Abstract
Recent advances in microfabrication technologies and advanced biomaterials have allowed for the development of in vitro platforms that recapitulate more physiologically relevant cellular components and function. Microengineered vascular systems are of particular importance for the efficient assessment of drug candidates to physiological barriers lining microvessels. This review highlights advances in the development of microengineered vascular structures with an emphasis on the potential impact on drug delivery studies. Specifically, this article examines the development of models for the studies on drug delivery to the central nervous system and cardiovascular system. We also discuss current challenges and future prospects of the development of microengineered vascular systems.
INTRODUCTION
The traditional paradigm of tissue engineering involves the combination of isolated patient cells and extracellular matrix proteins to produce an implantable substitute for damaged tissue. In particular, vascular tissue engineering pursuits include the development of cell based vascular grafts1, 2 and vascularized tissue implants.3–6 The introduction of microfluidic devices has broadened the traditional scope of tissue engineering to include microengineered tissue systems that attempt to reproduce relevant organ physiology. Microfluidic devices offer replicable and cost effective platforms7, 8 on which to study disease states and conduct preliminary drug screening and toxicology studies.9–12 Microfluidics have also been extended to point-of-care devices for patient-specific diagnoses.13–16 In this review, we will overview the key physiological components necessary for accurate in vitro vascular recapitulation and discuss the state-of-the-art microengineered technology available for the study of vascular systems. In vitro models of brain microvessels and arterial lining will be discussed with particular emphasis on the ability of these models to be used for preliminary drug studies.
Vascular Physiology in Relevance to Drug Discovery and Disease
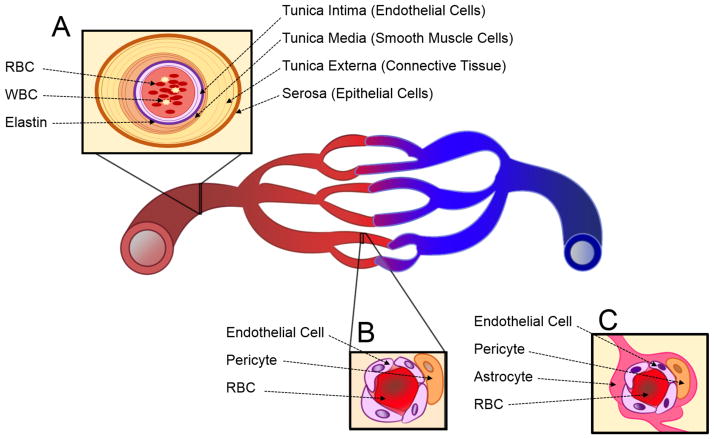
The design of reliable in vitro tissue systems requires an in-depth understanding of the in vivo physiology being recapitulated. Arteries and capillaries are distinguished by several key physiological differences (Figure 1). Arteries are thick walled, consisting of three distinct layers, and elastic to facilitate the circulation of blood from the heart to target tissues (Figure 1A). Capillaries are thin walled and consist of an endothelial cell monolayer supported by pericyte cells. This thin monolayer of cells facilitates efficient oxygen delivery, nutrient delivery, and waste removal from target tissues (Figure 1B). In both vascular systems, the relative cellular and extracellular components work together to maintain vascular functionality. Development of more physiologically relevant in vitro models to study vasculature has been a rising priority in drug discovery. In the arterial disease atherosclerosis, plaques can occur from the accumulation of foam cells, immune cells, platelets, extracellular matrix (ECM), and fats at damaged endothelial sites.17 To ensure physiological relevance to arterial vasculature, these components must be taken into consideration in the development of an in vitro model for atherosclerosis.18–22 Notable components that have been studied include endothelial cells, ECM, and platelets.21, 23, 24 The study of capillary disease manifestation, formation, and maintenance is also significant in the context of drug delivery. For example, the development of drugs for central nervous system (CNS) disorders is particularly challenging due to the specialized capillary microvessels of the blood brain barrier (BBB) (Figure 1C). The contributions of several relevant cell types, collectively called the neurovascular unit, are necessary for proper BBB function. Thus, the decision to exclude a component from a simplified in vitro model must be seriously considered in the context of each particular study.
Figure 1.
Schematic illustration of vascular systems: (A) an artery, (B) a general capillary, and (C) a neurovascular unit of central nervous system (CNS) capillary. RBC is red blood cell and WBC is white blood cell.
