ABSTRACT
Lung adenocarcinoma (LUAD) accounts for the most common histological subtype of lung cancer which remains the leading cause of cancer death worldwide. The discovery of more sensitive and specific novel target biomarkers for predicting the development and progression of LUAD is imperative. Flotillin-1 (Flot-1) has been reported to have important roles in the progression of several tumor types but not been reported in the progression of LUAD. Here, we demonstrated that the expression of flotillin-1 was upregulated in 5 LUAD cells. Moreover, multiple approaches were used to explore the tumorigenicity of flotillin-1 in LUAD cell lines. The expression levels of flotillin-1 were analyzed by immunoblotting after overexpression and siRNA-based knockdown. Cell proliferation, scratch wound healing, transwell migration and matrigel invasion and xenograft tumor growth assays were used to determine the role of flotillin-1 in LUAD progression. Downregulation of flotillin-1 reversed, whereas upregulation of flotillin-1 enhanced, the malignant phenotype of LUAD cells in vitro. Consistently, cells with flotillin-1 knockdown formed smaller tumors in nude mice than cells transfected with the empty vector. Furthermore, the control group demonstrated significantly more tumorigenic effects compared to the flotillin-1-silenced group in the xenograft model of LUAD. In all, there draws a conclusion that flotillin-1 is a tumorigenic protein that plays an important role in promoting the proliferation and tumorigenicity of LUAD, suggesting that flotillin-1 may represent a novel the therapeutic target to LUAD.
KEYWORDS: flotillin-1, lung adenocarcinoma, tumorigenicity, nude mouse model
Introduction
Although great efforts have been made toward its diagnosis and treatment, lung cancer still remains the leading cause of cancer death worldwide.1-3 This is a result of the poor prognosis of lung cancer patients, as half of them present with metastatic disease at the time of diagnosis. Lung adenocarcinoma (LUAD) is a common histological subtype of lung cancer, and its incidence rate has increased in the past decades,3-5 and the overall five-year survival rate of LUAD is merely to 16%.5,6 Therefore, the discovery of more sensitive and specific novel target biomarkers for predicting the development and progression of LUAD is mandatory, in turn leading to produce more valid treatments as well as improvements in diagnosis and prognosis.
Long known as a lipid raft protein, flotillin-1 oligomerizes to build microdomain scaffolds7 that are involved in molecular sorting,8,9 cytoskeletal dynamics,10 clathrin-independent endocytic pathways11-14 and phagosome trafficking.14-18 However, it also promotes cell proliferation19 and T cell activation,19,20 indicating its function in non-membrane compartments. Moreover, flotillin-1 functions as a regulatory signaling molecule that coordinates a variety of signal transduction processes.14,15,21 Recent studies have revealed that flotillin-1 has important roles in the progression of several tumor types, including hepatocellular carcinoma,22 neuroblastoma,23 breast cancer,24-26 tongue squamous cell cancer,27 esophageal squamous cell carcinoma,28-30 prostate cancer,19 NSCLC,5,31,32,33 renal cell carcinoma34 and bladder transitional cell carcinoma.35 Zhang SH et al. have reported that a high level of flotillin-1 expression is associated with disease progression and poor prognosis in patients with hepatocellular carcinoma.22 Consistently, our previous study revealed that upregulation of flotillin-1 expression in human LUAD tissues is significantly correlated with advanced clinical stage, lymph node metastasis, increased postoperative relapse and decreased overall survival.31 In addition, a study by Zhang X et al. further showed that the levels of flotillin-1 mRNA and protein expression are upregulated in NSCLC tissues and that the levels of flotillin-1 expression is correlated with the clinicopathological features and overall survival of the NSCLC patients.33 However, until now, the role of flotillin-1 in the development and evolution of LUAD remains unclear. This information is important for understanding not only the effects of tumors in patients as a whole but also the therapeutic impacts of effective treatments in general. Here, with a combination of overexpression and knockdown approaches, we found that flotillin-1 overexpression promoted the proliferation and metastasis of LUAD in vitro, whereas its downregulation inhibited the proliferation and metastasis of LUAD both in vitro and in vivo, thereby suggesting a tumorigenic role of flotillin-1.
Results
Flotillin-1 is upregulated in human LUAD cells
To evaluate the role of flotillin-1 in LUAD progression, western blotting was carried out to detect the expression levels of flotillin-1 in primary Human Bronchial Epithelial (HBE) cell and 5 LUAD cell lines (Fig. 1). As shown in Fig. 1, the flotillin-1 expression levels of H2009 cell and H1975 cell were relatively low among the 5 LUAD cell lines, while the flotillin-1 expression levels of A549 cell and LTEP-a-2 cell were relatively high among the 5 LUAD cell lines. Furthermore, flotillin-1 protein is upregulated in all 5 LUAD cell lines compared to HBE cell. To a certain extent, the results were supplement to our previous proteomics data,31 it is suggested that flotillin-1 probably plays an important role in the pathogenesis of LUAD.
Figure 1.
