Abstract
Retrograde intramedullary nailing for tibiotalocalcaneal arthrodesis is a salvage procedure reserved for severe cases of deformity. The aim of the present study was to compare the outcomes of this technique in patients with and without diabetes mellitus (DM). A total of 61 patients with and 56 without DM underwent retrograde intramedullary nailing and had a minimum follow-up period of 12 months. The overall incidence of complication was 45.2%; however, the overall incidence of complications between those with and without DM was not significantly different (odds ratio [OR] 0.79, 95% confidence interval [CI] 0.38 to 1.65, p = .54). Patients with DM had a significantly greater rate of superficial infections (OR 8.3, 95% CI 1.01 to 68.67, p = .03). However, no difference was seen in the rate of deep infection (OR 0.90, 95% CI 0.34 to 2.46, p = .83) or noninfectious complications (OR 0.50, 95% CI 0.23 to 1.13, p = .09). Successful limb salvage was achieved for 96.8% of the patients with DM and 94.7% of those without DM (p = .66). A femoral head allograft was used in 32 (27.4%) of 117 patients to substitute for an osseous void. Of the 32 patients who required a femoral head allograft, 21 (67.7%) experienced a complication compared with 32 (37.6%) of 85 patients who did not require a femoral head allograft (OR 3.16, 95% CI 1.35 to 7.41, p = .008). The incidence of patient satisfaction was 80% for patients with DM and 72% for those without DM (p = .36). Despite a high incidence of complications, limb salvage was accomplished in approximately 95% of patients with complicated deformities. Four patients (6.56%) with DM experienced a tibia fracture; therefore, we now routinely use a 300-mm-long nail for this reconstruction.
Keywords: Charcot foot, complication, fusion, neuropathy, outcomes, surgery
Severe hindfoot and ankle deformity can be physically and mentally debilitating for patients. Various etiologies, such as arthritis of the subtalar and ankle joints (whether degenerative, inflammatory, or post-traumatic), Charcot neuroarthropathy (CN), DM neuropathy of other etiology, failed ankle and/or hindfoot arthrodesis, failed total ankle arthroplasty, acquired or congenital rigid equinovarus deformities, and avascular necrosis of the talus, can result in deformity and impairment that could potentially require tibiotalocalcaneal arthrodesis (TTCA). Various methods have been used to stabilize an ankle/hindfoot arthrodesis, including plates, screws, external fixation, and intramedullary nails. The contraindications specific to using the intramedullary nail (IMN) technique include active infection, severe angular deformity of the distal tibia, severe osteoporosis, and an intact subtalar joint (1). TTCA has been used as a method for limb salvage to avoid major amputation, in particular, in diabetic patients with lower extremity complications (2–9). This technique has gained popularity over the years, in part owing to the increased rigidity of the construct compared with that of other fixation techniques. A construct of greater rigidity allows maintenance of proper alignment after correction. Multiple studies have shown biomechanically superior bending and torsional properties with the IMN compared with lag screws (10–12). Successful limb salvage has been reported using retrograde intramedullary nailing; however, the procedure can be fraught with many complications, such as nonunion rates ranging from 0% to 71% (2,3,5,7–9,13,14). A preliminary study of 40 patients from our institution demonstrated significant improvement in the American Orthopaedic Foot and Ankle Society scores in patients with and without diabetes mellitus (DM), despite high complication rates in both groups (14). The purpose of the present study was to expand the findings of the previous study and evaluate the success of this technique as a limb salvage procedure for severe hindfoot and ankle deformity, comparing patients with and without DM. Another goal of our study was to report on the lessons learned as a result of our ongoing experience with TTCA.
Patients and Methods
The investigational review board at our medical center designated our study as an exempt study. We reviewed the foot and ankle surgical database of the senior author (D.K.W.), searching for patients who had undergone arthrodesis of the ankle and hindfoot. A comprehensive foot and ankle registry was created in our division in January 2005, and every surgical patient was prospectivelyentered at surgery. Demographic data and the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes (arthritis of the ankle and foot, codes 715.7 and 716.7; CN, code 713.5; and deformity of the ankle or foot, code 736.7) and Current Procedural Terminology codes (codes 27870 [ankle arthrodesis], 28705 [pantalar arthrodesis], 28715 [triple arthrodesis], and 28725 [subtalar arthrodesis]) were entered into the spreadsheet (15,16). Postoperative complications were recorded prospectively in the comprehensive foot and ankle registry by categorically denoting the absence (code 0) or presence (code 1) of specific complications. Patients who underwent major arthrodesis were identified from the comprehensive foot and ankle registry, and the electronic medical records and digital radiographs were reviewed. All the procedures and postoperative follow-up evaluations were performed by the senior surgeon (D.K.W.) from January 1, 2005 until June 30, 2013.
After identifying the patients from the registry, data were extracted from the review of the electronic medical records and digital radiographs by one of us (B.R.M.). The following 3 retrograde intramedullary nailing systems were used: T2 Ankle Arthrodesis Nail (Stryker, Mahwah, NJ), Trigen Hindfoot Fusion Nail (Smith and Nephew, Memphis, TN), Versa Nail (Depuy Synthes, Warsaw, IN). The T2 Ankle Arthrodesis Nail (Stryker) was used exclusively for the last 77 reconstructions because of our preference for a 5° valgus nail. The primary indications for surgery are listed in Table 1.
Table 1.
Indications for surgery (N = 117 patients)
| Indications for Surgery | Patients With DM (n = 61; 52.14) | Patients Without DM (n = 56; 47.86) |
|---|---|---|
| Charcot neuroarthropathy | 44 (72.13) | 7 (12.5) |
| Osteoarthritis of ankle and subtalar joints | 8 (13.12) | 10 (17.86) |
| Traumatic arthritis | 6 (9.84) | 12 (21.43) |
| Acquired equinovarus deformity | 0 | 9 (16.07) |
| Failed total ankle replacement | 1 (1.64) | 2 (3.57) |
| Revision arthrodesis | 2 (3.28) | 11 (19.64) |
| Avascular necrosis of the talus | 0 | 5 (8.93) |
Abbreviation: DM, diabetes mellitus.
