Abstract
BACKGROUND & AIMS
Little is known about provider and health system factors that affect receipt of active therapy and outcomes of patients with hepatocellular carcinoma (HCC). We investigated patient, provider, and health system factors associated with receipt of active HCC therapy and overall survival.
METHODS
We performed a national, retrospective cohort study of all patients diagnosed with HCC from January 1, 2008 through December 31, 2010 (n = 3988) and followed through December 31 2014 who received care through the Veterans Administration (128 centers). Outcomes were receipt of active HCC therapy (liver transplantation, resection, local ablation, transarterial therapy, or sorafenib) and overall survival.
RESULTS
In adjusted analyses, receiving care at an academically affiliated Veterans Administration hospital (odds ratio [OR], 1.97; 95% confidence interval [CI], 1.60–2.41) or a multi-specialist evaluation (OR, 1.60; 95% CI, 1.15–2.21), but not review by a multidisciplinary tumor board (OR, 1.19; 95% CI, 0.98–1.46), was associated with a higher likelihood of receiving active HCC therapy. In time-varying Cox proportional hazards models, liver transplantation (hazard ratio [HR], 0.22; 95% CI, 0.16–0.31), liver resection (HR, 0.38; 95% CI, 0.28–0.52), ablative therapy (HR, 0.63; 95% CI, 0.52–0.76), and transarterial therapy (HR, 0.83; 95% CI, 0.74–0.92) were associated with reduced mortality. Subspecialist care by hepatologists (HR, 0.70; 95% CI, 0.63–0.78), medical oncologists (HR, 0.82; 95% CI, 0.74–0.91), or surgeons (HR, 0.79; 95% CI, 0.71–0.89) within 30 days of HCC diagnosis, and review by a multidisciplinary tumor board (HR, 0.83; 95% CI, 0.77–0.90), were associated with reduced mortality.
CONCLUSIONS
In a retrospective cohort study of almost 4000 patients with HCC cared for at VA centers, geographic, provider, and system differences in receipt of active HCC therapy are associated with patient survival. Multidisciplinary methods of care delivery for HCC should be prospectively evaluated and standardized to improve access to HCC therapy and optimize outcomes.
Keywords: Population, Liver Cancer, Quality, Risk Factor
The incidence of hepatocellular carcinoma (HCC) and associated mortality have been steadily on the rise.1,2 Liver cancer is currently the second leading cause of cancer mortality worldwide, is among the leading causes of death in cirrhosis, and is the fifth leading cause of cancer death in the US.1,3–5 The management of HCC differs from most other solid tumors in that: (1) this cancer arises in the setting of cirrhosis, which impacts therapeutic options and creates a competing risk for mortality; (2) durable cure can be obtained in a subset of patients with early stage disease; and (3) radiologic criteria may be sufficient for diagnosis in the absence of biopsy confirmation.6–8 Multiple and evolving treatment modalities offered by diverse treating specialties must be tailored to each HCC patient based not only on tumor stage but also on hepatic reserve and performance status. Furthermore, because death in patients with HCC generally results from progressive liver failure, often requiring careful temperance of treatment decisions, patients require collaborative or multidisciplinary care. In the community, the primary management of HCC may be directed by gastroenterologists, hepatologists, surgeons, or oncologists; however, it remains unclear to what extent care by a particular specialist and multidisciplinary care coordination affect treatment utilization and clinical outcomes.
Currently, most data on HCC outcomes in the US have been derived from the Surveillance, Epidemiology, and End Results (SEER)-Medicare data. These studies demonstrate that curative therapies for HCC are underutilized and care is geographically heterogeneous.9–12 While informative, SEER-Medicare linked data are limited by: (1) inclusion of older patients less likely to be candidates for transplantation and other curative therapies; (2) American Joint Committee on Cancer TNM staging that is rarely utilized for clinical decision-making; and (3) non-documentation of liver disease severity, a strong predictor of treatment and survival.13
Care delivery variables such as multidisciplinary tumor board review/discussion, clinician expertise, and geographic variation remain understudied in HCC. The Veterans Administration (VA) is the largest integrated provider of liver cancer-related care in the US, treatment that occurs in primary, secondary, and tertiary care facilities and in urban, suburban, and rural settings. The objectives of this study were to: (1) characterize the clinical presentation, treatment utilization, and survival in a diverse national sample of veterans, and (2) investigate associations between patient geographic, VA facility, and provider factors associated with receipt of active HCC therapy and overall survival. Specifically, we aimed to evaluate the impact of active HCC treatment on overall survival and identify whether care processes, such as having access to multidisciplinary tumor boards and treating HCC specialists (hepatologists, gastroenterologists, surgeons, oncologists), was associated with improved survival. We hypothesized that curative treatments, multidisciplinary tumor board review/discussion, and hepatology care were associated with improved survival in HCC.
Methods
Data were obtained as part of the Veterans Outcomes and Costs Associated with Liver disease (VOCAL) cohort study. Complementary detailed medical record review and administrative data sources were used for data collection for all eligible veterans nationally. The institutional review board at each of 7 VOCAL sites approved the study; manual chart review was performed centrally at the VA Connecticut Healthcare System.
Electronic Medical Record
Patients with potential HCC were initially identified by querying the VA Corporate Data Warehouse (CDW) for 1 inpatient or 2 outpatient International Classification of Diseases, 9th Revision, Clinical Modification (ICD9-CM) primary or secondary diagnosis codes for malignant neoplasm of the liver (ICD9-CM: 155.0, 155.2) from January 1, 2008 to December 31, 2010 and were followed through December 31, 2014. Diagnostic confirmation and tumor staging was obtained by manual chart abstraction of all cases performed by 2 trained individuals (R.M., K.D.) under physician supervision (T.H.T.). Information included the date and modality of the first image concerning for malignancy, official HCC diagnosis date, criteria upon which the HCC diagnosis was based, number and size(s) of tumors, presence or absence of macrovascular invasion and/or metastasis at the time of diagnosis, and whether the patients remained in VA care. Veteran performance status was assessed using the Eastern Cooperative Oncology Group (ECOG) performance status classification (coded as PS 0–2, PS 3–4).14 The date of HCC diagnosis was determined using the first date of imaging with contrast-enhanced computed tomography or magnetic resonance imaging that met diagnostic criteria for HCC,7 the date of the pathology report in cases where biopsies were performed, and the date of tumor board discussion (when available) in cases where imaging was equivocal. Tumor staging classification using the Barcelona Cancer Liver Clinic (BCLC) criteria was determined using medical record information. Milan criteria (accepted criteria for liver transplantation in the US) were defined as a single lesion ≤ 5 cm or up to 3 lesions all ≤ 3 cm with no macrovascular invasion.15
Patients were excluded for: a clinical diagnosis other than HCC; indeterminate lesions not meeting American Association for the Study of Liver Diseases (AASLD) imaging criteria16 for HCC; diagnosis date preceding January 1, 2008; or treatment initiation outside of the VA. Provider factors such as the types of specialty physicians (eg, surgery, hepatology, etc.) seen for HCC consultation within the first 30 days of diagnosis and multidisciplinary tumor board discussion were also recorded from manual chart review.