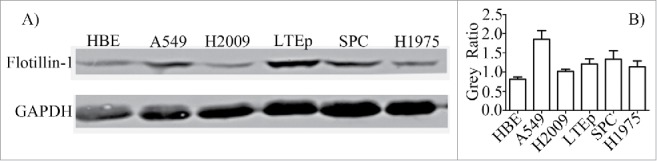
Flotillin-1 is upregulated in human LUAD cells. Western blotting analysis of flotillin-1 expression in human bronchial epithelial (HBE) cell and 5 different LUAD cell lines. GAPDH was used as a loading control. Flot-1 means flotillin-1. The levels of flotillin-1 in each cell were quantified and denoted as relative value [gray value of Flot-1/gray value of matched GAPDH] under immunoblotting data. The experiments were carried out 3 times.
Dysregulated flotillin-1 promotes the malignant phenotype of LUAD cells in vitro
To investigate whether flotillin-1 plays a role in the pathogenesis of LUAD, we selected normal HBE and relatively low levels of flotillin-1 expression H2009 cell and H1975 cell among LUAD cell lines (Fig. 1), then further established their corresponding derivates by stably overexpressing ectopic flotillin-1, and the derivates were confirmed with western blotting for 3 times (Fig. 2A). Through cell counting kit-8 (CCK-8) assay, we found that flotillin-1 overexpressed lung cells displayed higher proliferation rates than the corresponding lung cells with empty vector (Fig. 2B). Through scratch wound healing assay, we also found that the wound of flotillin-1 overexpressed lung cells healed quicker compared to the wound of the corresponding cells with empty vector (Fig. 2C). The following histogram in Fig. 2C shows that horizontal migrate rate (%) of flotillin-1 overexpressed lung cells was higher than that of the corresponding lung cells with empty vector during the monitor period. More importantly, we found that the number of penetrated flotillin-1 overexpressed lung cells is prominently much more than the number of penetrated corresponding lung cells with empty vector in both transwell migratory assay and transwell matrigel invasive assay (p < 0.05) (Fig. 2D to E). Moreover, H2009 cells and H1975 cells both with empty vector showed much better migratory and invasive ability than HBE cells with empty vector (p < 0.05) (Fig. 2E). Consistently, flotillin-1 overexpressed H2009 cells and H1975 cells displayed much better migratory and invasive ability compared to flotillin-1 overexpressed HBE cells (p < 0.05) (Fig. 2E). Surprisingly, flotillin-1 overexpressed HBE cells promoted proliferation, migratory ability and invasive ability compared to HBE cells with empty vector (Fig. 2B to E). In a whole, these data show that overexpressed of flotillin-1 significantly promotes the malignant phenotype of LUAD cells in vitro.
Figure 2.

Dysregulated Flotillin-1 Promotes the Malignant Phenotype of LUAD cells in vitro. (A) Selected normal HBE and relatively low levels of flotillin-1 expression H2009 and H1975 LUAD cell lines, and further established their corresponding derivates by stably overexpressing ectopic flotillin-1. Levels of flotillin-1 were analyzed by western blotting for 3 times. GAPDH was used as a loading control. (B) The quantification analytical line charts of the absorbance of indicated cells as determined by CCK-8 cell proliferation assay. The independent experiments were carried out 3 times, 4 repeated wells in every independent experiment. (C) The upper representative pictures of migrate distance at 0h, 24h, 40h as determined by scratch wound healing assay, 100X. The below column chart of horizontal migrate rate (%) was quantified using relative value [100* (0h wound distance - 24h/48h wound distance)/ 0h wound distance]. Each bar represents the mean standard deviation of 2 independent experiments. * means P<0.05. The independent experiments were carried out 2 times, 6 observing fields in every independent experiment. (D, E) The representative pictures (D) and quantification column chart (E) of migrated and invaded cells were analyzed using transwell assay, 200X. Flot-1 means flotillin-1. Each bar represents the mean standard deviation of 3 independent experiments. * means P<0.05, ** means P<0.01, *** means P<0.005.
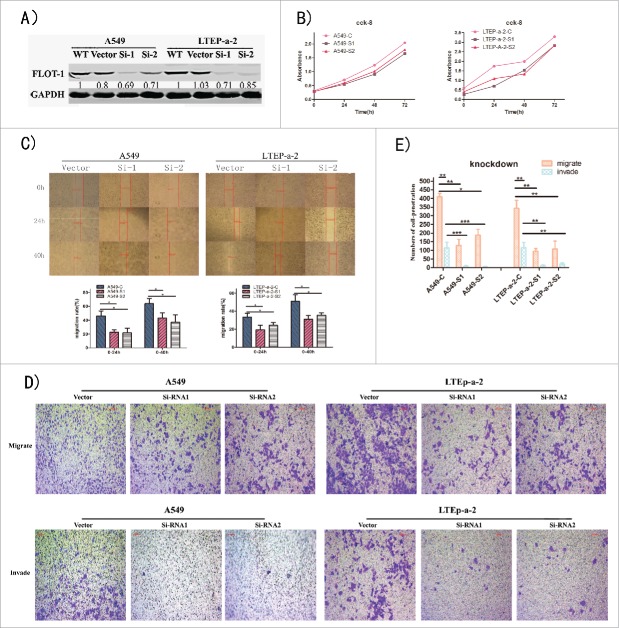
Downregulation of flotillin-1 inhibits the malignant phenotype of LUAD cells
To further evaluate the effect of flotillin-1 on the tumorigenic activity of LUAD cells, we chose the LUAD cell lines A549 and LTEP-a-2, which have high levels of flotillin-1 expression (Fig. 1). Their corresponding derivates with stable knockdown of flotillin-1 were established, and the derivates were checked with western blotting for 3 times (Fig. 3A). siRNA-based knockdown of flotillin-1 lung cells displayed significantly lower proliferation trend than the corresponding LUAD cells with empty vector (Fig. 3B). Through scratch wound healing assay, we found that the wound distance of flotillin-1 knocked-down LUAD cells was farther than the wound distance of the corresponding LUAD cells with empty vector at the monitor time (Fig. 3C). The following histogram in Fig. 3C shows that horizontal migrate rate (%) of flotillin-1 knocked-down LUAD cells was lower than that of the corresponding LUAD cells with empty vector during the monitor period. Moreover, the number of penetrated flotillin-1 knocked-down LUAD cells was transparently less than the number of penetrated corresponding LUAD cells with empty vector in transwell migratory assay as well as transwell matrigel invasive assay (p < 0.05) (Fig. 3D to E). These information shows that knockdown of flotillin-1 significantly inhibits the malignant phenotype of LUAD cells in vitro.
