Abstract
OBJECTIVES
Young women with hyperandrogenism have high risk of metabolic co-morbidities, including increased risk of nonalcoholic fatty liver disease (NAFLD). Whether testosterone (the predominant androgen) is associated with NAFLD independent of metabolic co-factors is unclear. Additionally, whether testosterone confers increased risk of NAFLD in women without hyperandrogenism is unknown.
METHODS
Among women in the prospective population-based multicenter Coronary Artery Risk Development in Young Adults (CARDIA) study, we assessed whether free testosterone levels measured at Year 2 (1987–1988) were associated with prevalent NAFLD at Year 25 (2010–2011) (n=1052). NAFLD was defined using noncontrast abdominal CT scan with liver attenuation≤40 Hounsfield units after excluding other causes of hepatic fat. The association of free testosterone with prevalent NAFLD was assessed by logistic regression.
RESULTS
Increasing quintiles of free testosterone were associated with prevalent NAFLD at Year 25 (adjusted odds ratio (AOR) 1.25, 95% confidence interval (CI) 1.04–1.50, P=0.015), independent of insulin resistance, body mass index, waist circumference, and serum lipids. Importantly, the association persisted among n=955 women without androgen excess (AOR 1.27, 95% CI 1.05–1.53, P=0.016). Visceral adipose tissue (VAT) volume partially mediated the association of free testosterone with NAFLD (mediating effect 41.0%, 95% CI 22–119%).
CONCLUSIONS
Increasing free testosterone is associated with prevalent NAFLD in middle age, even in women without androgen excess. Visceral adiposity appears to play an important role in the relationship between testosterone and NAFLD in women. Testosterone may provide a potential novel target for NAFLD therapeutics, and future studies in pre-menopausal women should consider the importance of testosterone as a risk factor for NAFLD.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) affects 25–30% of the United States population, with a global prevalence that continues to rise (1–3). NAFLD encompasses a spectrum of liver pathology including isolated steatosis and hepatocyte injury in the form ballooning with or without fibrosis, known as nonalcoholic steatohepatitis (NASH). Predictions indicate that NASH cirrhosis will soon become the leading cause of liver-related death globally, and leading indication for liver transplantation in the United States (3–6). Sex differences in NAFLD epidemiology are recognized. While isolated steatosis is more common in men, women have higher rates of NASH, with an estimated 15 million United States women affected (3,7,8). To prevent liver-related deaths due to NASH cirrhosis, there is an urgent need to understand key risk factors for NAFLD development and progression in women.
There is growing recognition that young women with hyperandrogenism represent a high-risk group for NAFLD (2). The mechanism by which androgens may promote hepatic steatosis in women is not well-defined, though increasing testosterone levels in middle-aged women are associated with higher visceral adipose tissue (VAT) volume, a known risk factor for NAFLD (2,9). Small studies among young hyperandrogenic women demonstrate higher prevalence of ultrasound-confirmed steatosis and liver enzyme elevation, independent of body mass index (BMI) and insulin resistance (10). Larger studies evaluating the association between androgens and NAFLD among pre-menopausal women are lacking. It remains unknown whether testosterone is associated with NAFLD independent of metabolic derangements, or whether testosterone is associated with NAFLD among women without hyperandrogenism.
Using data from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort, we aimed to evaluate the association of free testosterone measured in pre-menopausal years on risk of prevalent NAFLD in midlife. We hypothesized that testosterone levels would be associated with prevalent NAFLD in a broad population of women, including those without hyperandrogenism, independent of metabolic factors. If this association were identified, testosterone could represent a novel biomarker of NAFLD in pre-menopausal women, while also providing a potential novel target for therapeutic intervention.
METHODS
Study population
We utilized data from the CARDIA cohort (11,12), and the CARDIA Women’s Study (CWS) (13,14), an ancillary study to CARDIA. The CARDIA cohort is a multicenter community-based longitudinal study of cardiovascular disease in young African American and Caucasian adults aged 18–30 years. Participants were initially recruited in 1985–1986 from 4 cities across the United States (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA) with the intent to enroll equal proportions of African Americans and Caucasians. Subsequent follow-up occurred at Years 2, 5, 7, 10, 15, 20, and 25, with a 72% retention rate by Year 25. The initial cohort included n=5115 participants, of whom n=2790 (55%) were women. Participants were recruited by random-digit dialing from total communities, census tract information, or health-care plan as previously described (11). Institutional review board approval was obtained from all participating centers.
From the initial cohort of CARDIA women (n=2790) we included those that underwent computed tomography (CT) quantification of hepatic steatosis at the Year 25 follow-up examination (n=1805). We excluded women with other potential causes of hepatic steatosis including alcohol use>2 drinks/day (n=79), selfreport of other causes of chronic liver disease (n=18), those with HIV (n=4), and those with history of medication use associated with steatosis, including amiodarone, methotrexate, valproic acid, tamoxifen, steroids, diltiazem, or hormone replacement therapy (n=23). We then excluded women lacking Year 2 androgen measurements (n=629), for a final study sample of n=1052 (Figure 1). Baseline characteristics of women meeting inclusion and exclusion criteria were similar, with statistically significant, though not clinically meaningful differences for age, race, HDL, and triglyceride levels between groups (Supplementary Table 1 online).
Figure 1.
