Abstract
Asian and Pacific Islander (API) 30-day potentially preventable readmissions (PPRs) are understudied. Hawaii Health Information Corporation data from 2007–2012 statewide adult hospitalizations (N = 495,910) were used to compare API subgroup and White PPRs. Eight percent of hospitalizations were PPRs. Seventy-two percent of other Pacific Islanders, 60% of Native Hawaiians, and 52% of Whites with a PPR were 18 to 64 years, compared with 22% of Chinese and 21% of Japanese. In multivariable models including payer, hospital, discharge year, residence location, and comorbidity, PPR disparities existed for some API subpopulations 65+ years, including Native Hawaiian men (odds ratio [OR] = 1.14; 95% confidence interval [CI] = 1.04–1.24), Filipino men (OR = 1.19; 95% CI = 1.04–1.38), and other Pacific Islander men (OR = 1.30; 95% CI = 1.19–1.43) and women (OR = 1.23; 95% CI = 1.02–1.51) compared with Whites, while many API groups 18 to 64 years had significantly lower PPR odds. Distinct PPR characteristics across API subpopulations and age groups can inform policy and practice. Further research should determine why elderly API have higher PPR rates, while nonelderly rates are lower.
Keywords: readmissions, Asian, Native Hawaiian, Pacific Islander, inpatient, disparities
Potentially preventable hospital readmissions are common and expensive, accounting for almost 17% of Medicare hospital payments in the United States (Jencks, Williams, & Coleman, 2009; Robert Wood Johnson Foundation, 2013). These readmissions are thus the focus of recent major initiatives in the United States and internationally toward the dual goal of improving quality of care and reducing health care costs (Donzé, Lipsitz, Bates, & Schnipper, 2013; Jencks, 2010; Patient Protection and Affordable Care Act, 2010). Despite concern that these policies may disproportionately affect racial/ethnic minorities and the safety-net institutions that disproportionately serve racial/ethnic minorities, limited data exist regarding racial/ethnic differences in preventable readmissions (Hernandez & Curtis, 2011; Joynt, Orav, & Jha, 2011; Gilman et al., 2014). The lack of information about preventable readmissions by race/ethnicity, coupled with limited data about specific factors associated with disparities in readmissions by race/ethnicity, may lead to vulnerable racial/ethnic groups being less responsive to, or even excluded from, current quality improvement efforts (Hernandez & Curtis, 2011; Joynt et al., 2011). This could lead to a widening of racial/ethnic health gaps (Hernandez & Curtis, 2011) and is a particularly important issue to quantify given ongoing concern of inequitable burdens in readmission-related penalties for hospitals serving vulnerable communities (Gilman et al., 2014; Gu et al., 2014).
Asian and Pacific Islanders (API) include some of the fastest growing populations in the United States (U.S. Census Bureau, 2011), yet differences in preventable readmissions across heterogeneous API populations are extremely understudied. While a few recent studies considering race/ethnicity in avoidable readmissions have found significant racial/ethnic disparities, these studies either did not consider API subgroups (Allaudeen, Vidyarthi, Maselli, & Auerbach, 2011; Jencks et al., 2009; Joynt et al., 2011) or combined heterogeneous Asian and/or Pacific Islander subgroups into one group for analyses (Fleming, Gavin, Piatkowski, Chang, & Mukama, 2014; Vivo et al., 2014; Kroch, Duan, Martin, & Bankowitz, 2015). This analysis strategy can lead to misunderstanding of patterns and disparities (Ghosh, 2010). For instance, poor outcomes among some subgroups, such as Native Hawaiians or Filipinos, can be hidden by the strong health outcomes in other subgroups, such as the Japanese (Ghosh, 2010). Indeed, recent research combining all Asians into one race group found preventable readmission rates for acute heart failure (HF) to be similar for Asians compared with Whites (Fleming et al., 2014; Vivo et al., 2014). Previous scholarship suggests that this approach may conceal important differences.
This issue has important implications for the many ongoing efforts to reduce potentially preventable hospital readmissions. The Centers for Medicare and Medicaid Services (CMS) recently released guidance specifically for addressing readmissions in minority racial/ethnic groups (Betancourt, Tan-McGrory, & Kenst, 2015). Major recommendations include knowing specifically who is being readmitted and why; deploying a diverse, multidisciplinary team of caregivers to meet population needs; creating culturally appropriate systems of patient social support; and providing clear and relevant communication (Betancourt et al., 2015). Baseline quantitative information about which subgroups are readmitted at high rates and their descriptive characteristics are critical for meeting CMS recommendations, yet these data are currently missing for API subgroups. Even in the CMS report specifically on the topic of racial/ethnic differences in readmissions, none of the highlighted studies separated heterogeneous API groups (Betancourt et al., 2015). Yet only from such information can health care systems develop appropriate infrastructure to meet distinct cultural preferences, address unique communication needs, and meaningfully build on existing social support networks (Betancourt et al., 2015; Substance Abuse and Mental Health Services Administration [SAMHSA], 2006, chap. 4).
Additionally, recent literature reviews have identified successful intervention strategies for reducing readmissions (Hansen, Young, Hinami, Leung, & Williams, 2011; Kripalani, Theobald, Anctil, & Vasilevskis, 2014; Leppin et al., 2014). While some differences in outcomes by racial/ethnic groups have been evaluated, no studies considered the significant heterogeneity of API in intervention efficacy or relevance (Hansen et al., 2011; Kripalani et al., 2014; Leppin et al., 2014). Thus, the research gap for preventable readmissions among API subgroups not only precludes any urgency to address possible risk among distinct API populations (as rates, when combined, are typically the same as or better than those of Whites) but also limit recommendations for distinct API populations as well as API stakeholder inclusion in policy development and evaluation.
The study goal was to help address these research gaps, specifically to quantify differences in preventable readmissions across heterogeneous API racial/ethnic groups and to describe the API populations experiencing these preventable readmissions. API subgroups included Native Hawaiian, Japanese, Filipino, Chinese, and other Pacific Islanders. Our main study outcome was 30-day potentially preventable readmissions (PPRs). We also considered PPRs specifically for those with acute myocardial infarction (AMI), HF, and pneumonia as these were the initial focus of CMS Hospital Readmission Reduction Program (CMS, 2013) and we wanted to see if results from these specific readmission types would differ from PPRs generally. Our data were all adult, nonmaternity hospitalizations in Hawai‘i from 2007 to 2012. For maximum policy relevance, we specifically compared outcomes for those 18 to 64 years to those 65 years and older as most Americans have access to affordable, comprehensive health insurance through Medicare starting at age 65 years. Because Medicare plays a major role in PPR policy, and because studies have found distinct patterns of disparities for heterogeneous API groups on related outcomes for adults younger than 65 years compared with adults 65 years and older (Moy et al., 2013; Sentell et al., 2013; Sentell et al., 2015).
Hypotheses
We considered four study hypotheses. Our first was that API subgroups and Whites with PPRs would have distinct demographic and clinical characteristics that would be important for developing interventions focused on addressing PPR in these populations. Generally, we expected variation in demographic factors (especially age) and clinical factors (especially comorbidity), characteristics that might affect intervention design, payment, and stakeholder inclusion, but would be hidden if API subpopulations were only considered together. This hypothesis was based on several lines of evidence. While the overall population in the state of Hawai‘i has a high life expectancy, there is not an equitable pattern of health, longevity, and related social determinants of health within population subgroups (Andrade & McDermott, 2011). Overall, among Hawai‘i’s five major racial/ethnic groups (Native Hawaiian, Chinese, Japanese, Filipino, and White), Chinese and Japanese are the healthiest and longest lived, while Native Hawaiians typically report poorer health, have the shortest life expectancy, and the highest rates of poverty (Andrade & McDermott, 2011; Park, Braun, Horiuchi, Tottori, & Onaka, 2009). Filipinos experience some of the same health disparities as Native Hawaiians (e.g., high rates of preventable hospitalizations for heart disease), but not others (Park et al., 2009; Sentell et al., 2015). The health status and life expectancy of the White population in Hawai‘i tends to fall below that of the Chinese and Japanese, but above that of Native Hawaiians (Park et al., 2009). Other Pacific Islanders (e.g., Samoan, Micronesian, Tongan) are diverse and understudied, but evidence suggests that most, if not all, of these populations in Hawai‘i have poorer health and socioeconomic status compared with other racial/ethnic groups (Hagiwara, Miyamura, Yamata, & Sentell, 2016; Yuen, 2013).
In studies of nonmaternity hospitalizations in Hawai‘i, these differences manifest in distinct patterns among admitted patients. The more privileged groups, Japanese and Chinese, are, on average, hospitalized at older ages and are, thus, more likely to be covered by Medicare (Sentell et al., 2015). Interestingly, despite older ages at hospitalization, studies have found that Japanese and Chinese are hospitalized with fewer comorbidities than comparable White inpatients (Sentell et al., 2013; Sentell et al., 2015). While Native Hawaiians are hospitalized younger than Whites, they also have more comborbidities (Sentell et al., 2013; Sentell et al., 2015). Similar patterns of higher comborbidities and younger ages are seen for other Pacific Islanders (Hagiwara et al., 2016). While Hawai‘i has excellent population-level insurance coverage, Native Hawaiians and other Pacific Islanders, due to lower economic status, have higher rates of Medicaid than Whites (Sentell et al., 2013; Sentell et al., 2015).
Based on these lines of evidence, we predicted distinct demographic and clinical characteristics in PPRs with important implications for designing interventions. For instance, the most optimal clinical interventions and available funding to prevent a readmission in a 90-year old Japanese woman with Medicare and no comorbidities may be quite distinct from those to prevent a readmission in a 45-year-old Native Hawaiian man with multiple comorbidities and Medicaid.
Our second study hypothesis was that some API subgroups would be more likely to have a PPR compared with Whites even after accounting for the relevant demographic and access factors known to predict readmission that were available in the administrative data (Friedman & Basu, 2004). Specifically, we expected Native Hawaiians, Filipinos, and other Pacific Islanders to have higher rates of PPR than Whites when these were controlled. This is because Native Hawaiians, other Pacific Islanders, and Filipinos are more likely to have sociodemographic disadvantages compared with Whites (Andrade & McDermott, 2011), not all of which are readily measured or addressed in health care settings. This is relevant because such social factors are associated with readmissions in other research (Calvillo-King et al., 2013; Yam et al., 2010). The full range of an API patient’s social challenges may not always be considered in discharge planning (King et al., 2012); nor may the infrastructure of existing discharge programs always be sufficient to address the depth of identified social needs. Thus, key drivers of readmission may go undiscussed and/or unresolved (Bernheim & Ross, 2014; Enguidanos et al., 2015).
