Abstract
Rationale
Addiction involves maladaptive choice behavior in which immediate drug effects are valued more than delayed nondrug rewards.
Objectives and Methods
To model this behavior and extend our earlier work with the prescription opioid oxycodone, we allowed rats to choose between immediate intravenous delivery of the short-acting opioid remifentanil and delayed delivery of highly palatable food pellets. Treatment drugs were tested on a baseline where remifentanil was preferred over food.
Results
Treatment with a high dose of the opioid antagonist naltrexone decreased but did not reverse the preference for remifentanil. Treatment with the serotonin 5-HT2C agonist lorcaserin decreased remifentanil and food self-administration nonselectively. Across conditions in which the alternative to delayed food was either a moderate dose of oxycodone, a moderate or high dose of remifentanil, a smaller more immediate delivery of food, or timeout with no primary reinforcement, choice was determined by both the length of the delay and the nature of the alternative option. Delayed food was discounted most steeply when the alternative was a high dose of remifentanil, which was preferred over food when food was delayed by 30 s or more. Within-subject comparisons showed no evidence for trait-like impulsivity or sensitivity to delay across these conditions.
Conclusions
Choice was determined more by the current contingencies of reinforcement than by innate individual differences. This finding suggests that people might develop steep delay-discounting functions because of the contingencies in their environment, and it supports the use of contingency management to enhance the relative value of delayed non-drug reinforcers.
Keywords: Lorcaserin, Addiction, Intertemporal Choice, Concurrent Choice, Delay Discounting, Impulsivity
INTRODUCTION
The choice to take drugs even when it results in the loss of important nondrug commodities such as money, career and social relationships is a major feature of addiction (Heyman 2013; American Psychiatric Association 2013). This maladaptive choice behavior involves delay discounting, the tendency to place less value on rewards that are not received immediately (Bickel et al. 2014; Lamb et al. 2016). From this perspective, procedures that allow animals to choose between immediate drug injection and delayed delivery of a salient nondrug reward could provide a useful model of addiction (Huskinson et al. 2015; Maguire et al. 2013; Woolverton and Anderson 2006), with certain advantages over procedures that only involve drug reward or that only provide a choice between immediate drug and immediate nondrug reward.
We recently developed procedures in which rats chose between immediate injection of the prescription opioid oxycodone and delayed delivery of sweet food pellets (Secci et al. 2016). Rats preferred food over oxycodone when the food delay was short, but they switched to preferring oxycodone when the food delay was increased to 120 s. In the present study, we extended these results in several ways, using a procedure in which rats could choose between delayed food and immediate injection of the ultra-short acting opioid remifentanil. Remifentanil is highly reinforcing (Panlilio and Schindler 2000), and its ultra-short duration of action makes it well-suited for procedures involving repeated choices within daily testing sessions (Maguire et al. 2013; 2016).
First, we studied a range of conditions to better understand the interactive effects of food delay and drug dose on choice. In the previous study (Secci et al. 2016), we only determined delay-discounting functions for food under choice conditions with one dose of oxycodone; in the present study we did so with two different doses of remifentanil, including a dose that was maximally reinforcing under a progressive-ratio schedule (Panlilio and Schindler 2000). Also, since dose-effect functions for oxycodone were only determined in a condition where the nondrug alternative was food with a long delay, in the present study we compared remifentanil dose-effect functions when the nondrug alternative was food with a short delay, food with a moderate delay, or timeout with no food delivered.
Second, we studied the effects of treatment drugs under a range of conditions. Previously, we only studied the effects of the opioid antagonist naltrexone under a food-drug choice condition. Here, we compared the effects of naltrexone under food-drug choice, food-only and drug-only conditions. Also, to establish a more sensitive baseline for the testing of treatment drugs, we customized the food delay for each rat to achieve a criterion level of drug preference, rather than using the same long delay value for all subjects.
Third, we tested the effects of the serotonin 5-HT2C receptor agonist lorcaserin (Smith et al. 2008), which is prescribed as a treatment for obesity, but might have anti-addiction effects since it can decrease self-administration of nicotine (Briggs et al. 2016; Cousins et al. 2014; Higgins et al. 2013; Higgins et al. 2012; Levin et al. 2011), cocaine (Collins et al. 2016; Harvey-Lewis et al. 2016), methamphetamine (Gerak et al. 2016) and oxycodone (Neelakantan et al. 2017) in rats. With respect to choice behavior and opioid self-administration, agonists of 5-HT2C are also interesting because they decrease premature operant responding that is considered a form of impulsivity (Higgins et al. 2012; Navarra et al. 2008), and they block some effects of opioid administration and withdrawal (Wu et al. 2015; Zhang et al. 2016).
Finally, we compared delay discounting functions obtained when the alternative to delayed food was oxycodone, remifentanil, a smaller amount of more immediate food, or timeout with no primary reinforcement. These comparisons provide information about how delay discounting is affected by the nature of the alternative sources of reinforcement. Within-subject comparisons of these curves also provide an assessment of whether individual rats show a trait-like impulsivity or sensitivity to delay.
MATERIALS AND METHODS
Subjects
Sprague–Dawley rats (Charles River, Wilmington, MA, USA), 3–4 months old and 300–350 g at the start of the study were individually housed on a 12-h light/dark cycle, with experiments conducted in the light phase. Male rats were studied to allow direct comparisons with our previous results using choice procedures. All rats received 15–20 g/day laboratory chow 15 minutes after the daily training session. About one week before initial self-administration training with remifentanil, a catheter was implanted in the right jugular vein as previously described (Secci et al. 2016). Catheters were flushed before and after each session with 0.1 ml of gentamycin (0.4 mg/ml) and heparin (200 USP units/ml) in saline solution to help maintain patency. Facilities were fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All procedures were approved by the Animal Care and Use Committee of the Intramural Research Program of the National Institute on Drug Abuse and followed the guidelines of the National Research Council (2011).
Apparatus
Experimental chambers (30 × 24 × 29 cm; Coulbourn Instruments, Allentown, PA) enclosed in sound attenuation chests had two nosepoke holes and a central amber cuelight on one wall. Each nosepoke hole could be individually lit from within by a green LED. Food pellets (45 mg, ~58% sugar; Product #F0021; Bio- Serv, Frenchtown, NJ) were delivered between the nosepoke holes. Experimental events were controlled with Med-PC software (Med Associates, St Albans, VT). Self-administration drug solutions were delivered intravenously (IV) by injection pump (Med Associates, St Albans, VT, USA) in a volume of ~0.1 ml over ~2 sec. Remifentanil (μ-opioid agonist, Ultiva®, Mylan Institutional, Canonsburg, PA), oxycodone hydrochloride (μ-opioid agonist; Sigma-Aldrich, Natick, MA), lorcaserin (5HT2C receptor agonist; National Institute on Drug Abuse, Bethesda, MD) and naltrexone hydrochloride (opioid antagonist; Sigma-Aldrich, Natick, MA) were dissolved in saline vehicle. Intraperitoneal (IP) and subcutaneous (SC) treatment drugs were injected in a volume of 1 ml/kg body weight.
General Procedure
When a nosepoke hole was dark, it was inactive (i.e., responses in that hole had no programmed effect). When a nosepoke hole was lit from within, a response in that hole would immediately turn off all nosepoke lights and turn on the cuelight for a timeout period. During the timeout period, either an immediate IV injection (inj), delayed food, or no reinforcement was delivered, depending on the experiment and the stage of training. In food-drug choice experiments, the left hole produced timeout plus injection, and the right hole produced timeout plus a food pellet; for identification purposes, these holes will be referred to as the drug hole and food hole even for conditions where they were inactive or produced timeout with no food or drug delivery.
