Abstract
The long non-coding RNA (lncRNA) PCAT-1 resides in the chromosome 8q24 cancer-risk locus and acts as a vital oncogene during tumorigenesis and progression. However, how PCAT-1 is post-transcriptionally regulated, for example, by small ncRNAs, such as microRNAs (miRNAs) is largely unknown. Here, we report how miRNAs regulate PCAT-1 expression and also investigate the biological significance of this regulation in hepatocellular carcinoma (HCC). We found that miR-215, a P53-inducible miRNA, is a key regulator of PCAT-1 expression in HCC and identified an interaction between miR-215 and PCAT-1 in dual luciferase reporter gene assays. We also found that post-transcriptional silencing of PCAT-1 by miR-215 or PCAT-1 siRNAs significantly inhibited proliferation of HCC cells and, conversely, that inhibition of endogenous miR-215 up-regulated PCAT-1 expression and promoted cell viability. The tumor-suppressing role of miR-215 was further confirmed in an in vivo mouse HCC xenograft model. Of note, gene profiling assays suggested that the kinase CRK-like proto-oncogene, adaptor protein (CRKL), is a potential downstream target of the miR–215–PCAT-1 axis in HCC, and we demonstrated that CRKL silencing significantly suppresses cell proliferation. Taken together and considering the essential role of CRKL in cancer cells, we propose that the TP53–miR-215–PCAT-1–CRKL axis might represent an important regulatory pathway in HCC. In summary, our results highlight the involvement of several ncRNAs in HCC and thus provide critical insights into the molecular pathways operating in this malignancy.
Keywords: hepatocellular carcinoma; long noncoding RNA (long ncRNA, lncRNA); microRNA (miRNA); oncogene; p53; CRKL; PCAT-1; miR-215
Introduction
Hepatocellular carcinoma (HCC)3 is the sixth most prevalent human malignancy globally (1–3). Its morbidity approximately matched its mortality, indicating the aggressive nature of this malignant disease (1–3). Chronic infection with the hepatitis B or C viruses (HBV or HCV), exposure to dietary aflatoxin B, as well as alcohol abuse are major risk factors of HCC (2, 3). There are limited therapeutic options for this refractory disease. Most patients diagnosed at an advanced stage cannot receive surgical resection or liver transplantation (4, 5). Prevention, intervention, diagnosis, and treatment for HCC are far from satisfactory (4, 5). As a result, clarifying the underlying mechanism of HCC development and progression is urgently needed.
Long noncoding RNAs (lncRNAs) are functionally defined as transcripts >200 nucleotides in length with no protein-coding potential. Although more and more lncRNAs have been identified, the majority's biological functions are unclear (6–10). Indeed, lncRNAs may contribute diverse functional roles considering their expression across a variety of cellular- and tissue-specific contexts (6–10). Therefore, the exquisite regulation of lncRNA expression at either transcription or post-transcription levels can significantly impact disease development, i.e. malignant transformation. Our and others data declared the essential involvement of microRNAs (miRNAs) in post-transcription regulation of cancer-related lncRNAs, which may drive many important cancer phenotypes (11–16).
High-throughput sequencing of the poly(A)+ RNA (RNA-Seq) of prostate tissues and cells lines led to the discovery of a novel lncRNA PCAT-1 (17). PCAT-1 is evidently overexpressed in a subset of prostate cancers and may contribute to prostate cancer cell proliferation. The interaction between PCAT-1 and polycomb repressive complex 2 (PRC2) could transcriptionally regulate target genes of PRC2 and further indicates its important role in prostate cancer progression (17). It is also notable that PCAT-1 resides in the 8q24 “gene desert” locus, which is also prostate cancer susceptibility locus identified by genome-wide association studies (GWAS). Through integrative analyses of the lncRNA transcriptome with genomic data and single nucleotide polymorphism data from prostate cancer genome-wide association studies, Guo et al. (18) identified 45 candidate lncRNAs associated with risk to prostate cancer. Among these lncRNAs, PCAT-1 ranks the top hit. The risk-associated rs7463708 single nucleotide polymorphism increases binding of ONECUT2, a novel androgen receptor-interacting transcription factor, at a distal enhancer that loops to the PCAT-1 promoter, resulting in up-regulation of PCAT-1 upon prolonged androgen treatment (18). Besides the transcriptional regulation by the androgen receptor signaling, somatic copy-number alterations or structural variants may also contribute to PCAT-1 dysregulation in esophageal cancer (19). However, fine regulation of lncRNA PCAT-1 expression in HCC, especially at the post-transcriptional level, is still largely unknown. Two previous studies showed the role of lncRNA PCAT-1 in HCC (20, 21). Wen et al. (20) found that the increase of lncRNA PCAT-1 worsened HCC. Also, the increased expression of PCAT-1 was associated with advanced clinical parameters and poor overall survival of HCC patients (21). In this study, we for the first time declared the fine regulation of lncRNA PCAT-1 by the TP53-response miR-215 and its potential downstream effector, oncogene CRKL. Ectopic expression of miR-215 or PCAT-1 siRNA significantly inhibits expression, thereby suppressing HCC proliferation in vitro and in vivo.
Results
Candidate miRNAs targeting PCAT-1 at post-transcriptional level
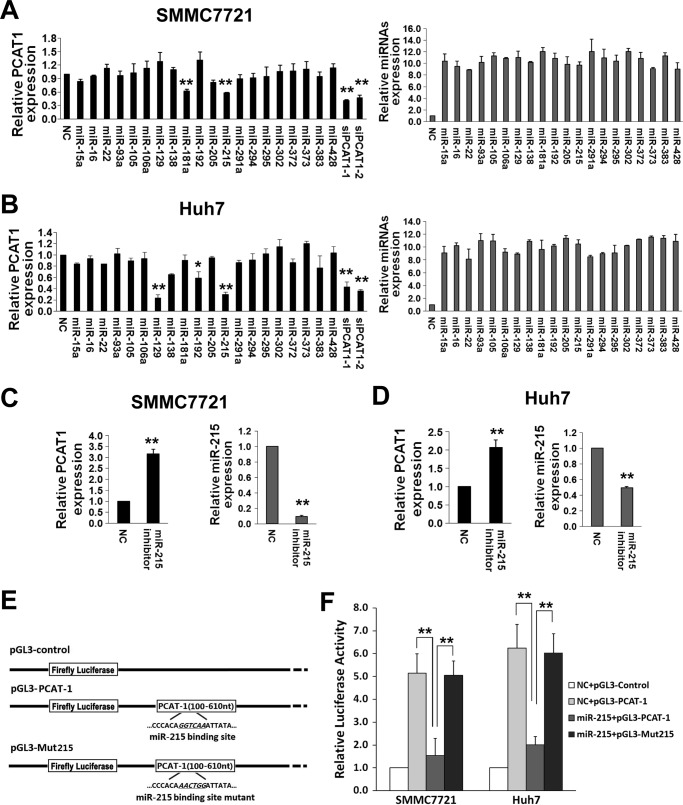
We predicted potential candidate miRNAs targeting lncRNA PCAT-1 by the miRCode software (22). Higher evolutionary conservation miRNA targeting sites across species might be more biologically important. Therefore, we examined 20 miRNA candidates (miR-15a, miR-16, miR-22, miR-93a, miR-105, miR-106a, miR-129, miR-138, miR-181a, miR-192, miR-205, miR-215, miR-291a, miR-294, miR-295, miR-302, miR-372, miR-373, miR-383, and miR-428), which show ≥56% conservation among 8 primate species excluding human in the current study. We first detected endogenous lncRNA PCAT-1 expression changes in SMMC7721 and Huh7 cells transfected with the RNA mimics of the 20 miRNAs and two PCAT-1 siRNAs (siPCAT-1-1 and siPCAT-1-2) as positive controls (Fig. 1, A and B). There was a 60∼70% decrease of PCAT-1 expression in HCC cells transfected with siPCAT-1-1 or siPCAT-1-2 compared with NC-RNA-transfected cells (both p < 0.01). In SMMC7721 cells, miR-181a and miR-215 can significantly down-regulate PCAT-1 expression (both p < 0.01). miR-129, miR-192, and miR-215 could significantly inhibit PCAT-1 expression in Huh7 cells (all p < 0.05). We only investigated miR-215 in the following studies considering the consistency in both cell lines. For further validation, inhibitors of miR-215 were delivered into SMMC7721 and Huh7 cell lines to antagonize endogenous miR-215 expression. Interestingly, there was significantly elevated PCAT-1 expression in HCC cells (both p < 0.01) (Fig. 1, C and D).