Our third study hypothesis concerned the role of comorbidities in explaining any API PPR disparities. Comorbidities are highly associated with readmissions (Ahmedani et al., 2015; Donzé et al., 2013; Zekry et al., 2012). Given the expected patterns of comorbidities noted above (i.e., higher comorbidities for Native Hawaiian and other Pacific Islanders compared with Whites despite younger ages, and lower comorbidities for Chinese and Japanese despite older ages), we considered specifically if comorbidity could explain any API disparities found in PPR rates. This hypothesis also followed from evidence of disparities for API populations in Agency for Healthcare Research and Quality (AHRQ)–defined potentially preventable hospitalizations for heart disease and diabetes compared with Whites even when comorbidity was considered (Sentell et al., 2013; Sentell et al., 2015). Our specific hypothesis was that comorbidity would be significant in multivariable models and reduce disparities, but that API PPR disparities would remain even when comorbidities were considered.
Our final study hypothesis was that we would see all these outcomes not only in an analysis of PPR generally but also in PPR specifically for AMI, HF, and pneumonia, diagnostic categories targeted by current CMS policy. We had no reason to assume that these three specific clinical areas would be distinct across factors mentioned above (e.g., age, comorbidities) for API subgroups so we felt the overall hypotheses would hold in these specific disease categories as well. This hypothesis was important to test as distinct patterns by PPR types could affect policy and intervention design.
New Contribution
Asian Americans and Pacific Islanders are understudied in health research generally and specifically around PPR. This study offers new, detailed information regarding potentially avoidable 30-day readmissions across API subgroups. Hawai‘i is home to approximately 25% of the U.S. Native Hawaiian or other Pacific Islander population and is more than 30% Asian (Hawai‘i Department of Health, 2008; U.S. Census Bureau, 2012a, 2012b). Thus, hospital data in Hawai‘i have unique detail about Asian, Native Hawaiian, and other Pacific Islander subgroups not captured in most population-based samples. This policy-relevant information will be useful both to other locations, such as California, where API populations are large, and also to locations, such as New York and Texas, where these populations are growing (U.S. Census Bureau, 2012a, 2012b). These research finding should be of interest to health services researchers, policy makers, managers, and/or practitioners both to understand health disparities among these growing populations in the United States and also to potentially provide insight into health disparities among potentially vulnerable communities. Study hypotheses are also relevant to the many ongoing projects focused on reducing PPR. Specifically, findings not just that groups vary, but how they vary specifically, can help focus existing interventions to be most relevant.
Method
Hawaii Health Information Corporation Data (HHIC) from 2007 to 2012 was used. HHIC provides Hawai‘i’s all-payer, all-visit, inpatient hospitalization data. HHIC collects patient-level inpatient discharge data including race/ethnicity, insurer, ICD-9 codes, age, and gender (HHIC, 2015) and includes a master patient identification variable that can track individuals across all hospitals in the state across years.
PPRs were identified using 3M’s PPR methodology, which allows hospitals to identify readmissions within 30 days of discharge that are considered potentially preventable, adjusting for critical factors (3M Health Information System Documentation Department, 2008). Quality improvement initiatives, pay for performance, and public report cards report 3M PPR rates (3M Health Information System Documentation Department, 2008). This method of PPR identification has been found to be particularly useful for quality improvement due to its inclusion of “preventability” (Mull et al., 2013). The 3M’s PPR method is extensively validated (3M Health Information System Documentation Department, 2008).
In the 3M methodology, PPRs are identified in readmission chains following an index admission. Certain hospitalizations cannot be identified as index admissions, such as a hospitalization ending in a transfer, one in which the patient died during the stay, or hospitalization for conditions, such as terminal cancer, for which readmissions are due to complex and/or extremely severe clinical factors and are unlikely to be avoidable given current technology. A hospitalization that is identified as a PPR cannot also be an index admission for a new chain, but can have another PPR following it in a chain.
From hospitalizations eligible to be index admission, the 3M PPR method creates “admission chains” of the number of PPRs that fall within 30 days of the relevant discharge and are clinically related to the index stay. In an example, for a readmission to be clinically related to an HF index admission, it would need to be for a diagnostic category defined by the 3M algorithm as both being clinically related to HF and considered potentially preventable (e.g., HF, major respiratory infection, cardiac arrest). For instance, a hospitalization for an appendectomy or a hip joint replacement during the 30-day period would not count as a PPR.
In the 3M terminology, an index admission with no PPR following is considered “Only Admission” and does not have a PPR chain. An index admission with at least one PPR is considered to have a PPR chain, which includes the index admission and the 1 or more preventable admissions following it. That PPR chain continues until the chain is broken, which can occur by a death, a non-PPR-relevant readmission, not having a PPR within 30-days of a discharge, a transfer, or a patient leaving against medical advice. Once a PPR chain is broken, an individual could start a new chain with a new index admission. In the HHIC data, which identify and track unique individuals across hospitals and years, PPR chains are measured across all hospitals and across the calendar year.
To simplify this complexity and allow us to focus most clearly on disparities in PPR, this study used a dichotomous measure of PPR based on index admission. The two choices for an eligible index admission were 0 = No PPR following that index admission (“Only Admission”) or 1 = At least 1 PPR following that index admission. We did not count the number of PPR in chains.
Sample
All non–pregnancy-related hospitalizations by any individual older than 18 years from December 2007–2012 were initially considered (N = 640,798). Each year contributed approximately equally to the study sample: 16.5% from 2007, 16.6% from 2008, 17.0% from 2009, 16.4% from 2010, 16.8% from 2011, and 16.7% from 2012. Hospitalizations at Tripler (the Department of Defense hospital; n = 51,205) were excluded as these hospitalizations did not report detailed API racial/ethnic data during the study period. After this exclusion, data from the remaining 24 hospitals in the state were included in the study sample, regardless of hospital size, as they all had eligible admissions. Hospitalizations were then excluded if they otherwise did not report race/ethnicity data (n = 11,647) or if they were not by a Hawai‘i resident because these individuals were unlikely to be followed in the Hawai‘i hospital data over time (n = 21,391). We also excluded hospitalizations not eligible to be considered “index admissions” for avoidable readmissions based on the 3M methods as described above (n = 60,644). One record with unknown gender was also excluded. After exclusions, the total number of eligible hospitalizations was 495,910 hospitalizations (77.4% of total hospitalizations) by 270,825 unique individuals.
From these hospitalizations, we used one index hospitalization per individual to ensure that multiple readmission chains by individuals in certain racial/ethnic group were not driving findings of disparities. To ensure we counted all individuals with a PPR, for any individual who had a PPR in the data, we took the first PPR index admission. For those without a PPR, we took the first index admission during the study period. Thus, all our analyses focus on the characteristics of unique individuals (n = 270,825) at their first eligible index admission. As described above, included index admissions were coded dichotomously 0 = No PPR follows, or 1 = At least 1 PPR follows.
PPR Types
We considered overall PPR and also for three conditions that were the initial focus for the CMS Hospital Readmission Reduction Program (CMS, 2013): acute myocardial infarction (AMI), heart failure (HF), and pneumonia. Based on previous research, we defined these conditions using ICD-9 codes from the index admission. The AMI sample was defined by ICD-9-CM: 410.xx excluding 410.x2 at index admission. The HF sample was defined by ICD-9-CM: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.xx at index admission. The Pneumonia sample was defined as ICD-9-CM codes 480.x, 481, 482.x as well as 4830, 4831, 4838, 485. xx, 486.xx, and 4870. The sample sizes for these subgroup analyses, using unique individuals at index admission, were: AMI = 17,793 index hospitalizations, HF = 26,089 index hospitalizations, and pneumonia = 24,103 index hospitalizations.
Race/Ethnicity
Racial/ethnic groups included were Chinese, Japanese, Filipino, Native Hawaiian, other Pacific Islander, Other race/ethnicity (e.g., African American), and White. The HHIC race/ethnicity variable was created from categories available consistently across all hospitals in Hawai‘i in the study period. Race/ethnicity data are typically provided by patient self-report at intake and include only one primary race. Mixed-race individuals are represented by self-report of their primary race of identification. Standardization of this variable across all Hawai‘i hospitals was confirmed prior to the study period (in 2006); high data quality was assured by HHIC using ongoing quality assurance, including clear specifications, data checks, and frequency reports (on monthly submissions) to identify discrepancies.
Age/Race/Gender Stratification
Previous work has found that racial/ethnic patterns of preventable hospitalizations among API groups vary by age group and gender (Moy et al., 2013; Sentell et al., 2013; Sentell et al., 2015). Thus, analyses were stratified by age group (18–64 years; 65+ years) and gender (female; male) to be more useful for clinical and policy considerations. Age group was assigned based on age at time of the included index hospitalization.
Control Variables
In the first multivariable model, we adjusted for payer (Medicare, Medicaid, Private, Department of Defense, Self-Pay, and Other/Miscellaneous), hospitalization admission type (AMI, HF, pneumonia, or other), and county of residence (Oahu, Hawai‘i Island, Kauai, and Maui) as access to care is often worse on the other islands, compared with the more urban Oahu. Our models also accounted for discharge year and clustering in the 24 hospitals represented in the study sample.
Comorbidity
Comorbidity was added in the final model and was measured with three variables: a comorbidity index, mental health diagnoses, and substance abuse diagnoses all chosen for previously identified relationships with readmissions (Ahmedani et al., 2015; Deswal, Petersen, Souchek, Ashton, & Wray, 2004; Donzé et al., 2013; Tang et al., 2014; Zekry et al., 2012). The comorbidity index was defined by the Elixhauser Index (Elixhauser, Steiner, Harris, & Coffey, 1998), a well-used measure of comorbidity developed with administrative data associated with readmissions in the elderly (Silverstein, Qin, Mercer, Fong, & Haydar, 2008) and shown to be valid in Asian populations (Chu, Ng, & Wu, 2010). Measurement of both substance abuse and mental health diagnoses followed previous research (Stranges, Levit, Stocks, & Santora, 2011). Specifically, having a mental health diagnosis was determined by ICD-9 295. xx-302.xx, 306.xx-314.xx, and 648.4x. Having a substance abuse diagnosis was determined by ICD-9 291.xx, 292.xx, 303.xx, 304.xx, 305.2x-305.9x, and 648.3x.
