Abstract
Objective
To measure carotid artery intima-media thickness (CIMT), a marker of subclinical atherosclerosis, in postmenopausal women with and without histories of preeclampsia, and to synthesize these results with those from prior studies of CIMT performed ≥10 years after preeclamptic pregnancies.
Patients and Methods
Forty women (median age 59 years) with histories of preeclampsia and 40 with histories of normotensive pregnancy (confirmed by medical record review), were selected from women who resided and delivered (1976–1982) in Olmsted County, MN. The participants were identified and recruited in 2014–2015 and CIMT was measured by B-mode ultrasound. Meta-analysis included CIMT studies that were performed ≥10 years after preeclamptic pregnancies, and which were identified through PubMED, EMBASE and Web of Science. Heterogeneity was assessed using the I2 statistic. Standardized mean difference was used as a measure of effect size.
Results
CIMT, expressed as a median (25th, 75th percentile), was greater in the preeclamptic compared to normotensive group, 0.80 mm (0.75, 0.85) versus 0.73 mm (0.70, 0.78), P=.004; the odds of having CIMT higher than threshold (0.77) was statistically significant after adjusting for confounding factors, OR 3.17 (95% CI: 1.10, 9.14). A meta-analysis of 10 studies conducted ≥10 years postpartum included 813 women with and 2,874 without histories of preeclampsia. CIMT was greater among women with histories of preeclampsia, with a standardized mean difference of 0.18, and 95% confidence interval of 0.05–0.30, P=.004.
Conclusion
Among women with histories of preeclampsia, CIMT may identify those with subclinical atherosclerosis, thus offering an opportunity for early intervention.
Keywords: cardiovascular risk, hypertensive pregnancy disorders, carotid intima-media thickness, preeclampsia
Introduction
Preeclampsia is a complex, multi-system, hypertensive pregnancy disorder traditionally defined as new-onset hypertension (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg) with proteinuria (≥300 mg/24 hours) after 20 weeks of gestation.1 Preeclampsia affects 2–7% of all pregnancies and not only increases the risk for both maternal and fetal morbidity and mortality,2 but also represents a risk factor for future hypertension, ischemic heart disease, stroke, and premature cardiovascular death in women.3 Factors contributing to the increased risk reflect a constellation of cardiometabolic parameters that may exist prior to pregnancy or occur during the pregnancy and persist thereafter,3 subsequently leading to asymptomatic atherosclerosis first, and clinical cardiovascular disease (CVD) events years to decades later.
Carotid artery intima-media thickness (CIMT) is a sex- and age-dependent measure of subclinical atherosclerosis4,5 that is evaluated using non-invasive, high-resolution ultrasound-based imaging of the combined thickness of the intima-media complex of the arterial wall.6 A recent systematic review of randomized controlled trials has suggested that CIMT may be a valid surrogate end point for cardiovascular events.7,8 Studies of the role of CIMT provide conflicting evidence regarding the association between preeclampsia and subclinical atherosclerosis. Disagreements as to the impact of preeclampsia on subclinical atherosclerosis, as defined by CIMT, may be due to the variety of CIMT methodologies used, incomplete or inaccurate classification of preeclampsia, and potentially further influenced by the paucity of postmenopausal data. The present study was designed to test the hypothesis that preeclampsia, as confirmed by chart review using accepted clinical criteria, is an independent risk factor for subclinical atherosclerosis, as defined by CIMT, among postmenopausal women. Given the limited and discrepant data as to the association between having a history of preeclampsia and CIMT, we also performed a meta-analysis incorporating prior studies that explored this association ≥ 10 years after the affected pregnancies.
METHODS
Study Design and Participants
This study was approved by the Institutional Review Boards at Mayo Clinic and Olmsted County Hospital, Rochester, MN. The Rochester Epidemiology Project (REP) medical records-linkage system9 was used to identify study subjects. The REP medical records-linkage system was established in 1966 to capture all health care information for the entire population of Olmsted County, MN. Details of the identification process and inclusion and exclusion criteria for our study participants have been reported previously.10 Briefly, study participants were recruited from a birth cohort consisting of women residents of Olmsted County, MN who delivered from a pregnancy lasting > 20 weeks (live or stillbirth) between January 1, 1976 and December 31, 1982. The medical records of women identified by Hospital International Classification of Diseases Adapted (HICDA) codes that might be indicative of a possible hypertensive pregnancy disorder were fully abstracted for demographic, socioeconomic, and clinical information at the time of each pregnancy. The current study group consisted of 40 consecutive women with histories of preeclampsia who fulfilled the inclusion criteria (no previous history of cardiovascular events and no current or previous diagnosis of cancer, except non-melanomatous skin cancer), and had no exclusionary criteria (a BMI >35 and ever smoking more than 100 cigarettes). The control group consisted of age- and parity-matched women (n=40) with histories of normotensive pregnancies. A history of preeclampsia was confirmed based on the standard definition:11 1) two or more blood pressure readings of a systolic blood pressure (SBP) > 140 mm Hg and/or a diastolic blood pressure (DBP) >90 mm Hg, taken at least 4 hours apart, after 20 weeks gestation, and 2) new onset proteinuria, as defined by a urine dipstick 1+, or proteinuria ≥300 mg per 24 hour urine, or a protein/creatinine ratio equivalent to ≥ 300 mg per 24 hours. Emergency room visits were not included in the assessment. As the primary focus of the study was to understand the potential preclinical vascular damage and mechanisms that place women with histories of preeclampsia at risk for subsequent CVD, all women with a medical record confirmed clinical diagnosis of previous CVD events, such as myocardial infarction, congestive heart failure, dysrhythmias, and stroke, were excluded. The participants were identified and recruited between April 1, 2014 and May 4, 2015. All participants gave written informed consent. They underwent physical exams, blood collections, and CIMT measurements at the time of their study visits.
