Summary
Cystic fibrosis is characterized by an overly exuberant neutrophilic inflammatory response to pathogens and other stimuli that starts very early in disease. The overwhelming nature of this response is a primary cause of remodeling and destruction of the airways, suggesting that anti-inflammatory therapies could be beneficial in CF. However, finding therapies that can effectively reduce the inflammatory response without compromising host defenses remains elusive. New approaches towards mapping inflammatory targets promise to aid in developing novel therapeutic strategies and improve outcomes in individuals with CF.
Keywords: Sputum, bronchoalveolar lavage, exhaled breath condensate, metabolomics
Introduction
Inflammation in cystic fibrosis (CF) is characterized by a marked and persistent influx of neutrophils into the airways. Despite the overwhelming nature of this inflammatory response, it remains insufficient to eradicate infection, resulting in a vicious cycle of infection, inflammation, and mucus hypersecretion/dehydration that causes progressive remodeling and destruction of the airways. This high degree of airway inflammation is responsible for much of the lung disease in CF, with concentrations of inflammatory biomarkers (particularly neutrophil elastase) the most predictive of disease progression1,2. Nevertheless, there are relatively few therapies developed to directly address airway inflammation in CF. This lack of treatment options reflects several challenges in developing effective anti-inflammatory therapies, including difficulties in measuring airway inflammation. This review will summarize the origins of airway inflammation in CF, current options for treatment, and how developments in measuring biomarkers of airway inflammation may lead to a new generation of anti-inflammatory treatments for CF.
Origins of CF Inflammation
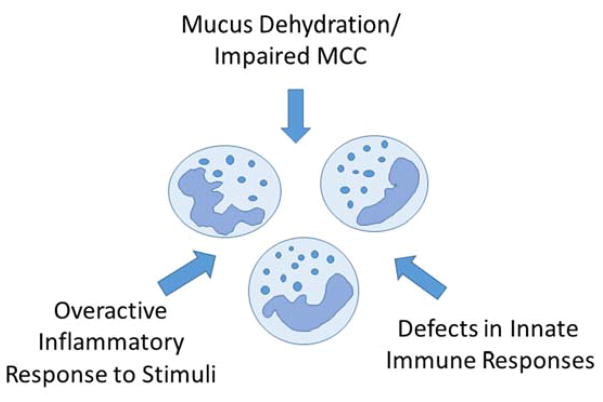
A key finding of studies of early CF lung disease is that airway inflammation begins at or very soon after birth. Neutrophils and neutrophil elastase can be detected in bronchoalveolar lavage (BAL) in patients diagnosed with CF by newborn screen as early as 3 months of age, and these inflammatory markers correlate with future development of bronchiectasis and gas trapping on CT scan1. This increase in inflammation does not appear to be solely a response to infection, since less than half of infants with neutrophil elastase detected in BAL fluid had an active pulmonary infection or history suggesting infection. These observations suggest that inflammation in CF airways is multifactorial (Figure 1) and can occur even in the absence of an infectious stimulus.
Figure 1.
Factors that contribute to the excessive inflammatory response in CF
Localized hypoxia in the CF lung could explain the early inflammation seen in the absence of obvious infection3. The gene mutated in CF, the cystic fibrosis transmembrane conductance regulator (CFTR), encodes a cAMP dependent anion channel that conducts chloride and bicarbonate and regulates the balance of chloride secretion and sodium absorption in the airway.4 Loss of CFTR channel activity produces a dehydrated airway surface environment where the total mass of salt and volume of water are inadequate to maintain mucus hydration, leading to defects in mucociliary clearance. The resulting thickened mucus and mucus plugging in the small airway create localized areas of hypoxia, which can trigger inflammatory responses5 including release of cytokines such as IL-1 and activation of the inflammatory cascade via binding to the IL-1 receptor6. The resultant increase in inflammation may then worsen hypoxia and contribute to a niche for anaerobic bacteria, thus further propagating the inflammatory cycle3.
While infection may not be required to initiate inflammation in CF, defects in immune responses to pathogens likely contribute to the excessive inflammatory environment. A number of mechanisms of immune dysregulation have been described in CF, including aberrant responses in inflammatory cells such as neutrophils and macrophages as well as altered signaling pathways in airway epithelia. These aspects of immune dysregulation were reviewed in detail in 2015 by Nichols and Chmiel in a previous volume of “Barriers to Normalcy”7 and will be only briefly summarized here. The mucus dehydration and impaired mucociliary clearance contribute to enhanced inflammatory responses, with failure to clear pathogens out of the airway leading to prolonged stimulation of inflammatory pathways8,9. Furthermore, there is evidence that CFTR may play a more direct role in regulation of inflammatory responses. For example, neutrophils isolated from patients with CF tend to undergo necrotic rather than apoptotic responses, releasing additional pro-inflammatory molecules such as High Mobility Group Box 1 (HMGB1) protein and metalloproteases10,11. There is also evidence that CFTR is involved in the acidification of phagosomes and bacterial killing in both neutrophils and macrophages12,13. Similarly, CF macrophages and monocytes also demonstrate defective immune response14.
Studies of animal models suggest that defects in innate immunity contribute to the excessive inflammatory responses in CF. CF pigs have decreased bacterial clearance and increased inflammation relative to unaffected litter mates after exposure to bacterial pathogens15. CFTR knockout ferrets16 also show abnormal bacterial clearance and enhanced inflammatory responses17. The mechanisms that underlie these defects are not fully defined, though there is evidence that defective bacterial clearance in the CF pig reflects altered airway acidification, likely related to loss of CFTR mediated bicarbonate secretion that alters the efficacy of antimicrobial peptides18.
Despite this evidence, the role of altered inflammatory responses directly related to CFTR deficiency (as opposed to secondary effects from defective mucociliary clearance) remains controversial. Systemic infection remains uncommon in CF despite high airway bacterial loads19, raising some questions about the clinical relevance of abnormalities observed in isolated CF inflammatory cells. Studies in animal models must also be interpreted with caution, since no animal model faithfully recapitulates all aspects of human disease. For example, the altered airway pH observed in pigs may not be present in human CF20, and airway acidification similar in magnitude to that of the CF pigs has been observed in asthma21,22, a disease that is not commonly associated with airway infection21,22.
Anti-inflammatory therapies in CF
Although the factors that contribute to inflammation in CF are not fully defined, the relevance of inflammation as a therapeutic target is unquestioned23. Nevertheless, despite intensive effort, limited therapies are available. Prednisone is perhaps the most canonical anti-inflammatory, and alternate day therapy with prednisone has been shown to increase forced vital capacity (FVC) in treated CF patients compared to placebo24. However, chronic use of systemic steroids is contraindicated due to their adverse effects including growth retardation, osteoporosis, cataracts, hyperglycemia and risk of opportunistic infection. High dose ibuprofen is a more targeted anti-inflammatory that has been shown to slow the rate of decline of FEV1 in two separate double blind, placebo controlled studies25,26, and this clinical benefit has been associated with a decrease in neutrophil migration to the lung27. Although trials with ibuprofen did not show a significant increase in adverse events between treatment and placebo groups, the perceived risk of gastrointestinal bleeding and renal toxicity coupled with the need to obtain serum levels to minimize these risks has inhibited widespread use of this drug.