Figure 3.
Downregulation of Flotillin-1 Inhibits the Malignant Phenotype of LUAD Cells in Vitro. (A) Stablely knocked down the flotillin-1 expression of A549 and LTEP-a-2 LUAD cell lines with 2 specific siRNA lentivirus, and established their corresponding derivates. Levels of flotillin-1 were analyzed by immunoblotting for 3 times. GAPDH was used as a loading control. (B) The quantification analytical line charts of the absorbance of indicated cells as determined by CCK-8 cell proliferation assay. The independent experiments were carried out 3 times, 4 repeated wells in every independent experiment. (C) The representative pictures of migrate distance at 0h, 24h, 40h as determined by scratch wound healing assay, 100X. The below column chart of horizontal migrate rate (%) was quantified using relative value [100* (24h/48h migrate distance - 0h migrate distance)/ 0h migrate distance]. Each bar represents the mean standard deviation of 2 independent experiments. * means P<0.05. The independent experiments were carried out 2 times, 6 observing fields in every independent experiment. (D, E) The representative pictures (D) and quantification column chart (E) of migrated and invaded cells were analyzed using transwell assay, 200X. Flot-1 means flotillin-1. Each bar represents the mean standard deviation of 3 independent experiments. * means P<0.05, ** means P<0.01,*** means P<0.005.
Collectively, these results suggest that flotillin-1 plays an important role in the malignant phenotype of LUAD cells in vitro.
Flotillin-1 contributes to the progression of malignant phenotype of LUAD in vivo
The ability of flotillin-1 to promote LUAD progression was further examined using an in vivo tumor model. Mice were subcutaneously inoculated with empty vector-transfected A549 cells (6 × 106 cells per mouse) in the left dorsal flank (Fig. 4A-a) and with flotillin-1-siRNA knocked-down A549 cells (6 × 106 cells per mouse) in the right dorsal flank (Fig. 4A-b). Solid tumors were visible by 15 days after implantation. The average time of tumor formation for the nude mice transfected with flotillin-1 knockdown cells (23.6 ± 2.50 days) was significantly longer than that for the control group (15 ± 2.78 days) (p < 0.05). The dissected tumors are shown in Fig. 4A-c. And the final tumor volumes of the control and flotillin-1 knockdown group in each mouse are shown in Fig. 4A-d. In addition, Fig. 4A-e shows that the speed of tumor growth with time in the flotillin-1 knockdown group was slower than that in the control group. Moreover, the final tumor volume in the flotillin-1 knockdown group were smaller than those in the control group (p < 0.05) (Fig. 4A-f), while the average tumor weight of the flotillin-1 knockdown group (0.47 ± 0.33 g) was lighter than that of the control group (1.89 ± 0.97 g) (p < 0.05) (Fig. 4A-g). Surprisingly, we observed that the control group of A549 cells infiltrated into the skin tissue as the yellow arrows showed in Fig. 4B-a (the right picture is the magnification of the red box of the left picture), indicating that tumor invasion occurred in flotillin-1 high expressed LUAD group. Furthermore, we also observed that A549 cells metastasized to lymph node as the white arrows showed in Fig. 4B-b on the control sides of the tumor-bearing mice, confirming that metastasis on the control sides occurred. The analyses of Fig. 4B-c and -d showed that the flotillin-1 knockdown tumors had a lower proportion of microvascular density and Ki67-positive cells than those of the primary tumors, but the percentage of F4/80-positive cells was not noticeably different.
Figure 4.

