Abstract
Background
To determine if a select subgroup of patients with combined liver and peritoneal colorectal metastases would derive oncologic benefit from surgical resection as a component of multimodality treatment.
Materials and Methods
We retrospectively compared 32 patients with combined colorectal peritoneal and liver metastases (CRLM) and 173 patients with peritoneal metastases only (CRPM) undergoing cytoreductive surgery with hyperthermic intraperitoneal chemoperfusion (CRS-HIPEC). Kaplan-Meier survival curves and multivariate Cox-regression models identified prognostic factors affecting survival.
Results
Major postoperative complications (Clavien-Dindo grades 3–5) occurred in 32% (CRLM) and 17% (CRPM) of patients (p=0.08). After an estimated median follow-up from surgery of 57 months, propensity score adjusted median progression-free survival was 5.1 months (CRLM) and 7.6 months (CRPM), while median overall survival was 13 months (CRLM) and 21 months (CRPM). Multivariate Cox-regression analysis of the CRLM group identified number of liver metastases to be the only independent predictor of poor survival (HR 2.3, P=0.03), with a dramatic decrease in survival in patients with more than 3 liver metastases.
Conclusions
Simultaneous resection of colorectal liver metastases at the time of CRS-HIPEC for peritoneal metastases may be associated with worse survival, especially in patients with more than 3 liver metastases.
Keywords: Colorectal cancer, Metastases, Cytoreductive surgery, HIPEC
INTRODUCTION
The peritoneum is the third most common site of colorectal metastases, following hepatic and pulmonary sites of dissemination.[1–6] A prospective analysis of 32 phase II/III NCCTG clinical trials of patients with metastatic colorectal cancer receiving “non-modern-era” systemic chemotherapy demonstrated median survival of 9–12 months and 5 year survival of 1.1%.[7] A significant improvement in median survival (15–30 months) has been achieved with the addition of “modern-era” chemotherapy and targeted agents. However, 5 year survival remains low (~10%), even with “modern-era” chemotherapy regimens, predominantly related to low complete response rates (~5%).[8–12]
The addition of surgical resection to systemic chemotherapy has been advocated to improve long-term survival in a larger subset of well-selected patients with colorectal metastases by achieving “mechanical complete response”. The success of this combined approach has been demonstrated in well-selected patients with isolated colorectal liver or lung metastases, with 5 year survival rates of 30–40% and 20–30%, respectively.[13, 14] This rationale has also been applied to patients with colorectal peritoneal metastases. A randomized controlled trial, a well-matched case-control study and numerous institutional case series have demonstrated improved median survival (22–63 months) and 5-year survival (20–51%) following cytoreductive surgery-hyperthermic intraperitoneal chemoperfusion (CRS-HIPEC) in well-selected patients with isolated colorectal peritoneal metastases.[15–20] However, certain prognostic factors have been attributed to poor survival following CRS-HIPEC, including the presence of synchronous liver metastases.[19, 21–23]
Combined surgical resection of liver metastases at the time of CRS-HIPEC for colorectal peritoneal metastases is controversial.[24] Maggiori and colleagues published a prospective, matched case-control study comparing CRS-HIPEC in patients with colorectal peritoneal metastases with and without synchronous liver metastases. Although they demonstrated worse survival in patients with synchronous liver disease, patients with < 3 liver lesions and limited peritoneal disease (peritoneal cancer index < 12) demonstrated 40 month median survival and 3 year survival over 60%, suggesting that long-term survival could be achieved in appropriately selected patients.[25]
We evaluated perioperative and oncologic outcomes following CRS-HIPEC for concurrent colorectal peritoneal and liver metastases in selected patients at a high-volume center.
MATERIALS AND METHODS
We performed a retrospective review of a prospectively maintained database of patients undergoing curative CRS-HIPEC for colorectal peritoneal metastases between 2005 and 2013. We identified a subgroup of patients with synchronous colorectal liver and peritoneal metastases (CRLM group; n=32) who underwent concurrent liver resection and/or radiofrequency ablation (RFA) at the time of CRS-HIPEC. We compared the CRLM group to the larger subset of patients undergoing CRS-HIPEC for colorectal peritoneal metastases alone (CRPM group; n=173). This study was approved by the Institutional Review Board at the University of Pittsburgh.
