Abstract
Continued seagrass declines in ecosystems with improved water quality may be driven by sediment stressors. One of the most cited examples of a seagrass ecosystem with declines is Cockburn Sound, Western Australia, where 75% of seagrasses (2169 ha) were lost in the 1960s–1980s due to poor water quality. Water quality has subsequently improved in Cockburn Sound, yet shoot density declines continue in some areas. Here, we investigated if sediment stressors (sulfide intrusion and heavy metals) contributed to declining Posidonia sinuosa shoot densities in Cockburn Sound. Seagrass δ34S were depleted at sites with a history of seagrass declines, indicating seagrasses at these sites were under sulfide stress. Heavy metals (Fe, Zn, Mn, Cr, Cu and Cd) in sediments and seagrasses did not show clear patterns with shoot density or biomass, and largely decreased from similar measurements in the late 1970s. However, seagrass cadmium concentrations were negatively correlated to seagrass biomass and shoot density. High cadmium concentrations interfere with sulfur metabolism in terrestrial plants, but impacts on seagrasses remain to be explored. Given that sulfide intrusion can prevent recolonization and drive seagrass declines, management plans in degraded seagrass ecosystems should include management of sediment stressors and water quality to provide comprehensive management.
Introduction
Seagrass declines threaten the substantial ecological and economic services that seagrass ecosystems provide. Seagrass loss has historically been linked to reduced water quality driven by disturbances such as coastal development and nutrient inputs1. These disturbances reduce light availability through inputs of fine sediments or by allowing the sudden growth of phytoplankton or epiphytic algal blooms. Given that seagrasses require relatively high light concentrations, reduced light availability can lead directly to sudden seagrass declines and dieback events2,3. In addition, reduced light availability limits the capacity of plants to oxygenate surrounding sediments through radial oxygen loss from roots4, which can increase the susceptibility of seagrasses to sulfide intrusion by limiting the diel cycling of sulfide and iron in the rhizosphere5. However, there are several examples of sudden seagrass diebacks in areas where water quality is good6–8. This suggests that other environmental factors likely play an equally important role in seagrass declines. Where water quality is improved but seagrass declines continue, belowground stressors likely drive seagrass declines and slow recovery8, yet our knowledge of the ecophysiological impacts of such stressors on seagrasses are limited.
The widespread (2169 ha) loss of seagrass in Cockburn Sound between the 1960’s and 1980’s3 was primarily attributed to low light availability driven by industrial inputs that lowered water quality, enhanced phytoplankton blooms and increased light attenuation9. As such, subsequent management projects have emphasized pressure response models linking seagrass loss with water quality. Since the 1980’s, water quality has considerably improved in Cockburn Sound and, in general, meets all environmental quality guidelines10. However, long-term seagrass monitoring shows significant declines in seagrass density at several sites in Cockburn and Warnbro Sounds, despite the improved water quality11. The decline in seagrass is patchy across the area, with sites around Garden Island and reference sites in Warnbro Sound showing the greatest rates of decline12. The juxtaposition between improved water quality but continued seagrass decline suggests that the current management model must be modified to account for drivers of seagrass loss other than poor water quality.
The intrusion of hydrogen sulfide from sediments has been implicated as a factor in seagrass declines globally7,8,13. Sediments are elevated in sulfides relative to seawater, and can be toxic to seagrasses14. The susceptibility of seagrasses to sulfide intrusion depends on the capacity for seagrasses to oxygenate the surrounding rhizosphere which can be influenced by physio-chemical conditions in sediments4. For example, an increase in sedimentary organic matter (OM) decreases oxygen availability and increases sulfate reduction through enhanced rates of microbial activity, thereby increasing the susceptibility of seagrasses to sulfide intrusion15,16. Elevated OM has been hypothesised as a driver of increased sulfide intrusion in temperate WA seagrasses including Posidonia sinuosa at Two Peoples Bay and Woodman Point17. Conversely, the presence of iron in sediments promotes the formation of iron-sulfide compounds like pyrite, reducing the possibility of sulfide intrusion into seagrass tissues18,19. However, the capacity for iron to buffer sulfide concentrations in sediments depends on reoxidation of FeS species formed, which can change appreciably over diel and seasonal periods5,20. Understanding differences in the composition and chemical nature of sediments, and how they change over time, will assist in identifying seagrass meadows that may be at greater risk of declines from sulfide intrusion. The isotopic ratio of sulfur (δ34S) can be used as an indicator for intrusion of sediment sulfides into seagrasses. More negative values indicate a higher contribution of sulfides from sediments (sediment average: −15‰ to −25‰), while a more positive value indicates that sulfate from the water column is the dominant S source for the plant (seawater average: +21‰)21.
Anthropogenic pollutants such as heavy metals have also previously been implicated as drivers of seagrass declines, particular in areas adjacent to coastal development22. Seagrasses can take up heavy metals from the water column through leaves, or from sediments through root uptake23. After inputs are reduced, tides or other hydrodynamic forces can remove pollutants over relatively short time scales (days-weeks), but heavy metals in sediments can persist for longer (years-decades) where mobility of the pollutants is limited24. The mobility of metals in marine sediments often depends on interactions with sulfides and iron, which changes depending on oxygen availability. Cationic heavy metals form solid phase sulfides when sulfide concentrations increase under anoxic conditions, but are remobilized when sediments become oxygenated25. Conversely, oxyanions are immobilized through sequestration with Fe(III) under oxygenated conditions, but become mobilised under reduced conditions when Fe(III) minerals are reduced to Fe(II)26. As such, metals may impact seagrasses even after inputs have ceased. Following uptake, metals can have varying effects on seagrass physiology and health. For example, seagrasses exposed to Cu and Zn show decreased photosynthetic efficiency22, while Cu, Ni, and Cr additions decreased leaf cell viability27. Long-term exposure to metals at slightly elevated concentrations may also decrease seagrass health, or lower seagrass resilience to other stressors.
We aimed to determine if sediment stressors (sulfide intrusion and heavy metals) were linked to continuing declines in shoot density of Posidonia sinuosa in Cockburn Sound. Specifically, we focused on the potential role of belowground stressors on P. sinuosa meadows across the region. We hypothesised that (i) sulfide intrusion from sediments into seagrass leaves (measured by δ34S) would be greatest at sites with highest rates of seagrass declines and not related to depth, (ii) seagrass δ34S would be positively correlated with sediment OM concentrations, (iii) seagrass δ34S would be negatively correlated with sediment iron concentrations and (iv) sites with seagrass declines would have higher concentrations of metals in sediments and seagrass tissues.
Results
Seagrass shoot density, biomass, and productivity
Posidonia sinuosa shoot density significantly differed across the study locations (F2,33 = 4.8, p = 0.015), with Warnbro Sound (WS) having lower densities than Eastern Banks (EB) and Garden Island (GI) (Table 1). Shoot density ranged from a minimum of 300 shoots m−2 at WS 3 m to a maximum of 2050 shoots m−2 at GI 2 m. Leaf biomass also significantly differed between locations (F2,33 = 7.46, p = 0.002), driven by lower biomass at WS sites. Root and rhizome biomass did not significantly differ across study locations, but significantly differed between depths (Roots: F3,32 = 6.7, p = 0.001; Rhizomes: F3,32 = 3.94, p = 0.017) driven by higher biomass at 2 m sites. There were no significant differences in above:belowground biomass ratios between locations or depths, showing changes in belowground biomass were not a result of a shift in biomass allocation. Productivity (leaf production) did not significantly differ between locations or depths (Table 1).
Table 1.