Figure 1.
Identification of candidate miRNAs targeting lncRNA PCAT-1 in HCC. A and B, miR-215 inhibits PCAT-1 expression. 20 nmol/liter mimics of miR-15a, miR-16, miR-22, miR-93a, miR-105, miR-106a, miR-129, miR-138, miR-181a, miR-192, miR-205, miR-215, miR-291a, miR-294, miR-295, miR-302, miR-372, miR-373, miR-383, and miR-428, siRNA duplexes (siPCAT-1-1, siPCAT-1-2) or NC RNA were transfected into SMMC7721 and Huh7 cells. lncRNA PCAT-1 expression was detected at 48 h after transfection (left, black columns). The transfection efficiency of different miRNAs was confirmed by qRT-PCR (right, gray columns). C and D, inhibition of miR-215 up-regulates PCAT-1 expression. 20 nmol/liter of miR-215 inhibitors and NC RNA were transfected into SMMC7721 and Huh7 HCC cells. All data of PCAT-1 expression were normalized to β-actin mRNA expression levels. All miRNA expression data were normalized to U6 small RNA expression. E, schematic constructions of pGL3-PCAT-1 and pGL3-Mut215. F, pGL3-PCAT-1 and pGL3-Mut215 were co-transfected into SMMC7721 and Huh7 cells with miR-215 mimics or NC RNA. Luciferase activity was detected at 48 h after transfection and normalized relative to the Renilla luciferase expression. Inhibition effects of miR-215 mimics on pGL3-PCAT-1 or pGL3-Mut215 are shown. All results of the mean of triplicate assays with standard deviation are presented. *, p < 0.05; **, p < 0.01.
miR-215 directly suppresses lncRNA PCAT-1 expression
To investigate the potential miRNA-lncRNA interactions, we first subcloned a 510-bp human PCAT-1 sequence after the firefly luciferase gene and named the construct as pGL3-PCAT-1 (Fig. 1E). Then SMMC7721 and Huh7 cells were co-transfected with this construct and miR-215 mimics or NC RNA. miR-215 suppressed a 70.0 or 67.7% luciferase activity compared with NC RNA in SMMC7721 or Huh7 cells (both p < 0.01)(Fig. 1F). We then introduced point substitutions disrupting the target sites of miR-215 in pGL3-PCAT-1 and named the construct as pGL3-Mut215 (Fig. 1E) After HCC cells were co-transfected with this construct and miR-215 mimics or NC RNA, we found that there was no significantly decreased luciferase activity caused by miR-215 (all p > 0.05) (Fig. 1F).
TP53 response–miR-215 inhibits HCC proliferation via suppressing lncRNA PCAT-1
Two previous studies demonstrated that miR-215 could be actively induced by TP53 in multiple cancers including lung cancer, colorectal cancer, and ostosarcoma (23, 24). However, it is still unclear if this exists in HCC. To test this, we examined miR-215 expression after overexpression of TP53 in both SMMC7721 and Huh7 cells (Fig. 2, A and B). We found that tumor suppressor TP53 does up-regulate miR-215 expression in HCC and, thus, down-regulation of lncRNA PCAT-1 (Fig. 2, A and B). Additionally, miR-215 inhibitors could rescue TP53-induced lncRNA PCAT-1 down-regulation in HCC cells (Fig. 2C and supplemental Fig. S1), which indicates that TP53 down-regulates PCAT-1 in a miR-215-dependent manner in HCC cells.
Figure 2.
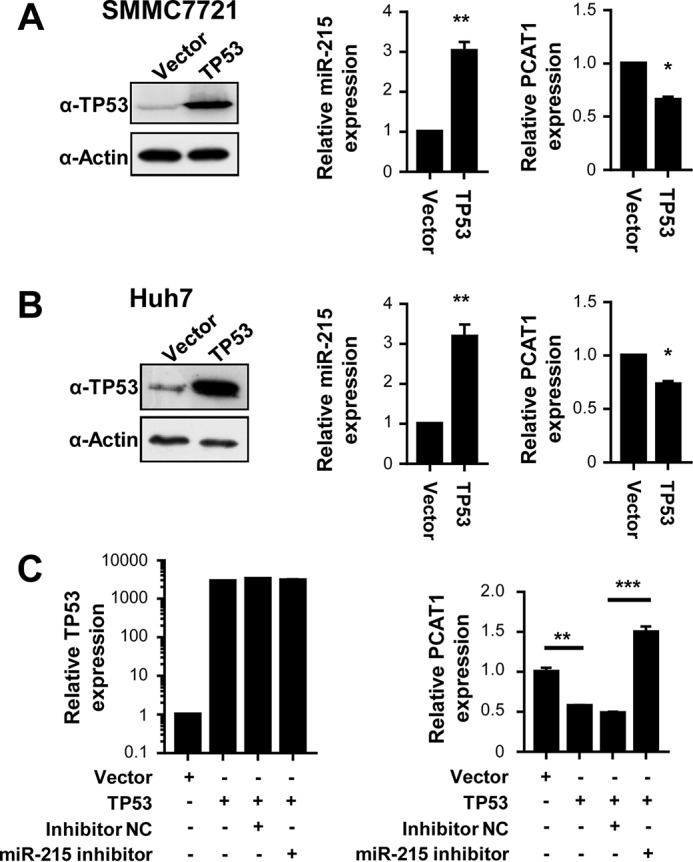
A and B, overexpression of TP53 induces miR-215 expression and suppresses lncRNA PCAT-1 expression in SMMC7721 cells (A) or Huh7 cells (B). C, miR-215 inhibitors rescue TP53-induced lncRNA PCAT-1 down-regulation in HCC cells. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
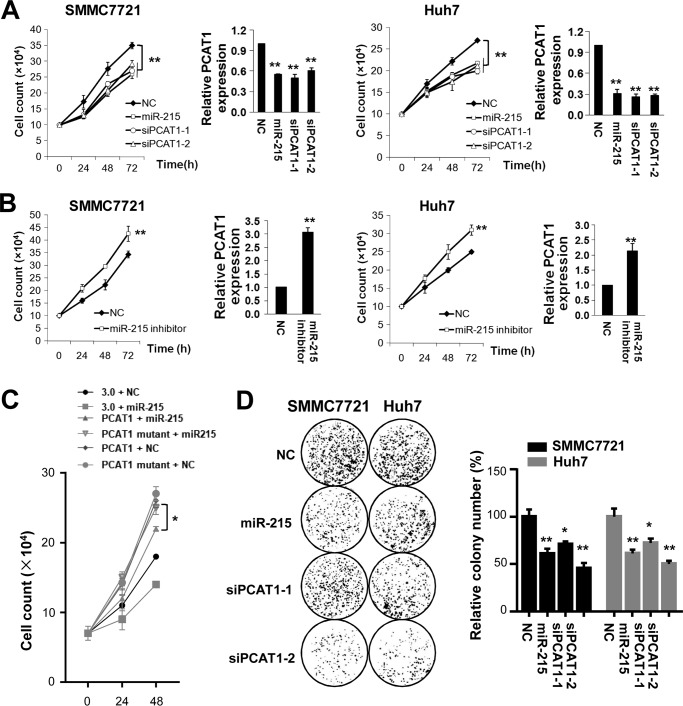
