Highlights
-
•
Auditory processing deficits in ASD correlate with social and behavioral symptoms.
-
•
Reduced interhemispheric connectivity is found with more severe sensory symptoms.
-
•
Verbal IQ is lower in individuals with reduced interhemispheric connectivity.
-
•
Thalamocortical overconnectivity may compensate for sensory and social deficits.
Abbreviations: ASD, autism spectrum disorders; ADI-R, Autism Diagnostic Interview Revised; ADOS, Autism Diagnostic Observation Schedule; MRI, magnetic resonance imaging; HG, Heschl’s Gyrus; IQ, intelligence quotient; SP, Sensory Profile; SRS, Social Responsiveness Scale; STG, superior temporal gyrus; TD, typically developing
Keywords: Auditory, Functional connectivity, Thalamus, Corpus callosum, fMRI, Autism spectrum disorder
Abstract
Autism spectrum disorder (ASD) is a complex and prevalent neurodevelopmental disorder characterized by social and communicative deficits, as well as repetitive behaviors and atypical sensitivity to sensory stimulation. Alterations in network connectivity are widely recognized, but their interplay with social and sensory symptoms remains largely unclear. Here, functional magnetic resonance imaging and diagnostic and behavioral assessments were used in a cohort of children and adolescents with ASD (n = 40) and matched typically developing (TD, n = 38) controls to examine the relation between auditory processing, interhemispheric and thalamocortical network connectivity, and social-behavioral symptom severity. We found that atypical processing of sounds was related to social, cognitive, and communicative impairments. Additionally, severity of sensory processing deficits and lower verbal IQ were related to reduced interhemispheric connectivity of auditory cortices in ASD. Increased connectivity between the thalamus and auditory cortex in ASD, however, was associated with reduced cognitive and behavioral symptomatology, suggesting that thalamocortical overconnectivity might reflect a compensatory mechanism in ASD. These findings provide novel evidence for links between auditory sensory deficits and impairments in social interaction and communication.
1. Introduction
Sensory symptoms are common in autism spectrum disorders (ASDs; Baranek et al., 2006, Leekam et al., 2007), with atypical processing of sound afflicting up to 65% of individuals with ASD (Bishop et al., 2013, Chang et al., 2012). Only since the adoption of the DSM-5 (American Psychiatric Association, 2013), however, has unusual reactivity to sensory stimuli become part of the diagnostic criteria for ASD. Previous studies have shown that auditory deficits are associated with more severe social-behavioral symptoms of autism (Jao Keehn et al., 2016, Stewart et al., 2016, Watson et al., 2011), and two recent functional magnetic resonance imaging studies (fMRI) have found atypically increased activation of sensory cortices in ASD in response to sounds that is related to the degree of sensory oversensitivity (Green et al., 2013, Green et al., 2016). Converging with these neuroimaging studies showing atypical responses of auditory cortex to sounds in ASD, genetic, post-mortem, molecular and animal model studies have also found atypical organization of auditory cortices in ASD (Figueiredo Anomal et al., 2015, Hoerder-Suabedissen et al., 2013, Stoner et al., 2014). The interaction between sensory symptoms, the social and behavioral manifestations of autism and alterations in functional brain organization, however, is not well understood. In this study, we therefore used fMRI and behavioral assessments to investigate how differences in functional connectivity of the auditory sensory network relate to atypical sensitivity to sounds, and to deficits in social cognition and communication in a cohort of children and adolescents with ASD.
Functional MRI has consistently revealed altered short and long-range connectivity in ASD (Anderson, 2014, Kana et al., 2011, Müller, 2014, Plitt et al., 2015, Vissers et al., 2012, Wass, 2011) but the organization of the auditory network is not well-studied even in healthy development. The auditory network can be characterized by connectivity between the thalamus – relaying auditory information from the periphery – and auditory cortex, and connectivity between the auditory cortices in the left and right hemisphere (interhemispheric connectivity). Atypical interhemispheric (Anderson et al., 2011, Lee et al., 2016, Zhu et al., 2014) and thalamocortical (Cerliani et al., 2015, Mizuno et al., 2006, Nair et al., 2015, Nair et al., 2013) connectivity are, therefore of particular relevance for understanding the neural underpinnings of auditory sensory processing abnormalities in ASD and how they relate to social and behavioral symptomatology.
Interhemispheric connectivity between left and right auditory cortex is established early in development, with bilateral auditory resting state networks being present from birth (Fransson et al., 2009, Van Den Heuvel et al., 2015) and even in utero (Thomason et al., 2013). While fMRI studies have found interhemispheric connectivity to be reduced in ASD (Anderson et al., 2011, Lee et al., 2016, Zhu et al., 2014), these studies have typically focused on networks involved in complex cognition, such as the default mode network (Damarla et al., 2010, Just et al., 2007, Kleinhans et al., 2008, Mason et al., 2008, Weng et al., 2010). The importance of interhemispheric connectivity for early sensory processing is much less well understood, although two recent studies in healthy adults suggest that increased interhemispheric information transfer is beneficial to speech perception and phonetic categorization (Elmer et al., 2016, Steinmann et al., 2014). Furthermore, reduced interhemispheric connectivity of the superior temporal gyri in ASD was one of the main findings in a study by Anderson et al. (2011), but its relationship to sensory and social symptoms has not been addressed. We hypothesized that reduced interhemispheric connectivity of auditory cortical regions would be replicated in our sample of children and adolescents with ASD, and that the reduction in connectivity would be associated with atypical sound processing, as well as more severe social and cognitive outcomes.
Secondly, we hypothesized that functional connectivity between thalamus and auditory cortices in ASD would be atypical and related to the severity of sensory symptoms and cognitive and communicative deficits. Thalamocortical projections begin to develop in utero, strengthen over the first years of life (Alcauter et al., 2014), and play an important role in early cortical differentiation (Kanold and Luhmann, 2010, O’Leary and Nakagawa, 2002). The thalamus not only relays information from the sensory periphery to cortex, but through top-down cortical modulations is also involved in attentional selection and suppression of sensory input (John et al., 2016). For example, a recent study using task-based fMRI (Green et al., 2016) showed increased activation of auditory and tactile cortices, emotional processing regions and the thalamus in a group of adolescents with ASD compared to TD controls when processing mildly aversive auditory and tactile stimuli. This increase in activation correlated with sensory symptom severity. The authors conclude that the increased activity might reflect a lack of attentional and emotional gating of aversive sensory stimuli. It is not clear how increased activation relates to strength of connectivity between two regions, but multiple other studies have found atypical connectivity between the thalamus and the temporal lobe in ASD (Cerliani et al., 2015, Nair et al., 2015, Nair et al., 2013). Mizuno et al. (2006) suggested that increased functional connectivity between the thalamus and cortex may serve to compensate for reduced long-range cortical connectivity in ASD. In line with these findings, Nair et al. (2015) found improved language and cognitive skills with increasing connectivity between the thalamus and the temporal lobe. Interestingly, increased input from the thalamus to maintain interhemispheric synchronization between sensory cortices has also been proposed in patients with agenesis of the corpus callosum, and in non-human species that lack a corpus callosum (Schmidt, 2003, Tyszka et al., 2011). We were therefore also interested in investigating the interaction between interhemispheric and thalamocortical connectivity in ASD and how different patterns of connectivity of the auditory network relate to atypical processing of sounds, and to deficits in social cognition and communication in ASD.