Mean seagrass shoot densities, biomass and productivity across Cockburn/Warnbro Sound.
| Site | Shoot density (shoots m−2) | Leaf biomass (g m−2) | Rhizome biomass (g m−2) | Root biomass (g m−2) | Productivity (mg sh day−1) |
|---|---|---|---|---|---|
| GI 2 m | 2050 (317) | 241.3 (32.0) | 263.5 (60.2) | 182.3 (27.4) | 1.03 (0.05) |
| GI 3 m | 1042 (102) | 88.9 (24.8) | 145.6 (41.7) | 49.8 (10.8) | 1.37 (0.07) |
| GI 5 m | 1450 (156) | 188.0 (19.1) | 300.4 (88.0) | 102.8 (19.6) | 1.43 (0.06) |
| GI 7 m | 858 (245) | 104.7 (20.1) | 130.8 (48.3) | 36.8 (14.4) | 1.22 (0.09) |
| EB 2 m | 1042 (158) | 94.2 (10.2) | 172.8 (41.8) | 24.6 (9.8) | 1.30 (0.09) |
| EB 7 m | 1425 (300) | 133.9 (34.3) | 267.0 (46.0) | 63.5 (33.7) | 1.05 (0.06) |
| EB 5 m | 1567 (82) | 162.5 (13.5) | 202.5 (54.2) | 72.6 (23.7) | 1.03 (0.04) |
| EB 3 m | 1758 (1000) | 154.5 (90.5) | 226.3 (32.1) | 84.7 (57.8) | 1.07 (0.05) |
| WS 2 m | 1308 (188) | 58.0 (13.2) | 321.7 (84.0) | 72.9 (25.6) | 1.16 (0.05) |
| WS 3 m | 300 (66) | 24.7 (9.1) | 17.3 (6.9) | 1.2 (0.8) | 1.48 (0.11) |
| WS 5 m | 1100 (292) | 105.8 (36.8) | 168.5 (42.4) | 67.2 (17.2) | 0.84 (0.05) |
| WS 7 m | 658 (129) | 89.9 (12.3) | 120.7 (62.1) | 40.0 (9.7) | 1.10 (0.05) |
GI = Garden Island, WS = Warnbro Sound. Means are calculated from three replicates, with standard errors provided in brackets.
Seagrass sulfur
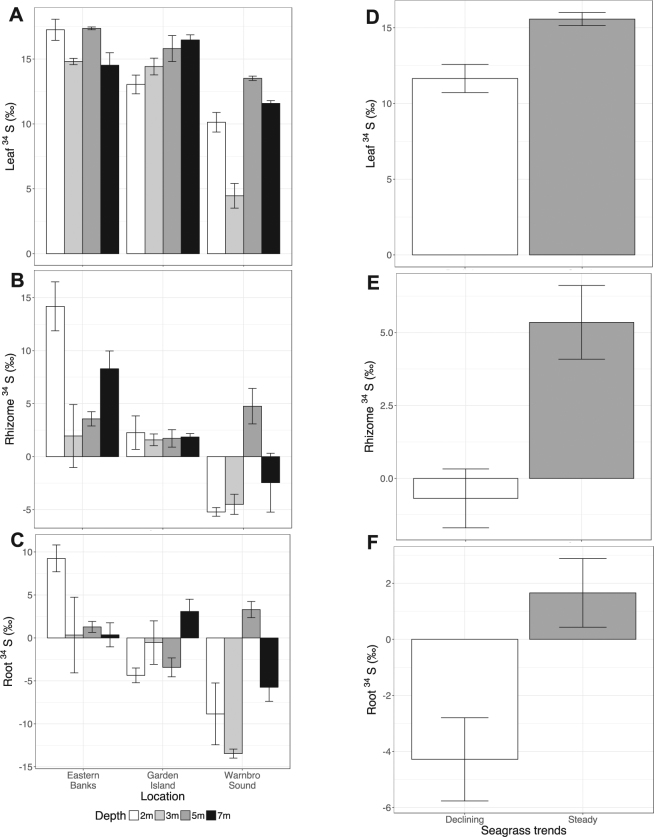
Seagrass δ34S values showed distinct differences across the study area (Table 2). Leaf δ34S significantly differed between Warnbro, Eastern Cockburn and Garden Island Sites (F2,33 = 19.8, p < 0.0001; Fig. 1A), but did not differ between depths (F3,32 = 2.52, p = 0.076). Rhizome δ34S significantly differed between locations (F2,33 = 11.9, p = 0.0001; Fig. 1B) but not between depths (F3,32 = 19.8, p = 0.434). Similarly, root δ34S significantly differed between locations (F2,33 = 6.6, p = 0.004; Fig. 1C) but not depths (F3,32 = 0.5, p = 0.68). Plants from Warnbro Sound consistently had the most depleted δ34S values in leaves, rhizomes, and roots, while Garden Island plants had more depleted δ34S signatures than Eastern Banks sites. Leaf δ34S was positively correlated to leaf biomass (R2 = 0.18, p = 0.005, n = 36), though this relationship was weak. Rhizome δ34S and root δ34S were not correlated to rhizome or root biomass respectively. Leaf δ34S was strongly correlated to rhizome δ34S (R2 = 0.40, p < 0.0001, n = 36). Rhizome δ34S was strongly correlated to root δ34S (R2 = 0.63 p < 0.0001, n = 36).
Table 2.
Mean seagrass total sulfur (% d.w.) and sulfur isotope composition (δ34S) values in leaves, roots and rhizomes across Cockburn/Warnbro Sound, plus comparison to similar data from other locations. Means are calculated from 3 replicates, with standard errors provided in brackets. *Root biomass was too low at WS 3 m for root S and δ34S in one replicate.
| Site | Leaf S (%) | Rhizome S (%) | Root S (%) | δ34S Leaf (‰) | δ34S Rhizome (‰) | δ34S Root (‰) |
|---|---|---|---|---|---|---|
| GI 2 m | 0.18 (0.009) | 0.49 (0.04) | 0.76 (0.07) | 13.04 (0.7) | 2.26 (1.6) | −4.36 (0.9) |
| GI 3 m | 0.18 (0.01) | 0.61 (0.02) | 0.53 (0.05) | 14.42 (0.6) | 1.58 (0.5) | −0.54 (2.5) |
| GI 5 m | 0.21 (0.04) | 0.53 (0.01) | 0.61 (0.05) | 15.80 (1.0) | 1.72 (0.8) | −3.43 (1.1) |
| GI 7 m | 0.20 (0.02) | 0.54 (0.04) | 0.54 (0.03) | 16.47 (0.4) | 1.85 (0.3) | 3.08 (1.4) |
| EB 2 m | 0.20 (0.009) | 0.52 (0.04) | 0.65 (0.1) | 14.81 (0.2) | 1.95 (3.0) | 0.33 (4.4) |
| EB 7 m | 0.17 (0.01) | 0.50 (0.01) | 0.55 (0.04) | 14.52 (1.0) | 8.29 (1.7) | 0.36 (1.4) |
| EB 5 m | 0.21 (0.01) | 0.45 (0.02) | 0.68 (0.03) | 17.36 (0.1) | 3.56 (0.7) | 1.28 (0.6) |
| EB 3 m | 0.22 (0.03) | 0.31 (0.02) | 0.40 (0.02) | 17.25 (0.8) | 14.18 (2.3) | 9.25 (1.6) |
| WS 2 m | 0.17 (0.003) | 0.78 (0.08) | 0.68 (0.07) | 10.13 (0.8) | −5.22 (0.4) | −8.85 (3.6) |
| WS 3 m | 0.20 (0.02) | 0.56 (0.04) | 1.12* | 4.46 (1.0) | −4.51 (0.9) | −13.47* |
| WS 5 m | 0.17 (0.02) | 0.49 (0.05) | 0.45 (0.01) | 13.51 (0.2) | 4.76 (1.7) | 3.30 (0.9) |
| WS 7 m | 0.20 (0.009) | 0.66 (0.1) | 0.81 (0.2) | 11.58 (0.2) | −2.46 (2.8) | −5.74 (1.6) |
| SW Aus.1 | 0.88 | 0.73 | 0.95 | 18.3 | 4.6 | 0.9 |
| Shark Bay2 | 0.85 | 0.99 | 0.78 | 12.47 | −3.17 | 1.62 |
| Global ave.3 | 0.60 | 0.60 | 0.8 | 12.40 | 5.10 | 0.10 |
1Holmer and Kendrick 2013; 2Cambridge et al. 2012; 3Holmer and Hasler-Sheetal 2014.