Statistical Analysis
Characteristics of patients with a PPR were summarized by descriptive statistics for each racial/ethnic subgroup based on their index admission and compared among subgroups using chi-square tests or Fisher’s exact tests (for categorical variables) and analysis of variance (ANOVA) or nonparametric Kruskal–Wallis tests, if the normality assumption was not satisfied (for continuous variables). As described above, we present the data once per unique individual so that the characteristics of individuals with multiple admissions do not misrepresent the underlying population responsible for these hospitalizations. (Thirty-eight percent of the study sample had more than one hospitalization during the study period.)
The first logistic regression model was then developed to estimate the likelihood of having a PPR by race/ethnicity adjusting for other factors using one visit per person. Specifically, we used generalized estimating equations logistic regression, which allowed us to address repeated measurements within hospitals. Multivariable adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were derived for each racial/ethnic group, age group, and gender combination compared with Whites of the same age group/gender combination. The control variables in the first model (to test Hypothesis 2) were payer and location of residence also considering year of discharge, hospitalization type, and hospital. The control variables in the second model (to test Hypothesis 3) additionally included all three comorbidity variables. To test Hypothesis 4, the same set of two models were run for each of the specific subsamples (AMI, HF, and pneumonia). All models included interactions for Age group * Ethnicity, Gender * Ethnicity, and Gender * Age group * Ethnicity to investigate comparison with Whites for combination of age group, gender, and ethnicity. All data analyses were performed in SAS 9.3 (Cary, NC, 2011). A two-tailed p value of less than .05 was regarded as statistically significant. The study was deemed exempt by the University of Hawai‘i Institutional Review Committee under Human Subjects Exemption 4.
Results
A total of 39,702 of the 495,910 hospitalizations (8.0%) were identified as PPR. These hospitalizations were by 25,338 individuals from the 270,825 individuals in the sample (9.2%). Table 1 shows the demographics of individuals studied (at their first eligible index visit). The largest studied racial/ethnic group was White (30.4%) followed by Japanese (18.6%), Native Hawaiian (15.9%), other race/ethnicity (13.4%), Filipino (12.9%), other Pacific Islander (4.5%), and Chinese (4.3%).
Table 1.
Sample Description From Hawaii Health Information Corporation Inpatient Data for All Individuals (at First Included Index Admission), January 2007 to November 2012 (n = 270,825).
| N | Percent | |
|---|---|---|
| Subsamples | ||
| Acute myocardial infarction sample | 17,793 | 6.6 |
| Heart failure sample | 26,089 | 9.6 |
| Pneumonia sample | 24,103 | 8.9 |
| Study sample characteristics | ||
| Potentially preventable readmissionsa | 25,338 | 9.4 |
| Race | ||
| Chinese | 11,636 | 4.3 |
| Filipino | 34,872 | 12.9 |
| Native Hawaiian | 43,122 | 15.9 |
| Japanese | 50,266 | 18.6 |
| Other Pacific Islander | 12,262 | 4.5 |
| Other raceb | 36,339 | 13.4 |
| White | 82,328 | 30.4 |
| Age group (years) | ||
| 18–64 | 182,945 | 67.6 |
| 65+ | 87,880 | 32.5 |
| Gender | ||
| Female | 170,107 | 62.8 |
| Male | 100,718 | 37.2 |
| Payer | ||
| Department of Defense | 5,740 | 2.1 |
| Medicaid/Quest | 49,438 | 18.3 |
| Medicare | 86,553 | 32.0 |
| Miscellaneousc | 3,978 | 1.5 |
| Self-pay | 8,855 | 3.3 |
| Private insurance | 116,261 | 42.9 |
| County | ||
| Hawai‘i | 41,276 | 15.2 |
| Kauai | 14,648 | 5.4 |
| Maui | 32,445 | 12.0 |
| Oahu | 182,456 | 67.4 |
| Substance use | ||
| No | 256,635 | 94.8 |
| Yes | 14,190 | 5.2 |
| Mental health | ||
| No | 243,165 | 89.8 |
| Yes | 27,660 | 10.2 |
| Age | 52.2 | 21.8 |
| Elixhauser Index | 1.71 | 1.79 |
Note. Individuals are only counted once in this table, regardless of the number of hospitalizations they had during the study period. Individuals who were hospitalized during the study period only for maternity-related conditions or for hospitalization types not eligible for potentially preventable readmission (PPR) analyses according to 3M metrics, and/or who were missing race/ethnicity data were excluded from this analysis.
Gives the number of individuals in the sample with at least one PPR.
“Other Pacific Islander” includes Samoans, Tongans, and Micronesian. “Other race” includes Hispanic, African American, and Puerto Rican.
Miscellaneous payer includes “No Fault” and Workers’ Compensation.
Hypothesis 1: Demographics and Clinical Characteristics
Table 2 shows demographic characteristics by race/ethnicity among those who have at least one PPR from their first index visit. The results from these analyses allow us to consider our first study hypothesis: API subgroups and Whites with PPRs will have distinct demographic and clinical characteristics. In terms of demographics, 71.9% of other Pacific Islanders and 60.3% of Native Hawaiians with a PPR were between 18 and 64 years, compared with 52.4% of Whites, 37.3% of Filipinos, 22.2% of Chinese, and 20.6% of Japanese (p < .0001). For clinical factors, the average comorbidity index scores as well as substance abuse and mental health diagnoses percentages varied significantly by race/ethnicity (p < .0001) as did both insurance type and location of residence (p < .0001).
Table 2.
Descriptive Results for Individuals With at Least One PPR From Hawaii Health Information Corporation Inpatient Data by Race/Ethnicity, January 2007 to November 2012 (n = 25,338).
| Chinese | Filipino | Native Hawaiian | Japanese | Other Pacific Islanders | Other race | White | Total | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Number of patients with at least one PPR | 1,160 | 3,157 | 4,000 | 5,480 | 1,127 | 2,617 | 7,779 | 25,338 | |
| Age group (years), n (%) | <.0001 | ||||||||
| 18–64 | 258 (22.2) | 1,176 (37.3) | 2,411 (60.3) | 1,129 (20.6) | 810 (71.9) | 1,473 (56.3) | 4,087 (52.4) | 11,344 (44.8) | |
| 65+ | 902 (77.8) | 1,981 (62.7) | 1,589 (39.7) | 4,351 (79.4) | 317 (28.1) | 1,144 (43.7) | 3,710 (47.6) | 13,994 (55.2) | |
| Gender, n (%) | <.0001 | ||||||||
| Female | 543 (46.8) | 1,547 (49.0) | 2,053 (51.3) | 2,720 (49.6) | 522 (46.3) | 1,284 (49.1) | 3,567 (45.7) | 12,236 (48.3) | |
| Male | 617 (53.2) | 1,610 (51.0) | 1,947 (48.7) | 2,760 (50.4) | 605 (53.7) | 1,333 (50.9) | 4,230 (54.3) | 13,102 (51.7) | |
| Payer, n (%) | <.0001 | ||||||||
| Department of Defense | 4 (0.3) | 23 (0.7) | 68 (1.7) | 33 (0.6) | 9 (0.8) | 83 (3.2) | 321 (4.1) | 541 (2.1) | <.0001 |
| Medicaid/Quest | 65 (5.6) | 283 (9.0) | 951 (23.8) | 181 (3.3) | 492 (43.7) | 602 (23.0) | 1,308 (16.8) | 3,882 (15.3) | <.0001 |
| Medicare | 909 (78.4) | 2,014 (63.8) | 1,933 (48.3) | 4,371 (79.8) | 358 (31.8) | 1,251 (47.8) | 3,982 (51.1) | 14,818 (58.5) | <.0001 |
| Miscellaneous | 4 (0.3) | 12 (0.4) | 25 (0.6) | 21 (0.4) | 11 (1.0) | 11 (0.4) | 76 (1.0) | 160 (0.6) | <.0001 |
| Self-pay | 16 (1.4) | 51 (1.6) | 57 (1.4) | 33 (0.6) | 66 (5.9) | 88 (3.4) | 232 (3.0) | 543 (2.1) | <.0001 |
| Private insurance | 162 (14.0) | 774 (24.5) | 966 (24.2) | 841 (15.3) | 191 (16.9) | 582 (22.2) | 1,878 (24.1) | 5,394 (21.3) | <.0001 |
| County, n (%) | <.0001 | ||||||||
| Hawai‘i | 34 (2.9) | 306 (9.7) | 785 (19.6) | 556 (10.1) | 59 (5.2) | 311 (11.9) | 1,562 (20.0) | 3,613 (14.3) | <.0001 |
| Kauai | 10 (0.9) | 192 (6.1) | 179 (4.5) | 243 (4.4) | 6 (0.5) | 97 (3.7) | 658 (8.4) | 1,385 (5.5) | <.0001 |
| Maui | 24 (2.1) | 361 (11.4) | 474 (11.9) | 318 (5.8) | 58 (5.1) | 195 (7.5) | 1,107 (14.2) | 2,537 (10.0) | <.0001 |
| Oahu | 1,092 (94.1) | 2,298 (72.8) | 2,562 (64.1) | 4,363 (79.6) | 1,004 (89.1) | 2,014 (77.0) | 4,470 (57.3) | 17,803 (70.3) | <.0001 |
| Substance use, n (%) | <.0001 | ||||||||
| No | 1,132 (97.6) | 3,030 (96.0) | 3,626 (90.7) | 5,316 (97.0) | 1,068 (94.8) | 2,356 (90.0) | 6,511 (83.5) | 23,039 (90.9) | |
| Yes | 28 (2.4) | 127 (4.0) | 374 (9.4) | 164 (3.0) | 59 (5.2) | 261 (10.0) | 1,286 (16.5) | 2,299 (9.1) | |
| Mental health, n (%) | <.0001 | ||||||||
| No | 1,013 (87.3) | 2,811 (89.0) | 3,315 (82.9) | 4,839 (88.3) | 1,032 (91.6) | 2,073 (79.2) | 5,582 (71.6) | 20,665 (81.6) | |
| Yes | 147 (12.7) | 346 (11.0) | 685 (17.1) | 641 (11.7) | 95 (8.4) | 544 (20.8) | 2,215 (28.4) | 4,673 (18.4) | |
| Age, M ± SD | 75.6 ± 16.2 | 67.1 ± 18.8 | 57.8 ± 18.2 | 75.6 ± 15.7 | 53.4 ± 17.5 | 59.3 ± 20.0 | 62.0 ± 19.0 | 64.9 ± 19.5 | <.0001 |
| Elixhauser Index, M ± SD | 3.53 ± 2.07 | 3.42 ± 2.02 | 3.61 ± 2.15 | 3.26 ± 1.86 | 3.51 ± 2.08 | 3.09 ± 2.02 | 2.99 ± 1.97 | 3.26 ± 2.01 | <.0001 |
Note. PPR = potentially preventable readmissions. These individuals are responsible for 39,702 PPRs. Individuals are only counted once in this table, regardless of the number of PPRs they had during the study period. Individuals who were hospitalized during the study period only for maternity-related conditions or for hospitalization types not eligible for PPR analyses according to 3M metrics, and/or who were missing race/ethnicity data were excluded from these analyses.