Data presented as n (%).
Postoperatively, the patients were generally seen at 1, 3, 6, and 12 weeks and subsequently at 3-month intervals. After 1 year, the patients were either seen every 6 months or annually depending on outcome. The postoperative protocol was standardized for patients according to the presence or absence of neuropathy. Patients with neuropathy were placed in a short-leg non-weightbearing cast for 12 weeks. Patients without neuropathy were placed in a short-leg non-weightbearing cast for 6 weeks, followed by a short-leg weightbearing cast or controlled ankle motion boot for an additional 6 weeks. All patients, regardless of the presence or absence of neuropathy, were eventually transitioned to removable walking boots until radiographic evidence of healing was verified. Those patients with DM were subsequently placed in a brace (molded ankle foot orthosis, double upright brace, or Charcot restraint orthotic walker) for ≥12 months postoperatively. The American Orthopaedic Foot and Ankle Society ankle hindfoot scores were also calculated at the final follow-up visit (17,18). During each postoperative visit, anteroposterior, lateral, and oblique radiographs were evaluated for osseous healing or manifestation of complications.
The present study included a consecutive series of 117 patients with a minimum follow-up duration of 52 (range 52 to 403) weeks. All the patients were >18 years old. During the study period,119 patients underwent surgery, but 2 did not have the requisite minimum follow-up period. One patient died of a myocardial infarction 10 weeks postoperatively, and 1 patient relocated 13 weeks after surgery. At their last follow-up evaluation, no complications were observed; however, they were excluded from the present study. The demographic data, including gender, age, body mass index, tobacco use, presence of DM neuropathy, presence of DM, presence of CN, length of surgery, length of follow-up, ambulatory status, need for ongoing bracing, postoperative infection, presence of previous ulceration, need for additional surgery, nonunion, and presence of DM arterial disease, were extracted from the electronic medical records. We also recorded the preoperative laboratory values, including fasting glucose, creatinine, and hemoglobin. Patients with DM or DM neuropathy also had the hemoglobin A1c measured within 1 month of surgery. Peripheral neuropathy was defined as a Michigan Neuropathy Screening Index score of ≥2.5 (19). Patients with abnormal findings on a pedal pulse examination underwent measurement of the ankle brachial index. Peripheral arterial disease was considered present if the ankle brachial index was <0.9 (20).
The analyzed outcomes included infectious and noninfectious complications. Superficial infections were defined as those treated with outpatient local wound care and oral antibiotics (20). Deep infections were defined as those that required hospital admission for treatment, intravenous antibiotics, and/or surgical debridement (20). Noninfectious complications included nonunion, the need for symptomatic hardware removal, and postoperative tibia fractures. Radiography was routinely used to assess for osseous union. If osseous union was not able to be assessed using radiography (owing to hardware considerations), computed tomography was performed to more accurately assess union. Patients who complained of pain at 6 months with intact hardware also underwent computed tomography. Union was defined as ≥50% osseous fusion of the ankle joint as seen on the radiographs or computed tomography scan. Additionally, nonunion was defined as failure to achieve osseous fusion by the 12-month follow-up evaluation or catastrophic hardware failure. An infected nonunion was considered an infectious complication rather than a noninfectious complication. An overall complication rate was determined by combining the infectious and noninfectious complications. Limb salvage was defined as preservation of the ankle joint, which equated to avoiding transtibial amputation.
Descriptive statistics are summarized as frequencies and percentages for categorical data or as the mean ± standard deviation or median and interquartile range for normally or non-normally distributed continuous data, as appropriate. Examination of normal distribution assumption for continuous data was determined using Q–Q plots and histograms. Pearson’s chi-square or Fisher’s exact test, as appropriate, was used to compare the frequency distribution of the categorical variables between the groups. A 2-sample t test or Wilcoxon Mann-Whitney test was performed to determine the differences between groups for the normally or non-normally distributed continuous data, respectively. Univariate logistic regression analysis was applied to assess the strength of the association between the predictor variable (DM) and the dichotomous outcome of interest (superficial infection, deep infection, infectious complication, noninfectious complication, and overall complication). The magnitude of associations between the potential predictor variables and outcome was quantified using the odds ratio (OR) and corresponding 95% confidence interval (CI). All tests were 2 sided, and the significance level was set to p = .05. All analyses were conducted using SAS, version 9.3, statistical software (SAS Institute, Cary, NC). Statistical analysis was performed by 2 of us (B.L.R., N.C.S.), both experienced in biostatistics.
Results
The mean follow-up duration was 159.8 ± 92.9 weeks for patients with DM and 155.1 ± 89.8 weeks for patients without DM. Our 2 groups of patients were similar with regard to age, gender, length of surgery, body mass index, tobacco use, previous surgery, previous foot ulcer, and rheumatoid arthritis (Table 2). Patients with DM had significantly greater serum glucose, creatinine, hemoglobin A1c levels and significantly lower hemoglobin levels than patients without DM. The patients with DM were also significantly more likely to have DM neuropathy, CN, and DM artery disease. Of the 61 diabetic patients, 9 (14.75%) had previously undergone solid organ transplantation versus none of our nondiabetic cohort (Table 2). The overall rate of complications (infectious and noninfectious) was not significantly different between the patients with and without DM (OR 0.79, 95% CI 0.38 to 1.65, p = .54). Patients with DM had an 8 times greater likelihood of superficial infection than patients without DM (OR 8.3, 95% CI 1.01 to 68.67, p = .03), but no significant differences were seen in the rate of deep infection or overall infection (Table 3). The incidence of noninfectious complications between the 2 groups was not significantly different (OR 0.50, 95% CI 0.23 to 1.13, p = .0877). Our 61 patients with DM experienced 15 noninfectious complications (24.6%), including 10 nonunions (16.39%), 4 tibia fractures (6.56%), and 1 hardware removal (1.64%) for a prominent screw. The control group of 56 patients without DM experienced 22 noninfectious complications (39.3%), including 14 nonunions (25%) and 8 symptomatic hardware removals (14.29%). More than 95% of the patients in both groups were ambulatory at the most recent follow-up examination (p = .60), although a trend was seen toward greater brace use in the patients with DM than in the control group (p = .0569). The incidence of limb salvage was also similar in the patients with (96.7%) and without (94.6%) DM (p = .6692). Of the patients with DM, 80% were satisfied with the outcome of surgery compared with 73% of the patients without DM (p = .3732). A femoral head allograft was used in 32 (27.4%) of 117 patients. Of the 32 patients who required a femoral head allograft to replace a deficient talus, 21 (65.6%) experienced an overall complication compared with 32 (37.6%) of the 85 patients who did not require a femoral head allograft (OR 3.16, 95% CI 1.35 to 7.41, p = .008). Of the 85 patients with DM neuropathy, 37 (43.5%) experienced a complication compared with 16 (50.0%) of the 32 patients without DM neuropathy (OR 0.77, 95% CI 0.34 to 1.74, p = .53).