Flotillin-1 Contributes to LUAD Progression of Malignant Phenotype of LUAD in Vivo. (A) Xenograft model in nude mice. Representative images of tumor-bearing mice in silencing group, (a) control group, (b) siRNA knocked-down (KD) group, (c) images of the tumors, (d) the final tumor volumes of control side and KD side per mouse, (e) mean tumor volume of control group and KD group on the monitor days, (f) the final tumor volume of control group and KD group, (g) the final tumor weight of control group and KD group; (B) H&E and IHC staining demonstrated that knockdown of flotillin-1 inhibited the malignant phenotype of LUAD cells in vivo. (a) The picture of LUAD cells of control group infiltrated into the skin dermis of nude mouse. The red arrow and the yellow arrow respectively show the epidermis and LUAD cells. (b) Metastasis was showed in the lymph node of tumor-bearing mice. The white arrow of the left picture shows the metastatic LUAD cells. The red arrow of the right picture shows the Flot-1-positive cells. (c) The representative pictures and (d) quantification column chart as indicated by the expression of CD31, Ki67, and F4/80-positive cells. Each bar represents the mean standard deviation of 3 independent experiments.*, P<0.05; **, P<0.01.
Taken together, these results indicate that flotillin-1 contributes to the tumorigenesis, metastasis and invasion of LUAD in vivo.
Discussion
Lung adenocarcinoma (LUAD) accounts for the majority of lung cancer cases.1-3 LUAD patients generally have no obvious clinical symptoms in the early phase of the disease, and they are usually with a low average age at disease onset. Moreover, it is difficult to find patients with early-stage LUAD, as hematogenous metastases have already occurred by the time they are diagnosed.3,29 It is equally important to note that the unimproved prognosis leads to only a 16% overall five-year survival rate of these lung cancer patients.5,6 Therefore, the discovery of novel target biomarkers for predicting the tumorigenesis and progression of LUAD is extremely necessary.
Flotillin-1, a protein marker of lipid rafts, is an important constituent of lipid rafts and serves as a signaling molecule that links the membrane receptors to signal transduction pathways14,15,21 in various types of cells. Recent studies have found that flotillin-1 is a useful indicator of tumor progression in hepatocellular carcinoma,22 neuroblastoma,23 breast cancer,24-26 tongue squamous cell cancer,27 esophageal squamous cell carcinoma,28-30 prostate cancer,19 NSCLC,5,31,32,33 renal cell carcinoma34 and bladder transitional cell carcinoma.35 However, until now, the role of flotillin-1 in the development and evolution of LUAD remains unclear. In agreement with these previous reports, our analysis of the plasma membrane proteome31 demonstrated that the upregulation of flotillin-1 expression in human LUAD tissues is significantly correlated with advanced clinical stage, lymph node metastasis, increased postoperative relapse and decreased overall survival. Therefore, delineating the role of flotillin-1 in the development and evolution of LUAD is essential.
Recently, Song LB et al. reported that flotillin-1 was markedly upregulated in both human breast cancer and esophageal squamous cell carcinoma cells and tissues. In addition, knockdown of flotillin-1 inhibited the proliferation and tumorigenesis of breast cancer and esophageal squamous cell carcinoma. However, the functional study that inhibiting the expression of flotillin-1 with siRNA and shRNA reduced the migration and invasion capacity of LUAD cells has never been reported. Consistent with these data, we demonstrated higher levels of flotillin-1 expression in LUAD cell lines compared to HBE (Fig. 1). In addition, with a combination of overexpressed and knockdown approaches, we found that overexpression of flotillin-1 promoted the proliferation, migration and invasion capacity of LUAD cells, whereas downregulation of flotillin-1 inhibited the proliferation, migration and invasion capacity of LUAD in vitro. Surprisingly, the HBE cell line with ectopic expression of flotillin-1 displayed a malignant phenotype compared to the control HBE cell line (Fig. 2C to E). Consistently, the xenograft tumors from the flotillin-1 knockdown cells were smaller, in both size and weight, than the xenograft tumors formed from the control cells. In addition, as the yellow arrows showed in Fig. 4B-a, the control group of LUAD cells infiltrated into the skin tissue (the right picture is the magnification of the red box of the left picture), confirming the invasion capacity of flotillin-1 high expression LUAD tumors. Similarly, as the white arrows showed in Fig. 4B-b, LUAD cells metastasized to lymph node. While lymph node status is a strong predictor of outcome for LUAD patients,36 therefore lymphatic metastasis indicated an important progression stage of flotillin-1 high expression LUAD. Moreover, the relatively low expression of flotillin-1 LUAD tumors displayed reduced Ki-67 proliferation index and metastasis capacity compared to the control tumors in vivo (Fig. 4B-c and -d), suggesting the specific effect of flotillin-1 in promoting proliferation and metastasis of LUAD. During tumor proliferation and metastasis, the tumor cells must acquire an adequate oxygen and blood supply. Consistently, our result suggests that the number of microvascular structures in tumors of the control groups was much higher than that in the flotillin-1 KD tumors (Fig. 4B-c and -d). On the whole, high expression of flotillin-1 promoted invasion and metastasis capacity of malignant phenotype in LUAD, suggesting an oncogenic role of flotillin-1.
Taken together, our findings provide new insights into the contribution of flotillin-1 to LUAD malignant phenotype, and suggest flotillin-1 as an attractive therapeutic target. Given the role of flotillin-1 in the tumorigenicity and progression of the malignant phenotype in LUAD cells, our results suggest that the level of flotillin-1 expression might be a useful index of the degree of malignancy and invasion and metastasis capacity of LUAD and could be used as a valuable clinical biomarker. Subsequent studies are needed to confirm the signaling pathways, such as the p533 and NF-κB signaling pathways,30 which may be activated by flotillin-1 to promote tumorigenesis. Identifying the precise role of flotillin-1 in the pathogenesis of LUAD will lead to a better understanding of the biological basis of cancer progression and enable the development of flotillin-1 as a new therapeutic target for LUAD. This information could then be used to determine whether flotillin-1 expression can serve as a therapeutic target and/or predict clinical outcome in patients with LUAD.
