stage II or III colon cancer reported that the presence of both lymphovascular and PNI was an independent poor prognostic factor for both overall and disease-free survival.20 Liebig at al. evaluated 269 patients with resected colon cancer and identified PNI as an independent prognostic factor for both cancer-specific overall survival and disease-free survival.21 None of these prior reports addressed the impact of adjuvant chemotherapy in patients with PNI.
Currently, the NCCN guideline recommends observation, enrollment in a clinical trial or adjuvant chemotherapy in the setting of PNI.24 The results of this study advocate for the administration of adjuvant chemotherapy for patients with T3 or T4 disease and PNI. A single institutional study by Suzuki et al. retrospectively reviewed 178 patients with stage I-III colon cancer who underwent curative surgery from 1999–2004, and reported PNI as a strong prognostic factor, with adjuvant chemotherapy attenuating the effects.25 Another small study of 509 patients who underwent curative surgery for pT3 or pT4 colon cancer from 1997 to 2001 reported significantly worse survival in patients with PNI as compared with patients without PNI, irrespective of adjuvant chemotherapy use.22 In this study, patients with PNI who received chemotherapy, had better survival compared with patients without PNI who did not receive chemotherapy. This likely reflects in part a selection bias due to the retrospective nature of the study, and highlights advancements in chemotherapy between the 2 studies.
To our knowledge, this represents the largest and most contemporary analysis of PNI in colon cancer. However, there are some important limitations which should be acknowledged. The NCDB is a large data set incorporating multiple institutions with the potential for coding inconsistencies and errors. As shown in Table 1, the cohorts who received chemotherapy differed in patient and disease demographics, illustrating a selective bias inherent to the retrospective nature of this study. However, multivariate analysis controlling for these factors was performed. Postoperative occurrences that may have influenced the treatment course were not captured in this study. Furthermore, the chemotherapy regimen and duration used was not captured by this data set, and thus outcomes specific to particular adjuvant regimens cannot be extrapolated.
Methods
Data
The National Cancer Data Base (NCDB) is a clinical oncology database, sourced from hospital registry data collected from over 1,500 Commission on Cancer accredited facilities across the United States. The NCDB captures approximately 80% of cancer cases in the United States from 1998 to 2012. This was a retrospective cohort study of clinical data from this registry from 2004–2012. The Penn State Health Institutional Review Board reviewed and approved the study.
Patient selection
The NCDB was reviewed for patients diagnosed with node-negative colon cancer. Patients with unknown nodal status were excluded. This analysis included pathological stage I and II adenocarcinoma of the large intestine, identified using histology ICD-O-3 code 8140/3. Patients with positive surgical margins, those who received neoadjuvant therapy, and those who underwent local excision or unspecified surgical procedures were also excluded.
Outcomes and covariates
The primary outcome assessed was survival in days from date of diagnosis. The presence of PNI as documented in pathology reports was coded within the database. Univariate analyses compared demographic data including age, sex, race, insurance type (private, Medicare, Medicaid and other government programs, unknown, not ensured), median income and the Charlson/Deyo comorbidity index (CCI), an index of 15 comorbidities including myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, diabetes, diabetes with chronic complications, hemiplegia or paraplegia, renal disease, moderate or severe liver disease and AIDS.26,27
Treatment facilities were stratified by facility type (community, comprehensive community, academic or research institution, other), and geographic region (Northeast, South, Midwest, West). Disease was characterized by the American Joint Committee on Cancer (AJCC) clinical stage, pathologic variables (regional lymph nodes sampled, positive regional lymph nodes, surgical margins, and pathological stage). Treatment was characterized by surgery type (partial or hemicolectomy, or total colectomy), and adjuvant therapy.
Statistical analysis
Statistical analyses were performed with STATA software (version 12.1, StataCorp, College Station, TX, USA). Patient demographics, disease characteristics, and treatment types were compared between groups using t-test for continuous variables, and chi square tests for categorical variables. Kaplan-Meier analyses were performed for each T stage and stratified by presence of neural invasion and receipt of chemotherapy and curves were compared using a log-rank test. Median survival time was computed based on the Kaplan-Meier analysis. A Cox proportional hazards model, controlling for patient, disease and treatment covariates, was performed.