Traditional Risk Factors
The diagnosis of hypertension was confirmed if a prior diagnosis and/or use of prescription anti-hypertensive medication were confirmed upon medical record review, or if a SBP≥ 140 mm Hg and/or DBP≥ 90 mm Hg was documented in the medical records on 2 separate occasions. Smoking was defined as never, past (>1 year ago), and current (including within the last 12 months). The diagnosis of dyslipidemia was confirmed if one or more of the following criteria were met: use of lipid-lowering drugs or laboratory measurements revealing a total cholesterol ≥ 200 mg/dL, triglycerides ≥ 150 mg/dL, or high density lipoprotein cholesterol (HDL) ≤ 50 mg/dL. Diabetes mellitus was diagnosed by either a HgA1c ≥ 6.5%, a fasting glucose > 126 mg/dL, or a physician diagnosis in the past, with or without the current use of glucose-lowering agents.
Blood Chemistries
Blood was collected from participants after an overnight fast. Total cholesterol, HDL, triglycerides, fasting blood glucose, and insulin levels were measured on a Roche Cobas Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN) and by using standard methods at the Mayo Clinic Medical Laboratories, Rochester, MN. Insulin resistance, a subnormal biological response to insulin, was estimated using a homeostasis model assessment, insulin resistance (HOMA-IR) score, calculated as (fasting glucose mg/dL× fasting insulin mIU/mL)/22.5. HOMA-IR is frequently used for insulin resistance assessment, both in clinical practice and in epidemiological studies,12 and requires a single plasma sample assayed for insulin and glucose. Current evidence suggests that persons with insulin resistance have increased risk of CVD events compared to those who are not insulin resistant.13 Insulin resistance was defined using the threshold (HOMA-IR≥ 2.73) previously determined by the National Health and Nutrition Examination Survey (NHANES) that consisted of 2,804 participants, representing the U.S. population.14
Measurement of Carotid Artery Intima-Media Thickness (CIMT)
CIMT images were acquired using a high-resolution B-mode ultrasound methodology obtained by a single sonographer using standardized imaging and processing protocols (patents 2005 and 2006), with participants in the supine position, as described previously.15–17 The jugular vein and carotid artery were imaged transversely, with the jugular vein stacked above the carotid artery. All images contained internal anatomical landmarks for reproducing probe angulation. The intima–media thickness of the far wall of the right common carotid artery, just distal to the carotid artery bulb, was determined at end diastole. CIMT was expressed as a mean (in millimeters, mm) of 70 to 100 standardized measurements between the intima–lumen and media–adventitia interfaces over a 10 mm length. An image analyst measured CIMT by automated computerized edge detection, using an in-house developed software package (patents 2005, 2006, 2011).15–17 This method standardizes the timing, location, and distance over which CIMT is measured, ensuring comparability within and across participants. Both the sonographer and analyst were blinded as to the women’s pregnancy histories. The threshold of 0.77 was used to categorize CIMT based on proposed sex- and age-adjusted reference limits for CIMT.5
Statistical Analysis
Descriptive statistics on demographic and clinical characteristics are reported as means with standard deviations, quartiles (median, 25th and 75th percentiles), or count and percentage, as appropriate. Group differences between women with histories of normotensive pregnancy and those with histories of preeclampsia were determined by the Student’s t test or Wilcoxon rank sum test for continuous variables, and the Chi-square test for categorical variables. There were no missing data for the variables of interest. The association of having a prior history of preeclampsia with increasing CIMT was analyzed with both ordinal and binary logistic regression analyses. Ordinal logistic regression used the proportional odds (PO) model, in which continuous CIMT values were transformed into rank-ordered responses. The PO model makes fewer distributional assumptions and thus is more robust to extreme values than linear regression.18 The binary logistic method used previously defined threshold to categorize CIMT.5 Pre-selected factors, including present day age, hypertension status, body mass index (BMI), dyslipidemia, a log-transformed HOMA-IR (for the PO model) and the established threshold for insulin resistance (for binary model) were tested as potential confounders, with separate and simultaneous adjustments in the models. For the three continuous adjustment variables (age, BMI and log HOMA) in the PO model, three knot splines were considered for possible improvement of fit. All data analyses were performed using SAS statistical software (version 9.4, SAS institute, Cary, NC), with significance determined based on an alpha level of .05.
Meta-analysis Methods
A systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews19 and Meta-analysis of Observational Studies in Epidemiology.20 The standardized protocol was specifically developed for the purpose of this review, was used by independent reviewers and is available from the study authors upon request.
Inclusion and exclusion criteria
Studies that compared CIMT among women who had preeclampsia and women who had pregnancies that were not complicated by preeclampsia were examined. Detailed diagnostic criteria used for the definition of preeclampsia are listed in Supplemental Table 1. Studies were eligible for inclusion if CIMT was measured in the common carotid artery. Studies that measured intima-media thickness in other arteries were excluded. Studies were included if CIMT was measured in non-pregnant women at least 10 years after delivery. Studies that combined preeclampsia with gestational hypertension and/or chronic hypertension in pregnancy were only eligible if data for the subset of women who developed preeclampsia could be obtained.