The most widely used therapy in CF with anti-inflammatory properties is azithromycin. Interest in azithromycin as a CF therapeutic stemmed from its benefit in diffuse panbronchiolitis28, a disease with many similarities to CF, and was thought to possibly relate to its antimicrobial activity against Pseudomonas aeruginosa growing in biofilms29. Indeed, the initial large study of chronic, low dose azithromycin in CF was targeted towards patients with persistent Pseudomonas infection. This study demonstrated that chronic azithromycin treatment led to improvement in FEV1, a decrease in exacerbations requiring antibiotic therapy, as well as improved quality of life (QOL) scores30,31. However, the clinical benefits occurred despite minimal impact on Pseudomonas bacterial density, suggesting that a different mechanism of action was responsible. Azithromycin has a number of anti-inflammatory effects, including reduction in neutrophil oxidative burst and increases neutrophil apoptosis32,33. In lung macrophages azithromycin also appears to inhibit apoptosis, stimulate phagocytosis of bacteria and cellular debris, as well as skew macrophage cytokine expression toward an anti-inflammatory phenotype34. Other anti-inflammatory effects of azithromycin include decreased mucin production with a resultant decrease in mucus viscosity, maintenance of tight junctions between epithelial cells and improvement of the integrity of the epithelial cell layer under inflammatory conditions33. These immunomodulatory effects may underlie the benefits of azithromycin more than its anti-Pseudomonal activity, and a large multi-center study demonstrated clinical benefit of chronic azithromycin in patients who did not have Pseudomonas infection35.
Given the extensive number of pathways identified as playing roles in CF airway inflammation, it may seem surprising that other anti-inflammatory therapies have not yet been developed. However, an effective anti-inflammatory for CF must manage a careful balancing act, providing sufficient potency to reduce inflammation induced lung damage without interfering with the ability to resolve infection. This balance can be difficult to achieve, as revealed by the Phase II clinical trial of BIIL 284 BS, a promising antagonist of the leukotriene B4 receptor known to play a significant role in CF airway inflammation. This trial was stopped early due to an increase in pulmonary adverse events in those receiving the active drug compared to placebo36. Further studies showed that treatment of CF mice with BIIL 284 BS interfered with their ability to resolve Pseudomonas aeruginosa respiratory infection37. These results showcase the difficulty of balancing a reduction in inflammation while not significantly increasing the bacterial burden with the use of anti-inflammatory therapies and emphasize the need for pre-clinical testing of novel therapeutics23.
Barriers to anti-inflammatory development: measuring airway inflammation
The dearth of effective anti-inflammatory therapies represents an ongoing barrier to normalcy in CF and suggests a need to identify new therapeutic targets. Testing of anti-inflammatory therapies and other treatments in CF has become increasingly challenging, as overall improvements in lung function and health make observing changes in traditional endpoints such as lung function or pulmonary exacerbations more difficult to assess without large and expensive trials38. However, accurately measuring airway inflammation directly as a marker of therapeutic activity can be difficult. Indeed, most of the major trials of anti-inflammatory therapies described above24–26,36 did not include an airway inflammation biomarker, with the exception of the azithromycin trial that demonstrated statistically significant though modest changes in neutrophil elastase30. Although treatment related reductions in airway inflammation biomarkers were often shown in smaller studies27,39, better biomarkers of airway inflammation are clearly needed to identify potential therapeutic targets and serve as surrogate markers of efficacy for clinical trials. This need is particularly great in young children to try and limit inflammation before the onset of lung damage. The challenges in developing better biomarkers reflect limitations in the primary methods to obtain airway samples: sputum collection, bronchoalveolar lavage, and assessments of exhaled breath (Figure 2, Table 2).
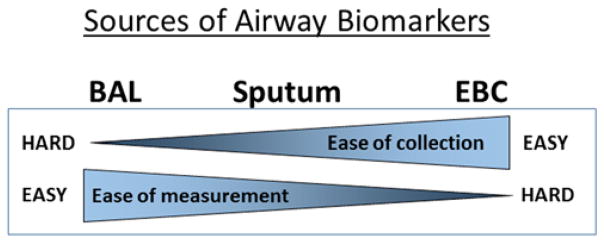
Figure 2.
Ease of collection and ease of biomarker measurement (including processing steps) are generally inversely related.
Table 2.
Comparison of airway samples for biomarker measurement
| Advantages | Disadvantages | Region sampled | Inflammatory biomarkers | |
|---|---|---|---|---|
| Sputum | Well established Non-invasive |
Requires immediate processing Difficult in young children |
Most affected large airways | Cell counts Cytokines/proteins Metabolites |
| BAL | Can be used in all subjects | Higher risk (anesthesia) Expensive |
Targeted smaller airways | Cell counts Cytokines/proteins Metabolites |
| EBC | Simple to collect Non-invasive |
Very low and variable biomarker concentrations | Small airways (may under-sample plugged airways) | Cytokines/proteins Metabolites |
Sputum
Historically, assessments of airway inflammation in CF (and other diseases) have been primarily based on analysis of biomarkers in sputum, in part reflecting the long experience and existence of standardized protocols for this airway sample. Given the intense airway inflammation that characterizes CF, it is no surprise that a multitude of inflammatory biomarkers are elevated in CF sputum, as summarized in several excellent reviews2,40. Among these biomarkers, sputum neutrophil elastase has emerged as one of the most predictive, with concentrations of sputum NE most highly correlated with lung function decline in large studies2,41,42.
However, the utility of sputum is limited somewhat by the need for specialized procedures to process samples that typically must be performed immediately after collection43. Spontaneously expectorated sputum likely arises from more affected regions of the lung, and concentrations of inflammatory markers can be influenced by regional variability in lung disease39 Furthermore, in general only older patients with more advanced disease can regularly expectorate sputum spontaneously. While sputum induction using hypertonic saline can be utilized to obtain samples from patients who do not spontaneously expectorate, many younger children have difficulty expectorating sputum even after induction44,45. Thus, sputum has a limited role in assessing—and by extension treating—airway inflammation in the youngest children.
Bronchoalveolar lavage fluid
For patients who cannot expectorate sputum, flexible bronchoscopy with BAL is considered the gold standard for airway biomarker assessment40. As with sputum, numerous inflammatory biomarkers are elevated in BAL fluid in children with CF including neutrophil counts, neutrophil elastase, pro-inflammatory cytokines such as interleukin-8, and others46–51. Several of these inflammatory biomarkers correlate with other aspects of disease severity including infection52, radiologic findings51,53,54, and infant lung function testing46,49. Like sputum, neutrophil elastase represents one of the most informative markers in BAL fluid, with elevated concentrations in infancy predictive of future bronchiectasis 1,55–57.