Administrative Data Sources
The CDW is a centralized VA administrative and clinical data repository that contains patient demographics, ICD9-CM diagnosis codes, current procedural terminology (CPT) codes, laboratory data, and pharmacy prescription records on all patients receiving care within the VA.17 Baseline clinical variables included patient demographics, ICD9-CM determined liver disease etiology, Charlson-Deyo comorbidity index, Model for End-Stage Liver Disease (MELD) score component laboratory tests, and serum alpha fetoprotein.18,19 Electronic Child-Turcotte-Pugh (CTP) score was determined using our validated algorithm.20 CPT codes from Procedural, Radiology, and fee-basis tables were used to identify liver resection, ablative, transarterial therapies, and hospice care. The fee-basis table for non-VA treatment in the CDW contains all treatments received outside of the VA for which a claim was generated by a third party. This includes claims for all treatments pre-authorized by the VA or outside emergency care not pre-authorized by the VA. A total of 95% of claims are processed within 200 days and 99% are processed within 2 years.21 Transplantation status was ascertained by cross-referencing the Organ Procurement and Transplantation Network Standard Transplant Analysis and Research file [Based on OPTN data as of June 1, 2015].22 Sorafenib prescriptions were determined via outpatient pharmacy records by using pharmacy release dates. Rurality and patient distance to the nearest VA medical center (VAMC) were determined using patient zip codes.23 Rurality codes were derived from the US Department of Agriculture 2013 Rural-Urban Continuum Codes.23
Ascertainment of Active HCC Therapy
All HCC treatments performed within the VA health care system during the follow-up period were recorded, including if they occurred at multiple VA facilities or if performed outside of the VA but paid by VA funds (“fee-basis”). The process outcome of interest was the overall percentage of patients receiving active HCC therapy and stratified by the BCLC staging system.24 Active HCC therapy was classified into the following categories: liver transplantation, resection, ablative therapy (microwave, radiofrequency, or cryo-ablation), transarterial therapy (chemoembolization or 90Y-radioembolization), sorafenib, systemic chemotherapy, or radiation. Interventional radiology treatments within VA facilities were identified using text-based Structured Query Language queries from radiology tables of the CDW (eg, “embolization,” “ablation”) with extensive manual review of the text-based “procedure description” to identify liver-specific procedure identifiers for each station (see Supplemental Methods). Surgical procedures within the VA were identified from inpatient procedure tables using CPT codes 47100 (wedge resection), 4712×/47130 (hepatectomy), and 4737×/47381 (intraoperative ablative techniques). Transplantation events were obtained from the Organ Procurement and Transplantation Network Standard Transplant Analysis and Research file. For patients who received HCC care within the VA but whose treatment was obtained outside of the VA and reimbursed by the VA (eg “fee-basis”), the fee-basis tables of the CDW were initially queried for all procedures (treatment-related or not) associated with ICD9 codes corresponding to malignant neoplasm of the liver (ICD9-CM: 155.0, 155.2). All CPT codes were then manually matched to individual procedures; CPT codes corresponding with known HCC treatments were then catalogued (Supplemental Methods). In the rare instances where CPT codes were not correctly classifying HCC treatment modalities (eg, codes present for intracranial embolization rather than trans-arterial embolization), study investigators reviewed and assigned procedures through manual review. We additionally performed a focused electronic medical record review and manually abstracted the first HCC therapy received (or no therapy received) among 223 patients (6% of cohort) to validate our treatment identification algorithms.
Outcomes
The clinical outcome of interest was overall 5-year survival. Death was ascertained using the VINCI Vital Status Master File (censoring as of December 31, 2014), which contains dates of death as recorded in all of the main federal mortality databases and has a sensitivity greater than 90% compared with the gold standard state death certificate registries.25
Statistical Analysis
Bivariate analyses were conducted with χ2 or Fisher’s exact tests for categorical variables, t tests, Kruskal-Wallis, and Wilcoxon rank sum tests for continuous variables where appropriate. Time-to-event data were compared using the log-rank test. A multivariable logistic regression model was fit to evaluate factors associated with not receiving active HCC therapy. Clinical and biological covariates known to affect the likelihood of treatment (eg, tumor stage, performance status, comorbidity) as well as covariates with a P value of <.15 in univariate analysis initially were selected for the multivariable model. The Vuong likelihood ratio test and the Homer-Lemeshow goodness-of-fit test were performed to evaluate model fit. For the outcome of mortality, time-varying Cox proportional hazards models were fit. Model 1 included active HCC therapy as the main time-varying exposure and was adjusted for race, Charlson-Deyo comorbidity, and presenting BCLC stage. Model 2 was fit with additional VA facility and provider factors to evaluate their effect on survival. In the time-varying analyses, we accounted for each distinct treatment modality the patient may have received, eg, chemoembolization, radiation, 90Y-radioembolization, etc. The total follow-up time for each patient was split into several observations accounting for each HCC therapy or no therapy where appropriate. Patients receiving more than 1 of the same type of HCC therapy (eg, TACE) were considered to be exposed to that treatment since the first treatment. Patients were censored at death or end of VA follow-up. Covariates for time-varying models were chosen if P was <.15 in univariable analyses. Spearman’s rho coefficients evaluated the correlation between proportion of active HCC therapy and median overall survival. Adjusted subgroup analyses were performed to evaluate the effect of fee-basis therapy (outside of the VA) on overall survival among patients with and without transplantation. A 2-sided P value of <.05 was defined to be statistically significant. Analyses were performed using Stata 13 (StataCorp, College Station, TX) and the geosphere package in R.26
Results
A total of 6827 patients were initially identified to have a malignant neoplasm of the liver by ICD9-CM diagnosis codes. After chart review, 1488 were excluded because of absence of HCC or presence of a non-HCC primary neoplasm (eg, cholangio-carcinoma, non-HCC metastatic disease); 1126 because of HCC diagnosis outside of the VA; 225 for management outside the VA, leaving the total study sample at N = 3988. Baseline characteristics of the sample stratified by CTP class are shown in Table 1. A total of 82% of patients had ICD9-CM-documented cirrhosis and nearly one half had CTP class B or C (decompensated) cirrhosis. The most common liver disease etiology was the combination of hepatitis C virus and alcohol (39%), followed by hepatitis C virus; 2.4% had HIV co-infection. The median MELD score was 10 (interquartile range [IQR] 7–13) overall; 17 (IQR 14–10) among patients with CTP class C. Most patients had ECOG performance status of ≤2. Nearly 36% of patients presented with early stage HCC (BCLC stages 0 or A). Among tumor characteristics, 36% of patients presented within Milan criteria for transplantation,16 18% had macrovascular invasion, and 7.2% had metastatic disease. Patients with advanced (CTP class B/C) cirrhosis presented with more advanced HCC and were more likely to have macrovascular invasion.
