Abstract
Many multicellular parasites seek out hosts by following trails of host-emitted chemicals. Host seeking is characteristic of endoparasites such as parasitic worms as well as ectoparasites such as mosquitoes and ticks. Many of these parasites use carbon dioxide (CO2), a respiration byproduct, in combination with host-specific chemicals for host location. Recent work has begun to elucidate the behavioral responses of parasites to CO2 and other host chemicals, and to unravel the mechanisms of these responses. Here we discuss recent findings that have greatly advanced our understanding of the chemosensory behaviors of host-seeking parasites. We focus primarily on well-studied parasites such as nematodes and insects, while also noting broadly relevant findings in a few less well-studied parasites.
Keywords: parasitic helminths, nematode, entomopathogenic nematodes, EPN, olfaction, ectoparasite, mosquito, chemosensation, host seeking
Chemosensory behaviors of parasitic helminths
Parasitic helminths comprise a large group of worms from a number of different phyla, including roundworms in the phylum Nematoda and flatworms in the phylum Platyhelminthes. Some parasitize humans, while others parasitize non-human animals or plants. Human-parasitic species cause extensive morbidity, mortality, and economic loss worldwide, and are responsible for some of the most common neglected tropical diseases [1]. Parasitic helminths utilize a number of different strategies for host infection: (i) reliance on passive host ingestion, (ii) transmission by intermediate vectors such as mosquitoes and flies, and (iii) engagement in active host-seeking and host-invasion behaviors. The latter group consists of some of the most devastating human-parasitic worms, including the skin-penetrating intestinal nematodes Strongyloides stercoralis, Ancylostoma duodenale and Necator americanus, as well as blood flukes in the genus Schistosoma. For parasitic worms that actively seek out hosts, chemosensation is a critical sensory modality, allowing the parasites to recognize appropriate hosts by detecting host-emitted chemicals.
Parasitic nematodes
The most well-studied of the helminths are the nematodes, which comprise a large and highly diverse phylum that includes both free-living species such as the model nematode Caenorhabditis elegans and parasitic species. Human parasitic nematodes infect over a quarter of the world’s population, and nematode parasites of livestock and plants cause economic and agricultural losses of billions of dollars each year [1,2]. The infective stages of many parasitic nematodes are developmentally-arrested larval stages that actively seek out hosts in which to complete their life cycles. The group of nematodes that host seek includes insect parasites, plant parasites, and skin-penetrating mammalian parasites.
The entomopathogenic nematodes (EPNs) comprise a guild of lethal parasites of insects that are of interest both as models for human parasitic nematodes and as biocontrol agents for a wide variety of insect pests and disease vectors. EPN infective juveniles (IJs) infect insect hosts either by entering through a natural body opening or by penetrating directly through the insect cuticle. EPNs rapidly kill their host, develop and reproduce inside the host cadaver until resources are depleted, and then exit as IJs to search for new hosts (Figure 1a–b). EPNs vary greatly in their host ranges; for example, Steinernema carpocapsae is a generalist that can infect over 250 different insect species under laboratory conditions, while Steinernema scapterisci is an orthopteran specialist whose only known natural host is the mole cricket [3]. EPN infective juveniles (IJs) are strongly attracted to live hosts, as well as carbon dioxide (CO2) and a wide variety of other host-derived volatiles (Figure 1c) [4–6]. Attraction to live insect hosts is greatly diminished in the absence of CO2, indicating that CO2 is an essential host-seeking cue for these parasites [5, 6]. In addition to chemotaxing toward hosts, some EPNs in the genus Steinernema exhibit jumping, a highly specialized host-seeking behavior in which the IJ springs into the air and onto passing hosts [7]. Steinernema IJs jump in response to complex odor blends emitted by live hosts, as well as CO2 and many individual host-derived odorants [5, 6].
Figure 1. Chemosensory responses of entomopathogenic nematodes (EPNs).
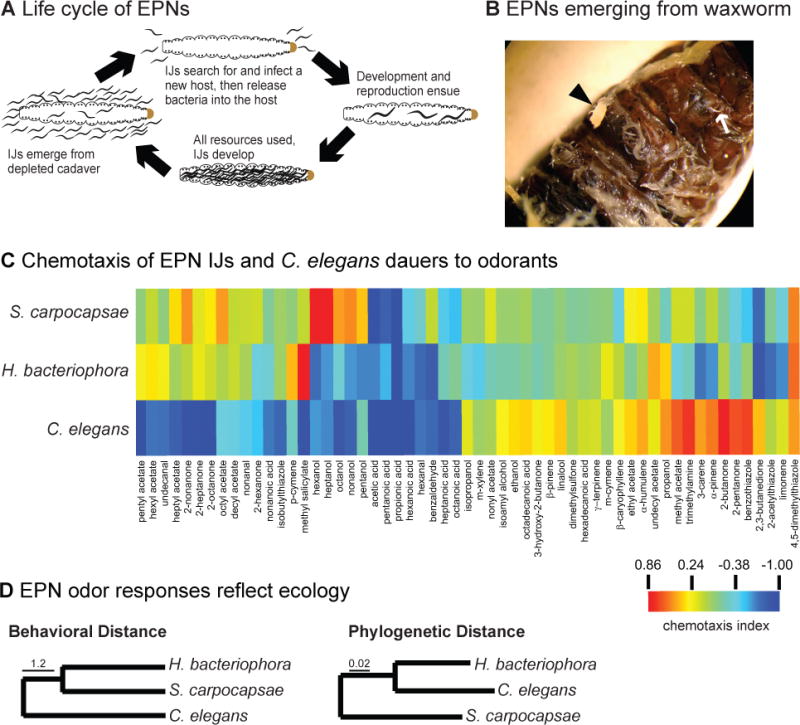
(a) Life cycle of EPNs. EPN IJs infect insect larvae either by entering through a natural body opening or by penetrating through the insect cuticle. IJs are associated with symbiotic bacteria, which they deposit into the host. The bacteria play an important role in overcoming the insect immune system. The nematodes develop and reproduce in the host cadaver for a few generations, feeding off the bacteria and digested insect tissue. New IJs then form and exit the cadaver to search for new hosts [106]. (b) IJs exiting a Galleria mellonella cadaver. Arrowhead indicates a clump of IJs; arrow indicates a single IJ. (c) The behavioral responses of H. bacteriophora IJs, S. carpocapsae IJs, and C. elegans dauers to the indicated odorants in a chemotaxis assay. The chemotaxis index ranges from 1 (perfect attraction) to −1 (perfect repulsion). Responses are color-coded according to the color scale on the lower right. (d) The odor response profiles of the EPNs are more similar to each other than to that of C. elegans despite their phylogenetic distance. Left, behavioral dendrogram of olfactory responses across species. Behavioral distance is based on the Euclidean distances between species based on their odor response profiles. Right, phylogenetic neighbor-joining tree. Branch lengths in the phylogenetic tree are proportional to genetic distances between taxa. Reprinted from [5] with permission. Abbreviations: EPNs, entomopathogenic nematodes; IJs, infective juveniles.