State-of-the-art Microengineered Technologies
Drug development is time consuming and risky. Only 1 in every 10,000 drug candidates make it to market, and those that do take well over a decade to develop.7 The need for animal in vivo verification in determining suitable drugs for clinical trials has remained unchanged. Meanwhile, a rise in in vitro profiling for drug candidates reflects the need to develop suitable in vitro models for various diseases.25 An abundant pool of microfabrication techniques are being applied for the development of microengineered tissue systems, including photolithography26–28, micromachining29–31, 3D printing32–34, paper printed devices35–37, thermoforming38–40, and wire/needle-based molding.41 With these technologies, microengineered tissue models can incorporate ECM scaffolds that provide more physiologically relevant microenvironments for cells and tissues.42, 43 These approaches provide more cost effective methodologies for evaluating drug efficacy than time-consuming animal tests, in which drug candidates are evaluated for an extended period of time before being dropped from the study.44 A high-throughput solution with physiological accuracy would then be desirable for identifying unsuitable drugs, which can reduce the number of animal tests, saving the cost and time. Although in vitro technology does not fully recapitulate the complexity of an in vivo system, it remains attractive as a cost effective alternative to highly variable in vivo studies.
MIRCROENGINEERED VASCULAR SYSTEMS
Microvasculature Platforms for Drug Screening: The Blood Brain Barrier
The BBB poses a significant challenge to CNS drug delivery by restricting the permeability of molecules from systemic circulation via a variety of specialized endothelial cell processes. Exposure to soluble cues from astrocyte cells induces increased expression of tight junction proteins between neighboring endothelial cells, resulting in limited paracellular diffusion of solutes. Direct interaction with pericyte cells causes the brain endothelial cells to have a decreased number of fenestra and pinocytic vesicles and to exhibit a specialized portfolio of cell surface receptors, culminating in a restriction of transcellular permeability.45–48 These specializations lead to significantly decreased permeability of the brain endothelium to circulating molecules. To promote the efficacy of systemically administered drugs, it is essential to assess the degree to which a drug candidate can effectively cross the BBB in the drug development process.
In vitro models of the BBB provide a cost effective platform for high throughput screening in the early stages of pharmaceutical studies. In order to be a predictive model, an in vitro model of the BBB would ideally recapitulate several key in vivo properties: physiologically relevant shear rates; acceptable barrier confluence indicated by transendothelial electrical resistance (TEER) measurements; relevant expression of tight junction molecules leading to restricted paracellular permeability; physiologically relevant cellular architecture; and functional expression of critical cell surface receptors. More importantly, an in vitro BBB model with these key features must be highly controllable, repeatable, robust, and easy to fabricate with standard methods. Several in vitro tissue systems have been developed for use as platforms to assess the ability of novel drug candidates to cross the BBB.48, 49 In particular, microfluidic models have demonstrated promise for use in the fields of drug development and disease study. The following sections compare microfluidic models to other available in vitro BBB models and discuss the challenges preventing wide spread adaptation of microfluidic models for use in drug screening and toxicology studies.
The simplest in vitro BBB models are static transwell cultures in which relevant cell types are cultured on the surfaces of a semi-permeable porous membrane. These models are easy to culture and readily facilitate TEER measurement with commercially available devices. Transwell cultures can be monocultures of endothelial cells, co-cultures of endothelial and astrocyte cells, or tri-cultures of endothelial, astrocyte and pericyte cells. Transwell models are currently the most widely utilized in vitro models of the BBB due to their ability to accommodate multiplexing for high throughput study. However, they lack several components of an ideal drug screening model discussed above. Specifically, static transwell designs do not allow for the direct interaction of cell types or the incorporation of relevant shear flow rates.49 A subset of static culture systems, such as spheroids and Matrigel angiogenesis assays,50 fall under the category of microtissue culture systems. These systems allow for detailed examination of direct cellular interaction on relevant length scales, but do not easily facilitate TEER measurement or flow incorporation.
