Abstract
PI3K signaling is frequently dysregulated in NSCLC-SQCC. In contrast to well characterized components of the PI3K signaling network contributing to the formation of SQCC, potential oncogenic effects of alterations in PIK3C2B are poorly understood. Here, a large cohort (n = 362) of NSCLC-SQCC was selectively screened for four reported somatic mutations in PIK3C2B via Sanger sequencing. In addition, two mutations leading to an amino acid exchange in the kinase domain (C1181, H1208R) were examined on a functional level. None of the mutations were identified in the cohort while well characterized hotspot PIK3CA mutations were observed at the expected frequency. Ultimately, kinase domain mutations in PI3KC2β were found to have no altering effect on downstream signaling. A set of SQCC tumors sequenced by The Cancer Genome Atlas (TCGA) equally indicates a lack of oncogenic potential of the kinase domain mutations or PIK3C2B in general. Taken together, this study suggests that PIK3C2B might only have a minor role in SQCC oncogenesis.
Introduction
Phosphoinositide-3-kinases (PI3Ks) are able to phosphorylate the inositol ring of three different phosphatidylinositol lipid substrates (PtdIns, PtdIns4P, PtdIns(4,5)P2), minor compounds on the cytosolic site of eukaryotic cell membranes. Following activation by upstream agonists such as receptor tyrosine kinases (RTKs) or G protein coupled receptors (GPCR), PI3Ks generate 3-phosphoinositides as second messengers. These 3-phosphinositides coordinate the function and localization of numerous effector proteins. Downstream pathways of those proteins control a broad range of different physiological functions as diverse as proliferation, migration, apoptosis and cell metabolism [1–5]. Eight different catalytic PI3K isoforms have been described that are subdivided into three different classes (class I, class II and class III). This classification is based on substrate specificity, associated co-factors and sequence homologies.
Because of its central role in intracellular signaling, dysregulation of the PI3K network belongs to the most common events in human cancers [6]. Prominent examples are loss of function mutations in PTEN, the main PI3K phosphatase and antagonist of PI3K signaling [7,8]. In regard to PI3K isoforms, there is plenty of evidence that alterations in class I alpha (p110α) promote oncogenesis. Somatic mutations clustered in PIK3CA hotspot regions are frequently found in a wide array of cancers [9,10] and their oncogenic potential is well documented in functional studies [11,12]. Aside from p110α, there are numerous publications linking further PI3K isoforms to tumorigenesis [13–15].
Among them is the class II isoform C2β. As class II PI3Ks were discovered based on sequence homologies with class I and class III instead of a functional context, the physiological role and downstream pathways of PI3KC2β remain enigmatic. Nevertheless, PI3KC2β has been repeatedly associated with various steps of oncogenesis in different cell lines. These range from enhanced cell migration [16] to an increase in chemotherapeutic resistance [17], anchorage-independent growth [18] and cell proliferation [19]. Moreover, a study characterizing the exomes of 31 non-small cell lung cancer (NSCLC) genomes found 4 missense mutations in PIK3C2B: c.349C>G (P117A), c.3542G>T (C1181F), c.3623A>G (H1208R) and c.4407G>T (L1469F). Two of them were located in a highly conserved region of the kinase domain (C1181 and H1208R) [20]. The frequency was even higher (3/12) when only considering the squamous cell carcinoma (SQCC) subtype.
Together with adenocarcinomas (ADC), SQCC comprise the majority of all non-small cell lung carcinomas (NSCLC) [21]. Recent efforts have been undertaken to unveil the underlying changes in the genome, transcriptome and proteome of these two histological subtypes. This led to growing evidence of distinct genomic alteration patterns. As for SQCC, oncogenesis appears to rely on alterations in squamous differentiation [22], oxidative stress response [23] and PI3K signaling [24]. According to a large genomic analysis, aberrant PI3K signaling is present in approximately half of all cases [25], mainly through alterations in PIK3CA and PTEN.
Given the high prevalence of PIK3C2B mutations in the small NSCLC cohort screened by Liu et.al. and the connection to multiple steps of cancer progression, PIK3C2B and the reported kinase domain mutations C1181F and H1208R were closer examined in regard to promote aberrant PI3K signaling in NSCLC-SQCC.
For this purpose, a cohort of 362 NSCLC-SQCC was selectively screened for all four reported alterations in PIK3C2B. To embed the sequencing results into a broader context, clinical outcomes of a set of SQCC sequenced by the cancer genome atlas (TCGA) [26,27] were analyzed with respect to alterations in PIK3C2B. Moreover, the functional impact of C1181F and H1208R was assessed in relation to its potential to hyper-activate downstream PI3K/ERK signaling in HEK293 cells.
Material and methods
DNA isolation from tumor samples
Punches from paraffin embedded NSCLC-SQCC tumor samples were provided by the Institute of Pathology and Tumor Tissue Bank, University of Bern. The SQCC cohort included 362 primary resected tumors and 29 corresponding mediastinal lymph node metastases diagnosed at the Institute of Pathology 2000–2013. In order to exclude pulmonary metastases of other SQCC, patients with previous SQCC of other organs were not included. The cohort comprised 52 females and 310 males with a median age of 69 years at the time of operation (range 43–85 years of age) and included all UICC 2009 pT stages (pT1a = 34, pT1b = 49, pT2a = 119, pT2b = 53, pT3 = 77, pT4 = 30) and UICC 2009 tumor stages (IA = 61, IB = 79, IIA = 73, IIB = 51, IIIA = 81, IIIB = 8, IV = 8). The study was approved by the Cantonal Ethics Commission of the Canton of Bern (KEK200/14), which waived the requirement for written informed consent. DNA was isolated from one or two paraffin punches per sample by using Qiaamp DNA MicroKIT kits (Qiagen, cat. no. 56304).
Sanger sequencing
Potentially mutated sites were amplified via AmpliTaq Gold DNA Polymerase (ThermoFischer, cat. no. N8080241) in a thermocycler (UNO II, Biometra). Cycling conditions consisted of an initial denaturation step at 95°C for 10 min and 30 cycles of denaturation (95°C, 30 sec), annealing (60°C, 30 sec) and extension (72°C, 40 sec). Primer sequences and amplification conditions for PIK3CA screening were adopted from Samuels et. al. [10].
