Abstract
Background
Kimura disease (KD) is a systemic soft-tissue disease that leads to formation of painless masses in lymph nodes, with the highest predilection for the head and neck and especially the parotid gland. KD lesions are characterized by marked eosinophil infiltration, production of IgE and increased expression of T-helper type 2 (Th2) cytokines (interleukin [IL]-4, IL-5, etc.). Skewing to a Th2 inflammation is also demonstrated in the peripheral blood, with elevated eosinophils and high IgE levels. It is thought that basophils may play important roles in orchestrating this Th2 inflammation via IL-4 production leading to the induction of IgE synthesis as well as eosinophil infiltration. However, there are no reports as yet on the role of basophils in KD.
Objective
The present study was performed to investigate the potential role of basophils in the pathogenesis of KD. In this context we also examined the expression of IL-4 in basophils in the KD lesions.
Methods
By immunohistochemistry using a monoclonal antibody against a basophil marker ProMBP1 we investigated the number and distribution of basophils in the KD lesions. By double immunohistochemistry we analyzed the colocalization of IL-4 in basophils.
Results
There was an increased number of basophils infiltrating the KD parotid gland lesions as compared to that in normal control parotid tissue. By double-immunofluorescence we found that approximately 7% of IL-4-positive cells in KD patients' parotid glands were basophils.
Conclusion
Basophils may also play a role in the pathogenesis of KD, leading to the induction of IgE synthesis and eosinophil infiltration.
Keywords: Kimura disease, Parotid gland, Basophils, Interleukin-4, Immunohistochemistry
INTRODUCTION
Kimura disease (KD) is a chronic inflammatory disease that leads to formation of painless masses in soft tissues and lymph nodes throughout the body [1]. The lesions are most common in the head and neck and especially in the parotid gland, where they are suggestive of tumors. The pathogenesis of KD remains unclear. However, KD is characterized by elevated eosinophils and high IgE levels in the peripheral blood, and infiltration of the lesional sites by eosinophils, T-helper type 2 (Th2) cells and mast cells. Th2 cytokines not only induce IgE synthesis, but also upregulate CC chemokines, which can then lead to the infiltration of eosinophils into the tissue. These inflammatory characteristics are important in the pathogenesis of KD [2,3].
Basophils are known to play a crucial role in IgE-mediated allergic diseases through the release of inflammatory mediators like interleukin (IL)-4 and IL-13 [4].
It is known that basophils produce IL-4, and that basophil-derived IL-4 and CD40 ligand directly induce class switching to IgE in B cells [5]. It is clear that Th2 cells play important roles in allergic diseases, but IL-4 is required for differentiation of naïve T cells into Th2 cells. Basophils have gained attention as sources of IL-4, especially as cells involved in the initial production of IL-4 that is necessary for induction of differentiation into Th2 cells [6,7,8]. However, the role of basophils in regulating the Th2 inflammation in KD, which is characterized by increased IgE production, Th2 cell and eosinophil infiltration, etc., has not been investigated.
In order to better define the role of basophils in the pathogenesis of KD, we investigated the presence and numbers of basophils n KD, as well as the expression of IL-4.
MATERIALS AND METHODS
Subjects
The subjects and tissue specimens were the same as those reported and employed in our earlier study [9]. Briefly, we obtained parotid gland tissue specimens from 6 male patients with KD (mean age ± standard error of the mean, 34.5 ± 6.9 years). Control parotid gland tissue specimens were obtained from 6 pleomorphic adenoma (nonneoplastic) surgical patients (5 male, 1 female patient; 49.3 ± 6.8 years). Some of the KD patients also had coexisting atopic dermatitis and allergic rhinitis (AR), while some of the control patients had coexisting AR. We performed histopathological and immunohistochemical analyses of the parotid gland tissue specimens (polymer-immunocomplex and double-immunofluorescence studies of buffered formalin [10%]-fixed, paraffin-embedded materials). The Ethics Committee of Tokyo Women's Medical University approved this study (approval number: 2144), and all patients granted written informed consent.