Table 2.
Demographic and preoperative variables (N = 117 patients)
| Variable | DM (n = 61) | No DM (n = 56) | p Value |
|---|---|---|---|
| Age (y) | 59.4 ± 12.3 | 56.9 ± 12.8 | .2831 |
| BMI (kg/m2) | 32.7 ± 8.0 | 32.2 ± 6.01 | .7310 |
| Charcot neuroarthropathy | 44 (72.1) | 7 (12.5) | <.0001 |
| DM | |||
| Type 1 | 12 (19.7) | NA | NA |
| Type 2 | 49 (80.3) | ||
| Insulin use | 39 (66.9) | NA | NA |
| Diabetes duration (y) | 18.75 ± 13.2 | NA | NA |
| Male gender | 31 (50.8) | 25 (45.5) | .5637 |
| Peripheral neuropathy | 58 (95.1) | 27 (48.2) | <.0001 |
| Peripheral arterial disease | 12 (19.7) | 1 (1.8) | .0023 |
| History of foot ulcer | 24 (39.3) | 14 (25.0) | .0979 |
| Renal disease (creatinine >1.4 mg/dL) | 21 (34.4) | 2 (3.6) | <.0001 |
| Rheumatoid arthritis | 3 (4.9) | 4 (7.1) | .7083 |
| Tobacco use | .1174 | ||
| 0 (never used) | 35 (57.4) | 38 (67.9) | |
| 1 (current) | 17 (27.9) | 16 (28.6) | |
| 2 (former) | 9 (14.8) | 2 (3.6) | |
| History of solid organ transplantation | 9 (14.75) | 0 | .0030 |
| Previous surgery | .4504 | ||
| 0 | 26 (42.62) | 20 (35.71) | |
| 1 | 34 (55.74) | 36 (64.29) | |
| 3 | 1 (1.64) | 0 | |
| Serum creatinine (mg/dL) | 1.1 ± 0.5 | 0.9 ± 0.4 | .0008* |
| Fasting glucose (mg/dL) | 153.2 ± 71.5 | 95.8 ± 13.0 | <.0001 |
| HbA1c (%) | 6.9 ± 1.7 | 5.8 ± 0.3 | .0094* |
| Hemoglobin | 12.4 ± 1.9 | 13.4 ± 1.6 | .0067 |
| ASA class | <.0001 | ||
| 1 | 0 | 1 (1.82) | |
| 2 | 3 (5.00) | 23 (41.82) | |
| 3 | 55 (91.67) | 31 (56.36) | |
| 4 | 2 (3.33) | 0 | |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; DM, diabetes mellitus; HbA1c, hemoglobin A1c; NA, not applicable.
Data presented as mean ± standard deviation or n (%).
Wilcoxon Mann-Whitney test.
Table 3.
Results (N = 117 patients)
| Variable | DM (n = 61) | No DM (n = 56) | OR (95% CI), p Value |
|---|---|---|---|
| Follow-up duration (wk) | 159.8 ± 92.9 | 155.1 ± 89.8 | .7783 |
| Ambulatory | 60 (98.4) | 54 (96.4) | .6060 |
| Brace use | 54 (88.5) | 42 (75.0) | .0569 |
| Use of external fixation | 15 (24.6) | 13 (23.2) | .8617 |
| Superficial (mild) infection | 8 (13.1) | 1 (1.8) | 8.3 (1.01 to 68.67), .0335 |
| Deep (major) infection | 10 (16.4) | 10 (17.9) | 0.90 (0.34 to 2.46), .8336 |
| Additional surgery | 21 (34.4) | 24 (42.9) | .3491 |
| Additional surgeries (n, % of patients) | 21 (34.4) | 24 (42.9) | .3491 |
| Nonunion | 10 (16.4) | 14 (25.0) | .2494 |
| Infectious complication (aggregate of minor and major infections) | 18 (29.5) | 12 (21.4) | 1.53 (0.66 to 3.67), .3174 |
| Noninfectious complication | 15 (24.6) | 22 (39.3) | 0.50 (0.23 to1.13), .0877 |
| Overall complications | 26 (42.6) | 27 (48.2) | 0.79 (0.38 to 1.65), .5439 |
| Below-the-knee amputation | 2 (3.3) | 3 (5.4) | .6692 |
| Length of surgery (min) | 173.5 ± 36.6 | 176.9 ± 39.9 | .6330 |
| Use of femoral head allograft | 16 (26.2) | 16 (28.6) | .7765 |
| Patient satisfaction | 49 (80.3) | 41 (73.2) | .3616 |
| AOFAS ankle hindfoot scale score | 58.8 ± 14.0 | 53.2 ± 17.0 | .0798 |
Abbreviations: AOFAS, American Orthopaedic Foot and Ankle Society; CI, confidence interval; DM, diabetes mellitus; OR, odds ratio.
Data presented as mean ± standard deviation or n (%).