Use of BAL fluid as a source of airway inflammation biomarkers is constrained by several limitations, including the time, expertise, and expense needed for the procedure58. Furthermore, bronchoscopy requires sedation, which carries both short term risks and increasing concerns about long term adverse outcomes 59. Due to these limitations, BAL has seen a limited role in clinical trials, though longer term observational studies that include BAL biomarkers such as AREST CF have provided significant insights into early disease51,60,61.
Exhaled breath
Many of the limitations of sputum and BAL can be overcome through use of exhaled breath, which contains both volatile and non-volatile compounds that could serve as inflammatory biomarkers. Exhaled biomarkers are often collected as exhaled breath condensate (EBC), and since collection only requires the subject to exhale through a chilled tube, EBC can be obtained simply and non-invasively even in young children62,63. Indeed, a number of airway inflammatory biomarkers that are informative in sputum or BAL fluid are also elevated in EBC from subjects with CF, including inflammatory cytokines63–66, 8-isoprostane64,67, nitrates64,68, leukotrienes69, and purines49,70, with measures of EBC leukotrienes and purines shown to track changes related to CF exacerbations69,70. EBC pH has also been shown to be decreased in subjects with CF and change with treatment of an exacerbation71–73. With specialized methods, EBC can even be collected from the youngest children during infant pulmonary function testing (iPFTs)74–76.
The ease of EBC collection is belied by difficulty in analysis, with EBC being described as “easy on patients” but “hard on scientists”77. Airway secretions in EBC arise from microaerosols generated during respiration, which represent a very low and highly variable fraction of the fluid volume of the condensate and may under-sample obstructed airways78,79. Therefore, extremely sensitive methods are typically needed to assess the low concentrations of most traditional biomarkers found in EBC, which ideally should also include a means to control for variable dilution78,80. Our own approach has been to utilize mass spectrometry to measure relevant biomarkers as well as urea as a dilution marker81–83, though there are other valid methods30,32. Failure to adequately address these challenges impacts the reproducibility and validity of EBC biomarkers and may limit their utility as effective measures of airway inflammation84.
Some of the limitations of EBC can be addressed by a focus on volatile biomarkers, which are not dependent on microaerosol generation for incorporation in exhaled breath. Several studies had shown that volatile organic carbon (VOC) profiles are altered in individuals with CF and could serve as inflammatory biomarkers85,86. One of the potentially exciting application of VOC profiling in CF is the development of electronic “nose” systems that could provide information on airway inflammation at the point of care87,88. However, current methods require sophisticated mathematical modeling to identify complex patterns in the detected VOCs, and the reproducibility of these signatures and their relationships to specific aspects of airway inflammation have not been established.
Non airway samples
The high levels of inflammation in the airways of individuals with CF translate into increases in systemic inflammatory biomarkers that could be assessed in serum or plasma, which are relatively easily obtained and analyze. Indeed, a large number of blood inflammatory markers are elevated relative in CF, including C-reactive protein89–92, immunoglobulin G90,93,94, cytokines91, tumor necrosis factor95, and transforming growth factor β96, many of which are altered with pulmonary exacerbation91,92,96,97. However, the potential contribution of non-pulmonary inflammation reduces the specificity of these biomarker for lung disease and limits applicability. There have also been small trials investigating the use of biomarkers in both saliva and urine as a surrogate for lung inflammation98,99.
Imaging
A number of small studies have been done using fluorodeoxyglucose (FDG) PET to quantify lung inflammation and follow response to treatment of CF exacerbations. FDG is concentrated in activated neutrophils which are recruited to sites of inflammation. The degree of inflammation can be estimated by the degree of FDG emission. A study following the kinetics of FDG movement to the lung showed that increased influx into the lung correlated with a more rapid decline in FEV1 over time100. Other studies have used FDG PET monitor changes in inflammation during antibiotic treatment of CF exacerbation. Patients underwent FDG PET on days 1 and 14 of treatment and degree of inflammation was determined using standard uptake values (SUV). This group found that over the course of 14 days of IV therapy the max SUV decreased101. Regular use of FDG PET CT is limited by radiation exposure, however, as low dose CT protocols improve this may become a useful technique to follow lung inflammation.
Novel strategies
Despite all we have learned about the inflammatory pathways involved in CF lung disease, numerous challenges remain in translating these findings into effective anti-inflammatories. Many signaling pathways are not easily amenable to pharmacological inhibition, often requiring biologic antibody based treatments that while effective can be expensive and difficult to administer102. Other pathways may be too vital to host defenses to serve as a viable therapeutic targets, as suggested by the outcomes of the BIIL 284 BS study36. Therefore, there remains an urgent need to identify new pathways that could serve as viable targets for anti-inflammatory development. The ideal pathway would have defined characteristics, including an involvement in early disease, a readily measurable biomarker of activity, and availability of a relatively simple pharmaceutical treatment. Perhaps most importantly, blockade of this pathway should reduce inflammation without interfering with the ability to resolve infection (Table 1).
Table 1.
Ideal inflammatory pathways to target
| 1. Involved in early disease |
| 2. Associated with a biomarker that is readily measured in a non-invasive sample |
| 3. Can be altered by a relatively simple pharmaceutical |
| 4. Inhibition reduces inflammation without limiting ability to contain infection |
Use of ‘omics strategies, particularly metabolomics, is well suited towards identifying pathways that meet these criteria. The changes in metabolite patterns associated with disease reflect cellular enzymatic activities, which are attractive as therapeutic targets since they can often be inhibited by small molecule therapeutics103. Furthermore, the identified metabolites can serve as biomarkers of pathway activity and drug effects, many of which can be readily measured using standard methods even in non-invasive sample such as EBC81,82,104. The potential metabolomics has been demonstrated in several studies that find CF specific metabolite patterns in sputum105, BALF106–108, blood109,110, and even EBC111,112.
Metabolomics studies can be particularly informative when interpreted in conjunction with other ‘omics evaluations. For example, one of the largest gene wide association studies to date in CF identified associations between disease severity and expression of the gene APIP113, which encodes an enzyme involved in the methionine salvage pathway, and metabolites associated with this pathway, including polyamines and free adenine, are associated with neutrophilic inflammation in CF107,108,114. Similarly, the lysophosphatidic acid receptor LPAR6 has been linked to CF lung disease in genomic studies115, while the lysophospholipid substrates of this receptor are elevated in CF bronchitis107,116. While such studies demonstrate the promise of metabolomics to identify biomarkers and therapeutic targets, further study is needed before the potential of these identified pathways is truly known.
Conclusions
The intense inflammation present in the airways of individuals with CF is one of the most significant causes of progressive lung disease. Until we have a cure for CF, development of effective anti-inflammatories needs to be a priority for the CF research community. New approaches using metabolomics and other strategies to map the inflammatory targets in CF hold promise in development of new therapies.