Table 1.
Baseline Demographics and Clinical Characteristics of the Study Sample at the Time of HCC Diagnosis
| Demographic and clinical characteristics | Total, n
(%) N = 3988 |
|---|---|
| Age at diagnosis, mean (SD) | 62 (8.0) |
| Male | 3960 (99) |
| Race | |
| White | 2154 (54) |
| Black | 924 (23) |
| Hispanic | 371 (9.4) |
| Asian/Alaskan Native/Hawaii/Pacific Islander | 85 (2.1) |
| Unknown | 454 (11) |
| Baseline cirrhosis | 3273 (82) |
| Etiology of liver diseasea | |
| HCV and alcohol | 1553 (39) |
| HCV | 894 (22) |
| Alcohol | 544 (14) |
| HCV & HBV co-infection | 205 (5.1) |
| HBV | 128 (3.2) |
| NASH | 144 (3.6) |
| Other/unknown | 520 (13) |
| HIV co-infection | 94 (2.4) |
| MELD score, median (IQR)] | 10 [7–13] |
| ECOG performance status | |
| 0–2 | 3753 (94) |
| 3–4 | 235 (5.9) |
| Barcelona liver clinic staging | |
| 0 | 228 (5.8) |
| A | 1191 (30) |
| B | 1263 (32) |
| C | 695 (17) |
| D | 576 (14) |
| Unknown | 35 (0.9) |
| Total tumor diameter | |
| ≤5 cm | 1663 (42) |
| > 5 cm | 994 (25) |
| Infiltrative disease (not quantified) | 371 (9.3) |
| Not reported | 960 (24) |
| Maximum tumor size | |
| ≤5 cm | 2345 (61) |
| > 5 cm | 1166 (29) |
| Infiltrative disease (not quantified) | 363 (9.1) |
| Not reported | 24 (0.6) |
| Number of lesions, median (IQR) | 2 [1,4] |
| Within Milan criteria | 1419 (36) |
| Baseline AFP ≥200 ng/mLb | 1343 (34) |
| Macrovascular invasion | 698 (17.5) |
| Metastatic disease | 288 (7.2) |
AFP, alpha fetoprotein; CTP, Child-Turcotte-Pugh class; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; IQR, interquartile range; SD, standard deviation.
Etiology of liver disease categories is not mutually exclusive.
Data missing for n = 68.
Data on patient geographic, VA facility, and provider factors are a presented in Table 2. Most veterans were urban-dwelling and half lived within 74 miles of the closest VA hospital. A total of 69% of patients received care at academically affiliated VAMCs. No differences were noted in CTP class by geographic region or among VAMCs with or without academic affiliation. A total of 51% of patients were treated at VAMCs located greater than 500 miles to the nearest VA transplant center. Within the first 30 days of HCC diagnosis, 46% of patients saw medical oncologists, 44% gastroenterologists, 42% hepatologists, and nearly 20% saw palliative care and surgery providers. A total of 54% were evaluated by more than 1 specialist and 34% were discussed at multidisciplinary tumor conference. Patients with CTP class B or C cirrhosis were less likely to see medical oncology, hepatology and surgery; however, they were more likely to see palliative care providers. Patients with CTP B or C cirrhosis were less likely to have cases reviewed at multidisciplinary tumor board (CTP A, 37%; CTP B, 31%; CTP C, 26%).
Table 2.
Patient Geographic, VA Facility and Provider Process of Care Characteristics of the HCC Cohort
| Patient geographic factors | Total, n (%) N = 3988 |
|---|---|
| Rurality | |
| Urban | 3438 (86) |
| Non-urban ≥ 20,000 pop. | 211 (5.3) |
| Non-urban 2,500–19,999 pop. | 284 (7.1) |
| Completely rural < 2,500 pop. | 52 (1.3) |
| Patient distance to VAMC | |
| 0–74 miles | 1936 (50) |
| 75–215 miles | 968 (25) |
| >215 miles | 967 (25) |
| VA facility and provider factors | |
| Region | |
| Northeast (VISN 1–4) | 534 (13) |
| Southeast (VISN 5–8) | 930 (23) |
| Midsouth (VISN 9, 15–17) | 922 (23) |
| Central (VISN 10–13) | 619 (16) |
| West (VISN 18–22) | 982 (25) |
| Academic center affiliation | 2750 (69) |
| Distance >500 miles to VATC | 2018 (51) |
| Specialist seen < 30 days of diagnosisa | |
| Medical oncology | 1835 (46) |
| Gastroenterology | 1767 (44) |
| Hepatology | 1685 (42) |
| Palliative care | 743 (19) |
| Surgery | 773 (19) |
| No specialist | 96 (2.4) |
| Evaluation by ≥1 specialist | 2155 (54) |
| Multidisciplinary tumor conference | 1366 (34) |
| Year of diagnosis | |
| 2008 | 1063 (26.7) |
| 2009 | 1396 (35.0) |
| 2010 | 1529 (38.3) |
pop., population; VA, Veterans Affairs; VAMC, VA medical center; VATC, VA transplant center; VISN, Veterans Integrated Service Network.
Comparator for each specialist is absence of evaluation by that specialist.
Per administrative identification of HCC-directed treatments, 74% of the cohort received active HCC therapy and 10% primarily entered hospice care. To confirm the accuracy of our administrative determination of receipt or non-receipt of therapy, we performed a validation of 223 cases (6%). Among the 170 patients administratively identified as having active HCC therapy, n = 168 (99%) were confirmed to have HCC therapy by chart review; the specificity for identifying each treatment modality was 92%. Among the 53 patients that we classified as having no therapy, n = 43 (81%) did not receive any therapy and 11 (19%) received therapy outside of the VA (the latter was not fee-based and was likely obtained through commercial insurance). Overall, the degree of agreement between our algorithm and manual chart abstraction was high: k = 0.85 (95% confidence interval [CI], 0.80–0.91). Based on the high correlation of administrative and manually obtained treatment data, we evaluated the first treatment (Figure 1) and all treatments (Supplementary Figure 1) received stratified by BCLC stage at cohort entry. The most common therapy first prescribed was transarterial therapy (54%), followed by sorafenib (27%) for the overall cohort and for BCLC Stages 0/A/B; sorafenib was the most commonly utilized initial therapy for BCLC stage C (63%). Among patients with potentially curable HCC (BCLC stages 0/A; n = 1419), only 25% received potentially curative therapies such as resection, transplantation, or ablative therapy. Even among patients with potentially curable HCC and good performance status (ECOG 1–2), 13% of patients did not receive active HCC therapy.
Figure 1.