Patients were excluded from undergoing CRS-HIPEC if they had extra-abdominal metastatic disease, poor performance status (ECOG 3–5), unresectable disease on preoperative imaging or intraoperative assessment and “significant” (as opposed to “limited”) progressive disease while on preoperative systemic chemotherapy (disease progression on imaging was defined as any increase in disease burden reported by the radiologist reviewing the imaging, while the distinction between “limited” versus “significant” disease progression was determined by the clinical judgement of the multidisciplinary team managing the patient based on whether the degree of progression was enough to preclude surgery). All patients in the CRLM group had pathologically confirmed parenchymal metastases; we excluded patients with extrinsic disease invading the liver or capsular disease only. Intraoperatively, volume of peritoneal disease was quantified by the peritoneal carcinomatosis index (PCI).[26] Cytoreductive Surgery (CRS) was performed in accordance with techniques described by Bao and Bartlett to achieve CC-0 (no residual macroscopic disease) or CC-1 (residual tumor nodule < 2.5 mm) resection.[27] Patients undergoing ≥ 3 organ resections or ≥ 2 visceral anastomoses were defined as having “extensive CRS” as opposed to “limited CRS.” A standard institutional protocol for HIPEC was initiated after CRS, with the closed technique and target intraperitoneal tissue temperature of 42°C. Mitomycin C 30 mg was added to the perfusate initially for 60 minutes followed by an additional 10 mg for a further 40 minutes. Postoperative morbidity was classified according to the Dindo-Clavien grading system.[28] For the purpose of analysis, grades 3–5 were considered major complications.
Statistical Analysis
Clinicopathologic, perioperative and oncologic outcomes between CRLM and CRPM groups were examined using Wilcoxon two-sample test or Fisher’s exact test when appropriate. Overall survival (OS) was calculated from the date of diagnosis of peritoneal metastases and also the date of surgery to the date of death. For patients presumably still alive at the time of analysis, follow-up was censored as of the date of last contact. Progression free survival (PFS) was calculated from the date of diagnosis of peritoneal metastases and also the date of surgery to the date of recurrence or progression or death. Kaplan-Meier method was used to estimate the survival distributions and Log-rank test was used to assess the difference. Survival function estimates and comparisons were also adjusted by propensity score weighting method to account for differences in confounding variables between the CRLM and CRPM groups. Variables in the propensity-scored model included, in order or importance, intraoperative PCI, pre-operative BMI and gender. The relationship of overall survival to patients’ characteristics was further assessed by Cox proportional hazards regression. The corresponding relative mortality rates are summarized as hazard ratios (HR), with HR > 1.0 corresponding to increased mortality. A significance level was set at 0.05 and all P values reported were two-sided. Statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC, USA).
RESULTS
CRLM Group
Thirty-two patients underwent CRS-HIPEC and concurrent liver resection and/or RFA for synchronous colorectal liver and peritoneal metastases. (Table 1) The majority of patients had a single liver metastasis (53.3%). Surgical treatments included surgical resection alone in 24 patients (75%), RFA alone in 6 patients (19%), and combined liver resection and RFA in 2 patients (6%). Among the patients who underwent liver resection, 69.2% had a non-anatomic wedge resection, 26.9% had a formal segmentectomy, and one patient had a bi-segmentectomy. Preoperative systemic chemotherapy was administered to 31 patients (96.9%); 48.4% had a partial response to therapy, 12.9% had stable disease and 35.5% had “limited” disease progression by radiographic imaging. KRAS mutations were identified in 31.3% of patients. Most common systemic therapy regimens consisted of 5-fluorouracil with oxaliplatin or irinotecan (80.7%), bevacizumab (67.7%) and cetuximab (9.7%). The median duration of preoperative chemotherapy was 6 months (range 3–12 months). There were no liver-specific post-operative complications including bile leaks and liver failure. There was one 60-day mortality (3.2%) as a result of post-operative sepsis and acute respiratory distress syndrome. Postoperative systemic chemotherapy was administered to 16 out of 23 patients (69.6%) in whom data was available. After a median follow-up of 60.9 months from surgery, seven patients (22.6%) developed liver recurrence, demonstrating a liver progression-free survival of 9.9 months.
Table 1.