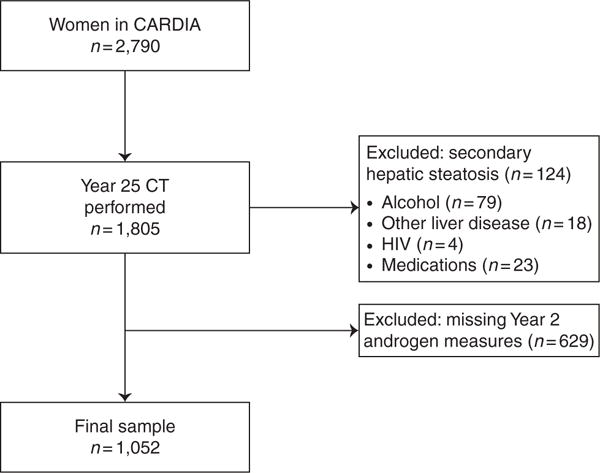
Cohort Selection Criteria.
Measurements
Androgens
Androgen levels were measured as part of the CARDIA Women’s Study from stored fasting serum samples collected at Year 2 (1987–1988), Year 10 (1997–1998), and Year 16 (2003–2004). Blood draws targeted the follicular phase of the menstrual cycle (13). Measurements included total testosterone, free testosterone, and sex hormone binding globulin (SHBG) performed at the Obstetrics and Gynecology Research and Diagnostic Laboratory at the University of Alabama, Birmingham. Total testosterone was measured with a competitive immunoassay (Beckman Coulter, Fullerton, CA) using direct chemiluminescence on the Beckman Access Automated System and SHBG was determined using equilibrium analysis. Total testosterone and SHBG levels were then used to calculate free testosterone, or biologically active testosterone levels (15). Free testosterone levels were measured at Year 2 (n=1049) and in a smaller sample of women at Year 10 (n=974) and Year 16 (n=778). There was a significant correlation between Year 2, 10 and 16 free testosterone measurements (correlation of 0.52 between Years 2 and 10, and 0.45 between Years 2 and 16, P-values <0.001). Year 2 free testosterone was chosen as the primary predictor because these values were available on the largest sample of women and reflect values obtained during pre-menopause, with median age of 28 years (IQR 25–30). Testosterone levels are known to decline with menopausal transition while levels remain relatively stable throughout a woman’s premenopausal years (16,17). In a sensitivity analysis, we evaluated the association of Year 10 free testosterone with prevalent NAFLD to confirm the consistency of estimates using another timepoint from pre-menopausal years. Year 16 values were also explored, although available in a much smaller subset of women (n=778), and also include women approaching peri-menopause (within 5 years of menopause) given a median age of 42 years (IQR 39–44). Quintiles of Year 2 free testosterone were used as the primary predictor after confirming the linear association of increasing quintiles (linear test of trend P=0.001). Free testosterone values by quintile were as follows: quintile 1: 0.01–0.1 ng/dl, quintile 2: 0.11–0.19 ng/dl, quintile 3: 0.20–0.27 ng/dl, quintile 4: 0.28–0.41 ng/dl, and quintile 5: 0.41–9.36 ng/dl. Most studies of androgens and NAFLD derive from women with androgen excess, therefore we also performed sensitivity analyses excluding hyperandrogenic women to assess whether free testosterone was associated with NAFLD among non-hyperandrogenic women. Identification of women with biochemical hyperandrogenism using Year 2 free testosterone has been previously defined in CARDIA using the 95% percentile values for non-oligomenorrheic, non-hirsute women (18), corresponding to a cutoff value of 0.64 ng/dl in our cohort.
Hepatic steatosis
Year 25 non-contrast multidetector abdominal CT scan was performed using GE models 750HD (64) at the Birmingham site, GE LightSpeed VCT (64) in Oakland (GE Healthcare, Waukesha, Wisconsin), and Siemens Sensation 64 at the Chicago and Minneapolis sites (Siemens Medical Solutions, Erlangen, Germany). Quality control and image analysis were performed at a core reading center (Wake Forest University Health Sciences, Winston-Salem, North Carolina). CT diagnosis of hepatic steatosis was defined as liver attenuation (LA) of ≤40 Hounsfield Units (HU). Mean LA values were determined from nine measurements on three CT slices of the right hepatic lobe. Prior studies using unenhanced CT scans have found LA values ≤40 HU to correlate with biopsy confirmed moderate-to-severe steatosis (>30%) (19–21). The characterization of LA in this cohort used a dedicated workflow within the National Institute of Health’s Center of Information Technology Medical Image Processing, Analysis, and Visualization application has been previously described (22). The interclass correlation coefficient between different readers on a random sample of 156 participants was 0.975 for LA, indicating high reproducibility of CT-measured LA in this cohort (22).
Covariates
Participant demographics, medical history, and alcohol use were obtained through standardized surveys as previously described in CARDIA (11). Race was defined as African American or Caucasian by self-report. Medication use was obtained by self-report and subsequent verification of medications brought to study visits. Standard protocols were employed for fasting serum collection and assays of plasma triglycerides (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), total cholesterol (TC), serum glucose and insulin levels (23,24). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the equation: [fasting glucose (mmol/l)×fasting insulin (mU/l)]/22.5. These serum measures were analyzed from baseline and Year 25. Diabetes history was defined as fasting glucose≥126 mg/dl or 2 h glucose tolerance test≥200 mg/dl or hemoglobin HbA1c≥6.5% or use of diabetic medications in the absence of pregnancy at any study visit in CARDIA. Certified technicians performed anthropometric measurements including weight, height and waist circumference using standardized protocols, and BMI calculated as weight (kg)/height (m)2. Waist circumference was measured midway between the iliac crest and bottom of the rib cage. These anthropomorphic measures were also analyzed from baseline and Year 25 visits. Visceral adiposity tissue (VAT) volume was measured on the Year 25 CT scan and reflects the sum of fat voxels within 10 mm set of slices centered at the L4–5 disk within the intraabdominal cavity. Interclass correlation coefficient for the inter-reader comparisons were 0.989 for VAT, and intra- and inter-reader error were 2.4% and 6.7%, respectively in 156 scans after blinded re-evaluation (25).
