Abstract
Endosomal maturation and transport constitutes a complex trafficking system present in all cell types. Neurons have adapted their endosomal system to meet their unique and complex needs. These adaptations include repurposing existing proteins to diversify endocytosis and trafficking, as well as preferential expression of certain regulators more highly in neurons than other cell types. These neuronal regulators include the family of Neuron-Specific Gene family members (Nsg), NEEP21 (Nsg1), and P19 (Nsg2). NEEP21/Nsg1 plays a role in the trafficking of multiple receptors, including the cell adhesion molecule L1/NgCAM, the neurotransmitter receptor GluA2, and β-APP. Recently, we showed that NEEP2/Nsg1 and P19/Nsg2 are not expressed in all neuronal cell types in vitro. However, it is not known where and when NEEP21/Nsg1 and P19/Nsg2 are expressed in vivo, and whether both proteins are always coexpressed. Here, we show that NEEP21/Nsg1 and P19/Nsg2 are present in both overlapping and distinct cell populations in the hippocampus, neocortex, and cerebellum during development. NEEP21/Nsg1 and P19/Nsg2 levels are highest during embryonic development, and expression persists in the juvenile mouse brain. In particular, a subset of layer V cortical neurons retains relatively high expression of both NEEP21/Nsg1 and P19/Nsg2 at postnatal day 16 as well as in the CA1-3 regions of the hippocampus. In the cerebellum, NEEP21/Nsg1 expression becomes largely restricted to Purkinje neurons in adulthood whereas P19/Nsg2 expression strikingly disappears from the cerebellum with age. This divergent and restricted expression likely reflects differential needs for this class of trafficking regulators in different neurons during different stages of maturation.
Keywords: endosome, neurodevelopment, neural-specific gene, Purkinje neuron, RRID: AB_2571866, RRID: AB_10896795, RRID: AB_882455, RRID: AB_2286684, RRID: AB_11205592, RRID: AB_2314065, RRID: AB_2138173, RRID: AB_477329, RRID: AB_2536181, RRID: AB_2535788, RRID: AB_2340686, RRID: AB_2534017, RRID: AB_2340462, RRID: AB_2534102, RRID: RGD_737891, RRID: SCR_007318
1 INTRODUCTION
Neurons are among the most morphologically complex cells in the body. This complexity manifests on two fronts. First, neurons are extremely large in size and extend axons and dendrites over long distances. A neuron’s soma is roughly the size of an epithelial cell, and neuronal axons can extend up to 1 m in length in humans. Second, neurons have a highly polarized morphology with distinct functional domains, axons and dendrites, which are molecularly distinct. Many proteins are found in one domain but not the other (Barnes & Polleux, 2009). This complexity requires both that proteins be transported over long distances during development and throughout life, and also that proteins need to be accurately sorted to the correct location in this very large cell (Winckler, 2016). These special requirements for protein transport have resulted in many neuronal adaptations in terms of cyto-skeleton and membrane transport in order to meet a neuron’s specific needs (Yap & Winckler, 2012). Lastly, neurons are postmitotic and among the longest-lived cells in the body. This long lifetime means that any problems due to mistrafficking or dysregulation of recycling and degradation have particularly devastating effects.
Many proteins are highly enriched or even specifically expressed in neurons. These include proteins that fundamentally underlie neuronal synaptic function, such as neurotransmitter receptors, but also cytoskeletal proteins and proteins regulating membrane transport. One such protein is Neuron Enriched Endosomal Protein of 21kDa (NEEP21/ Nsg1), a small single-pass transmembrane protein that is highly enriched in neurons (Ohnishi, Futamura, Kamino, & Nakamura, 2010; Sabéran-Djoneidi et al., 1998). Interestingly, NEEP21 is restricted to the somatodendritic domain (Steiner et al., 2002; Yap et al., 2008).
NEEP21 has been shown to play a critical role in the trafficking and polarization of a variety of proteins including the axonal cell adhesion molecule L1/NgCAM, β-APP, GluA2, and neurotensin receptors (Debaigt, Hirling, Steiner, Vincent, & Mazella, 2004; Norstrom, Zhang, Tanzi, & Sisodia, 2010; Steiner et al., 2002, 2005; Yap et al., 2008). When NEEP21 is knocked-down in cultured neurons, L1/NgCAM becomes mislocalized to the somatodendritic region and to LAMP2-positive endosomes (Yap et al., 2008). Missorting of cargo into LAMP2-positive endosomes in the absence of NEEP21 is also observed for neurotensin receptor and for GluA2. This dependence of correct protein trafficking on neuronal proteins specific to certain domains of the neuron highlights the complexity of the endosomal sorting machinery in neurons.
NEEP21 belongs to a family of endosomal proteins including Calcyon (Caly) and P19 (Nsg2) (Muthusamy et al., 2009). NEEP21 and P19 show approximately 50% amino acid sequence homology to each other, and 30% to Calcyon. NEEP21 and P19 were both identified as being highly enriched in the brain and developmentally regulated (Sabéran-Djoneidi et al., 1995, 1998). NEEP21 has been detected in rat brains at high levels up to P14, at which point the protein levels decline greatly (Steiner et al., 2002).
We recently showed that, surprisingly, NEEP21 and P19 were not expressed in all neurons cultured from embryonic rat hippocampus. Virtually, all CTIP2- and Satb2-positive neurons in hippocampal cultures expressed both NEEP21 and P19 robustly. In contrast, both NEEP21 and P19 were expressed at very low levels in Prox1-positive cells, which are likely derived from the dentate gyrus (Digilio et al., 2015). The differential expression found in cultured neurons thus prompted us to ask (a) if NEEP21 and P19 expression levels were similarly low in the dentate gyrus in vivo, (b) at what age NEEP21 and P19 expression disappeared, and (c) if NEEP21 and P19 expression profiles were always identical in vivo. In this study, we examined three brain regions: neocortex, hippocampus, and cerebellum for NEEP21 and P19 expression at several time points during development (E17), at early postnatal ages (P0, P8) and in young adults (P16). We find that NEEP21 and P19 are largely coexpressed with striking exceptions (intermediate progenitors and Purkinje neurons) where neurons selectively express only one of the two distinct endosomal proteins during development. Second, we find that expression of both proteins is broad earlier in development and restricts to small subpopulations where expression is maintained into adolescence and even adulthood.
2 | MATERIALS AND METHODS
2.1 | Animals
All animal use was approved by the University of Virginia IACUC. E17 mouse brains were harvested from a timed pregnant female C57BL/ 6NCrl mice from Charles River. E18 primary rat neurons were harvested from a timed pregnant female Sprague Dawley rat from Charles River (RRID: RGD_737891). All other mice were derived from WT C57BL6 mice after IVF for knockout NEEP21 mice from KOMP (RRID: SCR_007318). The NEEP21 allele is on chromosome 5 from nucleotides 38137193-38159467. The gene was targeted by KOMP and is cleaved from the 5′ UTR to the last exon of the coding region, exon 6, and replaced with LacZ and Neo genes. Mice aged P8 and above underwent cardiac perfusion before removal of the brain. For cardiac perfusions, mice were anesthetized with a 0.1 cc/25 gm dose of anesthetic (7 cc NaCl +2 cc Ketamine 100 mg/mL +1 cc Dexdomitor). Phosphate-buffered saline (PBS) was automatically pumped through the heart by use of a variable flow perfusion pump (Fisher Scientific, Cat #: 13-876-2) until there was no more blood in circulation. After this, 50–100 mL 4% paraformaldehyde (PFA) was pumped through to fix the brain. The brain was removed and stored in PFA at 4 °C for at least 24 hr.
2.2 | Cryosectioning
All brains were removed from PFA and placed in 30% sucrose at 4 °C for at least 24 hr before sectioning. Brains were removed from sucrose, patted dry with a kimwipe, and placed in Tissue Freezing Media (OCT Embedding Compound, VWR Catalog Number: 25608-930). Brains were frozen in isopentane surrounded by dry ice and cut immediately at –20 °C into 20 μm thick sections. Sections were placed on gelatin coated slides (1% gelatin, 0.05% chromium potassium sulfate) and kept at –80 °C.
2.3 | Immunohistochemistry
Sections were warmed to room temperature and washed with 1 X PBS 3 times for 5 min each. Antigen retrieval was performed for all antibodies by transferring slides to sodium citrate buffer (10 mM sodium citrate, pH 6.0) and microwaving until boiling. Sections were then cooled to room temperature, the sodium citrate buffer was replaced, and the sections were microwaved until boiling a second time. Sections were then rinsed three times with 1 X PBS and incubated with blocking solution (0.2% Triton X-100, 3% normal donkey serum) for 1 hour at room temperature. Sections were incubated with primary antibodies diluted in blocking buffer overnight at 4 °C. The next day, sections were washed with 1 X PBS three times and incubated with secondary antibodies for 1 hr at room temperature protected from light (secondary antibodies in antibody Table 1). Sections were then incubated with DAPI (4′,6-Diamindino-2-Phenylindole, Dihydrochloride, ThermoFisher Scientific, Cat 3: D1306) for 10 min at room temperature. Slides were washed three times with 1 X PBS and mounted with ProLong Diamond antifade mounting media (ThermoFisher Scientific, Cat: P36970). Antibodies used can be found in Table 1. Imaging was performed on a Zeiss AxioZoom Observer.Z1 with Apotome 3.1 structured illumination. All images were taken with a 40 × objective lens.
