Abstract
Wolbachia are endosymbiotic bacteria of arthropods and nematodes that can manipulate the reproduction of various host organisms to facilitate their own maternal transmission. Moreover, Wolbachia’s presence in host germ cells may contribute to the many cases of lateral gene transfer from Wolbachia to host genomes that have been described. A previous study in Chorthippus parallelus, a well-known orthopteroid forming a hybrid zone in the Pyrenees, identified Wolbachia sequences from two major supergroups in the genomes of infected and uninfected Chorthippus parallelus parallelus (Cpp) and Chorthippus parallelus erythropus (Cpe) subspecies. In this study, we map the Wolbachia genomic inserts to specific regions on the chromosomes of Cpp and Cpe by Fluorescent in situ Hybridization (FISH) using tyramides to increase the accuracy and detection of these insertions. Additionally, we consider some of the possible roles that these bacterial inserts play in the organization and function of the grasshopper genome, as well as how they can serve as markers for phylogenetic relationships of these organisms.
Keywords: Wolbachia, Horizontal gene transfer, Bacterial chromosome insertions, Hybrid zone, Chorthippus parallelus, FISH, Tyramides
INTRODUCTION
Chorthippus parallelus (Cp) is an orthopteroid insect of the family Acrididae (Zetterstedt 1821), which forms a hybrid zone in the Pyrenees where two of its subspecies, C. p. parallelus (Cpp) and C. p. erythropus (Cpe), meet. Cpp is widely distributed throughout the European continent, while Cpe is endemic to the Iberian Peninsula. It is estimated that the hybrid zone of Cp originated approximately 9,000 years ago (Hewitt 1988; Hewitt 2001) from two formerly allopatric subspecies during the last ice age (Hewitt, 1996; Gomez and Lunt 2007; Hewitt 1999, 2011). While F1 hybrid males produced in the laboratory are sterile in accordance with Haldane’s rule (Hewitt et al. 1987; Bella et al. 1990), hybrids inhabiting the contact zones are generally fit, viable and fertile after generations of coadaptation of genes from both pure species within a hybrid genome. Several studies have defined morphological, ethological, chromosomal, and molecular differences between the two subspecies that are used as markers of each Cp subspecies and their hybrids (Butlin and Hewitt 1985a, b; Gosálvez et al. 1988; Ritchie 1990; Cooper et al. 1995; Lunt et al. 1998; Ibrahim et al. 2002; Bella et al. 2007; see Shuker et al. 2005 for a review). Of all the reported markers, the cytogenetic X-linked ones are those that show the most striking differences between Cpp and Cpe subspecies: the distribution and/or composition of the heterochromatic regions differ between the Cpp and Cpe X chromosomes, including an extra Nucleolar Organizing Region (NOR) present only in Cpp (Gosálvez et al. 1988; Serrano et al. 1996; Bella et al. 1993, 2007).
The endosymbiont Wolbachia sp. (Rickettsiales) is a genus of obligate, intracellular alpha-proteobacteria that infects 40–52% of terrestrial arthropod species, as well as certain nematode species (Roman and Hammerstein 2012; Zug and Hammerstein 2012; Weinert et al. 2015). Generally, it has been classified as a single species, W. pipientis, with unique strains that form phylogenetically divergent clusters designated as supergroups (Werren 1997; Jeyaprakash and Hoy 2000; Bordenstein et al. 2009, Lindsey et al. 2016). For example, phylogenetic analyses of Wolbachia 16S rRNA gene in Cp demonstrated the existence of at least two different Wolbachia supergroups (B and F) infecting this grasshopper (Bella et al. 2010; Zabal-Aguirre et al. 2010).
The importance of Wolbachia in evolutionary biology is due in part to its ability to act as a reproductive isolation barrier (Bordenstein et al. 2001; Jaenike et al. 2006; Miller et al. 2010; Shropshire and Bordenstein 2016) and an evolutionary agent that can change host sex ratios and sex determination mechanisms (Wade 2001; Charlat et al. 2003, Werren et al. 2008). Such alterations span feminization of males, male killing, induction of parthenogenesis in non-parthenogenetic species, and cytoplasmic incompatibility (CI). CI results in embryonic lethality of offspring from an infected male mated to either an uninfected female or an infected female with a different Wolbachia strain and is the most common host modification produced by Wolbachia (reviewed in Werren et al. 2008; Serbus et al. 2008; LePage and Bordenstein 2013). CI-inducing Wolbachia present in both subspecies of Cp may contribute to the evolutionary dynamics of the hybrid zone by preventing hybridization of grasshoppers with incompatible Wolbachia strains (Zabal-Aguirre et al. 2010, Zabal-Aguirre et al. 2014). Furthermore, a previous study found that Wolbachia negatively affects chiasmata formation and induces spermatid malformation in hybrid males of this species; these cytogenetical effects may result in modifications of paternal chromatin that contribute to the pathology of CI (Sarasa et al. 2013).
Though exclusively transmitted through the maternal line, Wolbachia infect the sexual organs of both male and female arthropods, including the germline stem cells and stem cell niches (Frydman et al. 2006; Fast et al. 2011; Toomey et al. 2006; Toomey and Frydman 2014). As intracellular bacteria in such close proximity to the germline genome, it is not surprising that many cases of gene transfer from Wolbachia to its host have occurred (Aikawa et al. 2009; Kondo et al. 2002; Fenn et al. 2006; Bordenstein 2007; Nikoh et al. 2008; Klasson et al. 2009; Dunning Hotopp 2011; Brelsfoard et al. 2014; Klasson et al. 2014; Funkhouser-Jones et al. 2015). Some of the inserts are of considerable size or quantity (>100 kb) (Aikawa et al. 2009; Kondo et al. 2002; Nikoh et al. 2008; Funkhouser-Jones et al. 2015) and, in some cases, more than one insertion is present in the host genome (Dunning Hotopp 2011; Brelsfoard et al. 2014; Klasson et al. 2014; Funkhouser-Jones et al. 2015). We recently reported the first evidence for gene transfer from two discrete Wolbachia supergroups (B and F) into the nuclear genome of Cp, revealing that some inserts are subspecies-specific while others are present in both subspecies (Funkhouser-Jones et al. 2015). Here, we use FISH to cytogenetically localize and analyze these inserts in the chromosomes of both subspecies and discuss their possible origin in light of the evolutionary history of this organism and their contribution to its current genome organization.