Statistical analysis
Baseline (Year 0) and Year 25 characteristics were compared using chi-square and Mann-Whitney U tests where appropriate. Baseline measurements were selected for all analyses instead of Year 2 due to missing covariate measurements at Year 2, and good correlation between Year 0 and next available measurements. BMI and waist circumference were not measured at Year 2, though baseline and Year 5 measurements were highly correlated (correlation 0.860.89, P<0.001). HOMA-IR was performed at baseline, then not again until Year 7, although even baseline and Year 7 HOMA-IR values were significantly correlated (correlation 0.45, P<0.001). Lipid measurements were missing in 10–15% of the cohort at Year 2, though total cholesterol, LDL, HDL, and triglycerides at baseline and Year 2 were also highly correlated (correlation values of 0.61–0.68, P values <0.001).
Logistic regression was used to evaluate the association of quintiles of free testosterone at Year 2 and prevalent NAFLD at Year 25. Bivariate models assessed the association of covariates chosen a priori for clinical relevance and known associations with NAFLD. Covariates included age, race, and metabolic parameters including BMI, waist circumference, total cholesterol, LDL, HDL, triglycerides, and insulin resistance (HOMA-IR). To better capture the longitudinal effects of covariates on prevalent NAFLD, metabolic factors were analyzed at baseline as well as change from baseline to Year 25. The final multivariate model was developed using forward selection of covariates including those with two sided P-values<0.05, or strong biologic justification for inclusion. To evaluate the association of VAT (available only at Year 25) and NAFLD, the covariates identified in the final model were evaluated in a separate model using only Year 25 measurements. Finally, triglycerides, HOMA-IR and VAT volume were evaluated as mediators of free testosterone and NAFLD using the STATA mediation package (26), given their potential role along the causal pathway from testosterone to NAFLD in hyperandrogenic women (27). This approach employs regression analyses to capture direct and indirect mediating effects of key variables. Effect modification of these covariates was evaluated by adding the cross-product interaction terms to the final model, with P-values ≤0.10 used to identify statistically significant interactions. Analyses were performed using STATA 12.1 (StataCorp, College Station, TX).
RESULTS
Study cohort characteristics
Of the 1052 women meeting inclusion criteria, 49.6% were African American, with median ages at baseline and Year 25 of 26 years (IQR 23–28) and 51 years (IQR 48–53), respectively. Metabolic co-morbidities were more severe at Year 25 than at study entry including higher median BMI, waist circumference, HOMA-IR, total cholesterol, LDL, and triglycerides (Table 1). At study entry <1% of women had diabetes compared to nearly 13% with this diagnosis by Year 25. Among women with diabetes, 30.1% reported oral agent and/or insulin use. Most women (80.3%) had a history of hormonal contraception use.
Table 1.
Cohort characteristics by study year (n=1,052)
| Characteristic | Baseline (Year 0) | Year 25 | P value |
|---|---|---|---|
| Age, median years (IQR) | 26 (23–28) | 51 (48–53) | <0.001 |
| Race, n (%) | — | — | |
| African American | 522 (49.6) | ||
| Caucasian | 530 (50.4) | ||
| BMI, median kg/cm2 (IQR) | 23.2 (20.8–26.8) | 29.6 (24.6–35.4) | <0.001 |
| Waist circumference, median cm (IQR) | 71.8 (66.5–79.5) | 89.8 (78.3–101.0) | <0.001 |
| Year 25 Visceral adiposity volume, median cm3 (IQR) | — | 100.8 (66.5–148.3) | — |
| Total cholesterol median mg/dl (IQR) | 175 (156–199) | 195 (171–218) | <0.001 |
| HDL median mg/dl (IQR) | 55 (47–64) | 61 (51–73) | <0.001 |
| LDL median mg/dl (IQR) | 105 (88–128) | 110 (90–133) | 0.05 |
| Triglycerides median mg/dl (IQR) | 57 (43–74) | 84 (64–119) | <0.001 |
| Diabetes history, n (%) | 6 (0.6) | 133 (12.8) | <0.001 |
| HOMA-IR | 1.45 (1.18–1.89) | 1.95 (1.11–3.36) | <0.001 |
BMI, body mass index; HDL, high-density lipoprotien; HOMA-IR, Homeostatic model assessment of insulin resistance; IQR, interquartile range; LDL, low-density lipoprotien.
Eighty-nine (8.5%) women met CT definition for NAFLD at Year 25. Compared to women without NAFLD, women with NAFLD had higher baseline and Year 25 BMI, waist circumference, triglycerides, and HOMA-IR, and higher Year 2 free testosterone levels (P values≤0.01). At Year 25, NAFLD women also had lower HDL, higher VAT volume, and were more likely to have diabetes (P<0.001). Racial distribution was not significantly different between groups (Supplementary Table 2).