Hypothesis 2: Rates of Potentially Preventable Readmissions
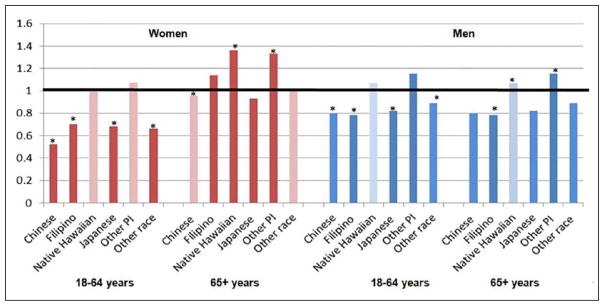
Our second study hypothesis was that some API subgroups would be more likely to have a PPR compared with Whites even after accounting for available demographic and access factors. Figure 1 shows the unadjusted OR for each race/ethnicity, gender, and age group combination compared with Whites. In this figure, disparities compared with Whites are only seen in some race/ethnicity/gender/age group combinations. The API groups with PPR disparities relative to Whites are Native Hawaiians and other Pacific Islanders.
Figure 1.
Unadjusted odds ratios (ORs) for potentially preventable hospitalization by Asian and Pacific Islander subgroups compared with Whites in bars (from index hospitalization; n = 270,825).
Note. An OR more than 1 indicates a higher rate of potentially preventable readmission compared with Whites and an OR below than 1 indicates a lower rate. Statistically significant differences (p < .05) are indicated by an asterisk. Statistically significant differences (p < .05) in unadjusted models are indicated with a darker color; significant results after multivariable adjustment (not including comorbidity) are indicated with an asterisk. In multivariable models adjusting for payer, county of residence, discharge year, admission type, hospital, and age group/gender/ethnicity interactions, but not the three comorbidity measures. Adjusted ORs for differences remaining significant were women 18 to 64 years: Chinese (OR = 0.55; 95% confidence interval [CI] = 0.40–0.76), Japanese (OR = 0.75; 95% CI = 0.65–0.89), other race (OR = 0.63; 95% CI = 0.53–0.77); women 65+ years: Chinese (OR = 0.90; 95% CI = 0.80–0.998), Native Hawaiian (OR = 1.26; 95% CI = 1.44–1.40), other Pacific Islander (OR = 1.31; 95% CI = 1.06–1.63); men 18 to 64 years: Chinese (OR = 0.79; 95% CI = 0.65–0.95), Filipino (OR = 0.74; 95% CI = 0.63–0.88), Japanese (OR = 0.83; 95% CI = 0.76–0.90), other race (OR = 0.84; 95% CI = 0.77–0.92), and men 65+ years: Filipino (OR = 1.21; 95% CI = 1.08–1.35), Native Hawaiian (OR = 1.27; 95% CI = 1.17–1.37]) and other Pacific Islander (OR = 1.45; 95% CI = 1.28–1.64).
The figure also shows significance from multivariable models considering age group, gender, payer, discharge year, hospitalization type, hospital, and county of residence, but not comorbidity, that tested our second study hypothesis. In multivariable models, significantly higher odds of a PPR compared with Whites remained for other Pacific Islander men and women 65+ years and were seen for both Native Hawaiian men and women 65+ years. Chinese women 65+ were significantly less likely to have a PPR than Whites as were Japanese, Chinese, and Filipino women and men 18 to 64 years. After adjustment, Filipino men 65+ years had significantly higher odds of PPR compared with Whites.
Hypothesis 3: Rates of Potentially Preventable Readmissions, Considering Comorbidity
In our final models (Table 3) accounting for payer, hospital, discharge year, residence location, admission type, and comorbidity, PPR disparities remained for some API subpopulations among those 65+ years, including Native Hawaiian men (OR = 1.14; 95% CI = 1.04–1.24), Filipino men (OR = 1.19; 95% CI = 1.04–1.38), and other Pacific Islander men (OR = 1.30; 95% CI = 1.19–1.43) and women (OR = 1.23; 95% CI = 1.02–1.51) compared with Whites. No other API group 65+ differed significantly from Whites.
Table 3.
Adjusted Odds Ratios (ORs) by Age Group/Gender for Potentially Preventable Hospitalization by Asian Pacific Islander Subgroup Compared With Whites for PPR Overall and for Acute Myocardial Infarction, Heart Failure, and Pneumonia Samples.
| Overall | Acute myocardial infarction | Heart failure | Pneumonia | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| N = 270,825 | n = 17,793 | n = 26,089 | n = 24,103 | |||||
|
|
|
|
|
|||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Female | ||||||||
| 18–65 years | ||||||||
| Chinese | 0.6195 | [0.4673, 0.8213] | 0.8409 | [0.2217, 3.1888] | 1.1295 | [0.7026, 1.8157] | 0.7447 | [0.385, 1.4406] |
| Filipino | 0.8021 | [0.71, 0.9062] | 0.7915 | [0.4471, 1.401] | 1.0298 | [0.7814, 1.3571] | 0.8012 | [0.6647, 0.9658] |
| Native Hawaiian | 0.9277 | [0.8476, 1.0153] | 0.8072 | [0.5487, 1.1875] | 0.9999 | [0.7628, 1.3106] | 0.8953 | [0.7297, 1.0984] |
| Japanese | 0.8056 | [0.7132, 0.9099] | 0.8302 | [0.468, 1.4726] | 0.8023 | [0.5251, 1.226] | 0.79 | [0.503, 1.2406] |
| Other PI | 0.8987 | [0.7945, 1.0165] | 0.8059 | [0.4935, 1.316] | 0.9851 | [0.6972, 1.3918] | 0.7208 | [0.4571, 1.1365] |
| Other race | 0.7206 | [0.6127, 0.8475] | 0.7254 | [0.4378, 1.2018] | 0.6952 | [0.5504, 0.8782] | 0.4925 | [0.3677, 0.6596] |
| 65+ years | ||||||||
| Chinese | 0.8926 | [0.8007, 0.995] | 0.691 | [0.5352, 0.892] | 1.0918 | [0.8864, 1.3449] | 1.1032 | [0.8552, 1.4232] |
| Filipino | 1.1487 | [0.9974, 1.3229] | 1.3295 | [1.0412, 1.6977] | 1.3608 | [1.15, 1.6102] | 0.9942 | [0.7927, 1.247] |
| Native Hawaiian | 1.1343 | [0.9965, 1.2913] | 1.0613 | [0.8319, 1.354] | 1.3044 | [1.1154, 1.5255] | 1.2121 | [0.941, 1.5612] |
| Japanese | 0.9948 | [0.8955, 1.105] | 1.0261 | [0.7661, 1.3743] | 0.9851 | [0.8262, 1.1744] | 0.9317 | [0.7449, 1.1652] |
| Other PI | 1.2376 | [1.0158, 1.508] | 1.0253 | [0.5442, 1.9315] | 1.2935 | [0.8903, 1.8794] | 1.5145 | [1.1353, 2.0205] |
| Other race | 0.9803 | [0.8363, 1.149] | 1.0362 | [0.8027, 1.3376] | 1.2702 | [1.023, 1.5771] | 1.3393 | [1.1108, 1.6147] |
| Male | ||||||||
| 18–65 years | ||||||||
| Chinese | 0.828 | [0.7064, 0.9707] | 0.7856 | [0.5008, 1.2325] | 1.1147 | [0.7601, 1.6349] | 0.7921 | [0.4272, 1.4687] |
| Filipino | 0.7551 | [0.6477, 0.8804] | 1.1961 | [0.8437, 1.6955] | 0.9628 | [0.7826, 1.1843] | 0.7857 | [0.6444, 0.9579] |
| Native Hawaiian | 0.8712 | [0.7989, 0.95] | 1.3563 | [1.0263, 1.7925] | 1.219 | [1.0347, 1.4361] | 0.9113 | [0.6654, 1.248] |
| Japanese | 0.8414 | [0.7709, 0.9183] | 0.9501 | [0.7264, 1.2426] | 0.9076 | [0.7663, 1.0748] | 0.7628 | [0.5525, 1.0533] |
| Other PI | 0.925 | [0.8732, 0.98] | 1.1098 | [0.9116, 1.351] | 1.2183 | [1.0151, 1.4622] | 0.9249 | [0.7052, 1.213] |
| Other race | 0.8588 | [0.7936, 0.9293] | 1.2998 | [0.9516, 1.7755] | 1.1473 | [0.9193, 1.4318] | 0.9388 | [0.7335, 1.2015] |
| 65+ years | ||||||||
| Chinese | 0.99 | [0.91, 1.0771] | 0.8716 | [0.4956, 1.5331] | 0.8825 | [0.7348, 1.0599] | 0.926 | [0.7698, 1.1139] |
| Filipino | 1.194 | [1.0352, 1.3773] | 1.0981 | [0.8733, 1.3808] | 1.1002 | [0.9502, 1.2739] | 1.0717 | [0.8605, 1.3347] |
| Native Hawaiian | 1.1368 | [1.0427, 1.2393] | 1.064 | [0.8276, 1.3678] | 1.1738 | [0.9596, 1.4357] | 0.9183 | [0.7697, 1.0956] |
| Japanese | 1.0896 | [0.9826, 1.2084] | 1.0141 | [0.8374, 1.2282] | 1.045 | [0.9295, 1.1748] | 0.8613 | [0.7408, 1.0014] |
| Other PI | 1.3032 | [1.1859, 1.432] | 1.1488 | [0.7773, 1.6977] | 1.0817 | [0.798, 1.4661] | 1.1088 | [0.8938, 1.3754] |
| Other race | 1.0594 | [0.9226, 1.2164] | 0.7328 | [0.534, 1.0056] | 1.1079 | [0.9373, 1.3096] | 0.9841 | [0.7824, 1.2379] |
Note. Model adjusts for comorbidity, substance use, mental health, payer, county of residence, discharge year, hospital, and age group/gender/ethnicity interactions (n = 270,825).