Conclusion
PNI is an independent poor prognostic factor in stage T3 and stage T4 colon cancer. Adjuvant chemotherapy in patients with T3–4N0 colon cancer is associated with improved survival in patients with PNI. Further studies are needed to characterize the benefits of specific chemotherapy regimens.
Disclosure of potential conflicts of interest
No potential conflicts of interest were declared.
Disclaimer
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC's NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Acknowledgments
The authors would like to acknowledge Kimberly Walker for her assistance in manuscript preparation.
References
- 1.No authors listed Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 1995; 345:939-44; PMID: 7715291; https://doi.org/ 10.1016/S0140-6736(95)90696-7 [DOI] [PubMed] [Google Scholar]
- 2.O'Connell MJ, Mailliard JA, Kahn MJ, Macdonald JS, Haller DG, Mayer RJ, Wieand HS. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 1997; 15:246-50; PMID: 8996149; https://doi.org/ 10.1200/JCO.1997.15.1.246 [DOI] [PubMed] [Google Scholar]
- 3.Yothers G, O'Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, Wolmark N. Oxaliplatin as adjuvant therapy for colon cancer: Updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011; 29:3768-74; PMID: 21859995; https://doi.org/ 10.1200/JCO.2011.36.4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolmark N, Fisher B, Rockette H, Redmond C, Wickerham DL, Fisher ER, Jones J, Glass A, Lerner H, Lawrence W, et al.. Postoperative adjuvant chemotherapy or BCG for colon cancer: Results from NSABP protocol C-01. J Natl Cancer Inst 1988; 80:30-6; PMID: 3276901; https://doi.org/ 10.1093/jnci/80.1.30 [DOI] [PubMed] [Google Scholar]
- 5.Wolmark N, Wieand HS, Hyams DM, Colangelo L, Dimitrov NV, Romond EH, Wexler M, Prager D, Cruz AB, Gordon PH, et al.. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst 2000; 92:388-96; PMID: 10699069; https://doi.org/ 10.1093/jnci/92.5.388 [DOI] [PubMed] [Google Scholar]
- 6.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, et al.. Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350:2343-51; PMID: 15175436; https://doi.org/ 10.1056/NEJMoa032709 [DOI] [PubMed] [Google Scholar]
- 7.Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27:3109-16; PMID: 19451431; https://doi.org/ 10.1200/JCO.2008.20.6771 [DOI] [PubMed] [Google Scholar]
- 8.Grem JL, Allegra CJ. Toxicity of levamisole and 5-fluorouracil in human colon carcinoma cells. J Natl Cancer Inst 1989; 81:1413-17; PMID: 2778828; https://doi.org/ 10.1093/jnci/81.18.1413 [DOI] [PubMed] [Google Scholar]
- 9.Haller DG, Catalano PJ, Macdonald JS, O'Rourke MA, Frontiera MS, Jackson DV, Mayer RJ. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: Final report of Intergroup 0089. J Clin Oncol 2005; 23:8671-8; PMID: 16314627; https://doi.org/ 10.1200/JCO.2004.00.5686 [DOI] [PubMed] [Google Scholar]
- 10.Chapuis PH, Dent OF, Fisher R, Newland RC, Pheils MT, Smyth E, Colquhoun K. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer. Br J Surg 1985; 72:698-702; PMID: 4041728; https://doi.org/ 10.1002/bjs.1800720909 [DOI] [PubMed] [Google Scholar]
- 11.Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Landmann RG, Guillem JG, Paty PB, Temple LK, Wong WD, et al.. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum 2008; 51:503-7; PMID: 18322753; https://doi.org/ 10.1007/s10350-008-9246-z [DOI] [PubMed] [Google Scholar]