MATERIAL AND METHODS
Sample collection
Male adult specimens of Cpp and Cpe were collected from the pure populations of Gabas (France) and Escarrilla (Spain), respectively, in both ends of the hybrid zone in the Pyrenees Mountains (Zabal-Aguirre et al. 2010). Grasshopper gonads were extracted and fixed in fresh absolute ethanol: glacial acetic acid (3:1) to be used to prepare slides, and the rest of the body was fixed in absolute ethanol for further DNA isolation. All materials were kept at −20°C until use.
DNA isolation and PCR identification of uninfected individuals
DNA extraction was carried out by the standard phenol-chloroform method (Sambrook et al. 1989). PCR amplifications were performed to analyze Wolbachia infection in 50 Cp specimens using the following Wolbachia 16S rRNA gene specific primers: 16SF 5′-ACTGCTACCTTGTTACGACTT-3′, 16SR 5′-TTGTAGCTTGCTATGGTATAACT-3′ (Zabal-Aguirre et al. 2010). PCR products were sequenced and homology searches were performed at http://blast.ncbi.nlm.nih.gov/Blast.cgi.
Generation of FISH probes
Three Wolbachia contigs from previous genome sequencing of uninfected Cpp grasshoppers (Funkhouser-Jones et al. 2015) were amplified using PCR in uninfected individuals. Cpar-Wb1 and its primers were previously described in Funkhouser-Jones et al. 2015. The following primers were designed to amplify Cpar-Wb2 and Cpar-Wb3: 1882contigF (5′-TCAGGGAGATCGAGTCAAGG-3′), and 1882contigR (5′-GGAAGACGATGATGGGTTTG-3′) for Cpar-Wb2 and 203contigF (5′-GCAGCTGAGGCTTATCTTGG-3′), 203contigR (5′-CCAATGCCGAAAACTTTCAT-3′) for Cpar-Wb3. PCR reactions were performed in 1X Buffer, 2 mM MgCl2, 2 μM dNTPs (Roche), 1.2 μM of each primer, 1.25 U of BIOTAQ DNA polymerase (Biotools), and 100 ng of genomic DNA. The PCR program started with a denaturation of 3 min at 95 °C, followed by 35 cycles of 30 s at 95 °C, 45 s at 56 °C and 3 min at 72 °C, and a final extension of 10 min at 72 °C. PCR products were run on 0.7% TAE agarose gels and purified using the illustra GFX PCR DNA and Gel Band Purification kit (GE Healthcare).
The purified PCR products were used to generate FISH probes with the DecaLabel DNA Labeling kit (Thermo Scientific), which is based on the random-primer method (Feinberg and Vogelstein 1983; 1984). The complete reaction was a modification of the kit protocol and consisted of 10 μl of decanucleotide, 5X Reaction Buffer, 1 μg of cDNA, and nuclease-free H2O to a final volume of 42 μl incubated at 100 °C for 10 min. We then added 1 mM dNTPs mix, 1.75 μl of Digoxigenin-11-dUTP (Roche), and 1 μl of Klenow enzyme and incubated at 30 °C for 2 hours. The probes were purified with the illustra GFX PCR DNA and Gel Band Purification kit (GE Healthcare), resuspended in 50 μl of H2O, and stored at −20 °C until further use.
Tyramide-coupled FISH
Chromosome slides were prepared from fixed male gonads to observe results over meiotic chromosomes in the larger stage of bivalents, diakinesis. Slides were prepared by the conventional technique of squashing, and the coverslip was removed after immersing the slides in liquid nitrogen. The squashed biological material was treated for 5 min with pepsin (50 μg/ml in 0.01 N HCl) at 37°C, followed by a 30 min incubation in 2% paraformaldehyde at room temperature. FISH coupled with tyramide amplification (FISH-TSA) was performed according to Krylov et al. 2007 and 2008, with the following minor changes: endogenous peroxidases were inactivated in 1% H2O2 for 30 min. After that, slides were dehydrated in a series of methanol washes (70%, 85%, 100%) and allowed to dry.
Chromosomes were denatured and hybridized with 50 μl of hybridization mixture under a cover glass for 5 min at 70°C. The hybridization mixture was composed of 2 μl of the labeled probe, 50% formamide, and 2X SSC (300 mM NaCl, 30 mM sodium citrate, pH 7.0). Slides were then left overnight in a wet chamber at 37°C. Posthybridization washing and visualization of FISH-TSA signal were also performed as described by Krylov et al. 2007 and 2008. Detection of probes with Antidigoxigenin-POD (Roche) was performed at a concentration of 1:2000 in blocking buffer (TNB-0.1 M Tris/HCl, 0.15 M NaCl, 0.5% Blocking reagent). The tyramide solution (Perkin Elmer) was incubated onto the slides for 5 min at a concentration of 1:50. Chromosomes were counterstained with 50 ng/μl of DAPI (4′,6-diamidino-2-phenylindole, Roche) diluted in Vectashield (Vector Laboratories). Results were observed in a digital image analysis platform based on Leica DMLB fluorescence microscope with two independent green and blue filters. Images were captured as tiff files using a cooled CCD Leica DF35 monochrome camera (Leica Microsystem) and final images were edited using Photoshop CS6 (Adobe). Six individuals from each subspecies and a minimum of 20 diakinesis stages per individual were analyzed.
C-Banding
The C-Banding technique was performed following the conventional protocol: 10 min incubation with 5% Ba(OH)2 (diluted in H2O), 5 min in H2O with 2 drops of acetic acid, and 30 min in 2X SSC at 60 °C, as described in Bella et al. (1993). After washing thoroughly, the C-bands were stained with propidium iodide (25 μg/ml diluted in 1X PBS) under a cover glass for 30 min. Finally, the slides were washed with 1X PBS, and mounted with Vectashield (Vector Laboratories). Results were observed and images captured employing the same platforms described above.