A comparison of the odor response profiles of Heterorhabditis bacteriophora and Steinernema carpocapsae IJs with C. elegans dauer larvae, which are analogous life stages, revealed that the parasitic species cluster together despite their phylogenetic distance (Figure 1d) [5]. This suggests an important role for chemosensation in the evolution of host range among EPNs. In future studies, it will be interesting to extend this type of comparison to include many more free-living and parasitic nematode species – including species with very different lifestyles, ecological niches, and host-seeking strategies – to better understand the relationship between ecology and chemosensory behavior. It will also be interesting to examine the relationship between chemosensory behavior and the evolution of proteins required for chemosensation, such as G proteincoupled receptors, G proteins, and guanylate cyclases.
In addition to responding to host-derived chemicals, EPNs respond to a number of other ecologically relevant chemical cues. For example, EPNs are attracted by odorants produced by insect-damaged plants as an indirect defense against predation [8–10]. However, some of the plant volatiles that attract EPNs also attract plant parasitic nematodes, suggesting an ecological cost to this plant defense mechanism [11]. EPNs also respond to ascarosides, a large family of small-molecule pheromones that regulate reproductive diapause as well as mating, aggregation, and chemotaxis behaviors in nematodes [12–16].
Skin-penetrating mammalian-parasitic nematodes such as the human threadworm Strongyloides stercoralis and the human hookworms Necator americanus and Ancylostoma caninum also engage in host-seeking and host-invasion behaviors. These nematodes are passed out of an infected host as eggs or newly hatched larvae in infested feces, develop into infective larvae in the feces, and then migrate into the environment in search of new hosts [17]. Infection of new hosts occurs by skin penetration, primarily through the skin of the feet [17]. Many mammalian-parasitic nematodes have highly specific host ranges. For example, Strongyloides fuelleborni kellyi is an exclusive parasite of humans, and Strongyloides ratti is a natural parasite of rats but not of other rodents [17, 18]. Chemosensation is thought to be an important component of host specificity: S. stercoralis is attracted to mammalian skin extract [19], and the closely related rat parasite S. ratti is attracted to some components of mammalian sera [20]. For S. stercoralis, a major component of skin attraction is the odorant urocanic acid, which is found in the skin of many mammals and is a potent attractant even at low concentrations [19]. Both S. stercoralis and S. ratti also navigate through sodium chloride gradients [21, 22]. Like the Strongyloides species, the dog hookworm Ancylostoma caninum is attracted to hydrophilic extracts of dog skin [23]. CO2 causes activation of A. caninum and S. stercoralis, and stimulates nictation (tail standing) behavior in A. caninum [23, 24]. In contrast to A. caninum and the Strongyloides species, chemoattraction to human skin has not been demonstrated for the human hookworms Necator americanus and Ancylostoma duodenale [25]. However, these species do display activation (increased locomotion) and penetration behaviors in response to chemosensory cues present in skin extract [26].
The fact that human hookworms have not been shown to display long-range host attraction is consistent with their foraging strategy: the infective larvae are thought to spend most of their time motionless, waiting for passing hosts [24, 26]. By contrast, Strongyloides species spend most of their foraging time actively searching for hosts [24]. This difference in foraging strategy is similar to the cruiser/ambusher distinction among EPNs: some EPNs are cruisers that actively chemotax through the soil searching for hosts, while others are ambushers that are thought to remain relatively stationary and primarily infect passing hosts [27]. However, ambushing EPNs are in fact capable of chemotaxing toward a wide variety of insect-derived odorants as well as live insects [5, 6], although they display less robust host attraction than cruising EPNs [6]. This suggests that ambushing EPNs exhibit long-range host seeking in response to appropriate chemosensory cues. The chemotaxis behavior of human hookworms has not been as thoroughly examined, so whether they are also capable of long-range host attraction is not yet clear.
Plant-parasitic nematodes use CO2 in combination with soluble and volatile host-specific chemicals to locate the roots of host plants [4, 11, 28]. In the case of the root-knot nematodes Meloidogyne incognita and Meloidogyne graminicola, infective larvae are attracted to host plants but show little or no attraction to non-host plants [28]. Moreover, when given a choice between a short route and a long route to reach a host plant, nematodes preferentially choose the short route; however, nematodes often travel the long route to reach a non-host plant. Thus, plant-parasitic nematodes respond differently to chemical blends produced by hosts versus non-hosts [28].
A number of other nematode parasites also display chemosensory behaviors. One example is the gastropod parasitic nematode Phasmarhabditis hermaphrodita. P. hermaphrodita shows attraction to mucosal extracts from slugs, indicating that it uses chemosensory cues to locate slug hosts [29–31]. However, attraction of P. hermaphrodita to potential hosts does not correlate with reproductive success inside the host, suggesting that other factors play an important role in host selection [30]. By contrast, the response of Steinernema carpocapsae to host volatiles following exposure to host cuticle is positively correlated with both host mortality and parasite reproduction inside the host, suggesting that at least some EPNs use chemosensory cues for host selection [32]. Thus, although P. hermaphrodita and S. carpocapsae have apparently similar life styles – both are lethal parasites of small invertebrates that engage in host seeking – the two species appear to rely on different mechanisms for appropriate host selection.
Insight into the cellular and molecular mechanisms underlying chemosensation in parasitic nematodes has come largely from studies of the free-living model nematode C. elegans. Despite profound differences in body size and lifestyle among nematodes, sensory neural anatomy and function are highly conserved across species [33]. Thus, many of the neural circuits and signaling pathways that operate in C. elegans are likely to operate similarly in parasitic nematodes. C. elegans shows directional chemotaxis in response to volatile chemicals such as alcohols, esters, and aldehydes; soluble chemicals such as salts and pheromones; and gases such as oxygen (O2) and CO2 [34]. Olfactory and gustatory responses are mediated primarily by neurons located in the main chemosensory organs of C. elegans, the paired amphid sensilla [34], while responses to O2 and CO2 are mediated by a distributed network of head and tail neurons [35–39]. Chemoreceptor genes in C. elegans are encoded by large families of G protein coupled receptors (GPCRs) and guanylate cyclases (GCs) [34]. Specific receptors have been identified for a few chemicals, including O2 [35, 40], ascarosides [41,42], and the odorant 2,3-butanedione [43]. The CO2 receptor has not yet been identified, although the receptor guanylate cyclase GCY-9 is required for CO2 response and may be a receptor for CO2 or a CO2 metabolite [39, 44].
In parasitic nematodes, chemosensory neuron function has been investigated in only a few cases. As in C. elegans, the amphidial ASE and ASH sensory neurons of S. stercoralis mediate chemoattraction and chemorepulsion to soluble chemicals, respectively [21, 45]. In addition, the BAG sensory neurons mediate CO2 repulsion by C. elegans adults and CO2 attraction by C. elegans dauers and EPN IJs, demonstrating that the neural basis of CO2 response is at least partly conserved across species regardless of whether CO2 is a repulsive or attractive cue [5, 37]. The molecular basis of chemoreception in parasitic nematodes has not yet been investigated. However, recent breakthroughs in the development of techniques for stable genetic transformation of S. stercoralis and S. ratti [46, 47] should enable new avenues of research into the molecular mechanisms that underlie chemosensation in these parasites.