TABLE 1.
All primary and secondary antibodies used as well as their dilution and reference information. All antibodies were diluted in blocking buffer described in materials and methods.
| Primary antibodies | ||||
|---|---|---|---|---|
| Target protein | Species | Dilution | Source, Cat. No | RRID |
| NEEP21 | Rabbit | 1:400 | ||
| P19 | Rabbit | 1:400 | Abcam, ab189513 | AB_2571866 |
| CTIP2 | Rat | 1:400 | Biogend, 650601 | AB_10896795 |
| SatB2 | Mouse | 1:400 | Abcam, ab51502 | AB_882455 |
| Sox2 | Goat | 1:100 | Santa Cruz, sc-17320 | AB_2286684 |
| NeuN | Guinea Pig | 1:1000 | Millipore, ABN90 | AB_11205592 |
| Calbindin | Mouse | 1:800 | Sigma-Aldrich, C9484 | AB_2314065 |
| MAP2 | Chicken | 1:20,000 | EnCor, CPCA-MAP2 | AB_2138173 |
| Parvalbumin | Mouse | 1:2000 | Sigma-Aldrich, P3088 | AB_477329 |
| Secondary antibodies | ||||
|---|---|---|---|---|
| Antibody | Fluorophore | Dilution | Source, Cat No. | RRID |
| Donkey anti Mouse | 647 | 1:400 | Life Technologies, A-31571 | AB_2536181 |
| Donkey anti Mouse | 488 | 1:400 | Life Technologies, A-21202 | AB_2535788 |
| Donkey anti Rat | 488 | 1:400 | Jackson Labs, 712-546-153 | AB_2340686 |
| Donkey anti Rabbit | 568 | 1:400 | Life Technologies, A10042 | AB_2534017 |
| Donkey anti Guinea Pig | 647 | 1:400 | Jackson Labs, 706-175-148 | AB_2340462 |
| Donkey anti Goat | 488 | 1:400 | Life Technologies, A-11055 | AB_2534102 |
2.4 | Primary neuronal culture and transfection
Primary rat neurons were dissected and cultured from E18 rat brains as our lab has previously described (Wisco et al., 2003). Briefly, hippocampi from E18 rat brains were dissected and dissociated, plated on coverslips, and cultured for 8–12 days. Transfections were performed as previously described by our lab (Yap et al., 2008). The P19-sh plasmid and the random control plasmid were purchased from SABiosciences, and the constructs are expressed from the U1 promoter cassette of the hMGFP vector under the control of the CMV promoter. Briefly, 8–12 DIV neurons were transfected using Lipofectamine 2000 and incubated for 72 hr before fixation.
2.5 | Characterization of antibodies
CTIP2 is a transcription factor that is specific to developing corticospinal motor neurons in the mouse embryonic neocortex (Arlotta et al., 2005), using an antibody to the same clone that was used to make the BioLegend antibody used in this study (RRID: AB_10896795) (Senawong et al., 2003). The antibody recognizes 150 amino acids located at the N-terminus of the protein (Senawong et al., 2003). The antibody was originally described as being specific in western blots showing CTIP2 coimmunoprecipitating with a binding partner, but no band in the control immunoprecipiptation (Senawong et al., 2003). This commercial antibody is now quality tested by BioLegend through western blotting and immunofluorescence. The antibody shows characteristic staining of layer IV and layer V neurons in the neocortex as had been described in the literature (Molyneaux, Arlotta, Menezes, & Macklis, 2007).
SatB2 is a transcription factor which marks postmitotic neurons in the upper layers of the neocortex (Britanova et al., 2008). The commercially available antibody that we use shows this stereotypical staining. The antibody used is commercially available through Abcam (RRID: AB_882455) and recognizes the C-terminal segment of SatB2. The antibody was shown to be specific on a western blot where it recognizes a single band for SatB2 which is not recognized upon shRNA knockdown (Asanoma et al., 2012).
NeuN, also known as Fox-3, is a transcription factor found broadly in CNS and PNS neurons. This particular antibody shows a broad distribution of neurons (Lacoste et al., 2014), and antibodies used against same clone commercially available from Millipore are also characteristically absent from Purkinje neurons (Kim, Adelstein, & Kawamoto, 2009) (RRID: AB_11205592). This antibody was shown to be specific to neurons in IHC where it does not co-localize with blood vessel markers (Lacoste et al., 2014). The antibody is a version of the A60 clone which has been shown to be specific to neurons in IHC through lack of staining in the corpus callosum and lack of colocalization with glial subtypes (Mullen, Buck, & Smith, 1992).
Calbindin is a calcium-binding protein present in a variety of CNS neurons. It is highly expressed in Purkinje cells, as shown through staining with the antibody available through Sigma Aldrich (Shakkottai et al., 2011) (RRID: AB_2314065). This antibody was verified in IHC by lack of staining after preadsorption with the peptide (Celio, 1990).
Sox2 is a transcription factor which marks progenitor cells in the mouse neocortex. The antibody is produced by Santa Cruz against the C-terminus end of Sox2 and recognizes a single band in mouse stem cell lysates (SantaCruz, RRID: AB_2286684). This antibody was shown to be specific by western blotting where it recognizes a single band (Chew et al., 2006). This antibody has been shown to specifically mark progenitor cells in the brain (Mathews et al., 2010), and our staining is consistent with previous literature showing Sox2 to be expressed in the VZ (Hutton & Pevny, 2011).
The Tbr2 chicken antibody was purchased through EMD Millipore (RRID: AB_10615604). This antibody was validated by IHC whereby it shows identical staining to a Tbr2Cre knock-in mouse (Vasistha et al., 2015). Tbr2 is a transcription factor that has been shown to be expressed in intermediate progenitors but not apical progenitors/stem cells (Sessa, Mao, Hadjantonakis, Klein, & Broccoli, 2008). In our sections, we see Tbr2 staining in the SVZ but not the VZ, consistent with this literature.
MAP2 is a microtubule bundling protein found at high levels in the somatodendritic region. MAP2 chicken antibody was purchased from EnCor Biotechnology (RRID: AB_2138173). This antibody shows a single band on western blots from rat and mouse brain lysates (EnCor). We have previously validated this antibody by showing colocalization with neuronal marker NeuN, and no colocalization with the glial marker GFAP in cultured neurons (Digilio et al., 2015). Here, we again find that the antibody specifically recognizes neuronal soma and dendrites (Figure 1b).
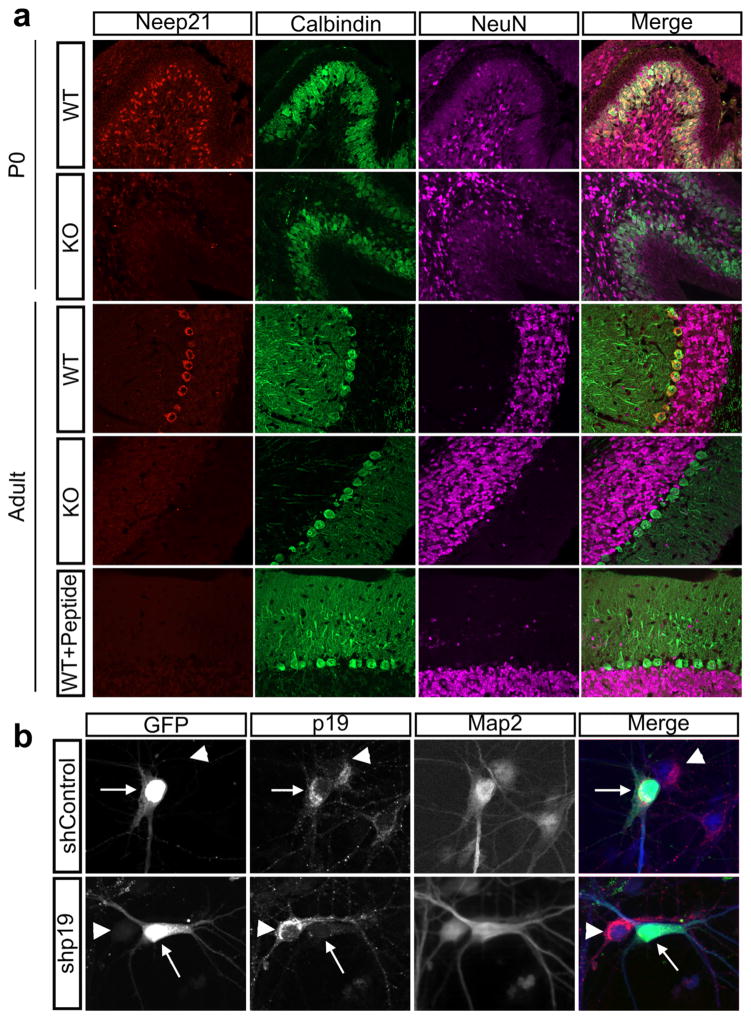
FIGURE 1.