Search strategy and selection
A biostatistician with expertise in conducting systematic reviews and meta-analyses (NMM) and vascular physiologist (TLW) developed the search strategy. Searches of PubMed, EMBASE and Web of Science through March 7, 2016 were performed for studies containing key words for CIMT and preeclampsia (Supplemental Appendix). There were no restrictions on publication language or status. Authors of relevant studies were contacted to obtain any missing data and to confirm information on the study methodology and the results. Authors of relevant abstracts were contacted to identify eligible unpublished datasets. Reference lists of papers that were included in the analysis were searched manually, as well as relevant reviews and editorials. Experts in the field were asked to provide information on potentially eligible studies.
Article Screening and Selection
Two reviewers (TLW, NMM) independently evaluated the eligibilities of all titles and abstracts, and performed full text screening to select articles for inclusion (detailed methodology is described in the Supplemental Appendix). Disagreements were resolved by consensus.
Data Abstraction
Two reviewers (TLW, NMM) independently abstracted the following data: 1) Study design, 2) Inclusion and exclusion criteria, 3) Criteria for a preeclampsia diagnosis, 4) Time period, 5) CIMT methodology and 6) CIMT measurements. Authors were contacted to clarify and confirm the accuracy of abstracted data. The handling of missing information is explained in the Supplemental Appendix.
Risk of bias
Risk of bias in individual studies was assessed according to the following criteria proposed by the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) working group:21 1) failure to develop and apply appropriate eligibility criteria (inclusion of control population), 2) flawed measurements of both exposure and outcome, 3) failure to adequately control for confounding variables, and 4) incomplete follow-up. Each reviewer independently evaluated the risk of bias within and across studies, and the overall quality of gathered evidence. An adapted version of the Newcastle-Ottawa tool for observational studies was also used.22
Statistical Analysis
The primary outcome was CIMT, expressed as means with standard deviations. The standardized mean difference (SMD) was used to examine differences between women with vs. without histories of preeclampsia. This measurement of effect size expresses the difference between group means in units of standard deviation, and was estimated by pooling individual trial results using random-effects models via the DerSimonian-Laird method (Review Manager 5.2). Heterogeneity was assessed using the Cochran q test and I2 statistic. A separate forest plot was constructed for each analysis showing the SMD (box), 95% confidence interval (CI) (lines), and weight (size of box) for each trial. The diamond shows the overall effect size. A P value <.05 was considered to be statistically significant. Analyses were performed in Review Manager (Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014.
Sensitivity analyses
Sensitivity analyses were conducted to examine the effects of 1) the exclusion of studies that included women with chronic hypertension at the time of pregnancy, 2) inclusion of studies performed in women ≥50 years old, 3) inclusion of measurements performed in the right vs. left common carotid arteries, if pooled measurements were not available, 4) measurements averaged for different artery locations (common carotid artery, bifurcation, internal carotid artery), and 5) the exclusion of study in which participants were followed for fewer than ten years postpartum, on average.
RESULTS
General Characteristics
Women with histories of normotensive pregnancies and preeclampsia were all non-Hispanic whites, and were comparable both in age at study visit and at index pregnancy by study design (Table 1). Women with histories of preeclampsia had greater BMI and waist circumferences, and were more likely to have current hypertension compared to women with histories of normotensive pregnancies. There were no group differences with respect to the frequency of using hormonal therapy, lipid-lowering agents, aspirin, or anti-inflammatory medications.
Table 1.
Characteristics of women with histories of normotensive and preeclamptic pregnancies
| Variable | Normotensive (n=40) |
Preeclampsia (n=40) |
P-value |
|---|---|---|---|
| Age at study consent | 59.7±4.5 | 59.4±4.8 | .78 |
| Age at 1st live birth | 24.3±3.4 | 24.2±3.7 | .90 |
| Anti-hypertensive medications | 5 (13%) | 23 (58%) | <.001 |
| Lipid lowering agents | 5 (13%) | 10 (25%) | .15 |
| Aspirin | 6 (15%) | 11 (28%) | .17 |
| Anti-inflammatory medications | 20 (50%) | 27 (68%) | .11 |
| Past or current hormone therapy | 17 (43%) | 17 (43%) | 1.00 |
| Tobacco Use: | .21 | ||
| . Never | 21 (53%) | 28 (70%) | |
| . Past | 15 (38%) | 8 (20%) | |
| . Current | 4 (10%) | 4 (10%) | |
| Clinical parameters | |||
| Body mass index (kg/m2) | 25.3 (23.1, 32.0) | 29.8 (25.9, 33.7) | .02 |
| Waist circumference (cm) | 85.3 (79.3, 99.6) | 98.0 (88.3, 104.0) | .009 |
| Systolic blood pressure (mm Hg) | 131.4±20.6 | 131.8±14.9 | .91 |
| Diastolic blood pressure (mm Hg) | 75.8±10.7 | 78.2±9.6 | .29 |
| Current hypertension | 8 (20%) | 24 (60%) | <.001 |
| Diabetes mellitus | 2 (5%) | 4 (10%) | .41 |
| Gestational diabetes mellitus | 2 (5%) | 2 (5%) | 1.00 |
| Hyperlipidemia | 29 (73%) | 32 (80%) | .43 |
| Blood chemistry | |||
| Total cholesterol (mg/dL) | 204.5 (182.0, 222.5) | 189.5 (168.0, 215.0) | .10 |
| LDL Cholesterol (mg/dL) | 123.0 (99.7, 136.4) | 106.1 (87.9, 124.3) | .09 |
| HDL Cholesterol (mg/dL) | 64.0 (50.5, 76.5) | 54.5 (41.0, 69.5) | .05 |
| Triglycerides (mg/dL) | 97.5 (72.0, 123.5) | 108.0 (85.0, 163.0) | .08 |
| If Fasting glucose (mg/dL) | 95.5 (91.0, 101.5) | 98.0 (91.5, 109.5) | .15 |
| Insulin (μIU/mL) | 4.6 (3.3, 6.0) | 7.1 (4.7, 14.8) | <.001 |
| HOMA Insulin resistance (HOMA-IR) | 1.1 (0.8, 1.5) | 1.8 (1.1, 4.0) | <.001 |
Continuous variables are reported as mean ± SD, median (IQR), or n (%)
Blood Chemistries
Blood lipids were in normative ranges and did not differ between the groups (Table 1). The fasting blood glucose was similar between groups, but circulating levels of insulin were higher in the preeclampsia group, resulting in a significantly higher calculated HOMA-IR for those in the preeclampsia group. However, no significant differences were observed in the rates of either gestational diabetes or diabetes mellitus between the groups.