CRLM Group: Clinical, Operative and Post-Operative Characteristics (n=32)
| Number of liver metastases; n (%)(n=30) | 1 lesion | 16 (53.3) |
| 2 lesions | 7 (23.3) | |
| ≥ 3 lesions | 7 (23.3) | |
| Diameter of largest liver lesion-cm; Median (range) | 2 (0.8–5.6) | |
| Distribution liver metastases; n (%) | Right lobe | 13 (40.6) |
| Left lobe | 13 (40.6) | |
| Bilobar | 6 (18.8) | |
| Extent liver resection; n (%) | RFA only | 6 (18.8) |
| Liver resection alone | 24 (75) | |
| Non-anatomic wedge resection | 18 (69.2) | |
| Segmentectomy | 7 (26.9) | |
| Bi-segmentectomy | 1 (3.9) | |
| Post-operative liver complications, n (%) | Bile leak | 0 |
| Liver failure | 0 | |
| Liver recurrence; n (%)(n=31) | 7 (22.6) | |
| Liver recurrence-free survival; months (n=31) | 9.9 [95% CI (1.58, 17.03)] | |
CRLM: colorectal liver and peritoneal metastases group; RFA: radiofrequency ablation
Comparison of CRLM and CRPM Groups
Patients in the CRLM group were statistically more likely to be male, have a higher preoperative BMI, higher PCI, more frequent ICU readmission, have a longer operative time and undergo “extensive CRS”. (Table 2) Major postoperative complications (Clavien-Dindo grades 3–5) occurred in 32% (CRLM group) and 17% (CRPM group) of patients (p=0.08). Moreover, cardiac complications and renal complications were statistically more common in the CRLM group than in CRPM group. There no difference between the two groups when comparing rates of complete cytoreduction (CC-0/1), postoperative bleeding, poorly-differentiated histology/high-grade disease, administration of chemotherapy, need for re-operation, and 30- or 60-day mortality. (Table 2)
Table 2.
Comparison of CRLM and CRPM Groups: Clinicopathologic and Perioperative Characteristics; Oncologic Outcomes
| CRLM (n=32) | CRPM (n=173) | p-value | |
|---|---|---|---|
| Demographics | |||
| Age at surgery- years; mean (SD) | 53.3 (12.9) | 54.6 (11.9) | 0.67 |
| Male gender; n (%) | 22 (68.8) | 78 (45.1) | 0.02 |
| Caucasian race; n (%) | 29 (93.6) (n=31) | 159 (91.9) | 0.4 |
| Pre-operative BMI- kg/m2; mean (SD) | 29.4 (5.9) | 26.9 (5.9) | 0.02 |
| ASA Score ≥ 3; n (%) | 19 (61.3) (n=31) | 101 (67.3) (n=150) | 0.11 |
| Tumor Characteristics | |||
| PCI Score; Mean (SD) | 13.7 (5.7) | 11.2 (5.9) | 0.03 |
| Mucinous histology; n (%) | 11 (42.3) (n=26) | 97 (60.6) (n=160) | 0.09 |
| Poorly differentiated; n (%) | 14 (48.3) (n=29) | 55 (38.2) (n=144) | 0.58 |
| High grade; n (%) | 13 (59.1) (n=22) | 55 (49.1) (n=112) | 0.60 |
| Operative Characteristics | |||
| EBL- ml; Mean (SD) | 618.8 (349.8) | 671.8 (593.1) | 0.58 |
| CC-score; n (%) | CC-0: 27 (84.4) CC-1: 5 (15.6) |
CC-0: 146 (84.4) CC-1: 27 (15.6) |
1.0 |
| Operative time- minutes mean (SD) | 520.9 (143.2) | 470.8 (138.2) | 0.05 |
| “Extensive CRS”; n (%) | 25 (78.1) | 71 (41) | 0.0002 |
| Postoperative Characteristics | |||
| Grade 3–5 Dindo-Clavien complication; n (%) | 10 (32.3) (n=31) | 26 (16.7) (n=156) | 0.08 |
| Hospital Length of Stay-days; mean (SD) | 16.0 (8.6) | 17.2 (25.6) | 0.07 |
| ICU length of stay- days; mean (SD) | 3.4 (7.6) | 2.3 (1.6) | 0.30 |
| ICU readmission rate; n (%) | 7 (21.9) | 14 (8.1) (n=172) | 0.03 |
| 30-day hospital readmission rate; n (%) | 6 (23.1) (n=26) | 43 (29.9) (n=144) | 0.64 |
| Reoperation rate; n (%) | 3 (9.4) | 20 (11.8) (n=169) | 1.0 |
| 30-day mortality; n (%) | 0 (n=29) | 1 (0.6) (n=161) | 1.0 |
| 60-day mortality; n (%) | 1 (3.5) (n=29) | 2 (1.2) (n=162) | 0.39 |
| Adjuvant chemotherapy; n (%) | 15 (68.2) (n=22) | 68 (63.6) (n=107) | 0.81 |
CRLM: colorectal liver and peritoneal metastases group; CRPM: colorectal peritoneal metastases group; n: number of patients; SD: standard deviation; BMI: body mass index; PCI: peritoneal cancer index; EBL: estimated blood loss; CC-score: completion of cytoreduction score; CRS: cytoreductive surgery; ICU: intensive care unit