Median free testosterone levels at Years 2 and 10 were 0.23 ng/dl (IQR 0.13–0.36) and 0.20 ng/dl (IQR 0.11–0.32), respectively, which were significantly correlated (ρ=0.52). This is consistent with the expected stability of free testosterone levels during premenopausal years (16,17). Free testosterone levels were lower by Year 16 with a median value of 0.15 ng/dl, reflecting decline in androgen production as women approach peri-menopausal years (16). Total testosterone and SHBG levels showed similar decline from Years 2 to 16 (test of trend P<0.001) (Table 2). Approximately 6% (n=65) of women in this study met criteria for hyperandrogenism using Year 2 free testosterone, which included n=50 women with a diagnosis of PCOS (18). Only n=24 women (2.3%) had history of spironolactone use, most of whom carried a diagnosis of PCOS. Hyperandrogenic women were slightly younger, more likely to be African American, and had higher baseline and Year 25 BMI and waist circumference than non-hyperandrogenic women. They also had higher levels of Year 25 LDL, HOMA-IR, and VAT volume, although these differences were not statistically significant (Supplementary Table 3).
Table 2.
Sex hormone measurements by study year
| Measurementa | Year 2 (n=1,049) |
Year 10 (n=974) |
Year 16 (n=778) |
|---|---|---|---|
| Age at study year, median years (IQR) | 28 (25–30) | 36 (33–38) | 42 (39–44) |
| Total testosterone, median ng/dl (IQR) | 37 (24–51) | 29 (19–43) | 21 (11–33) |
| Free testosterone, median ng/dl (IQR) | 0.23 (0.13–0.36) |
0.20 (0.11–0.32) |
0.15 (0.08–0.26) |
| Sex hormone binding globulin, median ng/dl (IQR) | 30 (22–43) | 27 (20–38) | 24 (18–36) |
IQR, interquartile range.
Test for trend P<0.001 for all reported measurements.
Free testosterone and prevalent NAFLD
We first evaluated whether free testosterone measured at Year 2 was associated with prevalent NAFLD at Year 25 in unadjusted analysis. Increasing quintiles of free testosterone conferred a nearly 30% increased odds of NAFLD (odds ratio (OR) 1.29, 95% confidence interval (CI) 1.10–1.51, P=0.001). Most metabolic co-factors were also associated with NAFLD on unadjusted analysis, including higher baseline BMI, waist circumference, triglycerides, as well as baseline HOMA-IR. Change from Year 0 to Year 25 BMI, waist circumference, triglycerides, HDL, and HOMA-IR were also associated with NAFLD (Table 3). There was a trend towards a protective effect of hormonal contraception on univariate analysis, although this was not significant on adjusted analysis (adjusted odds ratio (AOR) AOR 0.68, 95% CI 0.39–1.19, P=0.178).
Table 3.
Association of free testosterone with prevalent NAFLD at Year 25
| Characteristic | Univariate analysis | Multivariate analysisa | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | AOR (95% CI) | P-value | |
| Free testosterone at Year 2 (quintiles) | 1.29 (1.10–1.51) | 0.001 | 1.25 (1.04–1.50) | 0.015 |
| Age, per 5 years | 1.01 (0.75–1.37) | 0.933 | ||
| White vs. black race | 1.23 (0.79–1.90) | 0.357 | ||
| CARDIA center | 0.98 (0.81–1.20) | 0843 | ||
| Hormonal birth control use | 0.63 (0.39–1.03) | 0.066 | ||
| BMI (per 5 kg/m2) | 1.36 (1.14–1.62) | <0.001 | — | — |
| ΔBMI (per 5 kg/m2) | 2.01 (1.69–2.40) | <0.001 | — | — |
| Waist circumference (per 1 cm) | 1.04 (1.03–1.06) | <0.001 | 1.05 (1.02–1.08) | <0.001 |
| Δ Waist circumference (per 1 cm) | 1.08 (1.06–1.10) | <0.001 | 1.08 (1.05–1.10) | <0.001 |
| Triglycerides (per 20 mg/dl) | 1.15 (1.06–1.26) | 0.002 | 1.23 (1.09–1.39) | 0.001 |
| Δ Triglycerides (per 20 mg/dl) | 1.14 (1.08–1.20) | <0.001 | 1.09 (1.03–1.15) | 0.002 |
| Total cholesterol (per 10 mg/dl) | 1.01 (0.18–5.64) | 0.989 | ||
| A Total cholesterol (per 10 mg/dl) | 2.15 (0.68–6.85) | 0.193 | ||
| LDL (per 10 mg/dl) | 1.02 (0.95–1.09) | 0.659 | ||
| ΔLDL (per 10 mg/dl) | 1.02 (0.95–1.09) | 0.651 | ||
| HDL (per 5 mg/dl) | 0.93 (0.84–1.01) | 0.095 | ||
| ΔHDL (per 5 mg/dl) | 0.81 (0.74–0.88) | <0.001 | — | — |
| HOMA-IR (per unit) | 1.26 (1.04–1.55) | 0.018 | 0.81 (0.56–1.16) | 0.243 |
| Δ HOMA-IR (per unit) | 1.32 (1.23–1.42) | <0.001 | 1.11 (1.04–1.18) | 0.002 |
AOR, adjusted odds ratio; BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; CI, confidence interval; HDL, high-density lipoprotien; HOMA-IR, Homeostatic model assessment of insulin resistance; LDL, low-density lipoprotein; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio.