Figure 1.
Mean δ34S for seagrasses in Cockburn and Warnbro Sound. (A) Leaf, (B) Rhizome and (C) Root δ34S of seagrasses across three regions in Cockburn and Warnbro Sound. Colour of bars represent depth of sites (white = 2 m, light grey = 3 m, dark grey = 5 m, black = 7 m). Each bar represents the mean δ34S at each site, with error bars representing 1 standard error (n = 3). Mean (D) Leaf, (E) Rhizome and (F) Root δ34S grouped by long-term trends in shoot density at each site. White bars represent sites with a history of shoot declines, while grey bars are from sites with no shoot declines12. Each bar represents the mean δ34S, with error bars representing 1 standard error (n = 18).
Sites with a history of significant trends of shoot decline had significantly depleted leaf (F1,34 = 14.6, p = 0.0006; Fig. 1D), rhizome (F1,34 = 13.9, p = 0.0007; Fig. 1E) and root δ34S (F1,34 = 9.6, p = 0.004; Fig. 1F) values compared to sites with steady shoot density trends. Leaf, rhizome, and root δ34S from Garden Island and Warnbro Sound seagrasses was depleted relative to P. sinuosa from south-western Australia17, P. australis collected in Shark Bay28, and global averages for all seagrass species21. There was no relationship between sediment OM and δ34S in leaves (R2 = 0, p = 0.8, n = 36), rhizomes (R2 = 0.02, p = 0.4, n = 36), or roots (R2 = 0.03, p = 0.28, n = 36).
Leaf sulfur concentrations [S] showed no significant difference between sites, ranging from 0.17% at EB 7 m to 0.22% at EB 3 m (Table 2). Similarly, root [S] was not significantly different between locations or depths, with highest concentrations at WS 3 m (1.12%) and lowest at EB 3 m (0.4%). However, rhizome [S] was significantly higher in sites at Warnbro Sound sites (F2,33 = 8.0, p = 0.001), and was highest at WS 2 m (Table 2). Seagrass leaf, rhizome and root [S] in Cockburn/Warnbro Sound tended to be lower than comparable measurements of P. sinuosa in other SW Australia estuaries17, though root [S] values at WS 3 m were greater.
Sediment metal concentrations
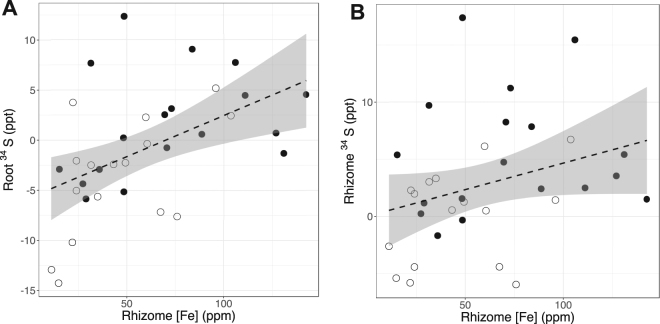
Sediment metal concentrations showed varying patterns between locations (Table 3). Sediment [Cd] was rarely above detectable levels, and showed no significant differences between locations. Sediment [Fe] was not significantly different between sites, but was significantly higher at deeper sites (F3,32 = 16.9, p < 0.0001). Sediment [Mn] was also significantly higher at deeper sites (F3,32 = 5.2, p = 0.005). Sediment [Cr] was significantly different between locations (F2,33 = 11.8, p = 0.0001), with highest values at Warnbro Sound sites. Sediment [Cr] was also greater at shallow sites (F3,32 = 7.1, p = 0.001). Sediment [Zn] concentrations were significantly higher in EB Sites, with concentrations above 5 ppm in EB 7 m, EB 5 m, and EB 3 m, and <2 ppm at all other sites. No sediment metal concentrations were significantly correlated to seagrass shoot density (all p > 0.05, n = 36). Root δ34S values across Warnbro/Cockburn Sounds was positively correlated to Fe concentrations in sediments (R2 = 0.14, p = 0.01, Fig. 2A), while rhizome δ34S values were positively correlated to rhizome [Fe] (R2 = 0.13, p = 0.04, n = 36, Fig. 2B).
Table 3.
Mean metal concentrations in sediments in Cockburn/Warnbro Sounds, plus comparable data collected from Warnbro Sound in 1977. Means are calculated from 3 replicates, with standard errors provided in brackets.
| Site | Sediment Fe (ppm) | Sediment Zn (ppm) | Sediment Mn (ppm) | Sediment Cr (ppm) | Sediment Cu (ppm) | Sediment Cd (ppm) |
|---|---|---|---|---|---|---|
| GI 2 m | 39.37 (1.3) | 1.62 (0.6) | 1.28 (0.01) | 0.92 (0.04) | 0.20 (0.01) | 0.004 (0.001) |
| GI 3 m | 62.23 (3.0) | 1.52 (0.3) | 1.46 (0.1) | 0.57 (0.01) | 0.43 (0.01) | 0.008 (0.002) |
| GI 5 m | 51.13 (2.1) | 1.25 (0.2) | 1.07 (0.03) | 0.69 (0.07) | 0.24 (0.02) | 0.005 (0.001) |
| GI 7 m | 79.43 (9.2) | 1.90 (0.3) | 1.28 (0.05) | 0.60 (0.05) | 0.35 (0.03) | 0.005 (0.0003) |
| EB 2 m | 34.80 (2.7) | 1.66 (0.07) | 0.87 (0.02) | 0.34 (0.006) | 0.24 (0.02) | 0.003 (0.001) |
| EB 7 m | 109.73 (3.7) | 6.78 (0.9) | 2.16 (0.03) | 0.27 (0.006) | 0.53 (0.01) | 0.008 (0.001) |
| EB 5 m | 64.80 (14.1) | 7.51 (2.3) | 1.14 (0.2) | 0.47 (0.009) | 0.39 (0.1) | 0.002 (0.001) |
| EB 3 m | 57.17 (0.8) | 5.51 (0.7) | 1.10 (0.01) | 0.67 (0.007) | 0.41 (0.06) | 0.006 (0.002) |
| WS 2 m | 31.70 (3.2) | 1.09 (0.8) | 1.23 (0.03) | 1.01 (0.02) | 0.13 (0.003) | 0.003 (0.001) |
| WS 3 m | 43.90 (5.5) | 0.48 (0.09) | 1.36 (0.05) | 0.94 (0.02) | 0.16 (0.007) | 0.004 (0.001) |
| WS 5 m | 83.20 (15.3) | 1.29 (0.5) | 1.90 (0.1) | 0.60 (0.07) | 0.41 (0.2) | 0.009 (0.002) |
| WS 7 m | 88.83 (3.9) | 1.14 (0.3) | 1.89 (0.09) | 0.57 (0.03) | 0.27 (0.02) | 0.006 (0.001) |
| Cockburn Sound 1977 1 | 3647.62 | 15.02 | 10.16 | 25.01 | 11.36 | 0.37 |
1Talbot and Chegwidden (1983).