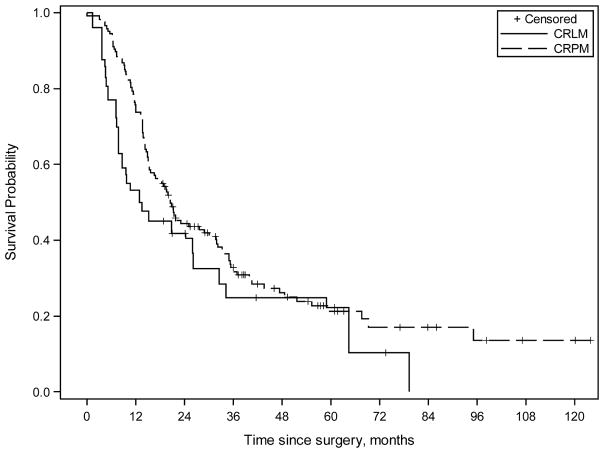
The estimated median follow-up calculated from surgery for the CRLM and CRPM groups was 60.9 months and 56.8 months, respectively. There was no difference in median progression-free survival (PFS) calculated from surgery between the CRLM and CRPM groups (8.2 vs. 7.6 months, p=0.9). Following propensity score adjustment, median PFS calculated from surgery for the CRLM and CRPM groups was 5.1 and 7.6 months, respectively (p=0.53). (Figure 1) The propensity score adjusted median PFS calculated form the date of diagnosis of peritoneal metastases for the CRLM and CRPM groups was 14.6 and 14.8 months, respectively (p=0.88). (Figure 2)
Figure 1.
Kaplan-Meier survival curves (adjusted results by using propensity score weighting method) comparing combined colorectal liver and peritoneal metastases group (CRLM) and colorectal peritoneal metastases group (CRPM) treated with multimodality therapy including systemic chemotherapy and cytoreductive surgery with hyperthermic intraperitoneal chemoperfusion. Propensity score adjusted estimated median progression-free survival (PFS) calculated from surgery was 5.1 months [95% CI 2.5, 9.1] for the CRLM group and 7.6 months [95% CI 6.1, 9.0] for the CRPM group (p=0.53).
Figure 2.
Kaplan-Meier survival curves (adjusted results by using propensity score weighting method) comparing combined colorectal liver and peritoneal metastases group (CRLM) and colorectal peritoneal metastases group (CRPM) treated with multimodality therapy including systemic chemotherapy and cytoreductive surgery with hyperthermic intraperitoneal chemoperfusion. Propensity score adjusted estimated median progression-free survival (PFS) calculated form the date of diagnosis of peritoneal metastases was 14.6 months [95% CI 8.4, 19.3] for the CRLM group and 14.8 months [95% CI 13.0, 18.8] for the CRPM group (p=0.88).
There median overall survival (OS) calculated from surgery for the CRLM and CRPM groups was 15.3 and 21.4 months, respectively (p=0.17) prior to propensity score adjustment, and 13 and 20.5 months following propensity score adjustment (p=0.16). (Figure 3) The propensity score adjusted survival probability at 3- and 5-years following surgery in the CRLM group was 24.8% and 22.2%, respectively. The propensity score adjusted median OS calculated form the date of diagnosis of peritoneal metastases for the CRLM and CRPM groups was 32.5 and 36.2 months (p=0.21), respectively. (Figure 4)
Figure 3.
Kaplan-Meier survival curves (adjusted results by using propensity score weighting method) comparing combined colorectal liver and peritoneal metastases group (CRLM) and colorectal peritoneal metastases group (CRPM) treated with multimodality therapy including systemic chemotherapy and cytoreductive surgery with hyperthermic intraperitoneal chemoperfusion. Propensity score adjusted estimated median overall survival (OS) calculated from surgery was 13 months [95% CI 7.4, 32.5] for the CRLM group and 20.5 months [95% CI 15.3, 30.1] for the CRPM group (p=0.16).
Figure 4.
Kaplan-Meier survival curves (adjusted results by using propensity score weighting method) comparing combined colorectal liver and peritoneal metastases group (CRLM) and colorectal peritoneal metastases group (CRPM) treated with multimodality therapy including systemic chemotherapy and cytoreductive surgery with hyperthermic intraperitoneal chemoperfusion. Propensity score adjusted estimated median overall survival (OS) calculated form the date of diagnosis of peritoneal metastases was 32.5 months [95% CI 15.0, 41.7] for the CRLM group and 36.2 months [95% CI 27.4, 43.6] for the CRPM group (p=0.21).