Suppression of PCAT-1 by miR-215 can significantly inhibit proliferation of SMMC7721 and Huh7 cells (Fig. 3A). Both miR-215 and PCAT-1 siRNAs can similarly inhibit viability of SMMC7721 cells at 72 h after transfection (miR-215, 25.7%; siPCAT-1-1, 22.9%; siPCAT-1-2, 16.8%; all p < 0.01). Similar results were observed in Huh7 cells at 72 h after RNA delivery (miR-215, 19.3%; siPCAT-1-1, 26.1%; siPCAT-1-2, 21.6%; all p < 0.01). On the contrary, miR-215 inhibitors could stimulate cell proliferation of both HCC cell lines (Fig. 3B). Antagonizing endogenous miR-215 expression led to a 23.9% increased SMMC7721 cell growth at 72 h (p < 0.05). Inhibition of endogenous miR-215 expression led to a 24.0% increased Huh7 proliferation at 72 h (p < 0.05). Overexpression of either lncRNA PCAT-1 or PCAT-1 mutant could promote HCC cell growth. However, in HCC cells whose PCAT-1 is not down-regulated (PCAT-1 mutant + miR-215), overexpression of miR-215 could not inhibit HCC cell proliferation (Fig. 3C), which indicates that miR-215 inhibits HCC cell viability mostly through suppression of PCAT-1. Colony formation assays also support the tumor suppressor nature of miR-215 (Fig. 3D). In line with the cell viability assays, miR-215 shows comparable inhibiting capability with both PCAT-1 siRNAs on colony formation (all p < 0.05).
Figure 3.
miR-215 inhibits cell proliferation of SMMC7721 or Huh7 cells. 20 nmol/liter of miR-215, NC RNA, or PCAT-1 siRNAs was transfected into HCC cells, respectively. A, overexpression of miR-215 or two PCAT-1 siRNAs (siPCAT-1-1 and siPCAT-1-2) inhibits HCC cell growth. Cell number was counted at 24, 48, and 72 h after transfection. B, inhibition of miR-215 promotes HCC cell growth. Cell number was counted at 24, 48, and 72 h after transfection. C, in HCC cells with lncRNA PCAT-1 not significantly down-regulated (PCAT-1 mutant + miR-215), overexpression of miR-215 could not inhibit HCC cell proliferation. D, colony formation assays. At the 10th day after transfection, the colony number in each well was counted. All results of the mean of triplicate assays with mean ± S.D. are presented. *, p < 0.05; **, p < 0.01.
miR-215 inhibits migration and invasion of HCC cells
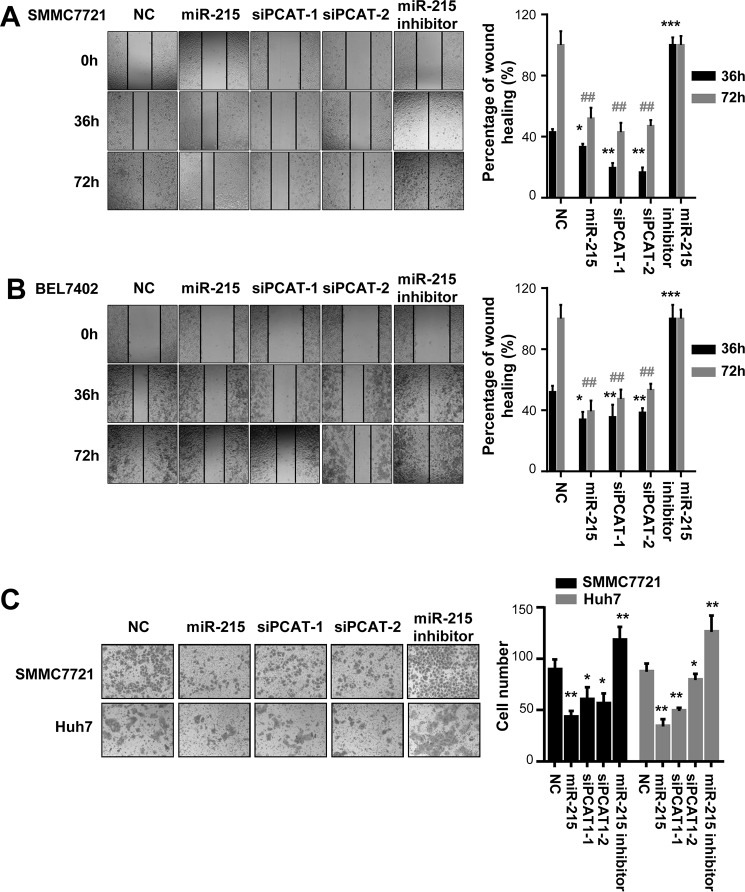
Because impacts of lncRNA PCAT-1 on HCC invasion and metastasis were still largely unclear, we examined how PCAT-1 siRNAs and miR-215 regulate migration and invasion of HCC cells. The wound-healing assays demonstrated that PCAT-1 siRNAs and miR-215 mimics impaired the motility of the HCC cells compared with control cells transfected with NC RNA (Fig. 4, A and B). On the contrary, miR-215 inhibitors (in-215) could significantly accelerate migration of HCC cells (Fig. 4, A and B).
Figure 4.
miR-215 reduces migration and invasion ability of HCC cells. Overexpression of miR-215 or two PCAT-1 siRNAs (siPCAT-1-1 and siPCAT-1-2) inhibits wound-healing in SMMC7721 and BEL7402 cells. However, suppression of miR-215 accelerates wound healing in SMMC7721 and BEL7402 cells. A and B, wound fields were observed directly after removal of inserts (0h) and cell migration was followed for 36 and 72 h in SMMC7721 cells (A) or for 36 and 72 h in BEL7402 cells (B). The wound-healing area in HCC cells is presented by a histogram. C, miR-215 inhibits the invasion abilities of SMMC7721 and Huh7 cells. Cells on the lower surface of the chamber were stained by crystal violet at 48 h after transfection. *, p < 0.05; **, p < 0.01; ##, p < 0.01.