Carotid Artery Intima-Media Thickness (CIMT)
CIMT was significantly greater in women with histories of preeclampsia, 0.80 mm (0.75, 0.85), compared to women with histories of normotensive pregnancy, 0.73 mm (0.70, 0.78) P=.004 (Figure 1). In the ordinal logistic model, the estimated odds ratio for higher CIMT was 3.33 (1.50–7.39) (P=.003) before, and 3.31 (1.32–8.27) (P=.01) after, adjustment for age, current hypertension, BMI, dyslipidemia, and log (HOMA-IR) in preeclampsia compared to the normotensive pregnancy group (Table 2). The spline fits to each of the continuous adjustments did not significantly improve the fit and did not significantly alter the results (data not shown). Using the binary logistic model, the relative odds of a CIMT value >0.77 were similarly more than 3-fold higher for women in the preeclampsia group, both before (OR: 3.46, 95% CI: 1.38–8.69, P=.008) and after adjustments (OR: 3.17, 95% CI: 1.10–9.14, P=.03) for potential confounding from conventional cardiovascular risk factors (age, current hypertension, BMI, dyslipidemia, and presence of insulin resistance) (Table 2).
Figure 1.
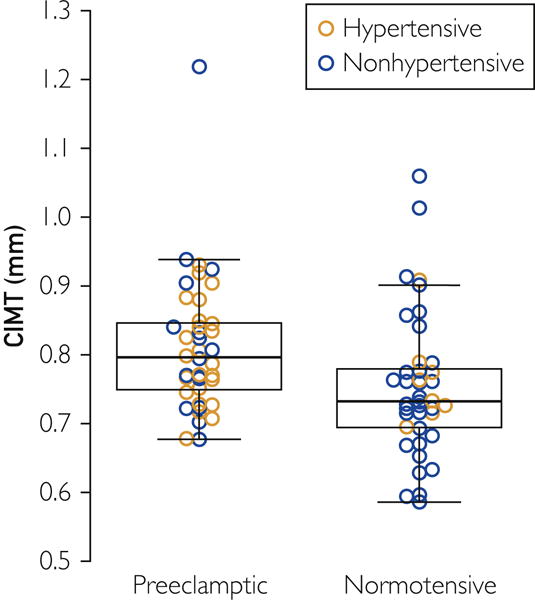
CIMT in women with histories of normotensive versus preeclamptic pregnancies. Filled triangles represent currently hypertensive, and open circles represent currently normotensive women. Black quadrangles represent the mean. The box represents the median and interquartile range; whiskers show the range. CIMT was greater in women with histories of preeclampsia compared to those with histories of normotensive pregnancies, P=.004.
Table 2.
Unadjusted and adjusted odds ratios for CIMT in women with a history of normotensive versus preeclamptic pregnancies
| Adjusting Model | Effect of Preeclampsia | |
|---|---|---|
| Ordinal Logistic OR (95% CI)* [P-value] |
Binary Logistic OR (95% CI)** [P-value] |
|
| None, unadjusted | 3.33 (1.50, 7.39) [.003] | 3.46 (1.38, 8.69) [.008] |
| Age, years | 3.50 (1.58, 7.79) [.002] | 3.55 (1.40, 8.97) [.008] |
| BMI, kg/m2 | 3.03 (1.35, 6.79) [.007] | 3.12 (1.22, 7.98) [.02] |
| Hypertension | 3.19 (1.34, 7.55) [.008] | 3.32 (1.22, 9.03) [.02] |
| Dyslipidemia | 3.34 (1.50, 7.43) [.003] | 3.38 (1.34, 8.53) [.01] |
| HOMA-IR† | 2.77 (1.18, 6.49) [.02] | 2.98 (1.15, 7.75) [.02] |
| Age + BMI + HTN + Dyslipidemia + HOMA-IR† | 3.31 (1.32, 8.27) [.01] | 3.17 (1.10, 9.14) [.03] |
BMI, body mass index, CI, confidence interval, OR, odds ratio; HOMA-IR, homeostasis model assessment-insulin resistance
Odds of higher CIMT measurement for women with histories of preeclampsia (n=40) vs. those with normotensive pregnancies (n=40) using ordinal logistic regression
Odds of CIMT > 0.77 for women with histories of preeclampsia (n=40) vs. those with normotensive pregnancies (n=40)
HOMA-IR was used as log transformed in ordinal and as categorical (>2.73) in binary model
Meta-analysis Results
We identified 234 potentially eligible articles (Figure 2). Full texts of 75 articles were reviewed, of which 10 were eligible for inclusion in the systematic review and meta-analysis. Study characteristics are shown in Table 3.
Figure 2.