Following amplification of the regions of interest, 5’ phosphates of the PCR products were degraded with rAPid alkaline phosphatase (Roche, cat. no. 4898133001) followed by 25 cycles of forward or reverse amplification at the same cycling conditions as indicated above (BigDye® Terminator v3.1 Cycle Sequencing Kit, Life Technologies, cat. no. 4337455). After DNA precipitation, amplicons were dissolved in Hi-Di formamide (Thermo-Scientific, cat. no. 4311320) and sequenced with an ABI3730 DNA analyzer (Applied Biosystems).
Primers
Primers of this project were purchased from Microsynth, designed with the Primer-Blast web tool (ncbi. nlm.nih.gov/tools/primer-blast/) and are depicted in Table 1.
Table 1.
| Sequencing Primers | ||
|---|---|---|
| Name | Forward | Reverse |
| PIK3C2B.Ex3 | CAGACCCCTCTCTCATCAGC | ACGAAGAGACTCCCCCATCT |
| PIK3C2B.Ex24 | CTGGAGTCCTTCCAAGCCAG | ACCGCTTGATGTTGCCAAAC |
| PIK3C2B.Ex31 | TCTGGAACAGTCCCCTTCCT | GGGCAGAAGCAGTTACCCTT |
| PIK3CA.Ex9 | GATTGGTTCTTTCCTGTCTCTG | CCACAAATATCAATTTACAACCATTG |
| Mutagenesis Primers | ||
| Name | Forward | Reverse |
| PIK3C2B.C1181F | TATCTACTCCTTCGCTGGCTGCT | AAGTTCTCCACAGCCTTCTCATACTC |
| PIK3C2B.H1208R | CACTGGTCGCATGTTCCA | GTCTTCAGCATGATGTTGTCGT |
| qPCR Primers | ||
| Name | Forward | Reverse |
| PIK3C2B.qPCR | CAGGCTTCAAGAGGCACTCA | TGGTCATCATTCACCGTCCG |
| HPRT.qPCR | TATGGCGACCCGCAGCCCT | CATCTCGAGCAAGACGTTCAG |
| TBP.qPCR | AGCGCAAGGGTTTCTGGTTT | CTGAATAGGCTGTGGGGTCA |
Plasmid engineering
A PIK3C2B expression vector with a C-terminal Myc-Tag was purchased from Origene (cat. no. NM-002646). Primers with an adequate nucleotide mismatch were designed to engineer C1181F and H1208R amino acid exchanges into the plasmid. The implemented changes in the base triplicates were: C1181F: TGC>TTC / H1208R: CAC>CGC
To facilitate ensuing ligation, primers were additionally phosphorylated at the 5’-end.
Site-directed mutagenesis was carried out with the Phusion Site-Directed Mutagenesis kit (Thermo Scientific, cat. no. F541). Mutations were incorporated by following the indicated cycling conditions: initial denaturation (98°C, 10 min) was followed by 25 cycles of annealing (C1181F: 69°C, H1208R: 64,5°C, 20 sec) and extension (72°C, 5 min). PCR products were ligated (Promega, cat. no. M180S) and successful engineering was tested via Sanger sequencing.
An empty control plasmid was created by removing the PI3KC2B open reading frame via restriction digestion with NheI (Promega, cat. no. R650A) and MluI (Promega, cat. no. R638A). Plasmid fragments were separated in 1% agarose gel and purified (Promega, cat. no. A9281). Afterwards, 5’-overhangs were blunted (NEB, cat. no. M0210S) and ligated (Promega, cat. no. M180S). Plasmid constructs were cloned into E. Coli XL-1 Blue bacteria.
Bacterial transformation
Competent E.Coli XL-1 Blue bacteria were transformed with 150 ng of target plasmid by applying a 42°C heat shock for 85 sec. After overnight culture in LB medium containing adequate antibiotic concentration (100 μg/ml ampicillin), clonally expanded plasmids were isolated with PureyieldTM Plasmid Miniprep/ Midiprep kits (Promega, cat. no. A1223/A2495).
Cell lines and culture
HEK293 cells were purchased from the American Type Culture Collection (ATCC). Cells were cultured in Dulbecco`s Modified Eagle Medium (Sigma Aldrich, cat. no. D5796) supplemented with 10% FBS (Gibco, cat. no. 10082147), 2 mM L-glutamine (Gibco, cat. no. 25030081) and 50.000 units of penicillin/streptomycin (Gibco, cat. no. 15140122). Cells were kept up to passage 50 or 3 months maximum.
Transient transfection
HEK293 cells were transfected at 50–60% confluency in different formats (6 well / 10 cm) with calcium phosphate. Appropriate amounts of plasmid DNA (6 well: 4 μg / 10 cm dishes: 30 μg) were thoroughly mixed with 1/10 Vol. of 2.5 M CaCl2 and 2x HEPES buffered saline (HBS, 40 mM HEPES, 10 mM D-Glucose, 10 mM KCl, 270 mM NaCl, 1,5 mM Na2HPO4). Subsequently, the transfection mix was added dropwise to HEK293 cells. After overnight exposure to the precipitate, medium was changed and cells were further cultivated for 48–72 h.
qPCR
RNA from transfected HEK293 cells was isolated with the RNeasy Mini kit (Qiagen, cat. no. 74104), followed by reverse transcription (Applied Biosystems, cat. no. 4368814). Quantitative PCRs were performed in a ViiA7 cycler (Applied Biosciences) using SybrSelect Mastermix (Applied Biosystems, cat. no. 4472908).
Expression of mRNA was normalized to TATA box binding protein (TBP) and hypoxanthine-guanine phosphoribosyl transferase (HPRT) housekeeping genes.