Histopathological analysis
Histopathological analyses were performed as described in our earlier manuscript [9]. Briefly, haematoxylin and eosin staining was performed to confirm the diagnosis of KD, while Congo red staining was used to determine the eosinophil counts in the KD and normal parotid glands.
Immunohistochemical analysis
The primary antibody employed in immunohistochemistry was a mouse anti-human basophil marker ProMBP-1 mAb (Clone No. J175-7D4; diluted 1:10; Bio-Legend, San Diego, CA, USA).
The detailed methods were as described in our earlier manuscript [9]. Briefly, 3-m sections were cut from paraffin-embedded tissue blocks were deparaffinized, rinsed in ethanol, rehydrated, and rinsed in phosphate-buffered saline (PBS; pH, 7.6). Antigen retrieval was performed by microwaving sections at pH 9.0 Tris-EDTA buffer (95℃, 400 W, 40 minutes). Nonspecific antibody binding was inhibited by treating the sections with 5% skim milk in PBS for 10 minutes at room temperature. Sections were subsequently incubated overnight at 4℃ with the primary antibody. Immunoreactivity was visualized by the polymer immunocomplex method. The chromogen was 3,3′-diaminobenzidine tetrahydrochloride, while hematoxylin was used for counterstaining. A specific control was included in each staining run. Mouse IgG1 (Clone DAK-GO1, DAKO, Glostrup, Copenhagen, DK) was used as a negative control.
Immunohistochemical identification of basophils as a source of IL-4 by double-immunofluorescence
Sections were simultaneously incubated with a mixture of the primary antibodies against IL-4 (diluted 1:20; Abnova Co., Taipei, Taiwan) and pro-form of major basic protein 1 (proMBP1), followed by their respective secondary antibodies such as donkey anti-rabbit IgG conjugated with Cy3TM (red signal; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and donkey anti-mouse IgG conjugated with Alexa Fluor488 (green signal; Molecular Probes, Eugene, OR, USA) (n = 6). Double-stained sections were observed with a Nikon ECLIPSE TS100 fluorescence microscope. A yellowish signal due to merging of red and green signals was considered to be the result of colocalization of IL-4 in basophils (identified by ProMBP1, a basophil cell marker).
Cell counting
Immunoreactive cells were counted in each of 10 randomly selected areas of 0.0625 mm2 per slide (i.e., one tissue section) using an objective micrometer under an Olympus light microscope at a magnification of 400 high-power field (HPF). The mean for 10 fields was used as the cell count for that section. After double-immunofluorescence staining, the proportion of cells immunoreactive for ProMBP1 among the cells immunoreactive for IL-4 was determined in 10 randomly selected areas per slide (i.e., one tissue section) at a magnification of 200 HPF with a Nikon ECLIPSE TS100 fluorescence microscope. Data are presented as the median (range).
Statistics
The Mann-Whitney U-test was used for statistical analysis of the immunohistochemical observations. A p value of less than 0.05 was considered statistically significant.
RESULTS
Subjects' characteristics
The age ranges of the KD and control patients showed no significant difference. The number of blood eosinophils (3,499.1/µL [1,207–8,628/µL]) (p < 0.01) in the KD patients was significantly higher than that (82.5/µL [31–290/µL]) in the controls. The serum total IgE level (5196.6 IU/mL [410–16,000 IU/mL]) (p < 0.01) in KD patients were significantly higher than that (125.0 IU/mL [6–595 IU/mL]) in the controls. The number of blood basophils (76.3/µL [39–127/µL]) tended to be higher in the KD patients than that (25.5/µL [20–111/µL]) in the controls, but without statistical significance.