Several techniques can be used to incorporate shear stress in an in vitro device. Cone plate viscometers can be added to transwell culture systems49 or cells can be seeded on a substrate for use in a parallel plate flow chamber.51 The Janigro Laboratory has designed several iterations of a novel dynamic in vitro BBB (DIV-BBB) device utilizing hollow polypropylene fibers to mimic microvessels. While this model reports the most physiological TEER values to date (1000 OΩ·cm2 compared to 2000–4000 O OΩ·cm2 for in vivo BBB) and has been successfully used in studying transendothelial trafficking of immune cells,52 many literature sources deem it unsuitable for high throughput pharmacological studies. Reasons cited include the large number of cells needed to load the device, the necessity of specific technical skills to establish the model and the inability to conduct non-destructive microscopy.53, 54
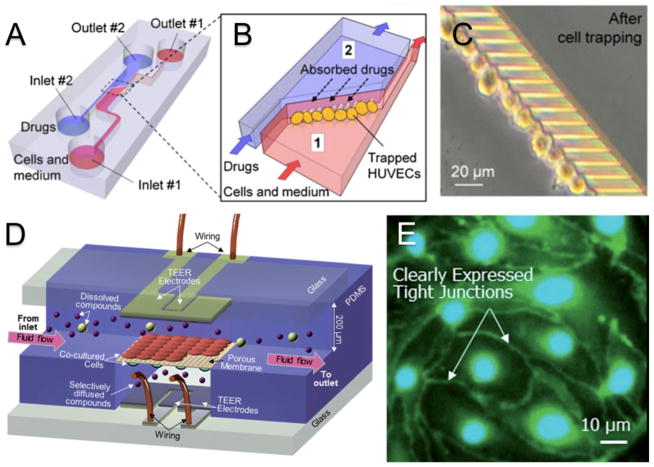
Recent advances in microfabrication techniques have allowed for the development of microtissue engineered BBB models capable of incorporating flow. Microfluidic models provide distinct advantages by allowing for nondestructive microscopy of cellular interactions on relevant length scales in the presence of physiologically relevant shear rates. Additionally, by downsizing the device setup, microfluidic devices require less cells and reagents, translating into better cost efficiency. In the most common microfluidic configurations, cells are cultured on semipermeable membranes at the interface of two microchannels made from polydimethylsiloxane (PDMS) that are supplied with media flow.55 These models have been redesigned and altered to include electrodes for TEER measurements.54, 56 Several variations of this general device structure have been published.53 Prabhakarpandian et al. developed a model with two side by side compartments separated by regularly spaced (3μm) pillars in which RBE4 endothelial cells seeded on the apical side are exposed to astrocyte conditioned media on the basolateral side to simulate the presence of astrocyte cells and induce the expression of relevant tight junction proteins.55 Incorporating platinum electrodes for TEER measurements, Griep et al. proposed a similar device by seeding hCMEC/D3 endothelial cells on a semipermeable membrane between two microchannels. This approach reported physiological barrier changes in TEER measurements in response to mechanical stimuli in the form of flow administration and biochemical stimuli in the form of TNF-α administration.56 Yeon et al. proposed a different model of the BBB in which culture time was greatly reduced. Human umbilical vein endothelial cells (HUVECs) were trapped in microholes via a pressure gradient and incubated with astrocyte conditioned media for as few as two hours before drug permeability studies were conducted (Figure 2A–C). Physiologically relevant shear rates were observed and immunofluorescence staining revealed a slightly discontinuous expression of tight junction proteins. The permeability for several drugs was shown to be comparable to those measured with other conventional methods.57 Booth and Kim have proposed the most comprehensively characterized microfluidic model to date (Figure 3C and 3D). In this model, b.END3 brain endothelial cells and C8-D1A astrocyte cells are cultured on either side of an ultrathin semipermeable membrane between to microfluidic flow chambers. Co-culture with astrocyte cells under flow conditions increased the TEER to over 250 OΩ·cm2 after three days of culture. Low permeability to tracker molecules was reported and verified by histological staining that showed a continuous membrane.54 While microfluidic BBB models provide a marked improvement over static culture models, they have yet to see wide spread implementation in the drug development process. This can be attributed to a number of factors including technological expertise needed for design and operation of devices, relatively high cost of model establishment, and insufficient multiplexing for high throughput studies.53
Figure 2.
In vitro models of the BBB for permeability studies. (A) Schematic representation of microfluidic cell trapping device for drug permeability studies across trapped HUVEC cells. (B) Detailed schematic experimental set up. Drugs are administered into microchannel 2 (blue) and transport across trapped HUVEC cell layer is quantified by measuring drug content in microchannel 1 (red). (C) HUVEC cells trapped in microholes are in close contact with each other. (D) Schematic depiction of a μBBB model. Endothelial (b.END3) and astrocyte (C8-D1A) cells are seeded on either side of polycarbonate membrane separating two microfluidic flow chambers. (E) Fluorescent staining of endothelial cell monolayer from μBBB devices clearly identifies expression of tight junction proteins. Reproduced from Yeon et al.57 and Booth et al.54 with permission.
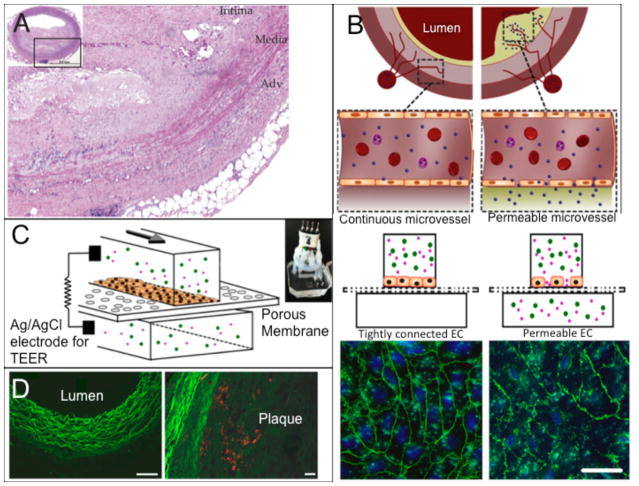
Figure 3.