Figure 2.
Scatterplots showing relationship between (A) rhizome [Fe] and root δ34S and (B) rhizome [Fe] and rhizome δ34S. Dashed lines represent linear model, while grey outline shows 95% confidence intervals. White dots denote replicates from sites with a history of seagrass declines, while black dots denote replicates from sites with steady seagrass trends12.
Seagrass metal content
Rhizome [Zn] was significantly higher at sites adjacent to Garden Island (F3,32 = 7.1, p = 0.002), primarily driven by high concentrations at GI 2 m and GI 7 m (Table 4). Rhizome [Fe] was not significantly different between locations (F2,33 = 2.8, p = 0.07), but was significantly greater at deeper sites (F3,32 = 6.7, p = 0.001) due to a positive correlation with sediment [Fe] (R2 = 0.27, p = 0.001, n = 36). Sediment [Fe] was weakly positively correlated with root δ34S (R2 = 0.17, p = 0.015) and rhizome [Fe] was weakly positively correlated with rhizome δ34S (R2 = 0.13, p = 0.04). Rhizome [Mn] was not significantly different between locations (F2,31 = 2.2, p = 0.13). Rhizome [Cr] were very low across Cockburn and Warnbro Sounds, despite the relatively high sediment [Cr]. Rhizome [Cr] was only above detectable limits at GI 3 m, GI 5 m, GI 7 m, and WS 5 m. Rhizome [Cd] was significantly higher at Warnbro Sound sites (F2,31 = 9.0, p = 0.0008), despite low sediment [Cd] across the entire study area.
Table 4.
Mean metal concentrations in rhizomes of seagrasses growing in Cockburn/Warnbro Sound. Means are calculated from 3 replicates, with standard errors provided in brackets.
| Site | Rhizome [Zn] (ppm) | Rhizome [Fe] (ppm) | Rhizome [Mn](ppm) | Rhizome [Cd] (ppm) | Rhizome [Cr] (ppm) |
|---|---|---|---|---|---|
| GI 2 m | 177.97 (14.6) | 23.90 (4.3) | 1.60 (0.1) | 0.16 (0.007) | 0.00 (0) |
| GI 3 m | 155.20 (24.0) | 35.63 (12.4) | 8.63 (6.3) | 0.25 (0.02) | 0.07 (0.07) |
| GI 5 m | 88.57 (8.1) | 42.50 (4.1) | 0.97 (0.2) | 0.14 (0.009) | 0.17 (0.09) |
| GI 7 m | 168.47 (16.3) | 100.53 (27.7) | 1.83 (0.1) | 0.24 (0.03) | 0.30 (0.1) |
| EB 2 m | 134.73 (19.7) | 56.03 (14.3) | 1.87 (0.7) | 0.24 (0.03) | 0.00 |
| EB 7 m | 79.93 (11.0) | 91.47 (19.7) | 1.17 (0.5) | 0.21 (0.03) | 0.00 |
| EB 5 m | 100.23 (10.4) | 94.97 (16.8) | 1.10 (0.2) | 0.19 (0.02) | 0.00 |
| EB 3 m | 49.63 (18.1) | 62.00 (22.5) | 0.87 (0.3) | 0.16 (0.01) | 0.03 (0.03) |
| WS 2 m | 124.37 (6.7) | 20.27 (2.8) | 0.97 (0.03) | 0.25 (0.01) | 0.00 |
| WS 3 m | 23.40* | 11.10* | 0.60* | 0.46* | 0.00* |
| WS 5 m | 77.23 (20.6) | 86.40 (13.5) | 2.40 (0.53) | 0.29 (0.07) | 0.17 (0.12) |
| WS 7 m | 106.47 (9.4) | 58.30 (13.6) | 1.17 (0.07) | 0.32 (0.04) | 0.00 |
| Cockburn Sound 1977 1 | 58.58 | 89.53 | N/A | 0.45 | N/A |
| Mediterranean Sea 2 | 44.10 | 170.40 | 8.50 | 1.12 | 0.54 |
*Root biomass was too low at WS 3 m for root S and δ34S in one replicate, 1Talbot and Chegwidden (1982); 2Tovar-Sanchez et al. (2010)
Rhizome [Cd] was significantly negatively correlated to seagrass shoot density (R2 = 0.20, p = 0.005, n = 36, Fig. 3), leaf biomass (R2 = 0.27, p = 0.002, n = 36), rhizome biomass (R2 = 0.22, p = 0.003, n = 36), and root biomass (R2 = 0.17, p = 0.008, n = 35). No other metals were correlated with changes in seagrass biomass, shoot density, or productivity (data not shown).
Figure 3.
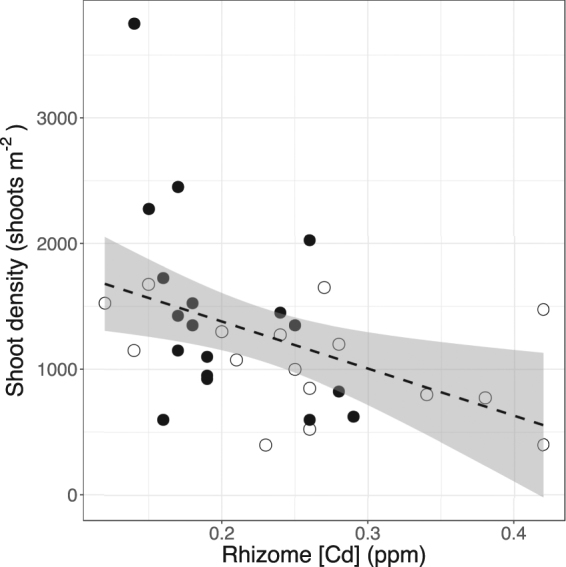
Scatterplots showing relationship rhizome cadmium concentrations and shoot densities. Dashed line represents linear model, grey outline shows 95% confidence interval. White dots denote replicates from sites with a history of seagrass declines, while black dots denote replicates from sites with steady seagrass trends12.
Rhizome [Cd] and [Fe] were typically lower than comparable rhizome metal concentrations measured in Cockburn Sound in 197729, and rhizome [Cd] from Mediterranean P. oceanica meadows30, while Rhizome [Zn] were higher. No comparable historical rhizome [Cr] or [Mn] data from Cockburn Sound is available, but concentrations in were lower than Mediterranean P. oceanica rhizomes30.
Discussion
Seagrasses in Warnbro Sound and at some locations in Cockburn Sound have tissues with depleted δ34S relative to nearby sites and consistent with sulfide intrusion. Seagrass δ34S was significantly depleted in leaves, rhizomes and roots at sites with recorded trends of reductions in shoot densities. Moreover, δ34S values at impacted sites were well below local, regional and global averages17,21, suggesting that these seagrasses are impacted by sediment stressors that could be contributing to the trend of declining shoot densities31. Seagrass management plans in Cockburn and Warnbro Sound focus exclusively on characterising the health of the ecosystem using above-ground seagrass metrics (e.g. shoot density) that have a cause-effect pathway directly related to increased light attenuation associated with historically poor water quality32. This approach discounts the role that sediment health has on the growth and mortality of seagrasses8,33. Though water quality caused the initial, widespread loss of seagrass in Cockburn Sound in the 1960s–1980s, recent declines are not related to water quality11. However, spatial patterns of seagrass loss are best explained by depleted δ34S; an indicator of sulfide intrusion that is a stressor that the existing seagrass management plans would not be able to address32,34.