Number and distribution of eosinophils
The number of eosinophils (Congo red staining) was significantly greater in the KD patients' parotid glands (median, 272.0/mm2; range, 24.8–589.6/mm2) than in the control subjects' parotid glands, which contained no detectable eosinophils (median, 0; range, 0–0; p < 0.01). Eosinophils were localized mainly between the lymphoid follicles.
Number and distribution of basophils
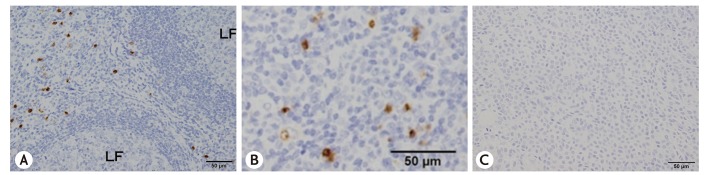
The number of ProMBP1-positive cells (i.e., basophils) was significantly greater in KD patients' parotid glands (median, 77.6/mm2; range, 16.0–140.0/mm2) compared with the control subjects' parotid glands (p < 0.01) (Figs. 1, 2). Basophils had infiltrated between the lymphoid follicles in KD (Fig. 2A, B). No basophils were detected in the control specimens.
Fig. 1. Numbers of proMBP1-positive cells in KD and normal parotid glands. Box plots represent the median values with 25% and 75% interquartiles. The error bars represent the 10th and 90th percentiles. Each group, n = 6. *p < 0.01. proMBP1, pro-form of major basic protein 1.
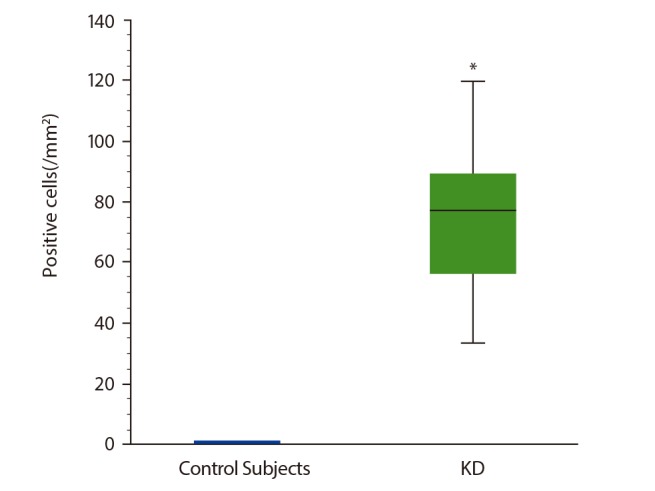
Fig. 2. Immunolocalization of proMBP1 in the parotid lesion of Kimura disease. (A) Low magnification and (B) high magnification. (C) Same tissue stained with control antibody. LF, lymphoid follicle; proMBP1, pro-form of major basic protein 1.
Percentage of IL-4-positive cells that were basophils
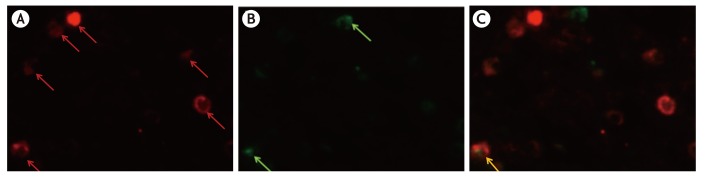
ProMBP1-positive cells (i.e., basophils) comprised 6.8% (median; range, 2.6%–17.8%) of the IL-4-positive cells in KD patients' parotid glands (Fig. 3). No IL-4 positive cells were detected in the control specimens.
Fig. 3. Immunohistochemical identification of interleukin (IL)-4-positive, proMBP1-positive cells in the Kimura disease parotid gland by the double-immunofluorescence method. Images staining positive for IL-4 (red) (A), proMBP1 (green) (B), and their merger (C). The yellow signal (arrow) in panel C indicates colocalization of IL-4 and proMBP1. proMBP1, pro-form of major basic protein 1.