Western blot
Proteins were extracted in RIPA buffer (20 mM Tris-base pH 8, 150 mM NaCl, 1% Triton-X-100, 0.1% SDS, 0.5% sodium deoxycholate) supplemented with 100 μM Na3VO4, 25 mM β-glycerophosphate, 1 mM NaF and cOmpleteTM Protease Inhibitor Cocktail (Roche, cat. no. 11836170001). Pierce BCA protein assay kit (Thermo Scientific, cat. no. 23225) was used to determine protein concentration. Subsequently, 20 μg of protein were separated via SDS-PAGE, transferred onto nitrocellulose membranes and blocked in Tris buffered saline (TBS, 130 mM NaCl, 30 mM Tris-Cl, pH 7.5) containing 5% Bovine Serum Albumin (BSA) for 2 h. Western blots were probed with rabbit anti-PI3KC2β polyclonal antibody (1/1000, described in [28]) rabbit anti-P-AKT Ser473 (1:1500, Cell signaling technology, cat. no. 4060L), rabbit anti-P-S6 Ser240/244 (1:2500, Cell signaling technology, cat. no. 5364L), mouse anti-pan-AKT(1:2000, Cell signaling technology, cat. no. 2920S) mouse anti-total-S6 (1:2000, Cell signaling technology, cat. no. 2317S), rabbit anti-P-ERK Thr202/Tyr204 (1500, Cell signaling technology, cat. no. 4370L), mouse anti-total-ERK (1:2000, Cell signaling technology, cat. no. 9107S) and mouse anti-β-actin antibody (1/2000, Sigma Aldrich, cat. no. A5316). Primary antibodies were detected by using goat anti-rabbit IR680 (1/10.000, cat. no. 926–68071, LiCor Bioscience) and goat anti-mouse IR800 (1/10.000, cat. no. 926–32210, LiCor Bioscience) antibodies and analyzed with a LI-COR OdysseySa imaging system.
Immunoprecipitation and lipid kinase assay
Lipid kinase activity of exogenously expressed PI3KC2β was measured with a bioluminescence based kit purchased from Promega (ADP-Glo-Kinase Assay, cat. no. V6930).
HEK293 cells were grown in 10 cm diameter dishes and transfected with plasmid constructs as described above. Cells were lysed for 20 minutes on ice by applying 2 ml lysis buffer (1% Triton X-100, TrisCl 50 mM pH 7.4, NaCl 150 nM, 1 mM EDTA), supplemented with 100 μM Na3VO4, 1 mM NaF, 20 mM β-glycerophosphate and cOmpleteTM Protease Inhibitor Cocktail. Then, lysates were centrifuged (16’000 g, 4°C, 30 min) to remove insoluble cellular debris and supernatant was incubated with anti-MycTag antibodies for 2 hours at 4°C under continuous agitation. Sepharose beads (GE Healthcare, cat. no. 17061801) were added to the mix, followed by further incubation under continuous agitation (1 h, 4°C). The resulting suspension was separated into different tubes (1/6, 2/6 and 3/6 of total volume). Antibody-protein complexes were then pooled down by centrifugation (4000 g, 4°C, 1 min). Finally, immunoprecipitates were washed 3 times in lysis buffer, followed by quick spin down (4000 g, 4°C, 1 min). Ensuing, sepharose pellets were re-suspended in kinase reaction buffer (40 mM Tris HCl pH 7.5, 20 mM MgCl2, 0.1 mg/ml BSA), supplemented with 0.2 mg/ml phosphatidylinositol substrate (PI, Sigma Aldrich, cat. no. 79403) and incubated on ice for 20 min. Enzymatic reaction was started after addition of 50 μM ATP and precipitates were incubated for 30 min at room temperature. Remaining experimental steps were carried out according to the manufacturer`s protocol. Luminescence was measured with a Modulus Microplate reader (Turner Biosystems).
Immunofluorescence
HEK293 cells were grown on glass coverslips. After 10% formalin fixation (10 min), coverslips were washed 3x10 min in phosphate buffered saline (1x PBS: 137 mM NaCl, 2.7 mM KCl, 18 mM KH2PO4, 10 mM Na2HPO4) and cells were subsequently permeabilized with a 1x PBS, 0.3% Triton-X100 solution. Following blocking with a 1% BSA, 0.2% gelatin, 0.05% saponin in 1x PBS solution and washing with a 0.1% BSA, 0.2% gelatin, 0.05% saponin in 1x PBS solution, fixed cells were treated overnight at 4°C with a mouse anti-MycTag (9E10 epitope) antibody diluted in an adequate buffer (0.1% BSA, 0.1% sodium azide, 0.3% triton X-100 in 1x PBS). Then, coverslips were rinsed 3 times in washing solution. Cells were further incubated with a fluorescent secondary anti-mouse-Alexa647 antibody (1:500, ThermoFischer, cat. no. A32728) to detect antigen-antibody complexes and counter-stained with DAPI (500 ng/ml, Sigma Aldrich, cat. no. 32670-25MG). Slides were scanned with a Pannoramic Midi II scanner (3DHISTECH Ltd.).
In silico meta-analysis
A SQCC data set sequenced by the TCGA and available on cbioportal [26,27] was analyzed in relation to PIK3C2B sequencing information. Raw data were visualized and formatted with Excel and Prism7 (GraphPad).
Statistical analyses
All experiments were performed in triplicates. Statistical analyses were conducted using Prism 7. The statistical test used is indicated in the legend of the figure. A value of p<0.05 was considered to be significant.
Results
Cohort validation
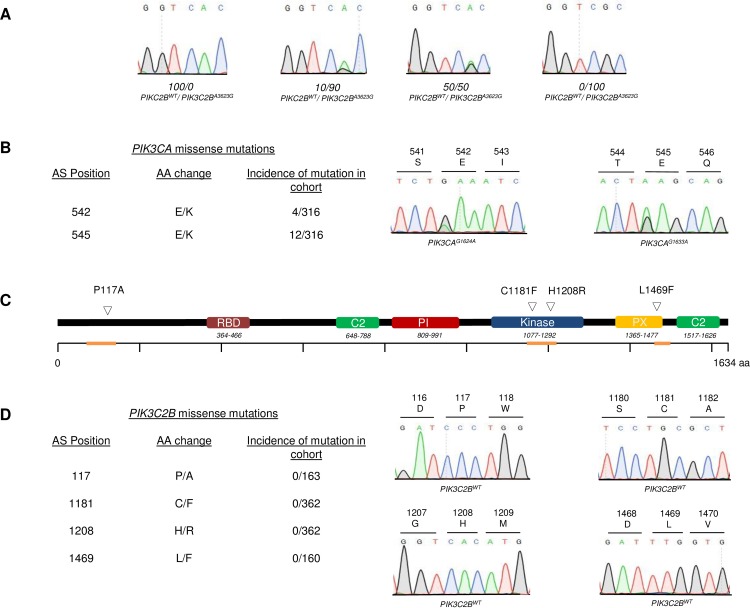
To ensure robust sequencing data, it had to be ascertained, that assay sensitivity was sufficient to detect tumor specific mutations and that the obtained cohort was representative. To validate the former, different ratios of PIK3C2BWT and PIK3C2BA3623G plasmids were analyzed via Sanger sequencing. Detection of 10% mutated plasmid combined with 90% wildtype plasmid was possible (Fig 1A).This result was considered to be sufficient for further analyses, as paraffin punches contained tumor fractions > 30%. To ensure that the cohort was representative, it was screened for the charge reversing hotspot mutations p110αE542K and p110αE545K. Both mutations were present in the cohort (Fig 1B). Relying on mutation data from the COSMIC database (cancer.sanger.ac.uk), a subsequent χ2 test revealed no significant difference between the observed and expected frequencies in the screened cohort (E542K p = 0.383, E545K p = 0.475).