The susceptibility of seagrasses to sulfide intrusion is governed by a variety of environmental factors. We expected sedimentary organic matter (OM) to be correlated to sulfide intrusion, but found no relationship between OM and leaf, rhizome or root δ34S. We predicted this relationship based on increased OM increasing sulfate reduction rates in sediments, a trend that has been observed in previous seagrass sediments enriched with OM13. However, other studies have shown that sulfide intrusion is not always related to sediment organic matter content16. The impact of organic loading on sulfide intrusion is likely dependent on a complex set of variable including the chemical composition of OM, microbial community functional traits, and the ability of the seagrasses to oxygenate their rhizosphere4,35. In addition, increases in water temperature could increase sulfide intrusion into seagrasses by enhancing sulfate reduction rates in sediments36 while simultaneously reducing the capacity of the plant to oxygenate the surrounding rhizosphere by increasing metabolic/respiration rates. Sulfide intrusion may become a greater driver of seagrass loss as temperatures increase from climate change, especially during extreme temperature events such as marine heatwaves37. Differences in seagrass δ34S could also be partially explained by differences in sediment δ34S across the study area17 that was not measured in this experiment. Previous studies have found acid-volatile sediment δ34S to be relatively stable in P. sinuosa meadows in Cockburn Sound17. However, sediment sulfide pools and δ34S can change appreciably over diel and seasonal cycles38,39. As such, future research focusing on drivers of sediment sulfide concentrations and intrusion into seagrass tissues in Cockburn and Warnbro Sounds over diel and seasonal time scales would provide useful information, with a particular focus on measuring nocturnal sulfide dynamics, given this is the period when seagrasses are more vulnerable to sulfide intrusion given there is no photosynthetic oxygen production and oxygen balance in seagrass tissues is driven by water column oxygen concentrations and current speeds40. In addition, future research could also explicitly examine any physiological damage that increased sulfide intrusion has on P. sinuosa and contribute to the development of a sediment-stress functional indicator for use in these meadows, similar to that developed in Halophila ovalis meadows41, which would aid in management efforts.
Sediment Fe was positively correlated to root and rhizome δ34S values, confirming our third hypothesis, and indicating that sediment Fe provides some protection against sulfide intrusion. Even small increases in sediment Fe can lessen sulfide intrusion by decreasing dissolved sulfide pools in sediments, particularly in calcium carbonate sediments with naturally low sediment Fe like in Cockburn Sound42. The subsequent role of sediment Fe would depend on reoxidation of any FeS products in order to maintain buffering capacity against a build-up of free sulfides in sediments5. Reoxidation of sediments in Cockburn Sound is relatively high, leading to low sulfate reduction rates and ensuring the buffering capacity of sedimentary Fe17.
Sediment Fe is currently 1–2 orders of magnitude lower in present day Cockburn Sound sediments compared to those measured in 197743. This difference may partially be explained by different extraction techniques; with previous studies extracting metals from sediments using an HCl/HNO3 extraction43 instead of the ethylenediaminetetraacetic acid (EDTA) extraction used in this study. EDTA extractions are considered more efficient in extracting some metals from sulfate-rich marine sediments than HCl44, and provides a more accurate estimate of metal bioavailability45. However, both techniques would differ in the pools of Fe species that they extract, with dilute HCl extractions targeting Fe(OH)3, FeCO3 and FeS, while EDTA extractions would target Fe(OH)3, FeOOH and Fe2O3 46. As such, the type of extractions used may contribute to differences measured between the two studies, with differences of ~20% previously observed in marine sediments45. In addition, a reduction of industrial inputs into the Sound, especially the loss of the iron refinery and steel mill, across this time period may also contribute to a reduction in sedimentary Fe. If so, the tighter regulation of coastal industry along Cockburn Sound may have concurrently improved growing conditions (by improving water quality) but left seagrass more vulnerable to sediment stressors, and contribute towards the lack of widespread seagrass recovery in the corresponding time period. Sediment Fe should be included in a suite a parameters to determine the sites where seagrasses are more vulnerable to sulfide intrusion, and addition of Fe to sediments could be used to alleviate sulfide stress for seagrasses in impacted areas or prior to restoration attempts19,42,47. In addition, further research into sediment Fe using sequential extraction techniques would provide the most comprehensive approach to assessing the mobility of Fe in sediments across the area, and the capacity for sediment Fe to act as a buffer against accumulation of sedimentary sulfides and subsequent intrusion into seagrass tissues.
Heavy metals were historically released into Cockburn Sound from surrounding industrial activity and are a concern as they have limited mobility in the environment48 and have known negative impacts on seagrasses22,49. We found lower concentrations of all metals in sediments relative to previous surveys in Cockburn Sound43. These differences again may result from different extraction techniques, or different metal speciation in sediments, which more detailed sequential extractions could reveal44. Sediment and seagrass metal concentrations did not generally show clear patterns at sites with and without seagrass declines, suggesting that heavy metals from industrial inputs are not contributing to the long-term declines of seagrass in Cockburn Sound and surrounding areas. One exception to this was seagrass Cd concentrations, which were elevated in Warnbro Sound sites and negatively correlated with seagrass biomass and shoot density (Fig. 3). Seagrasses have previously been used as bioindicators of environmental Cd concentrations50, and previous experiments have showed a limited effect of Cd uptake on leaf photosynthesis22 and accumulation in leaves of Halophila stipulacea 51. However, we are not aware of studies examining other physiological impacts on increased Cd uptake on seagrasses, including effects on sulfur metabolism. Increased Cd uptake in terrestrial angiosperms interferes with sulfur metabolism by increasing the activity of enzymes and expression of genes related to reductive sulfate assimilation, thereby increasing sulfide uptake through roots52. Cadmium uptake could play a similar role in seagrasses, and lead to an increase in vulnerability to sulfide intrusion.
Seagrass zinc concentrations were the only heavy metal concentrations to increase in seagrass rhizome relative to concentrations previously measured29, despite corresponding decreases in sediment Zn43. Seagrass Zn was particularly high at Garden Island sites (Table 4). Offshore sites in Cockburn Sound have previously been hypothesised to have higher Zn concentrations in sediments caused by fine contaminated sediments from industrial development accumulating offshore53. There was no strong correlation between Zn and shoot density or biomass, likely because Zn is an essential micronutrient for seagrasses23, with toxic effects only encountered in contaminated waters with high Zn concentrations (e.g. 570 µg l−1 22). However, seagrass tissues enriched in Zn can be transferred to associated invertebrates leading to bioaccumulation, particularly in detritivores and herbivores that directly consume dead or living seagrass tissues54. Metal concentrations were previously measured in sediment associated invertebrates29, and would provide a baseline for comparison for future studies of bioaccumulation of Zn in seagrass-associated invertebrates in Cockburn Sound.
Kendrick et al.3 hypothesised that the environment in Cockburn Sound had been “altered to an environment not suited to large-scale recolonization by Posidonia species”. Our data suggests that sulfide stress from sediments is contributing to this observed lack of seagrass recolonization (in spite of improved water quality), resulting in a phase shift in the benthic community of Cockburn Sound. Such phase shifts may be common after seagrass declines, particularly when declines have enriched sediments in OM from dead seagrass tissues. These organic matter inputs would alter oxygen and nutrient availability in sediments, potentially resulting in ‘legacy problems’ that prevent seagrass recovery even after initial drivers of loss are mediated. The loss of oxygen inputs from seagrass roots would also lead to a shift in the balance of benthic metabolism, favouring anaerobic respiration and more sulfidic conditions. We suggest that there is a need to integrate above- and below-ground measurements that include indicators in seagrasses, sediments, and the water column in seagrass management programs to provide a comprehensive assessment of seagrass health and potential pressures, even where water quality appears to be the sole driver of initial seagrass declines.