In a multivariate Cox-regression analysis of combined CRLM and CRPM groups, the presence of liver metastases was not a significant predictor of survival in either univariate or multivariate analysis. Subsequent multivariate Cox-regression analysis of the CRLM group alone demonstrated number of liver metastases to be the only independent predictor of poor survival (HR 2.3, p=0.03). (Table 3) The unadjusted estimated median OS and survival probability (by Kaplan-Meier method) were dramatically lower in patients with more than 3 liver metastases. (Table 4)
Table 3.
Multivariate analysis: Predictors of Survival in CRLM Group
| Variable | P-value | Hazard | 95% Confidence Limits | |
|---|---|---|---|---|
| Ratio | Lower | Upper | ||
| CC score- 1 vs. 0 | 0.4280 | 2.046 | 0.349 | 12.006 |
| “Extensive CRS”- Yes vs. No | 0.3282 | 2.279 | 0.437 | 11.887 |
| Moderately-poorly differentiated and/or high-grade vs. well differentiated and low-grade | 0.1288 | 4.468 | 0.647 | 30.835 |
| Age at surgery (per 5 unit increase) | 0.1638 | 1.224 | 0.921 | 1.625 |
| Number of liver metastases (per unit increase) | 0.0266 | 2.281 | 1.1 | 4.728 |
CRLM: colorectal liver and peritoneal metastases group; CC-score: completion of cytoreduction score; CRS: cytoreductive surgery
Table 4.
A. Estimated overall survival median (unadjusted; by Kaplan-Meier method) and B. Estimated survival probability by number of liver metastases; Calculated from surgery
| A. | ||||
|---|---|---|---|---|
| Number of liver metastases | Frequency (n) | Estimated Survival Median-months | 95% Confidence Interval | |
| Lower | Upper | |||
| <=3 | 27 | 20.7781 | 9.5671 | 34.2575 |
| > 3 | 3 | 5.1945 | 1.3151 | 26.137 |
| B. | ||||||
|---|---|---|---|---|---|---|
| Estimated survival probability (%) | Number of liver metastases | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 1 year | 0.91 | 0.81 | 0.63 | 0.37 | 0.14 | 0.02 |
| 3 years | 0.88 | 0.76 | 0.56 | 0.30 | 0.09 | 0.01 |
| 5 years | 0.85 | 0.71 | 0.48 | 0.22 | 0.05 | 0.00 |
The median time-interval from diagnosis of peritoneal metastases until CRS-HIPEC was 6 months for the CRPM group and 8 months for the CRLM group, and median time-interval from diagnosis of liver metastases until CRS-HIPEC was 3 months. Only 46 patients (25%) in the CRPM cohort underwent CRS-HIPEC within 3 months of diagnosis of PM and the median OS from surgery for this subgroup was 30.1 months (95% CI 18.4, 47.4 months). Similarly, in the CRLM cohort, only 12 patients (37%) underwent CRS-HIPEC within 6 months of diagnosis of PM (five patients within 3 months), and the median OS from surgery for this subgroup was 24.3 months (95% CI 4.8, 64.5 months).
DISCUSSION
Surgical resection and regional therapies, in combination with systemic chemotherapy, are now considered potentially curative therapeutic options for well-selected patients with isolated hepatic or pulmonary metastases from colorectal cancer.[13, 14] This approach has also been advocated for well-selected patients with colorectal peritoneal metastases, based on one randomized controlled trial and numerous single- and multi-institutional case series.[15, 16, 18–20, 23] However, the role for CRS-HIPEC in patients with combined hepatic and peritoneal metastases is less clear. In this study we report median survival (calculated from CRS-HIPEC) of 13 and 21 months for CRLM and CRPM subgroups respectively, and median survival (calculated from diagnosis of peritoneal metastases) of 32.5 and 36.2 months for CRLM and CRPM subgroups respectively. These data are consistent with our previously reported data; (a) Franko et al. (2008) compared outcomes following CRS-HIPEC in patients with CRPM undergoing multivisceral resection (≥ 2 organs resected) or not (<2 organs resected), demonstrating overall survival (calculated from CRS-HIPEC) of 15 months (multivisceral resection: 20 months vs. non-multivisceral resection: 14 months); and (b) Franko et al. (2010) subsequently compared survival in patients undergoing CRS-HIPEC for CRPM to those receiving systemic chemotherapy alone, reporting median survival of 35 months in the surgery group (calculated from diagnosis of peritoneal metastases).[18, 29] Moreover, in a multivariate Cox proportional hazards model of CRLM group, including completeness of resection, tumor differentiation/grade, extent of CRS and patient age, number of liver metastases was the only significant independent predictor of poor survival (HR 2.3) in this study.