(A) In vivo study exploring the effect of atherosclerotic lesions on microvessels surrounding an affected artery and on thrombotic complications from microvessel occlusion found dysfunctional endothelium in the surrounding microvessels. (B) Schematic of surrounding microvessels being permeable to nanoparticles due to disrupted endothelium. Fluorescent image shows the normal (left) and disrupted (right) adherens junctions. (C) A schematic of the microfluidic system studying nanoparticle translocation. (D) In vivo results from atherosclerotic aorta from a rabbit being treated with nanoparticles seen in red. Left is the lumen area and right shows the plaque with accumulation of nanoparticles. Reproduced from Sluimer et al. 64 and Kim et al.21 with permission.
Microvasculature Platforms for Drug Screening: Arterial Lining
Cardiovascular disease (CVD) accounted for more than 30% of all deaths in the United States in 2010.58 In atherosclerosis, one of the most common manifestations of CVD, the dysfunctional endothelium causes a chronic inflammatory response19 leading to increased endothelial permeability. The subsequent aggregation and introduction of platelets and leukocytes to the dysfunctional endothelium results in intimal thickening and occlusion.59 Though animal models for atherosclerosis have been used for drug development,60–62 microengineered in vitro vascular systems that can mimic microvessels in atherosclerotic regions (Figure 3A) have started to demonstrate potential for evaluating drug candidates. Estrada et al. were able to replicate atherosclerotic flow conditions to induce endothelial dysfunction. The model featured an elastic membrane on which human aortic endothelial cells (HAECs) were exposed to cyclic strains ranging from 5–10% and to oscillatory flow patterns with low shear stresses of approximately 1.3 dynes/cm2, which both are pertinent in atherosclerosis.63 Disturbed flow resulted in an increase in cell shape deformation and misalignment of nearly 60% in both cases. Kim et al. developed an endothelialized model that allowed the investigation of the relationship between the permeability of an endothelial monolayer and the nanoparticle translocation across the permeable endothelium (Figure 3B and 3C).21 This study focused on a potential pathway for drug delivery to plaque in atherosclerosis by mimicking the permeable endothelium of microvessels surrounding the arterial vessel or penetrating into the plaque.18, 64 The device was fabricated through assembling a porous elastic membrane with upper and lower channels. A monolayer of HUVECs was grown onto the porous polymer membrane and the monolayer permeability was measured using silver/silver chloride (Ag/AgCl)65, 66 electrodes on either side (Figure 3C). To mimic the permeable conditions of microvessels residing in atherosclerotic plaques, endothelial cells in the device were exposed to a combination of low shear stresses and the inflammatory mediator TNF-α. In addition, this study utilized two different nanoparticle types, Cy5.5-lipid-PLGA and Cy7-albumin, to examine the relationship between the endothelial permeability (via the albumin translocation) and the lipid-PLGA nanoparticle translocation in the in vitro microchip and an in vivo rabbit model of atherosclerosis. This work found that the nanoparticle translocation across the endothelial layer was highly dependent on the endothelial permeability in both the in vitro and in vivo models (Figure 3D). Such studies demonstrate the feasibility of microfluidic models for the examination of potential drug delivery mechanisms for CVD.