2. Materials and methods
2.1. Participants
High-functioning children and adolescents with ASD (n = 40) and typically developing control participants (n = 38) between the ages of 8–17 years were included in this study. Diagnoses of Autism Spectrum Disorder were confirmed with the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1989) and the Autism Diagnostic Interview Revised (ADI-R; Lord et al., 1994) based on criteria described by the DSM-5 (American Psychiatric Association, 2013). Participants with comorbid ASD-related medical conditions (e.g., Fragile-X syndrome, tuberous sclerosis, epilepsy), or other neurological conditions (e.g., Tourette syndrome), were excluded. Typically developing children were screened for any history of neurological, psychiatric, or developmental disorders. All participants were safety-screened for MRI contraindications (e.g., claustrophobia, ferrous material in body). Participants for this study were chosen from a larger cohort (ASD: n = 93, TD: n = 67), based on availability of both resting state data and the completed Sensory Profile Caregiver Questionnaire (SP; Dunn, 1999). 31 participants (18 ASD, 13 TD) did not have a complete Sensory Profile, and three ASD participants did not complete the resting state scan, and were thus excluded from this study. Motion during the resting state scan led to the exclusion of 29 participants (23 ASD, 6 TD; see criteria in section 2.3 below). Data from one TD participant was excluded due to sleepiness during the scan. FMRI data of five participants (1 ASD, 4 TD) were corrupted by scanning artifacts, four ASD participants did not meet diagnostic criteria, two TD participants were excluded due to ASD-related medical conditions, one TD participant was excluded because of a history of ASD in the family, one ASD participant was excluded because of a later disclosed seizure disorder, and five participants (3 ASD, 2 TD) were excluded due to structural brain abnormalities discovered after the MRI session. Groups of included subjects were matched on gender, handedness, nonverbal IQ, and in-scanner head motion (root-mean-square displacement [RMSD]) (Table 1). Informed assent and consent were acquired from all participants and their caregivers, and participants were compensated for their time. All study protocols were approved by the San Diego State University and University of California San Diego Institutional Review Boards.
Table 1.
Participant demographics.
| ASD (n = 40) |
TD (n = 38) |
χ2(1), p-value | ||||
|---|---|---|---|---|---|---|
| Gender | 8 female |
6 female |
0.23, p = 0.63 | |||
| Handedness | 7 left |
6 left |
0.04, p = 0.84 | |||
| Mean (SD) | Range | Mean (SD) | Range | t(76), p-value | % diff | |
| Age in years | 14.02 (2.76) | 9.20–18.00 | 13.66 (2.65) | 8.10–17.70 | 0.59, p = 0.56 | 0.026 |
| RMSD | 0.06 (0.03) | 0.02–0.12 | 0.06 (0.03) | 0.02–0.14 | 0.21, p = 0.83 | 0.024 |
| % Time points retained | 99.00 (2.00) | 94–100 | 99.00 (2.00) | 92–100 | 0.43, p = 0.67 | 0.002 |
| WASI-II | ||||||
| Verbal IQ | 102.90 (18.09) | 59–147 | 107.00 (9.57) | 87–127 | 1.24, p = 0.22 | 0.034 |
| Nonverbal IQ | 107.25 (20.94) | 53–145 | 103.68 (13.30) | 62–129 | 0.89, p = 0.38 | 0.034 |
| Full-scale IQ | 105.70 (18.70) | 61–141 | 105.66 (10.75) | 79–126 | 0.01, p = 0.99 | <0.001 |
| SRS | ||||||
| Cognition | 77.08 (13.18) | 45–105 | 43.18 (5.63) | 36–56 | 14.63, p < 0.001 | 0.785 |
| Communication | 80.73 (10.29) | 62–105 | 43.18 (4.76) | 36–55 | 20.45, p < 0.001 | 0.869 |
| SP Auditory | 3.41 (0.74) | 1.65–4.75 | 4.74 (0.28) | 3.90–5.00 | 10.38, p < 0.001 | 0.280 |
| ADOS | ||||||
| Social Interaction | 7.93 (2.31) | 3–13 | – | – | – | – |
| Communication | 3.95 (1.58) | 0–7 | – | – | – | – |
| Combined | 11.88 (3.23) | 7–19 | – | – | – | – |
| Stereotyped Behav/Restricted Interests | 2.15 (1.53) | 0–5 | – | – | – | – |
| ADI-R* | ||||||
| Social Interaction | 19.26 (4.40) | 10–28 | – | – | – | – |
| Communication | 13.34 (5.05) | 2–24 | – | – | – | – |
| Repetitive Behavior | 5.68 (2.30) | 1–12 | – | – | – | – |
2.2. Diagnostic measures and behavioral reports
The ADOS (Lord et al., 1989) and the ADI-R (Lord et al., 1994) were administered to the ASD participants, and the Wechsler Abbreviated Scale of Intelligence, 2nd edition (WASI-II; Wechsler, 2011), Social Responsiveness Scale (SRS; Constantino and Gruber, 2005), and Sensory Profile Caregiver Questionnaire (SP; Dunn, 1999) were administered or completed for all participants (see Table 1 for summary statistics). ADOS and ADI-R are standardized, semi-structured assessments that evaluate behaviors indicative of ASD symptomatology. Domain scores of each assessment relevant to the current study (ADOS Social Interaction and Communication Combined; ADOS Stereotyped Behaviors and Restricted Interests; ADI-R Social Interaction; ADI-R Communication; and ADI-R Repetitive Behaviors) were entered into correlational analyses.
The WASI-II (Wechsler, 2011) assesses overall cognitive capabilities. It was administered to all participants to obtain verbal, nonverbal, and full-scale IQ scores for initial groupwise matching, and verbal IQ scores for further analyses. The SRS (Constantino and Gruber, 2005) is a caregiver questionnaire that measures social abilities in children and adolescents, and is comprised of 5 social domains (Awareness, Cognition, Communication, Motivation, and Autism Mannerisms). Responses are scored on a 4-point Likert scale (1 = not true, 2 = sometimes true, 3 = often true, 4 = almost always true); higher scores indicate greater severity. Two specific domains of the SRS were examined in the current study: Social Cognition (SRS-COG), which assesses the ability to interpret social cues, and Social Communication (SRS-COM), which includes expressive forms of social communication.
The Sensory Profile (SP; Dunn, 1999) assesses sensory responsivity and reactivity as reported by a caregiver, and consists of 125 items that target several modalities including auditory, visual, tactile, and olfactory processing. Nine items are classified as specific to the auditory modality – 5 low threshold items measure hypersensitivity, and 4 high threshold items measure hyposensitivity (see Table 2 for specific items). Caregiver responses are scored on a 5-point Likert scale (1 = always, 2 = frequently, 3 = occasionally, 4 = seldom, 5 = never); lower scores indicate greater sensory symptomatology.
Table 2.
Auditory items of the Sensory Profile.
| Threshold | Auditory Processing Item |
|---|---|
| Low | Responds negatively to unexpected or loud noises |
| Low | Holds hands over ears to protect ears from sound |
| Low | Has trouble completing tasks when the radio is on |
| Low | Is distracted or has trouble functioning if there is a lot of noise around |
| Low | Can’t work with background noise |
| High | Appears to not hear what you say |
| High | Doesn’t respond when name is called but you know the child’s hearing is OK |
| High | Enjoys strange noises/seeks to make noise for noise’s sake |
| High | Seems oblivious within an active environment |
This questionnaire was used to measure auditory processing abnormalities (see subsections 2.4 and 4.4 for a brief discussion). An independent samples t-test assessed whether there were any differences in the resulting auditory Sensory Profile (A-SP) scores between the TD and ASD groups. Additionally, for the ASD group, the auditory Sensory Profile scores were correlated with the other available neuropsychological assessment scores (WASI-II, ADI-R, ADOS, and SRS) described above, in order to test for any relationships between atypical processing of sounds, and behavioral and social symptoms of ASD.
2.3. MRI acquisition and image preprocessing
Imaging data were acquired on a GE 3T Discovery MR750 scanner using an 8-channel head coil at the University of California San Diego Center for Functional MRI (CFMRI). A standard FSPGR T1-weighted sequence was used to acquire high-resolution structural images (172 slices; repetition time [TR] = 8.136 ms; echo time [TE] = 3.172 ms; flip angle = 8°; field of view [FOV] = 25.6 mm; matrix = 256 × 256; resolution = 1 mm3). Functional images were obtained using a single-shot gradient-recalled, echo-planar image pulse sequence. During the resting state scan, 180 whole-brain volumes were acquired (TR = 2000 ms; TE = 30 ms; slice thickness = 3.4 mm; flip angle = 90°; FOV = 22.0 mm; matrix = 64 × 64; in-plane resolution = 3.4 mm2) over the duration of 6 min. Participants were instructed: “Keep your eyes on the cross. Let your mind wander, relax, but please stay as still as you can. Do not to fall asleep.” Participants’ adherence to the instructions to remain awake, with eyes open, was monitored with an MR-compatible videocamera. A separate mock scan session prior to the actual scan acclimated the participants to the MR environment and allowed them to practice staying still.