Discussion
Using a retrograde ankle arthrodesis nail for TTCA, our overall rate of limb salvage in a complicated cohort of patients was 95%. The complexity of our diabetic cohort is illustrated by the high prevalence of DM neuropathy (95%), CN (72%), foot ulcers(40%), and DM artery disease (20%). Once a foot ulcer develops in a diabetic patient with CN, the risk of amputation increases by a factor of 12 (21). Of the control group of patients without DM, 50% had DM neuropathy and 12.5% had nondiabetic CN. Although CN involvement of the hindfoot and ankle is reported to be less common than in the midfoot, it is often more challenging to manage owing to the altered biomechanics associated with the deformity (8). Nonsurgical management remains the initial and mainstay treatment of CN, although hindfoot and ankle deformities can be difficult to brace owing to prominent malleoli (22). The primary goal of nonsurgical treatment with bracing and offloading is to maintain an ulcer-free, stable, plantigrade foot for ambulation. Progression of the deformity can result in severe ankle/hindfoot malalignment, resulting ininstability and an inability to brace the deformity. Prominent malleoli, secondary to varus, valgus, or talus collapse, can lead to ulceration, infection, and possible amputation. For certain patients, this progression seems to be unavoidable, and surgical reconstruction becomes warranted.
Successful outcomes have been well documented after TTCA with internal fixation in patients with CN or other hindfoot deformities, although the complications rates have been high (2,3). A multicenter study of 38 patients from 7 participating centers across North America and Europe reported that nonunion after previous ankle fusion was the most common indication for hindfoot and ankle nail implantation (23). Of the 38 patients,19 (50%) were retired or unemployed at surgery, and 19 were initially employed, with 13 of these patients taking sick leave as a result of their deformity. Eventually,15 of the 19 patients were able to return to their working status after their successful operation (23).
Jehan et al (1) performed a systematic review of 613 patients and reported a union rate 87%, complication rate of 56%, and amputation rate of 1.5%. Our overall union rate of 79.4% is comparable to the outcomes in that systematic review and the published data we reviewed for the present study (2–5,7–9,13,23–37) (Table 4). The published data review presented in Table 4 identified 342 diabetic patients with CN who underwent TTCA. The union rate was 79%, and the overall complication rate was 40%, findings remarkably similar to those from our series (Table 4). We used the ankle joint as the index joint for union owing to the difficulty in assessing subtalar union after TTCA. We agree with Coughlin et al (38), who reported that radiographic evaluation of the subtalar joint was not a reliable method for determining union. Despite our high complication rate of 45.3%, our limb salvage rate was 95% and patient satisfaction was reasonably high. One challenge that exists in comparing complication rates from study to study is the lack of consensus on the definition of a complication. Some studies have reported perioperative complications and postoperative complications, and others have reported only major complications that might necessitate additional surgery.
Table 4.
Systematic review of published data
| First Author | No. of Patients | Union (%) | Infection (%) | DM/CN Patients (n) | DM/CN Union (%) | DM/CN Infection (%) | Overall Complication (%) | Limb Salvage (%) |
|---|---|---|---|---|---|---|---|---|
| Kile (26), 1994 | 30 | 93 | 6.6 | 1 | NA | NA | NA | 93 |
| Moore (27), 1995 | 19 | 74 | 5 | 7 | 71 | NA | 11 | 100 |
| Pinzur (8), 1997 | 21 | 90.5 | 28.5 | 21 | 90.5 | 28.5 | 52 | 95 |
| Thordarson (28), 1999 | 12 | 100 | 0 | 1 | NA | NA | 58 | 100 |
| Stone (13), 2000 | 7 | 28.5 | 14 | 7 | 28.5 | 14 | NA | 86 |
| Chou (29), 2000 | 37 | 86 | 11 | 2 | NA | NA | 32 | 100 |
| Quill (30), 2003 | 82 | 97 | NA | 18 | NA | NA | NA | 100 |
| Mendicino (5), 2004 | 20 | 95 | 20 | 10 | 90 | 20 | 70 | 100 |
| Hammett (31), 2005 | 52 | 90 | 3.8 | 2 | NA | NA | 59 | 96 |
| Pinzur (9), 2005 | 9 | 100 | 11 | 9 | 100 | 20 | 22 | 100 |
| Caravaggi (2), 2006 | 14 | 71 | 29 | 14 | 71 | 29 | 29 | 93 |
| Pelton (7), 2006 | 33 | 88 | 3 | 10 | 80 | NA | 18 | 100 |
| Hockenbury (4), 2007 | 10 | 90 | 20 | 10 | 90 | 20 | 100 | 100 |
| Dalla Paola (3), 2007 | 18 | 78 | 0 | 18 | 78 | 0 | 17 | 100 |
| Niinimaki (32), 2007 | 34 | 76 | 12 | 3 | NA | 33 | 15 | 100 |
| Boer (33), 2007 | 50 | 100 | NA | 2 | 100 | NA | NA | 100 |
| Muckley (34), 2011 | 55 | 96 | 9 | NA | NA | NA | 25 | 100 |
| Caravaggi (35), 2012 | 45 | 87 | 31 | 45 | 87 | 31 | 53 | 87 |
| DeVries (36), 2012 | 154 | 94 | 28 | 67 | NA | NA | NA | 88 |
| DeVries (24), 2013 | 179 | NA | NA | 78 | NA | NA | NA | 88 |
| Rammelt (23), 2013 | 38 | 84 | 5 | 10 | NA | NA | 24 | 100 |
| Gross (37), 2014 | 30 | 86 | 10 | 7 | 57 | NA | 56 | 97 |
| Brodsky (25), 2014 | 30 | 97 | 10 | NA | NA | NA | 33 | 100 |
| Average (mean) | Total 979 | 86 | 13 | Total 342 | 79 | 22 | 40 | 97 |
Abbreviations: CN, Charcot neuroarthropathy; DM, diabetes mellitus; NA, not applicable.
A recent study by Bussewitz et al (39) concluded that in cases of complex pathologic entities with severe bone loss, a femoral head allograft was a suitable substitute for reconstructing the osseous void. They reported that the use of a bulk femoral head allograft could complicate healing, although the fusion rate was 84% (39). DeVries et al (24) reported that bone morphogenic protein-2 was associated with a nonsignificant enhanced limb salvage rate without an increased risk of complications. Another retrospective, comparative study by DeVries et al (40) evaluated the outcomes of IMN arthrodesis with or without external fixation augmentation. Major amputation was required in 22% of the IMN nail group and 29% of the IMN plus external fixation group, for an overall limb salvage rate 76% (40).