NEEP21 and P19 antibodies are specific to their intended targets. (a) WT cerebellar sections from P0 or P60 mouse brains stain positive for NEEP21, however there is no staining in the KO sections or when the primary antibody is incubated with its immunizing peptide for 1 hr before application. Calbindin marks Purkinje neurons, and NeuN marks granule neurons. (b) The P19 antibody purchased from Abcam is specific. Primary neurons were transfected with either shP19-GFP or shControl GFP (arrow) and fixed 72 hours later. P19 staining (red) is greatly diminished in the neuron expressing shP19 (arrow) as opposed to its neighboring untransfected cell (arrowhead). shControl did not diminish P19 staining (arrow)
Parvalbumin, a calcium binding protein, is used to identify subclasses of cortical interneurons (Rudy, Fishell, Lee, & Hjerling-Leffler, 2011; Rymar & Sadikot, 2007). The antibody used was raised in mouse against purified carp muscle Parvalbumin (Sigma, RRID: AB_477329). The antibody recognized a single band on western blots and does not react with other EF-hand family members (Sigma).
Antibody characterizations against the small endosomal proteins P19 and NEEP21 can be found in the results section. The P19 antibody was purchased from Abcam and is targeted against the C-terminus of the protein. It has been tested by Abcam on human neuronal cell line and rat brain lysates. Additionally, an immunocytochemistry image from a mouse neuron has been provided on the Abcam website and shows correct endosomal and trans-Golgi network staining. The NEEP21 antibody was raised in rabbit and is not commercially available (RRID: AB_2572209).
3 | RESULTS
3. | NEEP21 and P19 antibodies are specific
Recently, antibody specificity has become a concern in publications (Baker, 2015; Prassas & Diamandis, 2014), and we thus first validated the antibodies against NEEP21 and P19. We raised an anti-NEEP21 antibody in rabbits to the same peptide used previously in studies by Steiner et al. in 2002. Since, we wished to characterize the staining pattern in the mouse brain, we validated the anti-NEEP21 antibody on cryosections in two ways: by blocking with the immunizing peptide and by staining sections from the NEEP21/ Nsg1–/– mouse as controls. NEEP21 shows high staining in cerebellar Purkinje cells in P0 and adult brains, but this staining is absent in NEEP21 knockout sections as well as when the antibody was preincubated with the peptide, demonstrating antibody specificity (Figure 1a). To test the expression pattern of P19, we bought a commercially available rabbit monoclonal antibody to P19 from Abcam. Since no P19 knockout mouse exists, we were not able to validate the antibody on knockout sections. We were also not able to use peptide block to validate the anti-P19 antibody, since the amino acid sequence used for immunization was not disclosed by the company. Thus, we were unable to purchase or synthesize the peptide that this antibody was raised against. Therefore, to test antibody specificity we transfected dissociated E18 rat hippocampal neurons with short hairpin plasmid against P19 (shP19-GFP) and observed a distinct loss of staining in transfected cells versus nontransfected cells (Figure 1b). A sh-plasmid containing a random hairpin was used as a control and did not diminish P19 staining. Overall, our NEEP21 and P19 antibodies are specific to their respective targets.
3.2 | NEEP21 and P19 are similarly expressed in the hippocampus throughout development, but not uniformly in all regions
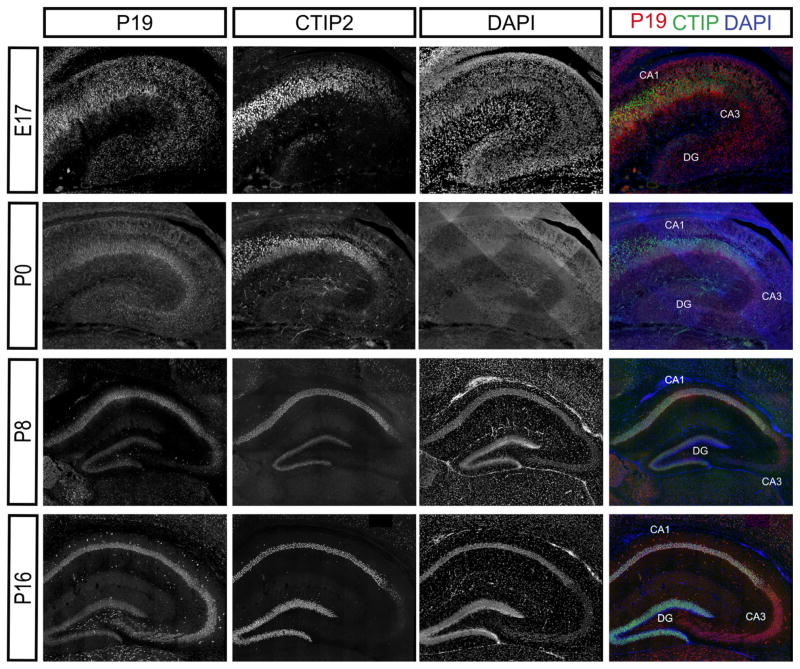
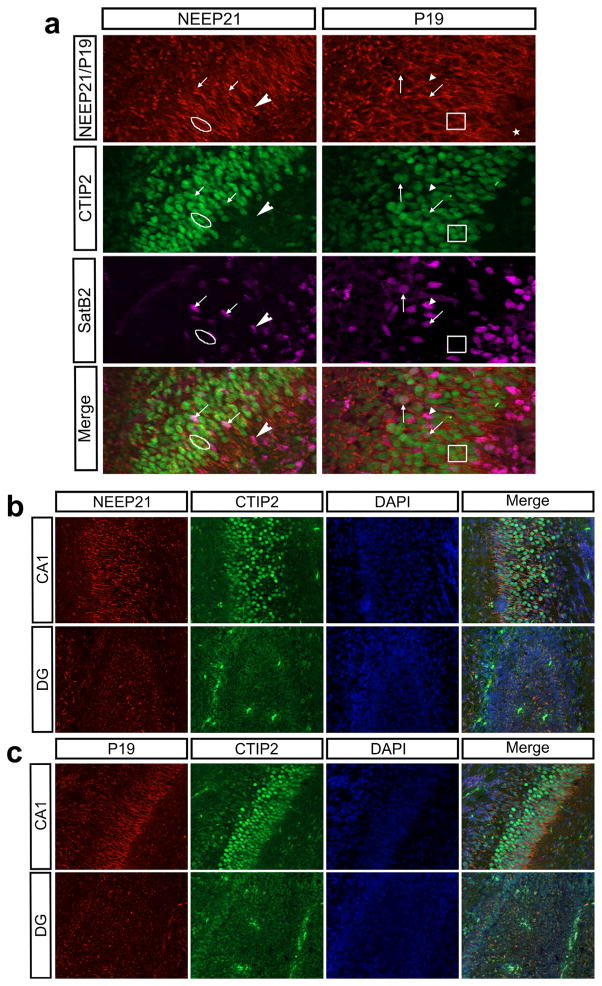
Given that NEEP21 and P19 are in the same family, we hypothesized that they would be similarly expressed. Indeed, NEEP21 and P19 are both expressed in the hippocampus throughout development (Figures 2 and 3). We did not observe any differences along the rostral-caudal axis in the neocortex (not shown) and are showing equivalent sections for all ages and markers. NEEP21 and P19 are present in CTIP2-positive, SatB2-positive, and CTIP2/SatB2-double positive hippocampal neurons in vitro (Digilio et al., 2015). Similarly, NEEP21 and P19 are both expressed highly in double-positive cells in vivo as well as in single positive CTIP2- or Satb2-neurons at E17 (Figure 4a). Interestingly, neurons in the CA1 region have higher expression of NEEP21 (Figure 4b) and P19 (Figure 4c) than neurons in the dentate gyrus (DG) at P0. This accords with our ex vivo data showing lower expression of NEEP21 and P19 in Prox1-positive cells, which are enriched in the DG. Unfortunately, anti-Prox1 antibodies did not produce convincing staining on cryosections.
FIGURE 2.
NEEP21 is expressed throughout the hippocampus during development. NEEP21 is highly expressed in CA1-CA3 regions of the hippocampus, but less highly expressed in the dentate gyrus (DG). CA1-CA2 and DG are marked by CTIP2, with CA3 showing characteristic lack of CTIP2
FIGURE 3.
P19 is expressed throughout the hippocampus during development. P19 is highly expressed in CA1-CA3 regions of the hippocampus throughout development. Ages and antibodies are indicated in the figure. CA1-CA2, DG marked by CTIP2. CA3 shows characteristic lack of CTIP2
FIGURE 4.