Acknowledgments
Sponsor: NIH/NHLBI R01-HL116228, NIH/NHLBI K23-HL089708
Footnotes
Conflicts of Interest: none declared
References
- 1.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, Murray CP, Stick SM, Investigators AC. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368(21):1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 2.Mayer-Hamblett N, Aitken ML, Accurso FJ, Kronmal RA, Konstan MW, Burns JL, Sagel SD, Ramsey BW. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am J Respir Crit Care Med. 2007;175(8):822–828. doi: 10.1164/rccm.200609-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery ST, Mall MA, Kicic A, Stick SM, Arest CF. Hypoxia and sterile inflammation in cystic fibrosis airways: mechanisms and potential therapies. Eur Respir J. 2017;49(1) doi: 10.1183/13993003.00903-2016. [DOI] [PubMed] [Google Scholar]
- 4.Boucher RC. An overview of the pathogenesis of cystic fibrosis lung disease. Adv Drug Deliv Rev. 2002;54(11):1359–1371. doi: 10.1016/s0169-409x(02)00144-8. [DOI] [PubMed] [Google Scholar]
- 5.Livraghi-Butrico A, Kelly EJ, Klem ER, Dang H, Wolfgang MC, Boucher RC, Randell SH, O’Neal WK. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunol. 2012;5(4):397–408. doi: 10.1038/mi.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritzsching B, Zhou-Suckow Z, Trojanek JB, Schubert SC, Schatterny J, Hirtz S, Agrawal R, Muley T, Kahn N, Sticht C, Gunkel N, Welte T, Randell SH, Langer F, Schnabel P, Herth FJ, Mall MA. Hypoxic epithelial necrosis triggers neutrophilic inflammation via IL-1 receptor signaling in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2015;191(8):902–913. doi: 10.1164/rccm.201409-1610OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols DP, Chmiel JF. Inflammation and its genesis in cystic fibrosis. Pediatr Pulmonol. 2015;50(Suppl 40):S39–56. doi: 10.1002/ppul.23242. [DOI] [PubMed] [Google Scholar]
- 8.Boucher RC. On the Pathogenesis of Acute Exacerbations of Mucoobstructive Lung Diseases. Annals of the American Thoracic Society. 2015;12(Suppl 2):S160–163. doi: 10.1513/AnnalsATS.201507-460AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261(1):5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 10.Rowe SM, Jackson PL, Liu G, Hardison M, Livraghi A, Solomon GM, McQuaid DB, Noerager BD, Gaggar A, Clancy JP, O’Neal W, Sorscher EJ, Abraham E, Blalock JE. Potential role of high-mobility group box 1 in cystic fibrosis airway disease. Am J Respir Crit Care Med. 2008;178(8):822–831. doi: 10.1164/rccm.200712-1894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gifford AM, Chalmers JD. The role of neutrophils in cystic fibrosis. Curr Opin Hematol. 2014;21(1):16–22. doi: 10.1097/MOH.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 12.Painter RG, Bonvillain RW, Valentine VG, Lombard GA, LaPlace SG, Nauseef WM, Wang G. The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J Leukoc Biol. 2008;83(6):1345–1353. doi: 10.1189/jlb.0907658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haggie PM, Verkman AS. Defective organellar acidification as a cause of cystic fibrosis lung disease: reexamination of a recurring hypothesis. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L859–867. doi: 10.1152/ajplung.00018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruscia EM, Bonfield TL. Cystic Fibrosis Lung Immunity: The Role of the Macrophage. J Innate Immun. 2016;8(6):550–563. doi: 10.1159/000446825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GAt, Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, McCray PB, Jr, Zabner J, Welsh MJ. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2(29):29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y, Kinyon JM, Lei-Butters DC, Griffin MA, Naumann P, Luo M, Ascher J, Wang K, Frana T, Wine JJ, Meyerholz DK, Engelhardt JF. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120(9):3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keiser NW, Birket SE, Evans IA, Tyler SR, Crooke AK, Sun X, Zhou W, Nellis JR, Stroebele EK, Chu KK, Tearney GJ, Stevens MJ, Harris JK, Rowe SM, Engelhardt JF. Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs. Am J Respir Cell Mol Biol. 2015;52(6):683–694. doi: 10.1165/rcmb.2014-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, Karp PH, Wohlford-Lenane CL, Heilmann KP, Leidinger MR, Allen PD, Zabner J, McCray PB, Jr, Ostedgaard LS, Stoltz DA, Randak CO, Welsh MJ. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351(6272):503–507. doi: 10.1126/science.aad5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahy JV, Keoghan MT, Crummy EJ, FitzGerald MX. Bacteraemia and fungaemia in adults with cystic fibrosis. The Journal of infection. 1991;22(3):241–245. doi: 10.1016/s0163-4453(05)80005-2. [DOI] [PubMed] [Google Scholar]
- 20.McShane D, Davies JC, Davies MG, Bush A, Geddes DM, Alton EW. Airway surface pH in subjects with cystic fibrosis. Eur Respir J. 2003;21(1):37–42. doi: 10.1183/09031936.03.00027603. [DOI] [PubMed] [Google Scholar]
- 21.Carpagnano GE, Foschino Barbaro MP, Resta O, Gramiccioni E, Valerio NV, Bracciale P, Valerio G. Exhaled markers in the monitoring of airways inflammation and its response to steroid’s treatment in mild persistent asthma. Eur J Pharmacol. 2005;519(1–2):175–181. doi: 10.1016/j.ejphar.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Tseliou E, Bessa V, Hillas G, Delimpoura V, Papadaki G, Roussos C, Papiris S, Bakakos P, Loukides S. Exhaled nitric oxide and exhaled breath condensate pH in severe refractory asthma. Chest. 2010;138(1):107–113. doi: 10.1378/chest.09-1257. [DOI] [PubMed] [Google Scholar]
- 23.Torphy TJ, Allen J, Cantin AM, Konstan MW, Accurso FJ, Joseloff E, Ratjen FA, Chmiel JF Anti-Inflammatory Therapy Working G. Considerations for the Conduct of Clinical Trials with Anti-inflammatory Agents in Cystic Fibrosis: A Cystic Fibrosis Foundation Workshop Report. Annals of the American Thoracic Society. 2015 doi: 10.1513/AnnalsATS.201506-361OT. [DOI] [PubMed] [Google Scholar]
- 24.Eigen H, Rosenstein BJ, FitzSimmons S, Schidlow DV. A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis. Cystic Fibrosis Foundation Prednisone Trial Group. J Pediatr. 1995;126(4):515–523. doi: 10.1016/s0022-3476(95)70343-8. [DOI] [PubMed] [Google Scholar]
