Abstract
Drug candidates against matrix metalloproteinases (MMPs) failed in the clinic in the past because their strong zinc-targeting warheads led to a lack of specificity. More recently, significant selectivity among MMPs was achieved by blocking the enzymes' specificity pockets, nearby exosites, and downstream domains. Scannevin and colleagues now elegantly twist the plot and achieve ultimate selectivity: They target MMP-9 by allosterically preventing activation of its zymogen.
Introduction
The matrix metalloproteinases (MMPs)2 are a family of zinc-dependent metallopeptidases belonging to the metzincin clan (1, 2). They are multidomain enzymes containing a prodomain that is proteolytically cleaved in one or more steps to activate the enzyme, a catalytic domain containing the reactive zinc ion, and several other structural and functional domains (1). MMPs were initially discovered 55 years ago as active factors in frog metamorphosis, in which they act as general degraders of extracellular matrix components (3). For decades, the few orthologs known in humans were also those that nonspecifically degraded extracellular matrix proteins, a process associated with metastasis and angiogenesis in cancer. As a result, these enzymes were viewed as promising anticancer targets, inspiring efforts to develop MMP inhibitors (MMPIs). The initial generations of MMPIs were small-molecule peptidomimetics equipped with a warhead that very strongly targeted the catalytic zinc ion (e.g. batimastat, ilomastat, marimastat, and prinomastat; Fig. 1 A), but these displayed low specificity. Discouragingly, they failed in clinical trials due to lack of efficacy (4); to date, the only approved MMPI is Periostat (doxycycline) for the treatment of chronic periodontitis, which displays only modest efficacy (5).
Figure 1.
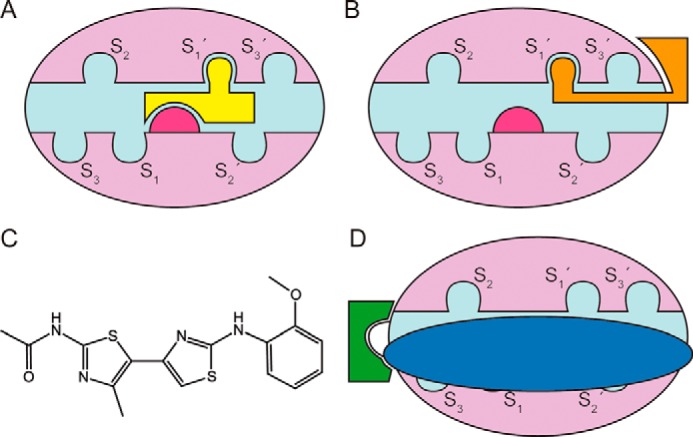
Mechanisms of MMP inhibitors. A, mode of action of initial MMPIs (yellow), which blocked the catalytic zinc ion (magenta) at the bottom of the active-site cleft (cyan). The cleft has subsites upstream (S1, S2, S3,…) and downstream (S1′, S2′, S3′,…) of the metal to accommodate the side chains of substrates. In MMPs, the primary specificity pocket is S1′. B, mode of action of current small-molecule MMPIs, which mostly bind to S1′ and/or exosites rather than the zinc. C, the chemical structure of compound JNJ0966. D, a novel approach by Scannevin et al. (9), in which the MMPI (green) targets the final activation point of the zymogen and prevents prodomain (blue) removal. This MMPI does not inhibit the mature MMP.
Over the decades, the known palette of MMP orthologs in humans reached 23, and their physiological functions expanded well beyond indiscriminate protein degradation to sophisticated (in)activation or shedding of a plethora of proteins, including growth factors, chemokines and other cytokines. It is now well established that MMPs contribute to various inflammatory, immune, infectious, and repair processes. As we've learned more about these vast functions, the basis for the failure of the initial zinc-targeting and therefore nonspecific MMPIs has become clear: General MMP inhibition leads to off-target effects and is thus not a viable strategy (6).
These clinical failures, combined with the increasing knowledge regarding the complexity of MMP biology, led to a period during which pharmaceutical companies discontinued their pipelines for the development of MMPIs. However, the field has gradually reemerged through the development of highly selective inhibitors for each of the biomedically relevant MMPs. This generation of small-molecule MMPIs does not possess strong zinc-binding groups; instead, they are designed to complement one of pockets within the active-site cleft, the specificity pocket, which diverges among MMPs. They also tackle secondary binding sites, exosites, and other domains downstream of the catalytic domain (Fig. 1B (1, 7)). A complementary approach targeting such sites is developing around antibody-based MMPIs, which may also be used for in vivo imaging (8). Cumulatively, these strategies have yielded potent, specific inhibitors of MMP-2, MMP-8, MMP-9, MMP-12, MMP-13, and MMP-14, but their clinical efficacy still remains to be proven (1, 7).