NEEP21 and P19 in the developing hippocampus. (a) CTIP2 positive (encircled/boxed), SatB2 positive (arrowhead), and double positive (arrow) neurons in the CA1 region of the hippocampus at E17 express NEEP21 and P19. (b, c) NEEP21 (b) and P19 (c) are both expressed at higher levels in the CA1 region of the hippocampus than in the dentate gyrus (DG) at P0. All imaging and processing was identical between brain regions
3.3 | NEEP21 and P19 are differentially expressed in the embryonic neocortex
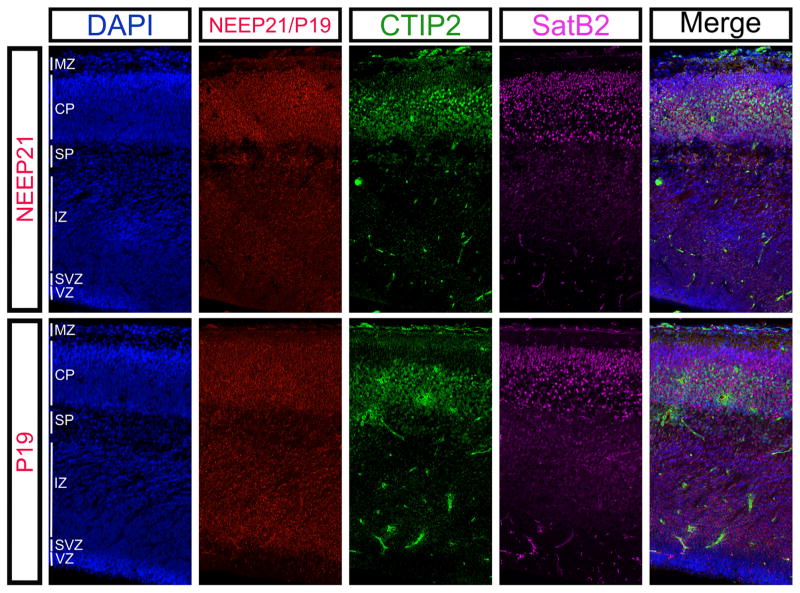
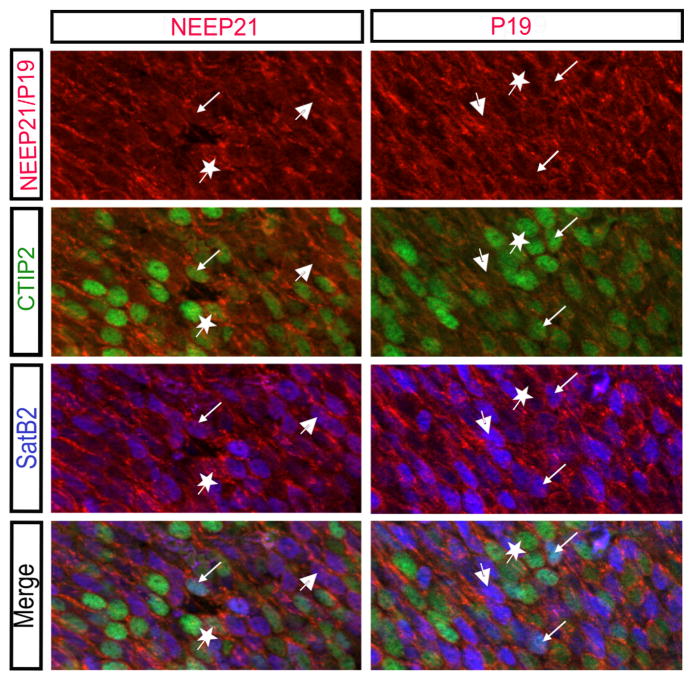
Given the similar NEEP21 and P19 expression in the hippocampus, we hypothesized that they would be expressed in similar spatial and temporal patterns in the developing neocortex. NEEP21 and P19 are both highly expressed in the cortical plate at E17.5 (Figure 5). However, many different cell types reside within the cortical plate, so we asked which cells expressed NEEP21 and P19. In particular, we focused on layer V neurons marked by CTIP2, and superficial layers marked by SatB2. Consistent with in vitro data (Digilio et al., 2015), NEEP21 and P19 are both highly expressed in CTIP2-positive, SatB2-positive, and CTIP2/Satb2-double positive cells in the E17 neocortex (Figure 6).
FIGURE 5.
Expression patterns of NEEP21 and P19 in the mouse embryonic cortical plate. NEEP21 and P19 are both expressed at high levels in the cortical plate (CP) at E17.5, as marked by SatB2- and CTIP2-positive neurons. CTIP2 marks layer V strongly and layer VI weakly at this age. NEEP21 is also highly expressed in the subplate (SP). P19 is expressed throughout the developing neocortex, and highly in the subventricular zone (SVZ) where NEEP21 is expressed at a much lower level
FIGURE 6.
NEEP21 and P19 are expressed in multiple cell types in the embryonic neocortex. NEEP21 and P19 are present in CTIP2 positive (star), SatB2 positive (large arrow), and double positive (small arrow) cells in the E17.5 neocortex
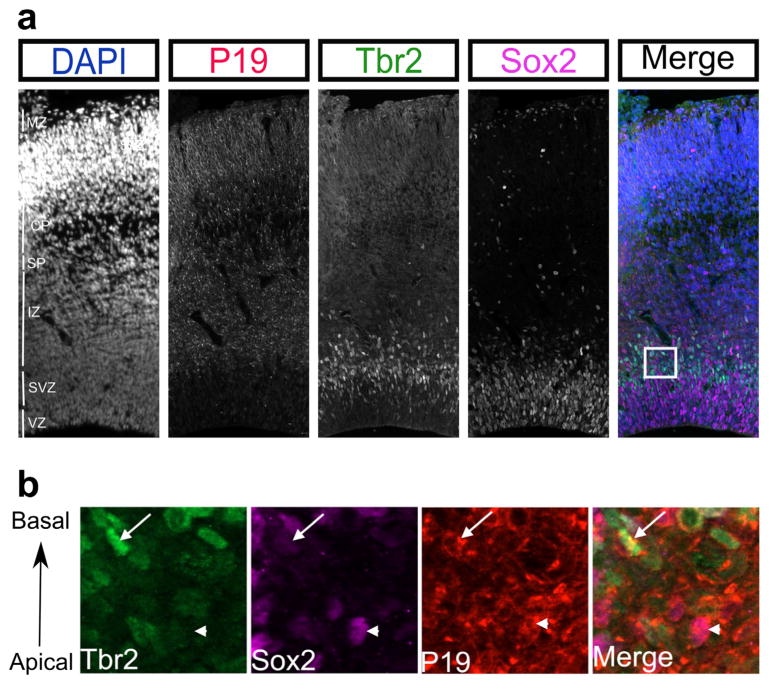
While NEEP21 and P19 both showed high expression in the cortical plate, they showed different expression patterns in the progenitor layers. NEEP21 was highly expressed in the cortical plate and subplate, with low expression in the intermediate, ventricular, and subventricular zones (Figure 5). By contrast, P19 expression is higher in the SVZ (Figure 7a). As the neocortex develops, newborn neurons migrate from the ventricular and subventricular zones to their final destination in the cortical plate with earlier born neurons forming the innermost layer. We sought to determine the identity of P19-positive cells in the progenitor layers by marking the SVZ with Tbr2 and the VZ with Sox2. Interestingly, all Sox2-positive cells in the VZ were lacking P19, however the basal population of Tbr2-positive intermediate progenitors were positive for P19. Examining the basal-most area of the SVZ more closely (Figure 7b), both Sox2 and Tbr2 expressing cells appeared positive for P19. These pools of progenitor cells were all at the basal edge of the SVZ, suggesting that newly born neurons that have yet to turn off Tbr2 and Sox2 express P19. Interestingly, the Tbr2-positive cells appears to be in metaphase while the Sox2-positive cell is in prophase indicating that P19 is expressed prior to the final mitotic division. Therefore, NEEP21 and P19 have similar expression patterns in the cortical plate, but differential expression in the lower zones of the developing neocortex.
FIGURE 7.