RESULTS
1) Wolbachia sequences are integrated into the genomes of both Cp subspecies
Our previous genomic study of Wolbachia-to-grasshopper gene transfer identified over 650 kb of Wolbachia DNA in the genome of uninfected Cpp, while cytological staining of Cp chromosomes verified that a 1.8 kb Wolbachia contig, Cpar-Wb1, was integrated into both the Cpp and Cpe genomes (Funkhouser-Jones et al. 2015). Here, positive PCR amplifications of the Cpar-Wb1 contig and two additional contigs, Cpar-Wb2 and Cpar-Wb3 (each 3kb in length, Table S1), in uninfected Cpp and Cpe grasshoppers confirm the integration of multiple Wolbachia sequences in the genomes of both subspecies of Cp
2) Tyramide-coupled FISH analysis corroborates a widespread representation of these three Wolbachia inserts in Cp chromosomes
Tyramide-coupled FISH analysis shows a widespread distribution of the three Wolbachia contigs in the Cpp and Cpe karyotypes (Figure 1, but see also Funkhouser-Jones et al. 2015 figures for complementary information). These data corroborate the PCR results and localize them to specific chromosomal locations. The FISH patterns reveal location similarities in both subspecies but also striking differences between Cpp and Cpe. It is remarkable that the majority of the hybridization signals in both subspecies are located in the telomeric regions, although interstitial signals in L1, M4, M5, and X chromosomes are also present (Figure 1). To properly identify the Cp chromosomes, C-banding technique was carried out after the tyramide-coupled FISH in Cpe and Cpp (Figure 2). Since C-banding reveals specific heterochromatic regions on each bivalent, it permits exact identification of chromosomes in both subspecies that are difficult to differentiate, such as chromosomes L1 and L2. It is of note that the X chromosome in the Cpe subspecies contains centromeric and interstitial constitutive heterochromatic regions, while the constitutive heterochromatin appears in centromeric and telomeric regions in the Cpp X chromosome. Nevertheless, the L2 and L3 chromosomes have the same constitutive heterochromatic pattern in both Cp subspecies: interstitial and centromeric heterochromatin in L2, and telomeric and centromeric in L3 (Figure 2), as previously described by Gosálvez et al. (1988), Bella et al. (1993; 2007) and Serrano et al. (1996).
Fig.1. Detection of Wolbachia inserts in chromosomes of uninfected individuals of both subspecies of Cp (Cp parallelus, Cpp; Cp erythropus, Cpe) by tyramide-coupled FISH.
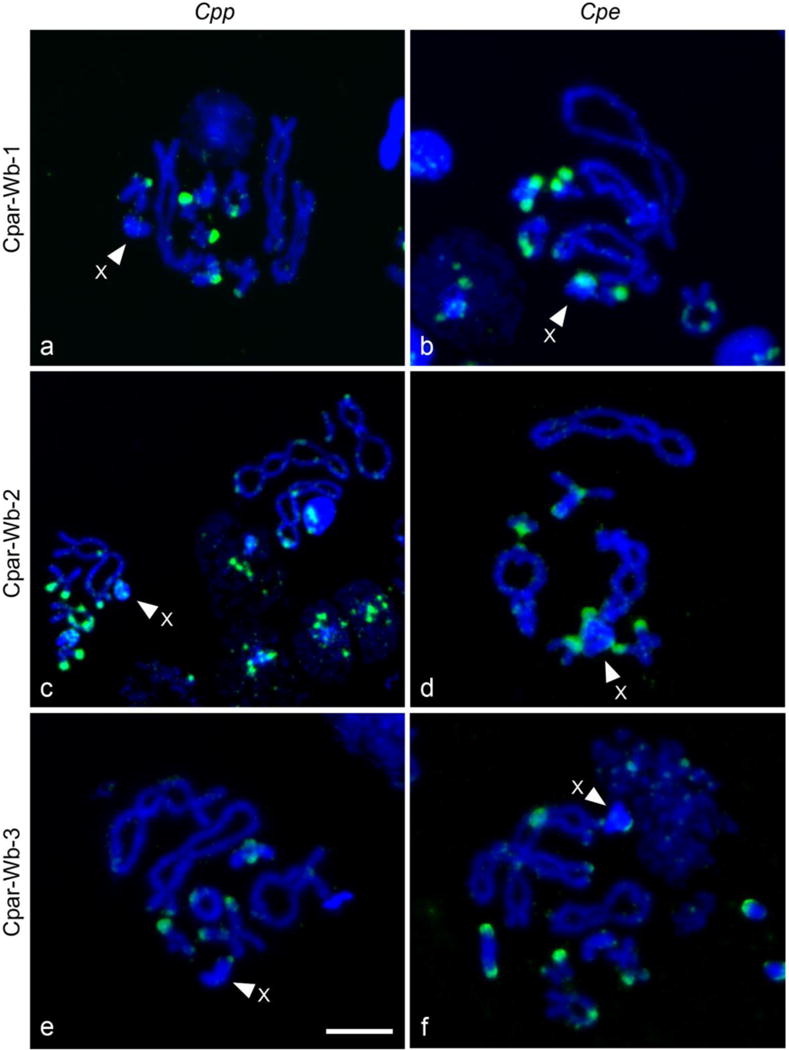
(a, b) Localization of the Cpar-Wb1 Wolbachia sequence in Cpp chromosomes and Cpe chromosomes, respectively. (b, c) Localization of the Cpar-Wb2 Wolbachia sequence. (e, f) Localization of the Cpar-Wb3 Wolbachia sequence. All the Wolbachia sequences are stained in green and chromosomes are counterstained with DAPI (blue). The X chromosome is indicated by an arrow. The signal analyses are performed in diakinesis stage of meiosis. Scale bar 40 μm.
Fig.2. C-Banding of Cpp and Cpe chromosomes after detection of Cpar-Wb1 and Cpar-Wb2 Wolbachia insert by tyramide-coupled FISH.