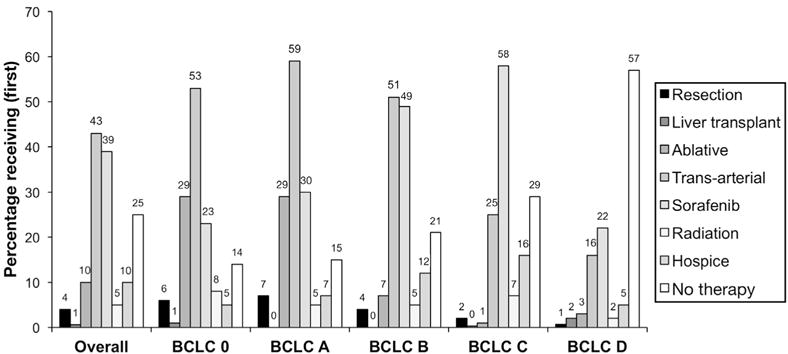
First HCC-directed therapy received. Percentage of veterans receiving hepatic resection, liver transplantation, ablative therapies (radio-frequency, microwave, or cryo-ablation), transarterial therapies (bland, chemo- or radio-embolization), hospice care, and no active therapy as first intervention stratified by BCLC stage.
Factors Associated With Active HCC Therapy
The characteristics associated with not receiving HCC therapy are shown in Table 3. In a multivariable model, demographic and clinical characteristics independently associated with lower odds of receiving HCC therapy were: older age (odds ratio [OR] 0.77; 95% CI, 0.68–0.86 for each 10-year increase), higher MELD scores (0.93; 95% CI, 0.92–0.96 for each 1-point increase), CTP class ≥B (OR 0.67; 95% CI, 0.54–0.83), ECOG performance status of 3–4 compared with 0–2 (OR 0.40; 95% CI, 0.27–0.56), baseline alphafetoprotein (AFP) ≥200 ng/mL (OR 0.80; 95% CI, 0.66–0.97), and macrovascular invasion (OR 0.60; 95% CI, 0.47–0.77). Facility rurality and patient distance to a VAMC had no significant impact on receipt of therapy. Patients receiving care at VA hospitals with an academic affiliation had higher odds of receiving active HCC therapy (OR 1.97; 95% CI, 1.60–2.41). Regional and provider differences were also evident. Compared with the reference category of the Northeast region, patients receiving care in the Midsouth had lower odds of receiving HCC therapy (OR 0.62; 95% CI, 0.44–0.85). Patients evaluated by general gastroenterology (OR 0.56; 95% CI, 0.43–0.72) and palliative care (OR 0.24; 95% CI, 0.18–0.31) had lower odds of receiving active HCC therapy compared with patients not seen by those specialists; by contrast, patients seen by medical oncology and surgery within 30 days were more likely to receive HCC therapy. Evaluation by more than 1 specialist (OR 1.60; 95% CI, 1.15–1.21) was associated with higher odds of active HCC therapy, whereas no specialist (hazard ratio [HR], 0.56; 95% CI, 0.29–1.08) and multidisciplinary tumor board did not reach statistical significance (HR, 1.19; 95% CI, 0.98–1.46).
Table 3.
Factors Associated With Receiving Active HCC Therapya in Multivariable Analysis (N = 3988)
| Adjusted |
|||
|---|---|---|---|
| OR | 95% CI | P value | |
| Patient demographic and clinical characteristics | |||
| Age at diagnosis (per 10-year increase) | 0.77 | 0.68–0.86 | <.001 |
| Charlson-Deyo comorbidity index | .040 | ||
| 0–2 | 1.00 | REF | |
| ≥ ≥3 | 1.24 | 1.01–1.52 | |
| MELD score (per 1-point increase) | 0.93 | 0.92–0.96 | <.001 |
| Child-Turcotte-Pugh class > B | 0.67 | 0.54–0.83 | .001 |
| ECOG performance status | <.001 | ||
| 0–2 | 1.00 | REF | |
| 3–4 | 0.40 | 0.27–0.56 | |
| Baseline AFP ≥200 ng/mL | 0.80 | 0.66–0.97 | .024 |
| Metastatic disease | 0.71 | 0.51–0.99 | .048 |
| Macrovascular invasion | 0.60 | 0.47–0.77 | <.001 |
| Within Milan criteria | 1.42 | 1.13–1.79 | .003 |
| Patient geographic factors | |||
| Rurality | .137 | ||
| Urban | 1.00 | REF | |
| Non-urban ≥20,000 | 1.41 | 0.92–2.15 | |
| Non-urban (2,500–19,999) Completely rural (<2,500) | 0.84 | 0.62–1.15 | |
| Patient distance to VA Medical Center | .337 | ||
| 0–74 miles | 1.00 | REF | |
| 75–215 miles | 0.85 | 0.69–1.08 | |
| >215 miles | 0.91 | 0.70–1.09 | |
| VA facility factors | |||
| Region | .032 | ||
| Northeast (VISN 1–4) | 1.00 | REF | |
| Southeast (VISN 5–8) | 0.85 | 0.61–1.18 | |
| Midsouth (VISN 9, 15–17) | 0.62 | 0.44–0.85 | |
| Central (VISN 10–13) | 0.80 | 0.56–1.15 | |
| West (VISN 18–22) | 0.81 | 0.59–1.13 | |
| Academic center affiliation (%) | 1.97 | 1.60–2.41 | <.001 |
| Provider factors | |||
| Specialist seen within 30 days of diagnosis, n (%)a | |||
| Hepatology | 1.20 | 0.91–1.57 | .202 |
| Medical oncology | 2.56 | 1.96–3.35 | <.001 |
| Surgery | 1.67 | 1.22–2.28 | .001 |
| Gastroenterology | 0.56 | 0.43–0.72 | <.001 |
| Palliative care | 0.24 | 0.18–0.31 | <.001 |
| No specialist | 0.56 | 0.29–1.08 | .083 |
| Evaluation by ≥1 specialist | 1.60 | 1.15–2.21 | .005 |
| Multidisciplinary tumor board, n (%) | 1.19 | 0.98–1.46 | .101 |
AFP, alpha fetoprotein; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; MELD, model for end stage liver disease; OR, odds ratio; VISN, Veterans Integrated Service Network.
Active HCC therapy is defined as receipt of any of the following during the course of follow-up: liver transplantation, resection, ablative/transarterial therapy, sorafenib.
Comparator for each specialist is absence of evaluation by that specialist. Year of diagnosis was evaluated as a covariate, was non-significant, and is not included in the model.