P19 is expressed in SVZ progenitors. (a) At E17.5, P19 is absent from the ventricular zone (VZ) progenitors as marked by Sox2, but highly expressed in the basal SVZ, as marked by Tbr2 positive cells. The area in the box is expanded in (b) and shows both Sox2 positive cells (arrowhead) and Tbr2 positive cells (arrow) can be found in the basal aspect of the SVZ. P19 is expressed in both of these cell types
3.4 | NEEP21 and P19 remain enriched in layer V neurons in the neocortex during development
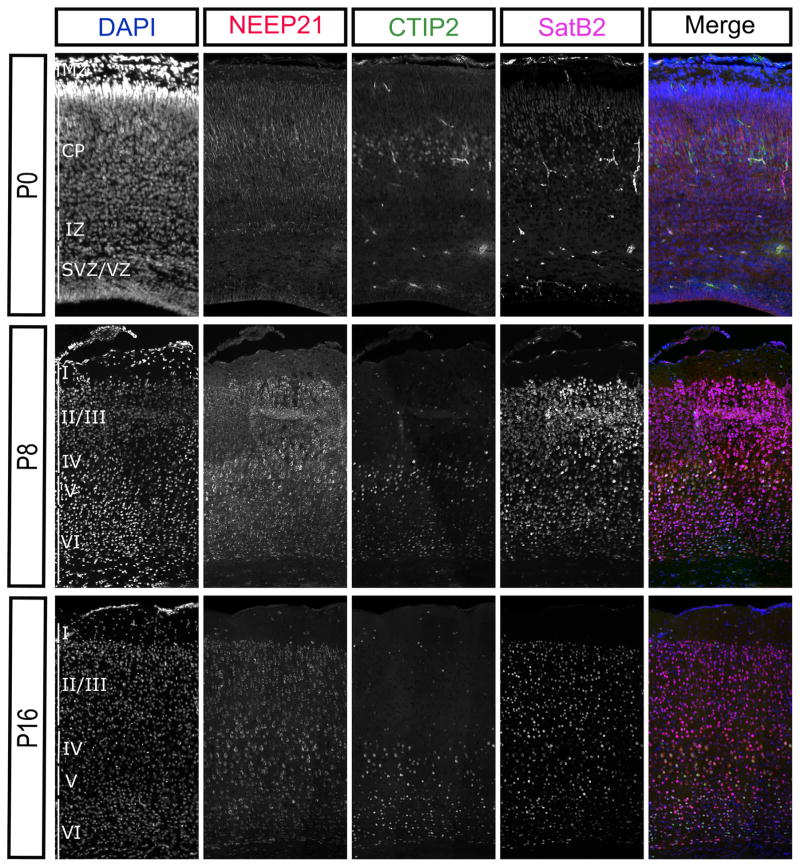
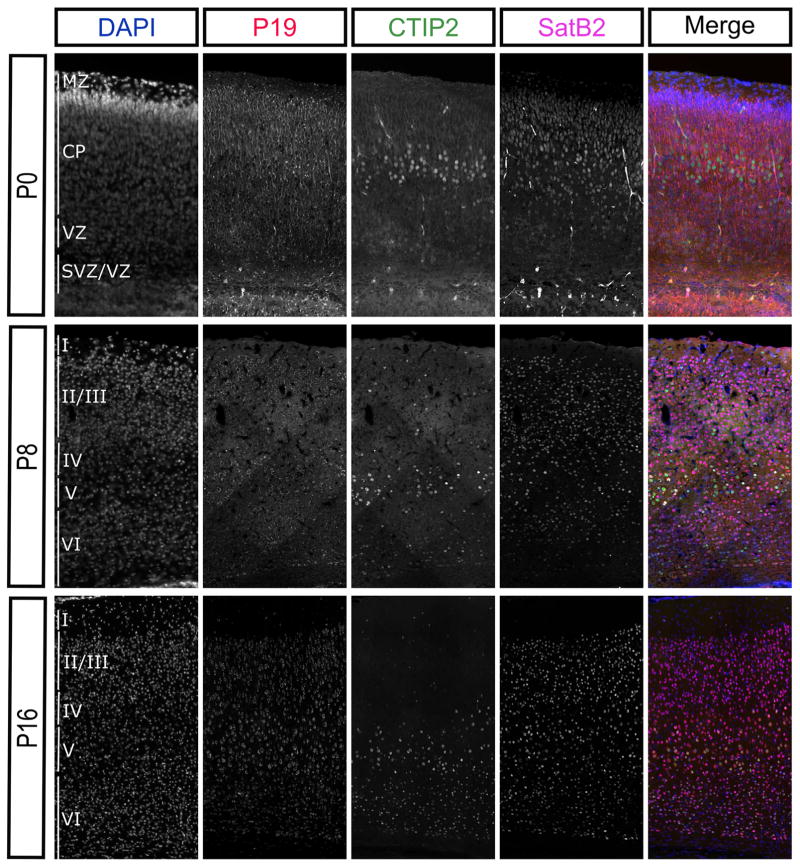
Clearly, NEEP21 and P19 are both highly expressed throughout the cortical plate during embryonic development (Figure 5). However, previous immunoblot studies have shown that expression levels of NEEP21 in the rat neocortex dramatically decrease postnatally (Steiner et al., 2002). Therefore, we tested whether the entire neocortex loses expression of NEEP21 and P19 during development or if the expression levels simply became restricted to certain cell types. We examined NEEP21 and P19 immunohistochemistry in the mouse neocortex through development using CTIP2 as a marker for deeper layer neurons and SatB2 as a marker of neurons in the superficial layers. Already by P8, NEEP21 and P19 expression levels were lower than they were in the P0 brain in a majority of neurons (Figures 8 and 9). Indeed, by P16 NEEP21 and P19 were expressed at low levels in all neurons in the neocortex but maintained high expression in layer V neurons. These neurons expressed CTIP2 or Satb2, and this expression is consistent along the rostral caudal axis of the neocortex. This shows that higher NEEP21 and P19 levels are maintained in a subpopulation of cortical neurons at later ages, as opposed to a general reduction in protein levels.
FIGURE 8.
NEEP21 expression remains enriched in layer V cortical neurons during development. NEEP21 is highly and broadly expressed throughout the cortical plate at P0, but is downregulated overall at P8 and P14, but remains relatively enriched in layer V
FIGURE 9.
P19 expression remains enriched in layer V cortical neurons during development. P19 is also broadly expressed in the P0 neocortex. Similarly, to NEEP21, P19 is downregulated overall at P8 and P14, but remains relatively enriched in layer V
3.5 | NEEP21 and P19 are expressed in some sets of interneurons in the neocortex
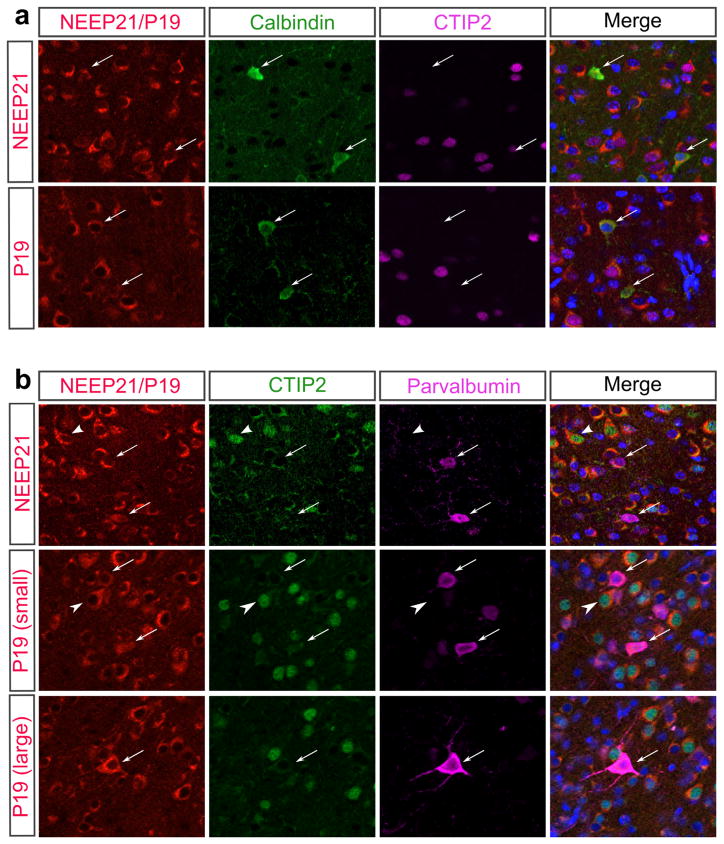
In vitro, we have seen that NEEP21 and P19 are both present in inter-neuron populations as well as subcortical projection neurons (Digilio et al., 2015). While we were unable to detect convincing staining using anti-GAD65 antibodies on tissue sections, we asked whether or not we could detect NEEP21 and P19 expression in other subsets of inter-neurons. Specifically, we looked at inhibitory interneurons that are positive for Calbindin or Parvalbumin at P8 when expression is already being restricted. Interestingly, both NEEP21 and P19 show expression in interneurons, but expression levels are not high in all populations of interneurons. Instead, we see a subset of Calbindin and Parvalbum positive neurons that express NEEP21 and P19 at either low or high levels (Figure 10). This shows that there is a range of NEEP21 and P19 expression levels in interneurons in the neocortex, with large interneurons showing higher levels of P19 (Figure 10B) and NEEP21 (not shown) than interneurons with smaller cell bodies at P8.
FIGURE 10.
NEEP21 and P19 have varied expression levels in interneurons. (a) NEEP21 and P19 are both expressed in Calbindin positive interneurons (arrows) in the P8 neocortex, and their expression levels vary between cells. (b) NEEP21 and P19 are both expressed in Parvalbumin positive interneurons (arrows). Expression levels are higher in larger Parvalbumin cells (larger) than small Parvalbumin cells (small). Arrows: Parvalbumin positive cells, Arrowheads: CTIP2 positive cells. DAPI staining is shown in blue in the merged image.