Individuals are only counted once in this table, regardless of the number of potentially preventable readmissions (PPRs) they had during the study period. Individuals who were hospitalized during the study period only for maternity-related conditions or for hospitalization types not eligible for PPR analyses according to 3M metrics, and/or who were missing race/ethnicity data were excluded from these analyses.
In those aged 18 to 64 years, Filipino men (OR = 0.76; 95% CI = 0.65–0.88) and women (OR = 0.80; 95% CI = 0.71–0.91), Native Hawaiian men (OR = 0.87; 95% CI = 0.80–0.95), Chinese men (OR = 0.83; 95% CI = 0.71–0.97) and women (OR = 0.89; 95% CI = 0.80–0.995), Japanese men (OR = 0.84; 95% CI = 0.77–0.92) and women (OR = 0.81; 95% CI = 0.71–0.91), and other Pacific Islander men (OR = 0.93; 95% CI = 0.87–0.98) were all significantly less likely to have a PPR than Whites once comorbidity and other factors were considered.
Other significant variables in the overall model can be seen in Appendix A. Those with a higher Elixhauser Index score, a substance abuse diagnosis, or a mental health diagnosis were significantly more likely to have a PPR compared with those who did not have such a diagnosis. Compared with those with private insurance, those with public insurance were significantly more likely to have a PPR. We also ran final models including an interaction between Elixhauser Index level and ethnicity (not shown). As this interaction was not significant (p = .09), the interaction was not included in our final model.
Hypothesis 4: AMI, HF, and Pneumonia Findings
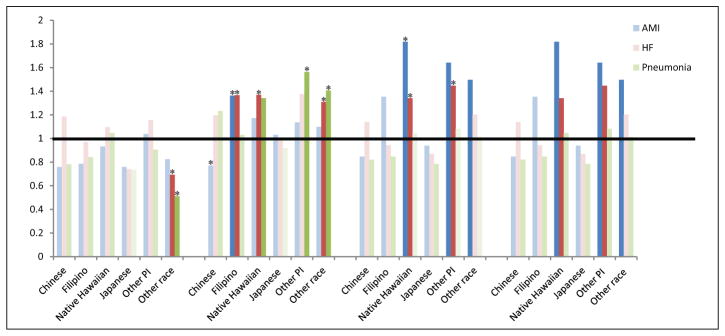
Finally, we hypothesized that the PPR patterns for those with an index admission for AMI, HF, and pneumonia would follow those of PPR generally. The number of patients in each sample is in Table 1. The unadjusted rates of PPR differed by race/ethnicity in unadjusted models for some API groups as seen in Appendix B. In multivariable models including comorbidity (Table 3), we found that for AMI, Filipino women 65+ years (OR = 1.33; 95% CI = 1.04–1.70) and Native Hawaiian men between 18 and 64 years (OR = 1.36; 95% CI = 1.03–1.79) had higher PPR odds compared with Whites, while Chinese women 65+ years had significantly lower PPR odds (OR = 0.69; 95% CI = 0.54–0.82). For HF, Filipino women 65+ years (OR = 1.36; 95% CI = 1.15–1.61) also had significantly higher PPR odds than Whites as did Native Hawaiian women (OR = 1.30; 95% CI = 1.12–1.53), Native Hawaiian men 18 to 64 years (OR = 1.22; 95% CI = 1.03–1.44) and other Pacific Islander men 18 to 64 years (OR = 1.22; 95% CI = 1.02–1.46). Among those with pneumonia, other Pacific Islander women 65+ years had higher PPR odds (OR = 1.51; 95% CI = 1.34–2.02), while Filipino women 18 to 65 years (OR = 0.81; 95% CI = 0.66–0.97) and men 18 to 65 years (OR = 0.79; 95% CI = 0.64–0.96) had significantly lower PPR odds compared with Whites. Significant model variables can be seen in Appendix A. In additional models considering an interaction between Elixhauser Index and ethnicity for specific PPR types, the interaction term was again not statistically significant in any model (0.27 for AMI; 0.48 for HF; 0.71 for pneumonia) and, thus, not included in final models.
For a comparison of what would be missing from analyses including only combined API populations, we ran our three final models (including comorbidity) using only a combined API population group compared with Whites. Neither the overall model (not shown) revealed significant difference between the combined API group and Whites in PPR odds (OR = 0.96; 95% CI = 0.90–1.02) nor did the AMI (OR = 1.05; 95% CI = 0.93–1.18) or Pneumonia models (OR = 0.93; 95% CI = 0.86–1.01). For HF, the combined API group had significantly higher PPR odds compared with Whites (OR = 1.10; 95% CI = 1.01–1.20).
Discussion
This study reveals important, previously unidentified patterns in 30-day PPRs for API subgroups. First, those hospitalized for PPR vary distinctly across API racial/ethnic groups in demographic, access, and clinical factors in ways that could affect the design and effectiveness of PPR-focused interventions. For instance, other Pacific Islanders, Native Hawaiians, and Filipinos with PPR include high percentage of working-age (18–64 years) adults, while the vast majority of Japanese and Chinese with PPR are elderly (65+ years). Second, our work reveals not only the demographics of those with PPR by API subgroup but also highlights some significant disparities. Many API populations, including Native Hawaiian and other Pacific Islander men 18 to 64 years and Filipino, other Pacific Islander, and Native Hawaiian men and women 65+ years had higher PPR rates compared with Whites in fully adjusted comparisons. Yet, in many groups, those younger than 65 years had significantly lower PPR odds than Whites. We also confirmed that these policy and program-relevant differences would have been hidden in analyses aggregating diverse API subpopulations. In combined analyses, API populations looked similar to Whites in three of our four final models, showing no indication of any PPR disparities or need for further research. Only in HF was there evidence for concern regarding higher API PPR rates. Yet the API groups most at risk for HF PPR (older Filipino and Native Hawaiian women and younger Native Hawaiian and other Pacific Islanders men) would have remained obscured.
Considering these findings in light of our specific study hypotheses, we find them to be mainly supported. Our first study hypothesis was that API subgroups and Whites with PPR would have distinct demographic and clinical characteristics. As expected, Chinese, Japanese, and Filipino patients were older than Whites, while Native Hawaiians and other Pacific Islanders were younger. Compared with Whites, more other Pacific Islanders and Native Hawaiians with PPR had Medicaid and more Chinese and Japanese had Medicare. Unexpectedly, we found that Whites with PPR had the lowest comorbidity index of all studied groups and that Filipinos with PPRs were covered by Medicare in higher percentages than Whites. Another interesting and unexpected finding was that Whites with a PPR had the highest percentages of substance abuse (16.5%) and mental health-related comorbidities (28.4%) among all studied racial/ethnic groups. For instance, in substance abuse diagnoses, Native Hawaiians (9.4%), other Pacific Islanders (5.2%), Filipinos (4.0%), Japanese (3.0%), and Chinese (2.4%) had much lower percentages. This highlights the fact that predictors of PPR vary by race/ethnicity, critical when designing policy to reduce PPR generally and PPR disparities in particular.
Differences across API groups in PPR would have typically been hidden not only because previous work has not disaggregated API subgroups but also because much of PPR research has focused on Medicare (Jencks et al., 2009). This is, in part, because Medicaid has fragmented, state-level variation making it challenging to do large, multistate research projects that might inform national policy (Regenstein & Andres, 2014; Trudnak et al., 2014). However, the Medicare focus excludes population-based data on most working-age adults, a research gap that is most likely to minimize the burden of PPR for groups with significant health disparities, such as Native Hawaiians and other Pacific Islanders, who manifest common chronic diseases at earlier ages (King et al., 2012; Mau, Sinclair, Saito, Baumhofer, & Kaholokula, 2009). Thus, in terms of designing interventions to address PPR disparities, it is important to consider that a large percentage of Native Hawaiians and other Pacific Islanders with PPR are working-age and have Medicaid. As the focus of the CMS readmission penalties are within Medicare payments and given the comprehensive data constraints mentioned above, not surprisingly many PPR-focused interventions have targeted older populations (Feltner et al., 2014; Leppin et al., 2014; Trudnak et al., 2014). Yet readmissions are quite costly to Medicaid, responsible for an estimated 12.5% of all Medicaid hospitalization payments (Trudnak et al., 2014) making further research on this topic valuable. A focus on the Medicaid population may necessitate distinct interventions. For instance, working-age adults may have different concerns, such as child or family caregiving responsibilities, that need to be addressed in their care and discharge planning. As 72% of other Pacific Islander and 60% Native Hawaiian PPRs was among nonelderly, efforts should be made to reduce this potentially large disease burden. While the PPR rate disparity was no long seen with Whites in multivariable models for these groups once comorbidity was controlled, this does not mean that this population should not be the focus of targeted interventions, given the potentially large impact of hospitalizations among this age group on productivity and support for their families. Also, existing Medicaid readmission research has often focused on those with comorbid substance abuse or mental illness (Regenstein & Andres, 2014). Thus, younger individuals covered by Medicaid may be excluded not only from PPR research studies but also PPR-focused interventions if they do not have a significant behavioral health comorbidity. In our study population, a focus on readmissions around substance abuse and mental health would incidentally prioritize Whites given their higher percentages with such diagnoses.
Our second study hypothesis was that some API subgroups would be more likely to have a PPR compared with Whites even after accounting for available, relevant demographic and access factors. Specifically, we expected Native Hawaiians, Filipinos, and other Pacific Islanders to have higher rates of PPR than Whites when these factors were controlled. This was supported. However, it is important to note that not all API groups had higher rates than Whites. Some API groups had lower rates of PPR than Whites, including many API groups aged 18 to 64 years and Chinese and Japanese patients.