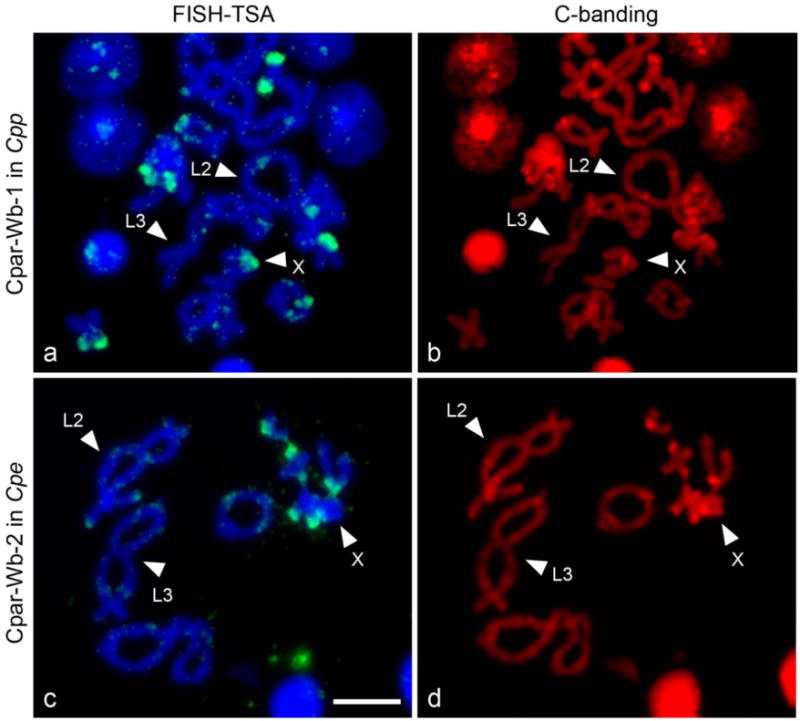
Green fluorescence reveals localization of Cpar-Wb1 Wolbachia insert in the Cpe chromosomes (a), and the Cpar-Wb2 insert in the Cpp chromosomes (c) counterstained with DAPI. The C bands appear in bright red (stained with propidium iodide), showing a distinctive pattern of constitutive heterochromatic regions for each subspecies, Cpp (b) and Cpe (d). These different heterochromatic regions allow the proper identification of the X chromosome and discriminate between the L2 and L3 chromosomes (all of them indicated with arrows). Scale bar 40 μm.
3) The integrated sequences of Wolbachia tend to accumulate in the constitutive heterochromatin of Cp
All information obtained with the tyramide-coupled FISH assays is summarized in Figure 3. Interestingly, there are several positions that show the three inserts in the same location, not only within the same subspecies, but also in both subspecies (for example the telomeric position in M4, M6, S7 and S8 chromosomes). In contrast, there are some chromosomes with a unique insert localization that is subspecies-specific (such as the interstitial localization of the Cpar-Wb2 insertion in the longer arm of L1 and L2 Cpp chromosomes). Although inserts show a clear tendency to accumulate in the constitutive heterochromatin, not all of the inserts’ signals appear in such regions, and not all the constitutive heterochromatic areas have insertions. It is noteworthy that the centromeres do not show insertions of any type, as opposed to the telomeric regions (Figure 3). Also of note is the different distribution patterns of these sequences in the X chromosomes. For example, in Cpe, the inserts localize together at the heterochromatic interstitial band of the X chromosome, which is not found in Cpp, even though the Cpar-Wb1 insert localizes in a nonheterochromatic interstitial position in both Cp X chromosomes. Also, in Cpp X chromosomes, we observe an irregular dotted signal for the Cpar-Wb2 insert and a telomeric signal for the Cpar-Wb3 sequence.
Fig.3. Ideogram of Cp parallelus and Cp erythropus showing the localization of Cpar-Wb1, Cpar-Wb2, and Cpar-Wb3 inserts.
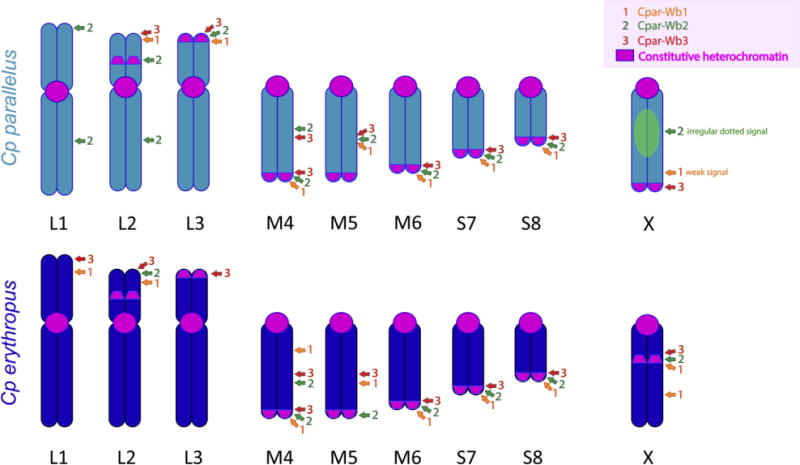
The constitutive heterochromatic regions are represented in pink along the different chromosomes of the two subspecies (Li, L2, L3, M4, M5, M6, S7, S8 and X). The localization of the Cpar-Wb1 insert is denoted in orange, while the Cpar-Wb2 and Cpar-Wb3 inserts are marked in green and red, respectively.
DISCUSSION
PCR amplification of the contigs used in this study in Cp individuals, regardless of infection status, corroborates our previous findings that the sequences are not false positives due to living Wolbachia or contamination (Funkhouser-Jones et al. 2015). This is also demonstrated by chromosomal FISH since the absence of non-specific hybridization signal (dispersed dots) either in the cytoplasm or on the chromosomes rules out a cytoplasmic Wolbachia infection.
The FISH approach with tyramides permits the physical mapping of the contigs since genomic studies can detect the insertions but do not show the distribution and localization of these sequences. The results here provide further information on i) the biology of Wolbachia and its frequent lateral transfer to its host, ii) the evolutionary history of the subspecies, and iii) tyramide-coupled FISH as a tool for further studies and models since this sensitive methodology reveals the position of small contigs of any source in a given karyotype. However, it is important to point out that the size or intensity of the FISH signals observed do not necessarily correlate with the number, size or orientation of the sequences detected, but do clearly indicate their presence.
Our previous phylogenetic analyses revealed that Wolbachia inserts in the Cp genome probably originated from relatively recent independent transfers from both B and F Wolbachia supergroups (Funkhouser-Jones et al, 2015). Though recombinant Wolbachia have been detected in the hybrid zone formed by these grasshoppers (Martinez-Rodriguez et al. 2016), it is unlikely that the insertions arose from a recombinant or “hybrid” B and F Wolbachia strain with genes from both supergroups transferring in a single event into the Cp genome (Funkhouser-Jones et al. 2015) due to the extensive number of redundant genes from both the B and F Wolbachia in the grasshopper nuclear genome (Funkhouser-Jones et al. 2015). The role that these bacterial sequences play in the organization and function of the grasshopper’s genome is uncertain. Altogether, the two Wolbachia supergroups (B and F) transferred at least 448 kb and 144 kb of DNA, respectively, into the host nuclear genome (Funkhouser-Jones et al. 2015). The contribution of these Wolbachia insertions represents around 4% of the global Cp genome, which is not negligible considering the giant size of the grasshopper genome: around 14.1 Gb (Lechner et al. 2013; R. Pereira, com. per.). However, our previous analysis did not allow us to distinguish whether these were large insertions or multiple smaller inserts spread throughout the genome. Our current results provide information on the physical mapping of these inserts and on the genomic and nuclear architecture of Cp (Figure 3). In absence of information of the expression of these inserts and given their preferential location in previously described C-heterochromatic regions [similar to Wolbachia insertions in the Drosophila ananassae genome (Klasson et al. 2014)], we presume that most of them probably do not a play a concrete role in grasshopper biology, unless they interrupt a coding sequence or interfere in a gene’s expression.