Methods
Study Sites and experimental design
Three locations (each with four sites) were sampled between March-May 2015: Eastern Banks (EB), Garden Island (GI), and Warnbro Sound (WS) (Fig. 4). At each location, sites were stratified by depth (2 m, 3 m, 5 m, and 7 m). Sites sampled had varying degrees of shoot declines based on historical monitoring data from the annual Cockburn Sound Management Council monitoring project12. Six sites (WS 2, 3, 5, 7 m; GI 3 and 5 m) had highly significant shoot declines, while the remaining six sites had no record of shoot declines (GI 2 and7m, EB 2, 3, 5, 7 m). Posidonia sinuosa was the dominant seagrass species at all sites.
Figure 4.
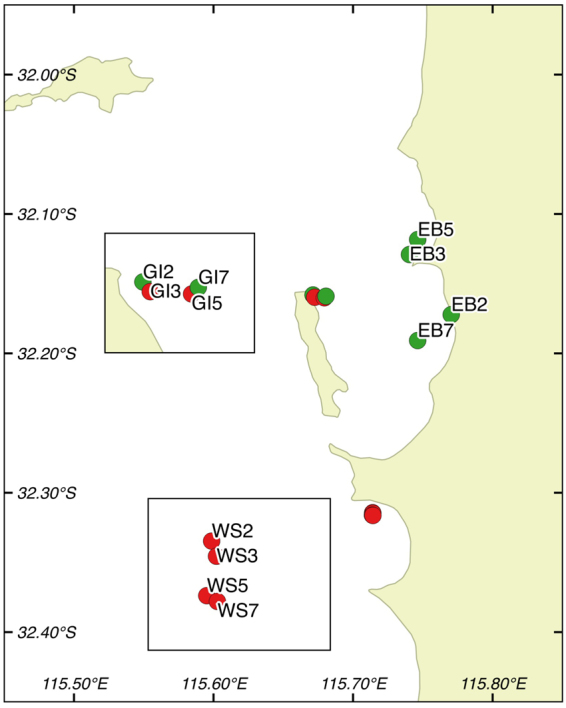
Map showing study area. GI = Garden Island, WS = Warnbro Sound, EB = Eastern Banks. Red icons denote sites with a history of seagrass declines, while green icons represent sites with steady shoot density trends12. Map was created with QGIS version 2.8.2 (Open Source Geospatial Foundation Project, http://qgis.osgeo.org).
Within each site, three 20 cm × 20 cm quadrats were set up. During the initial visit to each site, all P. sinuosa shoots within each quadrat were marked for productivity using a standard hole punching technique55. Sites were then revisited four weeks later, where quadrats with hole punched seagrasses were completely extracted using a 25-cm corer. Seagrasses were bagged, stored on ice and transported in dark to the laboratory for analysis. In addition, approximately 50 ml of sediment adjacent to each quadrat (surficial sediment to 10 cm) was collected using a 50 ml syringe with a cut-top. Sediment samples were transferred to 50 ml plastic tubes, stored on ice, and transported to the laboratory for analysis.
Seagrass biomass and productivity
Numbers of shoots within each quadrat (after extraction) were counted to determine shoot density. Adherent epiphytes and sediments were removed from seagrass leaves by gently scraping with a razor blade. Seagrasses were then sorted into leaves, rhizomes, and roots, dried at 60 °C and weighed for biomass. Seagrass productivity was measured using a standard technique55 that involved measuring the distance of the hole-punch above leaf sheaths on all seagrass leaves and weighing newly produced biomass after drying at 60 °C. This value was then averaged over 1 month to provide a productivity rate in mg sh day−1. Dried seagrass tissues were then ground finely using a ball mill for sulfur (S) analysis, or mortar and pestle for metal analysis.
Seagrass total sulfur and δ34S isotope ratios
Ground leaf, root, and rhizome samples were weighed into tin capsules together with vanadium pentoxide and analyzed by elemental analyzer combustion continuous flow isotope ratio mass spectroscopy (EA-C-CF-IRMS) at Iso-analytical, United Kingdom. The stable isotopic signatures were reported in standard delta notation (units per mill, ‰) as:
| 1 |
where R = 34S/32S. The international standard for δ34S is the Canyon Diablo Troilite (CDT) a meteorite of FeS used as a standard zero point for expression of sulfur isotopes. Average δ34S values can provide an indication of the relative intrusion of sulfides from sediments compared to sulfates from the water column. More negative δ34S values indicate a higher contribution of sulfides from sediments (sediment average: −15‰ to −25‰), while a more positive value indicates that sulfate from the water column is dominant S source for the plant (seawater average: +21‰)21.
Sediment characteristics and heavy metal analysis
Organic matter content of sediments was determined by the difference in sediment dry weight after combustion of samples at 550 °C for 8 hours56. Elemental analysis of sediment and seagrass rhizome extracts (Cd, Cr, Fe, Mn, Zn for seagrass, Cd, Cr, Fe, Mn, Zn, Cu for sediment) were performed on a PerkinElmer optima 5300DV inductively couple plasma optical emission spectrometer (ICP-OES) at the University of Western Australia. Sediment extracts were prepared by shaking 5 g of dried sediment with 45 ml 0.05 M EDTA solution44. EDTA is commonly used as an extractant of sediment metals, and is assumed to extract metals on exchange sites of both inorganic and organic complexes, providing a good representation of metals that are bioavailable44,45. Seagrass extracts were prepared by digesting 0.3 g of seagrass rhizome using a conc. HNO3/H2O2 digest57.
Statistical analysis
Linear regressions were used to determine relationships between seagrass biomass, seagrass S and δ34S content and seagrass metal concentrations. In addition, linear regression was used to investigate relationships between sediment OM/metal content, and seagrass biomass and seagrass elemental content. Differences in sediment and seagrass parameters between sites and depths were investigated using two-way ANOVAs. Data were square-root transformed where assumptions of heterogeneity were not met. All statistical analysis was performed using R version 3.2.258 and graphs produced using ggplot59.
Data accessibility
Data in this article are published onthe FigShare data Repository at https://figshare.com/s/3e705529e7c311830c33 (doi:10.6084/m9.figshare.5103340).
Acknowledgements
This work was supported by funding from the Cockburn Sound Management Council. MWF was supported through additional funding from the Department of Defence and the Robson and Robertson Research Fellowship provided by the University of Western Australia. GAK was partly funded by an ARC Linkage Grant (LP130100155). The authors are grateful to Andrea Zavala-Perez for assistance with field work and laboratory processing, and to Stephanie Turner (Department of Environment Regulation) for insightful comments.