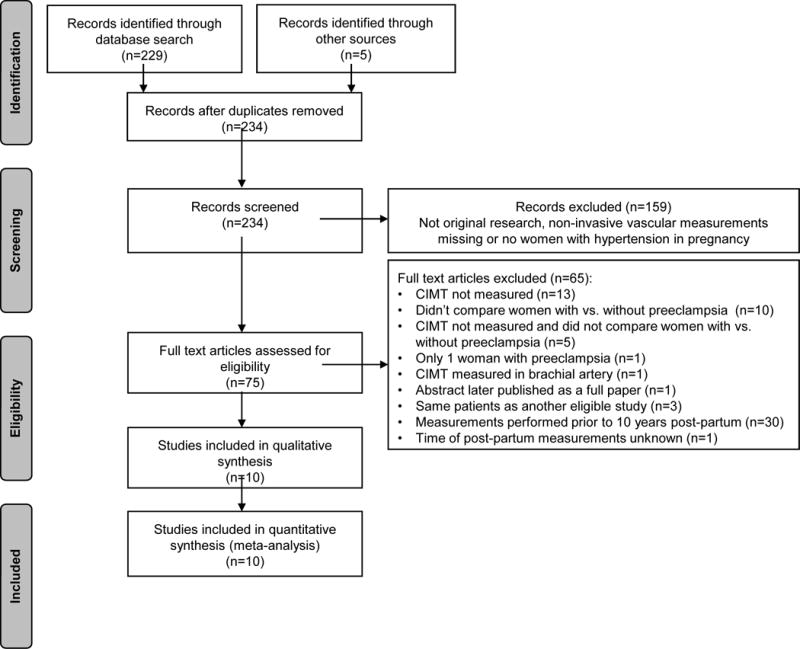
Flow chart of the studies that were evaluated and included in the meta-analysis
Table 3.
Summary of Studies
| Study | Type | Country | Sample Size | Age at the Time of the Study |
Time Post-partum |
Non-PE Group | Inclusion Criteria (at Index Pregnancy) |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| PE | Non-PE | All Primi- parous |
Only Non- smokers |
No Chronic Hypertension |
||||||
| Lazdam 2012 (un-published data from authors)23 | Cross-sectional | UK | Early: 58, Late: 75 | 101 | PE: 40 ± 5 non-PE: 41 ± 3 |
Early PE: 9 ± 2 Late PE: 10 ± 2 non-PE: 9 ± 2 |
No hypertension, proteinuria or IUGR in index pregnancy | − | − | + No chronic HTN at the time of pregnancy |
| Christensen 201624 | Nested cohort | Denmark | 20 | 20 | PE: 41 ± 3 non-PE: 41 ± 2 |
10 years | Previous normotensive pregnancy, no history of PE, GH, HELLP or eclampsia | − | Unclear | Unclear |
| Sandvik 201325 | Cross-sectional | Norway | 89 | 69 | PE: 38 ± 4 non-PE: 39 ± 5 |
10.9 ± 1.0 years | No preeclampsia | + | − | No essential HTN before 1st pregnancy |
| Akhter 201426 | Cross-sectional | Sweden | Severe PE: 42 | 44 | PE: 44 ± 3 non-PE: 44 ± 3 |
11 ± 5 years since last delivery | ≥1 normal pregnancy with term delivery of AGA infant | − | − | + |
| Andersgaard 201227 | Cross-sectional | Norway | 250 | 1,778 | No hypertension or proteinuria in previous pregnancies | − | − | − | ||
| McDonald 201328 | Nested cohort | Canada | 109 | 218 | PE: 49 (44–55) non-PE: 49 (45–56) median (IQR) |
median of 20 years after 1st pregnancy | No preeclampsia in any pregnancy | − | − | + |
| Brown 201529 | Cross-sectional | UK (Scotland) | 64 | 60 | 52 ± 8 | 10–30 years | Previous normotensive pregnancy | Unclear | − | + |
| Huakkamma 200930 | Cross-sectional | Finland | 35 | 489 | PE: 57 ± 7 non-PE: 57 ± 8 |
Healthy women, ≥ 1 birth, no history of GDM, PE, HTN, proteinuria before or during any pregnancy | − | − | − | |
| Garovic 2016 (current study) | Cross-sectional | USA | 40 | 40 | PE: 59 ± 5 non-PE: 60 ± 5 |
PE: 35 (33, 37) years non-PE: 35 (34, 37) years median (IQR) |
Previous normotensive pregnancy | − | − | + |
| Collen 201331 | Nested cohort | Sweden | 31 | 55 | PE: 65 ± 6 non-PE: 63 ± 5 |
PE: 39.5 years non-PE: 39.6 years |
Previous normal pregnancy | − | Unclear | Unclear |
“−“ indicates that the criteria were not met (i.e. study included multiparous women, smokers, or women with chronic hypertension at the time of the index pregnancy). “+” indicates that the criteria were met.