A large series of 179 reconstructions reported a limb salvage rate of 88%, identifying DM as the most notable risk factor associated with major amputation. Other factors associated with amputation were the need for revision surgery, older age, and preoperative ulceration (41). Our limb salvage rate of 95% is similar to that reported in the published data included in our review (Table 4). Of the 5 patients in our series who subsequently required below-the-knee amputation, 2 did so because of symptomatic nonunion with hardware failure and chronic pain and 3 because of persistent deep infection. All 5 of the patients who underwent amputation had DM neuropathy. However, the rate of complications was not significantly different when comparing patients with and without DM neuropathy.
Symptomatic stress reaction of the tibia is a known complication of retrograde IMN fixation, with rates as high as 10% in published studies (25). Noonan et al (42) reported on the biomechanical analysis of nail length and its effects on stress at the proximal tip of the nail. They attributed tibial stress reactions/fractures to the difference in the modulus of elasticity between the bone implant interface and the loss of ankle and subtalar joint motion (42). Four diabetic patients in our series (3.4%) experienced a tibia fracture at or above the proximal tip of the nail (Figs. 1 to 4). All of these fractures occurred in patients in whom we had used the shortest nail (150 mm), and all were successfully reconstructed by revising the 150-mm nail to the 300-mm nail (Figs. 1 to 4). We now routinely use a 300-mm nail for our TTCA procedures. This longer nail allows for stabilization of the nail in the isthmus of the tibial diaphysis, preventing valgus or varus toggling. To use the longer nail, free hand proximal cross-locking is necessary and surgeons must be comfortable with this technique.
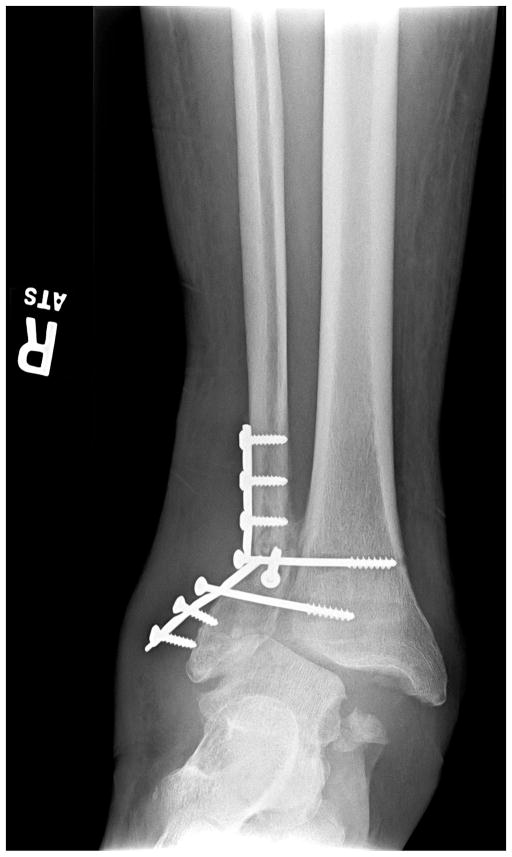
Fig. 1.
Anteroposterior radiograph of a 64-year-old male with type 1 diabetes for 40 years complicated by end-stage renal disease (requiring hemodialysis) and DM neuropathy. He had undergone open reduction internal fixation of an ankle fracture with subsequent hardware failure. A severe valgus deformity is present, although the skin is intact.
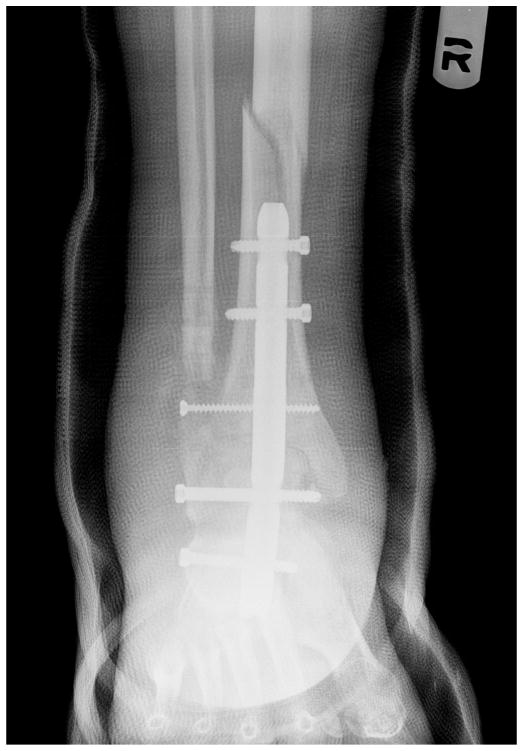
Fig. 4.
The tibia fracture was treated by revision surgery using a 300-mm retrograde nail. Subsequently, the tibia fracture healed, and the patient received a cadaveric renal transplant.
Three patients in our series presented with deep infections after 6 months, and ongoing vigilance must be used in this high-risk group of patients who undergo retrograde intramedullary nailing. Deep infections are considered surgical site infections if they present within 1 year of surgery in patients with implants. None of the 3 patients experienced any wound problems during the perioperative course. Another lesson learned was that fixation in the talus is important and maintenance of height is critical for patients undergoing TTCA (43). In patients with an avascular or deficient talus, we have used a femoral head allograft with good success. In rare cases, the distal tibia could be compromised, and a femoral head allograft can be used to reconstruct bone loss in the tibia (ie, after a complex tibial pilon fracture with nonunion). Approximately 25% of the patients in the present series required a femoral head allograft for bone loss, and use of a femoral head allograft was associated with a 3 times increased risk of a complication. At this point, we cannot state definitively whether this increased rate of complications resulted from the femoral head allograft or that the patients who required an allograft had more significant deformity.
