Abstract
Attention to a feature enhances the sensory representation of that feature. Although much has been learned about the properties of attentional modulation when attending to a single feature, the effectiveness of attending to multiple features is not well understood. We investigated this question in a series of experiments using a color detection task while varying the number of attended colors in a cueing paradigm. Observers were shown either a single cue, two cues, or no cue (baseline) before detecting a coherent color target. We measured detection threshold by varying the coherence level of the target. Compared to the baseline condition, we found consistent facilitation of detection performance in the one-cue and two-cue conditions, but performance in the two-cue condition was lower than that in the one-cue condition. In the final experiment, we presented a 50% valid cue to emulate the situation in which observers were only able to attend a single color in the two-cue condition, and found equivalent detection thresholds with the standard two-cue condition. These results indicate a limit in attending to two colors and further imply that observers could effectively attend a single color at a time. Such a limit is likely due to an inability to maintain multiple active attentional templates for colors.
Keywords: visual attention, feature, color, capacity
Introduction
Visual attention allows us to selectively process a limited set of visual stimuli from the multitude of sensory input. Voluntary attentional selection can be based on spatial locations (Carrasco, 2006; Posner, 1980) and non-spatial features (Egeth & Yantis, 1997; Theeuwes, 2010). Here, we focus on a particular type of non-spatial attention, namely feature-based attention, in which selection is based on specific values within a dimension (e.g., selecting the color red among other colors) without a change in focus of spatial attention (Maunsell & Treue, 2006; Scolari, Ester, & Serences, 2014).
It is now well-established that attending to a feature can enhance its early sensory representations, as shown by a variety of studies employing psychophysical (Boynton, Ciaramitaro, & Arman, 2006; Liu & Hou, 2011; Liu & Mance, 2011; Saenz, Buraĉas, & Boynton, 2003; White & Carrasco, 2011), neurophysiological (Cohen & Maunsell, 2011; Martinez-Trujillo & Treue, 2004) and brain imaging measures (Liu, Larsson, & Carrasco, 2007; Saenz, Buracas, & Boynton, 2002). An enhanced feature representation would be useful for other cognitive operations requiring the selection of that feature (e.g., during visual search for a specific feature). This body of work generally tested attention to a single feature, thus leaving open an important question regarding attentional capacity, i.e., how many features can be attended simultaneously? Answering this question would deepen our understanding of the mechanisms of attention and have practical implications on optimizing human performance in visually guided tasks.
Importantly, the question on “attentional capacity” is distinct from questions regarding the capacity to process multiple features (Shiffrin & Gardner, 1972; Townsend, 1990)1, or the storage capacity in short-term memory (Cowan, 2001). Instead, we focus on attentional templates/attentional sets, which have been theorized to underlie successful visual selection (Duncan & Humphreys, 1992; Folk, Remington, & Johnston, 1992; Wolfe, 2007). Specifically, our question concerns the limits in actively maintaining multiple attentional templates. This question has been addressed in visual search studies where the number of possible targets was varied. For example, Wolfe (2012) found that as the number of possible targets increased, search reaction time also increased (Wolfe, 2012). In particular, searching for two targets lead to lower performance than searching for a single target (Dombrowe, Donk, & Olivers, 2011; Stroud, Menneer, Cave, Donnelly, & Rayner, 2011). These results thus suggest that the number of active attentional templates is severely limited (possibly limited to one). However, other studies have found evidence that there could be multiple (at least two) active attentional templates (Adamo, Pun, & Ferber, 2010; Beck, Hollingworth, & Luck, 2012; Becker, Ravizza, & Peltier, 2015; Irons, Folk, & Remington, 2012; Moore & Weissman, 2010). No apparent consensus has emerged from these studies, likely due to the complex nature of the visual search task. First, search is inherently spatial as the locus of attention needs to be moved in space. In difficult searches and ones involving eye movements, search is likely serial, making it difficult to infer the number of concurrently active templates. Second, search performance is usually measured by reaction time, which reflects both attentional selection and post-selection decisional processes. These factors complicate the interpretation of results in terms of the quality of attentional templates.