Our study provides crucial evidence to support the hypothesis that flotillin-1 promotes the tumorigenicity and progression of the malignant phenotype in LUAD.
Materials and methods
Ethics statement
This study was approved by the Institute Research Ethics Committee of Central South University, China. Written informed consent was obtained from all participants in the study. All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of Central South University with the approval of the Scientific Investigation Board of Central South University.
Antibodies and reagents
Anti-flotillin-1 (Abcam, ab123512, 1:1000, the US), GAPDH (Abcam, ab181602, 1:2000, the US), goat anti-rabbit IgG (Millipor, AP132P, 1:8000, the US), Prestained Protein Ladder (Thermo Fisher Scientific, 26616, the US) were used in western blotting; Matrigel Basement Membrane Matrix is from BD Biocoat, the US. Cell counting kit-8 is from Beyotime. Puromicin, Mitomycin, Streptomycin and Penicillin are from Sigma, the US.
Cell culture
The lung adenocarcinoma (LUAD) cells lines A549, H2009, H1975 were purchased from the Shanghai Research Institute for Biological Science, the Chinese Academy of Sciences; normal human bronchial epithelial cells (HBE), LTEP-a-2, SPC-a-1 were kindly provided by Dr. Chaojun Duan of Central South University. The cells were grown in Dulbecco's Modified Eagle Medium (HyClone, Logan, UT) and RPMI 1640 (HyClone, Logan, UT) supplemented with 10% fetal bovine serum (Biowest S.A.S, South America).
Lentivirus and transfections
According to the human flotillin-1 coding sequence, the lentiviral pLenti-GFP-puro-Flot-shRNA constructs were established by ViGene (Shandong, China) and confirmed by sequencing. The control lentivirus was purchased from ViGene. To knockdown endogenous flotillin-1, two specific lentiviral siRNA sequences for flotillin-1 were as follows:
siRNA-1: 5′-CCCTCAATGTCAAGAGTGAAA-3′;
siRNA-2: 5′-ACAGAGAGATTACGAACTGAA-3′.
The effects of the siRNA were validated. In addition, the control vector containing hU6-MCS-Ubiquitin-EGFP-IRES-puromycin lentivirus was purchased from GeneChem (Shanghai, China). Cell lines stablely overexpressing flotillin-1 or knocked-down flotillin-1 were selected for 21 days with 3 μg/mL puromycin after infection for 72 hours. The transfected cells were cultured with 1.5 μg/mL puromycin in the subsequent experiments.
Western blotting
The cells were lysed in RIPA buffer, and protein concentrations were measured. An equal amount of protein from each sample was mixed with loading buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) separation, followed by blotting onto a 0.22 µm polyvinylidene difluoride (PVDF) membrane (Millipore, the US). The PVDF membranes were blocked with 5% nonfat dry milk or 5% BSA diluted with PBST for 2 hours at room temperature and then incubated with primary antibody at 4°C overnight followed by incubation with horseradish peroxidase-conjugated secondary antibody at room temperature for 1 hour. The signal was visualized with an enhanced chemiluminescence detection reagent (BeyoECL Plus, China) using FluorChem FC3 (BioTek, the US). GAPDH was detected simultaneously using anti-GAPDH rabbit monoclonal antibody as a loading control. The experiment was carried out three times.
Cell counting kit-8 proliferation assay
Cell proliferation assay was performed with the Cell Counting Kit-8. The cells (3 × 103) were uniformly seeded into four 96-well plates with DMEM containing 10% FBS. When the cells adhered to the plates, the medium was aspirated, and the cells were gently washed 3 times with cold PBS. Then, 200 μL complete DMEM containing the CCK-8 solution (10:1 dilution) was added to each well. After incubation for 2 hours, the absorbance was detected by a dual-wavelength spectrophotometric determination (λ = 450 nm and 650 nm) with an ultraviolet spectrophotometer (Bio Tek, Eon, the US). The treatments mentioned above were carried out 3 hours before detection at 0, 24, 48, 72 hour time points. The experiment was carried out in quintuplicate and repeated two times. The statistics were analyzed by paired student's t test.
Scratch wound healing assay
Cell migration was grossly evaluated using the scratch wound healing assay. Briefly, the cells were grown in DMEM containing 10% FBS overnight to a 95% to 100% confluency in a 6-well plate. The cell monolayers were wounded by dragging a 20 μL pipette tip across. The cells were washed 3 times to remove cellular debris, incubated with DMEM containing 0.5% FBS in the incubator and allowed to migrate for 24 or 40 hours. Images were taken 0, 24 and 40 hours after wounding using a Leica MD50-T inverted microscope (Germany). The distance migrated by the cell monolayer to close the wounded area during this time period was measured. Results were expressed as a horizontal migration rate (%)—that was quantificated using relative value [100* (0h wound distance – 24h/48h wound distance)/ 0h wound distance]. The independent experiment was carried out 2 times, and 6 observing fields in every independent experiment. The statistics were analyzed by paired student's t test.