Parasitic trematodes
Trematodes comprise a class of parasitic flatworms in the phylum Platyhelminthes. Trematodes in the genus Schistosoma are skin-penetrating parasites of mammals, commonly known as blood flukes [48]. Over 200 million people are estimated to be infected with human parasitic schistosomes, which cause the disease schistosomiasis [1]. Schistosomes have complex life cycles involving two host species, one of which is a mollusk and the other a vertebrate (Figure 2) [49]. During one phase of the life cycle, infective larvae called miracidia infect a molluscan host; during a different phase of the life cycle, infective larvae called cercariae infect a vertebrate host [49]. In contrast to human parasitic nematodes, which typically have very specific host ranges, some human parasitic schistosomes have broad host ranges. For example, Schistosoma mansoni infects humans, non-human primates, and rodents, and S. japonicum infects humans, cattle, dogs, pigs, rodents, and a number of other mammals [48].
Figure 2. Representative schistosome life cycle.
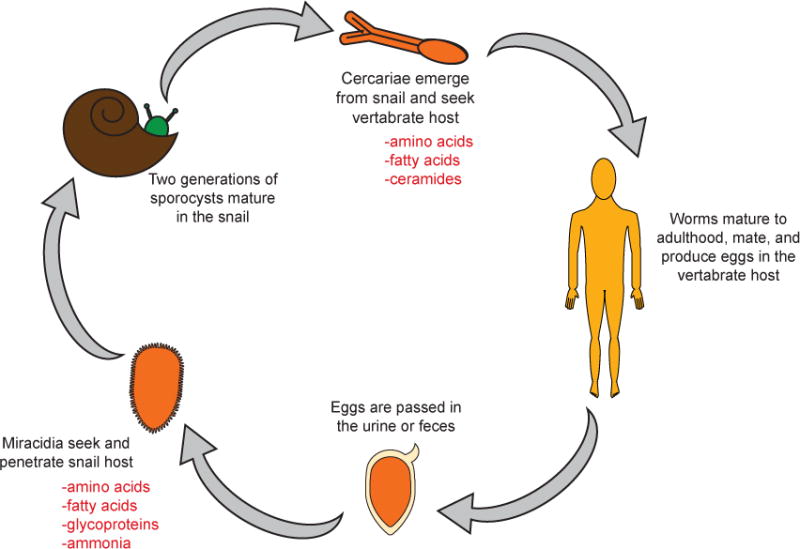
Eggs are passed in the urine or feces of the vertebrate host [49]. In the environment, the eggs hatch and release miracidia. Miracidia seek out snail hosts using temperature, gravity, light, and chemosensory cues; they then penetrate the snail. After multiple rounds of asexual reproduction in the snail, cercariae emerge from the snail and disperse. Cercariae orient themselves in the water column using temperature, gravity, and light [49]. When vertebrate hosts enter the water near cercariae, host skin compounds such as fatty acids and amino acids as well as warmth attract cercariae to the host and stimulate sustained attachment to and penetration of the host’s skin [49, 51, 53]. Within the host, cercariae use chemical gradients to guide migration to the intestines or bladder, pair, reproduce sexually, and lay eggs [54]. Some of the host-derived chemical cues for miracidia and cercariae are shown in red [49, 51–53].
Both molluscan-parasitic miracidia and vertebrate-parasitic cercariae demonstrate hostseeking behaviors in response to chemosensory cues (Figure 2). Within 1–3 hours of hatching, miracidia of many species exhibit long-range dispersal behaviors in which the larvae use phototactic and geotactic cues to migrate to snail habitats; during this time, miracidia appear to be insensitive to host chemosensory cues [49]. After 1–3 hours, miracidia exhibit short-range host-seeking behaviors and are capable of responding to snail-secreted chemicals [49]. Response to snail-derived odor clouds increases miracidial transmission success, confirming an important role for chemosensation in the host infection process [50]. The snail-derived cues that attract miracidia are not fully understood but are thought to include amino acids, fatty acids, ammonia, and a number of different glycoproteins [49].
Cercariae also rely on chemosensory cues for host finding and host invasion. For example, many schistosome cercariae are attracted to skin-derived chemicals such as fatty acids and amino acids; many of the same cues stimulate skin attachment and penetration behaviors [51, 52]. S. mansoni cercariae respond to ceramides, a class of lipids found on the outermost layer of human skin, with prolonged skin attachment [53]. Following infection, S. mansoni cercariae use chemosensory cues to migrate within host tissue [54]. In addition to schistosomes, other trematodes also engage in host-seeking behaviors, although the responses of these species to host chemosensory cues are less well understood [52].
Chemosensory behaviors of ectoparasites
Many ectoparasites rely on chemosensory cues to locate hosts and mates, select oviposition sites, and find food sources [55]. This includes ectoparasitic insects such as mosquitoes, bed bugs, kissing bugs, and tsetse flies, as well as non-insect invertebrates such as ticks. Ectoparasites that target humans transmit diseases such as malaria, dengue virus, West Nile virus, yellow fever, filariasis, trypanosomiasis, and leishmaniasis [55]. A better understanding of the chemosensory cues that attract and repel these parasites is therefore of great interest for the development of specific and effective traps and repellents.
Mosquitoes
Many mosquitoes track hosts over long distances – in some cases, as far as 70 meters – using host-emitted chemosensory cues [55, 56]. The anthropophilic species Anopheles gambiae, which transmits malaria, and Aedesaegypti, which transmits dengue and yellow fever, target human hosts using CO2 in combination with human skin and sweat odorants such as ammonia, lactic acid, and other carboxylic acids [55, 56]. The behavioral responses to human skin and sweat odorants are highly concentration- and mixture- dependent: some odorants are attractive only in combination with other odorants and at specific concentrations [57]. Odorants emitted by skin microbiota also attract An. gambiae [58] and enhance the effectiveness of traps containing human-derived odorants in some cases [59, 60]. In contrast to An. gambiae and Ae. aegypti, which specifically target humans, other species have much broader host ranges. For example, Culex quinquefasciatus blood-feeds from a broad range of mammalian and avian hosts and locates hosts using CO2 in combination with host odorants such as nonanal, which is secreted by both birds and mammals [61]. CO2 is critical for the long-range host-seeking behaviors of at least some mosquito species. For example, a brief encounter with CO2 but not skin odors induces a rapid upwind flight surge in Ae. aegypti [62], and exposure of Ae. aegypti and Cx. quinquefasciatus to odorants that block CO2-sensing neurons impairs their ability to locate hosts [63].