To achieve a more mechanistic understanding of the limit in feature-based attention, we used a psychophysical approach to examine the quality of feature representation when the locus of spatial attention is fixed (i.e., non-search task). A small number of studies have used this approach to test the limit of feature-based attention to motion directions. Two previous studies used directional cues to direct attention to motion and manipulated the reliability of the cue (Ball & Sekuler, 1981; Herrmann, Heeger, & Carrasco, 2012). A reliable cue indicated a narrow range of possible directions for an upcoming moving target, whereas an unreliable cue indicated a wide range of possible target directions. It was found that performance deteriorated as the cue became less reliable. This implies a limit in feature-based attention in that attention cannot be directed to more directions as effectively as to fewer directions. However, these studies do not provide a precise estimate of the limit of feature-based attention, nor were they designed to achieve such an objective. A recent study by us addressed this question by manipulating the number of discrete directional cues in a motion detection task (Liu, Becker, & Jigo, 2013). Compared to a baseline neutral condition, performance was improved when observers attended to a single direction, as well as when they attended to two orthogonal directions. However, there was a significant performance decrement when attending to two directions compared to attending to a single direction, thus revealing a limitation in our ability to attend to multiple directions.
An important question is whether this previously demonstrated limit is specific to the motion feature, or if it is a general property of feature-based attention. Here we extend this work by investigating attention to colors. A priori, color is an important visual feature and has been shown to be particularly effective in guiding attention (Motter & Belky, 1998; Williams, 1966). In addition, the aforementioned studies of visual search all examined the color feature. Hence, it is important to know whether results obtained for motion direction can be generalized to color. These considerations prompted us to investigate the limit of feature-based attention to color. To directly assess the quality of color representation during feature-based attention, we manipulated the number of color pre-cues and measured the detection threshold of a color target in a psychophysical task. This allowed us to assess changes in the sensitivity to color when observers attended one or two colors.
Experiment 1
In this experiment, we used a 2-interval forced choice (2-IFC) task to assess the behavioral consequence of attending one versus two colors. Observers viewed noisy color stimuli and were instructed to report the temporal interval that contained a coherent color target. Three cueing conditions were employed to manipulate feature-based attention. In the no-cue (baseline) condition, observers were provided with no prior information about the color target. Whereas the one-cue and two-cue conditions contained one and two pre-cues, respectively, that indicated the color target. These cues were always valid, thus prompting observers to attend to the cued colors.
Methods
Observers
Six observers (1 male and 5 female; mean age = 22 years; SD = 3) participated in the experiment and were naïve to its purpose (except one author, M.J.). All observers had normal or corrected-to-normal acuity and their color vision were assessed with the Dvorine Pseudo-Isochromatic Plates (Dvorine, 1953). Observers gave written informed consent under the study protocol approved by the Institutional Review Board at Michigan State University and were remunerated at a rate of $10/hour (except the author). We based our sample size on our previous study on feature-based attention to motion (Experiment 1 of Liu et al., 2013), which used a similar experimental design and analytical approach. The effect size for comparison between one-cue and no-cue condition in that experiment was 1.88. Assuming that cueing color feature would yield similar effects, we found that a sample size of 6 would yield a power of .90 given α=.05 for a paired-sample t-test (Faul, Erdfelder, Lang, & Buchner, 2007).
Apparatus
Visual stimuli were generated using MGL (http://justingardner.net/mgl), a set of OpenGL libraries running in MATLAB (Mathworks, Natick, MA), and displayed on a 21” CRT monitor with a refresh rate of 100 Hz and a resolution of 1024×768. Observers rested their heads on a chin rest positioned 68 cm away from the monitor.