3.6 | NEEP21 becomes restricted to Purkinje cells during cerebellar development
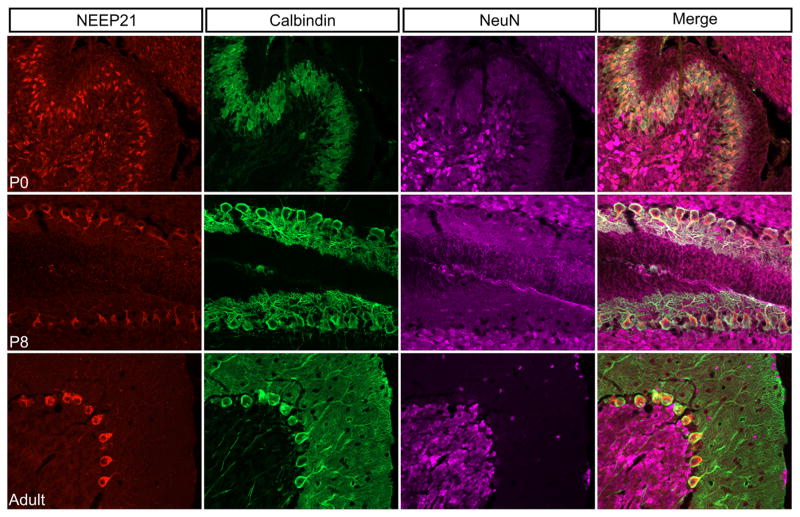
The cerebellum develops at a later time point than the neocortex, with much of it developing postnatally. Therefore, we stained mid-sagittal sections of cerebellum against NEEP21 in early development (at P0), mid-development (at P8) and fully developed (at P60). Initially, NEEP21 was expressed in both NeuN-positive granule neurons and Calbindin-positive Purkinje cells. However, by the end of cerebellar development at P60, NEEP21 expression had become restricted to only Calbindin-positive Purkinje cells and was essentially undetectable in NeuN-positive neurons (Figure 11).
FIGURE 11.
Expression pattern of NEEP21 in the cerebellum. NEEP21 is expressed ubiquitously in the developing P0 cerebellum. This includes Calbindin-positive Purkinje neurons as well as NeuN-positive granule neurons. However, it becomes restricted to Purkinje cells by P8. This exclusive expression in Purkinje cells is maintained throughout adulthood as shown in the P60 brain. NEEP21 is not detectable in granule neurons.
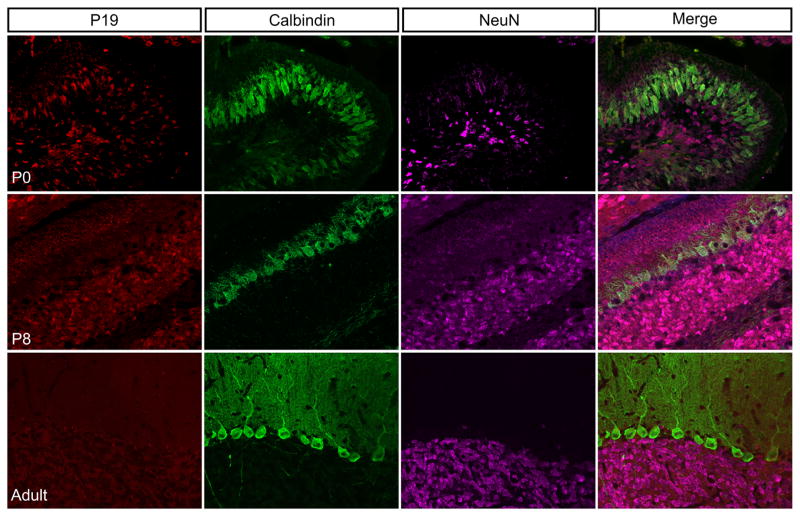
3.7 | P19 expression in the cerebellum decreases during development
NEEP21 and P19 were both expressed highly in the developing neo-cortex, therefore, we hypothesized that P19 would also be present in the developing cerebellum where NEEP21 was highly expressed. At P0, however, P19 was only expressed at fairly low levels in a subset of neurons, including some but not all developing Purkinje cells (marked by Calbindin expression) (Figure 12). By P8, P19 expression was very low in Purkinje cells but present in the external granule layer of neurons migrating past Purkinje cells to form the granule layer (Figure 12). This staining was noticeably different from NEEP21 staining in cerebellum at P8 where the Purkinje cells were the brightest NEEP21-expressing neurons. It should be noted that NEEP21 expression in the cerebellum at all times was much higher than P19 expression. NEEP21 images of the P8 cerebellum were taken with an exposure time of 40 ms and the P19 P8 images with an exposure time of 490 ms. This low expression of P19 was maintained throughout adulthood, and P19 was undetectable in either Calbindin-positive Purkinje cells or NeuN-positive neurons in the adult cerebellum, demonstrating a clear and surprising difference in expression between NEEP21 and P19 in the cerebellum (Figures 11 and 12).
FIGURE 12.
Expression pattern of P19 in the cerebellum. P19 is expressed broadly in the P0 cerebellum, but at much lower levels than NEEP21. P19 expression remains low throughout development, and is undetectable by adulthood
4 | DISCUSSION
In this work, we analyzed the protein expression levels of the related family members NEEP21/Nsg1 and P19/Nsg2 in developing mouse brain between E17 and P16. Initial papers describing NEEP21 and P19 expression levels described the two proteins as developmentally regulated. However, the spatial aspects of these changes were not explored and many questions were left unanswered including: Do expression levels decrease across the brain as a whole? Does expression become restricted? Do all neurons lose expression of these two protein? Here, we set out to answer these questions. We showed that NEEP21 and P19 are expressed in distinct spatial and temporal patterns that differ according to brain region by identifying two unexpected aspects of how the NEEP21 and P19 expression profiles change in time and space. First, we show that both proteins are expressed broadly in all brain regions early in development and then become more restricted in their expression at older ages. Contrary to previous reports, expression does not completely disappear but instead is simply reduced in most neurons while a subset of neurons retain expression. In particular, both NEEP21 and P19 remain expressed in layer V of the neocortex and in CA1 and CA3 of the hippocampus. The second conclusion is that there are some cell types where only one of the two proteins is expressed. P19 is expressed in the progenitor cells of the subventricular zone whereas NEEP21 is not expressed in this region. NEEP21, on the other hand, retains high expression in Purkinje neurons in P60 cerebellum whereas P19 is strikingly absent from the adult cerebellum. Our findings are summarized in Table 2.
TABLE 2.
Summary of spatial and temporal expression of NEEP21 and P19. More “+” signs indicate higher expression.
| E17
|
P0
|
P8
|
P16
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain area | subregion | NEEP21 | P19 | NEEP21 | P19 | NEEP21 | P19 | NEEP21 | P19 |
| Cortex: | Upper layers | +++ | +++ | +++ | +++ | − | − | − | − |
|
| |||||||||
| Layer V | +++ | +++ | +++ | +++ | ++ | ++ | ++ | ++ | |
|
| |||||||||
| Interneurons | ++ | ++ | |||||||
|
| |||||||||
| Subventricular zone | − | +++ | |||||||
|
| |||||||||
| Hippocampus | CA | +++ | +++ | +++ | +++ | ++ | ++ | ++ | ++ |
|
| |||||||||
| Dentate gyrus | + | + | + | + | + | + | + | + | |
|
| |||||||||
| Cerebellum | Granule layer | − | − | +++ | + | − | + | − | − |
|
| |||||||||
| Purkinje neurons | − | − | +++ | + | +++ | − | +++ | − | |
4.1 | NEEP21 and P19 are expressed highly in CA1 and CA3, but not in dentate gyrus throughout development
We showed that in the hippocampus, NEEP21 and P19 are widely expressed in the pyramidal cells of the CA1-3 regions with expression remaining high throughout development. This is consistent with the published role of NEEP21 in hippocampal LTP (Alberi et al., 2005; Steiner et al., 2005). The hippocampus is well known as a brain region responsible for learning and memory. These processes are dependent on the induction of long term depression (LTD) and long term potentiation (LTP) (Malenka & Bear, 2004; Sweatt, 2016). In LTP, AMPA receptors are recruited to the synapse and inserted into the membrane (Jurado, 2014; MacDougall & Fine, 2014). At the molecular level, NEEP21 has been shown to facilitate recycling of the AMPAR subunit GluR2 in hippocampal slices, and reduction of NEEP21 levels leads to a decrease in the surface levels of GluR2 containing synapses (Steiner et al., 2005). Interestingly, the two proteins were relatively less abundant in the granule cells of the DG. This observation agrees with expression of NEEP21 and P19 in hippocampal cultures (Digilio et al., 2015), and perhaps suggests a differential need for endosomal transport in CA1 and CA3 neurons versus DG neurons. One explanation for this is the anatomy of each hippocampal region. Pyramidal cells make up the majority of the CA1-3 regions of the hippocampus (Dale et al., 2015) and maintain extensive axons and dendritic arbors, while the DG is composed mainly of small granule neurons. We speculate that these large neurons might require specific neuronal endosomal regulators more than smaller, less expansive granule neurons do.