Data were processed and analyzed using Analysis of Functional NeuroImages (AFNI; Cox, 1996) and FMRI software library (FSL; Smith et al., 2004). Imaging data underwent a standard preprocessing pipeline of field map correction, slice-timing correction, motion correction, and spatial-smoothing with a Gaussian kernel of 6 mm FWHM. Structural images were normalized to the MNI152 template; functional images were coregistered to the structural images and resampled to 3 mm isotropic voxels. Functional time series data were bandpass-filtered (0.008 < f < 0.08 Hz) with a second-order Butterworth filter. White matter and lateral ventricles were segmented, and the average time series data from these segmentations were then extracted and served as nuisance regressors along with their first order derivatives. The 6 rigid-body motion parameters (3 rotation, 3 translation) and their first order derivatives were used as additional nuisance regressors. To minimize any effects of head motion (Power et al., 2012, Van Dijk et al., 2012), these parameters were also used to censor time points with motion exceeding 0.5 mm. No participants in the current study suffered >10% data loss due to censoring, and groups did not differ in the amount of censored time points (Table 1). Finally, global signal regression, a controversial tool used for noise reduction in functional connectivity data, was not implemented so as to avoid spurious deactivation effects (Fox et al., 2009, Jones et al., 2010, Murphy et al., 2009, Weissenbacher et al., 2009).
2.4. Functional connectivity analyses
Left and right hemisphere ROIs were created for auditory cortical regions by combining grey-matter masked Heschl’s Gyrus (HG) and superior temporal gyrus (STG) ROIs from the Harvard-Oxford atlas (Desikan et al., 2006), as shown in Fig. 1, and were resampled to EPI space. Average timecourses were extracted from the two auditory ROIs, and from left and right thalamus (as defined in the Harvard-Oxford atlas). Interhemispheric connectivity was assessed by correlating the timecourses from the left and right auditory cortical ROIs for each subject. All correlation coefficients were Fisher z-transformed. An independent samples t-test was used to test for differences in auditory interhemispheric connectivity between the TD and ASD groups. Similarly, average timecourses from left and right thalamus were Pearson correlated with those extracted from the left and right auditory cortical ROIs. A repeated measures ANOVA with group (TD, ASD) as a between subject factor revealed that there were no significant hemispheric differences in thalamocortical connectivity (F(2.34,178.15) = 2.78, ns, Greenhouse-Geisser sphericity corrected), nor any significant hemisphere by group interactions. The main effect of group was significant (F(1,76) = 8.02, p < 0.01). Thalamocortical connectivity was, therefore, averaged across the four possible ipsi- and contralateral comparisons for each subject. Differences in mean auditory thalamocortical connectivity between the TD and ASD groups were then assessed using an independent samples t-test. In a post-hoc test, the auditory cortical ROIs were split into primary (HG) and secondary (STG) ROIs to examine whether any differences found were driven by auditory regions implicated in more basic sensory (HG) vs. higher-order (STG) processing of sound.
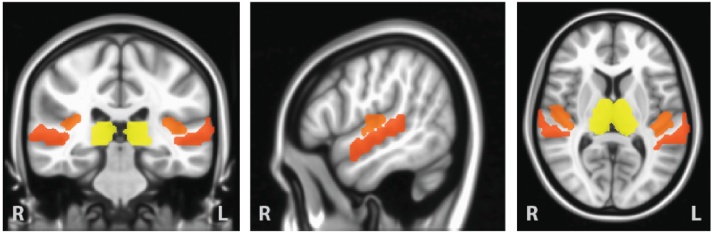
Fig. 1.
Auditory and thalamic regions of interest from the Harvard-Oxford anatomical atlas. Primary auditory regions (Heschl’s Gyrus) are shown in orange, secondary auditory regions (Superior Temporal Gyrus) are shown in red, and the thalamic ROIs are shown in yellow. Average timecourses for the functional connectivity analyses were derived from grey-matter masked left and right hemisphere ROIs separately. In an initial analyses step, average timecourses were derived from the combined “auditory” HG and STG ROIs. Post-hoc analyses then assessed whether results differed between primary and secondary auditory cortical regions.
Next, we assessed whether strength of interhemispheric and thalamocortical connectivity (as measured by the Fisher z-transformed correlation coefficients) was related to sensory and sensory-related social symptoms in ASD. Correlations were carried out across all participants irrespective of ASD diagnosis for verbal IQ (WASI-II) and the auditory Sensory Profile scores. The relationship between functional connectivity and the two relevant SRS subscales (Cognitive [SRS-COG] and Communicative [SRS-COM]) was assessed separately for the TD and ASD group, due to the design of the SRS scales and inherently non-overlapping distribution of scores between the two groups. Additional analyses including the two relevant ADOS subscales (Social Interaction and Communication Combined, and Stereotyped Behaviors and Restricted Interests), which were not available for the TD participants, were carried out for the ASD group only. In-scanner motion (as measured by RMSD) and age were partialled out in all correlation analyses as developmental effects on functional connectivity were not the focus of this study. A separate analysis of age effects revealed that the only significant correlation (partialling out motion) was between age and HG (but not STG) thalamocortical connectivity in the TD (HG: r(36) = −0.35, p < 0.05, STG: r(36) = −0.24, p = 0.15) but not in the ASD group (HG: r(38) = −0.08, p = 0.61, STG: r(38) = 0.11, p = 0.51).
Lastly, the relationship between interhemispheric and thalamocortical connectivity was assessed. Pearson correlations were performed to test whether atypical interhemispheric and thalamocortical connectivity were independent, or present in the same individuals, the latter suggesting a shared developmental origin or compensatory neural mechanisms. To test this further, participants with ASD were split into subgroups based on their pattern of interhemispheric and thalamocortical connectivity. A median-split on interhemispheric and thalamocortical connectivity yielded four subgroups: low interhemispheric – low thalamocortical connectivity (n = 12), low-high (n = 8), high-low (n = 8) and high–high (n = 12). The TD participants formed a fifth group. A one-way ANOVA tested for differences in auditory Sensory Profile scores between the five groups.