Adjusted for baseline and change in metabolic covariates from Year 0 to 25
ΔHDL, baseline and ΔBMI, and hormonal birth control use no longer significant on adjusted analysis (n=1,015).
Δ values reflect unit change from Years 0 to 25 to capture potential confounding effects of metabolic parameters over time.
On multivariate analyses, increasing quintiles of free testosterone remained independently associated with NAFLD (AOR 1.25, 95% CI 1.04–1.50, P=0.015), after adjustment for baseline and change in waist circumference, HOMA-IR, and triglycerides. BMI and HDL were no longer associated with NAFLD on adjusted analysis (Table 3). Although age and race have known associations with NAFLD risk (2), forcing these covariates into the adjusted model minimally affected free testosterone estimates (AOR 1.24, 95% CI 1.04–1.49, P=0.020). In a sensitivity analysis using the smaller number of women with Year 10 free testosterone (n=974), the strength of the association with prevalent NAFLD was similar on unadjusted analysis (OR 1.36, 95% CI 1.15–1.61, P<0.001) and after adjustment for the same metabolic parameters as the Year 2 model (AOR 1.22, 95% CI 1.00–1.48, P=0.050). Even fewer women had repeat hormone values at Year 16 (n=778), with similar association on unadjusted analysis (OR 1.45, 95% CI 1.14– 1.84, P<0.001). Although the direction of the association remained consistent on adjusted analysis, this was no longer significant (AOR 1.14, 95% CI 0.87–1.49, P=0.337). We then performed sensitivity analyses excluding the n=65 women (6.2%) meeting criteria for hyperandrogenism (18). Importantly, Year 2 free testosterone remained associated with prevalent NAFLD among non-hyperandrogenic women (AOR 1.27, 95% CI 1.05–1.53, P=0.016).
We further evaluated whether Year 2 free testosterone was associated with NAFLD when only considering baseline covariates, reflecting an age that preceded the development of metabolic co-morbidities in the majority of women. Year 2 free testosterone was strongly associated with prevalent NAFLD, independent of baseline triglycerides and waist circumference (AOR 1.24, 95% CI 1.05–1.46, P=0.013; Supplementary Table 4). Finally, we assessed the association of free testosterone with prevalent NAFLD using only Year 25 covariates, which reflect values obtained from the same year as NAFLD diagnosis. VAT volume was measured on Year 25 CT and used in place of waist circumference in Year 25 models given the high correlation of Year 25 waist circumference and VAT volume (correlation 0.74, P-value <0.001). On unadjusted analysis, most Year 25 metabolic covariates were associated with NAFLD, with VAT, HOMA-IR, and triglycerides remaining significant in the multivariate model (Supplementary Table 5a). Supplementary Table 5b demonstrates the effect of sequential adjustment of metabolic covariates on the association of free testosterone and NAFLD. This association was slightly attenuated with addition of Year 25 HOMA-IR and triglycerides (AOR 1.23, 95%CI 1.04–1.45, P=0.017), though inclusion of VAT resulted in a larger attenuation of the point estimate (AOR 1.17, 95% CI 0.98– 1.40, P=0.079).
To explore the mechanism by which testosterone may contribute to NAFLD we conducted formal testing for mediation using Year 25 covariates (26). As expected, VAT was confirmed to be a significant mediator, with 41.0% (95% CI 21.7–119.3%) of the observed association of free testosterone on prevalent NAFLD attributed to VAT volume. Year 25 triglyceride levels were also a statistically significant mediator, although with smaller estimated mediating effect of 12.1% (95% CI 2.8–37.8%). HOMA-IR did not appear to mediate the association of free testosterone and NAFLD (mediating effect of 12.9%, 95% CI −4.7–41.2%).
There was a statistically significant interaction between Year 25 HOMA-IR and free testosterone (P<0.001) suggesting that the association between free testosterone and NAFLD may vary by degree of insulin resistance. However, the association of free testosterone and NAFLD by level of HOMA-IR could not be further explored because all but 7 women with NAFLD also had insulin resistance (defined as HOMA-IR≥2) by Year 25. We also assessed for interactions between free testosterone and race given a higher proportion of African Americans in the subgroup of hyperandrogenic women (Supplementary Table 3), but no significant interaction was identified (P=0.14).
DISCUSSION
Using data from the large prospectively collected CARDIA cohort we identified an association of free testosterone measured in premenopausal women on risk of prevalent NAFLD in midlife. This association was independent of comprehensive metabolic parameters including anthropomorphic measures, lipids, and insulin resistance. Importantly, the association of free testosterone with prevalent NAFLD was apparent even in non-hyperandrogenic women, indicating a broader role of testosterone in the pathogenesis of NAFLD that extends beyond the well-recognized subgroup of women with androgen excess. Visceral adiposity was also identified as a strong, partial mediator of this association, suggesting a potential causal role of VAT along the pathway from androgens to NAFLD in women.
Prior data investigating androgens in NAFLD have largely focused on hyperandrogenic women with the common endocrinopathy known as Polycystic Ovary Syndrome (PCOS) (28). Indeed half of these hyperandrogenic women have imaging confirmed steatosis and concurrent liver enzyme elevation (29–31). The current study represents an important advance in our understanding of testosterone and NAFLD, by highlighting the association of pre-menopausal testosterone levels with subsequent risk of prevalent NAFLD, even in women without androgen excess. Although the onset of NAFLD could not be ascertained in our cohort, it is unlikely that women in their 20 s without metabolic co-morbidities had NAFLD at baseline, particularly during a time period that pre-dated the onset of the obesity and diabetes epidemics (1987–1988) (32). The strong correlation between Year 2 and Year 10 free testosterone measurements also supports a stability of androgen elevation during pre-menopausal years that may set the stage for NAFLD development in later life.