4.2 | NEEP21 and P19 expression is broad during development but persists into adulthood only in a subset of neuronal cell types with large dendritic arbors
Maintenance of NEEP21 expression in pyramidal neurons is also seen in the neocortex. The neocortex is composed of 6 layers with many different neuronal subtypes. Pyramidal cells are the primary cell type in cortical layers III and V, and these neurons have expanded dendritic arbors (Spruston, 2008). While the majority of neurons in the neocortex express NEEP21 and P19 at embryonic ages, layer V pyramidal neurons maintain expression of NEEP21 and P19 through development (Figures 8 and 9). This may suggest that NEEP21/P19 are important for excitatory pyramidal cells, especially those in layer V which can have very large dendritic trees, extending in some cases all the way to the pial surface.
However, expression of NEEP21 and P19 is also maintained in GABAergic interneurons positive for Calbindin or Parvalbumin. Interestingly, only a subset of Parvalbumin and Calbindin positive cells retain NEEP21 or P19. There are two types of Parvalbumin-positive interneurons in the neocortex: basket cells and chandelier cells (Rudy et al., 2011). Basket cells can vary in size, with large basket cells containing large axonal and dendritic arbors (Markram et al., 2004). Many large basket cells are dually positive for calbindin and parvalbumin. This characteristic, as well as their large size, leads us to hypothesize that these are the interneurons positive for NEEP21 or P19. However, additional studies will have to be done to further delineate the many inter-neuron subtypes and how NEEP21 and P19 are expressed in them.
NEEP21 is once again seen to be developmentally restricted in the cerebellum. Embryonically, all cerebellar cells contain NEEP21, but only Purkinje cells maintain NEEP21 into adulthood while granule cells lose expression (Figure 11). Granule neurons are much smaller than pyramidal neurons and Purkinje neurons, pointing perhaps to increased need for NEEP21 in larger neurons. This would be in line with the expression of NEEP21 in layer V cortical neurons and large interneurons.
4.3 | NEEP21 and P19 are largely co-expressed, but certain cell types express only one of the two proteins
Interestingly, NEEP21 and P19 expression patterns were not identical. NEEP21 and P19 are closely related family members, and we hypothesized that they would have the same expression pattern. This was true in culture (Digilio et al., 2015) as well as for most neurons in situ. However, there were a number of striking exceptions. For instance, the expression patterns diverged early in development (E17 neocortex). At this point during cortical development, P19 was highly expressed in the progenitor layers including the SVZ, which is well known for its role in neurogenesis, while NEEP21 was mainly restricted to the cortical plate. Radial glia reside in the VZ and can generate neurons and glia, whereas intermediate progenitor cells (IPCs) reside in the SVZ and produce only neurons (Englund et al., 2005; Miyata, Kawaguchi, Okano, & Ogawa, 2001; Noctor, Flint, Weissman, Dammerman, & Kriegstein, 2001; Paridaen & Huttner, 2014). IPCs express the transcription factor Tbr2, which is down-regulated once the cell’s neurogenic fate is determined (Englund et al., 2005; Ochiai et al., 2009). Interestingly, P19 was expressed in a subset of Tbr2 positive cells in the SVZ (Figure 7b). Even though P19 was first described as being enriched in neurons (Sabéran-Djoneidi et al., 1995), our data show expression of P19 in Tbr2-positive IPCs and thus point toward a potential role for P19 just prior to neural specification. NEEP21, on the other hand, is not expressed in the IPCs and may not function until later in neuronal maturation. This divergent expression suggests some nonredundant functions for NEEP21 and P19, a notion we will test in the future.
Whereas in the neocortex, NEEP21 and P19 had different expression patterns during development but eventually restricted similarly to layer V (Figures 8 and 9), they did not have overlapping expression patterns at any point during development in the cerebellum (Figures 11 and 12). P19 was expressed at low levels in many different cell types in the cerebellum at P0 whereas NEEP21 was expressed at a much higher levels. By P8, NEEP21 had overwhelmingly restricted expression to Purkinje cells, whereas P19 levels were low in Purkinje cells and higher in the migrating external granule layer. By P60, P19 was undetectable in the cerebellum, but NEEP21 levels remained high in Purkinje cells. This suggests non-overlapping roles for NEEP21 and P19 in the cerebellum, and specifically in Purkinje cells which maintain a high expression of NEEP21 even after development has completed.
Restriction of protein expression during development was also observed in the third Nsg family member, Calcyon (Caly). Caly was more highly expressed in younger rats as opposed to adult rats, similar to the reduction in NEEP21 and P19 protein levels (Heijtz, Alexeyenko, & Castellanos, 2007). This points to a key role in brain development for the Nsg family of proteins, and perhaps a continued requirement for these family members in a subset of large adult neurons. We were not able to carry out more detailed comparisons with Caly since the available antibodies do not recognize mouse Caly.
4.4 | Layer V cortical and Purkinje neurons – special needs for endosomal trafficking?
NEEP21 and P19 are both neuronal-enriched endosomal proteins. From their expression patterns, we can infer that they are important during development of the neocortex, hippocampus, and cerebellum. Consistent with this observation, we previously showed that NEEP21 regulates recycling pathways of the cell adhesion molecule L1. Other developmentally important guidance receptors might also be regulated by NEEP21 and this might explain why most neurons express Nsg family proteins developmentally. We have previously observed dendrite and axon growth defects in cultured neurons grown on L1 substrate when NEEP21 is knocked down, consistent with a role in process elaboration during development (Lasiecka, 2014).
In addition to high expression universally during development, NEEP21 remains enriched in distinct areas of the brain, including layer V cortical neurons, the CA1-3 region of the hippocampus, and Purkinje cells in the cerebellum. Considering the complex endosomal system that is present specifically in neurons, the restriction of these neuron-specific endosomal proteins suggests a high necessity for endosomal trafficking in these neurons. Indeed, layer V cortical neurons and Purkinje Cells have extraordinarily long axons, as well as complex dendritic trees. Complexity of neuronal processes and a large soma is a feature that is common to all pyramidal cells, as well as large basket cells. Thus, persistent expression of NEEP21 endosomal protein in these neuronal cell types suggests that these neurons might need additional proteins to maintain complex endosomal trafficking over long distances in large dendritic arbors. The function of P19 is very poorly understood and no functional data have been published. The differential expression suggests that P19 and NEEP21 might not perform redundant functions, at least in some cellular contexts, that is, IPCs for P19 and Purkinje neurons for NEEP21. More work is needed to understand the significance of the observed differential expression. The persistence of expression in the hippocampus, layer V projection neurons, and the very large Purkinje neurons raises many questions about what special needs these neuronal subclasses have that requires continued expression of this interesting, but poorly understood family of proteins.
Acknowledgments
Thank you to the Winckler lab for continuous input and advice.
Footnotes
CONFLICT OF INTEREST STATEMENT
There is no conflict of interest.
AUTHOR CONTRIBUTION
The authors take full responsibility for the accuracy of the data and have full access to all data. The authors take full responsibility for the integrity of the data. Study concept and design: KB, CCY, BW. Acquisition of data: CCY (Figure 1b), KB (all other figures). Analysis and interpretation of data: KB, CCY BW, NDD. Drafting of the manuscript: KB, BW. Critical revision of the manuscript for important intellectual content: KB, CCY, BW, NDD. Statistical analysis: N/ A. Obtained funding: BW, NDD. Administrative, technical, and material support: CCY, BW. Study supervision: CCY, BW, NDD.