Next, the impact of miR-215 on invasiveness of SMMC7721 and Huh7 cells was investigated using the Matrigel invasion assay system. Reduced invasion ability of HCC cells was observed after elevated expression of miR-215 (Fig. 4C). In line with this, PCAT-1 siRNAs can also inhibit the invasion of these HCC cells (Fig. 4C). We also confirmed this observation using inhibitors of miR-215 and found enhanced invasion ability of HCC cells transfected with its inhibitors (Fig. 4C).
miR-215-PCAT-1 axis modulates CRKL expression and CRKL-regulated cell growth
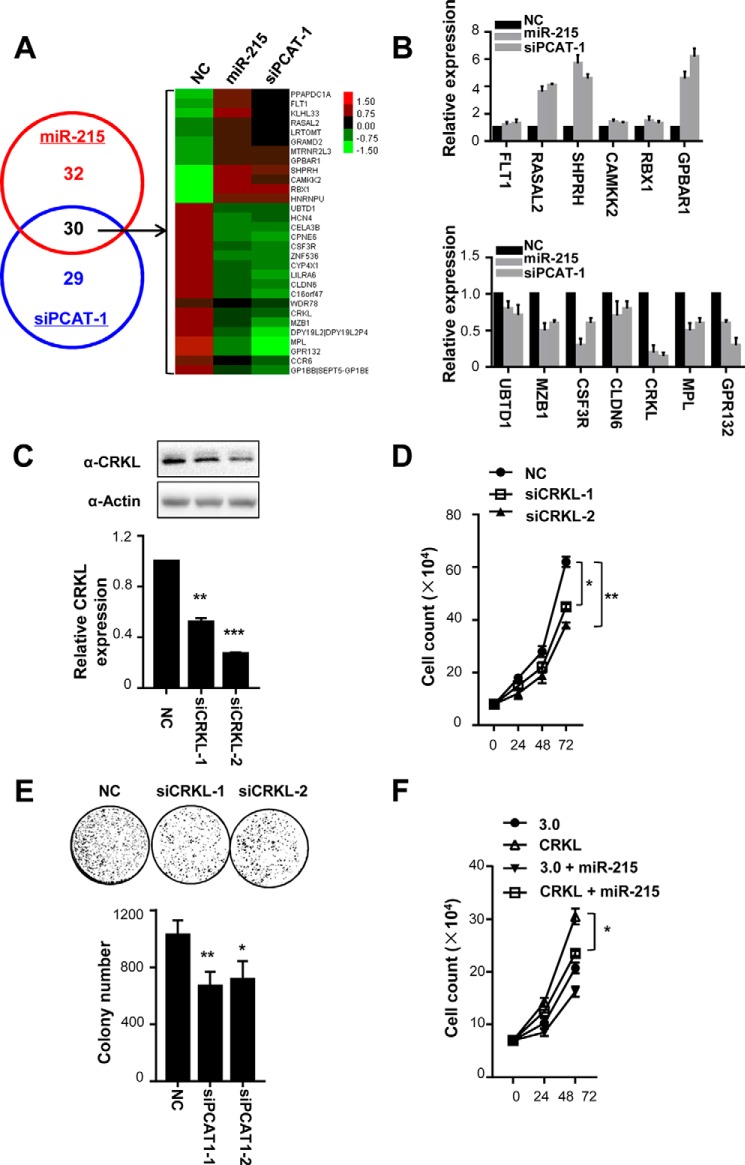
To further examine the downstream signaling pathway of the miR-215–PCAT-1 axis, we performed gene expression profiling of SMMC7721 cells transfected with NC RNA, miR-215 mimics, or siPCAT-1-1 (Fig. 5). miR-215 or siPCAT-1-1 caused 106 or 101 differentially expressed genes. Among them, a total of 30 genes were consistently de-regulated by any of these two small RNAs (Fig. 4A). Gene Ontology (GO) analyses indicate that most genes are ones coding protein-tyrosine kinases (GO:0004713).
Figure 5.
miR-215–PCAT-1 axis modulates CRKL expression and CRKL-regulated cell growth. A, dysegulated genes in SMMC7721 cells after delivery of miR-215 or siPCAT-1 using gene-profiling arrays (left). Cluster analysis of mRNAs expression profiles of the miR-215 group or the siPCAT-1 group versus the NC RNA group. Overexpression is coded in red, whereas decreased expression is indicated in green (right). B, candidate gene expression was validated using qRT-PCR. All data of gene expression were normalized to β-actin mRNA expression levels. C, protein expression of CRKL. D, inhibition of CRKL expression suppresses SMMC7721 cell proliferation. Left panel, different doses of NC RNA or CRKL siRNAs were transfected into SMMC7721 cells. Cell number was counted at 48 h after transfection. 30 nm NC RNA or CRKL siRNA was transfected into SMMC7721 cells. Cell number was counted at 24, 48, or 72 h after transfection. E, colony formation assays. 30 nm NC RNA or CRKL siRNA was transfected into SMMC7721 cells. After 10 days, colony number in each well was counted. F, overexpression of CRKL prevents anti-tumorigenic properties of miR-215 in HCC. *, p < 0.05; **, p < 0.01.
Thirteen genes identified by microarray profiling were then selectively validated using qRT-PCR (Fig. 5B). These genes are ones that have been reported to either be involved in cancer development or relatively high expression in SMMC7721 cells. Among these successfully validated downstream genes, we chose the oncogenic kinase CRKL as the candidate gene to examine (25–27). As shown in Fig. 5, C and D, and supplemental Fig. S2D, silencing CRKL expression could significantly suppress viability of HCC cells, in both dose- and time-dependent ways (all p < 0.05). Colony formation of HCC cells also support the oncogene nature of CRKL (Fig. 5E). In Fig. 5F, we found that overexpression of CRKL prevents the anti-tumorigenic properties of miR-215 in HCC.
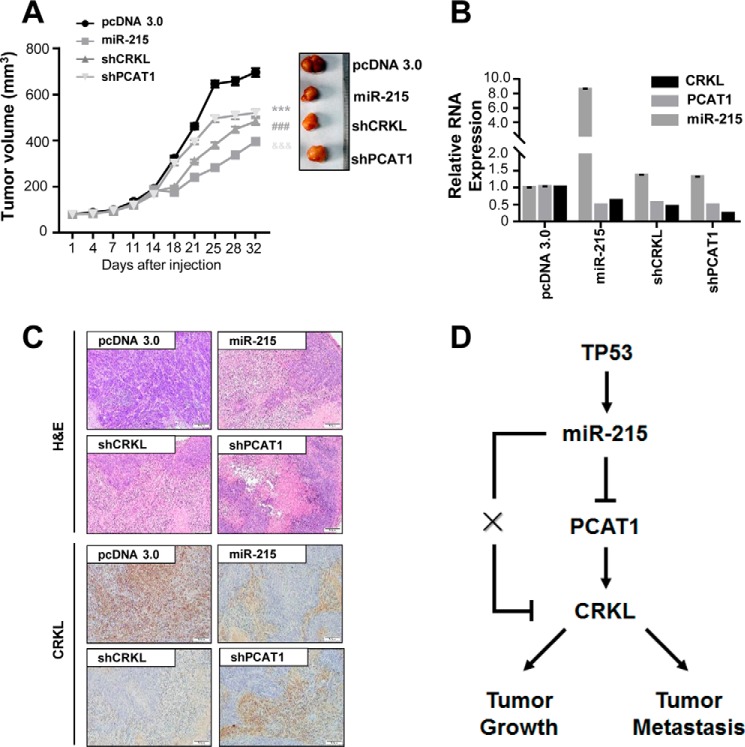
miR-215 inhibits HCC growth in vivo
We found that the growth of tumors from SMMC7721 xenografts with miR-215 up-regulation or knockdowns of PCAT-1 (shPCAT-1) and CRKL (shCRKL) was significantly inhibited compared with that of tumors formed from control xenografts (Fig. 6A). Expression of miR-215, lncRNA PCAT-1, and CRKL in xenografts was examined (Fig. 6B). The pathological detection with H&E staining further confirmed the HCC nature of SMMC7721 xenografts (Fig. 6C). Immunohistochemistry assay showed that the CRKL protein expression was obviously inhibited in tumor tissues with high miR-215 expression or low PCAT-1 expression (Fig. 6C). A working model summarizing relationships between TP53, miR-215, lncRNA PCAT-1, and CRKL is shown in Fig. 6D.