DISCUSSION
The present study demonstrated for the first time, that basophils infiltrate the parotid gland lesions of KD. The basophils were found to infiltrate between the lymphoid follicles. Also basophils infiltrating the KD parotid gland lesions were positive for IL-4 protein. Our findings indicate that the infiltrating basophils are functionally involved in the pathogenesis of the Th2 inflammation of KD by enhancing IgE production, eosinophil infiltration, etc., at the lesional sites.
Since the discovery of basophils, their functions were not well defined. There were various reasons for the inability to elucidate the functions, including the fact that: basophils account for only 0.5% of peripheral blood mononuclear cells. Moreover unlike mast cells, there are no animal models that are deficient only in basophils. Basophils have few specific surface markers and basophils were difficult to differentiate from mast cells. A number of basophil-specific antibodies, such as BSP-1 [10], 2D7 [11], 97A6 [12], and BB1 [13,14], have been established. However, although BSP-1 and 97A6 recognize basophil surface markers, they have problems such as inadequate sensitivity, also recognize mast cells, etc. [11,12]. 2D7 and BB1 specifically recognize basophils, but their ligands are unknown [11,13]. However, a monoclonal antibody against the proMBP1 was developed, and it is said to be specific for basophils [15].
We used monoclonal antibodies specific for tryptase and proMBP1 to perform double-immunofluorescence staining of the same tissue specimens, and we were able to clearly differentiate between tryptase-positive cells (mast cells) and proMBP1-positive cells (basophils) (data not shown). We therefore used proMBP1 to identify basophils. A study of 2D7-positive cells in the bronchial mucosa of asthma and proMBP1-positive cells in nasal polyps found very few of basophils infiltrating the lesional site [15]. In the asthmatic bronchial mucosa, basophils were only about one-tenth the number of mast cells [16]. The KD patients' parotid glands that we examined in this study contained a relatively large number of infiltrating basophils, i.e., 77.6/mm2 (median), which was about half of the number of infiltrating mast cells (data not shown).
The histopathological findings for KD are characterized by formation of lymphoid follicles with a germinal center. The lymphoid follicles contain large numbers of infiltrating dendritic cells and B lymphocytes [17]. In fact, IgE in KD lesional sites is localized in lymphoid follicles that contain many B lymphocytes, which are antibody-producing cells [18]. On the other hand, mainly eosinophils, mast cells and T lymphocytes infiltrate between the lymphoid follicles [2]. IL-4 and IL-5, which are Th2 cytokines, and eotaxin and RANTES, which are chemotactic factors for eosinophils, are also localized between the lymphoid follicles [2]. Cytokines known to induce Th2 inflammation include thymic stromal lymphopoietin (TSLP), IL-25, IL-33, etc. [19]. In KD, TSLP is the only one of these 3 cytokines that is increased in the lesional sites, and it is thought to play an important role in induction of Th2 inflammation in KD [9]. Furthermore, it was found that, in KD, macrophages between the lymphoid follicles produce mainly TSLP, while mast cells express the TSLP receptor [9]. Thus, it can be thought that TSLP-mediated interaction between the macrophages and mast cells localized between the lymphoid follicles is crucial for eliciting the Th2 inflammation seen in KD. Our present studies elucidated that many basophils are also infiltrating between the lymphoid follicles, suggesting that they may function as effector cells in those sites in the pathogenesis of the Th2 inflammation characterizing KD.
IL-4 and IL-13, which are Th2 cytokines, play central roles in allergic inflammation. IL-4 activates STAT6, leading to expression of GATA3 that mediates induction of increased differential proliferation of naïve T cells into Th2 cells that produce Th2 cytokines [20]. IL-4 also causes proliferation and activation of mast cells. IL-4 and IL-13 induce expression of CC chemokines by epithelial cells, thereby promoting infiltration of eosinophils and T cells into inflammatory sites. Moreover, IL-4 and IL-13 induce differentiation of B cells into IgE-producing cells. These 2 cytokines are thought to play important roles in the eosinophil, Th2 cell and mast cell infiltrations, as well as the increased IgE production, seen in KD lesions.