- 12.Faivre-Finn C, Bouvier-Benhamiche AM, Phelip JM, Manfredi S, Dancourt V, Faivre J. Colon cancer in France: Evidence for improvement in management and survival. Gut 2002; 51:60-4; PMID: 12077093; https://doi.org/ 10.1136/gut.51.1.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen VC, Baxter KJ, Love SB, Shepherd NA. Identification of objective pathological prognostic determinants and models of prognosis in Dukes' B colon cancer. Gut 2002; 51:65-9; PMID: 12077094; https://doi.org/ 10.1136/gut.51.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen HS, Sheen-Chen SM. Obstruction and perforation in colorectal adenocarcinoma: An analysis of prognosis and current trends. Surgery 2000; 127:370-6; PMID: 10776426; https://doi.org/ 10.1067/msy.2000.104674 [DOI] [PubMed] [Google Scholar]
- 15.Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, Benedetti J, Francini G, Shepherd LE, Francois Seitz J, et al.. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much?. J Clin Oncol 2004; 22:1797-806; PMID: 15067028; https://doi.org/ 10.1200/JCO.2004.09.059 [DOI] [PubMed] [Google Scholar]
- 16.Kumar A, Kennecke HF, Renouf DJ, Lim HJ, Gill S, Woods R, Speers C, Cheung WY. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer 2015; 121:527-34; PMID: 25332117; https://doi.org/ 10.1002/cncr.29072 [DOI] [PubMed] [Google Scholar]
- 17.O'Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, Smith MA. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 2011; 29:3381-8; PMID: 21788561; https://doi.org/ 10.1200/JCO.2010.34.3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cakar B, Varol U, Junushova B, Muslu U, Gursoy Oner P, Gokhan Surmeli Z, Cirak Y, Karaca B, Sezgin C, Karabulut B, et al.. Evaluation of the efficacy of adjuvant chemotherapy in patients with high-risk stage II colon cancer. J BUON 2013; 18:372-6; PMID: 23818348. [PubMed] [Google Scholar]
- 19.Nikberg M, Chabok A, Letocha H, Kindler C, Glimelius B, Smedh K. Lymphovascular and perineural invasion in stage II rectal cancer: A report from the Swedish colorectal cancer registry. Acta Oncol 2016; 55(12):1418-1424; PMID: 27732105; https://doi.org/ 10.1080/0284186X.2016.1230274 [DOI] [PubMed] [Google Scholar]
- 20.Huh JW, Lee JH, Kim HR, Kim YJ. Prognostic significance of lymphovascular or perineural invasion in patients with locally advanced colorectal cancer. Am J Surg 2013; 206:758-63; PMID: 23835209; https://doi.org/ 10.1016/j.amjsurg.2013.02.010 [DOI] [PubMed] [Google Scholar]
- 21.Liebig C, Ayala G, Wilks J, Verstovsek G, Liu H, Agarwal N, Berger DH, Albo D. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol 2009; 27:5131-7; PMID: 19738119; https://doi.org/ 10.1200/JCO.2009.22.4949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita S, Nakanisi Y, Taniguchi H, Yamamoto S, Akasu T, Moriya Y, Shimoda T. Cancer invasion to Auerbach's plexus is an important prognostic factor in patients with pT3-pT4 colorectal cancer. Dis Colon Rectum 2007; 50:1860-6; PMID: 17899273; https://doi.org/ 10.1007/s10350-007-9072-8 [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Huang X, Sun J, Gao P, Song Y, Chen X, Zhao J, Wang Z. Prognostic value of perineural invasion in colorectal cancer: A meta-analysis. J Gastrointest Surg 2015; 19:1113-22; PMID: 25663635; https://doi.org/ 10.1007/s11605-015-2761-z [DOI] [PubMed] [Google Scholar]
- 24.National Comprehensive Cancer Center (NCCN) NCCN Clinical Practice Guidelines in Oncology: Colon Cancer Version 2.2016. 2016; http://www.nccn.org/professionals/physician_gls/f_guidelines.asp [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T, Suwa K, Ogawa M, Eto K, Kawahara H, Fujita T, Ikegami M, Yanaga K. Adjuvant chemotherapy for the perineural invasion of colorectal cancer. J Surg Res 2015; 199:84-9; PMID: 25935467; https://doi.org/ 10.1016/j.jss.2015.03.101 [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987; 40:373-83; PMID: 3558716; https://doi.org/ 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 27.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45:613-19; PMID: 1607900; https://doi.org/ 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]