3. Results
3.1. Sensory profile
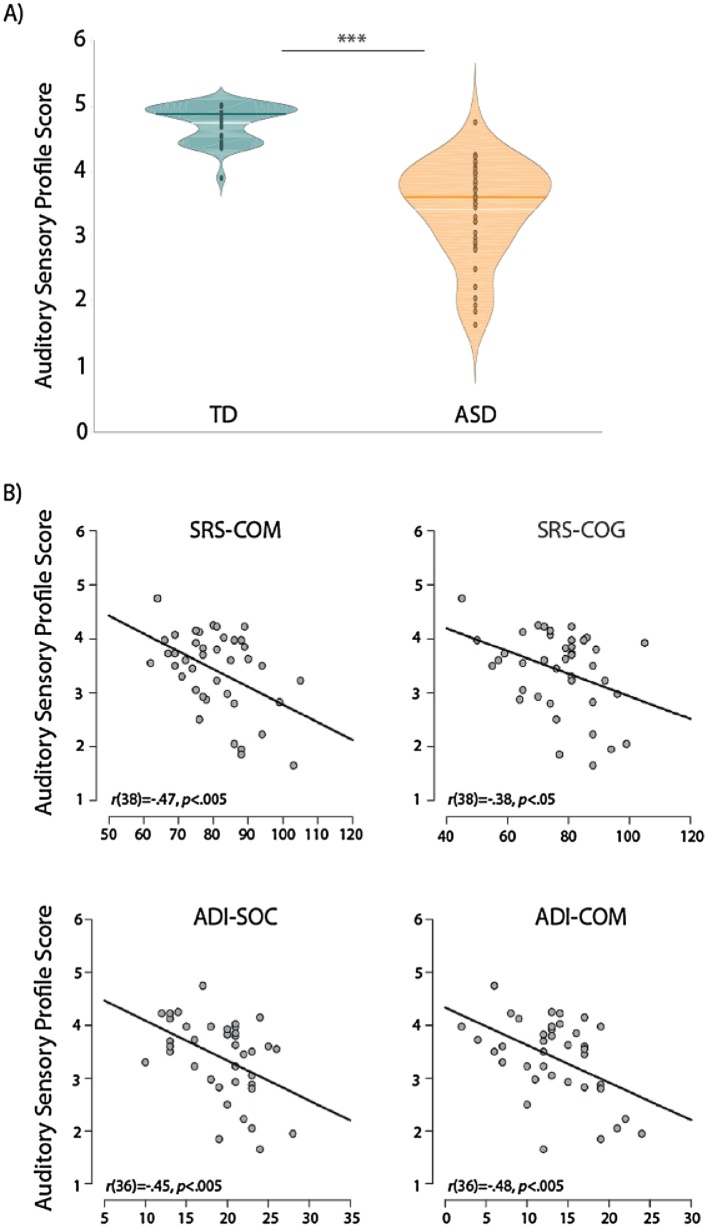
Clear differences in Sensory Profile auditory scores (mean of nine auditory items) were observed between the TD and ASD groups (t(76) = −10.38, p < 0.001; Fig. 2A). Additionally, while four of the items were designated as indexing “hypersensitivity” and five as measuring “hyposensitivity” to sound, there was a high positive correlation between the two categories (r(76) = 0.73, p < 0.001; this was true within the ASD group when assessed separately as well: r(38) = 0.48, p < 0.005). For this reason, we averaged across all nine items to yield an overall auditory Sensory Profile (A-SP) score for each subject that was subsequently used in all further analyses. Correlations with symptom severity measures were carried out for the ASD group only, showing significant relationships between auditory sensory deficits and higher SRS-COM (r(38) = −0.46, p < 0.005), SRS-COG (r(38) = −0.38, p < 0.05), ADI-R communication (ADI-COM: r(36) = −0.48, p < 0.005) and social interaction scores (ADI-SOC: r(36) = −0.45, p < 0.005; Fig. 2B). Note that lower A-SP scores indicate great sensory deficits. This suggests that atypical auditory processing in ASD as measured by the Sensory Profile is related to more severe sociocommunicative symptoms.
Fig. 2.
(A) Significant difference in auditory Sensory Profile scores (average of all auditory items) between the TD and ASD groups (*** indicated t(76) = −10.38, p < 0.001;,dark horizontal line = median, white horizontal line = mean). Lower scores indicate greater auditory processing deficits. (B) Significant correlations between auditory Sensory Profile scores and SRS and ADI-R subscales for the ASD group.
3.2. Interhemispheric connectivity
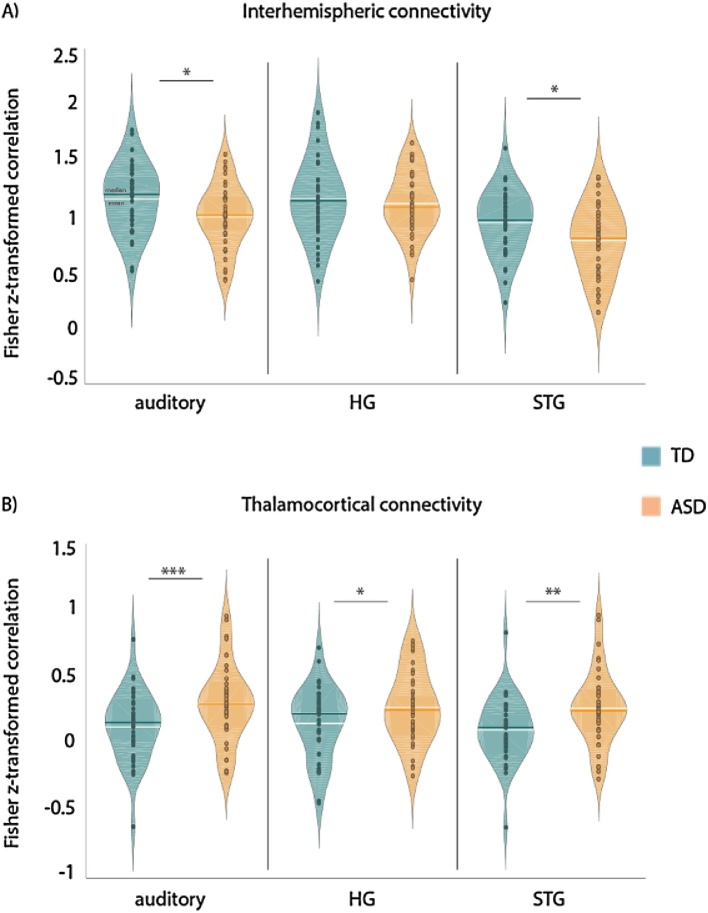
Interhemispheric connectivity of the left and right auditory combined ROI was significantly lower in the ASD than TD group (t(76) = −2.40, p < 0.05, Fig. 3A). A post-hoc split of the ROI into a primary auditory cortex (HG) ROI and a “secondary auditory” ROI covering the superior temporal gyrus (STG) revealed that this difference was driven by weaker interhemispheric connectivity of secondary auditory areas in ASD (t(76) = −2.37, p < 0.05), while interhemispheric connectivity of HG did not differ significantly between the ASD and TD groups (t(76) = −0.56, ns). Next, we assessed the relationship between the strength of interhemispheric connectivity, atypical auditory processing (as measured by the A-SP) and social-behavioral symptom severity.
Fig. 3.
Differences in interhemispheric (A) and thalamocortical (B) functional connectivity between the TD (green) and ASD (orange) groups, for the combined “auditory” ROI, and split into primary (HG) and secondary (STG) auditory cortical regions. Correlations were Fisher z-transformed, dark horizontal line = median, white horizontal line = mean, * p < 0.05, **p < 0.01, ***p < 0.005.
Interhemispheric connectivity between auditory regions was positively correlated (after partialling out effects of motion and age) with verbal IQ (r(74) = 0.27, p < 0.05), and negatively with the A-SP score (r(74) = 0.23, p < 0.05), indicating that reduced interhemispheric connectivity of auditory cortical areas was related to greater deficits in auditory sensory processing and lower verbal IQ. In order to assess whether the correlation of auditory interhemispheric connectivity with atypical sensory processing scores was modality specific, we also assessed the correlations between auditory interhemispheric connectivity and the average visual and tactile Sensory Profile scores of the same participants. Neither of these correlations was significant (visual: r(74) = 0.12, ns; tactile: r(74) = 0.13, ns). As there was a significant difference in A-SP scores between the TD and ASD groups (see section 3.1), we also performed the correlation analysis between interhemispheric connectivity and A-SP scores for the ASD group only. This correlation was not significant, suggesting that the significantly higher A-SP scores and significantly higher interhemispheric connectivity of the TD subjects drove the result seen for the whole group.
3.3. Thalamocortical connectivity
The ASD group showed significantly higher connectivity between auditory cortical regions and thalamus (t(76) = 2.77, p < 0.005, Fig. 3B). This was true for both HG (t(76) = 2.0, p < 0.05) and the STG ROI (t(76) = 2.71; p < 0.01). In order to assess how increased thalamocortical connectivity relates to ASD symptom severity, we correlated strength of connectivity with the A-SP scores and ADOS and SRS subscales. For the ASD participants, the strength of thalamocortical connectivity correlated negatively (partialling out effects of motion and age) with the ADOS repetitive behavior subscale (ADOS-REP: r(30) = −0.39, p < 0.05), the cognitive SRS subscale (SRS-COG: r(36) = −0.38, p < 0.05), and marginally with the communicative SRS subscale (SRS-COM: r(36) = −0.32, p = 0.054). SRS subscale scores were also available for the TD group, but correlations with strength of thalamocortical connectivity were not significant.