Figure 6.
miR-215 inhibits HCC growth in vivo. A, the growth of tumors from SMMC7721 xenografts with miR-215 up-regulation, silenced PCAT-1 (shPCAT-1) or silenced CRKL (shCRKL). B, expression of miR-215, lncRNA PCAT-1, and CRKL in xenografts was examined. C, H&E staining and CRKL immunohistochemical analyses of representative xenografts. D, a working model summarizing relationships between TP53, miR-215, lncRNA PCAT-1, and CRKL. **, p < 0.01.
Discussion
Cancer is a genetic disease that changes cellular information flow to modify cellular homeostasis and promote growth (6–10). Accumulated evidences demonstrated that lncRNAs and miRNAs play an important role during carcinogenesis (6–15). Although first identified in prostate cancer, lncRNA PCAT-1 has been shown to be involved in development of multiple cancers as an oncogene (19, 29–31). However, its fine-regulation at the post-transcriptional level and downstream signaling pathway in HCC were still unclear. To the best of our knowledge, we here for the first time revealed that miR-215 can suppress lncRNA PCAT-1 expression and HCC viability in vitro. Gene expression profiling data indicated that CRKL might be one of the key downstream effector genes in HCC. In line with this notion, in vivo xenografts results highlight that this miRNA-mediated epigenetic regulation might be therapeutically relevant for HCC.
It has been found that miR-215 function as tumor suppressors in multiple cancers including gastric cancer, colon cancer, breast cancer, lung cancer, renal cell carcinoma acute myeloid leukemia, and glioma (32–40). As one of the P53-inducable miRNAs (23, 24), miR-215 takes part in cancer development and progression through targeting YY-1, KDM1B, ZEB2, AKT1, XIAP, and RB1 (32–40). Interestingly, miR-215 is also a hypoxia-induced miRNA in glioma and vital for reprograming glioma-initiating cells to fit the hypoxic microenvironment via suppressing the expression of an epigenetic regulator KDM1B and modulating activities of multiple pathways. Glioma-initiating cells, which are critical for glioblastoma occurrence and recurrence, indicate the potential clinical value of miR-215 in glioma (34). Moreover, miR-215 is one of plasma miRNAs that can serve as prognostic markers for metastatic breast cancer and early detection markers of metastasis in breast cancer (41). Consistent with these observations, we revealed that miR-215 acts as a tumor suppressor via suppressing oncogene PCAT-1 expression in HCC.
CRKL encodes a protein kinase containing SH2 and SH3 domains, which has been shown to activate the RAS and JUN kinase signaling pathways. The kinase is vital in malignant transformation of multiple cancers, including HCC (25, 26, 28, 42, 43). Liu et al. applied in situ proximity ligation assay and determined that the novel interaction, CRKL–FLT1, has a high centrality ranking, and the expression of this interaction is strongly correlated with the migratory ability of HCC. They also found that knockdown of CRKL in HCC cells leads to a decrease in cell migration (28). Therefore, we speculate that the miR-215–PCAT-1 axis may interrupt CRKL and its downstream signaling pathway and, thus, suppress malignant phenomena of HCC cells. Interestingly, Tamura et al. (43) found that the TP53 target miR-200b/200c/429 miRNAs are negative regulators of the CRKL oncogene in cancer cells. Endogenous CRKL expression was decreased in cancer cells through the introduction of the TP53 family and endogenous TP53 activation, which is consistent to our results that TP53-response miR-215 can indirectly down-regulate CRKL expression via inhibiting lncRNA PCAT-1.
In all, we identified lncRNA PCAT-1 as a novel target gene of miR-215. This fine post-transcriptional regulation significantly impacts multiple malignant phenomena of HCC cells. The identification of CRKL as a potential downstream gene of the miR-215–PCAT-1 axis highlights the involvement of its downstream signaling pathways in HCC. Our findings highlight the interaction between miRNAs and lncRNA PCAT-1 during tumorigenesis and progression of HCC cells.
Experimental procedures
Cell culture and reagents
RPMI 1640 or DMEM with 10% fetal bovine serum (Hyclone) were used to culture SMMC7721 and Huh7 cells at 37 °C with 5% CO2. All miRNA mimics (miR-15a, miR-16, miR-22, miR-93a, miR-105, miR-106a, miR-129, miR-138, miR-181a, miR-192, miR-205, miR-215, miR-291a, miR-294, miR-295, miR-302, miR-372, miR-373, miR-383, and miR-428), miRNA inhibitors (miR-215), and small interfering RNA (siRNA) duplexes (siPCAT-1-1, siPCAT-1-2, siCRKL-1, and siCRKL-2) were products of Genepharma (Shanghai, China); siPCAT-1-1, 5′-GCAGAAACACCAAUGGAUATT-3′/5′-UAUCCAUUGGUGUUUCUGCTT-3′; siPCAT-1-2, 5′-AUACAUAAGACCAUGGAAATT-3′/5′-UUUCCAUGGUCUUAUGUAUTT-3′; siCRKL-1, 5′-UCUGCUCUACCAUGUUUAATT-3′/5′-UUAAACAUGGUAGAGCAGAGC-3′; siCRKL-2, 5′-UGAAAGUCACAAGGAUGAATT-3′/5′-UUCAUCCUUGUGACUUUCACG-3′. The negative control (NC) RNA duplex for miRNA mimics, miRNA inhibitors, or siRNAs (Genepharma) was nonhomologous to any human genome sequence. INTERFERin® (Polyplus) was used to transfect small RNAs. The TP53 CDS region was cloned into pcDNA3.1 vector as reported previously (44, 45) and transfected into HCC cells with Lipofectamine 2000 (Invitrogen).
Quantitative reverse transcription-PCR (qRT-PCR)
Each RNA sample was treated with RNase-free DNase to remove genomic DNA (Invitrogen) after its isolation from culture cells with TRIzol reagent (Invitrogen). These RNA samples were reverse transcribed into cDNAs using Revert Ace kit (TOYOBO, Osaka, Japan). Human U6 and miRNAs were examined with their specific stem-loop RT-PCR primers (Ribobio, Guangzhou, China). SYBR Green qRT-PCR was used to detect expression of PCAT-1 and other potential PCAT-1 downstream genes. The expression of individual genes was calculated relative to the β-actin expression (11–13).