The mechanism by which testosterone promotes NAFLD is likely multifactorial. Visceral adiposity, and to a lesser degree, hypertriglyceridemia, were found to be significant mediators of the association between testosterone and NAFLD, suggesting an important role of VAT in this relationship. These observations are consistent with clinical data noting that administration of synthetic testosterone increases visceral adiposity among post-menopausal women (33). Androgens have also been shown to directly regulate lipid metabolism within visceral and subcutaneous adipocytes in women (34). Rats exposed to high dose androgens during pre-natal periods demonstrate alterations in adipocyte lipolysis, hepatic lipid metabolism and glucose intolerance, and these metabolic derangements are sustained into puberty and adulthood (34,35). These data support the longstanding effects of early androgen exposure on subsequent risk of metabolic syndrome, and may help to explain the strong mediating effects of VAT and serum triglycerides identified in the current study. Surprisingly, insulin resistance was not found to be a significant mediator of the association of free testosterone and NAFLD although we did rely on HOMA-IR, a less sensitive measure of insulin resistance than gold standard euglycemic hyperinsulinemic clamp measurements (36).
Our findings raise the intriguing question as to whether antiandrogen therapy could help to prevent NAFLD in women, or reverse existing disease. In hyperandrogenic women, insulin resistance assessed by clamp measurements improves with spironolactone treatment, an androgen receptor antagonist (37). Spironolactone also increases glucose uptake in human adipocytes (38), supporting a role of androgens in peripheral insulin resistance. Impressively, data from mouse models of diet-induced diabetes and NAFLD found that spironolactone treatment decreased serum free fatty acids and hepatic triglycerides, improved serum lipids as well as NASH histology. Spironolactone further induced hepatocyte expression of lipogenic enzymes, supporting a more direct role of androgen receptors within hepatocytes on lipid metabolism (39). Likewise, short course spironolactone trials in humans have shown decreased visceral adiposity (40) and serum free fatty acids (41). Taken together, these data suggest that anti-androgen therapy may help to improve metabolic co-morbidities, and their role in NAFLD development and progression in women may warrant exploration.
While androgens are consistently pro-steatotic in women, it remains unknown why opposite trends are observed in men, with low testosterone conferring a higher risk of NAFLD (42–45). Testosterone replacement in male animal models of NAFLD has been shown to improve hepatic fat content (46,47) as well as other metabolic features in men such as visceral adiposity, insulin resistance, and dyslipidemia (48). Data on sex dimorphism and diabetes risk are also evident, with increasing levels of testosterone in men associated with reduced diabetes risk, and opposite findings observed in women (49). Further mechanistic studies of androgens in hepatic glucose and lipid homeostasis may yield innovative sex-based models of care in NAFLD. Testosterone may also provide a novel target for therapeutic intervention in men with NAFLD, albeit with potentially opposite goals of hormone modulation.
The current study has important strengths and some notable limitations. The CARDIA cohort includes a large, well-characterized population of Caucasian and African American women with detailed metabolic parameters followed for more than 2 decades, though lack of reported Hispanic ethnicity limits generalizability to Latina populations. Given the lack of liver pathology and hepatic enzyme measurements, the association of androgens with nonalcoholic steatohepatitis in women could not be addressed. CT imaging was also unavailable at baseline therefore the timing of NAFLD development could not be ascertained. However, as noted above we do believe our findings support an association of free testosterone on risk of developing NAFLD, as sex hormones were measured in healthy young women without metabolic disease, that were recruited prior to the onset of the obesity and diabetes epidemics. We defined NAFLD by liver attenuation (LA) cutoffs that were based on prior data showing excellent specificity, though lower sensitivity, for histologically confirmed NAFLD (19). Higher specificity minimizes the effect of measurement bias (50), although this comes at a cost of decreased NAFLD detection for those with mild degrees of steatosis, which likely explains the low prevalence of NAFLD in our cohort. Magnetic resonance imaging is more sensitive for detecting earlier grades of steatosis (51), and would have likely increased the number of women with NAFLD, though this imaging modality was not available. Although the current study was limited to free testosterone measurements in pre-menopausal women, a recent cross sectional study identified a strong and independent association of increasing quartiles of testosterone with hepatic steatosis in post-menopausal women (52). Therefore testosterone is likely an important risk factor for NAFLD among women across reproductive age. Finally, a notable strength of our study was the robust statistical approach to evaluate potential mediators, which allowed us to identify the significant role of VAT, and to a lesser degree, that of serum triglycerides, on the relationship between testosterone and NAFLD in women.
In conclusion, we identified a novel association of free testosterone measured in pre-menopausal years on subsequent risk of prevalent NAFLD in midlife within a large cohort of United States women. Importantly, this association was present even among women without androgen excess, suggesting a role of testosterone on NAFLD risk in a broader spectrum of women. Further mechanistic studies should explore androgen receptors in the liver including their role in glucose and lipid homeostasis, in an effort to expand targets for NAFLD therapeutics in women.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Nonalcoholic fatty liver disease (NAFLD) is common in hyperandrogenic women.