- 25.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332(13):848–854. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 26.Lands LC, Milner R, Cantin AM, Manson D, Corey M. High-dose ibuprofen in cystic fibrosis: Canadian safety and effectiveness trial. J Pediatr. 2007;151(3):249–254. doi: 10.1016/j.jpeds.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Konstan MW, Krenicky JE, Finney MR, Kirchner HL, Hilliard KA, Hilliard JB, Davis PB, Hoppel CL. Effect of ibuprofen on neutrophil migration in vivo in cystic fibrosis and healthy subjects. J Pharmacol Exp Ther. 2003;306(3):1086–1091. doi: 10.1124/jpet.103.052449. [DOI] [PubMed] [Google Scholar]
- 28.Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1829–1832. doi: 10.1164/ajrccm.157.6.9710075. [DOI] [PubMed] [Google Scholar]
- 29.Wozniak DJ, Keyser R. Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa. Chest. 2004;125(2 Suppl):62S–69S. doi: 10.1378/chest.125.2_suppl.62s. quiz 69S. [DOI] [PubMed] [Google Scholar]
- 30.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW, 3rd, Macrolide Study G. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290(13):1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 31.Southern KW, Barker PM, Solis-Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database Syst Rev. 2012;11:CD002203. doi: 10.1002/14651858.CD002203.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Culic O, Erakovic V, Cepelak I, Barisic K, Brajsa K, Ferencic Z, Galovic R, Glojnaric I, Manojlovic Z, Munic V, Novak-Mircetic R, Pavicic-Beljak V, Sucic M, Veljaca M, Zanic-Grubisic T, Parnham MJ. Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects. Eur J Pharmacol. 2002;450(3):277–289. doi: 10.1016/s0014-2999(02)02042-3. [DOI] [PubMed] [Google Scholar]
- 33.Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143(2):225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Hodge S, Reynolds PN. Low-dose azithromycin improves phagocytosis of bacteria by both alveolar and monocyte-derived macrophages in chronic obstructive pulmonary disease subjects. Respirology. 2012;17(5):802–807. doi: 10.1111/j.1440-1843.2012.02135.x. [DOI] [PubMed] [Google Scholar]
- 35.Saiman L, Anstead M, Mayer-Hamblett N, Lands LC, Kloster M, Hocevar-Trnka J, Goss CH, Rose LM, Burns JL, Marshall BC, Ratjen F, Group AZAS. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2010;303(17):1707–1715. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 36.Konstan MW, Doring G, Heltshe SL, Lands LC, Hilliard KA, Koker P, Bhattacharya S, Staab A, Hamilton A Investigators Coordinators of BIT. A randomized double blind, placebo controlled phase 2 trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of lung disease in children and adults with cystic fibrosis. J Cyst Fibros. 2014;13(2):148–155. doi: 10.1016/j.jcf.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doring G, Bragonzi A, Paroni M, Akturk FF, Cigana C, Schmidt A, Gilpin D, Heyder S, Born T, Smaczny C, Kohlhaufl M, Wagner TO, Loebinger MR, Bilton D, Tunney MM, Elborn JS, Pier GB, Konstan MW, Ulrich M. BIIL 284 reduces neutrophil numbers but increases P. aeruginosa bacteremia and inflammation in mouse lungs. J Cyst Fibros. 2014;13(2):156–163. doi: 10.1016/j.jcf.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson SJ, Mott LS, Esther CR, Jr, Stick SM, Hall GL. Novel end points for clinical trials in young children with cystic fibrosis. Expert review of respiratory medicine. 2013;7(3):231–243. doi: 10.1586/ers.13.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chmiel JF, Konstan MW, Accurso FJ, Lymp J, Mayer-Hamblett N, VanDevanter DR, Rose LM, Ramsey BW Assessment of Induced Sputum in Cystic Fibrosis Study G. Use of ibuprofen to assess inflammatory biomarkers in induced sputum: Implications for clinical trials in cystic fibrosis. J Cyst Fibros. 2015;14(6):720–726. doi: 10.1016/j.jcf.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in Cystic Fibrosis lung disease. Procedings of the American Thoracic Society. 2007;4:406–417. doi: 10.1513/pats.200703-044BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2012;186(9):857–865. doi: 10.1164/rccm.201203-0507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waters VJ, Stanojevic S, Sonneveld N, Klingel M, Grasemann H, Yau YC, Tullis E, Wilcox P, Freitag A, Chilvers M, Ratjen FA. Factors associated with response to treatment of pulmonary exacerbations in cystic fibrosis patients. J Cyst Fibros. 2015;14(6):755–762. doi: 10.1016/j.jcf.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Alexis NE, Hu SC, Zeman K, Alter T, Bennett WD. Induced sputum derives from the central airways: confirmation using a radiolabeled aerosol bolus delivery technique. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1964–1970. doi: 10.1164/ajrccm.164.10.2104051. [DOI] [PubMed] [Google Scholar]
- 44.Ho SA, Ball R, Morrison LJ, Brownlee KG, Conway SP. Clinical value of obtaining sputum and cough swab samples following inhaled hypertonic saline in children with cystic fibrosis. Pediatr Pulmonol. 2004;38(1):82–87. doi: 10.1002/ppul.20035. [DOI] [PubMed] [Google Scholar]
- 45.Ordonez CL, Kartashov AI, Wohl ME. Variability of markers of inflammation and infection in induced sputum in children with cystic fibrosis. J Pediatr. 2004;145(5):689–692. doi: 10.1016/j.jpeds.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 46.Dakin CJ, Numa AH, Wang HE, Morton JR, Vertzyas CC, Henry RL. Inflammation, Infection, and Pulmonary Function in Infants and Young Children with Cystic Fibrosis. American Journal of Respiratory and Critical Care Medicine. 2002;165(7):904–910. doi: 10.1164/ajrccm.165.7.2010139. [DOI] [PubMed] [Google Scholar]
- 47.Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, Hiatt P, McCoy K, Wilson CB, Inglis A, Smith A, Martin TR, Ramsey BW. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis*. Pediatric Pulmonology. 2001;32(5):356–366. doi: 10.1002/ppul.1144. [DOI] [PubMed] [Google Scholar]