Abbreviations: AGA, appropriate gestational age; GDM, gestational diabetes; GH, gestational hypertension; HELLP, hemolysis, elevated liver enzymes, low platelets; HTN, hypertension; IUGR, intrauterine growth restriction; PE, preeclampsia
Additional information regarding the diagnostic criteria for preeclampsia and exclusion criteria are reported in Supplemental Table 1 and 2, respectively. Ten studies included in the meta-analysis consisted of 3 nested cohort studies24,28,31 and 7 cross-sectional studies,23,25–27,29,30 including the current study. One study, in which the average postpartum interval was 9 ± 2 years, was also included, as many of the participants had been tested more than 10 years after delivery.23 These studies included 813 women who had histories of preeclampsia and 2,874 women with no histories of preeclampsia. CIMT was significantly higher among women with histories of preeclampsia in studies conducted at least 10 years post-partum, with a SMD of 0.18, and 95% CI of 0.05–0.30, P=.004 (Figure 3). The analysis revealed no statistically significant heterogeneity among the results of the respective studies (I2=35%, P=.13). This effect remained significant in sensitivity analyses of five studies (including the current study) that excluded women with chronic hypertension at the time of pregnancy23,26,28,29 (SMD: 0.27, 95% CI: 0.08–0.46, P=.005), and four studies (including the current study) that included women ≥ 50 years old29–31 (SMD: 0.27, 95% CI: 0.05–0.50, P=.02). Results were not different after excluding data for women with fewer than 10 years of post-partum follow-up in a study in which the average duration of follow-up was 9 ± 2 years (data provided from authors)23 (SMD: 0.19, 95% CI: 0.04–0.34, P=.01), or after excluding a study27 that was the largest and most influential (weight=23%), and presented CIMT as an age-adjusted mean with a 95% CI (SMD: 0.17, 95% CI: 0.02–0.33, P=.03). Detailed information about CIMT methodology for the included studies is provided in the Supplemental Table 3. One study reported separate values for the right and left common carotid arteries.24 Including the right or left artery did not alter the results of the meta-analysis (SMD: 0.16, 95% CI: 0.02–0.30, P=.03). Selecting different artery locations for three studies28–30 that included CIMT measurements of the bifurcation and/or internal carotid artery did not change the magnitude of the overall effect (SMD: 0.18, 95% CI: 0.07–0.29, P=.001). One study reported values for the common carotid and bifurcation,30 whereas the remaining studies included average values for the common and internal carotid arteries and the bifurcation.28,29
Figure 3.
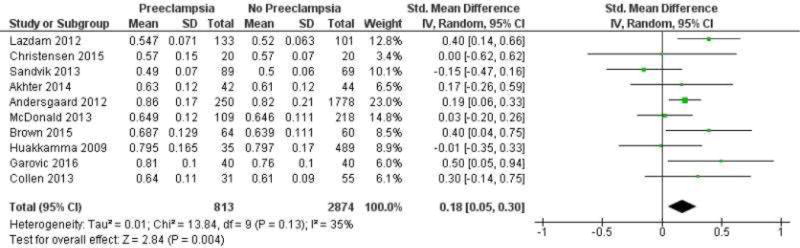
Meta-analysis of differences in CIMT between women with vs without histories of preeclampsia. Studies are listed according to the average amount of time, in increasing order, from pregnancy to CIMT measurements.
The risk of bias of individual studies is presented in Supplemental Table 4. Most information was derived from studies at moderate risk of bias (risk of bias across studies).
DISCUSSION
The results of the present study provide evidence that a history of preeclampsia is associated with subclinical atherosclerosis, as defined by CIMT, approximately 3 decades after the affected pregnancy in women with no histories of cardiovascular or cerebrovascular events. Women with and without histories of preeclampsia in the present study, by design, were closely age-matched, as CIMT increases with age.32,33 Further analysis indicated that the association between CIMT and having a history of preeclamptic pregnancy is independent of other known CVD risk factors. Women in the preeclampsia group had metabolic profiles consistent with elevated cardiovascular risk,34,35 including greater BMI and increased insulin resistance. However, accounting for these factors did not reduce the impact of preeclampsia on the risk of having an elevated CIMT in this age group. Similar results were obtained in a meta-analysis that included all trials that compared CIMT in women with histories of preeclampsia versus those without such histories, 10–40 years after their pregnancies. Taken together, these results suggest that measuring CIMT, a sensitive technique for quantifying subclinical atherosclerosis, may identify women with greater atherosclerotic burdens among those with histories of preeclampsia.
The emerging evidence that a history of preeclampsia is an independent risk factor for future CVD and cardiovascular risk factors.28,36–39 has been recognized by the AHA guidelines that identify preeclampsia as a risk factor for CVD40 and stroke41 in women. It remains unclear, however, as to how and when to screen former preeclamptic patients. Measuring CIMT may be helpful to detect subclinical atherosclerosis,42 but, as appropriately noted by Zoet et al.,3 “Few studies have investigated women with a history of preeclampsia in the fourth and fifth decade of life, when early signs of premature CVD are most likely to become apparent.” In our current study, the median age of the participants was 59 years. Median CIMT values among women with histories of preeclampsia were elevated compared to the population-based, age and sex adjusted 80th percentile, 0.80 mm (0.75, 0.85) vs. 0.73 (0.72–0.74).5 The CIMT values in women with normotensive pregnancies, 0.73 mm (0.70–0.78), were comparable to population based estimates. Measurements of coronary artery calcification (CAC) in the same women who underwent CIMT measurements in this study10 showed that having a history of preeclampsia was associated with increased odds of CAC. With the results of the current study showing an elevated CIMT after preeclamptic pregnancies, we postulated that the CAC and CIMT scores would be elevated in the same individuals. The correlation between CIMT and CAC in our participants was not statistically significant (crude, unadjusted Spearman coefficient: ρ=0.16, P=.15; Spearman partial coefficient, adjusted for the group effect, ρ=0.07, P=.54). These results suggest that these techniques image different aspects of subclinical atherosclerosis after preeclamptic pregnancies, as shown previously in healthy populations.43 Consistent with this assertion, certain studies suggest that these imaging techniques may predict clinical risk differently, such as the MESA study,44 which concluded that CAC was a stronger predictor for coronary outcomes, whereas CIMT was a stronger predictor of stroke. Our results set the stage for future studies that will explore the roles of CAC and CIMT in CVD risk prediction after preeclamptic pregnancies.