Fig 1. Screening of NSCLC-SQCC tumors for somatic PIK3C2B mutations.
A Chromatograms of different PIK3C2BWT / PIK3C2BA3623G ratios to determine maximal assay sensitivity B Table with incidence and position of detected hotspot alterations E542K and E545K in PIK3CA with representative chromatograms of screened genomic regions C Structural domains of PIK3C2B with localization of reported mutations P117A, C1181F, H1208R and L1469F. Genomic regions analyzed via Sanger sequencing are highlighted in orange D Table with incidence and position of found alterations in PIK3C2B with representative chromatograms.
PIK3C2B screening
After isolation of DNA from paraffin embedded tissue, samples were screened for the reported mutations via Sanger sequencing (screened regions indicated on Fig 1C in orange). The entire cohort of primary tumors and metastases was sequenced to detect the potential kinase domain mutations PIK3C2BG3542T and PIK3C2BA3623G. Eventually, neither could be identified, or any other mutation in the conserved catalytic and activation loop motifs in exon 25 of PIK3C2B (Fig 1D). Likewise, no alterations were found at amino acids positions 117 or 1469 (Fig 1D). The only observed sequential deviations were already documented SNPs in exon 3 and exon 25 of PIK3C2B (S1A Fig)
PIK3C2B in silico
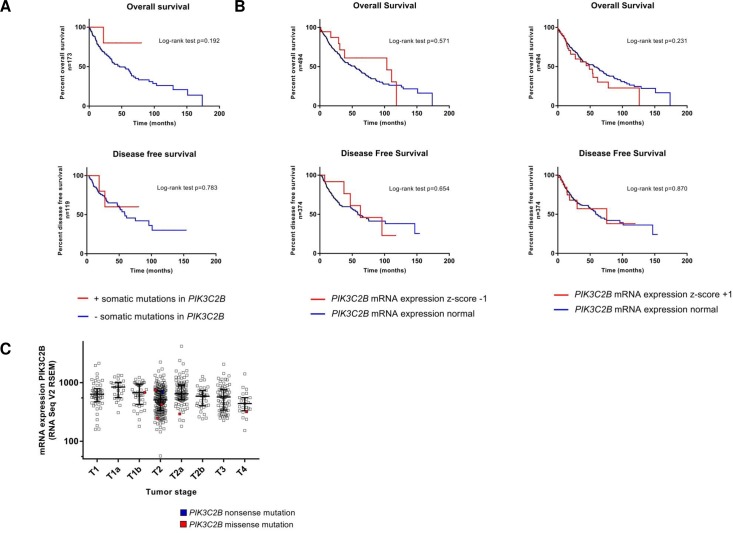
To put the results of the screening into a broader context, a set of 504 SQCC provided by the TCGA (cbioportal.org) was assessed in relation to PIK3C2B aberrations. As for somatic mutations, data were available for 177 tumors. Those harbored PIK3C2B alterations in 4% (7/177) of all cases, which were non-redundant and spread across the gene. Interestingly, previously described alterations P117A and H1208R were also found in the cohort. PIK3C2B mutations were not associated with a poorer overall or disease-free survival prognosis (Fig 2A). As for alterations in mRNA expression, a data set of 501 samples was available. Applying a z-score threshold of ± 1, the set was altered in 71/501 cases (upregulation in 50 cases, downregulation in 21 cases). Likewise, deviations in mRNA expression were not associated with significant deterioration of overall or disease-free survival (Fig 2B). Also, there was no observable pattern between American Joint Committee on Cancer (AJCC) tumor stages and the appearance of somatic mutations or mRNA expression levels (Fig 2C, n = 328). Protein expression level measured by reverse phase protein arrays (RPPA) was not altered in any of the samples after applying a z-score of ± 1(S1B Fig).
Fig 2. Meta-analysis of TCGA NSCLC-SQCC sequence data.
A Kaplan-Meier estimates of overall and disease free survival of patients with and without somatic mutations in PIK3C2B. Log rank test B Kaplan-Meier estimates of overall and disease free survival of patients with and without alterations in PIK3C2B mRNA expression. Z-score threshold ±1, RNA Seq V2 RSEM; log rank test C Scatter plot of PIK3C2B mRNA expression in all AJCC tumor stages. x-axis: AJCC tumor stages, y axis: RNA Seq V2 RSEM, log 10; log rank test.