- 48.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151(4):1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 49.Esther CR, Jr, Alexis NE, Clas ML, Lazarowski ER, Donaldson SH, Ribeiro CM, Moore CG, Davis SD, Boucher RC. Extracellular purines are biomarkers of neutrophilic airway inflammation. Eur Respir J. 2008;31(5):949–956. doi: 10.1183/09031936.00089807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF, Grimwood K, Wainwright C. Early airway infection, inflammation, and lung function in cystic fibrosis. Arch Dis Child. 2002;87(4):306–311. doi: 10.1136/adc.87.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stick SM, Brennan S, Murray C, Douglas T, von Ungern-Sternberg BS, Garratt LW, Gangell CL, De Klerk N, Linnane B, Ranganathan S, Robinson P, Robertson C, Sly PD Australian Respiratory Early Surveillance Team for Cystic F. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr. 2009;155(5):623–628. e621. doi: 10.1016/j.jpeds.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong DS, Hook SM, Jamsen KM, Nixon GM, Carzino R, Carlin JB, Robertson CF, Grimwood K. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol. 2005;40(6):500–510. doi: 10.1002/ppul.20294. [DOI] [PubMed] [Google Scholar]
- 53.Davis SD, Fordham LA, Brody AS, Noah TL, Retsch-Bogart GZ, Qaqish BF, Yankaskas BC, Johnson RC, Leigh MW. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med. 2007;175(9):943–950. doi: 10.1164/rccm.200603-343OC. [DOI] [PubMed] [Google Scholar]
- 54.Mott LS, Park J, Murray CP, Gangell CL, de Klerk NH, Robinson PJ, Robertson CF, Ranganathan SC, Sly PD, Stick SM on behalf of AC. Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax. 2011 doi: 10.1136/thoraxjnl-2011-200912. [DOI] [PubMed] [Google Scholar]
- 55.Tepper RS, Montgomery GL, Ackerman V, Eigen H. Longitudinal evaluation of pulmonary function in infants and very young children with cystic fibrosis. Pediatr Pulmonol. 1993;16(2):96–100. doi: 10.1002/ppul.1950160204. [DOI] [PubMed] [Google Scholar]
- 56.Garratt LW, Sutanto EN, Ling KM, Looi K, Iosifidis T, Martinovich KM, Shaw NC, Kicic-Starcevich E, Knight DA, Ranganathan S, Stick SM, Kicic A Australian Respiratory Early Surveillance Team for Cystic F. Matrix metalloproteinase activation by free neutrophil elastase contributes to bronchiectasis progression in early cystic fibrosis. Eur Respir J. 2015;46(2):384–394. doi: 10.1183/09031936.00212114. [DOI] [PubMed] [Google Scholar]
- 57.Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, Stick SM, Robinson PJ, Robertson CF, Ranganathan SC Australian Respiratory Early Surveillance Team for Cystic F. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180(2):146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 58.Effros RM, Dunning MB, III, Biller J, Shaker R. The promise and perils of exhaled breath condensates. American Journal of Physiology-Lung Cell Mol Physiol. 2004;287:L1073–L1080. doi: 10.1152/ajplung.00069.2004. [DOI] [PubMed] [Google Scholar]
- 59.DiMaggio C, Sun LS, Ing C, Li G. Pediatric anesthesia and neurodevelopmental impairments: a Bayesian meta-analysis. J Neurosurg Anesthesiol. 2012;24(4):376–381. doi: 10.1097/ANA.0b013e31826a038d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frayman KB, Armstrong DS, Carzino R, Ferkol TW, Grimwood K, Storch GA, Teo SM, Wylie KM, Ranganathan SC. The lower airway microbiota in early cystic fibrosis lung disease: a longitudinal analysis. Thorax. 2017 doi: 10.1136/thoraxjnl-2016-209279. [DOI] [PubMed] [Google Scholar]
- 61.Fayon M, Kent L, Bui S, Dupont L, Sermet I European Cystic Fibrosis Society Clinical Trial Network Standardisation C. Clinimetric properties of bronchoalveolar lavage inflammatory markers in cystic fibrosis. Eur Respir J. 2014;43(2):610–626. doi: 10.1183/09031936.00017713. [DOI] [PubMed] [Google Scholar]
- 62.Sagel SD. Noninvasive biomarkers of airway inflammation in cystic fibrosis. Curr Opin Pulm Med. 2003;9(6):516–521. doi: 10.1097/00063198-200311000-00012. [DOI] [PubMed] [Google Scholar]
- 63.Bodini A, D’Orazio C, Peroni D, Corradi M, Folesani G, Baraldi E, Assael BM, Boner A, Piacentini GL. Biomarkers of neutrophilic inflammation in exhaled air of cystic fibrosis children with bacterial airway infections. Pediatr Pulmonol. 2005;40(6):494–499. doi: 10.1002/ppul.20336. [DOI] [PubMed] [Google Scholar]
- 64.Robroeks CM, Rosias PP, van Vliet D, Jobsis Q, Yntema JB, Brackel HJ, Damoiseaux JG, den Hartog GM, Wodzig WK, Dompeling E. Biomarkers in exhaled breath condensate indicate presence and severity of cystic fibrosis in children. Pediatr Allergy Immunol. 2008;19(7):652–659. doi: 10.1111/j.1399-3038.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 65.Robroeks CM, Jobsis Q, Damoiseaux JG, Heijmans PH, Rosias PP, Hendriks HJ, Dompeling E. Cytokines in exhaled breath condensate of children with asthma and cystic fibrosis. Ann Allergy Asthma Immunol. 2006;96(2):349–355. doi: 10.1016/S1081-1206(10)61247-1. [DOI] [PubMed] [Google Scholar]
- 66.Bodini A, D’Orazio C, Peroni DG, Corradi M, Zerman L, Folesani G, Assael BM, Boner AL, Piacentini GL. IL-8 and pH values in exhaled condensate after antibiotics in cystic fibrosis children. Int J Immunopathol Pharmacol. 2007;20(3):467–472. doi: 10.1177/039463200702000305. [DOI] [PubMed] [Google Scholar]
- 67.Lucidi V, Ciabattoni G, Bella S, Barnes PJ, Montuschi P. Exhaled 8-isoprostane and prostaglandin E(2) in patients with stable and unstable cystic fibrosis. Free Radic Biol Med. 2008;45(6):913–919. doi: 10.1016/j.freeradbiomed.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 68.Dressel H, Muller F, Fischer R, Rommelt H, Hohlfeld JM, Behr J, Huber RM, Nowak D, Jorres RA. Independent information of nonspecific biomarkers in exhaled breath condensate. Respiration. 2010;80(5):401–409. doi: 10.1159/000319945. [DOI] [PubMed] [Google Scholar]
- 69.Carpagnano GE, Barnes PJ, Geddes DM, Hodson ME, Kharitonov SA. Increased leukotriene B4 and interleukin-6 in exhaled breath condensate in cystic fibrosis. Am J Respir Crit Care Med. 2003;167(8):1109–1112. doi: 10.1164/rccm.200203-179OC. [DOI] [PubMed] [Google Scholar]
- 70.Esther CR, Jr, Boysen G, Olsen BM, Collins LB, Ghio AJ, Swenberg JW, Boucher RC. Mass spectrometric analysis of biomarkers and dilution markers in exhaled breath condensate reveals elevated purines in asthma and cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L987–993. doi: 10.1152/ajplung.90512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ojoo JC, Mulrennan SA, Kastelik JA, Morice AH, Redington AE. Exhaled breath condensate pH and exhaled nitric oxide in allergic asthma and in cystic fibrosis. Thorax. 2005;60(1):22–26. doi: 10.1136/thx.2003.017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carpagnano GE, Barnes PJ, Francis J, Wilson N, Bush A, Kharitonov SA. Breath condensate pH in children with cystic fibrosis and asthma: a new noninvasive marker of airway inflammation? Chest. 2004;125(6):2005–2010. doi: 10.1378/chest.125.6.2005. [DOI] [PubMed] [Google Scholar]