Food-remifentanil experiments
Remifentanil-only, food-only and initial food-remifentanil choice training
Training was the same as the 'hungry/drug-first' training of Secci et al. (2016), but with IV remifentanil (4 µg/kg/inj) delivered instead of oxycodone. Briefly, experimentally naive rats (n=11) were initially trained with a remifentanil-only schedule in which remifentanil was available for responding in the drug hole, but the food hole was dark and inactive (4–10 sessions). Then, a second stage of remifentanil-only training (2–10 sessions) was conducted in which both holes were lit, the drug hole produced a timeout period with remifentanil delivery, and the food hole produced a timeout period with no reinforcement. Each of these remifentanil-only stages continued until a rat had two consecutive sessions with more than 50 injections/session and at least 90% of responses in the drug hole. Then, food-only training (4–10 sessions) was conducted in which the food hole was lit and produced timeout with 5-s delayed food, the drug hole was dark and inactive, and there was also automatic delivery (on a 60-s variable-time schedule) of timeout with 5-s delayed food; this food-only training continued until there were two consecutive sessions with 100 food reinforcers/session and at least 90% of responses in the food hole. Finally, the food-remifentanil choice schedule was introduced, in which both holes were lit and produced timeout with reinforcement, allowing rats to choose either remifentanil or 5-s delayed food. Under the initial food-only and remifentanil-only training conditions, the timeout duration was 20 s, and sessions lasted for 2 hrs, 100 pellets or 100 injections. In all subsequent conditions, the timeout duration was 150 s and sessions lasted 2 hrs, allowing up to 48 choice responses per session.
Delay and dose manipulations under the food-remifentanil choice schedule
The food delay was increased from 5 s to 15, 30, 60 and 120 s over sessions to obtain delay discounting functions; this was performed twice, once with a remifentanil dose of 4 µg/kg/inj, and once with a dose of 16 µg/kg/inj. In separate experiments, the remifentanil dose was varied by offering vehicle, 2, 4, 8, 16 and 32 µg/kg/inj in mixed order across subjects; this was performed under three conditions, with the food delay held constant at 5 s, with the food delay held constant at 60 s, and with timeout but no food delivered for responding in the food hole. Each condition in these dose and delay manipulations was maintained until the percentage of responses in the food hole was within 10 percentage points for two consecutive sessions, with no upward or downward trend across the last three sessions. The two delay manipulations and three dose manipulations were conducted in mixed order across subjects.
Treatment-drug experiments
A separate group of experimentally naive rats (n=10) was initially trained with the same remifentanil-only, food-only and food-remifentanil choice procedures described above, but with an initial training dose of 16 µg/kg instead of 4 µg/kg. After initial food-remifentanil choice training, the dose was decreased to 4 µg/kg and the food delay was adjusted over sessions so each rat maintained a baseline preference of about 90% for remifentanil; this was intended to increase sensitivity to treatment drugs by compensating for individual differences in delay discounting. Adjustment was accomplished by first exposing the rat to delays of 5, 15, 30, 60 and 120 s for one session each, then selecting the delay value for each session based on performance in the previous session. Once the delay was established for each rat, it remained the same throughout treatment drug testing. Each rat was also trained with vehicle substituted for remifentanil for 3–5 days, until at least 90% of responding occurred in the food hole, before returning to the baseline schedule. When the percentage of responses in the food hole was within 10 percentage points for two consecutive baseline sessions, lorcaserin (0, 0.1, 0.3, 1.7 or 3 mg/kg, IP, 15 min before session) and then naltrexone (0, 0.3, 1, 3, 10 or 30 mg/kg, SC, 5 min before session) were tested up to two times per week. Lorcaserin and naltrexone were also tested under a food-only condition (where responses in the drug hole produced no injection) and a remifentanil-only condition (where responses in the food-hole produced timeout but no food). Six rats were tested with the food-remifentanil choice condition first, and two each were tested with the food-only and remifentanil-only conditions first.
Food-food experiment
Smaller/sooner food versus larger/later food
The basic choice schedule was modified to give one food pellet 5 s after a response in the left hole (smaller/sooner option) or three food pellets delivered 5–120 s after a response in the right hole (larger/later option). The delay for the larger/later option was manipulated over sessions to obtain discounting functions as in the food-drug experiments. The subjects (n=8) for this experiment had been trained earlier with food-oxycodone choice and included 2 hungry/food-first rats, 2 sated/food-first rats, and 4 sated/drug-first rats from the Secci et al. (2016) study. Delay-discounting curves obtained with the food-oxycodone schedule in these rats are presented here to allow within-subjects comparisons between food-food and food-drug conditions.
Statistical analysis
Proc Mixed (SAS Institute, Cary, SC) was used to analyze the percentage of responses in the food hole, the numbers of times/session each hole was chosen, and the efficiency of responding. Paired comparisons were performed using the Holm-Bonferroni procedure to maintain familywise significance levels of .05. Efficiency of responding was calculated as the combined number of left-hole and right-hole choices, expressed as a percentage of 48 (the maximum possible per session). For calculation of reinforcers per session and percentage of maximum reinforcers, timeout with no food or drug delivery (in the food-only and remifentanil-only conditions, respectively) was considered a "reinforcer". Percentage measures were arcsine-root transformed for analysis, a standard procedure when values range between 0 and 100. To compare across experiments and to assess the within-subject stability of delay discounting, area under the curve was calculated for each delay function and correlated between conditions.
RESULTS
Choice between remifentanil and delayed food
Delay-discounting curves
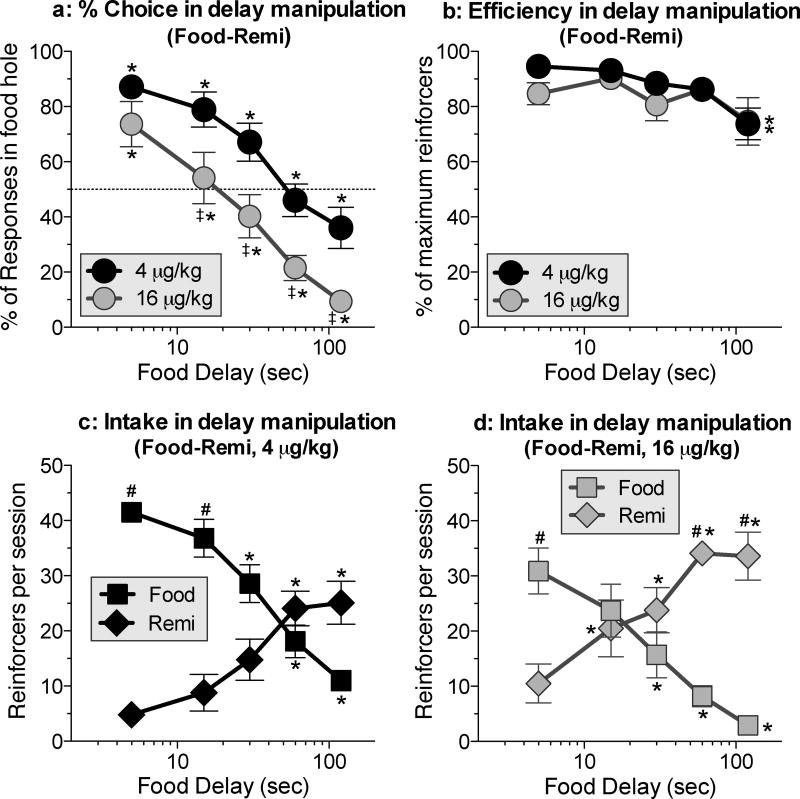
When the food delay was increased over sessions, the percentage of responses in the food hole decreased monotonically [Fig. 1a; Delay: F(1,9)=44.-02, P<.0001]. The delay-discounting curve was shifted down (towards remifentanil preference) at the higher dose of remifentanil [Fig. 1a; Dose: F(4,36)=33.02, P<.0001]. On average, the higher dose of remifentanil (Fig. 1a, gray symbols) was preferred over food when the delay was 30 s or longer, and the lower dose (Fig. 1a, black symbols) was preferred over food when the delay was 120 s.
Figure 1.