The present study had weaknesses that need to be acknowledged. The most obvious weakness of our study was the retrospective design. Even well-conducted retrospective studies will be subject to a large number of biases. The selection of a control group itself can introduce bias, and we attempted to minimize this by including all patients without DM as the control group rather than attempting to match them. Retrospective studies also rely on the accuracy of the medical records, and the data obtained for analysis will only be as good as the documentation in the medical record. We have attempted to minimize the measurement bias between the study and control groups by remaining consistent in our treatment. All patients received the same antibiotic prophylaxis according to the Surgical Care Improvement Project protocol. Our postoperative follow-up visits generally occurred at 1, 3, 6 and 12 weeks, give or take a few days. A trend was seen toward prolonged brace use for patients with DM, and this most likely resulted from the senior author’s (D.K.W.) concern for potential neuropathic failure in this high-risk group. We have attempted to minimize nonresponder bias, because we have not lost any patient to follow-up during the study period, with the exception of the 2 patients previously mentioned (and excluded). Nonetheless, our study was subject to this type of bias because some of the patients were followed up longer than others, and additional complications might have been detected with longer follow-up periods. Our outcome measures, the presence or absence of a postoperative complication, was assessed and treated consistently in each patient by the same attending physician. Therefore, interviewer bias was potentially present, because the senior author (D.K.W.) determined the outcomes. Many different risk factors play a role in postoperative complications, particularly for diabetic patients (ie, age, gender, neuropathy, and vascular disease). We attempted to address this using the proper statistical methods. The potential for selection bias existed because of the lessons learned by the senior author (D.K.W.) during the past decade. Patients with poorly controlled DM have an increased risk of postoperative infection (20). Consequently, we now delay elective surgery until the hemoglobin A1c level is ≤8% and active tobacco use has stopped. Although patient satisfaction was relatively high in the present study, some patients with successful limb salvage were not satisfied with the outcome. In patients with DM, successful limb salvage was achieved in 97% of the patients, but only 80% were satisfied (Table 3). Similarly, 95% of nondiabetic patients had successful limb salvage, although the patient satisfaction rate was only 72% (Table 3). We do not have a good explanation for this disparity. The mean American Orthopaedic Foot and Ankle Society ankle hindfoot scores in both groups was <60, implying some element of limited function. Patients without DM neuropathy might also experience greater rates of postreconstruction pain, possibly accounting for the lower satisfaction rate in this group.
Despite a high complication rate, this single-surgeon series with a mean follow-up of >2.5 years has demonstrated that diabetic patients with limb-threatening problems have a high likelihood of successful limb salvage with TTCA using a retrograde IMN. With the exception of an increased rate of superficial infections, patients with DM had outcomes similar to those patients without DM. The use of a femoral head allograft, although often necessary, increases the risk of complications by a factor of 3. From our experience, we now routinely use retrograde nails that are 300 mm long.
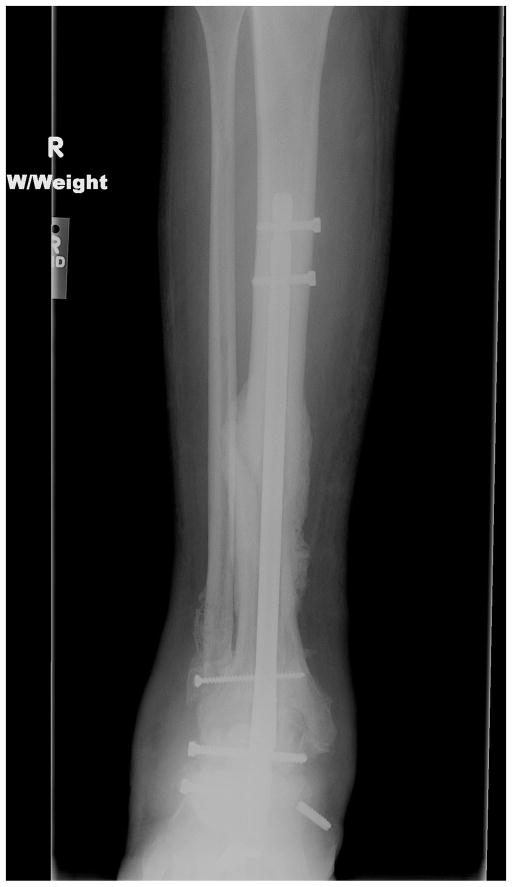
Fig. 2.
The patient was treated with hardware removal and tibiotalocalcaneal fusion using a 150-mm retrograde nail. A lateral transfibular approach was performed.
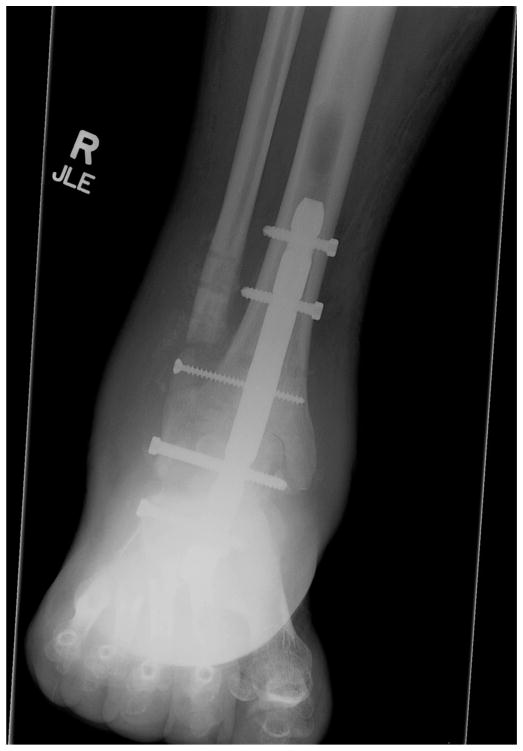
Fig. 3.
At 6 weeks after surgery, the patient fell and fractured his tibia, despite being in a short leg cast.
Footnotes
Level of Clinical Evidence: 3
Conflict of Interest: Dane K. Wukich receives royalties from Arthrex and serves as a consultant for Stryker.
Financial Disclosure: The Clinical and Translational Science Institute, University of Pittsburgh, provided statistical assistance for our report, and was supported by the National Institutes of Health (grant UL1-TR-000005).