Author Contributions
M.W.F. and G.A.K. conceived, designed and performed the experiment, analyzed the data and wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Orth R, et al. A global crisis for seagrass ecosystems. BioScience. 2006;56:987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2. [DOI] [Google Scholar]
- 2.Cambridge M, McComb A. The loss of seagrasses in Cockburn Sound, Western Australia. I. The time course and magnitude of seagrass decline in relation to industrial development. Aquatic Botany. 1984;20:229–243. doi: 10.1016/0304-3770(84)90089-5. [DOI] [Google Scholar]
- 3.Kendrick GA, et al. Changes in seagrass coverage in Cockburn Sound, Western Australia between 1967 and 1999. Aquatic Botany. 2002;73:75–87. doi: 10.1016/S0304-3770(02)00005-0. [DOI] [Google Scholar]
- 4.Brodersen KE, Nielsen DA, Ralph PJ, Kühl M. Oxic microshield and local pH enhancement protects Zostera muellerifrom sediment derived hydrogen sulphide. New Phytologist. 2014;205:1264–1276. doi: 10.1111/nph.13124. [DOI] [PubMed] [Google Scholar]
- 5.Pagès, A., Welsh, D. T., Robertson, D. & Panther, J. G. Diurnal shifts in co-distributions of sulfide and iron(II) and profiles of phosphate and ammonium in the rhizosphere of Zostera capricorni. Estuarine, Coastal and Shelf Science (2012).
- 6.Borum J, et al. Eelgrass fairy rings: sulfide as inhibiting agent. Marine Biology. 2013;161:351–358. doi: 10.1007/s00227-013-2340-3. [DOI] [Google Scholar]
- 7.Greve TM, Krause-Jensen D, Rasmussen MB, Christensen PB. Means of rapid eelgrass (Zostera marina L.) recolonisation in former dieback areas. Aquatic Botany. 2005;82:143–156. doi: 10.1016/j.aquabot.2005.03.004. [DOI] [Google Scholar]
- 8.Borum J, et al. The potential role of plant oxygen and sulphide dynamics in die-off events of the tropical seagrass. Thalassia testudinum. Journal of Ecology. 2005;93:148–158. doi: 10.1111/j.1365-2745.2004.00943.x. [DOI] [Google Scholar]
- 9.Cambridge M, Chiffings A, Brittan C, Moore L, McComb A. The loss of seagrass in Cockburn Sound, Western Australia II. Possible causes of seagrass decline. Aquatic Botany. 1986;24:269–285. doi: 10.1016/0304-3770(86)90062-8. [DOI] [Google Scholar]
- 10.Cockburn S. Management Council. State of Cockburn Sound report. 2015;2014:1–24. [Google Scholar]
- 11.Mohring, M. & Rule, M. Long-term trends in the condition of seagrass meadows in Cockburn and Warnbro Sounds. Report prepared for the Cockburn Sound Management Council. 1–94 (2013).
- 12.Mohring, M. & Rule, M. A Survey of Selected Seagrass Meadows in Cockburn Sound, Owen Anchorage and Warnbro Sound. Data Report prepared for Cockburn Sound Management Council. 1–49 (2014).
- 13.Frederiksen MS, Holmer M, Díaz-Almela E, Marbà N, Duarte CM. Sulfide invasion in the seagrass Posidonia oceanica at Mediterranean fish farms: assessment using stable sulfur isotopes. Marine Ecology Progress Series. 2007;345:93–104. doi: 10.3354/meps06990. [DOI] [Google Scholar]
- 14.Pedersen O, Binzer T, Borum J. Sulphide intrusion in eelgrass (Zostera marina L.) Plant, Cell & Environment. 2004;27:595–602. doi: 10.1111/j.1365-3040.2004.01173.x. [DOI] [Google Scholar]
- 15.Holmer M, Pedersen O, Ikejima K. Sulfur cycling and sulfide intrusion in mixed southeast Asian tropical seagrass meadows. Botanica Marina. 2006;49:91–102. doi: 10.1515/BOT.2006.013. [DOI] [Google Scholar]
- 16.Holmer M, et al. Sulfide intrusion in the tropical seagrasses Thalassia testudinum and Syringodium filiforme. Estuarine, Coastal and Shelf Science. 2009;85:319–326. doi: 10.1016/j.ecss.2009.08.015. [DOI] [Google Scholar]
- 17.Holmer M, Kendrick GA. High sulfide intrusion in five temperate seagrasses growing under contrasting sediment conditions. Estuaries and Coasts. 2013;36:116–126. doi: 10.1007/s12237-012-9550-7. [DOI] [Google Scholar]
- 18.Erskine JM, Koch MS. Sulfide effects on Thalassia testudinum carbon balance and adenylate energy charge. Aquatic Botany. 2000;67:275–285. doi: 10.1016/S0304-3770(00)00099-1. [DOI] [Google Scholar]
- 19.Marbà N, Calleja ML, Duarte CM, Álvarez E. Iron Additions Reduce Sulfide Intrusion and Reverse Seagrass (Posidonia oceanica) Decline in Carbonate Sediments. Ecosystems. 2007;10:745–756. doi: 10.1007/s10021-007-9053-8. [DOI] [Google Scholar]
- 20.Luther, G. W. & Church, T. M. Seasonal cycling of sulfur and iron in porewaters of a Delaware salt marsh. Marine Chemistry (1988).
- 21.Holmer M, Hasler-Sheetal H. Sulfide intrusion in seagrasses assessed by stable sulfur isotopes—a synthesis of current results. Front. Mar. Sci. 2014;1:64. doi: 10.3389/fmars.2014.00064. [DOI] [Google Scholar]
- 22.Macinnis-Ng CMO, Ralph PJ. Towards a more ecologically relevant assessment of the impact of heavy metals on the photosynthesis of the seagrass, Zostera capricorni. Marine Pollution Bulletin. 2002;45:100–106. doi: 10.1016/S0025-326X(01)00300-9. [DOI] [PubMed] [Google Scholar]
- 23.Luy N, Gobert S, Sartoretto S, Biondo R. Chemical contamination along the Mediterranean French coast using Posidonia oceanica (L.) Delile above-ground tissues: a multiple trace element study. Ecological Indicators. 2012;18:269–277. doi: 10.1016/j.ecolind.2011.11.005. [DOI] [Google Scholar]
- 24.Serrano O, Mateo MA, Renom P, Julià R. Characterization of soils beneath a Posidonia oceanica meadow. Geoderma. 2012;185–186:26–36. doi: 10.1016/j.geoderma.2012.03.020. [DOI] [Google Scholar]
- 25.Zhang, C. et al. Effects of sediment geochemical properties on heavy metal bioavailability. Environment International (2014). [DOI] [PubMed]
- 26.Bennett WW, et al. Inorganic arsenic and iron(II) distributions in sediment porewaters investigated by a combined DGT?colourimetric DET technique. Environ. Chem. 2012;9:31–40. doi: 10.1071/EN11074. [DOI] [Google Scholar]
- 27.Malea P, Adamakis I-DS, Kevrekidis T. Microtubule integrity and cell viability under metal (Cu, Ni and Cr) stress in the seagrass Cymodocea nodosa. Chemosphere. 2013;93:1035–1042. doi: 10.1016/j.chemosphere.2013.05.074. [DOI] [PubMed] [Google Scholar]
- 28.Cambridge ML, Fraser MW, Holmer M, Kuo J, Kendrick GA. Hydrogen sulfide intrusion in seagrasses from Shark Bay, Western Australia. Marine and Freshwater Research. 2012;63:1027–1038. doi: 10.1071/MF12022. [DOI] [Google Scholar]
- 29.Talbot V, Chegwidden A. Cadmium and other heavy metal concentrations in selected biota from Cockburn Sound, Western Australia. Marine and Freshwater Research. 1982;33:779. doi: 10.1071/MF9820779. [DOI] [Google Scholar]
- 30.Tovar-Sánchez A, Serón J, Marbà N, Arrieta JM, Duarte CM. Long-term records of trace metal content of western Mediterranean seagrass (Posidonia oceanica) meadows: Natural and anthropogenic contributions. J. Geophys. Res. 2010;115:G02006. [Google Scholar]
- 31.Fraser, M. W., Kendrick, G. A. & Zavala-Perez, A. Drivers of seagrass decline in Cockburn and Warnbro Sound. Report prepared for the Department of EnvironmentRegulation. 1–35 (2016).