Delay-discounting curves for food when the alternative was 4 or 16 µg/kg/inj of remifentanil. a: Percentage of responses in the food hole (i.e., percent choice) as a function of the food delay at each dose. (Details of within-curve differences: In the 4 µg/kg curve, all pairs of points differed from each other except 15 vs. 30, 15 vs. 5 and 60 vs. 120. In the 16 µg/kg curve, all pairs differed except 15 vs. 30 and 60 vs. 120.) b: Efficiency of responding during the delay-curve determinations at each dose. (Details of within-curve differences: In both curves, efficiency was lower at 120 compared to 5 and 15.) c: Intake (i.e., number of choices) of food and remifentanil during delay-curve determinations at 4 µg/kg/inj. (Details of within-curve differences: For remifentanil, 60 and 120 each differed from 5 and 15. For food, 30 differed from 5 and 15; and 60 and 120 each differed from 5, 15 and 30.) d: Intake of food and remifentanil during delay-curve determinations at 16 µg/kg/inj. (Details of within-curve differences: For remifentanil: 15 and 30 each differed from 5, and 60 and 120 each differed from 5 and 15. For food, 30 differed from 5, and 60 and 120 each differed from 5 and 15.) * differs within curve. ‡ differs between curves at the same delay. # differs between food and drug hole at same delay. P values for significant differences are <.05 in all figures.
Rates of food and drug intake during delay manipulations
As in the previous study, where rats chose between oxycodone and delayed food, rats obtained 80–95% of the maximum number of reinforcers at all delays except the longest, where there was a small but significant decrease in efficiency, to 74% [Fig. 1b; Delay: F(4,36)=4.06, P<.009]. As the food delay increased, drug intake (i.e., the number of times the drug hole was chosen; Figs. 1b and 1d, diamond symbols) increased and food intake (Figs. 1c and 1d, square symbols) decreased [Figs. 1c and 1d; Dose × delay interaction: F(10,57)=15.49, P<.0001]. The decreased efficiency of responding (Fig. 1b) at the longest delay appears to be due to slight asymmetries in the food and drug intake curves (Figs. 1a and 1d), with the number of drug reinforcers leveling off at the two longest delays.
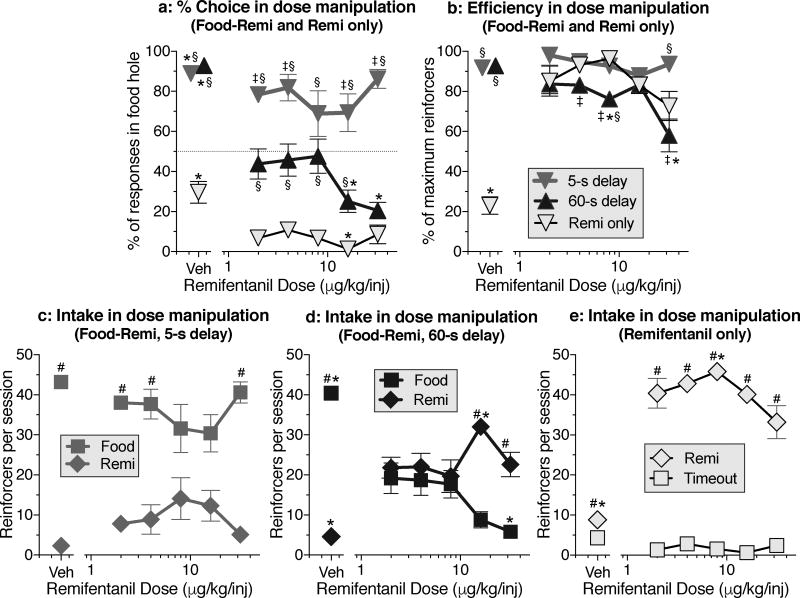
Remifentanil dose-effect curves
There were clear separations between the remifentanil dose-effect curves for percent choice obtained with a food delay of 5 s, with a food delay of 60 s, and with no food available (i.e., remifentanil only) [Fig. 2a; Dose × schedule interaction: F(10,57)=6.16, P<.0001]. Food was preferred over drug when the food delay was only 5 s (Fig. 2a, gray symbols), and drug was preferred in the remifentanil-only condition (Fig. 2a, outlined symbols), but the dose of remifentanil had little or no effect on choice within these two conditions. In contrast, choice was dose-dependent when the food delay was 60 s (Fig. 2a, black symbols), with food chosen about as often as drug when the dose was 2–8 µg/kg/inj, and remifentanil clearly preferred when the dose was 16–32 µg/kg/inj. Food was strongly preferred over vehicle injection regardless of whether the food delay was 5 s or 60 s (Fig. 2a, unconnected gray and black symbols). When neither drug nor food were available (i.e., in the vehicle condition of the remifentanil-only schedule, Fig. 2a, unconnected outlined symbols), 70% of responses occurred in the drug hole.
Figure 2.
Dose-effect curves for remifentanil when the alternative was food delayed by 5 s, food delayed by 60 s, or timeout with no food delivery (i.e., "Remi only"). a: Percent choice of the food hole as a function of remifentanil dose. (Details of within-curve differences: In the 5-s curve, vehicle differs from 2. In the 60-s curve, vehicle differs from all other doses, and 16 and 32 each differ from 8. In the remi-only curve, vehicle differs from 2 and 8, and 16 differs from all other doses except 32.) b: Efficiency of responding during the dose-effect determinations. (Details of within-curve differences: Within the 60-s curve, 8 differs from vehicle, and 32 differs from vehicle, 2 and 16. Within the remi-only curve, vehicle differs from all other doses.) c: Intake (i.e., number of choices) of food and remifentanil during dose-effect determinations at 5-s food delay. d: Intake of food and remifentanil during dose-effect determinations at 60-s food delay. (Details of within-curve differences: For remifentanil, vehicle differs from all other doses, and 16 differs from 8. For food, vehicle differs from all other doses, and 32 differs from 4 and 8.) e: Number of choices of remifentanil and timeout when food hole choices produced timeout with no food delivery. (Details of within-curve differences: For remifentanil, vehicle differs from all other doses, and 8 differs from 32.) * differs within curve. ‡ differs between 5-s and 60-s curves at the same dose. § differs from remifentanil-only condition at the same dose. # differs between food and drug hole at same dose.
Rates of food and drug intake during dose manipulations
The efficiency of responding was mostly high during the dose manipulations, but decreased when the food delay was 60 s and the highest dose of remifentanil was available [Fig 2b; Dose × schedule interaction: F(10,57)=51.18, P<.0001]. Dose-effect curves for food and drug intake showed very different profiles across the 5-s delay schedule (Fig. 2c), the 60-s delay schedule (Fig. 2d), and the remifentanil-only schedule (Fig. 2e) [Dose × schedule × hole interaction: F(10,57)=15.49, P<.0001]. With a 5-s delay, food intake (Fig. 2c, square symbols) was higher than drug intake (Fig. 2c, diamond symbols) at the lowest and highest doses, but not at the middle doses (Fig. 2c). With a 60-s delay (Fig. 2d), the food and drug holes were chosen about equally when the dose was between 2 and 8 µg/kg/inj, but the curves separated at higher doses due to increased number of injections at 16 µg/kg/inj and decreased number of food reinforcers at 32 µg/kg/inj. In the remifentanil-only schedule (Fig. 2e), there was a shallow inverted-U dose response curve in the drug hole, and very low rates in the food hole, which only produced timeout; when vehicle was substituted for drug in this schedule, response rates in both holes were low, but there was more responding in the drug hole than the food hole (Fig. 2e, unconnected diamond vs. unconnected square). When vehicle was substituted for drug in the food-remifentanil choice schedule with 5-s or 60-s delay (unconnected symbols in Figs. 2c and 2d), injection rates were low and food intake was high.