The location of the fluorescent signals reported here are consistent across all individuals analyzed. This makes false positives unlikely and also indicates that these insertions are stabilized at the population level. Some Wolbachia inserts are present at the same position on the chromosomes of both Cpp and Cpe, while other inserts are subspecies-specific (Fig. 3), indicating that insertion events likely occurred both before and after the divergence of the subspecies in allopatry. Cpp and Cpe-specific sequences seem to be fairly young since the two subspecies have diverged between 0.2 and 2 MYA (Cooper and Hewitt 1993; Lunt et al. 1998). If future analyses ascertain that hybrid-specific Wolbachia inserts originated independently, these would indicate even more recent transfer events given that the hybrid zone appeared approximately 9,000 years ago (Hewitt 1993; Shuker et al. 2005). This scenario seems plausible given that, with an annual generation, these approximately 9,000 generations of hybridization and recombination of parental genomes resulted in a different grasshopper hybrid genome as a consequence of the coadaptation of genomic elements from both subspecies. In the hybrid genomes, new Wolbachia transfers may have occurred, while previous insertions could have disappeared or translocated to a different chromosomal position. Further work analyzing individuals from a transect along the hybrid zone will serve to explore these possibilities and will also delineate clines for the new chromosomal markers described here and determine whether they are subject to selection processes.
Previous phylogenetic studies revealed that current Cp populations are infected by Wolbachia from B and F supergroups and that the F strains co-diverged with their grasshopper hosts in allopatry, as opposed to the more recent horizontal transmission of B strains (Zabal-Aguirre et al. 2010, 2014; Martinez-Rodriguez et al. 2016). This indicates that Wolbachia insertions present in the pure grasshopper genomes at the origin of the hybrid zone have further evolved, and perhaps have recombined, as part of the hybrid genome. The current distribution of the Wolbachia insertions analyzed here may reflect the history of the insertion events. For example, the Cpar-Wb2 insertion appears in fewer places (8 loci) in Cpe than the Cpar-Wb1 or Cpar-Wb3 contigs (10 loci each) but is overrepresented in Cpp (12, 8, and 9 loci, respectively, Fig. 3), whilst Cpar-Wb1 and Cpar-Wb3 appear in a similar number of positions in both subspecies (but not always in the same position). These results may indicate that all three inserts were present in the ancestral Cp lineage (before the divergence in allopatry), but we cannot determine if Cpar-Wb2 has been amplified in Cpp or partly eliminated in Cpe. Further studies are needed to fully understand the evolutionary history of these inserts.
The FISH localizations of the contigs assayed here does not completely correlate with the constitutive heterochromatin distribution described for Cpp and Cpe (Gosálvez et al. 1988; Bella et al. 1993, 2007; Serrano et al. 1996) (see Fig. 3): pericentromeric heterochromatin present in all the chromosomes of these organisms and the telomeric heterochromatin of chromosome pair 5 in both subspecies never show hybridization. The consistent absence of Wolbachia contigs in the pericentromeric areas is interesting and further studies in other grasshoppers and insects are necessary to confirm these observations, especially since we only found one previous reference of pericentromeric Wolbachia insertions in the literature (Klasson et al. 2014). Cpar-Wb3 localizes in the previously described telomeric heterochromatin, but it also appears in non-C-heterochromatic positions (in the short arms of L1 and L2 chromosomes in Cpe and in interstitial positions in the M4 and M5 chromosomes of both subspecies). However, certain non-C-heterochromatic regions in both chromosome complements show hybridization (see chromosomes L2 and L3, M4, M5 and X in Fig 3). While it seems that the insertions tend to accumulate in the telomeric regions of the chromosomes, there is not a clear pattern of colocalization of the insertions. In general, Cpar-Wb2 and Cpar-Wb3 contigs tend to map together, while all three contigs are localized together in some regions. These positions could be “hot spots” for the insertion of fragments of the Wolbachia genome or may indicate a single large insertion of Wolbachia DNA (including two or three of these contigs) in the Cp genome.
It is noteworthy that the X chromosome in Cpe subspecies contains centromeric and interstitial constitutive heterochromatic regions, while in Cpp subspecies the constitutive heterochromatin appears in centromeric and telomeric regions. Previous findings using C-banding followed by DNA-specific fluorescent stains proposed that the interstitial heterochromatic band characteristic of the Iberian endemic Cpe X chromosome does not derive from an inversion event involving the pericentromeric heterochromatin displayed by this chromosome or the telomeric heterochromatin only present in Cpp. They also indicate that the interstitial heterochromatic band specific to the X chromosome of Pyrenean Cpe originated recently, perhaps from the amplification and further heterochromatinization of certain unknown sequences (Bella et al. 1993, 2007). The co-localization of this heterochromatin with the three insertions makes these Wolbachia sequences possible candidates for the origin of this band. Our current results confirm a non-pericentromeric origin for this interstitial X-Cpe heterochromatic band, but it shares all three contigs with the telomeric heterochromatin displayed by the autosomes of both subspecies and Cpar-Wb3 with the telomeric heterochromatin of X-Cpp chromosome. The latter region is associated with a NOR only in the Cpp subspecies (Gosálvez et al. 1988), and it lacks the Cpar-Wb2 insert commonly present in the non-centromeric ends of the autosomes. However, the same contig appears associated with another NOR region in the short arm of the L2 chromosome of Cpp but not in the corresponding region (also with NOR) of Cpe, and interstitially appears as an irregular dotted signal in the X-Cpp chromosome. This is relevant given that most karyotypic differences in the distribution of Wolbachia insertions in these subspecies appear in the X-chromosomes, as has been repeatedly noted for other markers, which supports the postulated rapid evolution of the sexual chromosomes (van Doom and Kirkpatrick 2007; Qvarnström and Bailey 2009; Meiklejohn and Tao 2010; Palacios-Giménez et al. 2013) and the preferential accumulation of cytogenetic divergence in these chromosomes during allopatry (Gosálvez et al. 1988; Bella et al. 1993, 2007; Serrano et al. 1996). It is also noteworthy that a reported case of Wolbachia lateral transfer to a beetle strictly affects the X chromosome (Kondo et al. 2002; Nikoh et al. 2008), whilst in a tsetse fly, Glossina morsitans morsitans, the Wolbachia inserts appear distally in the heterochromatic X, Y and B-chromosomes (Brelsfoard et al. 2014), which is similar to what we observe in Cp.