Functional analysis of PI3KC2βC1181F/ PI3KC2βH1208R
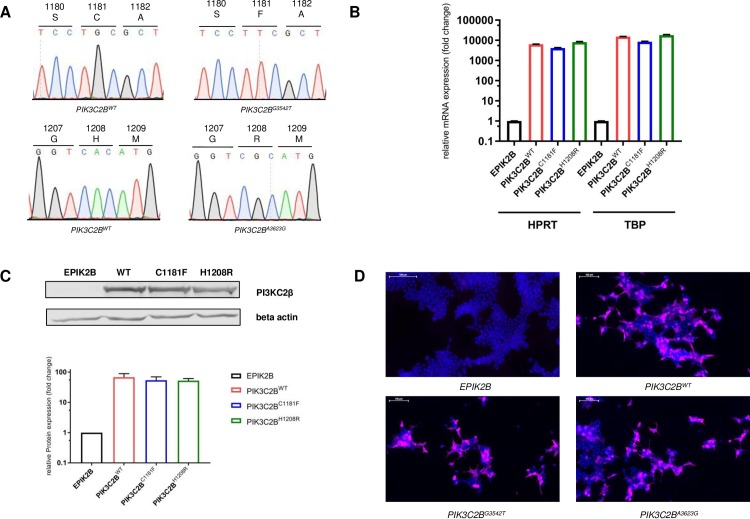
To assess the PI3KC2β mutations C1181F and H1208R on a functional level, they were cloned into a Myc-tagged PIK3C2B expression vector via site directed mutagenesis. Successful sequence alteration was verified by Sanger sequencing (Fig 3A). As a negative control, the ORF was removed to produce an empty backbone vector (EPIK2B). After CaCl2 transfection of HEK293 cells, PIK3C2B overexpression was examined on a transcriptional and translational level. Results of the conducted qPCRs and Western blots indicated strong overexpression on both levels (Fig 3B & 3C). Via immunofluorescence targeting the c-terminal Myc-tag of the protein, expression of exogenous PI3KC2β was witnessed in 65–75% HEK cells after transfection (Fig 3D).
Fig 3. PIK3C2B plasmid engineering and validation.
A Chromatograms of engineered PIK3C2B vectors B Relative mRNA expression of PIK3C2B after transfection of HEK293 cells (36h) with plasmid constructs. PIK3C2B expression was normalized to housekeeping genes hypoxanthin phosphoribosyltransferase 1 (HPRT) and TATA box binding protein (TBP). Means ± SEM; n = 3 independent experiments C Relative protein expression of PI3KC2β after transfection of HEK293 cells (36h) with plasmid constructs. Protein expression levels were normalized to empty vector EPIK2B. Means ± SEM; n = 3 independent experiments D Expression of exogenous PI3KC2β, visualized with immunofluorescence after transfection of HEK293 cells with plasmid constructs. Staining with DAPI (blue) and MycTag antibody (violet).
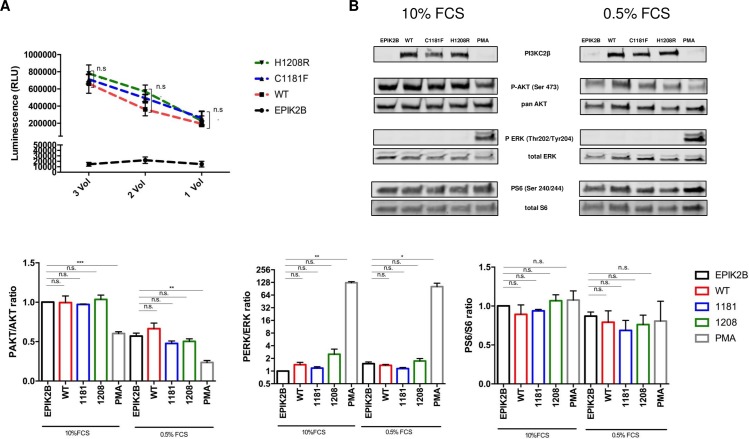
In addition, potential alterations in kinase activity caused by C1181F/ H1208R were measured. Following immunoprecipitation of exogenously expressed PI3KC2β variants from HEK293 cells with a MycTag antibody, kinase activity was measured with the ADP-Glo-Kinase Assay kit. Ultimately, no significant changes between PI3KC2βWT, PI3KC2βC1181F and PI3KC2βH1208R were detected (Fig 4A).
Fig 4. Functional Analysis of PI3KC2βWT, PI3KC2βC1181F and PI3KC2β H1208R.
A Lipid Kinase activity of PI3KC2βWT, PI3KC2βC1181F and PI3KC2β H1208R measured 36 hours after transfection of HEK293 cells with plasmid constructs. Exogenously expressed PI3KC2β was immunoprecipitated with sepharose beats. Different volumes (1x, 2x, 3x Vol.) of immunoprecipitates were exposed to 0.2mg/ml of PI substrate. Means ± SEM; n = 3 independent experiments; one-way ANOVA B Ratios of P-AKT/AKT, P-S6/S6 and P-ERK/ERK after transfection of HEK293 cells with plasmid constructs. 12h after transfection, cells were further kept in complete DMEM medium for 24h with 0.5% or 10% FCS. As a positive control for pathway alteration, cells were treated with 100nM PMA for 30 min. Protein expression levels were normalized to empty vector EPIK2B in unstarved conditions. Means ± SEM; n = 3 independent experiments; unpaired two tailed t-test.
Alterations in PI3K pathway activation were analyzed with Western blots. HEK293 cells were transfected with plasmid variants and subsequently kept at starved (0.5% FCS) or unstarved (10% FCS) conditions for 24 hours. Neither PI3KC2βC1181F nor PI3KC2βH1208R led to a significant increase in the phosphorylation of the pathway effector proteins AKT, S6 or ERK after transient overexpression compared to the wildtype allele (Fig 4B). As a positive control for pathway modulation, HEK cells treated with phorbol 12-myristate 13-acetate (PMA) were included (100nM, 30 min). Being a strong promotor of phosphokinase C, it led to the expected induction of ERK phosphorylation and a decrease in phosphorylation of AKT Ser473 (Fig 4B).
Discussion
Here, a large cohort of NSCLC-SQCC tumors was screened for four reported PIK3C2B missense mutations leading to amino acid exchanges (P117A, C1181F, H1208R, L1469F) [20]. In addition, kinase domain mutations C1181F and H1208R were assessed on a functional level.
The main focus lay on the two mutations in the kinase domain (C1181F, H1208R) bearing the highest potential for oncogenesis. None of these alterations were identified in 362 patient-derived tumor samples (Fig 1D). Assuming the frequency found in Liu et al., a complete absence of the mutations by chance in the significantly larger cohort had a p-value < 0.0001. The screening was then extended to L1469F, a mutation described in the phosphoinositide-binding domain (PX-domain). After screening the first half of the cohort, no mutations were found in 163 samples. Finally, the cohort was also screened for P117A, a mutation that occurred in the proline-rich domain of the protein. This region has been proposed to play a role in kinase activity regulation and clathrin binding [29]. There, the mutation was undetectable in 160 tested samples. The absence of any mutations in the cohort suggests that they might not confer a significant advantage in NSCLC-SQCC oncogenesis/signaling.