Effects of treatment drugs
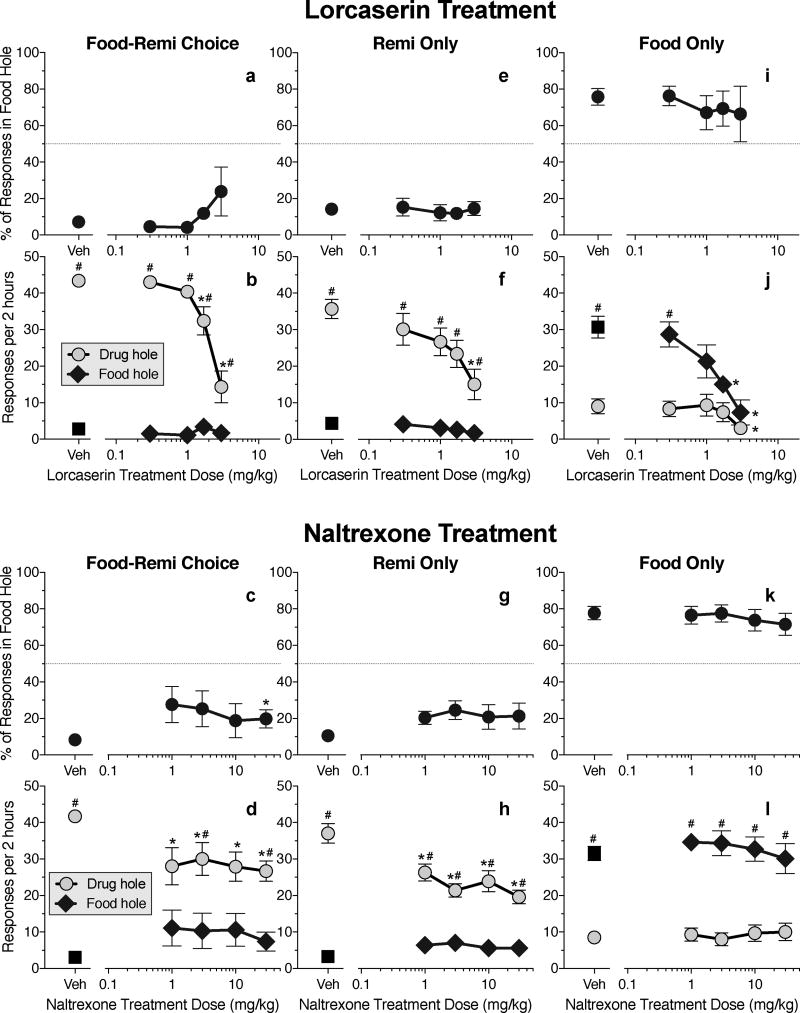
Food-remifentanil schedule with remifentanil preferred
When the food delay was individually adjusted to obtain a baseline with ~90% preference for the 4 µg/kg/inj dose of remifentanil in each rat, the mean (± SEM) delay value was 56.2 (± 9.5) s. Baseline preference was stable (range: 87–97% drug choice) throughout treatment-drug testing with the food-remifentanil schedule, without further adjustment of delay values. Lorcaserin treatment did not have a significant effect on percent choice of the food hole (Fig 3a), but it decreased remifentanil intake [Fig 3b; Dose × hole interaction: F(4,26)=12.82, P<.0001]. Treatment with the highest dose of naltrexone (30 mg/kg) increased choice of food by about 10 percentage points [Fig. 3c; Dose × hole interaction: F(4,24)=5.22, P<.004]; although this effect was only significant at the highest dose of naltrexone (due to between-subject variability at lower doses), the dose-effect curve was flat, and remifentanil was still preferred over food under all doses of naltrexone treatment. All four doses of naltrexone decreased remifentanil intake without affecting food intake [Fig. 3d; Dose × hole interaction F(4,24)=7.14, P<.0006].
Figure 3.
Effects of treatment drugs on choice between immediate remifentanil ("remi") and delayed food (food-remi choice), on self-administration of remifentanil when the alternative response produced only timeout (remi only), and on self-administration of delayed food when the alternative response produced only timeout (food only). a: Percent choice of the food hole as a function of lorcaserin dose in the food-remi choice schedule. b: Intake of food and remifentanil (choices per session) as a function of lorcaserin dose in the food-remi schedule. (Details of within-curve differences: For the drug hole, 1.7 differs from vehicle and 3, and 3 differs from all other doses.) c: Percent choice of the food hole as a function of naltrexone dose in the food-remi choice schedule. (Details of within-curve difference: 30 differs from vehicle.) d: Intake of food and remifentanil as a function of naltrexone dose in the food-remi choice schedule. (Details of within-curve differences: For the drug hole, all doses differ from vehicle.) e: Percent choice of the food hole as a function of lorcaserin dose in the remi-only schedule. f: Number of choices of the food and remifentanil holes as a function of lorcaserin dose in the remi-only schedule. (Details of within-curve differences: For the drug hole, 3 differs from vehicle.) g: Percent choice of the food hole as a function of naltrexone dose in the remi-only schedule. h: Number of choices of the food and remifentanil holes as a function of naltrexone dose in the remi-only schedule. (Details of within-curve differences: For the drug hole, all points differ from vehicle.) i: Percent choice of the food hole as a function of lorcaserin dose in the food-only schedule. j: Number of choices of the food and remifentanil holes as a function of lorcaserin dose in the food-only schedule. (Details of within-curve differences: For the food hole, 1.7 and 3 differ from vehicle and 0.3. For the drug hole, 3 differs from vehicle.) k: Percent choice of the food hole as a function of naltrexone dose in the food-only schedule. l: Number of choices of the food and remifentanil holes as a function of naltrexone dose in the food-only schedule. * differs within curve. # differs between food and drug holes at the same dose.
Remifentanil-only and food-only schedules
Lorcaserin and naltrexone did not affect percent choice under the remifentanil-only (Figs. 3e and 3g) or food-only (Figs. 3i and 3k) schedules. However, the highest dose of lorcaserin decreased drug intake in the remifentanil-only schedule [Fig. 3f; Dose: F(4,24)=4.71, P<.006; Hole: F(1,6)=80.2, P<.0001], and the two highest doses of lorcaserin decreased food intake in the food-only schedule [Fig. 3j; Dose × hole interaction: F(4,24)=4.51, P<.008]; the highest dose of lorcaserin also decreased nonreinforced responding in the drug hole in the food-only schedule (Fig. 3j, circle symbols). All four doses of naltrexone decreased drug intake in the remifentanil-only schedule [Fig. 3h; Dose × hole interaction: F(4,24)=9.89, P<.0001] but did not affect responding in either hole under the food-only schedule (Fig. 3l; Hole: F(1,6)=105.9, P<.0001).
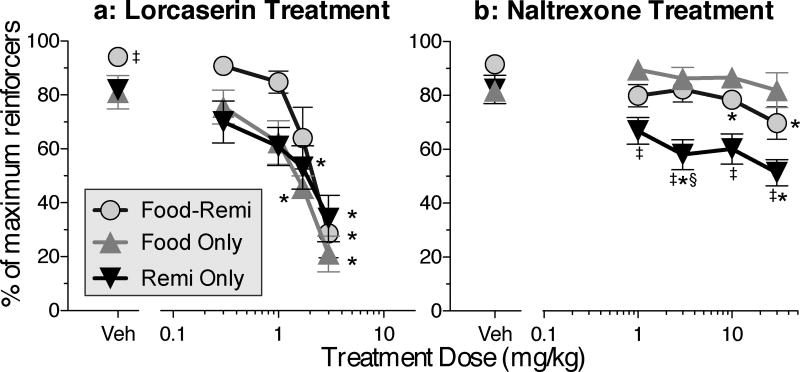
Efficiency
The higher doses of lorcaserin decreased the efficiency of responding in all three schedules [Fig. 4a; Dose: F(4,36)=32.97, P<.0001; Schedule: F(2,10)=6.93, P<.02]. All doses of naltrexone decreased efficiency in the remifentanil-only condition, and the two highest doses of naltrexone slightly decreased efficiency in the food-remifentanil schedule, but no dose of naltrexone affected efficiency in the food-only schedule [Fig. 4b; Dose × schedule interaction: F(8,35)=3.0, P<.02].
