Abstract
Transition-metal oxyhydrides are of considerable current interest due to the unique features of the hydride anion, most notably the absence of valence p orbitals. This feature distinguishes hydrides from all other anions, and gives rise to unprecedented properties in this new class of materials. Here we show via a high-pressure study of anion-ordered strontium vanadium oxyhydride SrVO2H that H− is extraordinarily compressible, and that pressure drives a transition from a Mott insulator to a metal at ~ 50 GPa. Density functional theory suggests that the band gap in the insulating state is reduced by pressure as a result of increased dispersion in the ab-plane due to enhanced Vdπ-Opπ-Vdπ overlap. Remarkably, dispersion along c is limited by the orthogonal Vdπ-H1s-Vdπ arrangement despite the greater c-axis compressibility, suggesting that the hydride anions act as π-blockers. The wider family of oxyhydrides may therefore give access to dimensionally reduced structures with novel electronic properties.
Incorporating hydride anions into transition metal oxides can dramatically affect their structural and electronic properties. Here the authors reveal a pressure-induced insulator-to-metal transition in SrVO2H and show that the compressibility of hydride anions without π-symmetry valence orbitals causes them to act as π-blockers.
Introduction
Transition-metal perovskite oxides, ABO3, have been the subject of intense study, primarily due to their flexible chemistry which allows a wide variety of different metal cations to be incorporated within the same architecture. This fact, combined with the strong inter-cation electronic and magnetic coupling transmitted via O 2p orbitals, makes the perovskite oxides ideal playgrounds to explore physical and chemical phenomena in transition-metal oxides1–5. The most common approach employed to tune the behavior and properties of perovskite oxides is chemical substitution, which typically involves cation substitutions on the A- or B-sites of the ABO3 framework6, 7. However, modification of the anion lattice, either through the introduction of anion vacancies8, 9, or by substituting non-oxide hetero-anions10–12, provides further opportunities to make dramatic changes to their physical behavior. For example, aliovalent anion doping allows the adjustment of metal oxidation states, the modification of the on-site electronic configuration of transition-metal centers and the tuning of inter-cation couplings, all of which can lead to the realization of novel electronic states.
Binary metal hydrides (e.g., NaH and CaH2) have proven particularly powerful reagents for modifying the anion lattice because they facilitate the de-intercalation of oxide anions. These low-temperature topochemical reactions13, 14 give access to metastable, oxygen-deficient phases that contain transition-metal cations in extremely low oxidation states (e.g., NiI, CoI, MnI, and RuII)15–18 and/or highly unusual local coordination geometries (e.g., the square-planar FeIIO4 units observed in the widely studied infinite-layer phase, SrFeO2)19. Reduction with metal hydrides can also bring about hydride-for-oxide anion exchange, yielding air-stable transition-metal oxyhydride phases, first observed during the conversion of LaSrCoO4 and Sr3Co2O7–x into LaSrCoO3H0.7 and Sr3Co2O4.33H0.84, respectively20, 21, and subsequently utilized to prepare ATiO3–xHx (A = Ba, Sr, Ca)22, 23 and Srn+1VnO2n+1Hn (n = 1, 2, ∞)24. Further transition-metal oxyhydride phases, such as SrCrO2H and LaSrMnO3.3H0.7 25–27, have been prepared via high-pressure synthesis routes.
The incorporation of hydride anions into extended oxide phases can modify the properties of host phases dramatically due to the strongly contrasting features of oxide and hydride anions, the most obvious of which is the charge. For example, hydride-for-oxide substitution leads to reduction of the metal and induces metallic conductivity in insulating AIITiO3 phases28, 29. In addition, the lower electronegativity of hydride compared to oxide means a higher degree of covalency in M–H bonds compared to M–O, leading, for example, to strong Co–H–Co coupling via the σ framework in LaSrCoO3H0.7, and hence to a high magnetic ordering temperature20, 30. The labile nature of H– in mixed oxide/hydride lattices also allows for facile anion-exchange reactions with nitride sources (NH3, for example), yielding mixed oxide-nitrides such as BaTiO3–xN2x/3 31, 32. In the context of the present paper, however, the most significant difference between the hydride and oxide anions (or indeed any other anion) is the absence of filled valence orbitals with π-symmetry. As a result, when hydride is substituted for oxide in an extended solid, exchange pathways of π-symmetry are effectively blocked, changing the orbital connectivity of a phase quite dramatically, particularly when the anions are in an ordered arrangement. Hydride-for-oxide anion substitution, along with a resultant anion order, therefore offers the intriguing prospect of preparing phases with electronic configurations and properties that contrast strongly with the parent oxide phases.
Reaction of cubic SrVO3 with CaH2 yields tetragonal SrVO2H, an oxide-hydride phase which adopts an anion-ordered, layered structure consisting of planes of vertex-linked VIIIO4 units separated by ordered SrH layers24. This hydride-for-oxide anion exchange converts the apex-linked VIVO6 units in SrVO3 into trans-VIIIO4H2 centers in SrVO2H, lifting the degeneracy of the vanadium 3d t 2g orbitals24. On-site electron–electron repulsion further splits the half-filled d xz/yz orbitals, leading to an insulating ground state that has been observed in photoemission and X-ray absorption spectroscopy measurements on thin film samples33. Neutron diffraction measurements reveal G-type antiferromagnetic order at relatively high temperature (T N > 300 K), indicating the presence of strong antiferromagnetic interactions. The strong super-exchange coupling via the oxide ligands (J 1) is unremarkable, and has been confirmed by recent computational studies from Wei et al. and Liu et al. (J 1 = 42.7 meV and 23.5 meV, respectively)34, 35. In the absence of π-symmetry orbitals on the H– bridging ligands, inter-layer coupling (J 2) might be expected to be very small. The computational work by both Wei and Liu noted above confirms that the inter-layer coupling is indeed substantially less than its intra-layer counterpart, although it is not negligible (J 2 = 5.7 meV and 1.9 meV, respectively). SrVO2H can therefore be viewed as a strongly correlated quasi-two-dimensional Mott insulator.