- 73.Tate S, MacGregor G, Davis M, Innes JA, Greening AP. Airways in cystic fibrosis are acidified: detection by exhaled breath condensate. Thorax. 2002;57(11):926–929. doi: 10.1136/thorax.57.11.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moeller A, Franklin P, Hall GL, Horak F, Jr, Wildhaber JH, Stick SM. Measuring exhaled breath condensates in infants. Pediatr Pulmonol. 2006;41(2):184–187. doi: 10.1002/ppul.20362. [DOI] [PubMed] [Google Scholar]
- 75.Muller WG, Morini F, Eaton S, Peters M, Jaffe A. Safety and feasibility of exhaled breath condensate collection in ventilated infants and children. Eur Respir J. 2006;28(3):479–485. doi: 10.1183/09031936.06.00063505. [DOI] [PubMed] [Google Scholar]
- 76.Patel K, Davis SD, Johnson R, Esther CR., Jr Exhaled breath condensate purines correlate with lung function in infants and preschoolers. Pediatr Pulmonol. 2012 doi: 10.1002/ppul.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hunt J. If it smells like a duck, it might be an asthma subphenotype. Am J Respir Crit Care Med. 2007;175(10):975–976. doi: 10.1164/rccm.200703-302ED. [DOI] [PubMed] [Google Scholar]
- 78.Effros RM, Biller J, Foss B, Hoagland K, Dunning MB, Castillo D, Bosbous M, Sun F, Shaker R. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am J Respir Crit Care Med. 2003;168(12):1500–1505. doi: 10.1164/rccm.200307-920OC. [DOI] [PubMed] [Google Scholar]
- 79.Effros RM, Hoagland KW, Bosbous M, Castillo D, Foss B, Dunning M, Gare M, Lin W, Sun F. Dilution of respiratory solutes in exhaled condensates. Am J Respir Crit Care Med. 2002;165(5):663–669. doi: 10.1164/ajrccm.165.5.2101018. [DOI] [PubMed] [Google Scholar]
- 80.Effros RM, Dunning MB, 3rd, Biller J, Shaker R. The promise and perils of exhaled breath condensates. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1073–1080. doi: 10.1152/ajplung.00069.2004. [DOI] [PubMed] [Google Scholar]
- 81.Esther CR, Jr, Olsen BM, Lin FC, Fine J, Boucher RC. Exhaled breath condensate adenosine tracks lung function changes in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304(7):L504–509. doi: 10.1152/ajplung.00344.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Esther CR, Jr, Boysen G, Olsen BM, Collins LB, Ghio AJ, Swenberg JW, Boucher RC. Mass spectrometric analysis of biomarkers and dilution markers in exhaled breath condensate reveals elevated purines in asthma and cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L987–993. doi: 10.1152/ajplung.90512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Esther CR, Jr, Jasin HM, Collins LB, Swenberg JA, Boysen G. A mass spectrometric method to simultaneously measure a biomarker and dilution marker in exhaled breath condensate. Rapid Commun Mass Spectrom. 2008;22(5):701–705. doi: 10.1002/rcm.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Horck M, Alonso A, Wesseling G, de Winter-de Groot K, van Aalderen W, Hendriks H, Winkens B, Rijkers G, Jobsis Q, Dompeling E. Biomarkers in Exhaled Breath Condensate Are Not Predictive for Pulmonary Exacerbations in Children with Cystic Fibrosis: Results of a One-Year Observational Study. PLoS One. 2016;11(4):e0152156. doi: 10.1371/journal.pone.0152156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robroeks CM, van Berkel JJ, Dallinga JW, Jobsis Q, Zimmermann LJ, Hendriks HJ, Wouters MF, van der Grinten CP, van de Kant KD, van Schooten FJ, Dompeling E. Metabolomics of volatile organic compounds in cystic fibrosis patients and controls. Pediatr Res. 2010;68(1):75–80. doi: 10.1203/PDR.0b013e3181df4ea0. [DOI] [PubMed] [Google Scholar]
- 86.Montuschi P, Paris D, Melck D, Lucidi V, Ciabattoni G, Raia V, Calabrese C, Bush A, Barnes PJ, Motta A. NMR spectroscopy metabolomic profiling of exhaled breath condensate in patients with stable and unstable cystic fibrosis. Thorax. 2012;67(3):222–228. doi: 10.1136/thoraxjnl-2011-200072. [DOI] [PubMed] [Google Scholar]
- 87.Paff T, van der Schee MP, Daniels JM, Pals G, Postmus PE, Sterk PJ, Haarman EG. Exhaled molecular profiles in the assessment of cystic fibrosis and primary ciliary dyskinesia. J Cyst Fibros. 2013;12(5):454–460. doi: 10.1016/j.jcf.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 88.Joensen O, Paff T, Haarman EG, Skovgaard IM, Jensen PO, Bjarnsholt T, Nielsen KG. Exhaled breath analysis using electronic nose in cystic fibrosis and primary ciliary dyskinesia patients with chronic pulmonary infections. PLoS One. 2014;9(12):e115584. doi: 10.1371/journal.pone.0115584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ngan DA, Wilcox PG, Aldaabil M, Li Y, Leipsic JA, Sin DD, Man SF. The relationship of systemic inflammation to prior hospitalization in adult patients with cystic fibrosis. BMC Pulm Med. 2012;12:3. doi: 10.1186/1471-2466-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Levy H, Kalish LA, Huntington I, Weller N, Gerard C, Silverman EK, Celedon JC, Pier GB, Weiss ST. Inflammatory markers of lung disease in adult patients with cystic fibrosis. Pediatr Pulmonol. 2007;42(3):256–262. doi: 10.1002/ppul.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nixon LS, Yung B, Bell SC, Stuart, Elborn J, Shale DJ. Circulating Immunoreactive Interleukin-6 in Cystic Fibrosis. American Journal of Respiratory and Critical Care Medicine. 1998;157(6):1764–1769. doi: 10.1164/ajrccm.157.6.9704086. [DOI] [PubMed] [Google Scholar]
- 92.Bell, Bowerman, Nixon, Macdonald, Elborn, Shale Metabolic and inflammatory responses to pulmonary exacerbation in adults with cystic fibrosis. European Journal of Clinical Investigation. 2000;30(6):553–559. doi: 10.1046/j.1365-2362.2000.00667.x. [DOI] [PubMed] [Google Scholar]
- 93.Proesmans M, Els C, Vermeulen F, De Boeck K. Change in IgG and evolution of lung function in children with cystic fibrosis. J Cyst Fibros. 2011;10(2):128–131. doi: 10.1016/j.jcf.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 94.Wheeler WB, Williams M, Matthews WJ, Jr, Colten HR. Progression of cystic fibrosis lung disease as a function of serum immunoglobulin G levels: A 5-year longitudinal study. The Journal of Pediatrics. 1984;104(5):695–699. doi: 10.1016/s0022-3476(84)80946-4. [DOI] [PubMed] [Google Scholar]