PCAT-1 reporter and mutant constructs
The pGL3-Control plasmid containing the firefly luciferase gene as a reporter (Promega) was digested with XbaI (Promega) and treated with mung bean nuclease (Promega) to form blunt ends. The sequence corresponding to the wild-type PCAT-1 (100–610 nucleotides) was amplified with Huh7 cDNA using PyrobestTM DNA Polymerase (TaKaRa). The PCR primer pair used was: 5′-CATCTGTACCCTTACAATTTG-3′/5′-GCGCACCCTTTGACCCTTGG-3′. The PCR products were ligated into the appropriately digested pGL3-Control. The resultant plasmid, designated pGL3-PCAT-1, was sequenced to confirm the orientation and integrity. The PCAT-1 reporter gene plasmid with the mutant miR-215-binding site was constructed with QuikChange Site-directed Mutagenesis kit (Stratagene) and named as pGL3-Mut215.
To address if miR-215 fails to inhibit proliferation in a model in which PCAT-1 is not down-regulated, we first cloned full-length lncRNA PCAT1 in the pcDNA3.1 expression construct named as PCAT1 (wild-type). The PCAT-1 expression construct with the mutant miR-205-binding site was constructed with a QuikChange Site-directed Mutagenesis kit (Stratagene) and named as PCAT1 mutant.
Dual luciferase reporter assays
SMMC7721 and Huh7 cells were seeded in 24-well plates and transfected with 20 nmol/liter of small RNAs (miR-215 mimics or NC RNAs) plus 50 ng of reporter constructs (pGL3-Control, pGL3-PCAT-1, or pGL3-Mut215) using LipofectamineTM 2000 (Invitrogen) when grown to 50% confluence. To standardize transfection efficiency, pRL-SV40 (1 ng) containing Renilla reniformis luciferase (Promega) was co-transfected. Both firefly luciferase activity and Renilla luciferase activities were examined at 48 h after transfection using a luciferase assay system (Promega). Three independent transfections were done for each luciferase construct (each in triplicate). Fold-changes were calculated by defining the activity of the pGL3-Control vector as 1.
Cell proliferation
SMMC7721 and Huh7 cells were seeded in 12-well plates and transfected with 20 nmol/liter of miR-215 mimics, PCAT-1 siRNAs (siPCAT-1-1 and siPCAT-1-2), miR-215 inhibitors, CRKL siRNAs (siCRKL-1 and siCRKL-2), or NC RNA (Genepharma), respectively. After harvesting by trypsin digestion, cells were washed twice with cold PBS, dyed with trypan blue, and counted under microscopy at 24, 48, and 72 h after transfection.
Colony formation assays
A total of 1,500 SMMC7721 or Huh7 cells were seeded into a 6-well cell culture plate and transfected with 20 nmol/liter of miR-215 mimics, siPCAT-1-1, siPCAT-1-2, siCRKL-1, siCRKL-2, or NC RNA, respectively. Cells were washed twice with cold PBS and fixed with 3.7% formaldehyde 10 days later. Colony number in each well was counted after cells were dyed with crystal violet.
Wound healing and transwell assays
The wound healing and transwell assays were performed as reported previously (46). In details, the SMMC7721 or Huh7 cell layer was scratched when reaching about 90% confluence. HCC cells were then continued to be cultured in a 37 °C CO2 incubator. The average extent of wound closure was quantified. During transwell assays, the transwell chambers were coated with 100 ml of BD Matrigel overnight in a cell incubator. SMMC7721 or Huh7 cells transfected with miR-215 mimics, PCAT-1 siRNAs (siPCAT-1-1 and siPCAT-1-2), miR-215 inhibitors (in-215), or NC RNA were added to upper transwell chambers (pore 8 mm, Corning). A medium containing 10% FBS (650 ml) was added to the lower wells. After 48 h incubation, cells were fixed and stained, and the nonmigratory cells were scraped from the upper part of the filter. Cells migrated to the lower wells through pores were stained with 0.2% crystal violet solution and counted.
Gene expression profiling
Huh7 cells were transfected with 20 nmol/liter of NC RNA, miR-215 mimics, or siPCAT-1-1 and total RNA was extracted. Gene profiling of these samples was measured using OneArray Plus chips (Phalanx Biotech Group). Differentially expressed genes between the NC RNA group and the miR-215 group, or the NC RNA group and the siPCAT-1-1 group were identified separately. The differentially expressed genes were identified following the criteria log2 (fold-change) ≥0.585 and p < 0.05. The gene profiling data have been deposited at the National Center for Biotechnology Institute Gene Expression Omnibus (GEO) repository under accession number GSE92648.
HCC xenograft
We next evaluated the tumor suppressor role of miR-215 in vivo via HCC xenografts. The miR-215 mature sequence was first cloned after the CMV promoter into the pcDNA3.1 vector. The plasmid was named as miR-215 and transfected into SMMC7721 cells for G418 (Geneticin) selection. We isolated a stable cell clone with relative high expression of miR-215. We purchased 5-week-old female nude BALB/c mice from Vital River Laboratory (Beijing, China). A total of 1 × 108 SMMC7721 cells with stable transfection of miR-215 or the pcDNA3.1 vector (NC) were inoculated subcutaneously into the fossa axillaris of 8 nude mice (n = 4 per group). We measured tumor volumes every day after tumor volumes equaled to or were greater than 50 mm3. All procedures involving mice were approved by the institutional Review Board of Cancer Hospital affiliated to Shandong University.
Histologic and immunohistochemical analyses
The HCC xenograft tissue sections were stained with H&E for histologic analyses. The slides were viewed and photographed. Formalin-fixed, paraffin-embedded HCC xenograft tissue samples were used for immunohistochemical analysis. Antibody against CRKL (Abcam) was used to determine proliferation abilities of tumor cells. Stained slides were read independently by two pathologists.
Statistics
Student's t test was used to calculate the difference between two groups. Differences between three or more groups were analyzed via one-way analysis of variance. A p value of less than 0.05 was used as the criterion of statistical significance. All analyses were performed with the SPSS software package (Version 16.0, SPSS Inc.).
Author contributions
M. Y. and Y. R. contributed to conception and design. Y. R., J. S., and J. L. contributed to acquisition of data, or analysis and interpretation of data. M. Y., Y. R., W. L., Z. Z., and J. Y. drafted the article.
Supplementary Material
Note added in proof
There were several errors in the version of this article that was published as a Paper in Press on September 8, 2017. In Fig. 6C, the miR-215 and shPCAT1 panels for the CRKL immunohistochemical analysis was inadvertently duplicated. The actin immunoblot in Fig. 5C was inadvertently used in supplemental Fig. S2F. There was also some textual overlap with Ge et al. (11). These errors have now been corrected and do not affect the results or conclusions of this work.
This work was supported by National Natural Science Foundation of China Grant 31671300, the National High-Tech Research and Development Program of China Grant 2015AA020950, and Taishan Scholars Program of Shandong Province (tsqn20161060). The authors declare that they have no conflicts of interest with the contents of this article.
The microarray data have been deposited at the National Center for Biotechnology Institute Gene Expression Omnibus (GEO) repository under accession number GSE92648.
This article contains supplemental Table S1 and Figs. S1 and S2.
- HCC
- hepatocellular carcinoma
- lncRNA
- long noncoding RNAs
- qRT
- quantitative RT
- NC
- negative control
- miRNA
- microRNA
- in-215
- miR-215 inhibitor.