The lack of any detected mutations led to a thorough validation of the employed assay and the cohort. The technical approach was challenged by mixing different ratios of wildtype and mutant plasmids. Ultimately, the sequencing assay was found to be sensitive enough to detect the mutations at a 1:9 ratio (Fig 1A). The obtained sensitivity was satisfying since the samples contained a minimum fraction of 30% tumor tissue. Next, the cohort was challenged by screening it for p110α mutations E542K and E545K. Both are charge reversing hotspot mutations that were proven to be oncogenic and frequently found in NSCLC-SQCC [25]. There, both mutations were detected at the expected frequency (Fig 1B) according to the COSMIC database. These two experiments showed that the absence of detected mutations was neither due to a technical issue nor to a singularity of the cohort.
To put the findings of the screening into a broader context, a NSCLC-SQCC dataset provided by the TCGA was analyzed in regard to PIK3C2B alterations. As for somatic mutations (n = 7/177), they were non-redundant, rare and did not change clinical outcomes (Fig 2A). Interestingly, P117A and H1208R were also present once in the cohort. One potential explanation could be that both are passenger mutations that are more frequent in the screened northern American cohorts. Alterations in mRNA expression (up or down) did likewise not change clinical outcomes (Fig 2B) and did not translate into overexpression at the protein level (S1A Fig). Also, neither somatic mutations nor mRNA expression levels of PIK3C2B were associated with a particular tumor stage (Fig 2C).
In line with the findings of the screening, transient overexpression of the reported protein variants PI3KC2βC1181F and PI3KC2βH1208R in HEK293 cells (Fig 3) did not reveal an effect of the mutations on lipid kinase activity (Fig 4A) or PI3K/ERK pathway activation (Fig 4B) in either direction. This shows that the two mutations do not confer any additional effect than the effect of the wildtype protein.
Taken together, the present data do not suggest a driver function of somatic PIK3C2B mutations in NSCLC-SCC and that aberrant PI3K pathway activation in NSCLC-SQCC occurs through alterations in more central compartments of the signaling axes like EGFR, PIK3CA and PTEN (25). Also, a recent study analyzed the mutational patterns in lung adenocarcinomas and squamous cell carcinomas [30]. In accordance with the aforementioned findings, PIK3C2B was not found to be significantly mutated in either. As a more promising approach, future studies could investigate alterations in PIK3C2B concomitantly with alterations in other, potentially redundant PI3K isoforms.
In terms of cancer genetics, evidence for oncogenic implications of somatic PIK3C2B alterations is scarce. The only exception is a single nucleotide polymorphism that has been reported to be significantly associated with prostate cancer risk [31]. So far, studies have mainly described amplifications of the genomic PIK3C2B locus. Gain at 1q32.1, the chromosomal region encoding for PIK3C2B and MDM4 has been reported in studies assessing copy-number alterations in glioblastoma multiforme [32,33]. In ovarian cancer, copy number gains of PIK3C2B have been reported as well [34].
Increased levels of cellular PI3KC2β have repeatedly been associated with oncogenesis. A study downregulating 779 kinases via RNAi in breast cancer cells (MCF7) ranked the siRNA targeting PI3KC2β as one of the top 20 to sensitize cells to tamoxifen [35]. Another in vitro study overexpressing PI3KC2β in oesophageal squamous cells (Eca109) reached a similar conclusion. Overexpression of PI3KC2β led to a 4-fold reduction in sensitivity to cisplatin. siRNA mediated down-regulation of the enzyme resulted in restoration of sensitivity to the drug [17]. Conversely though, promotion of resistance to thiopurines in leukemia cells through deletion of PI3KC2β has also been described [36]. The effect of PI3KC2β may thus be drug specific.
In vivo, overexpression of PI3KC2β in suprabasal and basal epidermal cell layers in mice did not affect epidermal growth and differentiation [37]. In the same study, mice with ubiquitous homozygous deletions of PIK3C2B were viable, fertile and without any reported phenotype.
Apart from cellular outcomes and phenotypes, the molecular consequences of PI3KC2β amplification on pathway signaling remain to be determined.
This task is complicated by the fact that PI3KC2β was discovered on the basis of sequence homologies rather than a functional context [38]. Assuming that PI3KC2β is able to generate PtdIns(3,4)P2 [28], several studies investigated the possibility that the isoform is able to activate AKT kinase, a cardinal node in diverse signaling cascades. So far, contrasting evidence is present in the literature. Silencing of PI3KC2β has been shown to reduce AKT activation in neuroblastoma models [18]. Conversely though, no effect on AKT phosphorylation was detected in epidermoid carcinoma cells (A-431) overexpressing PI3KC2β when compared to parental cells [39]. Another study described an attenuation of AKT phosphorylation in PI3KC2β overexpressing HEK293 cells [40]. To explain these seemingly contradictive effects on AKT activation, an indirect cross-talk mechanism with other signaling molecules not relying on the generation of specific phosphoinositides was proposed [41]. Consistent with this hypothesis, a recent study found an indirect, even tissue specific effect of AKT activation upon PI3KC2β inhibition [42].
PI3KC2β may fulfill context dependent tasks in different cell types. Hence, it could pose a considerable challenge to determine direct downstream targets and the exact physiological conditions under which PI3KC2β acts. Nevertheless, it is a necessary prerequisite to integrate it into the precise context of cancer formation as it does not appear to be a classic oncogene.
Supporting information
A Alterations in PI3KC2β protein expression measured with reverse-phase protein array (RPPA). Z-score threshold ±1 B Table with incidence rate and position of found PIK3C2B single nucleotide polymorphisms (SNP).