Survival Analysis
The total follow-up time for the study cohort was 6605 person-years, with a median survival of 1.1 years (IQR, 0.4–2.7) and overall 5-year survival of 11.9%. Median unadjusted overall survival stratified by BCLC stage is shown in Figure 2. Results of the multivariable survival models with active HCC therapy as a time-varying covariate are shown in Table 4. Model 1 included HCC therapy, patient demographics, and clinical covariates; whereas Model 2 was additionally adjusted for VA facility and provider factors. In both models, resection, transplantation, and liver-directed therapy were independently associated with improved survival and curative therapies (liver transplantation, resection, and ablative therapy) had an incrementally greater survival benefit than transarterial therapy, whereas sorafenib was associated with higher mortality. In Model 1, older age, race, Charlson-Deyo comorbidity, presenting BCLC stage, higher MELD, and CTP class were associated with increased mortality risk. After adjustment for facility and provider factors in Model 2, active HCC therapy, demographics, Charlson-Deyo comorbidity, and presenting BCLC stage remained independently associated with overall survival. However, these relationships were somewhat attenuated. Among facility and provider factors, region (overall P = .001), specialist seen within 30 days of diagnosis (hepatology: HR, 0.70; 95% CI, 0.63–0.78; medical oncology: HR, 0.82; 95% CI, 0.74–0.91; surgery: HR, 0.79; 95% CI, 0.71–0.89; palliative care, HR, 2.10; 95% CI, 1.87–2.36), and multidisciplinary tumor board (HR, 0.83; 95% CI, 0.77–0.90) were associated with overall survival. A moderate correlation was noted between the proportion of patients receiving active HCC treatment at each Veterans Integrated Service Network and overall median survival (Spearman ρ = 0.40, P = .02; Figure 3).
Figure 2.
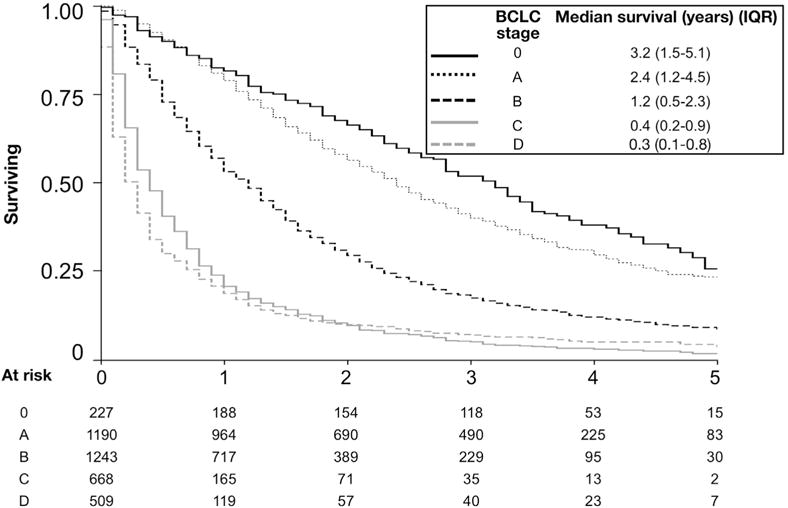
Unadjusted median survival stratified by BCLC stage. Kaplan-Meier survival analysis of 5-year overall survival shown.
Table 4.
Multivariable Analysis of Time-varying HCC Therapy, Patient, Facility, and Provider Factors on All-Cause Mortality
| Variable | Model 1a
|
Model 2b
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value value | |
| Active HCC therapyc | ||||||
| <.001 | <.001 | |||||
| No therapy | 1.00 | REF | 1.00 | REF | ||
| Liver transplantation | 0.18 | 0.13–0.25 | 0.22 | 0.16–0.31 | ||
| Resection | 0.31 | 0.13–0.25 | 0.38 | 0.28–0.52 | ||
| Ablative therapy | 0.50 | 0.42–0.60 | 0.63 | 0.52–0.76 | ||
| Transarterial therapy | 0.72 | 0.65–0.80 | 0.83 | 0.74–0.92 | ||
| Sorafenib | 1.70 | 1.54–1.86 | 1.99 | 1.80–2.20 | ||
| Patient demographic and clinical characteristics | ||||||
| Age at diagnosis (10-year increase) | 1.12 | 1.07–1.72 | <.001 | 1.07 | 1.03–1.13 | .002 |
| Race | ||||||
| White | 1.00 | REF | <.001 | 1.00 | REF | .002 |
| Black | 0.98 | 0.86–1.06 | 1.02 | 0.93–1.12 | ||
| Hispanic | 0.78 | 0.68–0.88 | 0.82 | 0.72–0.94 | ||
| Asian/Alaska/Hawaii/Pacific Islander | 0.85 | 0.66–1.10 | 0.90 | 0.69–1.16 | ||
| Charlson-Deyo comorbidity index | <.001 | <.001 | ||||
| 0–2 | 1.00 | REF | 1.00 | REF | ||
| ≥3 | 1.29 | 1.19–1.39 | 1.26 | 1.16–1.36 | ||
| Presenting BCLC Stage | <.001 | <.001 | ||||
| 0 | 1.00 | REF | 1.00 | REF | ||
| A | 1.13 | 0.94–1.35 | 1.13 | 0.94–1.36 | ||
| B | 1.71 | 1.43–2.05 | 1.63 | 1.36–1.96 | ||
| C | 2.92 | 2.41–3.54 | 2.50 | 2.05–3.05 | ||
| D | 2.88 | 2.36–3.51 | 2.40 | 1.96–2.93 | ||
| MELD score (1-point increase) | 1.04 | 1.03–1.05 | <.001 | 1.04 | 1.03–1.05 | <.001 |
| Child-Turcotte-Pugh class ≥ B | 1.57 | 1.44–1.71 | <.001 | 1.50 | 1.37–1.64 | <.001 |
| VA facility factors | ||||||
| Region | .001 | |||||
| Northeast (VISN 1–4) | 1.00 | REF | ||||
| Southeast (VISN 5–8) | 0.79 | 0.70–0.90 | ||||
| Midsouth (VISN 9, 15–17) | 0.95 | 0.83–1.08 | ||||
| Central (VISN 10–13) | 0.95 | 0.82–1.08 | ||||
| West (VISN 18–22) | 0.89 | 0.78–1.02 | ||||
| Academic center affiliation | 0.94 | 0.86–1.02 | .125 | |||
| Distance >500 miles to VATC | 1.01 | 0.93–1.09 | .838 | |||
| Provider factors | ||||||
| Specialist seen within 30 days of diagnosisd | ||||||
| Hepatology | 0.70 | 0.63–0.78 | <.001 | |||
| Medical oncology | 0.82 | 0.74–0.91 | <.001 | |||
| Surgery | 0.79 | 0.71–0.89 | <.001 | |||
| Gastroenterology | 1.02 | 0.93–1.13 | .673 | |||
| Palliative care | 2.10 | 1.87–2.36 | <.001 | |||
| No specialist | 0.89 | 0.65–1.21 | .447 | |||
| Evaluation by ≥1 specialist | 1.09 | 0.96–1.23 | .187 | |||
| Multidisciplinary tumor board | 0.83 | 0.77–0.90 | <.001 | |||
NOTE. Year of diagnosis was evaluated as a covariate, was non-significant, and is not included in the model.
BCLC, Barcelona clinic liver cancer; CI, confidence interval; HR, hazard ratio; MELD, model for end stage liver disease; VATC, VA transplant center; VISN, Veterans Integrated Service Network.
Model 1 includes HCC therapy, patient demographics, and clinical covariates including presenting BCLC stage.
Model 2 is additionally adjusted for VA facility and provider factors
Active HCC therapy=receipt of any of the following during the course of follow-up: liver transplantation, resection, ablative/transarterial therapy, sorafenib.