For the CRLM group in this study, we only included patients with intraparenchymal hepatic metastases (systemic dissemination) and excluded those with extrinsic disease invading the liver or capsular disease only (locoregional dissemination), since the biology and oncologic consequences of these two mechanisms for metastases are inherently different. The propensity-score adjusted overall survival calculated from the time of surgery in the CRLM group in our study was 13 months. While survival for the CRLM group in this study was lower than that reported in four recently published studies of combined resection of liver and peritoneal metastases (23–36 months), the retrospective nature of these studies makes direct comparisons difficult due to inherent differences in patient selection.[25, 30–32] Similarly, while the overall survival of our CRPM group was also lower (20.5 months) compared to these published series (15.8–49 months), it is similar to other studies, including Verwaal’s randomized control trial (22 months) and the multi-institutional case series reported by Glehen and colleagues (19 months).[19, 20] There are a number of potential reasons for differences in overall survival between studies, including variability in the distribution and extent of hepatic and peritoneal metastases, as well as lead-time bias associated with diagnosis of metastatic disease. The time-interval from diagnosis of peritoneal or liver metastases until surgery as well as the frequency and duration of preoperative systemic chemotherapy is unclear in most of the published series. In our study, median time-interval from diagnosis of peritoneal metastases until CRS-HIPEC was 6 months for the CRPM group and 8 months for the CRLM group, suggesting a relatively long period of non-surgical therapy prior to CRS-HIPEC in our patient population. Differences in duration of preoperative systemic chemotherapy could explain the lower overall survival in our patients when calculated from the time of surgery and similar overall survival when calculated from the time of diagnosis of peritoneal metastases, when comparing our study to other series. For example, only 46 patients in the CRPM cohort in our study underwent CRS-HIPEC within 3 months of diagnosis of PM and their median OS was 30.1 months, which is comparable to inclusion criteria and oncologic outcomes reported in published studies demonstrating better survival than our data. Similarly, only 12 patients in the CRLM cohort underwent CRS-HIPEC within 6 months of diagnosis of PM (five patients within 3 months), and the median OS for this subgroup was 24.3 months. This survival data is again more in-line with previously published studies of CRLM, and suggests differences in patient selection as a major determinant of survival disparity.
Despite propensity score adjustment, our data demonstrate worse, but statistically non-significant, PFS and OS in the CRLM group compared to the CRPM group. This is similar to data from the matched case-control study published by Maggiori and colleagues demonstrating a statistically significant decrease in survival following combined resection of liver and peritoneal metastases compared to resection of peritoneal metastases alone.[25] Based on our multivariate model, number of liver metastases was a significant independent negative prognostic factor for survival in the CRLM group. We found that the survival probability for patients with > 3 liver metastases was especially poor, suggesting a potential inflection point at which CRS-HIPEC may be contraindicated. Although our data would suggest that patients with more than 3 liver metastases may not benefit from this aggressive approach, given the small number of patients having > 3 liver metastases and the retrospective nature of this study we cannot draw any conclusive correlation between number of liver metastases and survival. Moreover, our study was not designed to provide an absolute cut-off for surgical decision making, rather our data provide an exploratory direction and magnitude of hazard ratios for each unit increase in liver lesions. We believe that the number of tumor metastases is a potential surrogate for a variety of poor prognostic factors including disease burden, ability to achieve complete resection and tumor biology, however the small sample size of our study precludes such granular analysis. Of note, in the combined cohort of CRPM and CRLM in our study, multivariate analysis did not demonstrate the presence of liver metastases to be an independent predictor of poor survival, supporting the fact that the mere presence of concurrent liver and peritoneal metastases should not be an absolute contraindication to CRS-HIPEC.
In our study, we found a higher rate of Dindo-Clavien grade ≥ 3 morbidity in the CRLM group however we feel that this was less likely to be related to the additional liver resection and more likely related to baseline differences in the groups including higher PCI, longer operative time and more “extensive CRS” in the CRLM group. Moreover, we found no correlation between PCI and the number of liver metastases and no liver-specific complications to suggest that the addition of liver resections contributed to excessive morbidity. On the other hand, Maggiori et al. reported a postoperative mortality rate of 8% in their combined liver and peritoneal cohort, major postoperative complication rate (Dindo-Clavien grade ≥ 3) of 51% and 5 patients with liver-specific complications.[25] There were important differences that probably contributed to the high complications in their study; they had more liver metastases (median 2; range 1–16) and they performed 12 major hepatectomies, compared to none in our study. Although they reported no statistically significant difference in the rate of major complications (Dindo-Clavien grade ≥ 3) between patients undergoing major versus nonmajor hepatectomies (67% vs. 44%; p=0.295), the clinical significance of this difference is quite real and may not have reached statistical threshold due to the small sample size. Similarly, when comparing their CRLM and CRPM groups, mortality rate was statistically higher (8% vs. 0%; p=0.051) and there was a strong clinical trend towards higher major complications (51% vs. 39%; p=0.246) in the CRLM group. This data should caution against performing major liver resections in combination with CRS-HIPEC.