Stimuli
Stimuli comprised of static arrays of 240 chromatic dots (size: 0.1°) that were drawn in an annulus (inner radius = 1°, outer radius = 5°) and centered on a central fixation disc (white; size: 0.3°; luminance: 14.8 cd/m2). On each trial, each dot was drawn in one of six isoluminant colors (see “Isoluminance task” below) that was selected from a pool of 7 colors (red, green, blue, yellow, purple, orange, or cyan), and randomly positioned within the annulus. During no-cue and one-cue conditions, the 6 colors were randomly selected on each trial. During the two-cue condition, the colors were pseudo-randomly selected such that the cued non-target color was excluded from the dot display. For example, if the target color was red and an observer was cued to “ red” and “green”, green dots were not presented on that trial. Observers were cued to the target color by colored discs (cues; size: 0.5°) that preceded the dot displays. Cues were positioned 1.5° to the left or right of fixation.
Color coherence
Color coherence refers to the proportion of dots drawn in a particular color (the target color) relative to the other five colors in the display (note that there were six colors in each dot stimulus). Numerically, coherence was defined by the following equation:
where Pt is the proportion of dots drawn in the target color and Pn is the proportion of dots drawn in the other five colors (noise) with the following constraint:
This ensured that the noise colors were equally proportioned after accounting for the target color. Displays with zero color coherence had an equal number of dots for each of the 6 colors (i.e., 40 dots per color) whereas in non-zero coherence displays, a disproportionately large number of dots were drawn in the target color. This measure of coherence is a color analog of motion coherence implemented in the classic random-dot motion stimulus (Newsome & Pare, 1988) and has been used in our previous study (Wang, Miller, & Liu, 2015).
Procedure
Isoluminance task
Prior to participating in the experiment, observers equated the perceived brightness across all 7 colors with heterochromatic flicker photometry (Kaiser, 1991; B. B. Lee, Martin, & Valberg, 1988). Observers viewed gray (luminance: 6.3 cd/m2) and chromatic square tiles (size: 1.8°×1.8°) that were arranged in a checkerboard pattern and constrained within an annulus (inner radius = 1.5°; outer radius = 6°) that was centered on a central fixation cross (white; size: 0.5°; luminance: 21.1 cd/m2). The gray and chromatic tiles flickered at 8 Hz in a counterphase fashion, and observers adjusted the luminance of the chromatic tiles until the flicker was minimized. The resulting luminance was an estimate of the color's isoluminance value relative to the constant gray. Thresholds for each of the 7 colors were obtained in separate blocks of 4 trials and the average value across the 4 trials served as the final luminance value for that color in the attention experiment.
Attention task
Observers performed a 2-IFC task (Figure 1a) at 6 fixed levels of color coherence. At trial onset, the fixation disc dimmed for 0.5 s (luminance: 4.2 cd/m2) to signal observers of the upcoming stimuli. During cued blocks (one-cue or two-cue), one or two cues appeared in this interval. During one-cue blocks, the cue was always drawn in the target color. During two-cue blocks, one cue was drawn in the target color while the other was drawn in the color that was absent from the display (see Stimuli). A 0.7-s fixation period followed the cue interval.
Figure 1.
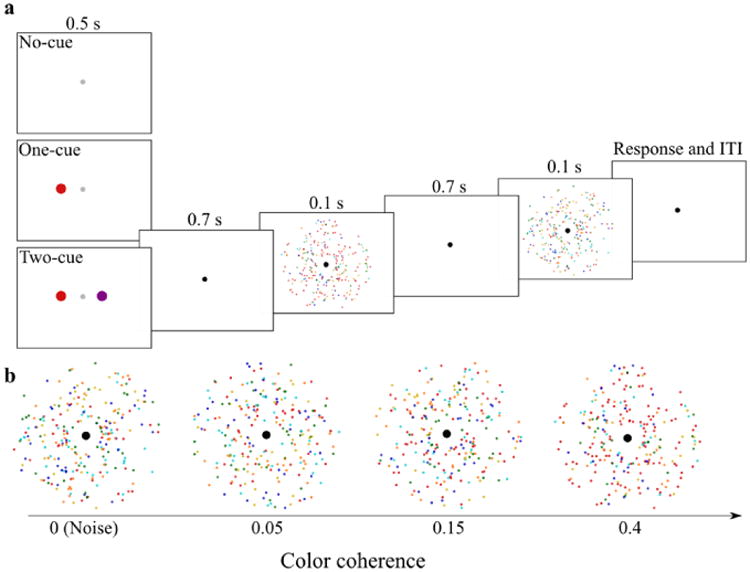
a) Trial sequence for Experiment 1. For ease of illustration, the stimuli are shown on a white background (instead of the black background in the actual experiment). b) Representative stimuli for varying levels of color coherence.