In this paper, we show how pressure can be used as a probe to investigate the structural and electronic properties of SrVO2H. In the structurally related perovskite phase, SrFeO2, an antiferromagnetically ordered Mott insulator at ambient pressure19, the application of external pressure enhances the interactions between the FeO2 layers, ultimately leading to a spin-state transition accompanied by antiferromagnetic-to-ferromagnetic and insulator-to-metal transitions36. Our motivation here is to establish how the presence of hydride ligands along the c-axis in SrVO2H influences the pressure dependence of the structural and electronic properties of the perovskite lattice.
Results
Pressure-dependent structural studies of SrVO2H
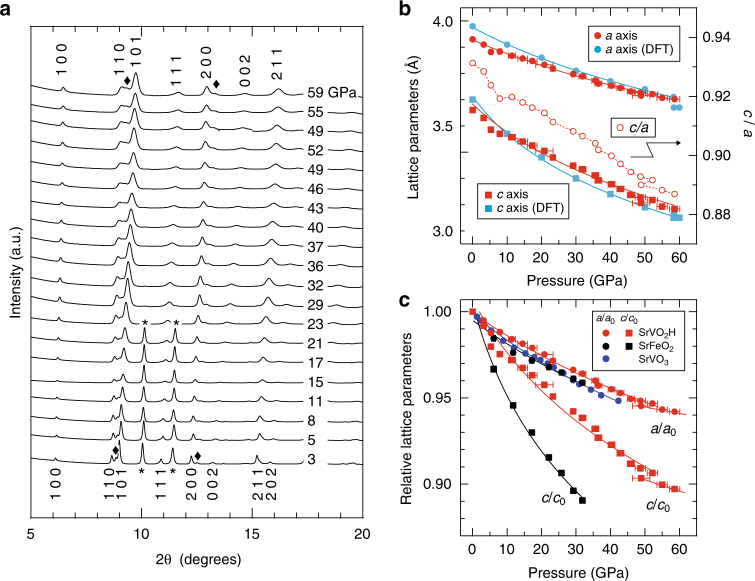
SrVO2H (Fig. 1b) was obtained from SrVO3 (Fig. 1c) by using a CaH2 reduction method. Powder synchrotron X-ray diffraction (XRD) data collected for SrVO2H (sample SrVO2H-A; Supplementary Note 1) at room temperature and a variety of applied pressures (Fig. 2a) can be readily indexed on the basis of a tetragonal unit cell, with no evidence of a symmetry-breaking structural transition up to the highest pressure measured (59 GPa). After releasing pressure, the pattern returned to the original ambient-pressure form with only slight peak broadening (Supplementary Fig. 2). The pressure dependence of the lattice parameters of SrVO2H is shown in Fig. 2b and 2c, where the analogous data collected from SrFeO2 36 and SrVO3 (Supplementary Fig. 3) are also shown for comparison. Linearized Birch-Murnaghan fits37 to these data allow the zero-pressure linear compressibility β of the a and c lattice parameters to be estimated (Supplementary Note 2). Fits to the data along a, which correspond to the compressibility of in-plane M–O bonds, yield similar β a values of 1.47(4) × 10−3 Pa–1 and 1.5(1) × 10−3 GPa–1, respectively, for SrVO2H and SrFeO2. These values are also similar to the isotropic compressibility of 1.72(4) × 10−3 GPa–1 for SrVO3, indicating an approximately equal M–O bond stiffness in all three phases. In contrast, the c-axis compressibility of β c = 3.7(2) × 10−3 GPa–1 for SrVO2H, while substantially lower than β c = 5.0(2) × 10−3 GPa–1 for SrFeO2 where no ligand is present along the Fe–Fe vector (Fig. 1a), is far larger than any of the values of β noted above, indicating that the V–H bonds in this phase are almost twice as compressible as the V–O bonds.
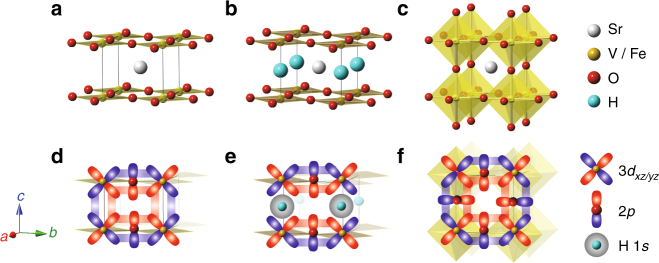
Fig. 1.
Crystal structures of perovskite related materials. a SrFeO2, b SrVO2H, and c SrVO3. White, yellow, red, and sky blue spheres, respectively, denote Sr, transition metal, O and H atoms. d–f represent the inter- and intra-layer orbital connectivity
Fig. 2.