-
✓
It is not known whether testosterone increases NAFLD risk independent of metabolic co-factors, or in women without hyperandrogenism.
WHAT IS NEW HERE
-
✓
Testosterone levels in healthy young women are independently associated with risk of NAFLD in midlife.
-
✓
Testosterone is associated with NAFLD risk, even in women without androgen excess.
Acknowledgments
This work was supported by UCSF Liver Center Grant Number P30 DK026743.
Financial support: The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN26820130 0027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), Johns Hopkins University School of Medicine (HHSN268200900041C) and Vanderbilt University Medical Center (R01HL098445). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging. This article has been reviewed by CARDIA for scientific content. The CWS is supported by the National Heart Lung and Blood Institute grant R01-HL065611 and contracts N01-HC-48047, N01-HC-48048, N01-HC-48049, and N01-HC-4805. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
Guarantor of the article: Monika Sarkar, MD, MAS.
Specific author contributions: Study idea, design, data analysis, primary manuscript preparation: Monika Sarkar; sex hormone content guidance, manuscript editing: Melissa Wellons; sex hormone content guidance, manuscript editing: Marcelle Cedars; data interpretation and manuscript editing: Lisa VanWagner; data interpretation and manuscript editing: Erica P. Gunderson; data interpretation and manuscript editing: Veeral Ajmera; sex hormone content guidance, manuscript editing: Laura Torchen; data interpretation and manuscript editing: David Siscovick; data interpretation and manuscript editing: J. Jeffrey Carr; data interpretation and manuscript editing: James G. Terry; data interpretation and manuscript editing: Mary Rinella; data interpretation and manuscript editing: Cora E. Lewis; Data interpretation and manuscript editing: Norah Terrault. All authors have approved the final draft manuscript.
CONFLICT OF INTEREST
Potential competing interests: None.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of non-alcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–95. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 5.Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology. 2016;64:19–22. doi: 10.1002/hep.28524. [DOI] [PubMed] [Google Scholar]
- 6.Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–53. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 7.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–24. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bambha K, Belt P, Abraham M, et al. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55:769–80. doi: 10.1002/hep.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen I, Powell LH, Kazlauskaite R, et al. Testosterone and visceral fat in midlife women: the Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring) 2010;18:604–10. doi: 10.1038/oby.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vassilatou E. Nonalcoholic fatty liver disease and polycystic ovary syndrome. World J Gastroenterol. 2014;20:8351–63. doi: 10.3748/wjg.v20.i26.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 12.Cutter GR, Burke GL, Dyer AR, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12:1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 13.Nair S, Slaughter JC, Terry JG, et al. Anti-mullerian hormone (AMH) is associated with natural menopause in a population-based sample: The CARDIA Women’s Study. Maturitas. 2015;81:493–8. doi: 10.1016/j.maturitas.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merkin SS, Azziz R, Seeman T, et al. Socioeconomic status and polycystic ovary syndrome. J Womens Health (Larchmt) 2011;20:413–9. doi: 10.1089/jwh.2010.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearlman WH, Crepy O, Murphy M. Testosterone-binding levels in the serum of women during the normal menstrual cycle, pregnancy, and the post-partum period. J Clin Endocrinol Metab. 1967;27:1012–8. doi: 10.1210/jcem-27-7-1012. [DOI] [PubMed] [Google Scholar]
- 16.Fritz M, Speroff L. Clinical Gynecology Endocrinology and Infertility. 8th. Lippincott Williams, and Wilkins; 2011. [Google Scholar]
- 17.Braunstein GD, Reitz RE, Buch A, et al. Testosterone reference ranges in normally cycling healthy premenopausal women. J Sex Med. 2011;8:2924–34. doi: 10.1111/j.1743-6109.2011.02380.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang ET, Ku IA, Shah SJ, et al. Polycystic ovary syndrome is associated with higher left ventricular mass index: the CARDIA women’s study. J Clin Endocrinol Metab. 2012;97:4656–62. doi: 10.1210/jc.2012-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–12. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 20.Zeb I, Li D, Nasir K, et al. Computed tomography scans in the evaluation of fatty liver disease in a population based study: the multi-ethnic study of atherosclerosis. Acad Radiol. 2012;19:811–8. doi: 10.1016/j.acra.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodama Y, Ng CS, Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–12. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 22.VanWagner LB, Ning H, Lewis CE, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis. 2014;235:599–605. doi: 10.1016/j.atherosclerosis.2014.05.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis CE, Funkhouser E, Raczynski JM, et al. Adverse effect of pregnancy on high density lipoprotein (HDL) cholesterol in young adult women. The CARDIA Study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 1996;144:247–54. doi: 10.1093/oxfordjournals.aje.a008919. [DOI] [PubMed] [Google Scholar]