Figure 4.
Efficiency of responding during the same sessions depicted in Figure 3. a: Efficiency after treatment with lorcaserin. (Details of within-curve differences: For food-remi choice, 3 differs from all doses except 1.7. For food only, 1.7 and 3 differ from all others. For remi only, 1.7 and 3 differ from vehicle.) b: Efficiency after treatment with naltrexone. (Details of within-curve differences: For food-remi choice, 10 and 30 differ from vehicle. For remi only, 3 and 30 differ from vehicle.) * differs within curve. ‡ differs from food-only at the same dose. § differs from food-remi choice at the same dose.
Choice between smaller/sooner food and larger/later food
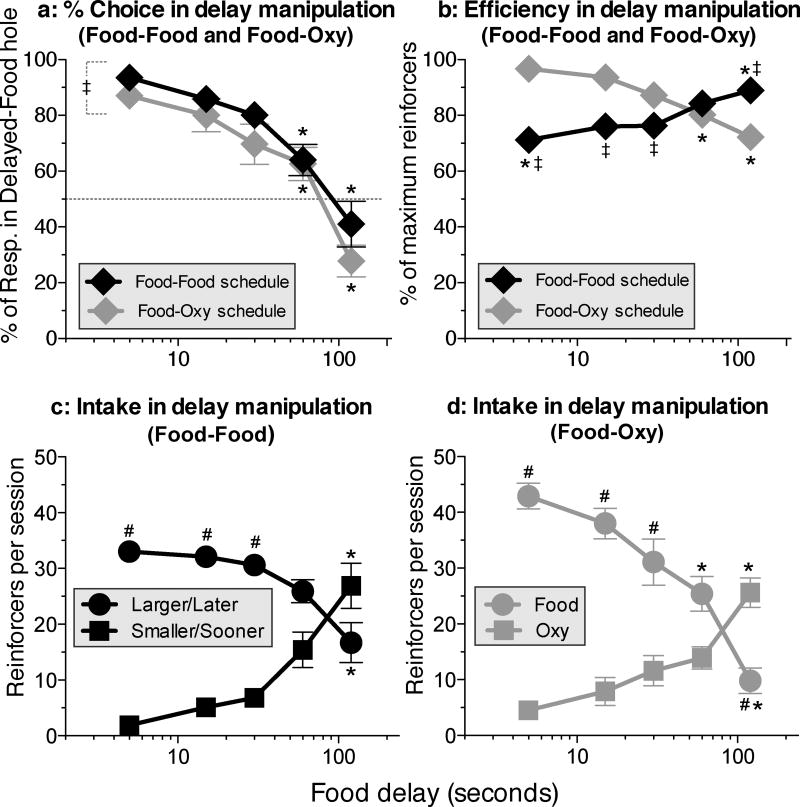
The overall shape of the delay curve obtained with choice between smaller/sooner food vs. larger/later food (Fig. 5a, black diamonds) was similar to the curves obtained with oxycodone vs. food in the same rats (Fig. 5a, gray diamonds), with a slight but significant downward shift (i.e., reduced percent choice of the delayed option) in the food-oxycodone condition [Fig. 5a; Schedule: F(1,7)=5.71, P<.05]. When the delay was 5 s for both the 1-pellet and 3-pellet options, the 3-pellet hole was strongly preferred (Fig. 5a, leftmost black diamond); but percent choice switched toward the 1-pellet (smaller/sooner) option as the delay for the 3-pellet (larger-later) option was increased [Fig. 5a; Delay: F(4,28)=46.0, P<.0001]. Efficiency increased as a function of delay in the food-food condition (Fig. 5b, black diamonds), but decreased as a function of delay in the food-oxycodone condition (Fig. 5b, gray diamonds) (Fig. 5b; Schedule × delay interaction: F(4,26)=12.43, P<.0001). The reinforcement-rate curves from the food-food condition (Fig. 5c) were similar to the curves obtained the oxycodone-food condition (Fig. 5d), showing a crossover to the immediate alternative as the delay increased (Hole × delay interaction: F(4,28)=17.2, P<.0001). However, there were fewer choices of the 3-pellet hole at 5-s delay in the food-food condition (Fig. 5c, black circles), compared to choices of the single-pellet hole at 5-s delay in the food-oxycodone condition (Fig. 5d, gray circles) (Figs. 5c and 5d; Schedule × delay interaction: F(4, 26=5.1, P<.004); this difference was probably due to satiation from the larger food option and is responsible for the upward slope of the food-food efficiency curve (Fig. 5b, black diamonds).
Figure 5.
Choice between larger/later food (3 pellets) and smaller/sooner food (1 pellet) in the food-food schedule, and choice between delayed food (1 pellet) and immediate injection of 50 µg/kg of oxycodone in the 'food-oxy' schedule in the same rats. a: Percentage of responses in the larger-later hole of the food-food schedule, or in the food hole of the food-oxy schedule. (Details of within-curve differences: For food-food and for food-oxy, 120 differs from all other delays, and 60 differs from 5.) b. Efficiency (Details of within-curve differences: For food-food, 120 differs from all other delays except 60, and 60 differs from 5 and 15. For food-oxy, 120 differs from all other delays except 60, and 5 differs from all other delays.) c: Intake (i.e., choices per session) for the larger/later food and smaller/sooner food holes. (Details of within-curve differences: Within the larger/later hole, and also within the smaller/sooner hole, 120 differs from all other delays except 60.) d: Choices per session of the delayed-food and immediate oxycodone holes under the food-oxy schedule. (Details of within-curve differences: For the oxycodone hole, 120 differs from all other delays. For the food hole, 120 differs from all other delays, and 60 differs from 5 and 15.) * differs within curve. ‡ differs between food-food and food-oxy curves at the same delay. # differs between larger/later hole and smaller/sooner hole at same delay, or between food hole and oxycodone hole at the same delay.
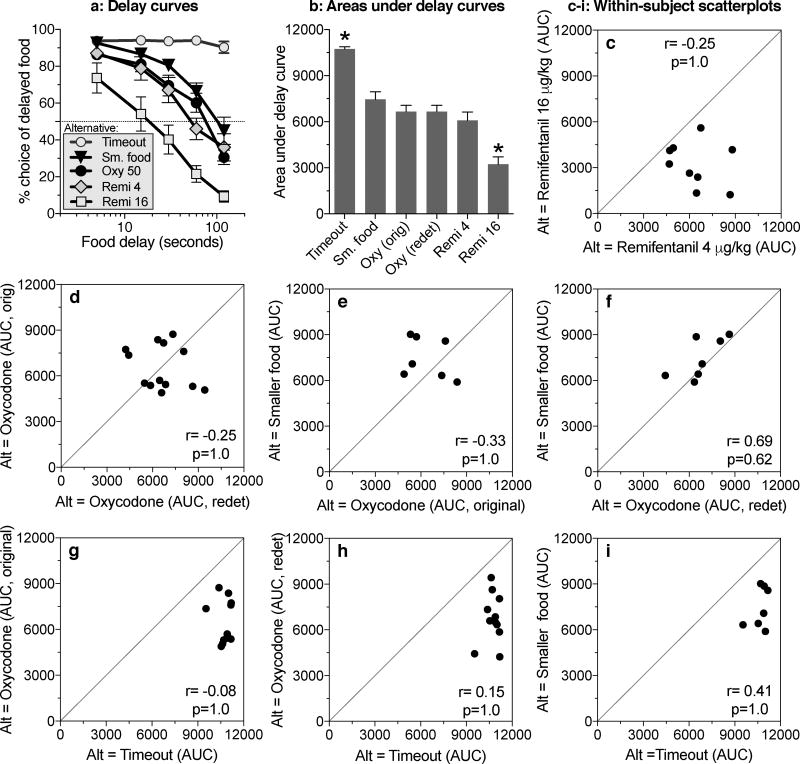
Between-experiment comparisons
Delay curves are shown in Fig. 6a for all conditions studied in this series of experiments. These data were used to calculate area under the curve, providing a single measure of choice for each subject in each condition, summarized across all delays (Fig. 6b). Area under the curve was affected by the nature of the alternative to delayed food [Fig. 6b; F(5,35)=28.85, P<.0001], with two noticeable effects. First, all of the alternatives that included primary reinforcement decreased choice of the delayed-food option relative to the timeout condition; and second, among these alternatives, the 16 µg/kg/inj dose of remifentanil produced the strongest decrease. The difference between the two remifentanil conditions (4 and 16 µg/kg/inj) produced a Cohen's d of 1.77, considered a 'very large' effect size (Sawilowsky 2009).