After a blood meal, female ectoparasitic mosquitoes seek out suitable aquatic environments in which to lay eggs. The host-seeking behavior of female mosquitoes is inhibited following a blood meal and a distinct but overlapping set of olfactory cues is used to locate oviposition sites [64–66]. In An. gambiae females, responses to indole and phenolic compounds are increased and responses to ammonia are decreased following a blood meal [66, 67]. Gravid Culex mosquitoes are attracted to the oviposition site odorants trimethylamine, nonanal, and 3-methylindole, and a synthetic blend of trimethylamine and nonanal was an effective trap for gravid females in field studies [65]. The changes in olfactory behavior that occur after blood feeding are at least partly a result of changes in the peripheral olfactory organs: the odor sensitivities of some olfactory neurons and the expression levels of some odorant receptors are altered following a blood meal [64, 66–68].
The primary olfactory organs of mosquitoes are the antennae and maxillary palps [55]. The surfaces of these organs are covered with sensory hairs called sensilla, which house the dendrites of the olfactory receptor neurons (ORNs); each sensillum contains the dendrites of one or a small number of ORNs. Electrophysiological recordings from mosquito ORNs have demonstrated that individual ORNs are tuned to overlapping subsets of odorants, and respond strongly to many odorants that are known host-seeking and oviposition cues [55]. Odorant detection in mosquitoes and other insects is mediated by members of the odorant receptor (OR), ionotropic glutamate receptor (IR), and gustatory receptor (GR) families [55, 69, 70]. Insect odorant receptors have been most thoroughly characterized in the model organism, Drosophila melanogaster. In D. melanogaster, each receptor is expressed in a small subset of ORNs, with a few exceptions [55, 71]. Members of both the OR and IR families function as odorant-gated heteromeric cation channels; in most cases, the channel consists of one ligand-specific receptor and one more broadly expressed co-receptor [71–75]. Many of the ORs are broadly tuned to acetate esters, alcohols, and ketones [76]. By contrast, IRs are more narrowly tuned to carboxylic acids and amines [71, 75].
Large-scale functional analyses of the An. gambiae OR family using both in vivo and heterologous expression systems revealed that many AgORs are strongly tuned to host and oviposition attractants such as 4-methylphenol, indole, and 1-octen-3-ol [77–79] (Figure 3a). To functionally compare the odorant receptor repertoires of An. gambiae and D. melanogaster, multidimensional odor spaces were constructed for each species in which each axis of the space corresponds to the responses conferred by a single OR in spikes/sec. A comparison of An. gambiae and D. melanogaster odor space revealed that esters are more widely distributed in D. melanogaster odor space and aromatics are more widely distributed in An. gambiae odor space (Figure 3b). Thus, the electrophysiological responses evoked by different esters differ more for D. melanogaster than An. gambiae, while the opposite is true for aromatics. These results suggest that mosquitoes may be better than fruit flies at discriminating among aromatics, some of which are emitted by human hosts, while fruit flies may be better at discriminating among esters, some of which are emitted by fruits [77].
Figure 3. Odor coding by the An. gambiae AgOR repertoire.
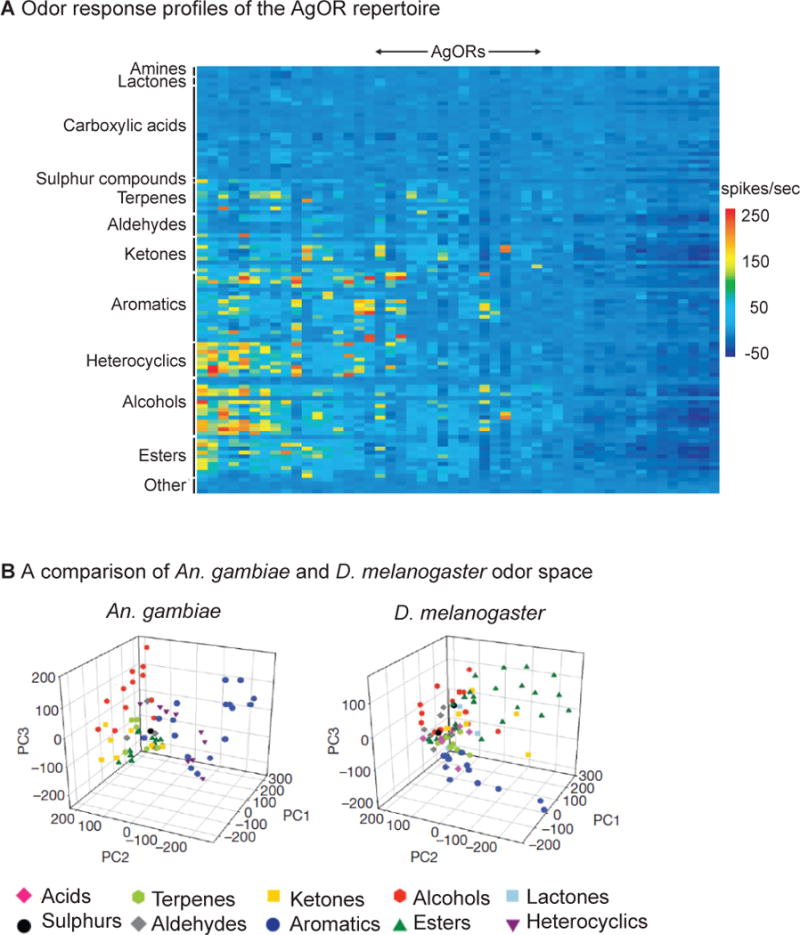
(a) Responses of AgORs to 110 odorants using the D. melanogaster ‘empty neuron’ system [76] for receptor decoding. The ‘empty neuron’ consists of an antennal olfactory neuron that lacks its endogenous odorant receptors. Odorant receptors of interest can be ectopically expressed in the ‘empty neuron’, and their odor responses can then be determined by single-unit electrophysiology [76]. AgOR response intensities are color-coded according to the scale on the right. (b) A comparison of An. gambiae and D. melanogaster odor space. For each species, the odor space is an n-dimensional space in which each axis represents the responses of one receptor in spikes/s, and n represents the total number of receptors tested. Each tested odorant was mapped to a particular position in this space based on the electrophysiological responses it elicited from each receptor in the ‘empty neuron’ system. To generate the graphs, principal component analysis was used to represent the n-dimensional odor spaces in three dimensions; the axes of the graphs correspond to the first three principle components. Left, An. gambiae odor space. Right, D. melanogaster odor space. Aromatics are more widely distributed in An. gambiae odor space, and esters are more widely distributed in D. melanogaster odor space. This suggests that mosquitoes may be better than fruit flies at discriminating among aromatics, some of which are emitted by human hosts, while fruit flies may be better at discriminating among esters, some of which are emitted by fruits. Reprinted from [77] with permission.
A number of ORs from other mosquito species that respond to host and oviposition attractants have also been characterized [55, 80, 81]. The mosquito IRs are less well characterized, although the An. gambiae IR AgIR76b was found to mediate behavioral responses to butylamine in mosquito larvae [82]. Given that many of the D. melanogaster IRs are tuned to odorants known to be important for mosquito host seeking such as carboxylic acids and amines [71, 75], a functional analysis of the mosquito IR repertoire will be of great interest.