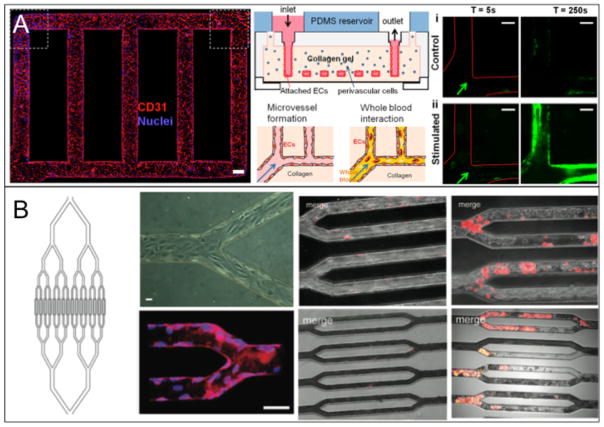
Occlusions that can arise from atherosclerosis and progress into thrombosis are part of an essential point of investigation in vascular disease prevention and treatment. Platelets that aggregate to form such occlusions are often exposed to a range of shear rates (greater than 4000s−1) or chemical factors that can cause activation and occlusion at inflamed sites.20 Recent approaches using endothelial cells cultured in three-dimensional vascularized microfluidic chips have demonstrated the ability to mimic dynamics of platelet aggregation. Zheng et al. developed a model that used a patterned collagen gel as a substrate for HUVECs to form a complete vascular network (Figure 4A).24 The network was used to study angiogenesis and whole blood-endothelium interactions with applications to thrombosis. Von Willebrand factor (VWF), a platelet binding protein that can be released from endothelial Weibel-Palade bodies, in combination with phorbol-12-myristate-13-acetate (PMA) was used in the thrombosis study to stimulate platelet aggregation. Aggregates grew over the course of 250 seconds to nearly occlude the PMA stimulated vessels while non-stimulated vessels did not form such aggregates. Tsai et al. featured a model using whole blood flowing through bifurcations in microfluidic devices seeded with either HUVECs or human lung microvascular endothelial cells (HLMVECs) (Figure 4B).23 In this study, HLMVECs were activated with the inflammatory mediator TNF-α to stimulate the coagulation of the blood, and HUVECs were used in conjunction with a toxin called STX2 to induce Hemolytic-uremic syndrome (HUS) to form thrombotic lesions. These approaches resulted in occlusion formation through platelet aggregation, showing the ability to reproduce multiple types of thrombus formations (Figure 4B). Both studies successfully stimulated thrombotic lesions to form in three-dimensional models, helping to elucidate the pathogenesis behind thrombotic complications in vascular disease.
Figure 4.
(A) A microvasculature system that shows occlusion due to aggregated platelets labeled with green CD41a. The endothelialized system where CD31 is shown in red and nuclei are shown in blue (left), a schematic of the entire system (middle) and the occlusion results for both stimulated and non-stimulated vasculature (right) are given. (B) A microvasculature-on-a-chip system that also shows occlusion in thrombotic environments. This study features HLMVECs and whole blood dyed with R6G that preferentially stains platelets and leukocytes. A schematic with endothelialized channels (left half) and platelet occlusion results (right half) are given. The results show occlusion progression with the effects of TNF-α activation for HLMVECs (top) and STX-2 activation for HUVECs (bottom). Reproduced from Zheng et al.24 and Tsai et al.23 with permission.
FUTURE PROSPECTS
Microengineered tissue systems have demonstrated potential for use in disease and drug related studies. Additionally, they provide a unique opportunity for detailed examination of phenomena that are hard to observe in vivo. In vitro systems are constantly questioned for their accuracy due to their inherent simplicity compared to the inherent complexity of in vivo models.7 Recent advances in microfabrication have allowed for the development of more physiologically relevant in vitro systems. These in vitro microsystems can provide a better solution to balance simplicity and physiological relevance.68 The balanced model systems have the potential to accelerate research towards the successful integration of different microsystems for expanded in vitro studies. Though the idea of integrating multiple in vitro micro-organ systems together is by no means a new one,69–72 the technical limitations preventing implementation of such a system have yet to be overcome. To a degree, microengineered organ systems have been developed to study disease relevant sub-organ systems.73–76 Recent improvements to microfluidic technologies may stir the development of better high throughput devices that can represent physiological function for greater impact in drug development.