Figure 6.
Between-experiment and within-subject comparisons of discounting curves. a: Percent choice of delayed food (mean ± SEM) in conditions where the alternative was: timeout with no reinforcement; immediate delivery of a smaller amount of food ('Sm. food'); immediate injection of 50 µg/kg of oxycodone ('Oxy 50'; from the 'redetermined' curve of Secci et al. 2016); immediate injection of 4 µg/kg of remifentanil ('Remi 4'); or immediate injection of 16 µg/kg of remifentanil ('Remi 16'). b: Area under the curve (mean +SEM) for the same conditions, plus the original ('orig') oxycodone curve obtained by Secci et al. (2016). c, d, e, f, g, h, i: Scatterplots assessing within-subject consistency of discounting curves for delayed food across the conditions with various alternative ('Alt') options. For each scatterplot, the Pearson correlation coefficient (r) and probability (p) values are reported numerically. * Areas under the delay curve for the 'Timeout' and 'Remi 16 µg/kg' conditions differ from all other conditions.
Within-subject comparisons
To assess within-subject consistency of delay discounting, scatterplots and correlations of area under the curve were produced for each pair of conditions that shared a group of subjects (Figs. 6c, d, e, f, g, h, i). These correlations were all nonsignificant, indicating that individual rats with strong discounting relative to other rats in one condition did not tend to show strong discounting under other conditions, or even under the same condition when tested twice (original vs. redetermined oxycodone curves).
DISCUSSION
The value of the food option was determined by how much the food was delayed, and also by the nature of the alternative option. That is, delayed food was chosen less often when the alternative was a high dose of remifentanil than when the alternative was a moderate dose of remifentanil, a moderate dose of oxycodone, or a smaller, more immediate delivery of food. This effect can be appreciated by considering the amount of delay required to produce 50% choice of the alternative reward in the delay curves: 95 s for smaller/sooner food, 75 s for oxycodone, 50 s for the moderate dose of remifentanil, and 20 s for the high dose of remifentanil. There was little or no discounting when the alternative to delayed food was timeout with no reinforcement. Although the comparison is complicated by the fact that the delayed option was three pellets in the food-food condition and one pellet in the food-drug conditions, the fact that 50% choice of smaller/sooner food occurred at delays that were comparable to those in the other conditions studied under our basic choice procedure is informative, given that 50% choice of smaller/sooner food often occurs at delays of less than 30 s under other procedures (e.g., Evenden and Ryan 1996; Peterson et al. 2015; Renda et al. 2016; Stein et al. 2015) and that there have been very few studies of choice between drugs and delayed food in rodents.
One of the goals of this series of experiments has been to develop streamlined procedures for studying choice between drug rewards and delayed nondrug rewards in rats, because 1) intravenous catheters can only be maintained for a limited period of time in rats, and 2) the procedures that have been used with nonhuman primates are extremely time-consuming (Woolverton and Anderson 2006). To this end, the same delayed nondrug reward (a single food pellet) was used for all delay curves except the food-food curve. Since the percent choice curve and the area under this curve could be affected by the nondelayed values of the drug and nondrug options, or by the difference between these two values, the shape of the curves obtained in these experiments might not generalize to other amounts of food. More complete information could be obtained by determining indifference points between delayed and nondelayed rewards over a wider range of values (Bickel et al. 2011; Huskinson et al. 2015; Mazur 1987). However, despite this limitation, the comparisons obtained with the streamlined procedure clearly demonstrate that the nature of the immediate drug option strongly affects the value of the delayed nondrug option.
Most studies of delay discounting in humans and animals have involved isomorphic situations, where the options involve different magnitudes of the same reinforcer, but a few studies have involved allomorphic situations, where two different reinforcers are available. Bickel et al. (2011) found a trend for cocaine addicts to discount delayed money more when the alternative was immediate cocaine than when the alternative was immediate money. Huskinson et al. (2015) found that rhesus monkeys discounted delayed food more steeply when the alternative was immediate cocaine than when the alternative was immediate food. These findings are consistent with the steep discounting of delayed food that we observed when the alternative to delayed food was immediate delivery of a 16 µg/kg/inj dose of remifentanil, and they are consistent with similar effects in rhesus monkeys (Maguire et al. 2013). These demonstrations that highly-salient rewards (food and money) can be discounted at different rates depending on the nature of the alternative option are potentially important and warrant further investigation, including study of the neural underpinnings (Platt et al. 2010).
Earlier we found that choice between oxycodone and 120-s delayed food was not substantially affected by the unit dose of oxycodone (Secci et al. 2016). However, choice between immediate remifentanil and delayed food was dose dependent in the present study. The sharp peak in the dose-effect function when rats chose between remifentanil and 60-s delayed food (Fig. 2d) agrees with the peaked dose-effect function obtained earlier in rats with a progressive ratio schedule of remifentanil self-administration, where the 16 µg/kg/inj dose maintained the highest breaking point, equivalent to the highest breaking point maintained by heroin (Panlilio and Schindler 2000). In the remifentanil-only condition, the rates of injection at the three highest doses (Fig. 2e; 0.38, 0.33 and 0.28 inj/min, respectively) closely match those obtained earlier with a simple one-response fixed-ratio (FR1) schedule with 5-s timeout (Fig. 1a of Panlilio & Schindler, 2000; 0.37, 0.33 and 0.25 inj/min, respectively). At lower doses (2 and 4 µg/kg/inj), spontaneous post-injection pauses in the FR1 schedule with 5-s timeout in the earlier study were shorter than 150 s, and consequently rates of intake were much higher (1.02 and 0.56 inj/min, respectively) than in the remifentanil-only condition (0.34 and 0.36 inj/min, respectively) of the present study, where all conditions included a 150-s timeout. Overall, these comparisons suggest that the food-drug choice schedule is akin to a progressive-ratio schedule in that it reflects the relative reinforcing efficacy of the drug and food options (Lenoir and Ahmed 2008; Richardson and Roberts 1996; Stafford et al. 1998), as opposed to a traditional FR1 schedule (with active and inactive response alternatives and short timeouts), which largely reflects pharmacokinetics (Panlilio et al. 2003; Panlilio et al. 2008).
Food and remifentanil intake functions during delay testing were slightly asymmetrical, with the number of injections leveling off as the number of food deliveries decreased between the 60 and 120 s delays (Figs 1c and 1d). Similar asymmetry was observed earlier with food-oxycodone choice (Secci et al. 2016). At the 16 µg/kg/inj dose of remifentanil, the leveling effect could be due to spontaneous post-injection pausing; at this dose, rats took 20 inj/hour under FR1 with 5-s timeout (Panlilio and Schindler 2000), about the same rate seen in the remifentanil-only schedule (Fig. 2e) and the choice schedule at 60-s and 120-s food delays (Fig. 1d). However, spontaneous post-injection pausing does not explain the asymmetry at the 4 µg/kg/inj dose; rats took 34 inj/hour at this dose under FR1 (Panlilio and Schindler 2000), much more than the 21 inj/hour seen in the remifentanil-only schedule (Fig. 2e) or the 12 inj/hour seen in the choice schedule at 60-s and 120-s food delays (Fig. 1c). In any case, despite the asymmetry, the overall efficiency of choice responding remained high and did not differ significantly between the 60-s delay and the 120-s delay (Fig. 1b).