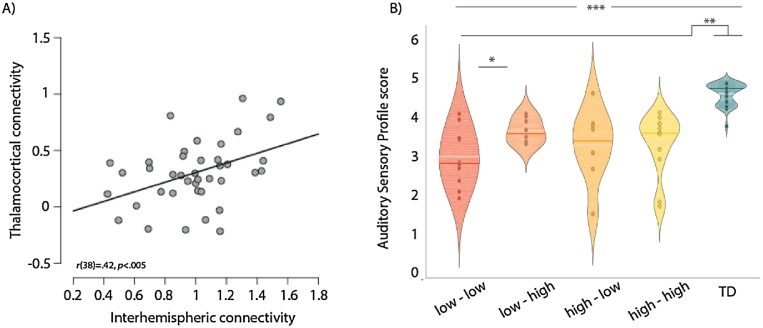
Increased thalamocortical connectivity was not related to auditory sensory deficits (A-SP scores) in the ASD group (r(36) = 0.18, ns). Together with the negative correlations of thalamocortical connectivity with the ADOS-REP, SRS-COG, and SRS-COM measures indicating improved functioning with higher thalamocortical connectivity, this suggests that atypically increased thalamocortical connectivity might reflect a compensatory mechanism. We next assessed whether the same ASD participants who showed increased auditory thalamocortical connectivity also showed increased interhemispheric connectivity between left and right auditory cortex. The strength of interhemispheric connectivity correlated positively (partialling out effects of motion and age) with the strength of thalamocortical connectivity in the ASD group (r(38) = 0.42, p < 0.005). Interestingly, this was not true for the TD group (r(36) = 0.28, ns). Despite the significant positive correlation in the ASD group, the pattern of connectivity seen in each subject varied, with some subjects also showing high thalamocortical connectivity but low interhemispheric connectivity, or low thalamocortical but high interhemispheric connectivity (Fig. 4A). To investigate the interaction between thalamocortical and interhemispheric connectivity of the auditory network further, we split the ASD participants by their pattern of interhemispheric and thalamocortical connectivity. This revealed a significant main effect of group (F(4,73) = 30.245, p < 0.001, Fig. 4). Post-hoc t-tests showed significant differences between the ASD and TD groups, but also between those subjects with low interhemispheric and low thalamocortical connectivity compared to the ASD subjects who had low interhemispheric but increased thalamocortical connectivity (t(18) = −2.53, p < 0.05).
Fig. 4.
(A) Positive correlation between interhemispheric (left and right auditory cortex) and auditory thalamocortical connectivity. (B) Auditory Sensory Profile scores for ASD subjects split by their pattern of interhemispheric and thalamocortical connectivity (low – low: low interhemispheric, low thalamocortical connectivity; low – high: low interhemispheric, high thalamocortical connectivity; high – low: high interhemispheric, low thalamocortical connectivity; high – high: high interhemispheric, high thalamocortical connectivity). Lower auditory Sensory Profile scores indicate more atypical sound processing. Dark horizontal line = median, white horizontal line = mean; * p < 0.05, **p < 0.01, ***p < 0.005.
4. Discussion
Many previous studies have reported altered sensory processing in autism (Marco et al., 2011), especially in the auditory domain (O’Connor, 2012). In the current study, we showed that this is reflected in Sensory Profile scores with greater deficits in the ASD compared to the TD group for the auditory modality. Importantly, these auditory deficits were related to symptom severity in ASD, particularly within the domain of social and communicative behavior. Greater auditory sensory deficits were also related to reductions in interhemispheric functional connectivity between auditory regions. Finally, increased auditory thalamocortical functional connectivity was not related to auditory sensory deficits, but was correlated with decreased repetitive behaviors and fewer social symptoms in the ASD group, suggesting a possible compensatory mechanism. Overall, these findings suggest that sensory impairments, specifically in the auditory domain, are related to core symptomatology in ASD.
4.1. Relationship between sensory and social features in ASD
The ability to acquire and filter incoming sounds is fundamental to the development of language and communication (Benasich et al., 2002), which are inherently social. Impairments in auditory processing may have cascading effects on higher-level social and communicative abilities. Our results showed that greater auditory sensory deficits in ASD were linked to increased symptom severity in several social domains including social interaction, social cognition, and communication and language. These findings suggest a connection between sensory and social features in ASD.
Atypical responses to auditory stimuli have been well documented in ASD (Dahlgren and Gillberg, 1989, Hilton et al., 2010, Tomchek and Dunn, 2007). On the one hand, individuals with ASD have been reported to show greater proficiency in tasks involving simple, low-level auditory stimuli (e.g., pitch discrimination) compared to their TD peers (Bonnel et al., 2010). On the other hand, there are reports of impairments in the processing of complex auditory stimuli often present in social environments such as speech in those with ASD (for a review, see O’Connor, 2012). Further auditory deficits in ASD have included weaker performance in auditory filtering tasks compared to TD (Lane et al., 2010, Tomchek and Dunn, 2007, Wiggins et al., 2009), as well as slower responses in orienting to social (and nonsocial) auditory stimuli than TD controls (Baranek et al., 2013, Dawson et al., 2004). These findings of atypical auditory processing, particularly in social contexts, may have an impact on language development and contribute to communicative difficulties (Tomchek et al., 2014). In our study, reported auditory abnormalities as measured by the Sensory Profile were highly related to the severity of social and communicative symptoms.
Our findings of increased auditory deficits associated with greater autism symptomatology are also consistent with recent evidence linking specific sensory abnormalities to socio-communicative deficits (Jao Keehn et al., 2016, Liss, 2006, Stewart et al., 2016, Watson et al., 2011), as well as stereotyped and restricted behaviors in children with ASD (Gabriels et al., 2008, Hilton et al., 2010, Kern et al., 2007a, Kern et al., 2007b, Wiggins et al., 2009). Although deficits in overall sensory processing have been correlated with impairments in social responsiveness and interaction (Baker et al., 2008, Ben-Sasson et al., 2009, Matsushima and Kato, 2013), this is to our knowledge the first study in ASD that has examined both auditory sensory processing abnormalities and intrinsic functional connectivity between auditory regions (see subsections 4.2 and 4.3), in relation to social features of autism.
4.2. Reduced interhemispheric connectivity in ASD
Analyses examining interhemispheric connectivity between auditory cortices showed weaker connectivity in ASD compared to TD participants, in accordance with our hypothesis. This finding was largely driven by altered connectivity between secondary auditory areas (STG), rather than primary regions (HG). Previous studies have provided similar evidence of decreased interhemispheric connectivity in STG (Anderson et al., 2011, Lo et al., 2011). While this region has been implicated in both abnormal auditory processing (Anderson et al., 2011) and social intelligence (Baron-Cohen et al., 1999), the relationship between reduced interhemispheric connectivity and sensory and social symptoms had not previously been tested directly. Our results show that lower interhemispheric connectivity was associated with greater sensory deficits in children with ASD, suggesting less robust communication between auditory cortical areas for processing sensory information.
It is important to note that modality-specific items on the Sensory Profile may not be exclusively sensory as many of the items concern behaviors that are social and sensory. Moreover, they may not exclusively tap into a single sensory modality (even when labeled “auditory,” “visual,” etc.) and are not age-normed. With this caveat in mind, interhemispheric functional connectivity in auditory areas was correlated with the A-SP scores, while correlations between interhemispheric connectivity and visual and tactile sensory processing scores were not significant. Combined, these results imply that the Sensory Profile can measure modality-specific deficits, and more importantly, that auditory processing impairments are reflected in reduced functional connectivity between the two hemispheres.
Our results further indicated that reduced interhemispheric connectivity between auditory cortical areas was correlated with lower verbal IQ. These findings are consistent with previous research examining neuropsychological outcomes in children and adolescents with corpus callosum abnormalities (CCA, which include agenesis, partial agenesis, hypoplasia, hyperplasia, and dysgenesis). In one study, CCA were frequently associated with intellectual disabilities, and 6.6% of the sample also presented with diagnoses of ASD (Margari et al., 2016). A multitude of studies investigating CCA in autism have reported a reduction in the total or partial volume of the corpus callosum (Aoki et al., 2013, Barbeau et al., 2015, Frazier and Hardan, 2009, Travers et al., 2012). These findings suggest that atypical structural development of the corpus callosum may impede interhemispheric functional interactions between brain regions.