LITERATURE CITED
- Alberi S, Boda B, Steiner P, Nikonenko I, Hirling H, Muller D. The endosomal protein NEEP21 regulates AMPA receptor-mediated synaptic transmission and plasticity in the hippocampus. Molecular and Cellular Neuroscience. 2005;29:313–319. doi: 10.1016/j.mcn.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, MacKlis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Asanoma K, Kubota K, Chakraborty D, Renaud SJ, Wake N, Fukushima K, Rumi MAK. SATB homeobox proteins regulate trophoblast stem cell renewal and differentiation. Journal of Biological Chemistry. 2012;287:2257–2268. doi: 10.1074/jbc.M111.287128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. Blame it on the antibodies. Nature. 2015;521:274–275. doi: 10.1038/521274a. [DOI] [PubMed] [Google Scholar]
- Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. The Annual Review of Neuroscience. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Tarabykin V. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Celio M. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- Chew J, Loh Y, Zhang W, Chen X, Tam W, Yeap L, Ng H. Reciprocal transcriptional regulation of complex in embryonic stem cells reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4 / Sox2 complex in embryonic stem cells. Molecular and Cellular Biology. 2006;25:6031–6046. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale E, Pehrson AL, Jeyarajah T, Li Y, Leiser SC, Smagin G, Olsen CK, Sanchez C. Effects of serotonin in the hippocampus: How SSRIs and multimodal antidepressants might regulate pyramidal cell function. CNS Spectrums. 2015:1–19. doi: 10.1017/S1092852915000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaigt C, Hirling H, Steiner P, Vincent JP, Mazella J. Crucial role of neuron-enriched endosomal protein of 21 kDa in sorting between degradation and recycling of internalized G-protein-coupled receptors. Journal of Biological Chemistry. 2004;279:35687–35691. doi: 10.1074/jbc.M402751200. [DOI] [PubMed] [Google Scholar]
- Digilio L, Yap CC, Winckler B. Ctip2-, Satb2-, Prox1-, and GAD65-Expressing neurons in rat cultures: Preponderance of single-and double-positive cells, and cell type-specific expression of neuron-specific gene family members, Nsg-1 (NEEP21) and Nsg-2 (P19) PLoS One. 2015;10:e0140010. doi: 10.1371/journal.pone.0140010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Charmaine L, Pham D, Ray DAM, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. Journal of Neuroscience. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijtz RD, Alexeyenko A, Castellanos FX. Calcyon mRNA expression in the frontal-striatal circuitry and its relationship to vesicular processes and ADHD. Behavioral and Brain Functions. 2007;3:33. doi: 10.1186/1744-9081-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton SR, Pevny LH. SOX2 expression levels distinguish between neural progenitor populations of the developing dorsal tel-encephalon. Developmental Biology. 2011;352:40–47. doi: 10.1016/j.ydbio.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Jurado S. The dendritic SNARE fusion machinery involved in AMPARs insertion during long-term potentiation. Frontiers in Cellular Neuroscience. 2014;8:407. doi: 10.3389/fncel.2014.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. Journal of Biological Chemistry. 2009;284:31052–31061. doi: 10.1074/jbc.M109.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste B, Comin CH, Ben-Zvi A, Kaeser PS, Xu X, Costa LF, Gu C. Sensory-related neural activity regulates the structure of vascular networks in the cerebral cortex. Neuron. 2014;83:1117–1130. doi: 10.1016/j.neuron.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasiecka ZM, Yap CC, Katz J, Winckler B. Maturational conversion of dendritic early endosomes and their roles in L1-mediated axon growth. Journal of Neuroscience. 2014;34:14633. doi: 10.1523/JNEUROSCI.1837-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall MJ, Fine A. The expression of long-term potentiation: reconciling the preists and the postivists. Philosophical transactions of the Royal Society of London. Series B Biological sciences. 2014;369:20130135. doi: 10.1098/rstb.2013.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nature Reviews Neuroscience. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Mathews EA, Morgenstern NA, Piatti VC, Zhao C, Jessberger S, Schinder AF, Gage FH. A distinctive layering pattern of mouse dentate granule cells is generated by developmental and adult neurogenesis. Journal of Comparative Neurology. 2010;518:4479–4490. doi: 10.1002/cne.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nature Reviews Neuroscience. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Muthusamy N, Ahmed SA, Rana BK, Navarre S, Kozlowski DJ, Liberles DA, Bergson C. Phylogenetic analysis of the neep21/calcyon/p19 family of endocytic proteins: Evidence for functional evolution in the vertebrate cns. Journal of Molecular Evolution. 2009;69:319–332. doi: 10.1007/s00239-009-9273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Norstrom EM, Zhang C, Tanzi R, Sisodia SS. Identification of NEEP21 as a ß-amyloid precursor protein-interacting protein in vivo that modulates amyloidogenic processing in vitro. Journal of Neuroscience. 2010;30:15677–15685. doi: 10.1523/JNEUROSCI.4464-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai W, Nakatani S, Takahara T, Kainuma M, Masaoka M, Minobe S, Miyata T. Periventricular notch activation and asymmetric Ngn2 and Tbr2 expression in pair-generated neocortical daughter cells. Molecular and Cellular Neuroscience. 2009;40:225–233. doi: 10.1016/j.mcn.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Ohnishi S, Futamura M, Kamino H, Nakamura Y. Identification of NEEP21, encoding neuron-enriched endosomal protein of 21 kDa, as a transcriptional target of tumor suppressor p53. International Journal of Oncology. 2010;37:1133–1141. doi: 10.3892/ijo_00000765. [DOI] [PubMed] [Google Scholar]
- Paridaen JT, Huttner WB. Neurogenesis during development of the vertebrate central nervous system. EMBO Reports. 2014;15:351–364. doi: 10.1002/embr.201438447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prassas I, Diamandis EP. Translational researchers beware! Unreliable commercial immunoassays (ELISAs) can jeopardize your research. Clinical Chemistry and Laboratory Medicine. 2014;52:765–766. doi: 10.1515/cclm-2013-1078. [DOI] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Developmental Neurobiology. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymar VV, Sadikot AF. Laminar fate of cortical GABAergic interneurons is dependent on both birthdate and phenotype. Journal of Comparative Neurology. 2007;501:369–380. doi: 10.1002/cne.21250. [DOI] [PubMed] [Google Scholar]
- Sabéran-Djoneidi D, Marey-Semper I, Picart R, Studler JM, Tougard C, Glowinski J, Lévi-Strauss M. A 19-kDa protein belonging to a new family is expressed in the Golgi apparatus of neural cells. Journal of Biological Chemistry. 1995;270:1888–1893. doi: 10.1074/jbc.270.4.1888. [DOI] [PubMed] [Google Scholar]
- Sabéran-Djoneidi D, Picart R, Escalier D, Gelman M, Barret A, Tougard C, Lévi-Strauss M. A 21-kDa polypeptide belonging to a new family of proteins is expressed in the Golgi apparatus of neural and germ cells. Journal of Biological Chemistry. 1998;273:3909–3914. doi: 10.1074/jbc.273.7.3909. [DOI] [PubMed] [Google Scholar]
- Senawong T, Peterson VJ, Avram D, Shepherd DM, Frye RA, Minucci S, Leid M. Involvement of the histone deacetylase SIRT1 in chicken ovalbumin upstream promoter transcription factor (COUP-TF)-interacting protein 2-mediated transcriptional repression. Journal of Biological Chemistry. 2003;278:43041–43050. doi: 10.1074/jbc.M307477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa A, Mao C, Hadjantonakis AK, Klein WH, Broccoli V. Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron. 2008;60:56–69. doi: 10.1016/j.neuron.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakkottai VG, do Carmo Costa M, Dell’orco JM, Sankaranarayanan A, Wulff H, Paulson HL. Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3. Journal of Neuroscience. 2011;31:13002–13014. doi: 10.1523/JNEUROSCI.2789-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nature Reviews Neuroscience. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Steiner P, Sarria JCF, Glauser L, Magnin S, Catsicas S, Hirling H. Modulation of receptor cycling by neuron-enriched endosomal protein of 21 kD. Journal of Cell Biology. 2002;157:1197–1209. doi: 10.1083/jcb.200202022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner P, Alberi S, Kulangara K, Yersin A, Sarria JCF, Regulier E, Hirling H. Interactions between NEEP21, GRIP1 and GluR2 regulate sorting and recycling of the glutamate receptor subunit GluR2. EMBO Journal. 2005;24:2873–2884. doi: 10.1038/sj.emboj.7600755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Neural plasticity and behavior - Sixty years of conceptual advances. Journal of Neurochemistry. 2016;139:1–21. doi: 10.1111/jnc.13580. [DOI] [PubMed] [Google Scholar]
- Vasistha NA, García-Moreno F, Arora S, Cheung AFP, Arnold SJ, Robertson EJ, Molnár Z. Cortical and clonal contribution of Tbr2 expressing progenitors in the developing mouse brain. Cerebral Cortex. 2015;25:3290–3302. doi: 10.1093/cercor/bhu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winckler B. Membrane trafficking mechanisms: Exocytosis and Endocytosis in Dendrites. In: Emoto K, Wong R, Huang E, Hoogenraad C, editors. Dendrites. Tokyo: Springer Japan; 2016. pp. 77–109. [Google Scholar]
- Wisco D, Anderson ED, Chang MC, Norden C, Boiko T, Folsch H, Winckler B. Uncovering multiple axonal targeting pathways in hippocampal neurons. Journal of Cell Biology. 2003;162:1317–1328. doi: 10.1083/jcb.200307069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap CC, Winckler B. Harnessing the power of the endosome to regulate neural development. Neuron. 2012;74:440–451. doi: 10.1016/j.neuron.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap CC, Wisco D, Kujala P, Lasiecka ZM, Cannon JT, Chang MC, Winckler B. The somatodendritic endosomal regulator NEEP21 facilitates axonal targeting of L1/NgCAM. Journal of Cell Biology. 2008;180:827–842. doi: 10.1083/jcb.200707143. [DOI] [PMC free article] [PubMed] [Google Scholar]