Naltrexone decreased opioid intake in the remifentanil-only and food-remifentanil schedules of the present study and in the food-oxycodone schedule of the previous study (Secci et al. 2016). Naltrexone did not significantly affect food intake in the food-only schedule or the food-remifentanil schedule, but it increased food intake in the food-oxycodone schedule. Thus, naltrexone reversed the preference for oxycodone over food, but produced only a slight decrease in preference for remifentanil over food. However, the fact that efficiency of responding was decreased substantially in the remifentanil-only schedule but not the food-remifentanil choice schedule indicates that there was a partial compensatory switching to food when the effects of remifentanil were blocked. Theoretically, if high-dose naltrexone treatment were continued for many sessions, choice of the drug hole should extinguish as in the food-only condition; this should be tested in the future and could provide a useful way to determine how variables such as training dose affect the persistence of drug choice. In the present study, the failure of acute naltrexone treatment to fully reverse the preference for remifentanil could be due to remifentanil having a higher reinforcing efficacy than oxycodone, thereby producing a conditioned preference or habitual responding that might only be reversed after extended experience with naltrexone under choice conditions. Notably, the rats that were used in the treatment-drug experiments were initially trained with 16 µg/kg remifentanil and showed steeper discounting of food in the food-remifentanil condition (90% drug choice at 56-sec delay, Fig. 3a) compared to those initially trained with 4 µg/kg (70% drug choice at 120-s delay, Fig. 1a, black symbols). The likelihood that there was a bias related to conditioned or habitual responding is also supported by the observation of 70% responding was in the drug hole when neither hole produced reinforcement.
Lorcaserin decreased remifentanil self-administration in the remifentanil-only condition and in the food-remifentanil condition. However, the same doses of lorcaserin also decreased food self-administration in the food-only condition. Lorcaserin decreased the efficiency of responding in all three of theses conditions, consistent with decreases in both food and drug reward, or a nonspecific disruption of operant behavior. In some previous studies, lorcaserin decreased nicotine self-administration at a dose that did not affect food self-administration (0.3 mg/kg) (Briggs et al. 2016; Levin et al. 2011), but in other studies nicotine and food self-administration were both decreased at about the same dose of lorcaserin (0.6 mg/kg) or other 5-HT2C agonists (Higgins et al. 2013; Higgins et al. 2012). In the recent study showing that lorcaserin doses of 1 mg/kg and higher decrease oxycodone self-administration (Neelakantan et al. 2017), the effects of lorcaserin on food self-administration were not tested. In previous studies of impulsivity, delay discounting was increased by serotonin depletion (Mobini et al. 2000) or treatment with the 5-HT2A/C agonist DOI (Hadamitzky et al. 2009), and decreased by the 5-HT2C/B agonist SER-082 (Talpos et al. 2006), but it was not affected by lorcaserin (0.1–0.6 mg/kg) in what appears to be the only previous study where lorcaserin was tested (Higgins et al. 2016). It has been proposed that 5-HT2A and 5-HT2C receptors have opposing effects, such that impulsivity can be decreased by either a 5-HT2A antagonist or a 5-HT2C agonist (Homberg 2012). However, there are clearly different forms of impulsivity (Talpos et al. 2006), and lorcaserin might affect premature operant responding more than it affects delay discounting (Higgins et al. 2016; Higgins et al. 2012).
The choice procedure used here is intended to model a specific kind of behavior that is a component of addiction, and it could be valuable to determine whether it is sensitive to manipulations that model other components of addiction. For example, long-access sessions increase the rate of drug intake (Lenoir et al. 2013), and the value of a self-administered drug can be increased by manipulating access conditions (Roberts et al. 2013) or making the drug function as a negative reinforcer (through withdrawal relief; Negus 2006). Procedures such as these might increase preference for immediate drug reward over delayed nondrug reward (i.e., increase the steepness of the delay discounting curves).
Humans who show steep delay discounting under one condition also tend to show steep discounting in other conditions (Bickel et al. 2011; Myerson et al. 2003; Odum 2011). Rats typically show consistency when retested with the same procedure (Renda and Madden 2016), but can be inconsistent across alternative forms of delay-discounting tests (Peterson et al. 2015), and individuals with the steepest discounting may be less extreme in later tests (Stein et al. 2015). In our procedure, we found no evidence for trait-like consistency when individual rats were tested under multiple conditions. Delay discounting has been proposed as a biomarker for addiction, and often predicts likelihood of success in maintaining abstinence, but trait vs. state distinctions in that relationship remain unclear (Bickel, 2014). Specifically, steep discounting might be genetically determined (Anokhin et al. 2011; Anokhin et al. 2015), or it might result from acute or chronic exposure to stress, drugs, or an environment with limited options for reward. Interestingly, extended experience with delayed reinforcement can produce lasting decreases in delay discounting in rats (Renda and Madden 2016). Our findings suggest that discounting can be strongly influenced by the current environment. Thus, the possibility that delay discounting behavior could be susceptible to change through therapeutic interventions (e.g., contingency management) that enhance the relative value of delayed non-drug reinforcers (Dugosh et al. 2016; Holtyn et al. 2014; Lussier et al. 2006) is important for future study at the behavioral and neurobiological levels.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Drug Abuse.
Footnotes
The authors declare no conflicts of interest.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of delay discounting in adolescence: a longitudinal twin study. Behav Genet. 2011;41:175–83. doi: 10.1007/s10519-010-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Grant JD, Mulligan RC, Heath AC. The genetics of impulsivity: evidence for the heritability of delay discounting. Biol Psychiatry. 2015;77:887–94. doi: 10.1016/j.biopsych.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: A candidate behavioral marker of addiction. Neuropharmacology. 2014;76(Pt B):518–27. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Landes RD, Christensen DR, Jackson L, Jones BA, Kurth-Nelson Z, Redish AD. Single-and cross-commodity discounting among cocaine addicts: the commodity and its temporal location determine discounting rate. Psychopharmacology (Berl) 2011;217:177–87. doi: 10.1007/s00213-011-2272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs SA, Hall BJ, Wells C, Slade S, Jaskowski P, Morrison M, Rezvani AH, Rose JE, Levin ED. Dextromethorphan interactions with histaminergic and serotonergic treatments to reduce nicotine self-administration in rats. Pharmacol Biochem Behav. 2016;142:1–7. doi: 10.1016/j.pbb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Gerak LR, Javors MA, France CP. Lorcaserin Reduces the Discriminative Stimulus and Reinforcing Effects of Cocaine in Rhesus Monkeys. J Pharmacol Exp Ther. 2016;356:85–95. doi: 10.1124/jpet.115.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins V, Rose JE, Levin ED. IV nicotine self-administration in rats using a consummatory operant licking response: sensitivity to serotonergic, glutaminergic and histaminergic drugs. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:200–5. doi: 10.1016/j.pnpbp.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugosh K, Abraham A, Seymour B, McLoyd K, Chalk M, Festinger D. A Systematic Review on the Use of Psychosocial Interventions in Conjunction With Medications for the Treatment of Opioid Addiction. J Addict Med. 2016;10:93–103. doi: 10.1097/ADM.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–70. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Collins GT, France CP. Effects of Lorcaserin on Cocaine and Methamphetamine Self-Administration and Reinstatement of Responding Previously Maintained by Cocaine in Rhesus Monkeys. J Pharmacol Exp Ther. 2016;359:383–391. doi: 10.1124/jpet.116.236307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadamitzky M, Feja M, Becker T, Koch M. Effects of acute systemic administration of serotonin2A/C receptor ligands in a delay-based decision-making task in rats. Behav Pharmacol. 2009;20:415–23. doi: 10.1097/FBP.0b013e3283305e11. [DOI] [PubMed] [Google Scholar]