The major strength of this study is the use of the unique population-based REP medical records-linkage system that allowed for confirmation of preeclamptic and normotensive pregnancies based on vigorous chart review using accepted clinical criteria. Furthermore, the CIMT methodology that we used is consistent with the accepted guidelines and recommendations, and is predictive of CVD.45 Women also were studied 3 decades after their pregnancies and after their reproductive ages, thus ensuring that the control group did not contain women who could still potentially develop preeclampsia. This study extends previous reports by addressing potential factors contributing to elevated CIMT in women with histories of preeclampsia. Taking these risk factors into consideration, we report that more women with histories of preeclampsia, but without prior diagnoses of cardiovascular events, were taking anti-hypertensive medications than age- and parity-matched women who had normotensive pregnancies only. However, despite the use of these medications, CIMT was greater in women with histories of preeclampsia. Finally, the meta-analysis of studies conducted 10–40 years post-partum provided supporting evidence for the association between preeclampsia and future elevated CIMT.
This study also has limitations. First, the number of women recruited from the REP who were participants in the prospective study was small. This limitation is somewhat offset by the fact that our study cohort was homogenous and consisted of participants matched by age and parity, with no previous cardiovascular events, and with comparable follow up period from their index pregnancies. In addition, our findings are further strengthened by the meta-analysis of prior studies of CIMT performed decades after preeclamptic pregnancies. The risk of bias across studies was moderate, suggesting that the estimated effect is likely to be close to the true effect. Second, all women were non–Hispanic white which limits the generalizability of these results to broader populations. However, use of a homogenous sample is beneficial in reducing variability due to genetic and cultural influences on cardiovascular risk parameters. Finally, the study evaluated women at one point in time many years after their incident pregnancies. Therefore, although it was possible to identify metabolic contributors to the development of accelerated CIMT following preeclampsia, a longitudinal evaluation of women following their affected pregnancies is needed to better target preventive and therapeutic approaches to reduce future CVD risk, including regular exercise and a healthy diet.46
These results, despite the study limitations, clearly indicate that postmenopausal women with histories of preeclampsia, compared to those without such histories, have significantly elevated CIMT. Future studies are needed to address the impact of detection of subclinical atherosclerosis by CIMT on incidence of CVD events in women with remote histories of preeclampsia.
CONCLUSION
Early recognition of subclinical atherosclerosis after preeclamptic pregnancies, as identified and quantified by measurements of CIMT, may offer an opportunity for early intervention, thus potentially modifying the course of CVD in women with histories of preeclampsia.
Supplementary Material
Acknowledgments
Financial support: This study was supported by National Institute of Health P50 AG044170, R01 AG034676, UL1 TR000135,1 a Mayo Clinic Clinical and Translational Science Award, the Department of Surgery, and the Mayo Foundation.
Abbreviations
- CAC
coronary artery calcification
- CI
confidence interval
- CIMT
carotid artery intima-media thickness
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- HDL
high density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment - insulin resistance
- PO
proportional odds
- SBP
systolic blood pressure
- SMD
standardized mean difference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None of the authors declare competing financial interests.
References
- 1.Milne F, Redman C, Walker J, et al. The pre-eclampsia community guideline (PRECOG): how to screen for and detect onset of pre-eclampsia in the community. BMJ. 2005;330(7491):576–580. doi: 10.1136/bmj.330.7491.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Zoet GA, Koster MP, Velthuis BK, et al. Determinants of future cardiovascular health in women with a history of preeclampsia. Maturitas. 2015;82(2):153–161. doi: 10.1016/j.maturitas.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Ciccone MM, Bilianou E, Balbarini A, et al. Task force on: ‘Early markers of atherosclerosis: influence of age and sex’. J Cardiovasc Med (Hagerstown) 2013;14(10):757–766. doi: 10.2459/JCM.0b013e328362078d. [DOI] [PubMed] [Google Scholar]
- 5.Tosetto A, Prati P, Baracchini C, Manara R, Rodeghiero F. Age-adjusted reference limits for carotid intima-media thickness as better indicator of vascular risk: population-based estimates from the VITA project. J Thromb Haemost. 2005;3(6):1224–1230. doi: 10.1111/j.1538-7836.2005.01440.x. [DOI] [PubMed] [Google Scholar]
- 6.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21(2):93–111. doi: 10.1016/j.echo.2007.11.011. quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 7.Peters SA, den Ruijter HM, Grobbee DE, Bots ML. Results from a carotid intima-media thickness trial as a decision tool for launching a large-scale morbidity and mortality trial. Circ Cardiovasc Imaging. 2013;6(1):20–25. doi: 10.1161/CIRCIMAGING.112.978114. [DOI] [PubMed] [Google Scholar]
- 8.Califf RM. Biomarkers, putative surrogates, surrogates, and decision making. Circ Cardiovasc Imaging. 2013;6(1):6–7. doi: 10.1161/CIRCIMAGING.112.982538. [DOI] [PubMed] [Google Scholar]
- 9.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White WM, Mielke MM, Araoz PA, et al. A history of preeclampsia is associated with a risk for coronary artery calcification 3 decades later. Am J Obstet Gynecol. 2016;214(4):519 e511–518. doi: 10.1016/j.ajog.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 12.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 13.Bonora E, Kiechl S, Willeit J, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007;30(2):318–324. doi: 10.2337/dc06-0919. [DOI] [PubMed] [Google Scholar]