Comparator for each specialist is absence of evaluation by that specialist. Comparator for no specialist is evaluation by any specialist.
Figure 3.
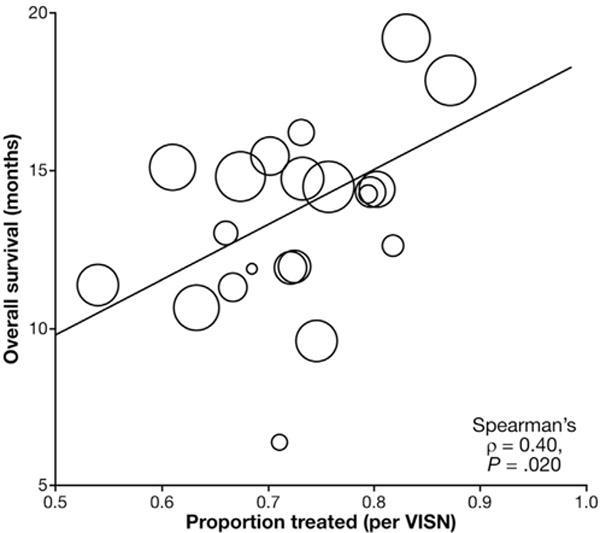
Correlation between receipt of active HCC therapy and overall survival at the regional level. Spearman correlation of the proportion of patients receiving active HCC therapy (defined as resection, transplantation, ablation, transarterial therapy, or sorafenib) (“Proportion treated”) and median overall survival (OS) in months grouped by Veterans Integrated Service Network region. The diameter of each circle is proportional to the number of HCC cases in each Veterans Integrated Service Network.
Subgroup Analyses: Effect of Fee Basis (Non-VA Therapy)
A total of 390 patients (10%) who received active HCC therapy excluding transplantation received fee-based HCC therapy outside of the VA. A total of 968 of 4565 (18%) treatments were fee based. Of the fee-based therapies, 1.6% (n = 16) were resection, 15% (n = 143) ablative, and 84% (n = 809) were trans-arterial (84%). In Cox proportional hazards models adjusted for BCLC stage, among patients who did not receive transplantation, fee-basis therapy was not significantly associated with mortality compared with VA treatment (HR, 0.93; 95% CI, 0.82–1.07; P = .318). Similarly, among the patients who went on to receive transplantation, fee-basis therapy was not associated with mortality compared with VA treatment (HR, 0.95; 95% CI, 0.54–1.67; P = .846).
Discussion
The VA is the largest single care provider to patients with chronic liver disease in the US. The detailed clinical data presented, including tumor size, staging, and performance status, overcome the limitations of many previous US studies with small sample sizes and with variable tumor stage reporting.10 In addition, to comprehensively examining clinical factors, we evaluated important facility, provider, and health-system level characteristics potentially guiding the delivery of HCC care and clinical outcomes among a diverse, national sample of patients receiving care at 128 medical centers (69% academic, 31% community-based). Findings from the VOCAL cohort of predominantly older males with significant medical comorbidities are important in light of the aging US population and a nearly 70% expected increase in cancer among older adults.27
It is not surprising that active HCC therapy was associated with prolonged survival, or that curative treatments (resection, transplantation, ablation) conferred the greatest survival benefit as shown in smaller, tertiary-care center settings.28–34 Expectedly, the biologic factors independently associated with no active HCC therapy were poor performance status, large tumor burden, decompensated liver disease, and high comorbidity. However, even among a subset of the fittest patients with potentially curable disease (BCLC stages 0-A, CTP A cirrhosis, and ECOG performance status 0–2) 13% did not receive any active treatment. Furthermore, only 25% of early stage (BCLC 0-A) patients initially received guideline-recommended therapy with curative intent, whereas the majority (54%) were initially treated only with palliative trans-arterial therapy. Though the precise reasons for these gaps in care need to be further investigated, process of care variables affected receipt of active therapy and survival. Receiving care at VAs with academic affiliations and multi-specialty evaluation were both associated with higher odds of receipt of active therapy.
Independent of tumor burden and medical comorbidity, receipt of HCC therapy and overall survival differed by region and provider specialty type. HCC in the US generally arises in patients with cirrhosis35 for whom optimizing therapy depends as much on tumor stage as on liver function, and multidisciplinary expert collaboration may lead to superior outcomes. Interestingly, we noted that hepatology care, while not associated with higher odds of receiving active therapy, was associated with a 30% mortality reduction. We can postulate that hepatologists, as experts in liver disease, are more likely to withhold treatment for patients with significant liver dysfunction and for those unlikely to benefit from tumor-directed care, and more likely to evaluate patients for curative therapies such as transplantation or resection. We noted that care by gastroenterology (non-hepatology) specialists was associated with lower odds of receipt of active HCC therapy (including transplantation, curative, and palliative therapies) and not associated with a survival benefit. Patients with access only to gastroenterology and not to hepatology providers were 32% less likely to receive care at academically affiliated VAs and 41% less likely to be reviewed at multidisciplinary tumor conferences. Thus, whether management by a hepatologist per se, or management at an institution with hepatology services, explains improved outcomes requires further study. The finding that multidisciplinary tumor board discussion was associated with a survival benefit has been noted in previous smaller investigations showing that multidisciplinary tumor board,36,37 multi-specialty-involvement,9,38 and receipt of care at hospitals with liver transplant programs and National Cancer Institute-designated Comprehensive Cancer Centers39 resulted in superior utilization of curative treatments and improved patient outcomes.40
Stage-specific median survival rates for BCLC 0/A/B/C/D patients in our cohort were 3.2, 2.4, 1.2, 0.4, and 0.3 years, respectively. Few comparable datasets exist to compare BCLC stage-specific survival and the impact of specific therapy in primary, secondary, and tertiary settings. The largest international prospective HCC registry, the global HCC BRIDGE study41 included 18,031 patients (13% from North America, 20% from Europe, and 67% from Asia) from 42 large academic centers. Compared with veterans, the North American patients in BRIDGE were similar in age and were mostly male (as in our cohort); however, 23% of North American BRIDGE patients had hepatitis B virus (HBV) compared with 3% in our study because of study site locations. Notably, more patients in the BRIDGE study had BCLC stage C disease (42%) compared with our study (17%). Because the BRIDGE study did not report stage-specific survival by region, it is challenging to compare the differences in median survival from our cohort (approximately 2.3 years lower for BCLC stage A, approximately 1 year lower for stages B and C, and comparable for stage D) as these differences could largely be explained by lower rates of cirrhosis in HBV-infected BRIDGE subjects with related higher utilization of curative therapies. Limited comparison can also be made to the US SEER-Medicare HCC cohort from 2000–2007 reported by Shaya et al.12 SEER-Medicare does not report BCLC staging because of the absence of data on liver disease severity and performance status, but the HRs we report for survival with resection, transplantation, and liver-directed therapy are highly comparable to those observed in SEER-Medicare enrollees. In later reports from the SEER registry,30 the overall 5-year survival rate for patients within Milan criteria of 39 months was slightly higher than the BCLC 0/A patients in our cohort (38months and 28 months, respectively) but some of this difference could be explained by lower median age, higher frequency of Asian patients (more likely to have noncirrhotic HCC caused by HBV), higher frequency of female patients, and biases related to requirements for histologic confirmation in that cohort. Again, the HRs for individual interventions in that study were highly comparable to the present study, with adjusted HRs associated with transplantation (0.12), resection (0.25), and locoregional therapy (0.54). Thus, our cohort appears to reflect treatment patterns and survival rates of other community-based North American cohorts.