4.3. Increased thalamocortical connectivity in ASD
ASD participants showed increased thalamocortical connectivity compared to TD participants for both primary and secondary auditory regions. These findings are consistent with previous studies demonstrating atypical thalamocortical connectivity in ASD (Nair et al., 2013, Nair et al., 2015). Unexpectedly, however, increased functional connectivity in ASD did not correlate with auditory Sensory Profile scores. Thalamocortical connectivity was inversely related to social symptomatology. While it could be argued that early abnormalities of auditory processing are likely to have downstream effects on social abilities, it is also conceivable that auditory thalamocortical overconnectivity is compensatory. A recent study demonstrated similar associations between increased temporal lobe thalamocortical connectivity and improved communication and language skills (Nair et al., 2015). This notion is substantiated by our findings of increased thalamocortical connectivity, which was related to fewer symptoms in social cognition and communication, as well as fewer repetitive behaviors within the ASD group only; there was no relationship between thalamocortical connectivity and social and communicative behavior in the TD group. Critically, the more atypical thalamocortical connectivity was in ASD, the more typical (closer to TD norms) the manifested symptoms were.
We further assessed the relation between interhemispheric and thalamocortical connectivity in ASD. Results indicated an overall significant positive correlation between interhemispheric and thalamocortical connectivity in which participants with increased interhemispheric connectivity also showed atypically high thalamocortical connectivity (high–high), and participants with atypically reduced interhemispheric connectivity also showed low thalamocortical connectivity (low–low). Some participants, however, had a more mixed pattern (low interhemispheric connectivity paired with high thalamocortical connectivity [low-high] and vice versa [high–low]). Importantly, auditory sensory deficits were more severe in the low–low ASD subgroup compared to the subgroup with low interhemispheric connectivity but high thalamocortical connectivity. This supports the hypothesis that in some cases of ASD increased auditory thalamocortical connectivity may reflect a compensatory mechanism for sensory processing when interhemispheric connectivity is reduced. One possible explanation for this mechanism is that with disrupted or reduced interhemispheric connectivity, thalamocortical connections strengthen to maintain synchrony between the two hemispheres (Schmidt, 2003, Tyszka et al., 2011). The sub-group of ASD participants with high interhemispheric and increased thalamocortical connectivity, on the other hand, did not show reduced sensory symptom severity. This suggests that the interaction between interhemispheric and thalamocortical connectivity is complex, and likely influenced by the developmental trajectory of other brain regions. It is plausible that increased thalamocortical connectivity is a compensatory response to a lack of interhemispheric synchronization – as observed in patients with agenesis of the corpus callosum – while increased thalamocortical connectivity in the presence of normal interhemispheric connectivity may reflect a different developmental trajectory that does not share the same compensatory function. Future studies are needed to unravel the complex interactions of brain network development by implementing longitudinal designs to track neural and behavioral development from infancy through early childhood.
4.4. Considerations and concerns
Several considerations and general concerns for the current study must be taken into account. Firstly, the constraints of scanning a clinical and developmental population such as ASD necessitated the inclusion of primarily high-functioning participants who were able to keep still during scanning. It remains unclear whether our findings would extend to lower functioning segments of the autism spectrum.
Secondly, it is important to consider that most of the significant correlations were based on parent-report measures, with the exception of repetitive behaviors, which were measured by the clinician’s observations during ADOS. Given such a context, there may be discrepancies between the observed sensory and social behaviors and the actual processing that contribute to the scores. These parent report measures are nevertheless informative, at least until a better means of directly recording neural and behavioral processing in realistic social environments becomes available.
Thirdly, unusual sensory responses have generally been characterized in the ASD literature as following one of two behavioral response patterns: hypersensitivity and hyposensitivity (Baranek et al., 2006, O’Neill and Jones, 1997, Rosenhall et al., 1999). Individuals who show hypersensitivity have a low threshold for, and are over-stimulated by sensory stimuli (e.g., avoidance of loud sounds); those who show hyposensitivity have a high threshold for, and are under-stimulated by sensory stimuli (e.g., diminished response to loud sounds). This distinction, however, was less clear in our sample assessed with the Sensory Profile, due to the high positive correlation between auditory hypersensitivity and hyposensitivity items, which could be ascribed to the tendency of some Sensory Profile items to be more social than sensory in nature (see subsection 4.2). Additionally, while some researchers have supported a prevalence of hyposensitive behaviors in children with ASD (Baranek et al., 2006, Baranek et al., 2013, Ben-Sasson et al., 2009, Rogers and Ozonoff, 2005), others have suggested coexisting response patterns of hypersensitivity and hyposensitivity, as well as reliable differences between ASD and TD for both (Hirstein et al., 2001, Kern et al., 2007a, Kern et al., 2007b). Future studies could examine the relation between auditory processing and thalamocortical functional connectivity—which would most likely reflect early auditory processing—more directly by assessing sensory thresholds with psychophysics.
4.5. Conclusions
Using diagnostic assessments, observational reports of sensory and social behaviors, and functional neuroimaging, we showed strong correlations between auditory sensory processing and symptom severity in ASD. Moreover, reduced interhemispheric functional connectivity in ASD was linked to increases in both sensory and social symptomatology, suggesting less robust communication between auditory cortices for processing and interpreting sensory input. Increased thalamocortical functional connectivity between thalamus and auditory cortices, however, was associated with decreased repetitive behaviors and social deficits, indicating a possible compensatory mechanism in ASD that may serve to ameliorate these symptoms. Together, these findings suggest that sensory deficits are linked to impairments in the core features of autism, specifically, social interaction and communication.
Funding
This work was supported by the National Institutes of Health – grants R01 MH081023 (RAM), R01 MH101173 (RAM), and K01 MH097972 (IF).
Conflict of interest
None.
Acknowledgements
The authors gratefully acknowledge the participants and parents, without whom the research would not have been possible.
Contributor Information
Annika C. Linke, Email: alinke@mail.sdsu.edu.