(TIF)
A Relative mRNA expression of PIK3C2B after transfection of PC9 cells (36h) with plasmid constructs. PIK3C2B expression was normalized to housekeeping genes hypoxanthin phosphoribosyltransferase 1 (HPRT) and TATA box binding protein (TBP). Means ± SEM; n = 2 independent experiments. Relative protein expression of PIK3C2β after transfection of PC9 cells (36h) with plasmid constructs. Mean ± SEM; n = 2 independent experiments B Transfection efficacy measured in PC9 cells via immunofluorescence. Cells were transfected with a GFP plasmid for 36h. Staining with DAPI (blue). Share of GFP+ cells: 10–20%.
(TIF)
Acknowledgments
We would like to acknowledge the Microscopy Imaging Center of the University of Bern (MIC). Mr. Kind is enrolled in the Graduate School for Cellular and Biomedical Sciences (GCB) of the University of Bern that we would like to acknowledge for the training provided. Furthermore, we would like to acknowledge Fabiana Jacob and the entire staff of the DCR-VPH for a helping hand with the sequencing part.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Swiss National Science Foundation grant (146464). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Castoria G, Migliaccio A, Bilancio A, Di Domenico M, de Falco A, Lombardi M, et al. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J. European Molecular Biology Organization; 2001;20: 6050–9. doi: 10.1093/emboj/20.21.6050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. Essential Role of Phosphoinositide 3-Kinase δ in Neutrophil Directional Movement. J Immunol. 2003;170 Available: http://www.jimmunol.org/content/170/5/2647 [DOI] [PubMed] [Google Scholar]
- 3.Graupera M, Guillermet-Guibert J, Foukas LC, Phng L-K, Cain RJ, Salpekar A, et al. Angiogenesis selectively requires the p110α isoform of PI3K to control endothelial cell migration. Nature. Nature Publishing Group; 2008;453: 662–666. doi: 10.1038/nature06892 [DOI] [PubMed] [Google Scholar]
- 4.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91: 231–41. Available: http://www.ncbi.nlm.nih.gov/pubmed/9346240 [DOI] [PubMed] [Google Scholar]
- 5.Makarov SS, Romashkova JA. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401: 86–90. doi: 10.1038/43474 [DOI] [PubMed] [Google Scholar]
- 6.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. Nature Publishing Group; 2014;13: 140–156. doi: 10.1038/nrd4204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95: 29–39. Available: http://www.ncbi.nlm.nih.gov/pubmed/9778245 [DOI] [PubMed] [Google Scholar]
- 8.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275: 1943–7. Available: http://www.ncbi.nlm.nih.gov/pubmed/9072974 [DOI] [PubMed] [Google Scholar]
- 9.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18: 77–82. Available: http://www.ncbi.nlm.nih.gov/pubmed/16357568 [DOI] [PubMed] [Google Scholar]
- 10.Samuels Y. High frequency of mutations of the PIK3CA gene in human cancers. Science (80-). 2004;304: 554 doi: 10.1126/science.1096502 [DOI] [PubMed] [Google Scholar]
- 11.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci. 2005;102: 802–807. doi: 10.1073/pnas.0408864102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao JJ, Liu Z, Wang L, Shin E, Loda MF, Roberts TM. The oncogenic properties of mutant p110 and p110 phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci. 2005;102: 18443–18448. doi: 10.1073/pnas.0508988102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piddock RE, Loughran N, Marlein CR, Robinson SD, Edwards DR, Yu S, et al. PI3Kδ and PI3Kγ isoforms have distinct functions in regulating pro-tumoural signalling in the multiple myeloma microenvironment. Blood Cancer J. 2017;7: e539 doi: 10.1038/bcj.2017.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda H, Hideshima T, Fulciniti M, Perrone G, Miura N, Yasui H, et al. PI3K/p110{delta} is a novel therapeutic target in multiple myeloma. Blood. American Society of Hematology; 2010;116: 1460–8. doi: 10.1182/blood-2009-06-222943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshioka K, Yoshida K, Cui H, Wakayama T, Takuwa N, Okamoto Y, et al. Endothelial PI3K-C2α, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat Med. 2012;18: 1560–1569. doi: 10.1038/nm.2928 [DOI] [PubMed] [Google Scholar]
- 16.Katso RM, Pardo OE, Palamidessi A, Franz CM, Marinov M, De Laurentiis A, et al. Phosphoinositide 3-Kinase C2beta Regulates Cytoskeletal Organization and Cell Migration via Rac-dependent Mechanisms. Mol Biol Cell. 2006;17: 3729–3744. doi: 10.1091/mbc.E05-11-1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Sun C, Zhang Y, Ji Z, Yang G. Phosphatidylinositol 3-Kinase-C2β Inhibits Cisplatin-Mediated Apoptosis via the Akt Pathway in Oesophageal Squamous Cell Carcinoma. J Int Med Res. 2011;39: 1319–1332. doi: 10.1177/147323001103900419 [DOI] [PubMed] [Google Scholar]
- 18.Russo A, Okur MN, Bosland M, O’Bryan JP. Phosphatidylinositol 3-kinase, class 2 beta (PI3KC2β) isoform contributes to neuroblastoma tumorigenesis. Cancer Lett. 2015;359: 262–268. doi: 10.1016/j.canlet.2015.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boller D, Doepfner KT, De Laurentiis A, Guerreiro AS, Marinov M, Shalaby T, et al. Targeting PI3KC2β impairs proliferation and survival in acute leukemia, brain tumours and neuroendocrine tumours. Anticancer Res. 2012;32: 3015–27. Available: http://www.ncbi.nlm.nih.gov/pubmed/22843869 [PubMed] [Google Scholar]