References
- 1. Laursen L. (2014) A preventable cancer. Nature 516, S2–S3 [DOI] [PubMed] [Google Scholar]
- 2. El-Serag H. B., and Rudolph K. L. (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132, 2557–2576 [DOI] [PubMed] [Google Scholar]
- 3. Maluccio M., and Covey A. (2012) Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 62, 394–399 [DOI] [PubMed] [Google Scholar]
- 4. Forner A., Llovet J. M., and Bruix J. (2012) Hepatocellular carcinoma. Lancet 379, 1245–1255 [DOI] [PubMed] [Google Scholar]
- 5. Wörns M. A., and Galle P. R. (2014) HCC therapies: lessons learned. Nat. Rev. Gastroenterol. Hepatol 11, 447–452 [DOI] [PubMed] [Google Scholar]
- 6. Evans J. R., Feng F. Y., and Chinnaiyan A. M. (2016) The bright side of dark matter: lncRNAs in cancer. J. Clin. Invest. 126, 2775–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmitt A. M., and Chang H. Y. (2016) Long noncoding RNAs in cancer pathways. Cancer Cell 29, 452–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goff L. A., and Rinn J. L. (2015) Linking RNA biology to lncRNAs. Genome Res. 25, 1456–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang X., Xie X., Xiao Y. F., Xie R., Hu C. J., Tang B., Li B. S., and Yang S. M. (2015) The emergence of long non-coding RNAs in the tumorigenesis of hepatocellular carcinoma. Cancer Lett. 360, 119–124 [DOI] [PubMed] [Google Scholar]
- 10. He Y., Meng X. M., Huang C., Wu B. M., Zhang L., Lv X. W., and Li J. (2014) Long noncoding RNAs: novel insights into hepatocelluar carcinoma. Cancer Lett. 344, 20–27 [DOI] [PubMed] [Google Scholar]
- 11. Ge Y., Yan X., Jin Y., Yang X., Yu X., Zhou L., Han S., Yuan Q., and Yang M. (2015) miRNA-192 [corrected] and miRNA-204 directly suppress lncRNA HOTTIP and interrupt GLS1-mediated glutaminolysis in hepatocellular carcinoma. PLoS Genet. 11, e1005726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang X., Ren Y., Yang X., Xiong X., Han S., Ge Y., Pan W., Zhou L., Yuan Q., and Yang M. (2015) miR-190a inhibits epithelial-mesenchymal transition of hepatoma cells via targeting the long non-coding RNA treRNA. FEBS Lett. 589, 4079–4087 [DOI] [PubMed] [Google Scholar]
- 13. Wang X., Li M., Wang Z., Han S., Tang X., Ge Y., Zhou L., Zhou C., Yuan Q., and Yang M. (2015) Silencing of long noncoding RNA MALAT1 by miR-101 and miR-217 inhibits proliferation, migration, and invasion of esophageal squamous cell carcinoma cells. J. Biol. Chem. 290, 3925–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang L., Shen H., Li X., Li Z., Liu Z., Xu J., Ma S., Zhao X., Bai X., Li M., Wang Q., and Ji J. (2016) miR-125a-5p decreases after long non-coding RNA HOTAIR knockdown to promote cancer cell apoptosis by releasing caspase 2. Cell Death Dis. 7, e2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirata H., Hinoda Y., Shahryari V., Deng G., Nakajima K., Tabatabai Z. L., Ishii N., and Dahiya R. (2015) Long noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and interacts with miR-205. Cancer Res. 75, 1322–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yuan S. X., Wang J., Yang F., Tao Q. F., Zhang J., Wang L. L., Yang Y., Liu H., Wang Z. G., Xu Q. G., Fan J., Liu L., Sun S. H., and Zhou W. P. (2016) Long noncoding RNA DANCR increases stemness features of hepatocellular carcinoma by derepression of CTNNB1. Hepatology 63, 499–511 [DOI] [PubMed] [Google Scholar]
- 17. Prensner J. R., Iyer M. K., Balbin O. A., Dhanasekaran S. M., Cao Q., Brenner J. C., Laxman B., Asangani I. A., Grasso C. S., Kominsky H. D., Cao X., Jing X., Wang X., Siddiqui J., Wei J. T., Robinson D., Iyer H. K., Palanisamy N., Maher C. A., and Chinnaiyan A. M. (2011) Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat. Biotechnol. 29, 742–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo H., Ahmed M., Zhang F., Yao C. Q., Li S., Liang Y., Hua J., Soares F., Sun Y., Langstein J., Li Y., Poon C., Bailey S. D., Desai K., Fei T., Li Q., et al. (2016) Modulation of long noncoding RNAs by risk SNPs underlying genetic predispositions to prostate cancer. Nat. Genet. 48, 1142–1150 [DOI] [PubMed] [Google Scholar]
- 19. Qin H. D., Liao X. Y., Chen Y. B., Huang S. Y., Xue W. Q., Li F. F., Ge X. S., Liu D. Q., Cai Q., Long J., Li X. Z., Hu Y. Z., Zhang S. D., Zhang L. J., et al. (2016) Genomic characterization of esophageal squamous cell carcinoma reveals critical genes underlying tumorigenesis and poor prognosis. Am. J. Hum. Genet. 98, 709–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wen J., Xu J., Sun Q., Xing C., and Yin W. (2016) Upregulation of long noncoding RNA PCAT-1 contributes to cell proliferation, migration and apoptosis in hepatocellular carcinoma. Mol. Med. Rep. 13, 4481–4486 [DOI] [PubMed] [Google Scholar]
- 21. Yan T. H., Yang H., Jiang J. H., Lu S. W., Peng C. X., Que H. X., Lu W. L., and Mao J. F. (2015) Prognostic significance of long non-coding RNA PCAT-1 expression in human hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 8, 4126–4131 [PMC free article] [PubMed] [Google Scholar]
- 22. Jeggari A., Marks D. S., and Larsson E. (2012) miRcode: a map of putative microRNA target sites in the long non-coding transcriptome. Bioinformatics 28, 2062–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Braun C. J., Zhang X., Savelyeva I., Wolff S., Moll U. M., Schepeler T., Ørntoft T. F., Andersen C. L., and Dobbelstein M. (2008) p53-Responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res. 68, 10094–10104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Georges S. A., Biery M. C., Kim S. Y., Schelter J. M., Guo J., Chang A. N., Jackson A. L., Carleton M. O., Linsley P. S., Cleary M. A., and Chau B. N. (2008) Coordinated regulation of cell cycle transcripts by p53-inducible microRNAs, miR-192 and miR-215. Cancer Res. 68, 10105–10112 [DOI] [PubMed] [Google Scholar]