Our third study hypothesis was that comorbidities would reduce, but not explain, PPR disparities for API subgroups compared with Whites. We found this to be true for several API groups 65+, including Native Hawaiian men, Filipino men, and other Pacific Islander men and women. Even when comorbidity was considered, these groups retained significantly higher PPR compared with Whites. After the inclusion of comorbidity, some interesting differences were seen. The ORs for Native Hawaiian women 65+ years no longer retained statistical significance, and the ORs became significantly lower for Native Hawaiians and other Pacific Islander men 18 to 64 compared with Whites. Comorbidity may help explain the PPR differences for these groups in particular. The distinct vulnerability patterns in PPR for many API populations compared with Whites before and after turning 65 remained in these final models. The apparent change in vulnerability for API populations 65+ is an important area for future study. It is possible that, because 65 is when most Americans become eligible for Medicare, this serves to change disparity patterns in PPR for API subgroups relative to Whites. Future research can help us determine if this is a cohort effect, because of relevant sociodemographic changes at age 65 years, such as unmet caregiving needs, and/or perhaps more limited access to Medicare in certain API populations.
Finally, we expected to see similar findings specifically for AMI, HF, and pneumonia, currently targeted in CMS initiatives to reduce PPR. Instead, we noted some important and distinct disparities in final models, especially for Filipino women 65+ and Native Hawaiian men 18 to 64 for both HF and AMI; for other Pacific Islander men 18 to 64 for AMI; and for other Pacific Islander women 65+ for Pneumonia, indicating specific API populations at risk. Considering the unique needs of these communities, particularly by specific type of readmissions, may be critical to efforts to reduce preventable readmissions. HF is an area of particularly high disparities for API populations relative to Whites and our findings support efforts to focus on, and resolve, these disparities (Mau et al., 2009; Sentell et al., 2015).
The higher risk of PPR among older, hospitalized Native Hawaiians, Filipinos, and other Pacific Islanders may be an important area to consider in designing interventions to address PPR. The reasons for these increased odds of PPR among these groups are likely multifaceted and include practical challenges, such as limited access to care generally and limited access to culturally or linguistically relevant care specifically, as well as underlying social determinants, such as higher poverty or lower health literacy, that can compromise preventive health care and disease management (King et al., 2012; Mau et al., 2009; Ursua et al., 2013). Additionally, innovative research has highlighted the role of psychosocial factors in health outcomes in API populations, including the role of acculturation and stress (Gomez, Kelsey, Glaser, Lee, & Sidney, 2004; Kaholokula, Nacapoy, Grandinetti, & Chang, 2008). Specific patterns of comorbidities themselves are distinct across API populations (Gomez et al., 2004) and may be important areas for research, especially for the Native Hawaiian population which saw the strongest effect from the inclusion of the comorbidity factors into multivariable models. Further understanding the health risk pathways by distinct API populations will provide insights into solutions (Gomez et al., 2004). For instance, while Filipinos are a large and rapidly growing immigrant group in the United States, many Filipinos intersect with a health care system that does not meet their unique cultural, linguistic, and mental health needs (Sanchez & Gaw, 2007). Many other Pacific Islander populations have similar issues (Andrade & McDermott, 2011; Hagiwara et al., 2016). Recent CMS guidance has highlighted the need to understand distinct patterns of readmission disparities for distinct racial/ethnic populations and to use this information to build appropriate infrastructure for culturally relevant care, communication, and discharge planning (Betancourt et al., 2015). Strategies for successfully building this infrastructure include members of the relevant population in program policy making and decision making, relevant racial/ethnic concordant providers, and/or training for nonconcordant providers around distinct population preferences and needs (Cooper & Powe, 2004; SAMHSA, 2006, chap. 4). Including diverse API populations, across age groups, in such work will help illuminate and resolve our findings of disparities.
Overall, this study suggests that differences in racial/ethnic groups may need to be considered in evaluating hospital performance. Early evidence from the CMS program shows that Hawai‘i has a high proportion of hospitals with lower-than-average readmission rates compared with the national average (Advisory Board, 2014). Despite these low rates, we still found important racial/ethnic disparities.
Limitations
This study has many strengths, including statewide data over a 6-year period, detailed data about disaggregated API subgroups, and a consideration of PPR among all adults, not just the Medicare population. However, it does have some limitations. First, this study’s focus on preventable readmissions is of particular value because readmissions are the focus of CMS efforts. However, the 3M PPR method does not measure readmission in exactly the same way that CMS does (Davies, Saynina, Schultz, McDonald, & Baker, 2003; Mull et al., 2013) and our data did not allow us to evaluate preventable admissions using the CMS methods. It may be useful to consider if similar findings are seen under the specific CMS readmissions metrics in future work. That said, one strength of the 3M method is the potentially “preventable” aspect (Mull et al., 2013), which may be particularly useful to consider in studies of racial/ethnic disparities toward efforts to resolve these inequities.
In future work, it would also be useful to consider other specific types of PPR, especially septicemia, as it was the most frequent reason for PPR in these data. Hospital-acquired infections have been a focus of Medicare policy since 2008 (McNair, Luft, & Bindman, 2009). Also, PPRs were measured across any hospital in Hawai‘i. Recent evidence suggests that it is important to consider hospitalizations across multiple facilities to fully understand preventable readmission patterns rather than focusing on just one hospital (Henke et al., 2015; Nasir et al., 2010). Thus, our ability to consider data across hospitals is a study strength as it allowed us a comprehensive portrait of readmission disparities. However, specific hospitals may be most concerned with their own readmission rates and rate disparities as these are the focus of the CMS penalties. Thus, there are likely to be logistical, administrative, and political challenges to implementing interventions designed to address PPRs across all hospitals. Also, it is possible that the patterns of disparities seen here across all hospitals might be different from those seen within any particular hospital.
There are limitations to the information available in the discharge data. We were not able to consider all factors that might predict readmission, including family characteristics, social support, and access to primary care (Friedman & Basu, 2004). We also lack detail regarding differential access to care by race/ethnicity beyond insurance status. Interactions by race/ethnicity in access factors, including insurance status, may help explain findings by age group and may be useful topics for future research. We included only patient characteristics at their first eligible visit. Certainly some characteristics may change during the study period. We also combined 6 years of data in our analyses, providing a large sample size to study heterogeneous API subpopulations. However, disparities may have changed over the years, especially given the focus on PPR during this time. Also, some have argued that current CMS policies have a disproportionate impact on safety-net hospitals by penalizing hospitals who have the highest risk patients with the most complex conditions (McNair et al., 2009). If these trends begin to exacerbate disparities by penalizing safety net hospitals and reducing their funding, in particular, it would be useful to consider the time trends for these disparities. Our findings can provide baseline data toward the importance of that goal, but again suggest the utility to consider more finely grained time trends in the future. Given the complexity of the analyses, our analyses considered having a PPR, or not dichotomously by race/ethnicity counting individuals only once from an index admission. The average number of PPR in each readmission chain and multiple PPR by race/ethnicity are also important topics for future research. Also, because of sample size limitations, the diverse populations that make up other Pacific Islanders (e.g., Samoan, Tongan, Micronesian) are combined even though they are heterogeneous and would likely show distinct patterns across study outcomes, and similarly with the “other” race categories, which included self-identified African Americans, Mexicans, and Puerto Ricans. Distinct PPR patterns for these diverse groups, while beyond the scope of this analysis, are important areas for future research, especially given the increased PPR odds we saw in these groups across several of our study outcomes. Also, other variables, including additional individual as well as neighborhood-level variables, may be useful to consider (Hu, Gonsahn, & Nerenz, 2014). Finally, Hawai‘i is a very specific location with unique market conditions and population-level characteristics. Whites, Asians, and Pacific Islanders in other locations may live within a different community, socioeconomic, and health contexts and may thus have different hospitalization and readmission patterns. Nevertheless, this study provides useful baseline data with which to make comparisons for PPR in locations where Asians and Pacific Islanders represent smaller, but often growing, populations.
Conclusions
Research on PPR has often excluded Asian American, Native Hawaiians, and Other Pacific Islanders due to sample size limitations and lack of appropriate data collection. Those that have included these populations typically have combined heterogeneous groups, often finding lower rates of PPR for that group compared with Whites (Fleming et al., 2014). This study shows that those findings may have hidden disparities as race/ethnicity was associated with preventable hospital readmissions for some API subgroups even in fully adjusted models. Particularly vulnerable are Native Hawaiians, Filipinos, and other Pacific Islanders 65+ years old. We also find distinct characteristics of those with a PPR by API group that should be considered in efforts to address these disparities.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research described was supported by National Institute on Minority Health and Health Disparities (NIMHD) Grant P20 MD000173 and P20 GM103466 and was also supported in part by NIMHD grants U54MD007584 and G12MD007601 and grant R01HS019990 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services.
Appendix A
Significant Variables From Multivariable Logistic Regression Analyses Predicting Potentially Preventable 30-Day Readmission.