- 32.Environmental Protection Authority. Environmental Quality Criteria Reference Document for Cockburn Sound (2015). 1–108 (2015).
- 33.Holmer M, Argyrou M, Dalsgaard T, Danovaro R. Effects of fish farm waste on Posidonia oceanica meadows: synthesis and provision of monitoring and management tools. Marine Pollution Bulletin. 2008;56:1618–1629. doi: 10.1016/j.marpolbul.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Lavery, P. S. & McMahon, K. Review of Cockburn Sound SEP Seagrass Monitoring Program (No. CMER 2011-04). Report prepared for the Cockburn Sound Management Council (2011).
- 35.Fraser, M. W., Statton, J., Hovey, R. K., Laverock, B. & Kendrick, G. Seagrass derived organic matter influences biogeochemistry, microbial communities, and seedling biomass partitioning in seagrass sediments. Plant and Soil #400, 133-146,. 10.1007/s11104-015-2721-0 (2015).
- 36.Garcias-Bonet N, Marbà N, Holmer M, Duarte CM. Effects of sediment sulfides on seagrass Posidonia oceanica meristematic activity. Marine Ecology Progress Series. 2008;372:1–6. doi: 10.3354/meps07714. [DOI] [Google Scholar]
- 37.Fraser MW, et al. Extreme climate events lower resilience of foundation seagrass at edge of biogeographical range. Journal of Ecology. 2014;102:1528–1536. doi: 10.1111/1365-2745.12300. [DOI] [Google Scholar]
- 38.Lee, K. S. & Dunton, K. H. Diurnal changes in pore water sulfide concentrations in the seagrass Thalassia testudinum beds: the effects of seagrasses on sulfide dynamics. Journal of Experimental Marine Biology and Ecology (2000). [DOI] [PubMed]
- 39.Holmer M, Duarte CM, Marbà N. Sulfur cycling and seagrass (Posidonia oceanica) status in carbonate sediments. Biogeochemistry. 2003;66:223–239. doi: 10.1023/B:BIOG.0000005326.35071.51. [DOI] [Google Scholar]
- 40.Pedersen O, Colmer TD, Borum J, Zavala-Perez A, Kendrick GA. Heat stress of two tropical seagrass species during low tides–impact on underwater net photosynthesis, dark respiration and diel in situ internal aeration. New Phytologist. 2016;210:1207–1218. doi: 10.1111/nph.13900. [DOI] [PubMed] [Google Scholar]
- 41.Kilminster K, Forbes V, Holmer M. Ecological Indicators. Ecological Indicators. 2014;36:280–289. doi: 10.1016/j.ecolind.2013.07.026. [DOI] [Google Scholar]
- 42.Holmer M, Duarte CM, Marbà N. Iron additions reduce sulfate reduction rates and improve seagrass growth on organic-enriched carbonate sediments. Ecosystems. 2005;8:721–730. doi: 10.1007/s10021-003-0180-6. [DOI] [Google Scholar]
- 43.Talbot V, Chegwidden A. Heavy metals in the sediments of Cockburn Sound, Western Australia, and its surrounding areas. Environmental Pollution Series B, Chemical and Physical. 1983;5:187–205. doi: 10.1016/0143-148X(83)90003-4. [DOI] [Google Scholar]
- 44.Leleyter L, Rousseau C, Biree L, Baraud F. Comparison of EDTA, HCl and sequential extraction procedures, for selected metals (Cu, Mn, Pb, Zn), in soils, riverine and marine sediments. Journal of Geochemical Exploration. 2012;116–117:51–59. doi: 10.1016/j.gexplo.2012.03.006. [DOI] [Google Scholar]
- 45.McCready S, Birch GF, Taylor SE. Extraction of heavy metals in Sydney Harbour sediments using 1M HCl and 0.05M EDTA and implications for sediment‐quality guidelines. Australian Journal of Earth Sciences. 2003;50:249–255. doi: 10.1046/j.1440-0952.2003.00994.x. [DOI] [Google Scholar]
- 46.Heron G, Crouzet C, Bourg A. Speciation of Fe (II) and Fe (III) in contaminated aquifer sediments using chemical extraction techniques. Environ. Sci. Technol. 1994;28:1698–1705. doi: 10.1021/es00058a023. [DOI] [PubMed] [Google Scholar]
- 47.Berner RA. Sedimentary pyrite formation: an update. Geochimica et Cosmochimica Acta. 1984;48:650–615. doi: 10.1016/0016-7037(84)90089-9. [DOI] [Google Scholar]
- 48.Rainbow PS. Biomonitoring of heavy metal availability in the marine environment. Marine Pollution Bulletin. 1995;31:183–192. doi: 10.1016/0025-326X(95)00116-5. [DOI] [Google Scholar]
- 49.Ralph, P. J. & Burchett, M. D. Photosynthetic response of Halophila ovalis to heavy metal stress. Environmental Pollution (1998).
- 50.Avelar M, et al. Iron, cadmium, and chromium in seagrass (Thalassia testudinum) from a coastal nature reserve in karstic Yucat?n. Environ Monit Assess. 2013;185:7591–7603. doi: 10.1007/s10661-013-3121-7. [DOI] [PubMed] [Google Scholar]
- 51.Malea, P. Uptake of Cadmium and the Effect on Viability of Leaf Cells in the Seagrass Halophila stipulacea (Forsk.) Aschers. Botanica Marina37 (1994).
- 52.Ernst W, Krauss GJ, Verkleij J, Wesenberg D. Interaction of heavy metals with the sulphur metabolism in angiosperms from an ecological point of view. Plant, Cell & Environment. 2008;31:123–143. doi: 10.1111/j.1365-3040.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 53.Skene, D., Ryan, D., Brooke, B. & Smith, J. The Geomorphology and Sediments of Cockburn Sound. Geoscience Australia (2005).
- 54.Barwick M, Maher W. Biotransference and biomagnification of selenium copper, cadmium, zinc, arsenic and lead in a temperate seagrass ecosystem from Lake Macquarie Estuary, NSW, Australia. Marine Environmental Research. 2003;56:471–502. doi: 10.1016/S0141-1136(03)00028-X. [DOI] [PubMed] [Google Scholar]
- 55.Short, F. & Duarte, C. In (eds Short, F. T., Short, C. A. & Coles, R. G.) 155–182 (Global seagrass research methods, 2001).
- 56.Fourqurean JW, et al. Seagrass ecosystems as a globally significant carbon stock. Nature Geoscience. 2012;5:505–509. doi: 10.1038/ngeo1477. [DOI] [Google Scholar]
- 57.Wheal MS, Fowles TO, Palmer LT. A cost-effective acid digestion method using closed polypropylene tubes for inductively coupled plasma optical emission spectrometry (ICP-OES) analysis of plant essential elements. Anal. Methods. 2011;3:2854–2863. doi: 10.1039/c1ay05430a. [DOI] [Google Scholar]
- 58.R Core Team. R Foundation for Statistical Computing. Vienna, Austria. R: A language and environment for statistical computing (2014).
- 59.Wickham, H. ggplot2: elegant graphics for data analysis. (Springer New York, 2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data in this article are published onthe FigShare data Repository at https://figshare.com/s/3e705529e7c311830c33 (doi:10.6084/m9.figshare.5103340).