- 95.Norman D, Elborn JS, Cordon SM, Rayner RJ, Wiseman MS, Hiller EJ, Shale DJ. Plasma tumour necrosis factor alpha in cystic fibrosis. Thorax. 1991;46(2):91–95. doi: 10.1136/thx.46.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harris WT, Muhlebach MS, Oster RA, Knowles MR, Clancy JP, Noah TL. Plasma TGF-beta(1) in pediatric cystic fibrosis: potential biomarker of lung disease and response to therapy. Pediatr Pulmonol. 2011;46(7):688–695. doi: 10.1002/ppul.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lagrange-Puget M, Durieu I, Ecochard R, Abbas-Chorfa F, Drai J, Steghens J-P, Pacheco Y, Vital-Durand D, Bellon G. Longitudinal study of oxidative status in 312 cystic fibrosis patients in stable state and during bronchial exacerbation. Pediatric Pulmonology. 2004;38(1):43–49. doi: 10.1002/ppul.20041. [DOI] [PubMed] [Google Scholar]
- 98.Patel N, Belcher J, Thorpe G, Forsyth NR, Spiteri MA. Measurement of C-reactive protein, procalcitonin and neutrophil elastase in saliva of COPD patients and healthy controls: correlation to self-reported wellbeing parameters. Respir Res. 2015;16:62. doi: 10.1186/s12931-015-0219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dickerhof N, Turner R, Khalilova I, Fantino E, Sly PD, Kettle AJ, Arest CF. Oxidized glutathione and uric acid as biomarkers of early cystic fibrosis lung disease. J Cyst Fibros. 2017;16(2):214–221. doi: 10.1016/j.jcf.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 100.Chen DL, Ferkol TW, Mintun MA, Pittman JE, Rosenbluth DB, Schuster DP. Quantifying pulmonary inflammation in cystic fibrosis with positron emission tomography. Am J Respir Crit Care Med. 2006;173(12):1363–1369. doi: 10.1164/rccm.200506-934OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amin R, Charron M, Grinblat L, Shammas A, Grasemann H, Graniel K, Ciet P, Tiddens H, Ratjen F. Cystic fibrosis: detecting changes in airway inflammation with FDG PET/CT. Radiology. 2012;264(3):868–875. doi: 10.1148/radiol.12111873. [DOI] [PubMed] [Google Scholar]
- 102.Shaughnessy AF. Monoclonal antibodies: magic bullets with a hefty price tag. Bmj. 2012;345:e8346. doi: 10.1136/bmj.e8346. [DOI] [PubMed] [Google Scholar]
- 103.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 104.Patel K, Davis SD, Johnson R, Esther CR., Jr Exhaled breath condensate purines correlate with lung function in infants and preschoolers. Pediatr Pulmonol. 2013;48(2):182–187. doi: 10.1002/ppul.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang J, Eiserich JP, Cross CE, Morrissey BM, Hammock BD. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radic Biol Med. 2012;53(1):160–171. doi: 10.1016/j.freeradbiomed.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wolak JE, Esther CR, Jr, O’Connell TM. Metabolomic analysis of bronchoalveolar lavage fluid from cystic fibrosis patients. Biomarkers. 2009;14(1):55–60. doi: 10.1080/13547500802688194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Esther CR, Jr, Turkovic L, Rosenow T, Muhlebach MS, Boucher RC, Ranganathan S, Stick SM, Arest CF. Metabolomic biomarkers predictive of early structural lung disease in cystic fibrosis. Eur Respir J. 2016;48(6):1612–1621. doi: 10.1183/13993003.00524-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Esther CR, Jr, Coakley RD, Henderson AG, Zhou YH, Wright FA, Boucher RC. Metabolomic Evaluation of Neutrophilic Airway Inflammation in Cystic Fibrosis. Chest. 2015;148(2):507–515. doi: 10.1378/chest.14-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Laguna TA, Reilly CS, Williams CB, Welchlin C, Wendt CH. Metabolomics analysis identifies novel plasma biomarkers of cystic fibrosis pulmonary exacerbation. Pediatr Pulmonol. 2015;50(9):869–877. doi: 10.1002/ppul.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Joseloff E, Sha W, Bell SC, Wetmore DR, Lawton KA, Milburn MV, Ryals JA, Guo L, Muhlebach MS. Serum metabolomics indicate altered cellular energy metabolism in children with cystic fibrosis. Pediatr Pulmonol. 2014;49(5):463–472. doi: 10.1002/ppul.22859. [DOI] [PubMed] [Google Scholar]
- 111.de Laurentiis G, Paris D, Melck D, Maniscalco M, Marsico S, Corso G, Motta A, Sofia M. Metabonomic analysis of exhaled breath condensate in adults by nuclear magnetic resonance spectroscopy. Eur Respir J. 2008;32(5):1175–1183. doi: 10.1183/09031936.00072408. [DOI] [PubMed] [Google Scholar]
- 112.Sofia M, Maniscalco M, de Laurentiis G, Paris D, Melck D, Motta A. Exploring airway diseases by NMR-based metabonomics: a review of application to exhaled breath condensate. J Biomed Biotechnol. 2011;2011:403260. doi: 10.1155/2011/403260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Corvol H, Blackman SM, Boelle PY, Gallins PJ, Pace RG, Stonebraker JR, Accurso FJ, Clement A, Collaco JM, Dang H, Dang AT, Franca A, Gong J, Guillot L, Keenan K, Li W, Lin F, Patrone MV, Raraigh KS, Sun L, Zhou YH, O’Neal WK, Sontag MK, Levy H, Durie PR, Rommens JM, Drumm ML, Wright FA, Strug LJ, Cutting GR, Knowles MR. Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nature communications. 2015;6:8382. doi: 10.1038/ncomms9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grasemann H, Shehnaz D, Enomoto M, Leadley M, Belik J, Ratjen F. L-ornithine derived polyamines in cystic fibrosis airways. PLoS One. 2012;7(10):e46618. doi: 10.1371/journal.pone.0046618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O’Neal WK, Gallins P, Pace RG, Dang H, Wolf WE, Jones LC, Guo X, Zhou YH, Madar V, Huang J, Liang L, Moffatt MF, Cutting GR, Drumm ML, Rommens JM, Strug LJ, Sun W, Stonebraker JR, Wright FA, Knowles MR. Gene expression in transformed lymphocytes reveals variation in endomembrane and HLA pathways modifying cystic fibrosis pulmonary phenotypes. American journal of human genetics. 2015;96(2):318–328. doi: 10.1016/j.ajhg.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guerrera IC, Astarita G, Jais JP, Sands D, Nowakowska A, Colas J, Sermet-Gaudelus I, Schuerenberg M, Piomelli D, Edelman A, Ollero M. A novel lipidomic strategy reveals plasma phospholipid signatures associated with respiratory disease severity in cystic fibrosis patients. PLoS One. 2009;4(11):e7735. doi: 10.1371/journal.pone.0007735. [DOI] [PMC free article] [PubMed] [Google Scholar]