High-pressure behavior of SrVO2H and SrFeO2. a Powder synchrotron XRD patterns of SrVO2H (sample SrVO2H-A) under various pressures at room temperature. All the patterns are indexed on the basis of a tetragonal unit cell. The filled diamond and asterisk symbols correspond to SrVO3 and rhenium from the gasket, respectively. b Pressure dependence of lattice parameters for the experimental (red) and the DFT-computed (sky blue) values of SrVO2H – note that some error bars are smaller than the width of the symbols. The decrease in pressure from 52 GPa to 49 GPa as the cell volume decreases suggests a phase transition to a denser phase. c Relative lattice parameters, a/a 0 and c/c 0, of SrVO2H (red), SrFeO2 (black), and SrVO3 (dark blue) as a function of pressure. Circles and squares correspond to the a and c axes, respectively. Solid lines in b and c represent linearized Birch-Murnaghan fits to the data
A pressure-induced insulator-to-metal transition in SrVO2H
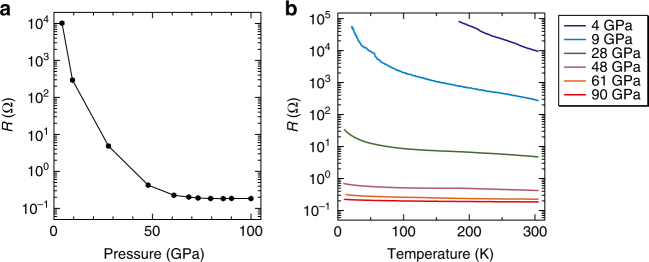
Figure 3 shows the resistivity of SrVO2H (sample SrVO2H-B; Supplementary Note 1) as a function of applied pressure. The observed resistivity of ~ 300 Ω∙cm at ambient pressure and 300 K is typical of a semiconductor and consistent with the Mott insulating picture proposed previously24. Application of up to 50 GPa pressure leads to a drop of four orders of magnitude in the resistivity of SrVO2H at 300 K, and a change in the temperature dependence, ΔR/ΔT, such that the resistance becomes essentially independent of temperature at this pressure (Supplementary Fig. 4), indicating an insulator-to-metal transition. This behavior is reproducible across different samples (Supplementary Fig. 5). We note that ΔR/ΔT does not become positive even at 90 GPa, as might be expected for a metallic phase. However, our measurements were performed on a non-sintered powder sample rather than a single crystal, so conduction across grain boundaries is likely to mask the metallic character. Similar features have been reported in previous studies36, 38. It should be noted that the absolute value of the resistivity is affected by the presence of impurities in the sample (such as SrVO3–x), but the essential features of the insulator-to-metal transition can be identified in all cases (Supplementary Fig. 6). The precise value of the critical pressure, P c, of the insulator-to-metal transition is not immediately obvious from the resistivity data, but a small but distinct anomaly is observed in the plot of lattice parameters vs. pressure just below 50 GPa (Fig. 2b, 2c). The discontinuity in the plot arises because at this point a reduction in the volume of the sample space causes a decrease in the measured pressure, an observation that is consistent with a phase transition to a denser state (details in Supplementary Note 3). Our analysis of the electronic structure to be discussed in the next section suggests that this denser state may indeed be metallic.
Fig. 3.
Electrical resistance for SrVO2H under pressure. a Pressure dependence of resistance R at 300 K (sample SrVO2H-B). b Temperature dependence of resistance R at various pressures
Ambient pressure electronic structure of SrVO2H
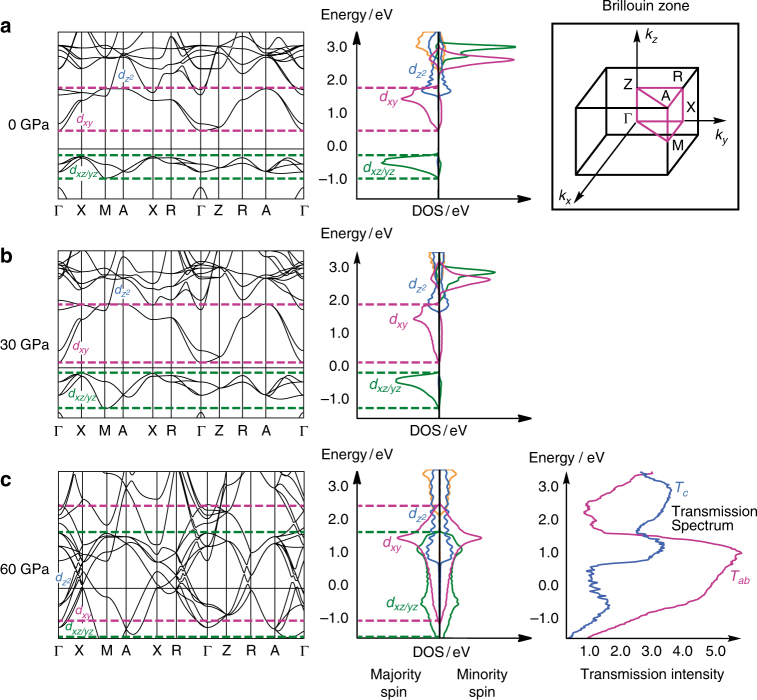
In order to explore the underlying origins of the physical measurements detailed above, we have turned to density functional theory. All calculations were performed at the PBE + U level, with a expansion of the unit cell as shown in Supplementary Fig. 8. We adopt a value of U eff = 2.0 eV for the Hubbard U parameter of vanadium, typical of similar calculations in the literature39–41. A full discussion of the influence of U eff on magnetic and structural properties, along with comparative data generated with the hybrid HSE06 functional, is presented in Supplementary Note 4, but our results are not strongly dependent on the chosen value, a point also noted by Wei et al. in their work34. The optimized cell parameters of the primitive unit cell at ambient pressure are a = b = 3.98 Å and c = 3.69 Å, in good agreement with the measured values of 3.93 Å and 3.67 Å. The spin densities on the vanadium centers confirm strong localization of the valence electrons, with ρ(V) = ± 1.73 being typical of a formally d 2 ion in an oxide lattice. The density of states (DOS) at ambient pressure and its projection onto selected valence orbitals is shown in Fig. 4a. The high spin moment on each metal center means that each of the metal 3d bands in the projected DOS (PDOS) is split into majority- and minority-spin components (shown to the left and right of the y-axes in Fig. 4, respectively). Of the vanadium-based 3d manifold, only the majority-spin 3d xz/yz orbitals lie below the Fermi level, E f, with their minority-spin counterparts being located ~ 3 eV above it. The strong splitting of the octahedral t 2g manifold arises because whilst the 3d xz and 3d yz orbitals are destabilized by only two in-plane V–O interactions, 3d xy is destabilized by four, and this anisotropy, combined with the finite U eff, leads to the emergence of a band gap and hence to the semiconducting properties of SrVO2H at ambient pressure. The band structure plot (Fig. 4a) highlights one further point that becomes significant in the context of the pressure-dependent properties: the dispersion of the bands just above and below E f (d xz/yz and d xy) is more prominent in the ab-plane (Γ→X→M, Z→R→A) than along the c-axis (M→A, X→R, Γ→Z). In direct contrast, the band is more strongly dispersed along c due to σ-type interaction via hydride ligands, but the bottom of this band lies ~ 1.6 eV above E f.