CO2 reception in many insects is mediated by conserved members of the GR family: Gr21a and Gr63a function as CO2 receptors in D. melanogaster, and three orthologs of these receptors function as CO2 receptors in mosquitoes and a number of other insects [83–86]. In D. melanogaster, the CO2 receptors are expressed in a specific class of antennal neurons; in mosquitoes, the CO2 receptors are expressed in maxillary palp neurons. The recent finding that certain odorants can interfere with CO2 receptor activation and disorient mosquitoes [63, 87] suggests that CO2 receptors may be powerful targets for mosquito control strategies. Accessory olfactory proteins such as odorant binding proteins (OBPs) also contribute to olfactory responses [55] and are likely to be useful targets for insect control. Interestingly, dengue virus was recently shown to increase the expression of some OBPs as well as a number of other olfactory proteins, raising the possibility that the virus might enhance mosquito blood-feeding or hostseeking behaviors [88].
Other ectoparasites
Other ectoparasitic insects and non-insect invertebrates locate hosts using many of the same volatile cues as mosquitoes. For example, human-parasitic ticks, bed bugs, kissing bugs, and tsetse flies are attracted to host odorants such as CO2, 1-octen-3-ol, indole, lactic acid, and 4-methylphenol [89–98]. For some ectoparasites, such as the fish louse Argulus coregoni, the role of olfaction in host-seeking behavior is developmental stage specific: young A. coregoni rely primarily on visual cues for host location, while adult A. coregoni rely on a combination of visual and olfactory cues [99]. Other species, such as the hedgehog parasitic tick Ixodes hexagonus, rely on subtle differences in host odor profiles for host selection. I. hexagonus is more attracted to the odor of sick hedgehogs than healthy hedgehogs and is more likely to parasitize sick hedgehogs [100]. The molecular basis of olfaction in many of these ectoparasites remains largely unexplored and will be an interesting avenue for future research.
Concluding remarks and future directions
A consistent theme across species that engage in host seeking is the reliance on a combination of general and specific host sensory cues. General cues typically include CO2 as well as nonchemosensory stimuli such as heat, while specific host cues are often a unique blend of host-derived odorants. The requirement for general versus specific cues varies greatly for different parasites, different parasite-host combinations, and different host-seeking behaviors. For example, the universal cue CO2 is sufficient to elicit hostseeking behaviors from EPNs [5, 6]. However, removing CO2 from the host odor blend abolishes attraction to some hosts but not others, indicating that the requirement for general versus specific host cues is host-dependent [5, 6]. Parasites also differ in whether they rely on CO2 for long-range versus short-range host-seeking behaviors. For example, in the case of Cx. quinquefasciatus, CO2 is more important for long-range host-seeking behavior, while host-specific odorants are more important for short-range landing behavior [101]. Similarly, Steinernema carpocapsae requires CO2 for long-range chemotaxis but not short-range jumping to waxworm odor [5]. By contrast, CO2 is a short-range activation cue for human hookworms in combination with warmth and/or moisture [25]. Thus, although general host cues such as CO2 are used by many parasites, the ways in which they are used for host seeking are often species-specific.
An important direction for future research will be to further investigate the extent to which chemosensory behavior contributes to host selection and the evolution of host specificity. Appropriate host selection is critical for endoparasites: many species have narrow host ranges, and infection of a non-host or dead-end host is often fatal. For a few parasites, attraction to host-derived chemicals has been shown to correlate with host suitability. For example, Steinernema carpocapsae is more attracted to hosts that support higher levels of mortality and parasite reproduction [32]. In the case of the human parasitic trematode Schistosoma haematobium, the miracidia are more attracted to hosts than sympatric nonhosts but are unable to discriminate hosts from allopatric non-hosts, suggesting that their chemosensory preferences have evolved to facilitate host selection [102]. However, for both species, long-range chemosensory behavior is not sufficient to ensure appropriate host selection: Steinernema carpocapsae is also attracted to non-host isopods [6], and the preference of Schistosoma haematobium miracidia for hosts over sympatric nonhosts is not absolute [102]. This suggests either that these parasites sometimes attempt to infect poor hosts, that host selection is further refined by host recognition and penetration behaviors, or both. Further work will be necessary to distinguish between these possibilities, and to investigate the relationship between host attraction and host suitability in other parasites.
Recent progress in understanding the behavioral responses to host odor cues and the mechanisms that underlie these responses has already led to the design of more specific and effective parasite control strategies. In the case of An. gambiae, the discovery that human skin microbiota play an important role in host seeking [58] led to the finding that the addition of some skin microbiota-derived odorants enhance the effectiveness of existing traps [59, 103], and the large-scale decoding of the AgOR repertoire [77] led to the finding that some of the odorants that strongly activate AgORs can increase trap effectiveness [104]. In addition, a better understanding of insect odorant receptor function led to the finding that the broad-spectrum insect repellent DEET directly modulates the odorant receptor complex [105], paving the way for future studies aimed at designing alternative insect repellents. The odor-driven behaviors of human endoparasites are much less well understood, but the possibility of developing chemically-baited traps or repellents for these parasites based on knowledge of the function and organization of their chemosensory systems is an exciting avenue for future research. Finally, a comparison of odorants that are attractants for different parasites reveals that many of the chemosensory cues that elicit host-seeking behaviors are conserved across phyla (Figure 4), raising the possibility that traps could be designed that target multiple phylogenetically diverse species of parasites. Multicellular parasites are a major cause of disease worldwide, and a better understanding of how they respond to host chemical cues at the molecular and cellular level should greatly facilitate the development of more effective control strategies.
Figure 4. Some of the common olfactory cues used by multicellular parasites for host finding.