A striking finding was that 24% of HCC patients received no HCC-specific care. There is strong internal validity of our data; recent data from Khalaf et al, in a smaller cohort of veterans with HCC, found a non-treatment rate of 34.5% with similar stage-specific survival for untreated patients.42 In SEER-Medicare patients in 2000–2007, fewer than 40% underwent any HCC-directed interventions, likely because of age and comorbidities.12 Later SEER registry data30 also demonstrate very low treatment rates, even for early stage HCC in US patients, with 43% of patients within Milan criteria not undergoing surgery, ablation, or transplantation. Thus, the non-treatment rates we observed are slightly lower than those observed in similar cohorts. Survival for untreated patients in our cohort was about 5 months, about 4 months less than reported in a recent European study,43 but also likely because of significant demographic differences across the study populations.
Although some of the reasons for care variation in HCC are not comprehensively measured with secondary data, our results suggest that access to multidisciplinary and expert care (eg, hepatology, surgery, interventional radiology, oncology) is critical for optimizing treatment choices and for maximizing survival, but that such access is non-uniform. Our data indicate that similar barriers to high-quality liver cancer care exist, as have been identified for complex cirrhosis care. Specifically, Kanwal et al44 identified hepatology clinician shortages, clustering of specialists around transplant centers, and inadequate care coordination between providers as key factors associated with poor cirrhosis care quality. These authors further contend that evidence-based metrics to guide quality improvement efforts could be used to improve the management of cirrhosis. Detailed national VA clinical and administrative data are a unique resource that may be tapped to facilitate development of a parsimonious set of evidence-based, patient-centered, liver cancer-specific quality measures. Such quality measures, which may be centered on timeliness and receipt of appropriate clinical care, patient survival, and/or patient-reported outcomes, could be applicable both within and outside the VA system.
In addition to quality measures, integrated health care systems such as the VA could also designate outcome-based HCC centers of excellence to allow patients and referring providers to obtain optimal evaluation and treatment. Alternatively, telehealth technologies can be harnessed to reduce geographic barriers to access with liver cancer-specific virtual tumor boards (VTBs). Several such VTBs have emerged within the VA system in the last several years. For instance, the study authors implemented a telehealth-based, regional, multidisciplinary VTB in Southeastern Pennsylvania in 2014 to improve access for rural Veterans to tertiary liver cancer care. In the first year of the regional VTB, access to expert body radiologist interpretation of magnetic resonance imaging and computed tomography imaging reduced diagnostic liver biopsy rates from 24% to 6% and access to the academically affiliated, hepatology-driven multidisciplinary team reduced the time to receipt of active HCC therapy for rural Veterans by nearly 30 days.20 Although telehealth-based VTB have strong potential to reduce diagnostic delays and to speed development of comprehensive treatment plans, significant barriers to implementation exist both within45 and outside the VA,46 including challenges related to timing, care coordination, patient travel/satisfaction, and expert/referrer compensation. Thus, optimization of efficient approaches to deliver expert HCC care for patients living in medically underserved or geographically remote areas requires further study.
Limitations
As with any observational cohort study, the potential for unmeasured confounding must be acknowledged. This was a study of mostly male US veterans who are older, may be more affected by medical comorbidities, and have worse outcomes than the general US population. However, these data serve to fill important knowledge gaps not addressed in clinical trials that enroll younger patients with no advanced liver disease. Although much of the tumor staging and receipt of therapy data were abstracted from chart review, ICD9-CM diagnosis codes were used to determine comorbidity and underlying liver disease that could possibly introduce misclassification bias. No information was collected on veterans who did not primarily receive VA care for HCC; they may have had more commercial insurance and different access to specialty care and transplantation. Although our algorithms were highly accurate in identifying active HCC therapy, we were somewhat limited in our ability to capture non-fee–based, non-VA care obtained through commercial insurance and Medicare. While we believe the impact is negligible (approximately 20% in our validation cohort), contamination by treated patients in our untreated group would result in an underestimation of the relative benefits of active HCC therapy on survival. Future studies should be performed linking VA data to Medicare data to more comprehensively evaluate the effect of care fragmentation on HCC outcomes. Though geographic and provider differences in care patterns and survival were noted, precise reasons for these differences (eg, provider expertise, patient access and adherence to treatment recommendations) were not elucidated in this study and should be explored further.
Conclusions
Among a large, national sample of veterans with HCC, we identified important demographic, clinical, and care delivery characteristics that affect receipt of active HCC therapy and overall survival. Delivery of curative therapies conferred the highest survival benefit and notable geographic and specialist variation was observed in the delivery of active treatment. Future studies should further evaluate modifiable health system and provider-specific barriers to delivering high-quality, multidisciplinary care in HCC to optimize patient outcomes.
Supplementary Material
EDITOR’S NOTES.
BACKGROUND AND CONTEXT
Liver cancer is on the rise in the United States and treatment options are complex. How patient access to any particular type of specialist or multidisciplinary tumor boards impacts treatment choice and outcomes has not been well studied.
NEW FINDINGS
Liver cancer care is highly variable across the country, but overall curative treatment options are underutilized. Involvement by a liver disease specialist (hepatologist) with liver cancer treatment decision-making, involvement by cancer specialists (oncologists) and surgeons, and having a case presented at a multidisciplinary board all have varying impact on patient survival.
LIMITATIONS
The study only evaluated outcomes among patients receiving care within the Veterans Administration. Patients were mostly male and some patients received dual care in the community that could not be effectively captured.
IMPACT
This is the largest US-based cohort for whom Barcelona Clinic Liver Cancer stage-specific outcomes have been described, identifying certain processes of care that are associated with improved liver cancer outcomes that should be tested prospectively.
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs of the US Government. The content is the responsibility of the authors alone and does not necessarily reflect the views of or imply endorsement by the US Government.
Funding
This work was supported by unrestricted research funds from Bayer Healthcare Pharmaceuticals and the VA HIV, Hepatitis and Public Health Pathogens Programs.