While systemic chemotherapy alone for treatment of metastatic colorectal cancer demonstrates good median survival with the use of modern multidrug, sequential regimens, long-term survival remains low (~5–10% 5 year survival).[7–10, 12] This is highlighted by a recent pooled analysis of two systemic chemotherapy trials (NCCTG N9741 and NCCTG N9841) of metastatic colorectal cancer in which post hoc comparison between subgroups with and without peritoneal metastases demonstrated 5-year survival of 6% (no peritoneal metastases) and 4.1% (with peritoneal metastases) despite median survival of 17.6 months (no peritoneal metastases) and 12.7 months (with peritoneal metastases).[33] Patients in both groups in this study received multimodality therapy including systemic chemotherapy, surgical resection and regional chemoperfusion. While the median survival for patients with CRPM and CRLM was not much better than that reported in clinical trials of systemic chemotherapy alone, our data demonstrate 3- and, 5-year propensity score adjusted overall survival probability calculated from surgery in the CRLM group of 24.8% and 22.2% respectively, which is higher than the published long-term survival from systemic chemotherapy alone. This suggests that the addition of CRS-HIPEC to a multimodality approach may provide long-term survival in a larger number of patients when compared to systemic chemotherapy alone.
Limitations in our study include the small sample size in the CRLM group and non-randomization of the two comparative groups. We also recognize that our findings are not generalizable to all patients with concurrent colorectal liver and peritoneal metastases since our cohort included patients with limited hepatic disease requiring no more than segmental resections. Additionally, patients in the CRLM and CRPM groups were not formally matched however propensity score adjustment was performed to mitigate bias associated with baseline differences between the groups. Finally, we can only infer any potential benefit from the addition of CRS-HIPEC to multimodality therapy since direct comparison to a chemotherapy alone arm was not performed.
CONCLUSIONS
We found that the presence of liver metastases was not an independent predictor of poor survival when the CRLM and CRPM groups were analyzed together. Simultaneous resection of colorectal liver metastases at the time of CRS-HIPEC for peritoneal metastases may be associated with worse survival, especially in patients with more than 3 liver metastases.
Acknowledgments
We would like to acknowledge Sam Pakraftar and Sara Alhelo for database support.
Sources of support: Dr. Downs-Canner is supported by grant number T32CA113263 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This work was also partially funded by generous support from Valarie Koch and the New Era Cap Company.
Footnotes
Disclosure of conflicts: None
Conceived and designed the study: SDC, HAC, DLB; Data Collection: HLJ, LR, Analyzed the data: HAC, YS; Wrote the paper: SDC, HAC, MPH, SAA, JFP, DLB, HJZ.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elias D, Honore C, Dumont F, Ducreux M, Boige V, Malka D, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Annals of surgery. 2011;254(2):289–93. doi: 10.1097/SLA.0b013e31822638f6. [DOI] [PubMed] [Google Scholar]
- 2.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. The British journal of surgery. 2002;89(12):1545–50. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 3.Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Annals of surgery. 2006;243(2):212–22. doi: 10.1097/01.sla.0000197702.46394.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludeman L, Shepherd NA. Serosal involvement in gastrointestinal cancer: its assessment and significance. Histopathology. 2005;47(2):123–31. doi: 10.1111/j.1365-2559.2005.02189.x. [DOI] [PubMed] [Google Scholar]
- 5.Mohan HM, O’Connor DB, O’Riordan JM, Winter DC. Prognostic significance of detection of microscopic peritoneal disease in colorectal cancer: a systematic review. Surgical oncology. 2013;22(2):e1–6. doi: 10.1016/j.suronc.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358–63. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 7.Dy GK, Hobday TJ, Nelson G, Windschitl HE, O’Connell MJ, Alberts SR, et al. Long-term survivors of metastatic colorectal cancer treated with systemic chemotherapy alone: a North Central Cancer Treatment Group review of 3811 patients, N0144. Clinical colorectal cancer. 2009;8(2):88–93. doi: 10.3816/CCC.2009.n.014. [DOI] [PubMed] [Google Scholar]
- 8.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 9.Golfinopoulos V, Salanti G, Pavlidis N, Ioannidis JP. Survival and disease-progression benefits with treatment regimens for advanced colorectal cancer: a meta-analysis. The Lancet Oncology. 2007;8(10):898–911. doi: 10.1016/S1470-2045(07)70281-4. [DOI] [PubMed] [Google Scholar]