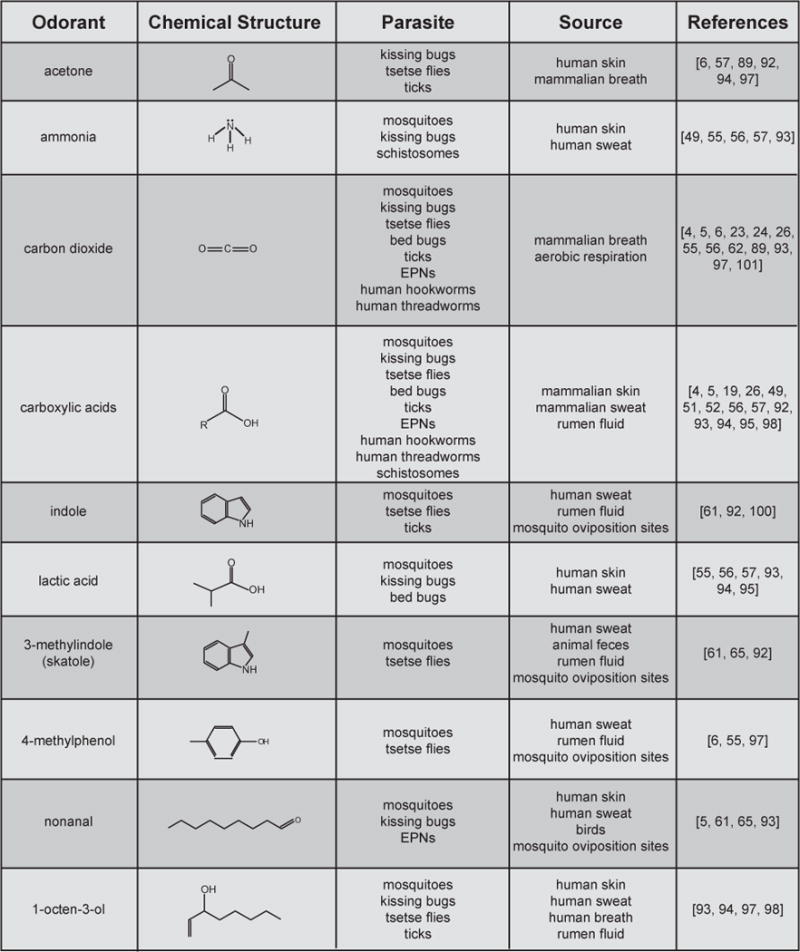
Only odorants used by more than one family are shown. Many of the odorants listed are common in nature, so sources listed are representative but not exhaustive. Any chemical compound (R) containing a carboxyl group, as shown, is considered a carboxylic acid.
Acknowledgments
We thank Michelle Castelletto for valuable comments on the manuscript.
References
- 1.Lustigman S, et al. A research agenda for helminth diseases of humans: The problem of helminthiases. PLoS Negl Trop Dis. 2012;6:e1582. doi: 10.1371/journal.pntd.0001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasser JN, Freckman DW. A world perspective on nematology: the role of the society. In: Veech JA, Dickerson DW, editors. Vistas on Nematology. Society of Nematologists; Hyatsville, MD: 1987. pp. 7–14. [Google Scholar]
- 3.Nguyen KB, et al. Steinernematidae: species descriptions. In: Nguyen KB, Hunt DJ, editors. Entomopathogenic nematodes: Systematics, phylogeny, and bacterial symbionts. Brill; Leiden: 2007. pp. 121–609. (Nematology monographs and perspectives). [Google Scholar]
- 4.Rasmann S, et al. Ecology and evolution of soil nematode chemotaxis. J Chem Ecol. 2012 doi: 10.1007/s10886-012-0118-6. [DOI] [PubMed] [Google Scholar]
- 5.Hallem EA, et al. A sensory code for host seeking in parasitic nematodes. Curr Biol. 2011;21:377–383. doi: 10.1016/j.cub.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dillman AR, et al. Olfaction shapes host-parasite interactions in parasitic nematodes. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1211436109. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell JF, Kaya HK. How and why a parasitic nematode jumps. Nature. 1999;397:485–486. [Google Scholar]
- 8.Rasmann S, et al. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature. 2005;434:732–737. doi: 10.1038/nature03451. [DOI] [PubMed] [Google Scholar]
- 9.Ali JG, et al. Subterranean herbivore-induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. J Chem Ecol. 2010;36:361–368. doi: 10.1007/s10886-010-9773-7. [DOI] [PubMed] [Google Scholar]
- 10.Kollner TG, et al. A maize (e)-beta-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell. 2008;20:482–494. doi: 10.1105/tpc.107.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali JG, et al. Constitutive and induced subterranean plant volatiles attract both entomopathogenic and plant parasitic nematodes. J Ecol. 2011;99:26–35. [Google Scholar]
- 12.Choe A, et al. Ascaroside signaling is widely conserved among nematodes. Curr Biol. 2012;22:772–780. doi: 10.1016/j.cub.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivasan J, et al. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasan J, et al. A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 2012;10:e1001237. doi: 10.1371/journal.pbio.1001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noguez JH, et al. A novel ascaroside controls the parasitic life cycle of the entomopathogenic nematode Heterorhabditis bacteriophora. ACS Chem Biol. 2012 doi: 10.1021/cb300056q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edison AS. Caenorhabditis elegans pheromones regulate multiple complex behaviors. Current opinion in neurobiology. 2009;19:378–388. doi: 10.1016/j.conb.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viney ME, Lok JB. Strongyloides spp. WormBook; 2007. www.WormBook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viney ME. The biology and genomics of Strongyloides. Med Microbiol Immunol. 2006;195:49–54. doi: 10.1007/s00430-006-0013-2. [DOI] [PubMed] [Google Scholar]
- 19.Safer D, et al. Urocanic acid is a major chemoattractant for the skin-penetrating parasitic nematode Strongyloides stercoralis. Proc Natl Acad Sci USA. 2007;104:1627–1630. doi: 10.1073/pnas.0610193104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koga M, Tada I. Strongyloides ratti: Chemotactic responses of third- stage larvae to selected serum proteins and albumins. J Helminthol. 2000;74:247–252. [PubMed] [Google Scholar]
- 21.Forbes WM, et al. Chemoattraction and chemorepulsion of Strongyloides stercoralis infective larvae on a sodium chloride gradient is mediated by amphidial neuron pairs ASE and ASH, respectively. Vet Parasitol. 2004;120:189–198. doi: 10.1016/j.vetpar.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Tobata-Kudo H, et al. Chemokinetic behavior of the infective third-stage larvae of Strongyloides ratti on a sodium chloride gradient. Parasitol Int. 2000;49:183–188. doi: 10.1016/s1383-5769(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 23.Granzer M, Hass W. Host-finding and host recognition of infective Ancylostoma caninum larvae. Int J Parasitol. 1991;21:429–440. doi: 10.1016/0020-7519(91)90100-l. [DOI] [PubMed] [Google Scholar]
- 24.Sciacca J, et al. Response to carbon dioxide by the infective larvae of three species of parasitic nematodes. Parasitol Int. 2002;51:53–62. doi: 10.1016/s1383-5769(01)00105-2. [DOI] [PubMed] [Google Scholar]
- 25.Haas W, et al. Infective larvae of the human hookworms Necator americanus and Ancylostoma duodenale differ in their orientation behaviour when crawling on surfaces. Parasitol Res. 2005;95:25–29. doi: 10.1007/s00436-004-1256-8. [DOI] [PubMed] [Google Scholar]
- 26.Haas W, et al. Behavioural strategies used by the hookworms Necator americanus and Ancylostoma duodenale to find, recognize and invade the human host. Parasitol Res. 2005;95:30–39. doi: 10.1007/s00436-004-1257-7. [DOI] [PubMed] [Google Scholar]
- 27.Lewis EE. Behavioral ecology. In: Gauger R, editor. Entomopathogenicnematology. CAB International; New York: 2002. pp. 205–223. [Google Scholar]
- 28.Reynolds AM, et al. Chemotaxis can take plant-parasitic nematodes to the source of a chemo-attractant via the shortest possible routes. J R Soc Interface. 2011;8:568–577. doi: 10.1098/rsif.2010.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rae RG, et al. The chemotactic response of Phasmarhabditis hermaphrodita (Nematoda: Rhabditida) to cues of Deroceras reticulatum. Nematol. 2006;8:197–200. [Google Scholar]
- 30.Rae RG, et al. Chemoattraction and host preference of the gastropod parasitic nematode Phasmarhabditis hermaphrodita. J Parasitol. 2009;95:517–526. doi: 10.1645/GE-1637.1. [DOI] [PubMed] [Google Scholar]
- 31.Hapca S, et al. Movement of the parasitic nematode Phasmarhabditis hermaphrodita in the presence of mucus from the host slug deroceras reticulatum. Biological Control. 2007;41:223–229. [Google Scholar]
- 32.Lewis EE, et al. Host recognition behavior predicts host suitability in the entomopathogenic nematode Steinernema carpocapsae (Rhabditida: Steinernema) Parasitol. 1996;113:573–579. doi: 10.1017/s0031182000067627. [DOI] [PubMed] [Google Scholar]