With the current data, it is not possible to know if certain C-heterochromatic regions in some way attract repeated Wolbachia insertions or if the insertions serve as foci for the generation of heterochromatin. Further studies in other organisms would help to ascertain this possible origin for certain heterochromatic regions. This is relevant given the wide distribution of Wolbachia in terrestrial arthropods and nematodes (Le Page and Bordenstein 2013) and the ubiquity of lateral transfers from Wolbachia to its host.
Supplementary Material
Table S1. Contigs of Wolbachia genomic inserts used for FISH probes
Acknowledgments
This work was supported by the Spanish Ministerio de Economía y Competitividad (CGL2012-35007 grant) with the collaboration of Chromacell S.L. and by NIH Award R21 HD086833 and NSF Award IOS 1456778 to S.R.B. We are grateful to the people who helped in the collection and handling of the grasshoppers and to the other members of our group in the Genetics Unit at the Universidad Autónoma de Madrid. We also express our gratitude to the Aragón Government, Spain, and the French Parc National des Pyrénées for permission to collect the grasshoppers. We thank Prof. JM Szymura for showing us the Tyramide-coupled FISH to map small DNA sequences and to Prof. V Krylov for his help to develop this technique in our lab.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- Aikawa T, Anbutsu H, Nikoh N, Kikuchi T, Shibata F, Fukatsu T. Longicorn beetle that vectors pinewood nematode carries many Wolbachia genes on an autosome. Proc Royal Soc B. 2009;276:3791–3798. doi: 10.1098/rspb.2009.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bella JL, Hewitt GM, Gosálvez J. Meiotic imbalance in laboratory produced hybrid males of Chorthippus parallelus parallelus and Chorthippus parallelus erythropus. Gens Res. 1990;56:43–48. doi: 10.1017/S001667230002886X. [DOI] [Google Scholar]
- Bella JL, Serrano L, Hewitt GM, Gosálvez J. Heterochromatin heterogeneity and rapid divergence of the sex-chromosomes in Chorthippus parallelus parallelus and C. p. erythropus (Orthoptera) Genome. 1993;36:542–547. doi: 10.1139/g93-074. [DOI] [PubMed] [Google Scholar]
- Bella JL, Serrano L, Orellana J, Mason PL. The origin of the Chorthippus parallelus hybrid zone: chromosomal evidence of multiple refugia for Iberian populations. J Evol Biol. 2007;20:568–576. doi: 10.1111/j.1420-9101.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- Bella JL, Martínez-Rodriguez P, Arroyo-Yebras F, Bernal A, Sarasa J, Fernández-Calvín B, Mason PL, Zabal-Aguirre M. Wolbachia infection in the Chorthippus parallelus hybrid zone: evidence for its role as a reproductive barrier. J Orthopt Res. 2010;19:205–212. doi: 10.1665/034.019.0206. [DOI] [Google Scholar]
- Bordenstein SR, O’Hara FP, Werren JH. Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasona. Nature. 2001;409:708–710. doi: 10.1038/35055543. [DOI] [PubMed] [Google Scholar]
- Bordenstein SR. Evolutionary genomics: transdomain gene transfers. Current Biol. 2007;17:R935–R936. doi: 10.1016/j.cub.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Bordenstein SR, Paraskevopoulos C, Dunning Hotopp JC, Sapountzis P, Lo N, Bandi C, Tettelin H, Werren JH, Bourtzis K. Parasitism and mutualism in Wolbachia: What the phylogenomic trees can and cannot say. Mol Bio Evol. 2009;26:231–241. doi: 10.1093/molbev/msn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelsfoard C, Tsiamis G, Falchetto M, Gomulski LM, Telleria E, Alam U, Doudoumis V, Scolari F, Benoit JB, Swain M, Takac P, Malacrida AR, Bourtzis K, Aksoy S. Presence of extensive Wolbachia symbiont insertions discovered in the genome of its host Glossina morsitans morsitans. PLoS Negl Trop Dis. 2014;8:e2728. doi: 10.1371/journal.pntd.0002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucker RM, Bordenstein SR. Speciation by symbiosis. Trends Ecol Evol. 2012;27:443–51. doi: 10.1016/j.tree.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Butlin RK, Hewitt GM. A hybrid zone between Chorthippus parallelus parallelus and Chorthippus parallelus erythropus (Orthoptera, Acrididae) – Morphological and Electrophoretic Characters. Biol J Linn Soc Lond. 1985a;26:269–285. doi: 10.1111/j.1095-8312.1985.tb01636.x. [DOI] [Google Scholar]
- Butlin RK, Hewitt GM. A hybrid zone between Chorthippus parallelus parallelus and Chorthippus parallelus erythropus (Orthoptera, Acrididae) – Behavioral Characters. Biol J Linn Soc Lond. 1985b;26:287–299. doi: 10.1111/j.1095-8312.1985.tb01637.x. [DOI] [Google Scholar]
- Charlat S, Hurst GD, Merçot H. Evolutionary consequences of Wolbachia infections. Trends Genet. 2003;19:217–223. doi: 10.1016/S0168-9525(03)00024-6. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Hewitt GM. Nuclear DNA sequence divergence between parapatric subspecies of the grasshopper Chorthippus parallelus. Insect Mol Biology. 1993;2:185–194. doi: 10.1111/j.1365-2583.1993.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Ibrahim KM, Hewitt GM. Postglacial expansion and genome subdivision In the European grasshopper Chorthippus parallelus. Mol Ecol. 1995;4:49–60. doi: 10.1111/j.1365-294X.1995.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA. Genetics and speciation. Nature. 1992;355:511–515. doi: 10.1038/355511a0. [DOI] [PubMed] [Google Scholar]