Fig. 4.
Density functional theory for SrVO2H. DOS and band structure, a at ambient pressure, b at 30 GPa, and c at 60 GPa. The transmission spectrum along the c-axis (T c, blue line) and along the V–O–V bisector (T ab, purple) is also shown in plot c for the high-pressure state. Majority- and minority-spin states in the DOS are shown to the left and right of the axis, respectively. The first Brillouin zone for the tetragonal lattice is shown in the inset
Pressure dependence of the electronic structure
The variation of optimized lattice parameters as a function of applied external pressure is displayed alongside the experimental data in Fig. 2b (sky blue lines). Whilst the computed lattice parameters are marginally more sensitive to pressure than those measured experimentally, the agreement between experiment and theory is striking, both in terms of the absolute values and in the differentiation between a and c, where the compressibility along the latter is approximately double that along the former. The corresponding PDOS plots and band structure diagrams for selected pressures are summarized in Fig. 4b (30 GPa) and Fig. 4c (60 GPa). As the pressure increases to 30 GPa, the most conspicuous change in the DOS is that the vacant majority-spin d xy band broadens substantially (the dashed purple lines in Fig. 4 mark the upper and lower limits of this band), and the corresponding band structure confirms that this arises primarily due to increased dispersion in the ab-plane (Γ→X→M, Z→R→A). The occupied d xz/yz band also broadens, albeit to a lesser extent, and again this can be traced to greater dispersion in the ab-plane rather than along c (compare changes in dispersion along X→M vs. Γ→Z). At 60 GPa, the broadening is sufficient to induce overlap between the occupied d xz/yz and unoccupied d xy bands, resulting in the complete loss of spin density and the emergence of a metallic state with an associated discontinuity in the optimized lattice parameters, consistent with the features observed in the crystallographic and resistivity data around 50 GPa. Even at 60 GPa, it is clear that it is the dispersion in the ab-plane, and not along c, which is the driving force for the transition to a metallic state: a clear band gap is maintained along Γ→Z and Μ→A. To reinforce this point, we have mimicked the effects of an anisotropic pressure source by varying either a or c while keeping the other constant (see Supplementary Fig. 11). If we maintain a at its ambient pressure value of 3.93 Å but reduce c to its 60 GPa-optimized value of 3.29 Å, we note that the dispersion of the d xz/yz band increases, but critically not to the extent that the band gap closes: the system remains an insulator. Conversely if we maintain c at its ambient-pressure value of 3.69 Å but contract a to its 60 GPa value of 3.67 Å, the band gap closes and a metallic state does emerge. From this, we conclude that it is the contraction in the ab-plane and not along c that drives the formation of the metallic state, despite the fact that the magnitude of the contraction in ab is smaller in absolute terms. To reinforce this point, we have explicitly computed the energy-dependent transmission spectrum for current flow both along the c-axis and in the ab-plane for the 60 GPa structure (Fig. 4c, right). The major peak in T ab is coincident with the broad d xy band that spans the Fermi level, while transmission along c (T c) becomes significant only above ~ 0.8 eV, coincident with the bottom of the band. As a result, transmission at the Fermi level is approximately five times stronger in the ab-plane than along c, indicating that the metallic behavior is strongly two-dimensional. In summary, the emergence of metallic behavior appears not to be connected directly to the very substantial compression along c, which simply reflects the intrinsically higher compressibility of the hydride ion vs. oxide, rather than any increased delocalization of itinerant electrons along this axis. The high-pressure metallic state can instead be considered as a quasi-two-dimensional metal with a conduction band made up mainly of the overlapping d xz/yz and d xy bands with substantial dispersion only in the ab-plane. Finally we note that the precise window in which the insulator-to-metal transition is predicted to take place is strongly dependent on chosen methodology: a higher value of U eff pushes the transition to higher pressures, as does the hybrid HSE06 functional (see Supplementary Note 4 for a full discussion). We do not, therefore, claim to be able to pinpoint the critical pressure exactly, but rather note that the pressure-induced dispersion of the d xy band is the driving force in all cases. Thus unlike the absolute value of the critical pressure, our conclusions regarding the dimensionality of the metallic behavior are not strongly dependent on the chosen methodology.