- 33.Ashton FT, et al. Chemo-and thermosensory neurons: structure and function in animal parasitic nematodes. Vet Parasitol. 1999;84:297–316. doi: 10.1016/s0304-4017(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 34.Hart AC, Chao MY. From odors to behaviors. Caenorhabditis elegans. 2010 [PubMed] [Google Scholar]
- 35.Gray JM, et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 36.Chang AJ, et al. A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS Biol. 2006;4:e274. doi: 10.1371/journal.pbio.0040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hallem EA, Sternberg PW. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bretscher AJ, et al. Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron. 2011;69:1099–1113. doi: 10.1016/j.neuron.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallem EA, et al. Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc Natl Acad Sci USA. 2011;108:254–259. doi: 10.1073/pnas.1017354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmer M, et al. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61:865–879. doi: 10.1016/j.neuron.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGrath PT, et al. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature. 2011;477:321–325. doi: 10.1038/nature10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim K, et al. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science. 2009;326:994–998. doi: 10.1126/science.1176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sengupta P, et al. ODR-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell. 1996;84:899–909. doi: 10.1016/s0092-8674(00)81068-5. [DOI] [PubMed] [Google Scholar]
- 44.Brandt JP, et al. A single gene target of an ets-family transcription factor determines neuronal CO2-chemosensitivity. PLoS ONE. 2012;7:e34014. doi: 10.1371/journal.pone.0034014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ketschek AR, et al. Amphidial neurons ADL and ASH initiate sodium dodecyl sulphate avoidance responses in the infective larva of the dog hookworm Anclyostoma caninum. Int J Parasitol. 2004;34:1333–1336. doi: 10.1016/j.ijpara.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Li X, et al. Transgenesis in the parasitic nematode Strongyloides ratti. Mol Biochem Parasitol. 2011;179:114–119. doi: 10.1016/j.molbiopara.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lok JB. Nucleic acid transfection and transgenesis in parasitic nematodes. Parasitology. 2011:1–15. doi: 10.1017/S0031182011001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han ZG, et al. Schistosoma genomics: new perspectives on schistosome biology and host-parasite interaction. Annu Rev Genomics Hum Genet. 2009;10:211–240. doi: 10.1146/annurev-genom-082908-150036. [DOI] [PubMed] [Google Scholar]
- 49.Sukhdeo SC, Sukhdeo MVK. Trematode behaviours and the perceptual worlds of parasites. Can J Zool. 2004:292–315. [Google Scholar]
- 50.Hertel J, et al. Snail odour-clouds: spreading and contribution to the transmission success of Trichobilharzia ocellata (Trematoda, Digenea) miracidia. Oecologia. 2006;147:173–180. doi: 10.1007/s00442-005-0239-5. [DOI] [PubMed] [Google Scholar]
- 51.Haeberlein S, Haas W. Chemical attractants of human skin for swimming Schistosoma mansoni cercariae. Parasitol Res. 2008;102:657–662. doi: 10.1007/s00436-007-0807-1. [DOI] [PubMed] [Google Scholar]
- 52.Haas W. Parasitic worms: strategies of host finding, recognition and invasion. Zoology. 2003;106:349–364. doi: 10.1078/0944-2006-00125. [DOI] [PubMed] [Google Scholar]
- 53.Haas W, et al. Schistosoma mansoni: human skin ceramides are a chemical cue for host recognition of cercariae. Exp Parasitol. 2008;120:94–97. doi: 10.1016/j.exppara.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Grabe K, Haas W. Navigation within host tissues: Schistosoma mansoni and Trichobilharzia ocellata schistosomula respond to chemical gradients. Int J Parasitol. 2004;34:927–934. doi: 10.1016/j.ijpara.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 55.Carey AF, Carlson JR. Insect olfaction from model systems to disease control. Proc Natl Acad Sci U S A. 2011;108:12987–12995. doi: 10.1073/pnas.1103472108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smallegange RC, et al. Sweaty skin: an invitation to bite? Trends in parasitology. 2011;27:143–148. doi: 10.1016/j.pt.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Qiu YT, et al. Behavioural responses of Anopheles gambiae sensu stricto to components of human breath, sweat and urine depend on mixture composition and concentration. Medical and veterinary entomology. 2011;25:247–255. doi: 10.1111/j.1365-2915.2010.00924.x. [DOI] [PubMed] [Google Scholar]
- 58.Verhulst NO, et al. Cultured skin microbiota attracts malaria mosquitoes. Malaria J. 2009;8:302. doi: 10.1186/1475-2875-8-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verhulst NO, et al. Improvement of a synthetic lure for Anopheles gambiae using compounds produced by human skin microbiota. Malar J. 2011;10:28. doi: 10.1186/1475-2875-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verhulst NO, et al. Human skin microbiota and their volatiles as odour baits for the malaria mosquito Anopheles gambiae s.s. Entomologia Experimental et Applicata. 2011;139:170–179. [Google Scholar]
- 61.Syed Z, Leal WS. Acute olfactory response of Culex mosquitoes to a human-and bird-derived attractant. Proc Natl Acad Sci U S A. 2009;106:18803–18808. doi: 10.1073/pnas.0906932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dekker T, Carde RT. Moment-to-moment flight manoeuvres of the female yellow fever mosquito (Aedes aegypti L.) in response to plumes of carbon dioxide and human skin odour. J Exp Biol. 2011;214:3480–3494. doi: 10.1242/jeb.055186. [DOI] [PubMed] [Google Scholar]
- 63.Turner SL, et al. Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature. 2011;474:87–91. doi: 10.1038/nature10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takken W, et al. Inhibition of host-seeking response and olfactory responsiveness in Anopheles gambiae following blood feeding. J Insect Physiol. 2001;47:303–310. doi: 10.1016/s0022-1910(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 65.Leal WS, et al. Reverse and conventional chemical ecology approaches for the development of oviposition attractants for Culex mosquitoes. PLoS ONE. 2008;3:e3045. doi: 10.1371/journal.pone.0003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siju KP, et al. Influence of blood meal on the responsiveness of olfactory receptor neurons in antennal sensilla trichodea of the yellow fever mosquito, Aedes aegypti. J Insect Physiol. 2010;56:659–665. doi: 10.1016/j.jinsphys.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Qiu YT, et al. Olfactory coding in antennal neurons of the malaria mosquito, Anopheles gambiae. Chemical senses. 2006;31:845–863. doi: 10.1093/chemse/bjl027. [DOI] [PubMed] [Google Scholar]