- Dillon RJ, Webster G, Weightman AJ, Dillon VM, Blanford S, Charnley AK. Composition of Acridid gut bacterial communities as revealed by 16S rRNA gene analysis. J Invert Pathol. 2008;97:265–272. doi: 10.1016/j.jip.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Dunning Hotopp JC. Horizontal gene transfer between bacteria and animals. Trends Genet. 2011;27:157–163. doi: 10.1016/j.tig.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast EM, Toomey ME, Panaram K, Desjardins D, Kolaczyk ED, Frydman HM. Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science. 2011;334:990–992. doi: 10.1126/science.1209609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Addendum Anal Biochem. 1984;137:266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fenn K, Conlon C, Jones M, Quail MA, Holroyd NE, Parkhill J, Blaxter M. Phylogenetic relationships of the Wolbachia of nematodes and arthropods. PLoS Pathog. 2006;2:e94. doi: 10.1371/journal.ppat.0020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman HM, Li JM, Robson DN, Wieschaus E. Somatic stem cell tropism in Wolbachia. Nature. 2006;441:509–512. doi: 10.1038/nature04756. [DOI] [PubMed] [Google Scholar]
- Funkhouser-Jones LJ, Sehnert SR, Martínez-Rodriguez P, Toribio R, Pita M, Bella JL, Bordenstein SR. Wolbachia co-infection in a hybrid zone: discovery of horizontal gene transfers from two Wolbachia supergroups into an animal genome. PeerJ. 2015;3:e1479. doi: 10.7717/peeij.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A, Lunt DH. Refugia within refugia: patterns of phylogeographic concordance in the Iberian Peninsula. In: Weiss S, Ferrand N, editors. Phylogeography of Southern European Refugia. Springer; Dordrecht: 2007. pp. 155–188. [DOI] [Google Scholar]
- Gosálvez J, López-Fernández C, Bella JL, Butlin RK, Hewitt GM. A hybrid zone between Chorthippus parallelus parallelus and Chorthippus parallelus erythropus (Orthoptera: Acrididae): chromosomal differentiation. Genome. 1988;30:656–663. doi: 10.1139/g88-111. [DOI] [Google Scholar]
- Hewitt GM. Hybrid zones-natural laboratories for evolutionary studies. Trends Ecol Evol. 1988;3:158–167. doi: 10.1016/0169-5347(88)90033-X. [DOI] [PubMed] [Google Scholar]
- Hewitt GM. After the ice – parallelus meets erythropus in the Pyrenees. In: Harrison RG, editor. Hybrid Zones and the Evolutionary Process. Oxford University Press; Oxford; 1993. pp. 140–164. [Google Scholar]
- Hewitt GM. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc Lond. 1996;58:247–276. doi: 10.1111/j.1095-8312.1996.tb01434.x. [DOI] [Google Scholar]
- Hewitt GM. Post-glacial re-colonization of European biota. Biol J Linn Soc Lond. 1999;68:87–112. doi: 10.1111/j.1095-8312.1999.tb01160.x. [DOI] [Google Scholar]
- Hewitt GM. Speciation, hybrid zones and phylogeography – or seeing genes in space and time. Mol Ecol. 2001;10:537–549. doi: 10.1046/j.1365-294x.2001.01202.x. [DOI] [PubMed] [Google Scholar]
- Hewitt GM. Quaternary phylogeography: the roots of hybrid zones. Genetica. 2011;139:617–638. doi: 10.1007/s10709-011-9547-3. [DOI] [PubMed] [Google Scholar]
- Hewitt GM, Butlin RK, East TM. Testicular dysfunction in hybrids between parapatric subspecies of the grasshopper Chorthippus parallelus. Biol J Linn Soc. 1987;31:25–34. doi: 10.1111/j.1095-8312.1987.tb01978.x. [DOI] [Google Scholar]
- Ibrahim KM, Cooper SJB, Hewitt GM. Testing for recombination in a short nuclear DNA sequence of the European meadow grasshopper, Chorthippus parallelus. Mol Ecol. 2002;11:583–590. doi: 10.1046/j.0962-1083.2001.01441.x. [DOI] [PubMed] [Google Scholar]
- Jaenike J, Dyer KA, Cornish C, Minhas MS. Asymmetrical reinforcement and Wolbachia infection in Drosophila. PLOS Bio. 2006;4:e325. doi: 10.1371/journal.pbio.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash A, Hoy MA. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol Biol. 2000;9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- Kondo N, Nikoh N, Ijichi N, Shimada M, Fukatsu T. Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect. Proc Natl Acad Sci USA. 2002;99:14280–14285. doi: 10.1073/pnas.222228199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasson L, Kambris Z, Cook PE, Walker T, Sinkins SP. Horizontal gene transfer between Wolbachia and the mosquito Aedes aegypti. BMC Genomics. 2009;10:33–10. doi: 10.1186/1471-2164-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasson L, Kumar N, Bromley R, Sieber K, Flowers M, Ott SH, Tallon LJ, Andersson SG, Dunning Hotopp JC. Extensive duplication of the Wolbachia DNA in chromosome four of Drosophila ananassae. BMC Genomics. 2014;15:1097. doi: 10.1186/1471-2164-15-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylov V, Tlapakova T, Macha J. Localization of the single copy gene Mdh2 on Xenopus tropicalis chromosomes by FISH-TSA. Cytogenet Genome Res. 2007;116:110–112. doi: 10.1159/000097427. [DOI] [PubMed] [Google Scholar]
- Krylov V, Tlapakova T, Macha J, Curlej J, Ryban L, Chrenek P. Localization of human coagulation factor VIII (hFVIII) in transgenic rabbit by FISH-TSA: identification of transgene copy number and transmission to the next generation. Folia Biol (Praha) 2008;54:121–124. doi:FB2008A0020. [PubMed] [Google Scholar]
- Lechner M, Marz M, Ihling C, Sinz A, Stadler PF, Krauss V. The correlation of genome size and DNA methylation rate in metazoans. Theory in Biosciences. 2013;132:47–60. doi: 10.1007/s12064-012-0167-y. [DOI] [PubMed] [Google Scholar]
- LePage D, Bordenstein SR. Wolbachia: Can we save lives with a great pandemic? Trends Parasitol. 2013;29:385–93. doi: 10.1016/j.pt.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey AR, Bordenstein SR, Newton IL, Rasgon JL. Wolbachia pipientis should not be split into multiple species: A response to Ramírez-Puebla et al., “Species in Wolbachia? Proposal for the designation of ‘Candidatus Wolbachia bourtzisii’, ‘Candidatus Wolbachia onchocercicola’;, ‘Candidatus Wolbachia blaxteri’, ‘Candidatus Wolbachia brugii’, ‘Candidatus Wolbachia taylori’, ‘Candidatus Wolbachia collembolicola’ and ‘Candidatus Wolbachia multihospitum’ for the different species within Wolbachia supergroups”. Syst Appl Microbiol. 2016;39:220–2. doi: 10.1016/j.syapm.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt D, Ibrahim KM, Hewitt GM. mtDNA phylogeography and postglacial patterns of subdivision in the meadow grasshopper Chorthippus parallelus. Heredity. 1998;80:633–641. doi: 10.1046/j.1365-2540.1998.00311.x. [DOI] [PubMed] [Google Scholar]