- 24.Bild DE, Jacobs DR, Liu K, et al. Seven-year trends in plasma low-density-lipoprotein-cholesterol in young adults: the CARDIA Study. Ann Epidemiol. 1996;6:235–45. doi: 10.1016/1047-2797(96)00005-1. [DOI] [PubMed] [Google Scholar]
- 25.Van Wagner LB, Wilcox JE, Colangelo LA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology. 2015;62:773–83. doi: 10.1002/hep.27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hicks R, Tingly D. Causal mediation analysis. Stata J. 11:605–619. [Google Scholar]
- 27.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley CE, Brown AJ, Diehl AM, et al. Review of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. World J Gastroenterol. 2014;20:14172–84. doi: 10.3748/wjg.v20.i39.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones H, Sprung VS, Pugh CJ, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:3709–16. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- 30.Cerda C, Perez-Ayuso RM, Riquelme A, et al. Nonalcoholic fatty liver disease in women with polycystic ovary syndrome. J Hepatol. 2007;47:412–7. doi: 10.1016/j.jhep.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Vassilatou E, Lafoyianni S, Vryonidou A, et al. Increased androgen bioavailability is associated with non-alcoholic fatty liver disease in women with polycystic ovary syndrome. Hum Reprod. 2010;25:212–20. doi: 10.1093/humrep/dep380. [DOI] [PubMed] [Google Scholar]
- 32.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovejoy JC, Bray GA, Bourgeois MO, et al. Exogenous androgens influence body composition and regional body fat distribution in obese postmenopausal women–a clinical research center study. J Clin Endocrinol Metab. 1996;81:2198–203. doi: 10.1210/jcem.81.6.8964851. [DOI] [PubMed] [Google Scholar]
- 34.Corbould A. Effects of androgens on insulin action in women: is androgen excess a component of female metabolic syndrome? Diabetes Metab Res Rev. 2008;24:520–32. doi: 10.1002/dmrr.872. [DOI] [PubMed] [Google Scholar]
- 35.Abruzzese GA, Heber MF, Ferreira SR, et al. Prenatal hyperandrogenism induces alterations that affect liver lipid metabolism. J Endocrinol. 2016;230:67–79. doi: 10.1530/JOE-15-0471. [DOI] [PubMed] [Google Scholar]
- 36.Singh B, Saxena A. Surrogate markers of insulin resistance: A review. World J Diabetes. 2010;1:36–47. doi: 10.4239/wjd.v1.i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mo ghetti P, Tosi F, Castello R, et al. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metab. 1996;81:952–60. doi: 10.1210/jcem.81.3.8772557. [DOI] [PubMed] [Google Scholar]
- 38.Corbould A. Effects of spironolactone on glucose transport and interleukin-6 secretion in adipose cells of women. Horm Metab Res. 2007;39:915–8. doi: 10.1055/s-2007-993156. [DOI] [PubMed] [Google Scholar]
- 39.Wada T, Kenmochi H, Miyashita Y, et al. Spironolactone improves glucose and lipid metabolism by ameliorating hepatic steatosis and inflammation and suppressing enhanced gluconeogenesis induced by high-fat and highfructose diet. Endocrinology. 2010;151:2040–9. doi: 10.1210/en.2009-0869. [DOI] [PubMed] [Google Scholar]
- 40.Karashima S, Yoneda T, Kometani M, et al. Comparison of eplerenone and spironolactone for the treatment of primary aldosteronism. Hypertens Res. 2016;39:133–7. doi: 10.1038/hr.2015.129. [DOI] [PubMed] [Google Scholar]
- 41.Muneyyirci-Delale O, Kaplan J, Joulak I, et al. Serum free fatty acid levels in PCOS patients treated with glucophage, magnesium oxide and spironolactone. Gynecol Endocrinol. 2013;29:474–7. doi: 10.3109/09513590.2013.769515. [DOI] [PubMed] [Google Scholar]
- 42.Shen M, Shi H. Sex Hormones and Their Receptors Regulate Liver Energy Homeostasis. Int J Endocrinol. 2015;2015:294–278. doi: 10.1155/2015/294278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim S, Kwon H, Park JH, et al. A low level of serum total testosterone is independently associated with nonalcoholic fatty liver disease. BMC Gastroenterol. 2012;12:69. doi: 10.1186/1471-230X-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoyos CM, Yee BJ, Phillips CL, et al. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur J Endocrinol. 2012;167:531–41. doi: 10.1530/EJE-12-0525. [DOI] [PubMed] [Google Scholar]
- 45.Adams LA, Feldstein A, Lindor KD, et al. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology. 2004;39:909–14. doi: 10.1002/hep.20140. [DOI] [PubMed] [Google Scholar]
- 46.Kelly DM, Nettleship JE, Akhtar S, et al. Testosterone suppresses the expression of regulatory enzymes of fatty acid synthesis and protects against hepatic steatosis in cholesterol-fed androgen deficient mice. Life Sci. 2014;109:95–103. doi: 10.1016/j.lfs.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Nikolaenko L, Jia Y, Wang C, et al. Testosterone replacement ameliorates nonalcoholic fatty liver disease in castrated male rats. Endocrinology. 2014;155:417–28. doi: 10.1210/en.2013-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yassin A, Almehmadi Y, Saad F, et al. Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clin Endocrinol (Oxf) 2016;84:107–14. doi: 10.1111/cen.12936. [DOI] [PubMed] [Google Scholar]
- 49.Ding EL, Song Y, Malik VS, et al. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–99. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 50.Copeland KT, Checkoway H, McMichael AJ, et al. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol. 1977;105:488–95. doi: 10.1093/oxfordjournals.aje.a112408. [DOI] [PubMed] [Google Scholar]
- 51.Imajo K, Kessoku T, Honda Y, et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626–637 e627. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 52.Lazo M, Zeb I, Nasir K, et al. Association Between Endogenous Sex Hormones and Liver Fat in a Multiethnic Study of Atherosclerosis. Clin Gastroenterol Hepatol. 2015;13:1686–93. doi: 10.1016/j.cgh.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