Discussion
At first sight the pressure-induced insulator-metal transition in SrVO2H appears remarkably similar to that in SrFeO2 previously reported by some of us36 – both phases are antiferromagnetic insulators at ambient pressure, both undergo strongly anisotropic lattice contractions on the application of pressure (Δc > Δa) and both eventually become metallic, at 34 GPa and 50 GPa, respectively. A closer inspection however reveals profound differences between the two systems. In the case of SrFeO2, the transition from insulating to metallic behavior is intimately connected to a change in spin state from S = 2 at ambient pressure to S = 1 above the critical pressure. The ambient-pressure electronic structure has been discussed at some length, and it is the orbital of Fe that is doubly occupied 42, 43. The band gap is primarily a consequence of the large spin polarization that splits the majority- and minority-spin manifolds. Above P c (34 GPa) however, the relative destabilization of the Fe–O orbital as the Fe–O bond lengths are compressed leads to a local configuration (S = 1)44. At even higher pressures, the compression along c leads to repulsions between the orbitals in adjacent layers, causing a partial transfer of electron density out of the band into d xy, and therefore to a substantial increase in the DOS at E f. The result is a metallic state where the electron density distribution is approximately isotropic36. In contrast, our calculations on the SrVO2H system suggest that the greater compressibility along the c-axis is not directly related to the emergence of metallic behavior: even at the high-pressure limit the inter-layer V–V separation is too large to afford significant direct Vdπ–Vdπ overlap. The very different behavior of SrFeO2 and SrVO2H arises simply because the electronic configuration is very different (d 6 vs. d 2) and the band gap in the latter is determined by the different number of Vdπ–O2pπ–Vdπ interactions experienced by the 3d xy and 3d xz/yz orbitals (four and two, respectively). The hydride ions in SrVO2H can therefore be viewed as π-orbital blockers in the sense that they have no π-symmetry valence orbitals to interact with the vanadium π-symmetry d-orbitals, 3d xz/yz. The metallic behavior then emerges because the compression in the ab-plane, although smaller in magnitude than that along the c-axis, increases the Vdπ–O2pπ–Vdπ interactions and hence the dispersion of the 3d xz/yz and the 3d xy bands, to the extent that they overlap and form a single continuous band. Schematic orbital connectivities for SrFeO2, SrVO2H and SrVO3 are shown in Fig. 2d–f. In short, when SrVO3 is converted to SrVO2H, the 3-dimensional Vdπ–O2pπ–Vdπ network is reduced to two dimensions as a result of the π-blocking SrH layer, with dramatic consequences for the physical properties simply because the band gap falls in the middle of the Vdπ band.
Insulator-to-metal transitions in strongly correlated systems have been a central subject in condensed matter physics for many decades, and the transitions of a large number of oxides with different d-electron counts have been studied in some detail45. The physics of low d-electron count systems (d 1 or d 2 systems such as VO2, V2O3, Ti2O3, and REVO3 where RE = rare earth, for example) is, however, complicated because the splitting of the Mdπ manifold is very sensitive to the subtle details of the distortions and tilting of the MO6 octahedra. In contrast, the highly anisotropic coordination environment of vanadium in SrVO2H simplifies the problem by causing a substantial zeroth-order splitting of this manifold, and the unusual pressure-dependent electronic properties of SrVO2H highlight the importance of the π-blocking nature of the hydride anion. We note that the use of π-blockers differs markedly from conventional strategies for reducing dimensionality, where bulky blocking layers are typically used to separate (super)conducting layers. These observations provide a strong motivation for the synthesis of new transition-metal oxyhydride phases with novel and elaborate anion-ordering schemes. Some closely related phases such as Sr2VO3H and Sr3V2O5H2 (Supplementary Fig. 14) are already known24, 27, and similar pressure-induced insulator-to-metal transitions might be anticipated in these S = 1 chains and ladders. Wider studies of the oxyhydride family will undoubtedly increase our ability to control π-orbital connectivity, in reduced phases and hence engineer materials with novel properties.
Methods
Sample preparation
Powder samples of SrVO3 were obtained by hydrogen reduction from Sr2V2O7, as described previously46. Two powder samples of SrVO2H (referred to as SrVO2H-A and SrVO2H-B) were synthesized by a topochemical reaction between SrVO3 and CaH2, as reported previously24. The principal difference between the two reduced samples is that SrVO2H-A contains small amounts of SrVO3–x which were observed to adversely affect the resistance measurements. A detailed discussion of samples SrVO2H-A and SrVO2H-B is presented in Supplementary Note 1.
High-pressure X-ray diffraction
Powder synchrotron XRD experiments for SrVO2H-A under high pressures were performed at room temperature using the NE1A synchrotron beam line of the Photon Factory–Advanced Ring for Pulse X-rays (PF–AR) at the High Energy Accelerator Research Organization (KEK). Powder samples were loaded into a 100 μm hole of pre-indented rhenium gasket of the diamond anvil cell. Daphne oil 7373 was used as a pressure transmitting medium. The volume of the sample space was varied using a screw. At each volume, the fluorescence shift of ruby chips was used to measure the pressure. To estimate the pressure distribution along the sample, several ruby chips were placed inside the hole at different distances from its center. The pressure gradient increased with pressure, but did not exceed ±3 GPa at 59 GPa. The incident X-ray beam was monochromatized to a wavelength of 0.4173 Å. The beam was collimated to a diameter of about 50 μm at 0–21 GPa and 30 μm at 23−59 GPa. Due to the relatively large size of beam, peaks from the rhenium gasket were obvious in the pressure range 0–21 GPa. High-pressure experiments on SrVO3 were conducted in the same manner, with a wavelength of 0.4186 Å and a beam collimated to 50 μm in diameter.