References
- 1.McClendon MT, Stupp SI. Tubular hydrogels of circumferentially aligned nanofibers to encapsulate and orient vascular cells. Biomaterials. 2012;33:5713–5722. doi: 10.1016/j.biomaterials.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li S, Sengupta D, Chien S. Vascular tissue engineering: from in vitro to in situ. Wiley Interdiscip Rev Syst Biol Med. 2014;6:61–76. doi: 10.1002/wsbm.1246. [DOI] [PubMed] [Google Scholar]
- 3.Juhas M, Engelmayr GC, Jr, Fontanella AN, Palmer GM, Bursac N. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proc Natl Acad Sci U S A. 2014;111:5508–5513. doi: 10.1073/pnas.1402723111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooper RC, Hernandez KA, Boyko T, et al. Fabrication and In Vivo Microanastomosis of Vascularized Tissue-Engineered Constructs. Tissue Eng Part A. 2014 doi: 10.1089/ten.tea.2013.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black AF, Berthod F, L’Heureux N, Germain L, Auger FA. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 1998;12:1331–1340. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- 6.Yeatts AB, Both SK, Yang W, et al. In vivo bone regeneration using tubular perfusion system bioreactor cultured nanofibrous scaffolds. Tissue Eng Part A. 2014;20:139–146. doi: 10.1089/ten.tea.2013.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esch MB, King TL, Shuler ML. The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng. 2011;13:55–72. doi: 10.1146/annurev-bioeng-071910-124629. [DOI] [PubMed] [Google Scholar]
- 8.Moraes C, Mehta G, Lesher-Perez SC, Takayama S. Organs-on-a-chip: a focus on compartmentalized microdevices. Ann Biomed Eng. 2012;40:1211–1227. doi: 10.1007/s10439-011-0455-6. [DOI] [PubMed] [Google Scholar]
- 9.Wong KH, Chan JM, Kamm RD, Tien J. Microfluidic models of vascular functions. Annu Rev Biomed Eng. 2012;14:205–230. doi: 10.1146/annurev-bioeng-071811-150052. [DOI] [PubMed] [Google Scholar]
- 10.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71:185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Price GM, Tien J. Methods for forming human microvascular tubes in vitro and measuring their macromolecular permeability. Methods Mol Biol. 2011;671:281–293. doi: 10.1007/978-1-59745-551-0_17. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip. 2013;13:1489–1500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 13.Harris LF, Rainey P, Castro-Lopez V, O’Donnell JS, Killard AJ. A microfluidic anti-Factor Xa assay device for point of care monitoring of anticoagulation therapy. Analyst. 2013;138:4769–4776. doi: 10.1039/c3an00401e. [DOI] [PubMed] [Google Scholar]
- 14.Lee W, Jung J, Hahn YK, et al. A centrifugally actuated point-of-care testing system for the surface acoustic wave immunosensing of cardiac troponin I. Analyst. 2013;138:2558–2566. doi: 10.1039/c3an00182b. [DOI] [PubMed] [Google Scholar]
- 15.Sista R, Hua Z, Thwar P, et al. Development of a digital microfluidic platform for point of care testing. Lab Chip. 2008;8:2091–2104. doi: 10.1039/b814922d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chin CD, Linder V, Sia SK. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip. 2012;12:2118–2134. doi: 10.1039/c2lc21204h. [DOI] [PubMed] [Google Scholar]
- 17.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz RS, Burke A, Farb A, et al. Microemboli and microvascular obstruction in acute coronary thrombosis and sudden coronary death: relation to epicardial plaque histopathology. J Am Coll Cardiol. 2009;54:2167–2173. doi: 10.1016/j.jacc.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 19.Sarah Jane George CL. Pathogenesis of Atherosclerosis. In: Sarah Jane George JJ, editor. Atherosclerosis: Molecular and Cellular Mechanisms. Wiley-VCH; 2010. pp. 1–20. [Google Scholar]
- 20.Li M, Hotaling NA, Ku DN, Forest CR. Microfluidic thrombosis under multiple shear rates and antiplatelet therapy doses. PLoS One. 2014;9:e82493. doi: 10.1371/journal.pone.0082493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y, Lobatto ME, Kawahara T, et al. Probing nanoparticle translocation across the permeable endothelium in experimental atherosclerosis. Proc Natl Acad Sci U S A. 2014;111:1078–1083. doi: 10.1073/pnas.1322725111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai M, Kita A, Leach J, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122:408–418. doi: 10.1172/JCI58753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, Chen J, Craven M, et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A. 2012;109:9342–9347. doi: 10.1073/pnas.1201240109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowes J, Brown AJ, Hamon J, et al. Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nat Rev Drug Discov. 2012;11:909–922. doi: 10.1038/nrd3845. [DOI] [PubMed] [Google Scholar]
- 26.Harriott LR. Limits of lithography. Proceedings of the IEEE. 2001;89:366–374. [Google Scholar]