Abbreviations used in this paper
- BCLC
Barcelona Cancer Liver Clinic
- CDW
corporate data warehouse
- CI
confidence interval
- CPT
current procedural terminology
- CTP
Child-Turcotte-Pugh
- ECOG
Eastern Cooperative Oncology Group
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- ICD9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- IQR
interquartile range
- MELD
Model for End-Stage Liver Disease
- OR
odds ratio
- SEER
Surveillance, Epidemiology, and End Results
- VAMC
VA medical center
- VOCAL
Veterans Outcomes and Costs Associated with Liver disease
- VTB
virtual tumor board
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2017.02.040.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–243. vii–x. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Njei B, Rotman Y, Ditah I, et al. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61:191–199. doi: 10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0 Cancer incidence and mortality worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. Available at: http://globocan.iarc.fr/ (accessed June 24, 2016) [Google Scholar]
- 4.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 6.American College of Radiology. Liver imaging reporting and data system, version 2014. Available at: http://www.acr.org/Quality-Safety/Resources/LIRADS (accessed February 29, 2016)
- 7.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong T, Gow P, Fink M, et al. Novel population-based study finding higher than reported hepatocellular carcinoma incidence suggests an updated approach is needed. Hepatology. 2016;63:1205–1212. doi: 10.1002/hep.28267. [DOI] [PubMed] [Google Scholar]
- 9.Chirikov VV, Mullins CD, Hanna N, et al. Multispecialist care and mortality in hepatocellular carcinoma. Am J Clin Oncol. 2015;38:557–563. doi: 10.1097/COC.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 10.Hyder O, Dodson RM, Nathan H, et al. Referral patterns and treatment choices for patients with hepatocellular carcinoma: a United States population-based study. J Am Coll Surg. 2013;217:896–906. doi: 10.1016/j.jamcollsurg.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah SA, Smith JK, Li Y, et al. Underutilization of therapy for hepatocellular carcinoma in the medicare population. Cancer. 2011;117:1019–1026. doi: 10.1002/cncr.25683. [DOI] [PubMed] [Google Scholar]
- 12.Shaya FT, Breunig IM, Seal B, et al. Comparative and cost effectiveness of treatment modalities for hepatocellular carcinoma in SEER-Medicare. Pharmacoeconomics. 2014;32:63–74. doi: 10.1007/s40273-013-0109-7. [DOI] [PubMed] [Google Scholar]
- 13.Altekruse SF, McGlynn KA, Dickie LA, et al. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992–2008. Hepatology. 2012;55:476–482. doi: 10.1002/hep.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 15.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Raoul J-L, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Department of Veterans Affairs. Corporate Data Warehouse information. Available at: https://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm Accessed May 3, 2017.
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical co-morbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology (Baltimore, Md) 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 20.Egert EM, JR, Watts MM, Booty J, et al. A regional multidisciplinary liver tumor board improves access to hepatocellular carcinoma treatment for patients geographically distant from tertiary medical center. [abstract] Poster of distinction presented at the 2015 Liver Meeting of the American Association for the Study of Liver Diseases; San Francisco, CA. 2015. [Google Scholar]
- 21.Gidwani R, Hong J, Murrell S. Fee Basis Data: A Guide for Researchers. Menlo Park, CA VA Palo Alto: Health Economics Resource Center; Nov, 2015. [Google Scholar]
- 22.Organ Procurement and Transplantation Network. Organ Procurement and Transplantation Network Standard Transplant Analysis and Research (UNOS STAR) file. Based on OPTN data as of June 1, 2015. Available at: https://optn.transplant.hrsa.gov/ Accessed May 3, 2017.
- 23.US Department of Agriculture. Rural-urban continuum codes. Available at: http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx (accessed August 1, 2016)
- 24.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 25.Sohn MW, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hijmans RJ. Geosphere: spherical trigonometry. R package version 1.5-1. Available at: http://cran.r-project.org/package=geosphere (accessed August 1, 2016)
- 27.Prince MJ, Wu F, Guo Y, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- 28.Seror O, Nault JC, Nahon P, et al. Is segmental trans-arterial yttrium 90 radiation a curative option for solitary hepatocellular carcinoma </= 5 cm? Hepatology. 2015;61:406–407. doi: 10.1002/hep.27174. [DOI] [PubMed] [Google Scholar]
- 29.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 30.Devaki P, Wong RJ, Marupakula V, et al. Approximately one-half of patients with early-stage hepatocellular carcinoma meeting Milan criteria did not receive local tumor destructive or curative surgery in the post-MELD exception era. Cancer. 2014;120:1725–1732. doi: 10.1002/cncr.28639. [DOI] [PubMed] [Google Scholar]
- 31.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 32.Cho YK, Kim JK, Kim MY, et al. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453–459. doi: 10.1002/hep.22648. [DOI] [PubMed] [Google Scholar]
- 33.Cabibbo G, Enea M, Attanasio M, et al. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 34.Bruix J, Sherman M. American Association for the Study of Liver Diseases Practice Guidelines. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 36.Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21:1287–1295. doi: 10.1245/s10434-013-3413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang TT, Sawhney R, Monto A, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford) 2008;10:405–411. doi: 10.1080/13651820802356572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau K, Salami A, Barden G, et al. The effect of a regional hepatopancreaticobiliary surgical program on clinical volume, quality of cancer care, and outcomes in the Veterans Affairs system. JAMA Surgery. 2014;149:1153–1161. doi: 10.1001/jamasurg.2014.1711. [DOI] [PubMed] [Google Scholar]
- 39.Sanoff HK, Chang Y, Stavas JM, et al. Effectiveness of initial transarterial chemoembolization for hepatocellular carcinoma among Medicare beneficiaries. J Natl Compr Canc Netw. 2015;13:1102–1110. doi: 10.6004/jnccn.2015.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Saghir NS, Keating NL, Carlson RW. Tumor boards: optimizing the structure and improving efficiency of multidisciplinary management of patients with cancer worldwide. Am Soc Clin Oncol Educ Book. 2014:e461–e466. doi: 10.14694/EdBook_AM.2014.34.e461. [DOI] [PubMed] [Google Scholar]
- 41.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khalaf N, Ying J, Mittal S, et al. Natural history of untreated hepatocellular carcinoma in a US cohort and the role of cancer surveillance. Clin Gastroenterol Hepatol. 2017;15:273–281 e1. doi: 10.1016/j.cgh.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 43.Giannini EG, Farinati F, Ciccarese F, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology. 2015;61:184–190. doi: 10.1002/hep.27443. [DOI] [PubMed] [Google Scholar]
- 44.Kanwal F, Volk M, Singal A, et al. Improving quality of health care for patients with cirrhosis. Gastroenterology. 2014;147:1204–1207. doi: 10.1053/j.gastro.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 45.Stevenson L, Ball S, Haverhals LM, et al. Evaluation of a national telemedicine initiative in the Veterans Health Administration factors associated with successful implementation. J Telemed Telecare. 2016 Nov 30;:pii. doi: 10.1177/1357633X16677676. 1357633X16677676 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Shea CM, Teal R, Haynes-Maslow L, et al. Assessing the feasibility of a virtual tumor board program: a case study. J Healthc Manag. 2014;59:177–193. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