Transwell migration assay
Cells treated with 20 μg/mL mitomycin for 2 hours before digesting the cells with trypsin. Cells (4 × 104) were seeded in the upper chambers of the Transwell inserts (Corning Falcon) with serum-free DMEM. The lower chambers were filled with 600μL DMEM containing 10% FBS as a chemoattractant. After incubation at 37 °C for 18 hours (overexpressed cell lines) or 22 hours (knockdown cell lines), the inserts were soaked in PBS for 5 minutes. The PBS was aspirated, and subsequently the migrated cells on the lower surface of the filter were fixed with 4% paraformaldehyde for 20 minutes and stained with 0.5% crystalviolet solution for 30 minutes in the dark. The upper chambers were dried with cotton swabs before taking pictures using a Leica DMI3000B inverted microscope (Germany). The number of migrated cells was counted from 8 random microscopic fields. The experiment was carried out three times. The statistics were analyzed by one-way ANOVA.
Transwell matrigel invasion assay
Cells treated with 20 μg/mL mitomycin for 2 hours before digesting the cells with trypsin. Cells (4 × 104) in serum-free DMEM were seeded into the upper chambers of the 24-well 8-μm pore size Transwell inserts (Corning Falcon) precoated with Matrigel (BD Biosciences). The subsequent procedures were the same as those for the Transwell migration assay.
Xenografted tumor model, immunohistochemistry, and H&E staining
BALB/c nude male mice (3- to 4-weeks-old) were obtained from the SLUADk Scene of Laboratory Animal Co, LTD (Changsha, China) and maintained under specific pathogen-free conditions in the Laboratory Animal Center of Central South University. For the xenografted tumor model, nude mice were randomly divided into 2 groups (n = 8/group). After 4 days, the mice were subcutaneously inoculated. Tumor growth was monitored every 3 days. Tumor volume was estimated using the equation V = (L × W2)/2, where L is the length and W is the width of the xenograft; length and width were measured with calipers. After injection for 30 days, the mice were sacrificed by cervical dislocation; the tumors and lymph nodes were excised and embedded in paraffin. Serial 6.0 μm-thick sections were cut and subjected to immunohistochemistry (IHC) and H&E staining. After deparaffinization, the sections were immunohistochemically analyzed using antibodies against F4/80 (Abcam, ab100790, 1:600, the US), Ki-67 (Cell Signaling Technology, 9449S, 1:200, the US), CD31 (Cell Signaling Technology, 3528S, 1:400, the US), flotillin-1(Cell Signaling Technology, 18634, 1:200, the US). The proliferation index was quantified from the proportion of Ki67-positive cells, the microvascular density was estimated by assessing the CD31-positive microvasculars, and the macrophage index was estimated by assessing the percentage of F4/80-positive cells. Serial 6.0 μm-thick sections were counterstained with Mayer's hematoxylin solution. Images were captured using a Leica DMI3000B inverted microscope (Germany).
Statistical analysis
Statistical analyses were performed using the SPSS 17.0 Windows software. Single comparisons between two groups were done using Student's t test. The data from each experimental group were compared using one-way ANOVA. The data for the quantitative results are expressed as the mean ± SD. Graphical plotting and testing of differences of means were achieved using GraphPad Prism 5. P-values of 0.05 or less were considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Role of the funding source
The funding bodies had no role in the design of the study, collection, analysis and interpretation of the data or the writing of the manuscript.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81372516, 81570776) and the Hunan Provincial Natural Science Foundation of China (No. 2016JJ5013).