R. Joanne Jao Keehn, Email: rjao@mail.sdsu.edu.
References
- Alcauter S., Lin W., Smith J.K., Short S.J., Goldman B.D., Reznick J.S., Gilmore J.H., Gao W. Development of thalamocortical connectivity during infancy and its cognitive correlations. J. Neurosci. 2014;34:9067–9075. doi: 10.1523/JNEUROSCI.0796-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . 5th Edition. American Psychiatric Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Anderson J.S., Druzgal T.J., Froehlich A., DuBray M.B., Lange N., Alexander A.L., Abildskov T., Nielsen J.A., Cariello A.N., Cooperrider J.R., Bigler E.D., Lainhart J.E. Decreased interhemispheric functional connectivity in autism. Cereb. Cortex. 2011;21:1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.S. Comprehensive Guide to Autism. Springer New York; New York, NY: 2014. Cortical underconnectivity hypothesis in autism: evidence from functional connectivity MRI; pp. 1457–1471. [Google Scholar]
- Aoki Y., Abe O., Nippashi Y., Yamasue H. Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Mol. Autism. 2013;4:25. doi: 10.1186/2040-2392-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A.E.Z., Lane A., Angley M.T., Young R.L. The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: a pilot study. J. Autism Dev. Disord. 2008;38:867–875. doi: 10.1007/s10803-007-0459-0. [DOI] [PubMed] [Google Scholar]
- Baranek G.T., David F.J., Poe M.D., Stone W.L., Watson L.R. Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. J. Child Psychol. Psychiatry. All. Discip. 2006;47:591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Baranek G.T., Watson L.R., Boyd B.A., Poe M.D., David F.J., McGuire L. Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Dev. Psychopathol. 2013;25:307–320. doi: 10.1017/S0954579412001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau E.B., Lewis J.D., Doyon J., Benali H., Zeffiro T.A., Mottron L. A greater involvement of posterior brain areas in interhemispheric transfer in autism: FMRI, DWI and behavioral evidences. NeuroImage Clin. 2015;8:267–280. doi: 10.1016/j.nicl.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S., Ring H.A., Wheelwright S., Bullmore E.T., Brammer M.J., Simmons A., Williams S.C. Social intelligence in the normal and autistic brain: an fMRI study. Eur. J. Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A., Hen L., Fluss R., Cermak S.A., Engel-Yeger B., Gal E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J. Autism Dev. Disord. 2009;39:1–11. doi: 10.1007/s10803-008-0593-3. [DOI] [PubMed] [Google Scholar]
- Benasich A.A., Thomas J.J., Choudhury N., Leppänen P.H.T. The importance of rapid auditory processing abilities to early language development: evidence from converging methodologies. Dev. Psychobiol. 2002;40:278–292. doi: 10.1002/dev.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S.L., Hus V., Duncan A., Huerta M., Gotham K., Pickles A., Kreiger A., Buja A., Lund S., Lord C. Subcategories of restricted and repetitive behaviors in children with autism spectrum disorders. J. Autism Dev. Disord. 2013;43:1287–1297. doi: 10.1007/s10803-012-1671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnel A., McAdams S., Smith B., Berthiaume C., Bertone A., Ciocca V., Burack J.A., Mottron L. Enhanced pure-tone pitch discrimination among persons with autism but not Asperger syndrome. Neuropsychologia. 2010;48:2465–2475. doi: 10.1016/j.neuropsychologia.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Cerliani L., Mennes M., Thomas R.M., Di Martino A., Thioux M., Keysers C. Increased functional connectivity between subcortical and cortical resting-state networks in autism spectrum disorder. JAMA Psychiatry. 2015;72:1–11. doi: 10.1001/jamapsychiatry.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M.C., Parham L.D., Blanche E.I., Schell A., Chou C.P., Dawson M., Clark F. Autonomic and behavioral responses of children with autism to auditory stimuli. Am. J. Occup. Ther. 2012;66:567–576. doi: 10.5014/ajot.2012.004242. [DOI] [PubMed] [Google Scholar]
- Constantino J., Gruber C. Western Psychological Services; Los Angeles, CA: 2005. Social Responsiveness Scale (SRS) [Google Scholar]
- Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dahlgren S.O., Gillberg C. Symptoms in the first two years of life. A preliminary population study of infantile autism. Eur. Arch. Psychiatry Neurol. Sci. 1989;238:169–174. doi: 10.1007/BF00451006. [DOI] [PubMed] [Google Scholar]
- Damarla S.R., Keller T.A., Kana R.K., Cherkassky V.L., Williams D.L., Minshew N.J., Just M.A. Cortical underconnectivity coupled with preserved visuospatial cognition in autism: evidence from an fMRI study of an embedded figures task. Autism Res. 2010;3:273–279. doi: 10.1002/aur.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Toth K., Abbott R., Osterling J., Munson J., Estes A., Liaw J. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev. Psychol. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dunn W. Psychological Corporation; San Antonio, TX: 1999. Sensory Profile. [Google Scholar]
- Elmer S., Hänggi J., Jäncke L. Interhemispheric transcallosal connectivity between the left and right planum temporale predicts musicianship, performance in temporal speech processing, and functional specialization. Brain Struct. Funct. 2016;221:331–344. doi: 10.1007/s00429-014-0910-x. [DOI] [PubMed] [Google Scholar]
- Figueiredo Anomal R., De Villers-Sidani E., Brandão J.A., Diniz R., Costa M.R., Romcy-Pereira R.N., Lebedev M., Desai N.S., Kulesza R.J. Impaired processing in the primary auditory cortex of an animal model of autism. Front. Syst. Neurosci. 2015;9 doi: 10.3389/fnsys.2015.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Skiöld B., Engström M., Hallberg B., Mosskin M., Åden U., Lagercrantz H., Blennow M. Spontaneous brain activity in the newborn brain during natural sleep-an fMRI study in infants born at full term. Pediatr. Res. 2009;66:301–305. doi: 10.1203/PDR.0b013e3181b1bd84. [DOI] [PubMed] [Google Scholar]
- Frazier T.W., Hardan A.Y. A meta-analysis of the corpus callosum in autism. Biol. Psychiatry. 2009;66:935–941. doi: 10.1016/j.biopsych.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriels R.L., Agnew J.A., Miller L.J., Gralla J., Pan Z., Goldson E., Ledbetter J.C., Dinkins J.P., Hooks E. Is there a relationship between restricted, repetitive, stereotyped behaviors and interests and abnormal sensory response in children with autism spectrum disorders? Res. Autism Spectr. Disord. 2008;2:660–670. [Google Scholar]
- Green S.A., Rudie J.D., Colich N.L., Wood J.J., Shirinyan D., Hernandez L., Tottenham N., Dapretto M., Bookheimer S.Y. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:1158–1172. doi: 10.1016/j.jaac.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S.A., Hernandez L., Bookheimer S.Y., Dapretto M. Reduced modulation of thalamocortical connectivity during exposure to sensory stimuli in ASD. Autism Res. 2016 doi: 10.1002/aur.1726. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton C.L., Harper J.D., Kueker R.H., Lang A.R., Abbacchi A.M., Todorov A., Lavesser P.D. Sensory responsiveness as a predictor of social severity in children with high functioning autism spectrum disorders. J. Autism Dev. Disord. 2010;40:937–945. doi: 10.1007/s10803-010-0944-8. [DOI] [PubMed] [Google Scholar]
- Hirstein W., Iversen P., Ramachandran V.S. 2001. Autonomic Responses of Autistic Children to Objects and People. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A., Oeschger F.M., Krishnan M.L., Belgard T.G., Wang W.Z., Lee S., Webber C., Petretto E., Edwards A.D., Molnár Z. Expression profiling of mouse subplate reveals a dynamic gene network and disease association with autism and schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3555–3560. doi: 10.1073/pnas.1218510110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao Keehn R.J., Sanchez S.S., Stewart C.R., Zhao W., Grenesko-Stevens E.L., Keehn B., Müller R.-A. Impaired downregulation of visual cortex during auditory processing is associated with autism symptomatology in children and adolescents with autism spectrum disorder. Autism Res. 2016:1–14. doi: 10.1002/aur.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John Y.J., Zikopoulos B., Bullock D., Barbas H. The emotional gatekeeper: a computational model of attentional selection and suppression through the pathway from the amygdala to the inhibitory thalamic reticular nucleus. PLoS Comput. Biol. 2016;12:e1004722. doi: 10.1371/journal.pcbi.1004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.B., Bandettini P.A., Kenworthy L., Case L.K., Milleville S.C., Martin A., Birn R.M. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. Neuroimage. 2010;49:401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just M.A., Cherkassky V.L., Keller T.A., Kana R.K., Minshew N.J. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb. Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana R.K., Libero L.E., Moore M.S. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys. Life Rev. 2011;8:410–437. doi: 10.1016/j.plrev.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Kanold P.O., Luhmann H.J. The subplate and early cortical circuits. Annu. Rev. Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- Kern J.K., Garver C.R., Carmody T., Andrews A.A., Trivedi M.H., Mehta J.A. Examining sensory quadrants in autism. Res. Autism Spectr. Disord. 2007;1:185–193. [Google Scholar]