Cells that produce IL-4 include Th2 cells, mast cells, basophils, eosinophils, etc. Mast cells are considered to be the main source of IL-4 in perennial AR and asthma [21,22]. In addition, eosinophils are thought to be the main cells producing IL-4 in nasal polyps [23]. In fact, immunohistochemical studies indicated that in KD IL-4 is localized mainly in mast cells and T cells [2].
On the other hand, upon activation basophils are known to immediately produce large quantities of IL-4 [24]. It is also known that basophils are an important source of the IL-4 that initiates differentiation of naïve T cells into Th2 cells in the early stage of allergic inflammation [6,7,8]. However, all the in vivo research showing roles of basophils in IL-4 production and induction of Th2 cells from naïve T cells was conducted in animals [6,7,8,24]. In our present study, we confirmed in vivo that IL-4 is localized in basophils in the parotid lesions of human KD patients.
This is the first report to document that the parotid gland lesions of KD patients are infiltrated by a relatively large number of basophils. We also showed that some of the infiltrating basophils are positive for IL-4 protein, suggesting that those cells are actively functioning in the lesional sites. Based on these findings, we postulate that IL-4 produced by those basophils contributes to the pathogenesis of KD.
ACKNOWLEDGEMENTS
The authors wish to express their gratitude to Miss N. Abo and Mrs. N. Sakayori for their valuable technical assistance.
References
- 1.Kimura T, Yoshimura S, Ishikawa E. On the unusual granulation combined with hyperplastic changes of lymphatic tissues. Trans Soc Pathol Jpn. 1948;37:179–180. [Google Scholar]
- 2.Kimura Y, Pawankar R, Aoki M, Niimi Y, Kawana S. Mast cells and T cells in Kimura's disease express increased levels of interleukin-4, interleukin-5, eotaxin and RANTES. Clin Exp Allergy. 2002;32:1787–1793. doi: 10.1046/j.1365-2222.2002.01552.x. [DOI] [PubMed] [Google Scholar]
- 3.Ohta N, Fukase S, Suzuki Y, Ito T, Yoshitake H, Aoyagi M. Increase of Th2 and Tc1 cells in patients with Kimura's disease. Auris Nasus Larynx. 2011;38:77–82. doi: 10.1016/j.anl.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Bieneman AP, Chichester KL, Chen YH, Schroeder JT. Toll-like receptor 2 ligands activate human basophils for both IgE-dependent and IgE-independent secretion. J Allergy Clin Immunol. 2005;115:295–301. doi: 10.1016/j.jaci.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Yanagihara Y, Kajiwara K, Basaki Y, Ikizawa K, Ebisawa M, Ra C, Tachimoto H, Saito H. Cultured basophils but not cultured mast cells induce human IgE synthesis in B cells after immunologic stimulation. Clin Exp Immunol. 1998;111:136–143. doi: 10.1046/j.1365-2249.1998.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 8.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakitani E, Nonaka M, Shibata N, Furukawa T, Yoshihara T. Increased expression of thymic stromal lymphopoietin and its receptor in Kimura's disease. ORL J Otorhinolaryngol Relat Spec. 2015;77:44–54. doi: 10.1159/000371424. [DOI] [PubMed] [Google Scholar]
- 10.Bodger MP, Mounsey GL, Nelson J, Fitzgerald PH. A monoclonal antibody reacting with human basophils. Blood. 1987;69:1414–1418. [PubMed] [Google Scholar]
- 11.Kepley CL, Craig SS, Schwartz LB. Identification and partial characterization of a unique marker for human basophils. J Immunol. 1995;154:6548–6555. [PubMed] [Google Scholar]