High-pressure resistivity
Four-probe ac resistance measurements were carried out using Pt electrodes. Three measurements were done on sample SrVO2H-A up to 50 GPa and on sample SrVO2H-B up to 100 GPa, all in the temperature range 8 K < T < 300 K. NaCl was used as a pressure transmitting medium. The sample-gasket cavity was coated with an insulating anhydrous gypsum (CaSO4) with epoxy. The initial sectional area and the distance between probes were about 40 × 40 μm2 and 30 μm, respectively. The applied pressure was calibrated with fluorescence manometer on ruby chips placed around the sample.
Density functional theory
The geometry optimizations described here were performed using the VASP software package (VASP 5.3)47, with the PBE density functional. In order to investigate different magnetic configurations it proved necessary to consider a expansion of the primitive unit cell (Supplementary Fig. 8). A plane-wave cutoff of 600 eV was used and the Brillouin zone was sampled on an 8 × 8 × 7 Γ-centered grid. The effect of strong correlations is typically introduced through the effective Hubbard U value (U eff). A survey of the literature indicates that U eff values up to 5.0 eV have been adopted for vanadium39–41. In the present study, we adopt a value of U eff = 2.0 eV, although the dependence of the optimized lattice parameters on U eff is weak (Supplementary Table 2). The potential pressure dependence of U eff in SrFeO2 has been discussed, and Rahman et al. have suggested that its value should decrease approximately linearly with pressure from its ambient-pressure value44. Whilst systematic reduction of our chosen value of 2.0 eV to 0.0 eV in the present case reduces the absolute value of the critical pressure of insulator-to-metal transition, it does not influence the underlying physics in any substantial way. We have also considered the hybrid HSE06 functional48 with 25% Hartree-Fock exchange and a range constant, ω, of 0.2. These calculations were done as single points using the geometries optimized at the PBE + U (U eff = 2.0 eV) level. An insulating state is retained with HSE06 even at a structure optimized under 90 GPa of pressure, but the closing of the band gap due to broadening of the d xy conduction band is clearly discernable. Transport analysis was performed using the ATK software package (ATK 2016.4)49, 50 with the same PBE density functional and U eff = 2.0 eV used in the VASP calculations. A localized double-ζ basis set, extended with single polarization function (DZP) was used to describe all atoms. The Brillouin zone was sampled on a 21 × 21 × 21 Monkhorst-Pack grid.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Acknowledgements
The work was supported by CREST and JSPS KAKENHI (JP16H06438, JP16H6439, JP16H6440, JP16K21724, and JP16H06033). High-pressure synchrotron radiation experiments were performed under the approval of the Photon Factory Program Advisory Committee (No. 2015G012). We thank Taku Okada and Takumi Kikegawa for their support of the high-pressure XRD measurement. MAP thanks CONACYT and the Balliol College, Oxford for a scholarship. J.E.M. and V.A. acknowledge financial support from the EPSRC (EP/K021435/1).
Author contributions
T.Y., D.Z., J.E.M., H.K., and M.A.H. designed the research. M.A.P. and N.I. synthesized the material. T.Y. collected high-pressure XRD data and analyzed. T.K. and K.Y. measured the high-pressure resistivity. D.Z. and V.A. performed the electronic structure analysis. All the authors discussed the results. T.Y., D.Z., H.K., J.E.M., and M.A.H. wrote the manuscript, with comments from all the authors.
Competing interests
The authors declare no competing financial interests.
Footnotes
Takafumi Yamamoto and Dihao Zeng contributed equally to this work.
Electronic supplementary material
Supplementary Information accompanies this paper at doi:10.1038/s41467-017-01301-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
John E. McGrady, Email: john.mcgrady@chem.ox.ac.uk
Hiroshi Kageyama, Email: kage@scl.kyoto-u.ac.jp.
Michael A. Hayward, Email: michael.hayward@chem.ox.ac.uk
References
- 1.Schooley JF, Hosler WR, Cohen ML. Superconductivity in semiconducting SrTiO3. Phys. Rev. Lett. 1964;12:474–475. doi: 10.1103/PhysRevLett.12.474. [DOI] [Google Scholar]
- 2.Moritomo Y, Asamitsu A, Kuwahara H, Tokura Y. Giant magnetoresistance of manganese oxides with a layered perovskite structure. Nature. 1996;380:141–144. doi: 10.1038/380141a0. [DOI] [Google Scholar]
- 3.Kobayashi KI, Kimura T, Sawada H, Terakura K, Tokura Y. Room-temperature magnetoresistance in an oxide material with an ordered double-perovskite structure. Nature. 1998;395:677–680. doi: 10.1038/27167. [DOI] [Google Scholar]