- 25. Cheung H. W., Du J., Boehm J. S., He F., Weir B. A., Wang X., Butaney M., Sequist L. V., Luo B., Engelman J. A., Root D. E., Meyerson M., Golub T. R., Jänne P. A., and Hahn W. C. (2011) Amplification of CRKL induces transformation and epidermal growth factor receptor inhibitor resistance in human non-small cell lung cancers. Cancer Discov 1, 608–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yeung C. L., Ngo V. N., Grohar P. J., Arnaldez F. I., Asante A., Wan X., Khan J., Hewitt S. M., Khanna C., Staudt L. M., and Helman L. J. (2013) Loss-of-function screen in rhabdomyosarcoma identifies CRKL-YES as a critical signal for tumor growth. Oncogene 32, 5429–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim Y. H., Kwei K. A., Girard L., Salari K., Kao J., Pacyna-Gengelbach M., Wang P., Hernandez-Boussard T., Gazdar A. F., Petersen I., Minna J. D., and Pollack J. R. (2010) Genomic and functional analysis identifies CRKL as an oncogene amplified in lung cancer. Oncogene 29, 1421–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu C. H., Chen T. C., Chau G. Y., Jan Y. H., Chen C. H., Hsu C. N., Lin K. T., Juang Y. L., Lu P. J., Cheng H. C., Chen M. H., Chang C. F., Ting Y. S., Kao C. Y., Hsiao M., and Huang C. Y. (2013) Analysis of protein-protein interactions in cross-talk pathways reveals CRKL protein as a novel prognostic marker in hepatocellular carcinoma. Mol. Cell. Proteomics 12, 1335–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prensner J. R., Chen W., Iyer M. K., Cao Q., Ma T., Han S., Sahu A., Malik R., Wilder-Romans K., Navone N., Logothetis C. J., Araujo J. C., Pisters L. L., Tewari A. K., Canman C. E., et al. (2014) PCAT-1, a long noncoding RNA, regulates BRCA2 and controls homologous recombination in cancer. Cancer Res. 74, 1651–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi W. H., Wu Q. Q., Li S. Q., Yang T. X., Liu Z. H., Tong Y. S., Tuo L., Wang S., and Cao X. F. (2015) Upregulation of the long noncoding RNA PCAT-1 correlates with advanced clinical stage and poor prognosis in esophageal squamous carcinoma. Tumour Biol. 36, 2501–2507 [DOI] [PubMed] [Google Scholar]
- 31. Liu L., Liu Y., Zhuang C., Xu W., Fu X., Lv Z., Wu H., Mou L., Zhao G., Cai Z., and Huang W. (2015) Inducing cell growth arrest and apoptosis by silencing long non-coding RNA PCAT-1 in human bladder cancer. Tumour Biol. 36, 7685–7689 [DOI] [PubMed] [Google Scholar]
- 32. Chen Z., Han S., Huang W., Wu J., Liu Y., Cai S., He Y., Wu S., and Song W. (2016) MicroRNA-215 suppresses cell proliferation, migration and invasion of colon cancer by repressing Yin-Yang 1. Biochem. Biophys. Res. Commun. 479, 482–488 [DOI] [PubMed] [Google Scholar]
- 33. Wang Y. X., Zhang T. J., Yang D. Q., Yao D. M., Yang L., Zhou J. D., Deng Z. Q., Ma J. C., Guo H., Wen X. M., Lin J., and Qian J. (2016) Reduced miR-215 expression predicts poor prognosis in patients with acute myeloid leukemia. Jpn. J. Clin. Oncol 46, 350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hu J., Sun T., Wang H., Chen Z., Wang S., Yuan L., Liu T., Li H. R., Wang P., Feng Y., Wang Q., McLendon R. E., Friedman A. H., Keir S. T., Bigner D. D., Rathmell J., Fu X. D., Li Q. J., Wang H., and Wang X. F. (2016) miR-215 is induced post-transcriptionally via HIF-Drosha complex and mediates glioma-initiating cell adaptation to hypoxia by targeting KDM1B. Cancer Cell 29, 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ge G., Zhang W., Niu L., Yan Y., Ren Y., and Zou Y. (2016) miR-215 functions as a tumor suppressor in epithelial ovarian cancer through regulation of the X-chromosome-linked inhibitor of apoptosis. Oncol Rep. 35, 1816–1822 [DOI] [PubMed] [Google Scholar]
- 36. Yao J., Zhang P., Li J., and Xu W. (2017) MicroRNA-215 acts as a tumor suppressor in breast cancer by targeting AKT serine/threonine kinase 1. Oncol. Lett. 14, 1097–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ye M., Zhang J., Zhang J., Miao Q., Yao L., and Zhang J. (2015) Curcumin promotes apoptosis by activating the p53-miR-192–5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 357, 196–205 [DOI] [PubMed] [Google Scholar]
- 38. Deng Y., Huang Z., Xu Y., Jin J., Zhuo W., Zhang C., Zhang X., Shen M., Yan X., Wang L., Wang X., Kang Y., Si J., and Zhou T. (2014) MiR-215 modulates gastric cancer cell proliferation by targeting RB1. Cancer Lett. 342, 27–35 [DOI] [PubMed] [Google Scholar]
- 39. Khella H. W., Bakhet M., Allo G., Jewett M. A., Girgis A. H., Latif A., Girgis H., Von Both I., Bjarnason G. A., and Yousef G. M. (2013) miR-192, miR-194 and miR-215: a convergent microRNA network suppressing tumor progression in renal cell carcinoma. Carcinogenesis 34, 2231–2239 [DOI] [PubMed] [Google Scholar]
- 40. White N. M., Khella H. W., Grigull J., Adzovic S., Youssef Y. M., Honey R. J., Stewart R., Pace K. T., Bjarnason G. A., Jewett M. A., Evans A. J., Gabril M., and Yousef G. M. (2011) miRNA profiling in metastatic renal cell carcinoma reveals a tumour-suppressor effect for miR-215. Br. J. Cancer 105, 1741–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Madhavan D., Peng C., Wallwiener M., Zucknick M., Nees J., Schott S., Rudolph A., Riethdorf S., Trumpp A., Pantel K., Sohn C., Chang-Claude J., Schneeweiss A., and Burwinkel B. (2016) Circulating miRNAs with prognostic value in metastatic breast cancer and for early detection of metastasis. Carcinogenesis 37, 461–470 [DOI] [PubMed] [Google Scholar]
- 42. Seo J. H., Wood L. J., Agarwal A., O'Hare T., Elsea C. R., Griswold I. J., Deininger M. W., Imamoto A., and Druker B. J. (2010) A specific need for CRKL in p210BCR-ABL-induced transformation of mouse hematopoietic progenitors. Cancer Res. 70, 7325–7335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tamura M., Sasaki Y., Kobashi K., Takeda K., Nakagaki T., Idogawa M., and Tokino T. (2015) CRKL oncogene is downregulated by p53 through miR-200s. Cancer Sci. 106, 1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang M., Guo Y., Zhang X., Miao X., Tan W., Sun T., Zhao D., Yu D., Liu J., and Lin D. (2007) Interaction of P53 Arg72Pro and MDM2 T309G polymorphisms and their associations with risk of gastric cardia cancer. Carcinogenesis 28, 1996–2001 [DOI] [PubMed] [Google Scholar]
- 45. Ren Y., Li R. Q., Cai Y. R., Xia T., Yang M., and Xu F. J. (2016) Effective codelivery of lncRNA and pDNA by pullulan-based nanovectors for promising therapy of hepatocellular carcinoma. Adv. Funct. Materials 40, 7314–7325 [Google Scholar]
- 46. Yang X., Yu D., Ren Y., Wei J., Pan W., Zhou C., Zhou L., Liu Y., and Yang M. (2016) Integrative functional genomics implicates EPB41 dysregulation in hepatocellular carcinoma risk. Am. J. Hum. Genet. 99, 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.