- Harvey-Lewis C, Li Z, Higgins GA, Fletcher PJ. The 5-HT(2C) receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology. 2016;101:237–45. doi: 10.1016/j.neuropharm.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Heyman GM. Addiction and choice: theory and new data. Front Psychiatry. 2013;4:31. doi: 10.3389/fpsyt.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Altherr EB, MacMillan C, Fletcher PJ, Pratt WE. Lorcaserin and CP-809101 reduce motor impulsivity and reinstatement of food seeking behavior in male rats: Implications for understanding the anti-obesity property of 5-HT2C receptor agonists. Psychopharmacology (Berl) 2016;233:2841–56. doi: 10.1007/s00213-016-4329-3. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Lau W, de Lannoy IA, Lee DK, Izhakova J, Coen K, Le AD, Fletcher PJ. Evaluation of chemically diverse 5-HT(2)c receptor agonists on behaviours motivated by food and nicotine and on side effect profiles. Psychopharmacology (Berl) 2013;226:475–90. doi: 10.1007/s00213-012-2919-2. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ. The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology. 2012;37:1177–91. doi: 10.1038/npp.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtyn AF, Koffarnus MN, DeFulio A, Sigurdsson SO, Strain EC, Schwartz RP, Silverman K. Employment-based abstinence reinforcement promotes opiate and cocaine abstinence in out-of-treatment injection drug users. J Appl Behav Anal. 2014;47:681–93. doi: 10.1002/jaba.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR. Serotonin and decision making processes. Neurosci Biobehav Rev. 2012;36:218–36. doi: 10.1016/j.neubiorev.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Huskinson SL, Woolverton WL, Green L, Myerson J, Freeman KB. Delay discounting of food by rhesus monkeys: Cocaine and food choice in isomorphic and allomorphic situations. Exp Clin Psychopharmacol. 2015;23:184–93. doi: 10.1037/pha0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Maguire DR, Ginsburg BC, Pinkston JW, France CP. Determinants of choice, and vulnerability and recovery in addiction. Behav Processes. 2016;127:35–42. doi: 10.1016/j.beproc.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Supply of a nondrug substitute reduces escalated heroin consumption. Neuropsychopharmacology. 2008;33:2272–82. doi: 10.1038/sj.npp.1301602. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–20. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Johnson JE, Slade S, Wells C, Cauley M, Petro A, Rose JE. Lorcaserin, a 5-HT2C agonist, decreases nicotine self-administration in female rats. J Pharmacol Exp Ther. 2011;338:890–6. doi: 10.1124/jpet.111.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, France CP. Delay discounting of food and remifentanil in rhesus monkeys. Psychopharmacology (Berl) 2013;229:323–30. doi: 10.1007/s00213-013-3121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, France CP. Delay discounting of the mu-opioid receptor agonist remifentanil in rhesus monkeys. Behav Pharmacol. 2016;27:148–54. doi: 10.1097/FBP.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Al-Ruwaitea AS, Ho MY, Bradshaw CM, Szabadi E. Effect of central 5-hydroxytryptamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology (Berl) 2000;149:313–8. doi: 10.1007/s002130000385. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Scott Hanson J, Holt DD, Estle SJ. Discounting delayed and probabilistic rewards: Processes and traits. Journal of Economic Psychology. 2003;24:619–635. [Google Scholar]
- National Research Council. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research National. Academies Press; Washington DC: 2011. [PubMed] [Google Scholar]
- Navarra R, Comery TA, Graf R, Rosenzweig-Lipson S, Day M. The 5-HT(2C) receptor agonist WAY-163909 decreases impulsivity in the 5-choice serial reaction time test. Behav Brain Res. 2008;188:412–5. doi: 10.1016/j.bbr.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Neelakantan H, Holliday ED, Fox RG, Stutz SJ, Comer SD, Haney M, Anastasio NC, Moeller FG, Cunningham KA. Lorcaserin suppresses oxycodone self-administration and relapse vulnerability in rats. ACS Chem Neurosci. 2017 doi: 10.1021/acschemneuro.6b00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–23. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- Odum AL. Delay discounting: trait variable? Behav Processes. 2011;87:1–9. doi: 10.1016/j.beproc.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Katz JL, Pickens RW, Schindler CW. Variability of drug self-administration in rats. Psychopharmacology (Berl) 2003;167:9–19. doi: 10.1007/s00213-002-1366-x. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Schindler CW. Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology (Berl) 2000;150:61–6. doi: 10.1007/s002130000415. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW. A stimulus-control account of regulated drug intake in rats. Psychopharmacology (Berl) 2008;196:441–50. doi: 10.1007/s00213-007-0978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JR, Hill CC, Kirkpatrick K. Measurement of impulsive choice in rats: same- and alternate-form test-retest reliability and temporal tracking. J Exp Anal Behav. 2015;103:166–79. doi: 10.1002/jeab.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ML, Watson KK, Hayden BY, Shepherd SV, Klein JT. Frontiers in Neuroscience Neuroeconomics: Implications for Understanding the Neurobiology of Addiction. In: Kuhn CM, Koob GF, editors. Advances in the Neuroscience of Addiction. CRC Press/Taylor & Francis Llc.; Boca Raton (FL): 2010. [PubMed] [Google Scholar]
- Renda CR, Madden GJ. Impulsive choice and pre-exposure to delays: III. Four-month test-retest outcomes in male wistar rats. Behav Processes. 2016;126:108–12. doi: 10.1016/j.beproc.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Gabriele A, Zimmer BA. Conflation of cocaine seeking and cocaine taking responses in IV self-administration experiments in rats: methodological and interpretational considerations. Neurosci Biobehav Rev. 2013;37:2026–36. doi: 10.1016/j.neubiorev.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawilowsky SS. New Effect Size Rules of Thumb. Journal of Modern Applied Statistical Methods. 2009;8:597–599. [Google Scholar]
- Secci ME, Factor JA, Schindler CW, Panlilio LV. Choice between delayed food and immediate oxycodone in rats. Psychopharmacology (Berl) 2016;233:3977–3989. doi: 10.1007/s00213-016-4429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BM, Smith JM, Tsai JH, Schultz JA, Gilson CA, Estrada SA, Chen RR, Park DM, Prieto EB, Gallardo CS, Sengupta D, Dosa PI, Covel JA, Ren A, Webb RR, Beeley NR, Martin M, Morgan M, Espitia S, Saldana HR, Bjenning C, Whelan KT, Grottick AJ, Menzaghi F, Thomsen WJ. Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J Med Chem. 2008;51:305–13. doi: 10.1021/jm0709034. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 1998;139:169–84. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stein JS, Renda CR, Barker SM, Liston KJ, Shahan TA, Madden GJ. Impulsive choice predicts anxiety-like behavior, but not alcohol or sucrose consumption, in male Long-Evans rats. Alcohol Clin Exp Res. 2015;39:932–40. doi: 10.1111/acer.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpos JC, Wilkinson LS, Robbins TW. A comparison of multiple 5-HT receptors in two tasks measuring impulsivity. J Psychopharmacol. 2006;20:47–58. doi: 10.1177/0269881105056639. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Anderson KG. Effects of delay to reinforcement on the choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 2006;186:99–106. doi: 10.1007/s00213-006-0355-x. [DOI] [PubMed] [Google Scholar]
- Wu X, Pang G, Zhang YM, Li G, Xu S, Dong L, Stackman RW, Jr, Zhang G. Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in heroin-treated mice. Neurosci Lett. 2015;607:23–8. doi: 10.1016/j.neulet.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Wu X, Zhang YM, Liu H, Jiang Q, Pang G, Tao X, Dong L, Stackman RW., Jr Activation of serotonin 5-HT(2C) receptor suppresses behavioral sensitization and naloxone-precipitated withdrawal symptoms in morphine-dependent mice. Neuropharmacology. 2016;101:246–54. doi: 10.1016/j.neuropharm.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]