- 12.Bühring HJ, Simmons PJ, Pudney M, Müller R, Jarrossay D, van Agthoven A, Willheim M, Brugger W, Valent P, Kanz L. The monoclonal antibody 97A6 defines a novel surface antigen expressed on human basophils and their multipotent and unipotent progenitors. Blood. 1999;94:2343–2356. [PubMed] [Google Scholar]
- 13.McEuen AR, Buckley MG, Compton SJ, Walls AF. Development and characterization of a monoclonal antibody specific for human basophils and the identification of a unique secretory product of basophil activation. Lab Invest. 1999;79:27–38. [PubMed] [Google Scholar]
- 14.McEuen AR, Calafat J, Compton SJ, Easom NJ, Buckley MG, Knol EF, Walls AF. Mass, charge, and subcellular localization of a unique secretory product identified by the basophil-specific antibody BB1. J Allergy Clin Immunol. 2001;107:842–848. doi: 10.1067/mai.2001.114650. [DOI] [PubMed] [Google Scholar]
- 15.Plager DA, Weiss EA, Kephart GM, Mocharla RM, Matsumoto R, Checkel JL, Schwartz LB, Gleich GJ, Leiferman KM. Identification of basophils by a mAb directed against pro-major basic protein 1. J Allergy Clin Immunol. 2006;117:626–634. doi: 10.1016/j.jaci.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Macfarlane AJ, Kon OM, Smith SJ, Zeibecoglou K, Khan LN, Barata LT, McEuen AR, Buckley MG, Walls AF, Meng Q, Humbert M, Barnes NC, Robinson DS, Ying S, Kay AB. Basophils, eosinophils, and mast cells in atopic and nonatopic asthma and in late-phase allergic reactions in the lung and skin. J Allergy Clin Immunol. 2000;105(1 Pt 1):99–107. doi: 10.1016/s0091-6749(00)90184-2. [DOI] [PubMed] [Google Scholar]
- 17.Aguzzi A, Krautler NJ. Characterizing follicular dendritic cells: a progress report. Eur J Immunol. 2010;40:2134–2138. doi: 10.1002/eji.201040765. [DOI] [PubMed] [Google Scholar]
- 18.Takenaka T, Okuda M, Usami A, Kawabori S, Ogami Y. Histological and immunological studies on eosinophilic granuloma of soft tissue, so-called Kimura's disease. Clin Allergy. 1976;6:27–39. doi: 10.1111/j.1365-2222.1976.tb01409.x. [DOI] [PubMed] [Google Scholar]
- 19.Byers DE. Defining the roles of IL-33, thymic stromal lymphopoietin, and IL-25 in human asthma. Am J Respir Crit Care Med. 2014;190:715–716. doi: 10.1164/rccm.201408-1539ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou M, Ouyang W. The function role of GATA-3 in Th1 and Th2 differentiation. Immunol Res. 2003;28:25–37. doi: 10.1385/IR:28:1:25. [DOI] [PubMed] [Google Scholar]
- 21.Bradding P, Feather IH, Wilson S, Bardin PG, Heusser CH, Holgate ST, Howarth PH. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J Immunol. 1993;151:3853–3865. [PubMed] [Google Scholar]
- 22.Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 23.Nonaka M, Nonaka R, Woolley K, Adelroth E, Miura K, Okhawara Y, Glibetic M, Nakano K, O'Byrne P, Dolovich J, et al. Distinct immunohistochemical localization of IL-4 in human inflamed airway tissues. IL-4 is localized to eosinophils in vivo and is released by peripheral blood eosinophils. J Immunol. 1995;155:3234–3244. [PubMed] [Google Scholar]
- 24.van Panhuys N, Prout M, Forbes E, Min B, Paul WE, Le Gros G. Basophils are the major producers of IL-4 during primary helminth infection. J Immunol. 2011;186:2719–2728. doi: 10.4049/jimmunol.1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]