References
- 1.Jehan S, Shakeel M, Bing AJ, Hill SO. The success of tibiotalocalcaneal arthrodesis with intramedullary nailing—a systematic review of the literature. Acta Orthop Belg. 2011;77:644–651. [PubMed] [Google Scholar]
- 2.Caravaggi C, Cimmino M, Caruso S, Dalla Noce S. Intramedullary compressive nail fixation for the treatment of severe Charcot deformity of the ankle and rear foot. J Foot Ankle Surg. 2006;45:20–24. doi: 10.1053/j.jfas.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Dalla Paola L, Volpe A, Varotto D, Postorino A, Brocco E, Senesi A, Merico M, De Vido D, Da Ros R, Assaloni R. Use of a retrograde nail for ankle arthrodesis in Charcot neuroarthropathy: a limb salvage procedure. Foot Ankle Int. 2007;28:967–970. doi: 10.3113/FAI.2007.0967. [DOI] [PubMed] [Google Scholar]
- 4.Hockenbury RT, Gruttadauria M, McKinney I. Use of implantable bone growth stimulation in Charcot ankle arthrodesis. Foot Ankle Int. 2007;28:971–976. doi: 10.3113/FAI.2007.0971. [DOI] [PubMed] [Google Scholar]
- 5.Mendicino RW, Catanzariti AR, Saltrick KR, Dombek MF, Tullis BL, Statler TK, Johnson BM. Tibiotalocalcaneal arthrodesis with retrograde intramedullary nailing. J Foot Ankle Surg. 2004;43:82–86. doi: 10.1053/j.jfas.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Papa J, Myerson M, Girard P. Salvage, with arthrodesis, in intractable diabetic neuropathic arthropathy of the foot and ankle. J Bone Joint Surg Am. 1993;75:1056–1066. doi: 10.2106/00004623-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Pelton K, Hofer JK, Thordarson DB. Tibiotalocalcaneal arthrodesis using a dynamically locked retrograde intramedullary nail. Foot Ankle Int. 2006;27:759–763. doi: 10.1177/107110070602701001. [DOI] [PubMed] [Google Scholar]
- 8.Pinzur MS, Kelikian A. Charcot ankle fusion with a retrograde locked intramedullary nail. Foot Ankle Int. 1997;18:699–704. doi: 10.1177/107110079701801104. [DOI] [PubMed] [Google Scholar]
- 9.Pinzur MS, Noonan T. Ankle arthrodesis with a retrograde femoral nail for Charcot ankle arthropathy. Foot Ankle Int. 2005;26:545–549. doi: 10.1177/107110070502600709. [DOI] [PubMed] [Google Scholar]
- 10.Berend ME, Glisson RR, Nunley JA. A biomechanical comparison of intramedullary nail and crossed lag screw fixation for tibiotalocalcaneal arthrodesis. Foot Ankle Int. 1997;18:639–643. doi: 10.1177/107110079701801007. [DOI] [PubMed] [Google Scholar]
- 11.Fleming SS, Moore TJ, Hutton WC. Biomechanical analysis of hindfoot fixation using an intramedullary rod. J Southern Orthop Assoc. 1998;7:19–26. [PubMed] [Google Scholar]
- 12.Millett PJ, O’Malley MJ, Tolo ET, Gallina J, Fealy S, Helfet DL. Tibiotalocalcaneal fusion with a retrograde intramedullary nail: clinical and functional outcomes. Am J Orthop. 2002;31:531–536. [PubMed] [Google Scholar]
- 13.Stone NC, Daniels TR. Midfoot and hindfoot arthrodeses in diabetic Charcot arthropathy. Can J Surg. 2000;43:449–455. [PMC free article] [PubMed] [Google Scholar]
- 14.Wukich DK, Shen JY, Ramirez CP, Irrgang JJ. Retrograde ankle arthrodesis using an intramedullary nail: a comparison of patients with and without diabetes mellitus. J Foot Ankle Surg. 2011;50:299–306. doi: 10.1053/j.jfas.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Current Procedural Terminology. American Medical Association; Chicago: 2014. [Google Scholar]
- 16.International Classification of Diseases. 9. World Health Organization; Geneva, Switzerland: 2011. [Google Scholar]
- 17.Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15:349–353. doi: 10.1177/107110079401500701. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim T, Beiri A, Azzabi M, Best AJ, Taylor GJ, Menon DK. Reliability and validity of the subjective component of the American Orthopaedic Foot and Ankle Society clinical rating scales. J Foot Ankle Surg. 2007;46:65–74. doi: 10.1053/j.jfas.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Suder NC, Wukich DK. Prevalence of diabetic neuropathy in patients undergoing foot and ankle surgery. Foot Ankle Spec. 2012;5:97–101. doi: 10.1177/1938640011434502. [DOI] [PubMed] [Google Scholar]
- 20.Wukich DK, Crim BE, Frykberg RG, Rosario BL. Neuropathy and poorly controlled diabetes increase the rate of surgical site infection after foot and ankle surgery. J Bone Joint Surg Am. 2014;96:832–839. doi: 10.2106/JBJS.L.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sohn MW, Stuck RM, Pinzur M, Lee TA, Budiman-Mak E. Lower-extremity amputation risk after Charcot arthropathy and diabetic foot ulcer. Diabetes Care. 2010;33:98–100. doi: 10.2337/dc09-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers LC, Frykberg RG, Armstrong DG, Boulton AJ, Edmonds M, Van GH, Hartemann A, Game F, Jeffcoate W, Jirkovska A, Jude E, Morbach S, Morrison WB, Pinzur M, Pitocco D, Sanders L, Wukich DK, Uccioli L. The Charcot foot in diabetes. Diabetes Care. 2011;34:2123–2129. doi: 10.2337/dc11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rammelt S, Pyrc J, Agren PH, Hartsock LA, Cronier P, Friscia DA, Hansen ST, Schaser K, Ljungqvist J, Sands AK. Tibiotalocalcaneal fusion using the hindfoot arthrodesis nail: a multicenter study. Foot Ankle Int. 2013;34:1245–1255. doi: 10.1177/1071100713487526. [DOI] [PubMed] [Google Scholar]