- 27.Huh D, Kim HJ, Fraser JP, et al. Microfabrication of human organs-on-chips. Nat Protoc. 2013;8:2135–2157. doi: 10.1038/nprot.2013.137. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Hirdes D, Folch A. Gray-scale photolithography using microfluidic photomasks. Proc Natl Acad Sci U S A. 2003;100:1499–1504. doi: 10.1073/pnas.0435755100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giridhar MS, Seong K, Schulzgen A, Khulbe P, Peyghambarian N, Mansuripur M. Femtosecond pulsed laser micromachining of glass substrates with application to microfluidic devices. Appl Opt. 2004;43:4584–4589. doi: 10.1364/ao.43.004584. [DOI] [PubMed] [Google Scholar]
- 30.Teixidor D, Thepsonthi T, Ciurana J, Özel T. Nanosecond pulsed laser micromachining of PMMA-based microfluidic channels. Journal of Manufacturing Processes. 2012;14:435–442. [Google Scholar]
- 31.Wilson ME, Kota N, Kim Y, et al. Fabrication of circular microfluidic channels by combining mechanical micromilling and soft lithography. Lab Chip. 2011;11:1550–1555. doi: 10.1039/c0lc00561d. [DOI] [PubMed] [Google Scholar]
- 32.Bertassoni LE, Cecconi M, Manoharan V, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip. 2014;14:2202–2211. doi: 10.1039/c4lc00030g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee V, Lanzi A, Ngo H, Yoo S-S, Vincent P, Dai G. Generation of Multi-scale Vascular Network System Within 3D Hydrogel Using 3D Bio-printing Technology. Cellular and Molecular Bioengineering. 2014:1–13. doi: 10.1007/s12195-014-0340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitson PJ, Rosnes MH, Sans V, Dragone V, Cronin L. Configurable 3D-Printed millifluidic and microfluidic ‘lab on a chip’ reactionware devices. Lab Chip. 2012;12:3267–3271. doi: 10.1039/c2lc40761b. [DOI] [PubMed] [Google Scholar]
- 35.Grimes A, Breslauer DN, Long M, Pegan J, Lee LP, Khine M. Shrinky-Dink microfluidics: rapid generation of deep and rounded patterns. Lab Chip. 2008;8:170–172. doi: 10.1039/b711622e. [DOI] [PubMed] [Google Scholar]
- 36.Yetisen AK, Akram MS, Lowe CR. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip. 2013;13:2210–2251. doi: 10.1039/c3lc50169h. [DOI] [PubMed] [Google Scholar]
- 37.Yuen PK, Goral VN. Low-cost rapid prototyping of flexible microfluidic devices using a desktop digital craft cutter. Lab Chip. 2010;10:384–387. doi: 10.1039/b918089c. [DOI] [PubMed] [Google Scholar]
- 38.Disch A, Mueller C, Reinecke H. Low cost production of disposable microfluidics by blister packaging technology. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:6323–6326. doi: 10.1109/IEMBS.2007.4353801. [DOI] [PubMed] [Google Scholar]
- 39.Focke M, Kosse D, Muller C, Reinecke H, Zengerle R, von Stetten F. Lab-on-a-Foil: microfluidics on thin and flexible films. Lab Chip. 2010;10:1365–1386. doi: 10.1039/c001195a. [DOI] [PubMed] [Google Scholar]
- 40.Mathur A, Roy SS, McLaughlin JA. Transferring vertically aligned carbon nanotubes onto a polymeric substrate using a hot embossing technique for microfluidic applications. J R Soc Interface. 2010;7:1129–1133. doi: 10.1098/rsif.2009.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tien J. Microfluidic approaches for engineering vasculature. Current Opinion in Chemical Engineering. 2014;3:36–41. [Google Scholar]
- 42.Mu X, Zheng W, Xiao L, Zhang W, Jiang X. Engineering a 3D vascular network in hydrogel for mimicking a nephron. Lab Chip. 2013;13:1612–1618. doi: 10.1039/c3lc41342j. [DOI] [PubMed] [Google Scholar]
- 43.Tien J, Nelson CM. Microstructured Extracellular Matrices in Tissue Engineering and Development: An Update. Ann Biomed Eng. 2013 doi: 10.1007/s10439-013-0912-5. [DOI] [PubMed] [Google Scholar]
- 44.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 45.Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis. 2013;36:437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 46.Groothuis DR. The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol. 2000;2:45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bicker J, Alves G, Fortuna A, Falcao A. Blood-brain barrier models and their relevance for a successful development of CNS drug delivery systems: A review. Eur J Pharm Biopharm. 2014 doi: 10.1016/j.ejpb.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Cucullo L, Aumayr B, Rapp E, Janigro D. Drug delivery and in vitro models of the blood-brain barrier. Curr Opin Drug Discov Devel. 2005;8:89–99. [PubMed] [Google Scholar]