- Kern J.K., Trivedi M.H., Grannemann B.D., Garver C.R., Johnson D.G., Andrews A.A., Savla J.S., Mehta J.A., Schroeder J.L. Sensory correlations in autism. Autism. 2007;11:123–134. doi: 10.1177/1362361307075702. [DOI] [PubMed] [Google Scholar]
- Kleinhans N.M., Richards T., Sterling L., Stegbauer K.C., Mahurin R., Johnson L.C., Greenson J., Dawson G., Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Lane A.E., Young R.L., Baker A.E.Z., Angley M.T. Sensory processing subtypes in autism: association with adaptive behavior. J. Autism Dev. Disord. 2010;40:112–122. doi: 10.1007/s10803-009-0840-2. [DOI] [PubMed] [Google Scholar]
- Lee J.M., Kyeong S., Kim E., Cheon K.-A. Abnormalities of inter- and intra-hemispheric functional connectivity in autism spectrum disorders: a study using the autism brain imaging data exchange database. Front. Neurosci. 2016;10:191. doi: 10.3389/fnins.2016.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam S.R., Nieto C., Libby S.J., Wing L., Gould J. Describing the sensory abnormalities of children and adults with autism. J. Autism Dev. Disord. 2007;37:894–910. doi: 10.1007/s10803-006-0218-7. [DOI] [PubMed] [Google Scholar]
- Liss M. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006;10:155–172. doi: 10.1177/1362361306062021. [DOI] [PubMed] [Google Scholar]
- Lo Y.C., Soong W.T., Gau S.S.F., Wu Y.Y., Lai M.C., Yeh F.C., Chiang W.Y., Kuo L.W., Jaw F.S., Tseng W.Y.I. The loss of asymmetry and reduced interhemispheric connectivity in adolescents with autism: a study using diffusion spectrum imaging tractography. Psychiatry Res. – Neuroimaging. 2011;192:60–66. doi: 10.1016/j.pscychresns.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., Goode S., Heemsbergen J., Jordan H., Mawhood L., Schopler E. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J. Autism Dev. Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Müller R.-A. Comprehensive Guide to Autism. Springer New York; New York, NY: 2014. Anatomical and functional connectivity in autism spectrum disorders; pp. 49–75. [Google Scholar]
- Marco E.J., Hinkley L.B.N., Hill S.S., Nagarajan S. Sensory processing in autism: a review of neuropsychologic findings. Pediatr. Res. 2011;69:48–54. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margari L., Palumbi R., Campa M.G., Operto F.F., Buttiglione M., Craig F., Matricardi S., Verrotti A. Clinical manifestations in children and adolescents with corpus callosum abnormalities. J. Neurol. 2016 doi: 10.1007/s00415-016-8225-x. [DOI] [PubMed] [Google Scholar]
- Mason R.A., Williams D.L., Kana R.K., Minshew N., Just M.A. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K., Kato T. Social interaction and atypical sensory processing in children with autism spectrum disorders. Hong Kong J. Occup. Ther. 2013;23:89–96. [Google Scholar]
- Mizuno A., Villalobos M.E., Davies M.M., Dahl B.C., Müller R.-A. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104:160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A., Treiber J.M., Shukla D.K., Shih P., Müller R.-A. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013;136:1942–1955. doi: 10.1093/brain/awt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A., Carper R.A., Abbott A.E., Chen C.P., Solders S., Nakutin S., Datko M.C., Fishman I., Müller R.A. Regional specificity of aberrant thalamocortical connectivity in autism. Hum. Brain Mapp. 2015;36:4497–4511. doi: 10.1002/hbm.22938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor K. Auditory processing in autism spectrum disorder: a review. Neurosci. Biobehav. Rev. 2012;36:836–854. doi: 10.1016/j.neubiorev.2011.11.008. [DOI] [PubMed] [Google Scholar]
- O’Leary D.D., Nakagawa Y. Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex. Curr. Opin. Neurobiol. 2002;12:14–25. doi: 10.1016/s0959-4388(02)00285-4. [DOI] [PubMed] [Google Scholar]
- O’Neill M., Jones R.S.P. Sensory-perceptual abnormalities in autism: a case for more research? J. Autism Dev. Disord. 1997;27:283–293. doi: 10.1023/a:1025850431170. [DOI] [PubMed] [Google Scholar]
- Plitt M., Barnes K.A., Martin A. Functional connectivity classification of autism identifies highly predictive brain features but falls short of biomarker standards. NeuroImage Clin. 2015;7:359–366. doi: 10.1016/j.nicl.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S.J., Ozonoff S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J. Child Psychol. Psychiatry. 2005;46:1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Rosenhall U., Nordin V., Sandström M., Ahlsén G., Gillberg C. Autism and hearing loss. J. Autism Dev. Disord. 1999;29:349–357. doi: 10.1023/a:1023022709710. [DOI] [PubMed] [Google Scholar]
- Schmidt M.F. Pattern of interhemispheric synchronization in HVc during singing correlates with key transitions in the song pattern. J. Neurophysiol. 2003;90:3931–3949. doi: 10.1152/jn.00003.2003. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Steinmann S., Leicht G., Mulert C. Interhemispheric auditory connectivity: structure and function related to auditory verbal hallucinations. Front. Hum. Neurosci. 2014;8:55. doi: 10.3389/fnhum.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C.R., Sanchez S.S., Grenesko E.L., Brown C.M., Chen C.P., Keehn B., Velasquez F., Lincoln A.J., Müller R.-A. Sensory symptoms and processing of nonverbal auditory and visual stimuli in children with autism spectrum disorder. J. Autism Dev. Disord. 2016;45:1590–1601. doi: 10.1007/s10803-015-2367-z. [DOI] [PubMed] [Google Scholar]
- Stoner R., Chow M.L., Boyle M.P., Sunkin S.M., Mouton P.R., Roy S., Wynshaw-Boris A., Colamarino S.A., Lein E.S., Courchesne E. Patches of disorganization in the neocortex of children with autism. N. Engl. J. Med. 2014;370:1209–1219. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Dassanayake M.T., Shen S., Katkuri Y., Alexis M., Anderson A.L., Yeo L., Mody S., Hernandez-Andrade E., Hassan S.S., Studholme C., Jeong J.-W., Romero R. Cross-hemispheric functional connectivity in the human fetal brain. Sci. Transl. Med. 2013;5:173ra24. doi: 10.1126/scitranslmed.3004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchek S.D., Dunn W. Sensory processing in children with and without autism: a comparative study using the short sensory profile. Am. J. Occup. Ther. 2007;61:190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- Tomchek S.D., Huebner R.A., Dunn W. Patterns of sensory processing in children with an autism spectrum disorder. Res. Autism Spectr. Disord. 2014;8:1214–1224. [Google Scholar]
- Travers B.G., Adluru N., Ennis C., Tromp D.P.M., Destiche D., Doran S., Bigler E.D., Lange N., Lainhart J.E., Alexander A.L. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res. 2012;5:289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyszka J.M., Kennedy D.P., Adolphs R., Paul L.K. Intact bilateral resting-state networks in the absence of the corpus callosum. J. Neurosci. 2011;31:15154–15162. doi: 10.1523/JNEUROSCI.1453-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel M.P., Kersbergen K.J., De Reus M.A., Keunen K., Kahn R.S., Groenendaal F., De Vries L.S., Benders M.J.N.L. The neonatal connectome during preterm brain development. Cereb. Cortex. 2015;25:3000–3013. doi: 10.1093/cercor/bhu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers M.E., Cohen M.X., Geurts H.M. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci. Biobehav. Rev. 2012;36:604–625. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Wass S. Distortions and disconnections: disrupted brain connectivity in autism. Brain Cogn. 2011;75:18–28. doi: 10.1016/j.bandc.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Watson L.R., Patten E., Baranek G.T., Poe M., Boyd B.A., Freuler A., Lorenzi J. Differential associations between sensory response patterns and language, social, and communication measures in children with autism or other developmental disabilities. J. Speech Lang. Hear. Res. 2011;54:1562–1576. doi: 10.1044/1092-4388(2011/10-0029). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. second edition (WASI-II) Psychological Corporation; San Antonio, TX: 2011. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Weissenbacher A., Kasess C., Gerstl F., Lanzenberger R., Moser E., Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: A quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Weng S.-J., Wiggins J.L., Peltier S.J., Carrasco M., Risi S., Lord C., Monk C.S. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins L.D., Robins D.L., Bakeman R., Adamson L.B. Brief report: sensory abnormalities as distinguishing symptoms of autism spectrum disorders in young children. J. Autism Dev. Disord. 2009;39:1087–1091. doi: 10.1007/s10803-009-0711-x. [DOI] [PubMed] [Google Scholar]
- Zhu H., Fan Y., Guo H., Huang D., He S. Reduced interhemispheric functional connectivity of children with autism spectrum disorder: evidence from functional near infrared spectroscopy studies. Biomed. Opt. Express. 2014;5:1262–1274. doi: 10.1364/BOE.5.001262. [DOI] [PMC free article] [PubMed] [Google Scholar]