References
- 1.Parkin DM. Global cancer statistics in the year (2000). LANCET ONCOLOGY 2001;2(10):596-596. doi: 10.1016/S1470-2045(01)00486-7. PMID:11905707 [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Parkin DM, Li LD, Chen YD, and Bray F. Estimation and projection of the national profile of cancer mortality in China: 1991–2005. BRITISH JOURNAL OF CANCER. 2004;11:2157-66. doi: 10.1038/sj.bjc.6601813. PMID:15150609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi YX, Zhu T, Zou T, Zhuo W, and Chen YX. Prognostic and predictive values of CDK1 and MAD2L1 in lung adenocarcinoma. Oncotarget. 2016;7:85235-43. doi: 10.18632/oncotarget.13252. PMID:27835911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, Murray T, and Xu JQ. Cancer statistics 2006. CA-A CANCER JOURNAL FOR CLINICIANS. 2006;56:106-30. doi: 10.3322/canjclin.56.2.106. PMID:16514137 [DOI] [PubMed] [Google Scholar]
- 5.Little AG, Gay E, Greer Gaspar, Laurie E, and Stewart AK. National survey of non-small cell lung cancer in the United States: Epidemiology,pathology and patterns of care. LUNG CANCER 2007;57:253-60. doi: 10.1016/j.lungcan.2007.03.012. PMID:17451842 [DOI] [PubMed] [Google Scholar]
- 6.Howe HL, Wingo PA, Thun MJ, Ries LAG, and Rosenberg HM. Re: Annual report to the nation on the status of cancer (1973 through 1998), featuring cancer with recent increasing trends- Response. JOURNAL OF THE NATIONAL CANCER INSTITUTE. 2001;93:1656-1656. doi: 10.1093/jnci/93.21.1656-a. PMID:11390532 [DOI] [PubMed] [Google Scholar]
- 7.Solis GP, Hoegg M, Munderloh C, Schrock Y, and Malaga-Trillo E. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J. 2007;403:313-22. doi: 10.1042/BJ20061686. PMID:17206938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge L, Qi W, Wang LJ, Miao HH, Qu YX, Li BL, and Song BL. Flotillins play an essential role in Niemann-Pick C1-like 1-mediated cholesterol uptake. PNAS. 2011;108;551-6. doi: 10.1073/pnas.1014434108. PMID:21187433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langhorst MF, StuermerCA Reuter Aand. Scaffolding microdomains and beyond: the function of reggie/flotillin proteins. Cell Mol Life Sci. 2005;62:2228-40. doi: 10.1007/s00018-005-5166-4. PMID:16091845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludwig A, Otto GP, Riento K, Hams E, and Fallon PG. Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. The Journal of Cell Biology. 2010;191:771-81. doi: 10.1083/jcb.201005140. PMID:21059848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frick M, Bright NA, Riento K, Bray A, and Merrified CL. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr Biol. 2007;17:1151-56. doi: 10.1016/j.cub.2007.05.078. PMID:17600709 [DOI] [PubMed] [Google Scholar]
- 12.Riento K, Frick M, Schafer I, and Nichols BJ. Endocytosis of flotillin-1 and flotillin-2 is regulated by Fyn kinase. Journal of Cell Science. 2009;122:912-8. doi: 10.1242/jcs.039024. PMID:19258392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols BJ. GM1-containing lipid rafts are depleted within clathrin-coated pits. Curr Biol. 2003;13:686-90. doi: 10.1016/S0960-9822(03)00209-4. PMID:12699627 [DOI] [PubMed] [Google Scholar]
- 14.Li R, Liu P, Wan Y, Chen T, and Wang Q, Mettbach U, Baluska F, Samaj J, Fang X, Lucas WJ, et al.. A Membrane Microdomain-Associated Protein, Arabidopsis Flot1, Is Involved in a Clathrin-Independent Endocytic Pathway and Is Required for Seedling Development. The Plant Cell. 2012;24:2105-22. doi: 10.1105/tpc.112.095695. PMID:22589463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glebov OO, Bright NA, and Nichols BJ. flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. NATURE CELL BIOLOGY. 2006;1:16-46. doi: 10.1038/ncb1342. PMID:16341206 [DOI] [PubMed] [Google Scholar]
- 16.Cremona ML, Matthies HJG, Pau K, Bowton E, Speed N, Lute BJ, Anderson M, Sen N, Robertson SD, Vaughan RA, et al.. flotillins is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat Neurosci. 2011;14:469-77. doi: 10.1038/nn.2781. PMID:21399631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walton JR, Frey HA, Vandre DD, Kwiek JJ, and Ishikawa, Tomoko. Expression of flotillins in the human placenta: potential implications for placental transcytosis. Histochemistry and Cell Biology. 2013;139:487-500. doi: 10.1007/s00418-012-1040-2. PMID:23064789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babuke T, Ruonala M, Meister M, Amaddii M, and Genzler C. Hetero-oligomerization of reggie-1/flotillin-2 and reggie-2/flotillin-1 is required for their endocytosis. Cell Signal. 2009;21:1287-97. doi: 10.1016/j.cellsig.2009.03.012. PMID:19318123 [DOI] [PubMed] [Google Scholar]