- 49.Naik P, Cucullo L. In vitro blood-brain barrier models: current and perspective technologies. J Pharm Sci. 2012;101:1337–1354. doi: 10.1002/jps.23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Urich E, Patsch C, Aigner S, Graf M, Iacone R, Freskgard PO. Multicellular Self-Assembled Spheroidal Model of the Blood Brain Barrier. Scientific Reports. 2013:3. doi: 10.1038/srep01500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lane WO, Jantzen AE, Carlon TA, et al. Parallel-plate flow chamber and continuous flow circuit to evaluate endothelial progenitor cells under laminar flow shear stress. J Vis Exp. 2012 doi: 10.3791/3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cucullo L, Marchi N, Hossain M, Janigro D. A dynamic in vitro BBB model for the study of immune cell trafficking into the central nervous system. J Cereb Blood Flow Metab. 2011;31:767–777. doi: 10.1038/jcbfm.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilhelm I, Krizbai IA. In Vitro Models of the Blood-Brain Barrier for the Study of Drug Delivery to the Brain. Mol Pharm. 2014 doi: 10.1021/mp500046f. [DOI] [PubMed] [Google Scholar]
- 54.Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (muBBB) Lab Chip. 2012;12:1784–1792. doi: 10.1039/c2lc40094d. [DOI] [PubMed] [Google Scholar]
- 55.Prabhakarpandian B, Shen MC, Nichols JB, et al. SyM-BBB: a microfluidic Blood Brain Barrier model. Lab Chip. 2013;13:1093–1101. doi: 10.1039/c2lc41208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griep LM, Wolbers F, de Wagenaar B, et al. BBB on chip: microfluidic platform to mechanically and biochemically modulate blood-brain barrier function. Biomed Microdevices. 2013;15:145–150. doi: 10.1007/s10544-012-9699-7. [DOI] [PubMed] [Google Scholar]
- 57.Yeon JH, Na D, Choi K, Ryu SW, Choi C, Park JK. Reliable permeability assay system in a microfluidic device mimicking cerebral vasculatures. Biomed Microdevices. 2012;14:1141–1148. doi: 10.1007/s10544-012-9680-5. [DOI] [PubMed] [Google Scholar]
- 58.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 60.Daugherty A. Mouse models of atherosclerosis. Am J Med Sci. 2002;323:3–10. doi: 10.1097/00000441-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Daugherty A, Lu H, Howatt DA, Rateri DL. Modes of defining atherosclerosis in mouse models: relative merits and evolving standards. Methods Mol Biol. 2009;573:1–15. doi: 10.1007/978-1-60761-247-6_1. [DOI] [PubMed] [Google Scholar]
- 62.Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Estrada R, Giridharan GA, Nguyen MD, Prabhu SD, Sethu P. Microfluidic endothelial cell culture model to replicate disturbed flow conditions seen in atherosclerosis susceptible regions. Biomicrofluidics. 2011;5:32006–3200611. doi: 10.1063/1.3608137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sluimer JC, Kolodgie FD, Bijnens AP, et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol. 2009;53:1517–1527. doi: 10.1016/j.jacc.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brian J, Polka AS, Mijares Geraldine, William MacCrehanb MG. Ag/AgCl microelectrodes with improved stability for microfluidics. Sensors and Actuators B. 2006;114:239–247. [Google Scholar]
- 66.Douville NJ, Tung YC, Li R, Wang JD, El-Sayed ME, Takayama S. Fabrication of two-layered channel system with embedded electrodes to measure resistance across epithelial and endothelial barriers. Anal Chem. 2010;82:2505–2511. doi: 10.1021/ac9029345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valencia PM, Farokhzad OC, Karnik R, Langer R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat Nanotechnol. 2012;7:623–629. doi: 10.1038/nnano.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blinder YJ, Mooney DJ, Levenberg S. Engineering approaches for inducing blood vessel formation. Current Opinion in Chemical Engineering. 2014;3:56–61. [Google Scholar]
- 69.Wagner I, Materne EM, Brincker S, et al. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab Chip. 2013 doi: 10.1039/c3lc50234a. [DOI] [PubMed] [Google Scholar]
- 70.Williamson A, Singh S, Fernekorn U, Schober A. The future of the patient-specific Body-on-a-chip. Lab Chip. 2013 doi: 10.1039/c3lc50237f. [DOI] [PubMed] [Google Scholar]
- 71.Sung JH, Esch MB, Prot JM, et al. Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab Chip. 2013;13:1201–1212. doi: 10.1039/c3lc41017j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Esch MB, Sung JH, Yang J, et al. On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic ‘body-on-a-chip’ devices. Biomed Microdevices. 2012;14:895–906. doi: 10.1007/s10544-012-9669-0. [DOI] [PubMed] [Google Scholar]
- 73.Grosberg A, Nesmith AP, Goss JA, Brigham MD, McCain ML, Parker KK. Muscle on a chip: in vitro contractility assays for smooth and striated muscle. Journal of pharmacological and toxicological methods. 2012;65:126–135. doi: 10.1016/j.vascn.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim HJ, Huh D, Hamilton G, Ingber DE. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip. 2012;12:2165–2174. doi: 10.1039/c2lc40074j. [DOI] [PubMed] [Google Scholar]
- 76.Mao S, Gao D, Liu W, Wei H, Lin JM. Imitation of drug metabolism in human liver and cytotoxicity assay using a microfluidic device coupled to mass spectrometric detection. Lab Chip. 2012;12:219–226. doi: 10.1039/c1lc20678h. [DOI] [PubMed] [Google Scholar]