The work of Scannevin and colleagues (9) introduces a novel strategy: binding the zymogen. Like many peptidases, MMPs are kept in a latent state by an N-terminal prodomain spanning 66–91 residues, which sterically blocks the access of substrates to the active-site cleft. It operates following a mechanism dubbed “cysteine switch,” which features a conserved cysteine residue that binds the catalytic zinc (10). This prodomain is removed during maturation in vivo through sequential limited proteolysis by other peptidases and autolysis, which release the competent enzyme at its physiological site of action. As one role of MMPs in cancer is associated with excessive activity, blocking zymogen activation could provide a fairly targeted mechanism to disrupt cancer development. The conceptually simple but elegant working hypothesis, then, of Scannevin et al. (9), is that repressing activation of the zymogen could be an alternate approach to preventing activity. Using high-throughput screening employing the ThermoFluor approach, which reports on protein stability variations due to ligand binding, the authors identified compound JNJ0966 (Fig. 1C), which strongly bound to the zymogen of MMP-9 and prevented generation of active MMP-9. It did not inhibit the mature enzyme or any of mature MMP-1, MMP-2, MMP-3, and MMP-14 and did not prevent activation of the highly related zymogen of MMP-2. Further activity, gel and immunoblotting assays demonstrated that JNJ0966 treatment leads to accumulation of a partially processed protein consistent with initial cleavage after Glu-59 but not the final cleavage at Arg-106–Phe-107.
To identify the molecular determinants of this inhibition, the authors solved the crystal structure of the complex between JNJ0966 and a construct of proMMP-9 spanning the prodomain and the catalytic domain and compared it with the unbound protein. They found that JNJ0966 binds to a structural pocket close to the final activation cleavage point, thus disrupting the structure of segment Phe-107–Thr-109, which becomes disordered and can no longer be processed (Fig. 1D). In contrast, the active site—including the catalytic zinc and the prodomain segment blocking it—is not affected. The authors validated the importance of this site by testing the activation of several MMP-9 constructs with mutations in the binding pocket. In addition, activity of JNJ0966 against MMP-9 and its potential clinical utility were validated in a mouse model for human neuroinflammatory disorders such as multiple sclerosis.
Taken together, this work describes a hitherto unknown pharmacological approach to metallopeptidase inhibition and paves the way for the development of the next generation of drugs. By designing specific binders for each of the MMP zymogens, this strategy could be of general applicability and yield very specific drugs without off-target effects as they would not interact, in principle, with other MMPs or proteins. The approach could even be expanded to peptidases from different classes that are likewise regulated through zymogen-mediated latency and any other bioactive protein that is activated from a latent proprotein.
Acknowledgments
The Structural Biology Unit of IBMB is a “María de Maeztu” Unit of Excellence of the Spanish Ministry of Economy, Innovation and Competitiveness.
This work was supported in part by grants from Spanish and Catalan agencies (BFU2015-64487-R; MDM-2014-0435, and 2017SGR3). The author declares that he has no conflicts of interest with the contents of this article.
- MMP
- matrix metalloproteinase
- MMPI
- MMP inhibitor.
References
- 1. Tallant C., Marrero A., and Gomis-Rüth F. X. (2010) Matrix metalloproteinases: Fold and function of their catalytic domains. Biochim. Biophys. Acta 1803, 20–28 [DOI] [PubMed] [Google Scholar]
- 2. Overall C. M., and López-Otín C. (2002) Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat. Rev. Cancer 2, 657–672 [DOI] [PubMed] [Google Scholar]
- 3. Gross J., and Lapière C. M. (1962) Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc. Natl. Acad. Sci. U.S.A. 48, 1014–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zucker S., Cao J., and Chen W. T. (2000) Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene 19, 6642–6650 [DOI] [PubMed] [Google Scholar]
- 5. Caton J., and Ryan M. E. (2011) Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD). Pharmacol. Res. 63, 114–120 [DOI] [PubMed] [Google Scholar]
- 6. Dufour A., and Overall C. M. (2013) Missing the target: Matrix metalloproteinase antitargets in inflammation and cancer. Trends Pharmacol. Sci. 34, 233–242 [DOI] [PubMed] [Google Scholar]
- 7. Fields G. B. (2015) New strategies for targeting matrix metalloproteinases. Matrix Biol. 44–46, 239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sela-Passwell N., Kikkeri R., Dym O., Rozenberg H., Margalit R., Arad-Yellin R., Eisenstein M., Brenner O., Shoham T., Danon T., Shanzer A., and Sagi I. (2011) Antibodies targeting the catalytic zinc complex of activated matrix metalloproteinases show therapeutic potential. Nat. Med. 18, 143–147 [DOI] [PubMed] [Google Scholar]
- 9. Scannevin R. H., Alexander R., Haarlander T. M., Burke S. L., Singer M., Hou C., Zhang Y. M., Maguire D., Spurlino J., Deckman I., Carroll K. I., Lewandowski F., Devine E., Dzordzorme K., Tounge B., Milligan C., Bayoumy S., Williams R., Schalk-Hihi C., Leonard K., Jackson P., Todd M., Kuo L. C., and Rhodes K. J. (2017) Discovery of a highly selective chemical inhibitor of matrix metalloproteinase-9 (MMP-9) that allosterically inhibits zymogen activation. J. Biol. Chem. 292, 17963–17974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Wart H. E., and Birkedal-Hansen H. (1990) The cysteine switch : A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. U.S.A. 87, 5578–5582 [DOI] [PMC free article] [PubMed] [Google Scholar]