- 24.DeVries JG, Nguyen M, Berlet GC, Hyer CF. The effect of recombinant bone morphogenetic protein-2 in revision tibiotalocalcaneal arthrodesis: utilization of the retrograde arthrodesis intramedullary nail database. J Foot Ankle Surg. 2012;51:426–432. doi: 10.1053/j.jfas.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Brodsky JW, Verschae G, Tenenbaum S. Surgical correction of severe deformity of the ankle and hindfoot by arthrodesis using a compressing retrograde intramedullary nail. Foot Ankle Int. 2014;35:360–367. doi: 10.1177/1071100714523270. [DOI] [PubMed] [Google Scholar]
- 26.Kile TA, Donnelly RE, Gehrke JC, Werner ME, Johnson KA. Tibiotalocalcaneal arthrodesis with an intramedullary device. Foot Ankle Int. 1994;15:669–673. doi: 10.1177/107110079401501208. [DOI] [PubMed] [Google Scholar]
- 27.Moore TJ, Prince R, Pochatko D, Smith JW, Fleming S. Retrograde intramedullary nailing for ankle arthrodesis. Foot Ankle Int. 1995;16:433–436. doi: 10.1177/107110079501600710. [DOI] [PubMed] [Google Scholar]
- 28.Thordarson DB, Chang D. Stress fractures and tibial cortical hypertrophy after tibiotalocalcaneal arthrodesis with an intramedullary nail. Foot Ankle Int. 1999;20:497–500. doi: 10.1177/107110079902000806. [DOI] [PubMed] [Google Scholar]
- 29.Chou LB, Mann RA, Yaszay B. Tibiotalocalcaneal arthrodesis. Foot Ankle Int. 2000;21:804–808. doi: 10.1177/107110070002101002. [DOI] [PubMed] [Google Scholar]
- 30.Quill G. Tibiotalocalcaneal arthrodesis with medullary rod fixation. Tech Foot Ankle Surg. 2003;2:135–143. [Google Scholar]
- 31.Hammett R, Hepple S, Forster B, Winson I. Tibiotalocalcaneal (hindfoot) arthrodesis by retrograde intramedullary nailing using a curved locking nail: the results of 52 procedures. Foot Ankle Int. 2005;26:810–815. doi: 10.1177/107110070502601004. [DOI] [PubMed] [Google Scholar]
- 32.Niinimaki TT, Klemola TM, Leppilahti JI. Tibiotalocalcaneal arthrodesis with a compressive retrograde intramedullary nail: a report of 34 consecutive patients. Foot Ankle Int. 2007;28:431–434. doi: 10.3113/FAI.2007.0431. [DOI] [PubMed] [Google Scholar]
- 33.Boer R, Mader K, Pennig D, Verheyen CC. Tibiotalocalcaneal arthrodesis using a reamed retrograde locking nail. Clin Orthop Relat Res. 2007;463:151–156. [PubMed] [Google Scholar]
- 34.Muckley T, Klos K, Drechsel T, Beimel C, Gras F, Hofmann GO. Short-term outcome of retrograde tibiotalocalcaneal arthrodesis with a curved intramedullary nail. Foot Ankle Int. 2011;32:47–56. doi: 10.3113/FAI.2011.0047. [DOI] [PubMed] [Google Scholar]
- 35.Caravaggi CM, Sganzaroli AB, Galenda P, Balaudo M, Gherardi P, Simonetti D, Ferraresi R, Farnetti A, Morandi A. Long-term follow-up of tibiocalcaneal arthrodesis in diabetic patients with early chronic Charcot osteoarthropathy. J Foot Ankle Surg. 2012;51:408–411. doi: 10.1053/j.jfas.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 36.DeVries JG, Berlet GC, Hyer CF. Union rate of tibiotalocalcaneal nails with internal or external bone stimulation. Foot Ankle Int. 2012;33:969–978. doi: 10.3113/FAI.2012.0969. [DOI] [PubMed] [Google Scholar]
- 37.Gross JB, Belleville R, Nespola A, Poircuitte JM, Coudane H, Mainard D, Galois L. Influencing factors of functional result and bone union in tibiotalocalcaneal arthrodesis with intramedullary locking nail: a retrospective series of 30 cases. Eur J Orthop Surg Traumatol. 2014;24:627–633. doi: 10.1007/s00590-013-1347-2. [DOI] [PubMed] [Google Scholar]
- 38.Coughlin MJ, Grimes JS, Traughber PD, Jones CP. Comparison of radiographs and CT scans in the prospective evaluation of the fusion of hindfoot arthrodesis. Foot Ankle Int. 2006;27:780–787. doi: 10.1177/107110070602701004. [DOI] [PubMed] [Google Scholar]
- 39.Bussewitz B, DeVries JG, Dujela M, McAlister JE, Hyer CF, Berlet GC. Retrograde intramedullary nail with femoral head allograft for large deficit tibiotalocalcaneal arthrodesis. Foot Ankle Int. 2014;35:706–711. doi: 10.1177/1071100714531231. [DOI] [PubMed] [Google Scholar]
- 40.DeVries JG, Berlet GC, Hyer CF. A retrospective comparative analysis of Charcot ankle stabilization using an intramedullary rod with or without application of circular external fixator—utilization of the retrograde arthrodesis intramedullary nail database. J Foot Ankle Surg. 2012;51:420–425. doi: 10.1053/j.jfas.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 41.DeVries JG, Berlet GC, Hyer CF. Predictive risk assessment for major amputation after tibiotalocalcaneal arthrodesis. Foot Ankle Int. 2013;34:846–850. doi: 10.1177/1071100712472488. [DOI] [PubMed] [Google Scholar]
- 42.Noonan T, Pinzur M, Paxinos O, Havey R, Patwardhin A. Tibiotalocalcaneal arthrodesis with a retrograde intramedullary nail: a biomechanical analysis of the effect of nail length. Foot Ankle Int. 2005;26:304–308. doi: 10.1177/107110070502600406. [DOI] [PubMed] [Google Scholar]
- 43.Devries JG, Philbin TM, Hyer CF. Retrograde intramedullary nail arthrodesis for avascular necrosis of the talus. Foot Ankle Int. 2010;31:965–972. doi: 10.3113/FAI.2010.0965. [DOI] [PubMed] [Google Scholar]