- 14.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196(2):696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Hodis HN, Mack WJ, LaBree L, et al. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Vitamin E Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106(12):1453–1459. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- 16.Selzer RH, Hodis HN, Kwong-Fu H, et al. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111(1):1–11. doi: 10.1016/0021-9150(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 17.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154(1):185–193. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 18.Harrell FEJ, editor. Regression modeling strategies: With application to linear models, logistic regression, and survival analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009:339b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JGS, editor. Chapter 22 Overview of Reviews In Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Ltd; Chichester, UK: 2008. [Google Scholar]
- 22.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. University of Ottawa: OttaOttawa Hospital Research Institute; (accessed September 2016) [Google Scholar]
- 23.Lazdam M, de la Horra A, Diesch J, et al. Unique blood pressure characteristics in mother and offspring after early onset preeclampsia. Hypertension. 2012;60(5):1338–1345. doi: 10.1161/HYPERTENSIONAHA.112.198366. [DOI] [PubMed] [Google Scholar]
- 24.Christensen M, Kronborg CS, Eldrup N, Rossen NB, Knudsen UB. Preeclampsia and cardiovascular disease risk assessment - Do arterial stiffness and atherosclerosis uncover increased risk ten years after delivery? Pregnancy Hypertens. 2016;6(2):110–114. doi: 10.1016/j.preghy.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Sandvik MK, Leirgul E, Nygard O, et al. Preeclampsia in healthy women and endothelial dysfunction 10 years later. Am J Obstet Gynecol. 2013;209(6):569 e561–569 e510. doi: 10.1016/j.ajog.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Akhter T, Larsson M, Wikstrom AK, Naessen T. Thicknesses of individual layers of artery wall indicate increased cardiovascular risk in severe pre-eclampsia. Ultrasound Obstet Gynecol. 2014;43(6):675–680. doi: 10.1002/uog.13289. [DOI] [PubMed] [Google Scholar]
- 27.Andersgaard AB, Acharya G, Mathiesen EB, Johnsen SH, Straume B, Oian P. Recurrence and long-term maternal health risks of hypertensive disorders of pregnancy: a population-based study. Am J Obstet Gynecol. 2012;206(2):143 e141–148. doi: 10.1016/j.ajog.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 28.McDonald SD, Ray J, Teo K, et al. Measures of cardiovascular risk and subclinical atherosclerosis in a cohort of women with a remote history of preeclampsia. Atherosclerosis. 2013;229(1):234–239. doi: 10.1016/j.atherosclerosis.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Brown CE, Flynn J, Carty DM, Scotland G, Delles C. Vascular Consequences of Pre-Eclampsia. J Hypertens. 2015;33(Suppl):1e46. [Google Scholar]
- 30.Haukkamaa L, Moilanen L, Kattainen A, et al. Pre-eclampsia is a risk factor of carotid artery atherosclerosis. Cerebrovasc Dis. 2009;27(6):599–607. doi: 10.1159/000216834. [DOI] [PubMed] [Google Scholar]
- 31.Collen AC, Hellgren M, Gustafsson H, Johansson MC, Manhem K. Cardiovascular and metabolic characteristics 40 years after hypertensive pregnancies: a long-term follow-up study of mothers. J Hypertens. 2013;31(4):758–765. doi: 10.1097/HJH.0b013e32835e2a9b. [DOI] [PubMed] [Google Scholar]
- 32.Jarauta E, Mateo-Gallego R, Bea A, Burillo E, Calmarza P, Civeira F. Carotid intima-media thickness in subjects with no cardiovascular risk factors. Rev Esp Cardiol. 2010;63(1):97–102. doi: 10.1016/s1885-5857(10)70014-1. [DOI] [PubMed] [Google Scholar]
- 33.Lim TK, Lim E, Dwivedi G, Kooner J, Senior R. Normal value of carotid intima-media thickness–a surrogate marker of atherosclerosis: quantitative assessment by B-mode carotid ultrasound. J Am Soc Echocardiogr. 2008;21(2):112–116. doi: 10.1016/j.echo.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Paramsothy P, Knopp RH, Bertoni AG, et al. Association of combinations of lipid parameters with carotid intima-media thickness and coronary artery calcium in the MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56(13):1034–1041. doi: 10.1016/j.jacc.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 35.Wild RA, Wu C, Curb JD, et al. Coronary heart disease events in the Women’s Health Initiative hormone trials: effect modification by metabolic syndrome: a nested case-control study within the Women’s Health Initiative randomized clinical trials. Menopause. 2013;20(3):254–260. doi: 10.1097/GME.0b013e31826f80e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seely EW, Tsigas E, Rich-Edwards JW. Preeclampsia and future cardiovascular disease in women: How good are the data and how can we manage our patients? Semin Perinatol. 2015;39(4):276–283. doi: 10.1053/j.semperi.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125(11):1367–1380. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357(9273):2002–2006. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 39.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28(1):1–19. doi: 10.1007/s10654-013-9762-6. [DOI] [PubMed] [Google Scholar]
- 40.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update. Circulation. 2011;123(11):1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(5):1545–1588. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milic NM, Milin-Lazovic J, Weissgerber TL, Trajkovic G, White WM, Garovic VD. Preclinical atherosclerosis at the time of pre-eclamptic pregnancy and up to 10 years postpartum: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;49(1):110–115. doi: 10.1002/uog.17367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mersich B, Rig OJ, Z LE, Studinger P, Visontai Z, Kollai M. Carotid artery stiffening does not explain baroreflex impairment in pre-eclampsia. Clin Sci (Lond) 2004;107(4):407–413. doi: 10.1042/CS20040137. [DOI] [PubMed] [Google Scholar]
- 44.Zeb I, Budoff MJ. MESA: the NIH-sponsored study that validates atherosclerosis imaging for primary prevention. Curr Atheroscler Rep. 2011;13(5):353–358. doi: 10.1007/s11883-011-0191-2. [DOI] [PubMed] [Google Scholar]
- 45.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128(4):262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 46.Scicchitano P, Cameli M, Maiello M, et al. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J Funct Foods. 2014:611–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