| Overalla | AMI | HF | Pneumonia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||||
| Ethnicity | ||||||||||||
| Chinese | 0.8205 | 0.7423 | 0.907 | 0.7942 | 0.4503 | 1.4009 | 1.0495 | 0.8998 | 1.224 | 0.8811 | 0.6991 | 1.1104 |
| Filipino | 0.9547 | 0.8654 | 1.0533 | 1.0843 | 0.9135 | 1.287 | 1.1038 | 1.0146 | 1.2008 | 0.905 | 0.8219 | 0.9965 |
| Native Hawaiian | 1.0104 | 0.9545 | 1.0695 | 1.0545 | 0.9116 | 1.2197 | 1.1688 | 1.0465 | 1.3054 | 0.9762 | 0.8818 | 1.0807 |
| Japanese | 0.9258 | 0.8679 | 0.9876 | 0.9518 | 0.783 | 1.157 | 0.9305 | 0.8169 | 1.0598 | 0.8339 | 0.7225 | 0.9625 |
| Other PI | 1.0761 | 0.9995 | 1.1585 | 1.0131 | 0.8749 | 1.1731 | 1.1383 | 0.9259 | 1.3995 | 1.0286 | 0.868 | 1.219 |
| Other race | 0.8954 | 0.8369 | 0.9578 | 0.9199 | 0.7979 | 1.0604 | 1.0293 | 0.9397 | 1.1275 | 0.8835 | 0.7828 | 0.9971 |
| White | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Gender | ||||||||||||
| Female | 0.7016 | 0.6162 | 0.7987 | 1.0174 | 0.8624 | 1.2002 | 1.0153 | 0.9445 | 1.0914 | 0.9151 | 0.8403 | 0.9964 |
| Male | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Payer | ||||||||||||
| Department of Defense | 1.6571 | 1.442 | 1.9044 | 1.6679 | 1.1344 | 2.4523 | 2.247 | 1.8401 | 2.7439 | 2.1458 | 1.5874 | 2.9008 |
| Medicaid/Quest | 1.6348 | 1.4348 | 1.8627 | 1.8154 | 1.5512 | 2.1246 | 1.6759 | 1.4193 | 1.9789 | 1.5748 | 1.2936 | 1.917 |
| Medicare | 2.3306 | 2.0812 | 2.61 | 1.8256 | 1.5127 | 2.2033 | 1.8472 | 1.5412 | 2.2139 | 1.7539 | 1.4623 | 2.1037 |
| Miscellaneous | 0.8133 | 0.7218 | 0.9164 | 0.8587 | 0.3853 | 1.9139 | 0.6887 | 0.338 | 1.4032 | 0.8271 | 0.3957 | 1.7289 |
| Self-pay | 1.0266 | 0.9301 | 1.1332 | 0.8164 | 0.5386 | 1.2374 | 0.7757 | 0.6085 | 0.9889 | 0.9115 | 0.6515 | 1.2752 |
| Private insurance | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| County | ||||||||||||
| Hawai‘i | 0.8846 | 0.7332 | 1.0672 | 0.8393 | 0.7144 | 0.9859 | 0.9012 | 0.7482 | 1.0856 | 0.7987 | 0.6667 | 0.9569 |
| Kauai | 1.0401 | 0.8771 | 1.2332 | 0.8646 | 0.7377 | 1.0132 | 0.9451 | 0.7739 | 1.1542 | 0.9555 | 0.8399 | 1.0869 |
| Maui | 0.9034 | 0.7812 | 1.0447 | 0.8229 | 0.5804 | 1.1666 | 0.7566 | 0.6314 | 0.9065 | 0.6725 | 0.5921 | 0.7637 |
| Oahu | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Substance use | ||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.1353 | 1.0226 | 1.2604 | 0.827 | 0.6238 | 1.0966 | 1.012 | 0.8484 | 1.207 | 0.8781 | 0.7211 | 1.0693 |
| Mental health | ||||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.5615 | 1.3677 | 1.7828 | 1.0486 | 0.8763 | 1.2546 | 0.9674 | 0.8813 | 1.0619 | 1.0627 | 0.9325 | 1.211 |
| Age (years) | ||||||||||||
| <65 | 0.9407 | 0.8262 | 1.0711 | 0.9746 | 0.7946 | 1.1953 | 1.0879 | 0.9424 | 1.2558 | 0.901 | 0.8096 | 1.0027 |
| 65+ | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Elixhauser Index | 1.3634 | 1.3196 | 1.4086 | 1.1954 | 1.1666 | 1.225 | 1.1632 | 1.1416 | 1.1852 | 1.1956 | 1.1771 | 1.2143 |
|
| ||||||||||||
| Interactions | p Value | |||||||||||
|
| ||||||||||||
| Ethnicity * Older than 65 years | .05 | .85 | .67 | .13 | ||||||||
| Ethnicity * Gender | .52 | .71 | .19 | .31 | ||||||||
| Ethnicity * Gender * Older than 65 years | .43 | .13 | .57 | .21 | ||||||||
Note. CI = confidence interval; OR = odds ratio; AMI = acute myocardial infarction; HF = heart failure.
In the overall model, hospitalization disease type was statistically significant: OR [95% CI] = 1.51 [1.41, 1.63] for AMI versus Others; 1.59 [1.46, 1.73] for HF versus Others; 1.36 [1.28, 1.45] for Pneumonia versus Others.
Appendix B
Unadjusted Odds Ratios (ORs) for Potentially Preventable Hospitalization by Asian and Pacific Islander (API) Subgroups compared With Whites for Acute Myocardial Infarction (AMI), Heart Failure (HF), and Pneumonia Samples
Note. An OR more than 1 indicates a higher rate of potentially preventable readmission (PPR) compared with Whites and an OR less than 1 indicates a lower rate. Statistically significant (p < .05) differences in unadjusted models are indicated by darker shading. Significant differences from the multivariable model are indicated with an asterisk. In multivariable models adjusting for all study variables except the three comorbidity measures (payer, county of residence, discharge year, admission type, hospital, and age group/gender/ethnicity interactions).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 3M Health Information System Documentation Department. Potentially preventable readmissions classification system: Methodology overview. 2008 Retrieved from https://www.illinois.gov/hfs/SiteCollectionDocuments/3MPotentiallyPreventableReadmissions.pdf.
- Advisory Board. Where hospitals have the most readmissions; 364 hospitals have higher-than-average readmission rates, data show. 2014 Jan 6; Retrieved from http://www.advisory.com/daily-briefing/2014/01/06/where-hospitals-have-the-most-readmissions.
- Ahmedani BK, Solberg LI, Copeland LA, Fang-Hollingsworth Y, Stewart C, Hu J, … Simon GE. Psychiatric comorbidity and 30-day readmissions after hospitalization for heart failure, AMI, and pneumonia. Psychiatric Services. 2015;66:134–140. doi: 10.1176/appi.ps.201300518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaudeen N, Vidyarthi A, Maselli J, Auerbach A. Redefining readmission risk factors for general medicine patients. Journal of Hospital Medicine. 2011;6:54–60. doi: 10.1002/jhm.805. [DOI] [PubMed] [Google Scholar]
- Andrade N, McDermott J, editors. People and cultures of Hawai‘i: The evolution of culture and ethnicity. Honolulu: University of Hawai‘i Press; 2011. [Google Scholar]
- Bernheim SM, Ross JS. Hospital discharge and the transition home for poor patients: “I knew I couldn’t do what they were asking me. Journal of General Internal Medicine. 2014;29:269–270. doi: 10.1007/s11606-013-2698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt JR, Tan-McGrory A, Kenst KS. Guide to preventing readmissions among racially and ethnically diverse Medicare beneficiaries. Baltimore, MD: Centers for Medicare & Medicaid Services, Office of Minority Health; 2015. Sep, (Prepared by the Disparities Solutions Center, Mongan Institute for Health Policy at Massachusetts General Hospital) [Google Scholar]
- Calvillo-King L, Arnold D, Eubank KJ, Lo M, Yunyongying P, Stieglitz H, … Halm EA. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: Systematic review. Journal of General Internal Medicine. 2013;28:269–282. doi: 10.1007/s11606-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services. Readmissions Reduction Program. Baltimore, MD: Author; 2013. Retrieved from www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. [Google Scholar]
- Chu YT, Ng YY, Wu SC. Comparison of different comorbidity measures for use with administrative data in predicting short- and long-term mortality. BMC Health Services Research. 2010;10:140. doi: 10.1186/1472-6963-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LA, Powe NR. Disparities in patient experiences, health care processes, and outcomes: The role of patient–provider racial, ethnic, and language concordance. New York, NY: Commonwealth Fund; 2004. Jul, Retrieved from http://www.commonwealthfund.org/programs/minority/cooper_raceconcordance_753.pdf. [Google Scholar]
- Davies S, Saynina O, Schultz E, McDonald KM, Baker LC. Implications of metric choice for common applications of readmission metrics. Health Services Research Journal. 2013;48(6 Pt 1):1978–1995. doi: 10.1111/1475-6773.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deswal A, Petersen NJ, Souchek J, Ashton CM, Wray NP. Impact of race on health care utilization and outcomes in veterans with congestive heart failure. Journal of American College of Cardiology. 2004;43:778–784. doi: 10.1016/j.jacc.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Donzé J, Lipsitz S, Bates DW, Schnipper JL. Causes and patterns of readmissions in patients with common comorbidities: Retrospective cohort study. British Medical Journal. 2013;347:f7171. doi: 10.1136/bmj.f7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Medical Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Enguidanos S, Coulourides Kogan AM, Schreibeis-Baum H, Lendon J, Lorenz K. “Because I Was Sick”: Seriously ill veterans’ perspectives on reason for 30-day readmissions. Journal of the American Geriatric Society. 2015;63:537–542. doi: 10.1111/jgs.13238. [DOI] [PubMed] [Google Scholar]
- Feltner C, Jones CD, Cené CW, Zheng ZJ, Sueta CA, Coker-Schwimmer EJ, … Jonas DE. Transitional care interventions to prevent readmissions for persons with heart failure: A systematic review and meta-analysis. Annals of Internal Medicine. 2014;160:774–784. doi: 10.7326/M14-0083. [DOI] [PubMed] [Google Scholar]
- Fleming LM, Gavin M, Piatkowski G, Chang JD, Mukamal KJ. Derivation and validation of a 30-day heart failure readmission model. American Journal of Cardiology. 2014;114:1379–1382. doi: 10.1016/j.amjcard.2014.07.071. [DOI] [PubMed] [Google Scholar]
- Friedman B, Basu J. The rate and cost of hospital readmissions for preventable conditions. Medical Care Research and Review. 2004;61:225–240. doi: 10.1177/1077558704263799. [DOI] [PubMed] [Google Scholar]
- Ghosh C. A national health agenda for Asian Americans and Pacific Islanders. Journal of the American Medical Association. 2010;304:1381–1382. doi: 10.1001/jama.2010.1358. [DOI] [PubMed] [Google Scholar]
- Gilman M, Adams EK, Hockenberry JM, Wilson IB, Milstein AS, Becker ER. California safety-net hospitals likely to be penalized by ACA value, readmission, and meaningful-use programs. Health Affairs. 2014;33:1314–1322. doi: 10.1377/hlthaff.2014.0138. [DOI] [PubMed] [Google Scholar]
- Gomez SL, Kelsey JL, Glaser SL, Lee MM, Sidney S. Immigration and acculturation in relation to health and health-related risk factors among specific Asian subgroups in a health maintenance organization. American Journal of Public Health. 2004;94:1977–1984. doi: 10.2105/ajph.94.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Koenig L, Faerberg J, Steinberg CR, Vaz C, Wheatley MP. The Medicare Hospital Readmissions Reduction Program: Potential unintended consequences for hospitals serving vulnerable populations. Health Services Research Journal. 2014;49:818–837. doi: 10.1111/1475-6773.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara MK, Miyamura J, Yamada S, Sentell T. Younger and sicker: Comparing Micronesians to other ethnicities in Hawai‘i. American Journal of Public Health. 2016;3:485–491. doi: 10.2105/AJPH.2015.302921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: A systematic review. Annals of Internal Medicine. 2011;155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- Hawai‘i Department of Health. Gender, age, ethnicity, and poverty by county— Population of Hawai‘i, Hawai‘i Health Survey (HHS) 2008. 2009 Retrieved from http://health.hawaii.gov/hhs/files/2013/05/hhs08t11.pdf.