- 4.Azuma M, et al. Colossal negative thermal expansion in BiNiO3 induced by intermetallic charge transfer. Nat. Commun. 2011;2:347. doi: 10.1038/ncomms1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boris AV, et al. Dimensionality control of electronic phase transitions in nickel-oxide superlattices. Science. 2011;332:937–940. doi: 10.1126/science.1202647. [DOI] [PubMed] [Google Scholar]
- 6.Sleight AW, Gillson JL, Bierstedt PE. High-temperature superconductivity in BaPb1–xBixO3 system. Solid State Commun. 1975;17:27–28. doi: 10.1016/0038-1098(75)90327-0. [DOI] [Google Scholar]
- 7.Alonso JA, Martínez-Lope MJ, Casais MT, Aranda MAG, Fernández-Díaz MT. Metal-insulator transitions, structural and microstructural evolution of RNiO3 (R = Sm, Eu, Gd, Dy, Ho, Y) Perovskites: evidence for room-temperature charge disproportionation in monoclinic HoNiO3 and YNiO3. J. Am. Chem. Soc. 1999;121:4754–4762. doi: 10.1021/ja984015x. [DOI] [Google Scholar]
- 8.Hiroi Z, Takano M. Absence of superconductivity in the doped antiferromagnetic spin-ladder compound (La,Sr)CuO2.5. Nature. 1995;377:41–43. doi: 10.1038/377041a0. [DOI] [Google Scholar]
- 9.Le Toquin R, Paulus W, Cousson A, Prestipino C, Lamberti C. Time-resolved in situ studies of oxygen intercalation into SrCoO2.5, performed by neutron diffraction and X-ray absorption spectroscopy. J. Am. Chem. Soc. 2006;128:13161–13174. doi: 10.1021/ja063207m. [DOI] [PubMed] [Google Scholar]
- 10.Jansen M, Letschert H. Inorganic yellow-red pigments without toxic metals. Nature. 2000;404:980–982. doi: 10.1038/35010082. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi Y, Tian M, Eguchi M, Mallouk TE. Ion-exchangeable, electronically conducting layered perovskite oxyfluorides. J. Am. Chem. Soc. 2009;131:9849–9855. doi: 10.1021/ja9040829. [DOI] [PubMed] [Google Scholar]
- 12.Tsujimoto Y, Sathish C, Matsushita Y, Yamaura K, Uchikoshi T. New members of layered oxychloride perovskites with square planar coordination: Sr2MO2Cl2 (M = Mn, Ni) and Ba2PdO2Cl2. Chem. Commun. 2014;50:5915–5918. doi: 10.1039/C4CC01422G. [DOI] [PubMed] [Google Scholar]
- 13.Hayward MA, Green MA, Rosseinsky MJ, Sloan J. Sodium hydride as a powerful reducing agent for topotactic oxide deintercalation: synthesis and characterization of the nickel(I) oxide LaNiO2. J. Am. Chem. Soc. 1999;121:8843–8854. doi: 10.1021/ja991573i. [DOI] [Google Scholar]
- 14.Yamamoto T, Kageyama H. Hydride reductions of transition metal oxides. Chem. Lett. 2013;42:946–953. doi: 10.1246/cl.130581. [DOI] [Google Scholar]
- 15.Hayward M, Rosseinsky M. Synthesis of the infinite layer Ni(I) phase NdNiO2+x by low temperature reduction of NdNiO3 with sodium hydride. Solid State Sci. 2003;5:839–850. doi: 10.1016/S1293-2558(03)00111-0. [DOI] [Google Scholar]
- 16.Seddon J, Suard E, Hayward M. The topotactic reduction of YBaCo2O5 and LaBaCo2O5 – square-planar Co(I) in an extended oxide. J. Am. Chem. Soc. 2010;132:2802–2810. doi: 10.1021/ja910103d. [DOI] [PubMed] [Google Scholar]
- 17.Dixon E, Hadermann J, Ramos S, Goodwin AL, Hayward MA. Mn(I) in an extended oxide - the synthesis and characterization of La1–xCaxMnO2+d (0.6 < x < 1) J. Am. Chem. Soc. 2011;133:18397–18405. doi: 10.1021/ja207616c. [DOI] [PubMed] [Google Scholar]
- 18.Denis Romero F, et al. SrFe0.5Ru0.5O2: square-planar Ru2+ in an extended oxide. J. Am. Chem. Soc. 2013;135:1838–1844. doi: 10.1021/ja309798e. [DOI] [PubMed] [Google Scholar]
- 19.Tsujimoto Y, et al. Infinite-layer iron oxide with a square-planar coordination. Nature. 2007;450:1062–1065. doi: 10.1038/nature06382. [DOI] [PubMed] [Google Scholar]
- 20.Hayward MA, et al. The hydride anion in an extended transition metal oxide array–LaSrCoO3H0.7. Science. 2002;295:1882–1884. doi: 10.1126/science.1068321. [DOI] [PubMed] [Google Scholar]
- 21.Helps R, Rees N, Hayward MA. Sr3Co2O4.33H0.84 – an extended transition metal oxide-hydride. Inorg. Chem. 2010;49:11062–11068. doi: 10.1021/ic101613b. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi Y, et al. An oxyhydride of BaTiO3 exhibiting hydride exchange and electronic conductivity. Nat. Mater. 2012;11:507–511. doi: 10.1038/nmat3302. [DOI] [PubMed] [Google Scholar]
- 23.Sakaguchi T, et al. Oxyhydrides of (Ca,Sr,Ba)TiO3 perovskite solid solutions. Inorg. Chem. 2012;51:11371–11376. doi: 10.1021/ic300859n. [DOI] [PubMed] [Google Scholar]
- 24.Denis Romero F, et al. Strontium vanadium oxide-hydrides: ‘square-planar’ two-electron phases. Angew. Chem. Int. Ed. 2014;53:7556–7559. doi: 10.1002/anie.201403536. [DOI] [PubMed] [Google Scholar]
- 25.Tassel C, et al. Direct synthesis of chromium perovskite oxyhydride with a high magnetic transition temperature. Angew. Chem. Int. Ed. 2014;53:10377–10380. doi: 10.1002/anie.201405453. [DOI] [PubMed] [Google Scholar]
- 26.Tassel C, et al. High-pressure synthesis of manganese oxyhydride with partial anion order. Angew. Chem. Int. Ed. 2016;55:9667–9670. doi: 10.1002/anie.201605123. [DOI] [PubMed] [Google Scholar]