- Martínez P, Del Castillo P, Bella JL. Cytological detection of Wolbachia in squashed and paraffin embedded insect tissues. Biotech Histochem. 2009;84:347–353. doi: 10.3109/10520290902903381. [DOI] [PubMed] [Google Scholar]
- Martinez-Rodriguez P, Hernández-Pérez M, Bella JL. Detection of Spiroplasma and Wolbachia in the bacterial gonad community of Chorthippus parallelus. Microb Ecol. 2013;66:211–223. doi: 10.1007/s00248-013-0226-z. [DOI] [PubMed] [Google Scholar]
- Martínez-Rodríguez P, Arroyo-Yebras F, Bella JL. Understanding Wolbachia acquisition and codivergence of hosts and their associated bacteria: Wolbachia infection in the Chorthippus parallelus hybrid zone. bioRxiv. 2016 doi: 10.1101/044784. [DOI] [Google Scholar]
- Meiklejohn CD, Tao Y. Genetic conflict and sex chromosome evolution. Trends Ecol Evol. 2010;25:215–223. doi: 10.1016/j.tree.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WJ, Ehrman L, Schneider D. Infectious speciation revisited: Impact of symbiont-depletion on female fitness and mating behavior of Drosophila paulistorum. PLOS Pathog. 2010;6:e1001214. doi: 10.1371/journal.ppat.1001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoh N, Tanaka K, Shibata F, Kondo N, Hizume M, Shimada M, Fukatsu T. Wolbachia genome integrated in an insect chromosome: Evolution and fate of laterally transferred endosymbiont genes. Genome Res. 2008;18:272–280. doi: 10.1101/gr.7144908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Giménez OM, Castillo ER, Martí DA, Cabral-de-Mello DC. Tracking the evolution of sex chromosome systems in Melanoplinae grasshoppers through chromosomal mapping of repetitive DNA sequences. BMC Evol Biol. 2013;13:167. doi: 10.1186/1471-2148-13-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman Z, Hammerstein P. Still a Host of Hosts for Wolbachia: Analysis of Recent Data Suggests That 40% of Terrestrial Arthropod Species Are Infected. Plos One. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvarnström A, Bailey RI. Speciation through evolution of sex-linked genes. Heredity. 2009;102:4–15. doi: 10.1038/hdy.2008.93. [DOI] [PubMed] [Google Scholar]
- Ritchie MG. Are differences in song responsible for assortative mating between subspecies of the grasshopper Chorthippus-parallelus (Orthoptera, Acrididae) Anim Behav. 1990;39:685–691. doi: 10.1016/S0003-3472(05)80379-3. [DOI] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1989. [Google Scholar]
- Sarasa J, Bernal A, Fernández-Calvín B, Bella JL. Wolbachia Induced Cytogenetical Effects as Evidenced in Chorthippus parallelus (Orthoptera) Cytogenet Genome Res. 2013;139:36–43. doi: 10.1159/000341572. [DOI] [PubMed] [Google Scholar]
- Serbus LR, Casper-Lindley C, Landmann F, Sullivan W. The genetics and cell biology of Wolbachia-Host interactions. Annu Rev Genet. 2008;42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- Serrano L, García de la Vega C, Bella JL, López-Fernández C, Hewitt GM, Gosálvez J. A hybrid zone between two subspecies of Chorthippus parallelus. X-chromosome variation through a contact zone. J Evol Biol. 1996;9:173–184. doi: 10.1046/j.1420-9101.1996.9020173.x. [DOI] [Google Scholar]
- Shuker DM, King TM, Bella JL, Butlin RK. The genetic basis of speciation in a grasshopper hybrid zone. In: Fellowes M, Holloway G, Rolff J, editors. Insect Evolutionary Ecology. CABI Publishing, Oxford University Press; Oxford; 2005. pp. 427–454. [Google Scholar]
- Shropshire JD, Bordenstein SR. Speciation by symbiosis: the microbiome and behavior. mBio. 2016;7:e01785–15. doi: 10.1128/mBio.01785-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey ME, Panaram K, Fast EM, Beatty C, Frydman HM. Evolutionary conserved Wolbachia-encoded factors control pattern of stem-cell niche tropism in Drosophila ovaries and favor infection. Proc Natl Acad Sci USA. 2013;110:10788–10793. doi: 10.1073/pnas.1301524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomey ME, Frydman HM. Extreme divergence of Wolbachia tropism for the stem-cell-niche in the Drosophila testis. PLOS Pathog. 2014;10:e1004577. doi: 10.1371/journal.ppat.1004577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn GS, Kirkpatrick M. Turnover of sex chromosomes induced by sexual conflict. Nature. 2007;449:909–12. doi: 10.1038/nature06178. [DOI] [PubMed] [Google Scholar]
- Wade MJ. Evolution: Infectious speciation. Nature. 2001;409:675–677. doi: 10.1038/35055648. [DOI] [PubMed] [Google Scholar]
- Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc Royal Soc B. 2015;282:20150249. doi: 10.1098/rspb.2015.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH. Biology of Wolbachia. Ann Rev Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nature Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Zabal-Aguirre M, Arroyo F, Bella JL. Distribution of Wolbachia infection in Chorthippus parallelus populations within and beyond a Pyrenean hybrid zone. Heredity. 2010;104:174–184. doi: 10.1038/hdy.2009.106. [DOI] [PubMed] [Google Scholar]
- Zabal-Aguirre M, Arroyo F, García-Hurtado J, de la Torre J, Hewitt GM, Bella JL. Wolbachia effects in natural populations of Chorthippus parallelus from the Pyrenean hybrid zone. J Evol Biol. 2014;27:1136–1148. doi: 10.1111/jeb.12389. [DOI] [PubMed] [Google Scholar]
- Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS One. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Contigs of Wolbachia genomic inserts used for FISH probes