- 68.Fox AN, et al. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proc Natl Acad Sci USA. 2001;98:14693–14697. doi: 10.1073/pnas.261432998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benton R, et al. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Croset V, et al. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genetics. 2010;6:e1001064. doi: 10.1371/journal.pgen.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abuin L, et al. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69:44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benton R, et al. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sato K, et al. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 74.Wicher D, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–U1010. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 75.Silbering AF, et al. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J Neurosci. 2011;31:13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 77.Carey AF, et al. Odorant reception in the malaria mosquito Anopheles gambiae. Nature. 2010;464:66–71. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hallem EA, et al. Olfaction: mosquito receptor for human-sweat odorant. Nature. 2004;427:212–213. doi: 10.1038/427212a. [DOI] [PubMed] [Google Scholar]
- 79.Wang G, et al. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci U S A. 2010;107:4418–4423. doi: 10.1073/pnas.0913392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scialo F, et al. Molecular and functional characterization of the odorant receptor2 (OR2) in the tiger mosquito Aedes albopictus. PLoS ONE. 2012;7:e36538. doi: 10.1371/journal.pone.0036538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grant AJ, Dickens JC. Functional characterization of the octenol receptor neuron on the maxillary palps of the yellow fever mosquito, Aedes aegypti. PLoS ONE. 2011;6:e21785. doi: 10.1371/journal.pone.0021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu C, et al. Distinct olfactory signaling mechanisms in the malaria vector mosquito. Anopheles gambiae PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu T, et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol. 2007;17:1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jones WD, et al. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 85.Kwon JY, et al. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robertson HM, Kent LB. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J Insect Sci. 2009;9:19. doi: 10.1673/031.009.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- 88.Sim S, et al. Dengue virus infection of the Aedes aegypti salivary gland and chemosensory apparatus induces genes that modulate infection and bloodfeeding behavior. PLoS Pathog. 2012;8:e1002631. doi: 10.1371/journal.ppat.1002631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McMahon C, Guerin PM. Attraction of the tropical bont tick, Amblyomma variegatum, to human breath and to the breath components acetone, NO and CO2. Die Naturwissenschaften. 2002;89:311–315. doi: 10.1007/s00114-002-0317-z. [DOI] [PubMed] [Google Scholar]
- 90.Harraca V, et al. Characterization of the antennal olfactory system of the bed bug (Cimex lectularius) Chemical senses. 2010;35:195–204. doi: 10.1093/chemse/bjp096. [DOI] [PubMed] [Google Scholar]
- 91.Harraca V, et al. Smelling your way to food: Can bed bugs use our odour? J Exp Biol. 2012;215:623–629. doi: 10.1242/jeb.065748. [DOI] [PubMed] [Google Scholar]
- 92.Harraca V, et al. Olfactory and behavioural responses of tsetse flies, Glossina spp., to rumen metabolites. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195:815–824. doi: 10.1007/s00359-009-0459-y. [DOI] [PubMed] [Google Scholar]
- 93.Guerenstein PG, Lazzari CR. Host-seeking: how triatomines acquire and make use of information to find blood. Acta Trop. 2009;110:148–158. doi: 10.1016/j.actatropica.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 94.Ortiz MI, Molina J. Preliminary evidence of Rhodnius prolixus (Hemiptera: Triatominae) attraction to human skin odour extracts. Acta Trop. 2010;113:174–179. doi: 10.1016/j.actatropica.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 95.Anderson JF, et al. A carbon dioxide, heat and chemical lure trap for the bedbug, Cimex lectularius. Med Vet Entomol. 2009;23:99–105. doi: 10.1111/j.1365-2915.2008.00790.x. [DOI] [PubMed] [Google Scholar]
- 96.Wang C, et al. Bed bug (Heteroptera: Cimicidae) attraction to pitfall traps baited with carbon dioxide, heat, and chemical lure. J Econ Entomol. 2009;102:1580–1585. doi: 10.1603/029.102.0423. [DOI] [PubMed] [Google Scholar]
- 97.Gibson G, Torr SJ. Visual and olfactory responses of haematophagous Diptera to host stimuli. Med Vet Entomol. 1999;13:2–23. doi: 10.1046/j.1365-2915.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- 98.Osterkamp J, et al. Host-odor recognition in two tick species is coded in a blend of vertebrate volatiles. J Comp Physiol A. 1999;185:59–67. doi: 10.1007/s003590050366. [DOI] [PubMed] [Google Scholar]
- 99.Mikheev VN, et al. Tuning host specifcity during the ontogeny of a fish ectoparasite: behavioural responses to host-induced cues. Parasitol Res. 2004;92:220–224. doi: 10.1007/s00436-003-1044-x. [DOI] [PubMed] [Google Scholar]
- 100.Bunnell T, et al. The fecal odor of sick hedgehogs (Erinaceus europaeus) mediates olfactory attraction of the tick Ixodes hexagonus. J Chem Ecol. 2011;37:340–347. doi: 10.1007/s10886-011-9936-1. [DOI] [PubMed] [Google Scholar]
- 101.Lacey ES, Carde RT. Activation, orientation and landing of female Culex quinquefasciatus in response to carbon dioxide and odour from human feet: 3-D flight analysis in a wind tunnel. Med Vet Entomol. 2011;25:94–103. doi: 10.1111/j.1365-2915.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- 102.Allan F, et al. Host choice and penetration by Schistosoma haematobium miracidia. J Helminth. 2009;83:33–38. doi: 10.1017/S0022149X08073628. [DOI] [PubMed] [Google Scholar]
- 103.Mukabana WR, et al. A novel synthetic odorant blend for trapping of malaria and other African mosquito species. J Chem Ecol. 2012;38:235–244. doi: 10.1007/s10886-012-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smallegange RC, et al. Identification of candidate volatiles that affect the behavioural response of the malaria mosquito Anopheles gambiae sensu stricto to an active kairomone blend: laboratory and semi-field assays. Physiol Entomol. 2012;37:60–71. [Google Scholar]
- 105.Pellegrino M, et al. A natural polymorphism alters odour and deet sensitivity in an insect odorant receptor. Nature. 2011;478:511–514. doi: 10.1038/nature10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dowds BCA, Peters A. Virulence mechanisms. In: Gaugler R, editor. Entomopathogenic nematology. CAB International; New York: 2002. pp. 79–98. [Google Scholar]