- 27.Bang J, et al. Hydrogen ordering and new polymorph of layered perovskite oxyhydrides: Sr2VO4–xHx. J. Am. Chem. Soc. 2014;136:7221–7224. doi: 10.1021/ja502277r. [DOI] [PubMed] [Google Scholar]
- 28.Yajima T, et al. Epitaxial thin films of ATiO3–xHx (A = Ba, Sr, Ca) with metallic conductivity. J. Am. Chem. Soc. 2012;134:8782–8785. doi: 10.1021/ja302465c. [DOI] [PubMed] [Google Scholar]
- 29.Bouilly G, et al. Electrical properties of epitaxial thin films of oxyhydrides ATiO3–xHx (A = Ba and Sr) Chem. Mater. 2015;27:6354–6359. doi: 10.1021/acs.chemmater.5b02374. [DOI] [Google Scholar]
- 30.Bridges CA, Darling GR, Hayward MA, Rosseinsky MJ. Electronic structure, magnetic ordering, and formation pathway of the transition metal oxide hydride LaSrCoO3H0.7. J. Am. Chem. Soc. 2005;127:5996–6011. doi: 10.1021/ja042683e. [DOI] [PubMed] [Google Scholar]
- 31.Yajima T, et al. A labile hydride strategy for the synthesis of heavily nitridized BaTiO3. Nat. Chem. 2015;7:1017–1023. doi: 10.1038/nchem.2370. [DOI] [PubMed] [Google Scholar]
- 32.Masuda N, et al. Hydride in BaTiO2. 5H0. 5: a labile ligand in solid state chemistry. J. Am. Chem. Soc. 2015;137:15315–15321. doi: 10.1021/jacs.5b10255. [DOI] [PubMed] [Google Scholar]
- 33.Katayama T, et al. Epitaxial growth and electronic structure of oxyhydride SrVO2H thin films. J. Appl. Phys. 2016;120:085305. doi: 10.1063/1.4961446. [DOI] [Google Scholar]
- 34.Wei Y, et al. The effect of hydrogen ordering on the electronic and magnetic properties of the strontium vanadium oxyhydride. J. Phys. Condens. Matter. 2015;27:206001. doi: 10.1088/0953-8984/27/20/206001. [DOI] [PubMed] [Google Scholar]
- 35.Liu K, Hou YS, Gong XG, Xiang HJ. Orbital delocalization and enhancement of magnetic interactions in perovskite oxyhydrides. Sci. Rep. 2016;6:19653. doi: 10.1038/srep19653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawakami T, et al. Spin transition in a four-coordinate iron oxide. Nat. Chem. 2009;1:371–376. doi: 10.1038/nchem.289. [DOI] [PubMed] [Google Scholar]
- 37.Hazen, R. M. & Downs, R. T. High-Temperature and High-Pressure Crystal Chemistry (Reviews in Mineralogy & Geochemistry Vol. 41) Part I, Chapter 2, 35-60 (Mineralogical Society of America / Geochemical Society, 2001).
- 38.Yamamoto T, et al. Pressure-induced structural, magnetic, and transport transitions in the two-legged ladder Sr3Fe2O5. J. Am. Chem. Soc. 2011;133:6036–6043. doi: 10.1021/ja200410z. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Maxisch T, Ceder G. Oxidation energies of transition metal oxides within the GGA + U framework. Phys. Rev. B. 2006;73:195107. doi: 10.1103/PhysRevB.73.195107. [DOI] [Google Scholar]
- 40.Wei J, Ji H, Guo WH, Nevidomskyy AH, Natelson D. Hydrogen stabilization of metallic vanadium dioxide in single-crystal nanobeams. Nat. Nanotechnol. 2012;7:357–362. doi: 10.1038/nnano.2012.70. [DOI] [Google Scholar]
- 41.Huffman TJ, et al. Anisotropic infrared response of vanadium dioxide microcrystals. Phys. Rev. B. 2013;87:115121. doi: 10.1103/PhysRevB.87.115121. [DOI] [Google Scholar]
- 42.Pruneda JM, Íñiguez J, Canadell E, Kageyama H, Takano M. Structural and electronic properties of SrFeO2 from first principles. Phys. Rev. B. 2008;78:115101. doi: 10.1103/PhysRevB.78.115101. [DOI] [Google Scholar]
- 43.Xiang HJ, Wei S–H, Whangbo M–H. Origin of the structural and magnetic anomalies of the layered compound SrFeO2: a density functional investigation. Phys. Rev. Lett. 2008;100:167207. doi: 10.1103/PhysRevLett.100.167207. [DOI] [PubMed] [Google Scholar]
- 44.Rahman M, Nie YZ, Guo GH. Electronic structures and magnetism of SrFeO2 under pressure: a first-principles study. Inorg. Chem. 2013;52:12529–12534. doi: 10.1021/ic401615r. [DOI] [PubMed] [Google Scholar]
- 45.Imada M, Fujimori A, Tokura Y. Metal-insulator transitions. Rev. Mod. Phys. 1998;70:1039–1263. doi: 10.1103/RevModPhys.70.1039. [DOI] [Google Scholar]
- 46.Rey MJ, et al. Preparation and structure of the compounds SrVO3 and Sr2VO4. J. Solid State Chem. 1990;86:101–108. doi: 10.1016/0022-4596(90)90119-I. [DOI] [Google Scholar]
- 47.Kresse G, Furthmuller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169–11186. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 48.Krukau A, Vydrov O, Izmaylov A, Scuseria G. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006;125:224106. doi: 10.1063/1.2404663. [DOI] [PubMed] [Google Scholar]
- 49.Brandbyge M, Mozos J-L, Ordejon P, Taylor J, Stokbro K. Density-functional method for nonequilibrium electron transport. Phys. Rev. B. 2002;65:165401. doi: 10.1103/PhysRevB.65.165401. [DOI] [Google Scholar]
- 50.Taylor J, Guo H, Wang J. Ab initio modeling of quantum transport properties of molecular electronic devices. Phys. Rev. B. 2001;63:245407. doi: 10.1103/PhysRevB.63.245407. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.