- 19.Santamaria A, Castellanos E, Gomez V, Benedit P, and Renau-Piqueras J. PTOV1 Enables the Nuclear Translocation and Mitogenic Activity of flotillin-1, a Major Protein of Lipid Rafts. Molecular and Cellular Biology. 2005;25:1900-11. doi: 10.1128/MCB.25.5.1900-1911.2005. PMID:15713644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Affentranger S, Martinelli S, Hahn J, Rossy J, Niggli V. Dynamic reorganization of flotillins in chemokine-stimulated human T-lymphocytes. BMC Cell Biol. 2011;12:28. doi: 10.1186/1471-2121-12-28. PMID:21696602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandur PD, Dirksen M, Moore KB, Moody SA. Xenopus flotillin1, a novel gene highly expressed in the dorsal nervous system. Developmental Dynamics. 2004;231:881-7. doi: 10.1002/dvdy.20191. PMID:15517583 [DOI] [PubMed] [Google Scholar]
- 22.Zhang SH, Wang CJ, Shi L, Li XH, Zhou J, Song LB, Liao WT. High Expression of FLOT1 Is Associated with Progression and Poor Prognosis in Hepatocellular Carcinoma. PLoS One. 2013;8:e64709. doi: 10.1371/journal.pone.0064709. PMID:23840303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arata T, Takamasa U, Reiko K, Kazuki S, Junko T, Miki O. flotillin-1 Regulates Oncogenic Signaling in Neuroblastoma Cells by Regulating ALK Membrane Association. Cancer Research. 2014;74:3790. doi: 10.1158/0008-5472.CAN-14-0241. PMID:24830726 [DOI] [PubMed] [Google Scholar]
- 24.Lin C, Wu Z, Lin X, Yu C, Shi T, Zeng Y, Wang X, Li J, Song L. Knockdown of FLOT1 Impairs Cell Proliferation and Tumorigenicity in Breast Cancer through Upregulation of FOXO3a. Clinical Cancer Research. 2011;17:3089-99. doi: 10.1158/1078-0432.CCR-10-3068. PMID:21447726 [DOI] [PubMed] [Google Scholar]
- 25.Li L, Luo J, Wang B, Wang D, Xie X, Yuan L, Guo J, Xi S, Gao J, Lin X, et al.. Microrna-124 targets flotillin-1 to regulate proliferation and migration in breast cancer. MOL CANCER. 2013;12:163. doi: 10.1186/1476-4598-12-163. PMID:24330780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurrle N, Ockenga W, Meister M, Vollner F, Kuhne S, John BA, Banning A, Tikkanen R. Phosphatidylinositol 3-Kinase dependent upregulation of the epidermal growth factor receptor upon flotillin-1 depletion in breast cancer cells. BMC CANCER. 2013;13:575. doi: 10.1186/1471-2407-13-575. PMID:24304721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Zhang Y, Chen SW, Li FJ, Zhuang SM, Wang LP, Zhang J, Song M. Prognostic significance of Flotillin1 expression in clinically N0 tongue squamous cell cancer. Int J Clin Exp Pathol. 2014;7:996-1003. PMID:24695539 [PMC free article] [PubMed] [Google Scholar]
- 28.Gong H, Song L, Lin C, Liu A, Lin X, Wu J, Li M, Li J. Downregulation of miR-138 Sustains NF-kB Activation and Promotes Lipid Raft Formation in Esophageal Squamous Cell Carcinoma. CLIN CANCER RES. 2013;19:1083-93. doi: 10.1158/1078-0432.CCR-12-3169. PMID:23319823 [DOI] [PubMed] [Google Scholar]
- 29.Lin C, Liu A, Zhu J, Zhang X, Wu G, Ren P, Wu J, Li M, Li J, Song L. miR-508 sustains phosphoinositide signalling and promotes aggressive phenotype of oesophageal squamous cell carcinoma. Nat Commun. 2014;5:4620. doi: 10.1038/ncomms5620. PMID:25099196 [DOI] [PubMed] [Google Scholar]
- 30.Song LB, Gong H, Lin CY, Wang CJ, Liu LP, Wu JH, Li MF, Li J. Flotillin-1 Promotes Tumor Necrosis Factor-α Receptor Signaling and Activation of NF-κB in Esophageal Squamous Cell Carcinoma Cells. Gastroenterology. 2012;143. 995-1005. doi: 10.1053/j.gastro.2012.06.033. PMID:22732732 [DOI] [PubMed] [Google Scholar]
- 31.Zhang PF, Zeng GQ, Hu R, Li C, and Yi H. Identification of flotillin-1 as a novel biomarker for lymph node metastasis and prognosis of lung adenocarcinoma by quantitative plasma membrane proteome analysis. Journal of Proteomics. 2012;77:202-14. doi: 10.1016/j.jprot.2012.08.021. PMID:22982323 [DOI] [PubMed] [Google Scholar]
- 32.Arkhipova KA, Sheyderman AN, Laktionov KK, Mochalnikova VV, Zborovskaya IB. Simultaneous expression of flotillin-1, flotillin-2, stomatin and caveolin-1 in non-small cell lung cancer and soft tissue sarcomas. BMC Cancer. 2014;14:100. doi: 10.1186/1471-2407-14-100. PMID:24533441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Wang R, Liu S, Zhang J, Luo J, Zhang B, Zhang X. Abnormal expression of FLOT1 correlates with tumor progression and poor survival in patients with non-small cell lung cancer. TUMOR BIOL. 2014;35:3311-15. doi: 10.1007/s13277-013-1434-3. PMID:24277378 [DOI] [PubMed] [Google Scholar]
- 34.Raimondo F, Cifola I, Rocco F, Kienle MG, Mocarelli P, Brambilla PM, Valerio DT, Magni F, Perego R, Bianchi C, et al.. Caveolin-1 and flotillin-1 Differential Expression in Clinical Samples of Renal Cell Carcinoma. The Open Proteomics Journal. 2008;1:87-98. doi: 10.2174/1875039700801010087 [DOI] [Google Scholar]
- 35.Guan Y, Song H, Zhang G, Ai X. Overexpression of flotillin-1 is involved in proliferation and recurrence of bladder transitional cell carcinoma. Oncol Rep. 2014;42:748-54. PMID:24890092; doi: 10.3892/or.2014.3221 [DOI] [PubMed] [Google Scholar]
- 36.Liu YF, Chen YH, Li MY, Zhang PF, Peng F, Li GQ, Xiao ZQ, Chen ZC. Quantitative proteomic analysis identifying three annexins as lymph node metastasis-related proteins in lung adenocarcinoma. Med Oncol. 2012;29:174-84. doi: 10.1007/s12032-010-9761-3. PMID:21132403 [DOI] [PubMed] [Google Scholar]