- Hawaii Health Information Corporation Inpatient Data. Data specifications 2015. 2015 Retrieved from http://hhic.org/files/2015_Data_Specs_0829141.pdf.
- Henke RM, Karaca Z, Lin H, Wier LM, Marder W, Wong HS. Patient factors contributing to variation in same-hospital readmission rate. Medical Care Research and Review. 2015;72:338–358. doi: 10.1177/1077558715577478. [DOI] [PubMed] [Google Scholar]
- Hernandez AF, Curtis LH. Minding the gap between efforts to reduce readmissions and disparities. Journal of the American Medical Association. 2011;305:715–716. doi: 10.1001/jama.2011.167. [DOI] [PubMed] [Google Scholar]
- Hu J, Gonsahn MD, Nerenz DR. Socioeconomic status and readmissions: Evidence from an urban teaching hospital. Health Affairs. 2014;33:778–785. doi: 10.1377/hlthaff.2013.0816. [DOI] [PubMed] [Google Scholar]
- Jencks S. Defragmenting care. Annals of Internal Medicine. 2010;153:757–758. doi: 10.7326/0003-4819-153-11-201012070-00010. [DOI] [PubMed] [Google Scholar]
- Jencks S, Williams M, Coleman E. Rehospitalizations among patients in the Medicare fee-for-service program. New England Journal of Medicine. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. Journal of the American Medical Association. 2011;305:675–681. doi: 10.1001/jama.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaholokula JK, Nacapoy AH, Grandinetti A, Chang HK. Association between acculturation modes and type 2 diabetes among Native Hawaiians. Diabetes Care. 2008;31:698–700. doi: 10.2337/dc07-1560. [DOI] [PubMed] [Google Scholar]
- King GL, McNeely MJ, Thorpe LE, Mau ML, Ko J, Liu LL, … Chow EA. Understanding and addressing unique needs of diabetes in Asian Americans, Native Hawaiians, and Pacific Islanders. Diabetes Care. 2012;35:1181–1188. doi: 10.2337/dc12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripalani S, Theobald CN, Anctil B, Vasilevskis EE. Reducing hospital readmission: Current strategies and future directions. Annual Review of Medicine. 2014;65:471–485. doi: 10.1146/annurev-med-022613-090415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroch E, Duan M, Martin J, Bankowitz RA. Patient factors predictive of hospital readmissions within 30 days. Journal for Healthcare Quality. 2015;38:106–115. doi: 10.1097/JHQ.0000000000000003. [DOI] [PubMed] [Google Scholar]
- Leppin AL, Gionfriddo MR, Kessler M, Brito JP, Mair FS, Gallacher K, … Montori VM. Preventing 30-day hospital readmissions: A systematic review and meta-analysis of randomized trials. Journal of the American Medical Association Internal Medicine. 2014;174:1095–1107. doi: 10.1001/jamainternmed.2014.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mau M, Sinclair K, Saito E, Baumhofer KN, Kaholokula JK. Cardiometabolic health disparities in Native Hawaiians and other Pacific Islanders. Epidemiological Reviews. 2009;31:113–129. doi: 10.1093/ajerev/mxp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair PD, Luft HS, Bindman AB. Medicare’s policy not to pay for treating hospital-acquired conditions: The impact. Health Affairs. 2009;28:1485–1493. doi: 10.1377/hlthaff.28.5.1485. [DOI] [PubMed] [Google Scholar]
- Moy E, Mau M, Raetzman S, Barrett M, Miyamura JB, Chaves KH, … Andrews R. Ethnic differences in potentially preventable hospitalizations among Asian American, Native Hawaiians and Other Pacific Islanders: Implications for reducing health care disparities. Ethnicity & Disease. 2013;23:6–11. [PubMed] [Google Scholar]
- Mull HJ, Chen Q, O’Brien WJ, Shwartz M, Borzecki AM, Hanchate A, … Rosen AK. Comparing 2 methods of assessing 30-day readmissions: What is the impact on hospital profiling in the Veterans health administration? Medical Care. 2013;51:589–596. doi: 10.1097/MLR.0b013e31829019a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir K, Lin Z, Bueno H, Normand SL, Drye EE, Keenan PS, … Krumholz HM. Is same-hospital readmission rate a good surrogate for all-hospital readmission rate? Medical Care. 2010;48:477–481. doi: 10.1097/MLR.0b013e3181d5fb24. [DOI] [PubMed] [Google Scholar]
- Park CB, Braun KL, Horiuchi BY, Tottori C, Onaka AT. Longevity disparities in multiethnic Hawai‘i: An analysis of 2000 life tables. Public Health Reports. 2009;124:579–584. doi: 10.1177/003335490912400415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient Protection and Affordable Care Act of 2010, Pub. L. No. 111–148 S
- Regenstein M, Andres E. Reducing hospital readmissions among Medicaid patients: A review of the literature. Quality Management in Health Care. 2014;23:203–225. doi: 10.1097/QMH.0000000000000043. [DOI] [PubMed] [Google Scholar]
- Robert Wood Johnson Foundation. Reform in action: How the U.S. health care system can reduce avoidable readmissions: Insights from the Robert Wood Johnson Foundation and Aligning Forces for Quality. 2013 Feb; Retrieved from http://www.rwjf.org/en/research-publications/find-rwjf-research/2013/02/reform-in-action-how-the-u-s-health-caresystem-can-reduce-avo.html.
- Substance Abuse and Mental Health Services Administration. Substance abuse: Administrative issues in outpatient treatment. Rockville, MD: Center for Substance Abuse Treatment; 2006. Preparing a program to treat diverse clients. Treatment Improvement Protocol (TIP) Series No. 46. [PubMed] [Google Scholar]
- Sanchez F, Gaw A. Mental health care of Filipino Americans. Psychiatric Services. 2007;58:810–815. doi: 10.1176/ps.2007.58.6.810. [DOI] [PubMed] [Google Scholar]
- Sentell T, Miyamura J, Ahn HJ, Chen JJ, Seto T, Juarez D. Potentially preventable hospitalizations for congestive heart failure among Asian Americans and Pacific Islanders in Hawai‘i. Journal of Immigrant and Minority Health. 2015;17:1289–1297. doi: 10.1007/s10903-014-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentell TL, Ahn HJ, Juarez DT, Tseng CW, Chen JJ, Salvail FR, … Mau MM. Comparison of potentially preventable hospitalizations related to diabetes among Native Hawaiian, Chinese, Filipino, and Japanese elderly compared with whites, Hawai‘i, December 2006-December 2010. Preventing Chronic Disease. 2013;10:E123. doi: 10.5888/pcd10.120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein MD, Qin H, Mercer SQ, Fong J, Haydar Z. Risk factors for 30-day hospital readmission in patients ≥65 years of age. Baylor University Medical Center Proceedings. 2008;21:363–372. doi: 10.1080/08998280.2008.11928429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges E, Levit K, Stocks C, Santora P. Statistical Brief No. 117. Rockville, MD: Agency for Health Care Policy and Research; 2011. State variation in inpatient hospitalizations for mental health and substance abuse conditions, 2002–2008. [PubMed] [Google Scholar]
- Tang VL, Halm EA, Fine MJ, Johnson CS, Anzueto A, Mortensen EM. Predictors of rehospitalization after admission for pneumonia in the veterans affairs healthcare system. Journal of Hospital Medicine. 2014;9:379–383. doi: 10.1002/jhm.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudnak T, Kelley D, Zerzan J, Griffith K, Jiang HJ, Fairbrother GL. Medicaid admissions and readmissions: Understanding the prevalence, payment, and most common diagnoses. Health Affairs. 2014;33:1337–1344. doi: 10.1377/hlthaff.2013.0632. [DOI] [PubMed] [Google Scholar]
- Ursua RA, Islam NS, Aguilar DE, Wyatt LC, Tandon SD, Abesamis-Mendoza N, … Trinh-Shevrin C. Predictors of hypertension among Filipino immigrants in the Northeast US. Journal of Community Health. 2013;38:847–855. doi: 10.1007/s10900-013-9689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. Overview of race and Hispanic origin: 2010. 2010 Census Briefs. 2011 Mar; Retrieved from http://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf.
- U.S. Census Bureau. The Asian population: 2010. 2010 Census Briefs. 2012a Mar; Retrieved from http://www.census.gov/prod/cen2010/briefs/c2010br-11.pdf.
- U.S. Census Bureau. The Native Hawaiian and Other Pacific Islander population: 2010. 2010 Census Briefs. 2012b May; Retrieved from http://www.census.gov/prod/cen2010/briefs/c2010br-12.pdf.
- Vivo RP, Krim SR, Liang L, Neely M, Hernandez AF, Eapen ZJ, … Fonarow GC. Short- and long-term rehospitalization and mortality for heart failure in 4 racial/ethnic populations. Journal of the American Heart Association. 2014;3(5):e001134. doi: 10.1161/JAHA.114.001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam CH, Wong EL, Chan FW, Leung MC, Wong FY, Cheung AW, … Yeoh EK. Avoidable readmission in Hong Kong—System, clinician, patient or social factor? BMC Health Services Research. 2010;10:311. doi: 10.1186/1472-6963-10-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen S. Which ethnic group makes the most money? Hawai‘i Business Magazine. 2013 Oct; Retrieved from http://www.hawaiibusiness.com/which-ethnic-group-makes-the-most-money/
- Zekry D, Loures Valle BH, Graf C, Michel JP, Gold G, Krause KH, … Herrmann FR. Prospective comparison of 6 comorbidity indices as predictors of 1-year post-hospital discharge institutionalization, readmission, and mortality in elderly individuals. Journal American Medical Directors Association. 2012;13:272–278. doi: 10.1016/j.jamda.2010.11.011. [DOI] [PubMed] [Google Scholar]