- 20.Liu P, Morrison C, Wang L, Xiong D, Vedell P, Cui P, et al. Identification of somatic mutations in non-small cell lung carcinomas using whole-exome sequencing. Carcinogenesis. 2012;33: 1270–1276. doi: 10.1093/carcin/bgs148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pesch B, Kendzia B, Gustavsson P, Jöckel K-H, Johnen G, Pohlabeln H, et al. Cigarette smoking and lung cancer-relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer. Wiley Subscription Services, Inc., A Wiley Company; 2012;131: 1210–1219. doi: 10.1002/ijc.27339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41: 1238–1242. doi: 10.1038/ng.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. Meyerson M, editor. PLoS Med. 2006;3: e420 doi: 10.1371/journal.pmed.0030420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rekhtman N, Paik PK, Arcila ME, Tafe LJ, Oxnard GR, Moreira AL, et al. Clarifying the Spectrum of Driver Oncogene Mutations in Biomarker-Verified Squamous Carcinoma of Lung: Lack of EGFR/KRAS and Presence of PIK3CA/AKT1 Mutations. Clin Cancer Res. 2012;18: 1167–1176. doi: 10.1158/1078-0432.CCR-11-2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammerman PS, Lawrence MS, Voet D, Jing R, Cibulskis K, Sivachenko A, et al. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489: 519–525. doi: 10.1038/nature11404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data: Figure 1. Cancer Discov. 2012;2: 401–404. doi: 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci Signal. 2013;6: pl1–pl1. doi: 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arcaro A, Volinia S, Zvelebil MJ, Stein R, Watton SJ, Layton MJ, et al. Human phosphoinositide 3-kinase C2beta, the role of calcium and the C2 domain in enzyme activity. J Biol Chem. 1998;273: 33082–90. Available: http://www.ncbi.nlm.nih.gov/pubmed/9830063 [DOI] [PubMed] [Google Scholar]
- 29.Wheeler M, Domin J. The N-terminus of phosphoinositide 3-kinase-C2? regulates lipid kinase activity and binding to clathrin. J Cell Physiol. 2006;206: 586–593. doi: 10.1002/jcp.20507 [DOI] [PubMed] [Google Scholar]
- 30.Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48: 607–616. doi: 10.1038/ng.3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koutros S, Schumacher FR, Hayes RB, Ma J, Huang W- Y, Albanes D, et al. Pooled Analysis of Phosphatidylinositol 3-Kinase Pathway Variants and Risk of Prostate Cancer. Cancer Res. 2010;70: 2389–2396. doi: 10.1158/0008-5472.CAN-09-3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumihito N, Joel L, Anne W, Young Ho K, Jian H, Catherine L, et al. Intratumoral patterns of genomic imbalance in glioblastoma. Brain Pathol. 2010;20: 936–44. doi: 10.1111/j.1750-3639.2010.00395.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao SK, Edwards J, Joshi AD, Siu I-M, Riggins GJ. A survey of glioblastoma genomic amplifications and deletions. J Neurooncol. 2010;96: 169–179. doi: 10.1007/s11060-009-9959-4 [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Huang J, Yang N, Greshock J, Liang S, Hasegawa K, et al. Integrative Genomic Analysis of Phosphatidylinositol 3’-Kinase Family Identifies PIK3R3 as a Potential Therapeutic Target in Epithelial Ovarian Cancer. Clin Cancer Res. 2007;13: 5314–5321. doi: 10.1158/1078-0432.CCR-06-2660 [DOI] [PubMed] [Google Scholar]
- 35.Iorns E, Lord CJ, Ashworth A. Parallel RNAi and compound screens identify the PDK1 pathway as a target for tamoxifen sensitization. Biochem J. 2009;417: 361–371. doi: 10.1042/BJ20081682 [DOI] [PubMed] [Google Scholar]
- 36.Diouf B, Cheng Q, Krynetskaia NF, Yang W, Cheok M, Pei D, et al. Somatic deletions of genes regulating MSH2 protein stability cause DNA mismatch repair deficiency and drug resistance in human leukemia cells. Nat Med. 2011;17: 1298–1303. doi: 10.1038/nm.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harada K, Truong AB, Cai T, Khavari PA. The class II phosphoinositide 3-kinase C2beta is not essential for epidermal differentiation. Mol Cell Biol. American Society for Microbiology (ASM); 2005;25: 11122–30. doi: 10.1128/MCB.25.24.11122-11130.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown RA, Ho LKF, Weber-Hall SJ, Shipley JM, Fry MJ. Identification and cDNA Cloning of a Novel Mammalian C2 Domain-Containing Phosphoinositide 3-Kinase, HsC2-PI3K. Biochem Biophys Res Commun. 1997;233: 537–544. doi: 10.1006/bbrc.1997.6495 [DOI] [PubMed] [Google Scholar]
- 39.Katso RM, Pardo OE, Palamidessi A, Franz CM, Marinov M, De Laurentiis A, et al. Phosphoinositide 3-Kinase C2beta Regulates Cytoskeletal Organization and Cell Migration via Rac-dependent Mechanisms. Mol Biol Cell. 2006;17: 3729–3744. doi: 10.1091/mbc.E05-11-1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domin J, Harper L, Aubyn D, Wheeler M, Florey O, Haskard D, et al. The class II phosphoinositide 3-kinase PI3K-C2beta regulates cell migration by a PtdIns3P dependent mechanism. J Cell Physiol. 2005;205: 452–62. doi: 10.1002/jcp.20478 [DOI] [PubMed] [Google Scholar]
- 41.Falasca M, Maffucci T. Regulation and cellular functions of class II phosphoinositide 3-kinases. Biochem J. 2012;443: 587–601. doi: 10.1042/BJ20120008 [DOI] [PubMed] [Google Scholar]
- 42.Alliouachene S, Bilanges B, Chicanne G, Anderson KE, Pearce W, Ali K, et al. Inactivation of the Class II PI3K-C2β Potentiates Insulin Signaling and Sensitivity. Cell Rep. Elsevier; 2015;13: 1881–94. doi: 10.1016/j.celrep.2015.10.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A Alterations in PI3KC2β protein expression measured with reverse-phase protein array (RPPA). Z-score threshold ±1 B Table with incidence rate and position of found PIK3C2B single nucleotide polymorphisms (SNP).
(TIF)
A Relative mRNA expression of PIK3C2B after transfection of PC9 cells (36h) with plasmid constructs. PIK3C2B expression was normalized to housekeeping genes hypoxanthin phosphoribosyltransferase 1 (HPRT) and TATA box binding protein (TBP). Means ± SEM; n = 2 independent experiments. Relative protein expression of PIK3C2β after transfection of PC9 cells (36h) with plasmid constructs. Mean ± SEM; n = 2 independent experiments B Transfection efficacy measured in PC9 cells via immunofluorescence. Cells were transfected with a GFP plasmid for 36h. Staining with DAPI (blue). Share of GFP+ cells: 10–20%.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.