Myelin oligodendrocyte glycoprotein immunoglobulin G (anti-MOG-IgG) has been recently found to be associated with some forms of idiopathic inflammatory demyelinating CNS disorders. In a preliminary study from India, recurrent optic neuritis and isolated longitudinally extensive transverse myelitis were identified as the common phenotypes.1 The clinical and radiologic features of this newly discovered subset of autoimmune disorders are only beginning to be understood. In this context, we would like to draw attention to an unusual and hitherto unreported association noticed in a patient with anti-MOG-IgG–associated myelitis.
Methods.
Case.
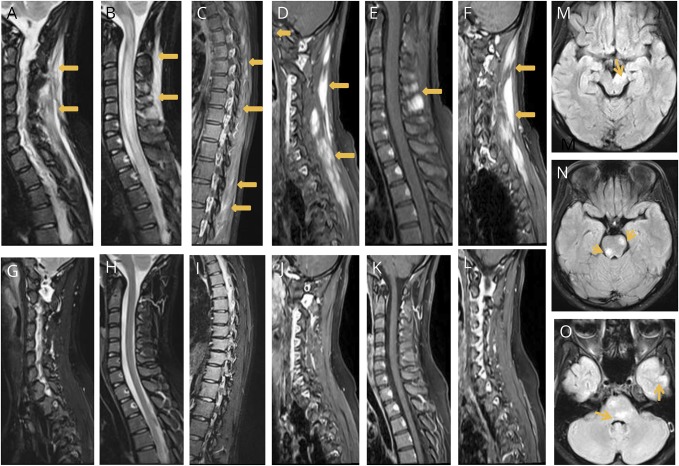
A 22-year-old woman developed rapidly worsening paraplegia with urinary retention without any preceding illness. There was no myalgia or muscle tenderness at the time of examination. Routine investigations including erythrocyte sedimentation rate, C-reactive protein, and serum creatine phosphokinase were normal. Antinuclear antibody was negative. An MRI of the spine revealed longitudinally extensive myelitis involving the entire cord (figure, B). The posterior paraspinal muscles in relation to cervical, upper thoracic, and lumbar vertebrae showed hyperintense signals on T2TIRM (turbo inversion recovery magnitude) sequences with postcontrast (gadolinium) enhancement (figure, A–F). An MRI of the brain showed nonenhancing patchy lesions in the brain stem, cerebellum, bilateral frontal, and left temporal cortex (figure, G and H). Electrophysiologic examination (including F-wave studies and paraspinal muscle electromyography) was unremarkable. Cell-based assay for MOG IgG was positive (titer 1: 8,192, range 1: 128-1: 655362), while serum aquaporin 4 IgG was negative (assays2 were performed at coauthors' laboratory in Japan). CSF examination was unremarkable except for a mild increase in protein (59 mg/dL; normal range 15–45 mg/dL), and oligoclonal bands were absent. Investigations including imaging were performed before treatment. Patient recovered completely after receiving IV methyl prednisolone for 5 days followed by an oral taper of prednisolone over 6 weeks. A repeat scan done after an interval of 3 months (figure, F–K) revealed a complete resolution of spinal cord lesions and paraspinal muscle hyperintensity. Currently, she remains well nearly 18 months after disease onset.
Figure. MRI of anti-MOG-IgG–associated myelitis.
MRI—Sagital T2 TIRM sequences of the cervical (A and B) and thoracic spine (C), showing hyperintense signals selectively involving the paraspinal muscles (arrows) and longitudinally extensive hyperintense intrameduallry lesion extending from the cervical medullary junction into the dorsal cord; gadolinium (GADAVIST, 0.5 M) enhancement (D–F) of the paraspinal muscles (arrows) noted. MRI scans (G–L) show complete resolution of lesions 3 months later. Brain MRI showed lesions (arrows) in the right cerebral peduncle (M), multiple lesions in the pons (N and O), and temporal cortex (O). TIRM = turbo inversion recovery magnitude.
Discussion.
This young woman had anti-MOG–IgG–associated longitudinal extensive transverse myelitis accompanied by striking paraspinal muscle signal changes on MRI that extended from cervical to lumbar regions. These lesions were asymptomatic, did not involve subcutaneous tissue (ruling out injury from external causes), and were not present in other groups of muscles screened. The muscle abnormality was maximally seen in cervical and upper dorsal regions, corresponding to the sites of maximal cord abnormality. It completely disappeared with resolution of spinal cord lesions. The hyperintense signals seen in paraspinal muscles on T2 turbo inversion recovery magnitude (TIRM) sequences suggested muscle edema, and the contrast enhancement was in keeping with an inflammatory muscle disease.3 However, MOG is a myelin protein solely expressed at the outermost surface of myelin sheaths and oligodendrocyte membranes. Besides, a primary/concommitant muscle disease selectively involving paraspinal muscles was unlikely.
Involvement of extraneural tissue has been described previously in anti-MOG-IgG–associated disease. Perineural enhancement that extended into the orbital soft tissue surrounding inflammed optic nerve segments has been reported in patients with anti-MOG-IgG–related optic neuritis.4,5 These changes were seen before receiving steroids and particularly in the first attack of optic neuritis.5 Our patient's findings may be of similar nature, involving paraspinal muscles adjoining affected spinal cord segments. It is possible that the selective and transient involvement of paraspinal muscles is causally linked with the spinal cord inflammation. Alternatively, we hypothesize that a parallel (second) antibody with a different target and possibly affecting vascular permeability may be involved.
Our current understanding of the spectrum of anti-MOG–IgG–associated disorders is limited, and larger cohorts are needed to fully understand the spectrum of clinical and radiologic manifestations of these disorders.
Footnotes
Author contributions: Dr. Lekha Pandit: study concept, design, acquisition of data, and analysis and interpretation. Dr. Sharik Mustafa: study design and concept, acquisition of data, and critical revision of the draft. Dr. Raghuraj Uppor: contributed to data aquisition and data analysis. Dr. Ichiro Nakashima, Dr. Toshiyuki Takahashi, and Dr. Kimhiko Kaneko: analysis and interpretation.
Study funding: No targeted funding reported.
Disclosure: L. Pandit, S. Mustafa, and R. Uppoor report no disclosures. I. Nakashima received travel funding and/or speaker honoraria from Mitsubishi Tanabe Pharma and Novartis Pharma; is an editorial board member for Multiple Sclerosis International; and received research support from LSI Medicine Corporation. T. Takahashi received research support from Cosmic corporation. K. Kaneko reports no disclosures. Go to Neurology.org/nn for full disclosure forms. The Article Processing Charge was funded by the authors.
References
- 1.Pandit L, Sato D, Mustafa S, et al. . Relapsing optic neuritis and isolated transverse myelitis are the predominant clinical phenotypes for patients with antibodies to myelin oligodendrocyte glycoprotein in India. Mult Scler J Exp Trans Clinic 2016;2:2055217316675634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato DK, Callegaro D, Lana-Peixoto MA, et al. . Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014;82:474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.May DA, Disler DG, Jones EA, et al. . Abnormal signal intensity in Skeletal muscle at MR imaging: patterns, pearls, and pitfalls. Radiographics 2000;20:S295–S315. [DOI] [PubMed] [Google Scholar]
- 4.Jarius S, Ruprecht K, Kleiter I, et al. . MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflamm 2016;13:280 doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SM, Woodhall MR, Kim JS, et al. . Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm 2015;2:e163 doi: 10.1212/NXI.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]