- 10.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. The Lancet Oncology. 2014;15(10):1065–75. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 11.Dy GK, Krook JE, Green EM, Sargent DJ, Delaunoit T, Morton RF, et al. Impact of complete response to chemotherapy on overall survival in advanced colorectal cancer: results from Intergroup N9741. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(23):3469–74. doi: 10.1200/JCO.2007.10.7128. [DOI] [PubMed] [Google Scholar]
- 12.Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(21):2240–7. doi: 10.1200/JCO.2013.53.2473. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Kye BH, Lee JI, Lee SC, Lee YS, Lee IK, et al. Surgical resection for lung metastases from colorectal cancer. Journal of the Korean Society of Coloproctology. 2010;26(5):354–8. doi: 10.3393/jksc.2010.26.5.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25(29):4575–80. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 15.Cao C, Yan TD, Black D, Morris DL. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Annals of surgical oncology. 2009;16(8):2152–65. doi: 10.1245/s10434-009-0487-4. [DOI] [PubMed] [Google Scholar]
- 16.Chua TC, Morris DL, Saxena A, Esquivel J, Liauw W, Doerfer J, et al. Influence of modern systemic therapies as adjunct to cytoreduction and perioperative intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis: a multicenter study. Annals of surgical oncology. 2011;18(6):1560–7. doi: 10.1245/s10434-010-1522-1. [DOI] [PubMed] [Google Scholar]
- 17.Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(5):681–5. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 18.Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ., 3rd Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116(16):3756–62. doi: 10.1002/cncr.25116. [DOI] [PubMed] [Google Scholar]
- 19.Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22(16):3284–92. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Annals of surgical oncology. 2008;15(9):2426–32. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 21.Cavaliere F, De Simone M, Virzi S, Deraco M, Rossi CR, Garofalo A, et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2011;37(2):148–54. doi: 10.1016/j.ejso.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 22.da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. Journal of the American College of Surgeons. 2006;203(6):878–86. doi: 10.1016/j.jamcollsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(1):63–8. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 24.de Cuba EM, Kwakman R, Knol DL, Bonjer HJ, Meijer GA, Te Velde EA. Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases: Systematic review of all literature and meta-analysis of observational studies. Cancer treatment reviews. 2013;39(4):321–7. doi: 10.1016/j.ctrv.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Maggiori L, Goere D, Viana B, Tzanis D, Dumont F, Honore C, et al. Should Patients With Peritoneal Carcinomatosis of Colorectal Origin With Synchronous Liver Metastases Be Treated With a Curative Intent? A Case-Control Study. Annals of surgery. 2013;258(1):116–21. doi: 10.1097/SLA.0b013e3182778089. [DOI] [PubMed] [Google Scholar]
- 26.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer treatment and research. 1996;82:359–74. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 27.Bao P, Bartlett D. Surgical techniques in visceral resection and peritonectomy procedures. Cancer journal. 2009;15(3):204–11. doi: 10.1097/PPO.0b013e3181a9c6f0. [DOI] [PubMed] [Google Scholar]
- 28.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Annals of surgery. 2009;250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 29.Franko J, Gusani NJ, Holtzman MP, Ahrendt SA, Jones HL, Zeh HJ, 3rd, et al. Multivisceral resection does not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Annals of surgical oncology. 2008;15(11):3065–72. doi: 10.1245/s10434-008-0105-x. [DOI] [PubMed] [Google Scholar]
- 30.Chua TC, Yan TD, Zhao J, Morris DL. Peritoneal carcinomatosis and liver metastases from colorectal cancer treated with cytoreductive surgery perioperative intraperitoneal chemotherapy and liver resection. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2009;35(12):1299–305. doi: 10.1016/j.ejso.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 31.Kianmanesh R, Scaringi S, Sabate JM, Castel B, Pons-Kerjean N, Coffin B, et al. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Annals of surgery. 2007;245(4):597–603. doi: 10.1097/01.sla.0000255561.87771.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varban O, Levine EA, Stewart JH, McCoy TP, Shen P. Outcomes associated with cytoreductive surgery and intraperitoneal hyperthermic chemotherapy in colorectal cancer patients with peritoneal surface disease and hepatic metastases. Cancer. 2009;115(15):3427–36. doi: 10.1002/cncr.24385. [DOI] [PubMed] [Google Scholar]
- 33.Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(3):263–7. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]