After the fixation period, two intervals of chromatic stimuli were displayed. Each interval lasted 0.1 s and was separated by a 0.7-s fixation period. One interval contained a zero-coherence stimulus while the other contained a color target at one of six possible coherence levels (0.025, 0.05, 0.1, 0.15, 0.2, or 0.4, Figure 1b). These coherence values were chosen because they met the criteria of: producing numbers of target and noise dots that were integers that summed to 240 total dots; and adequately sampling the range of the psychometric function, based on pilot data. Following the second chromatic stimulus, observers reported the interval that contained the coherent color target by using “1” or “2” on the keyboard's numeric keypad for the first and second interval, respectively. We explicitly instructed observers to report the interval that they perceived to contain a dominant color (i.e., a color that was disproportionately represented). An inter-trial interval that varied between 1 and 1.5 s followed the observer's response.
The task was performed in blocks of 48 trials with cue condition (no-cue, one-cue, and two-cue) held constant in each block. Within a block, target color, coherence level, and the location of the target-colored cue (left or right of fixation) were randomized. Each observer performed 14 blocks (672 trials) of each cue condition with their order pseudo-randomized such that each occurred once every three blocks. The experiment spanned two hour-long sessions that were completed on separate days.
Training
Prior to the main experiment, observers familiarized themselves with the task in a separate practice session. In this session, observers performed blocks of each cueing condition until their performance increased monotonically as a function of color coherence. On average, observers performed 1.7 blocks of each cueing condition (5 blocks total; SD: 3). The practice session always took place on a different day.
Analysis
For each observer, performance was assessed separately for each cueing condition and fit with a Weibull function:
where P(c) represents performance as a function of color coherence, γ is the lower asymptote, γ is the deviation from 1 at the upper asymptote, c is color coherence, α is the range of the Weibull function, and β is its slope. The function was fit using maximum-likelihood estimation as implemented in the Palamedes Toolbox (Prins & Kingdon, 2009).
Performance (P(c)) was evaluated as the proportion of correct responses. When fitting performance, γ was fixed at 0.5 and γ was constrained between 0 and 0.1. Color coherence threshold was evaluated at a proportion correct of 0.75 and planned t-tests were conducted between the thresholds for each cueing condition.
Results and Discussion
To visualize overall task performance, we fit the aggregate data across observers for each cue (Figure 2a). One and two cues improved performance relative to baseline (no-cue), as evidenced by a leftward shift of both psychometric functions. To quantify these effects, we fit the Weibull function to individual observer data and obtained threshold estimates for each observer. Group-averaged thresholds are shown in Figure 2b and individual thresholds were compared with planned t-tests (Figure 2b). Color coherence thresholds were significantly lower for one- (t(5)=4.2, p<0.01) and two-cue conditions (t(5)=3.5, p<0.05) relative to baseline. In addition, one-cue threshold was lower than two-cue (t(5)=4.5, p<0.01). We also separated our data, conditioning on whether the target occurred in the first or second interval, and computed separate thresholds for all conditions. A 2-way repeated-measures ANOVA with cue condition (no, one, two) and target interval (first, second) as factors revealed a main effect of cue condition (F(2,10)=11.8, p<0.01) but no main effect or interaction for target interval (both p>0.1). Therefore, the effect we observed was consistent for targets occurring in both intervals.
Figure 2.
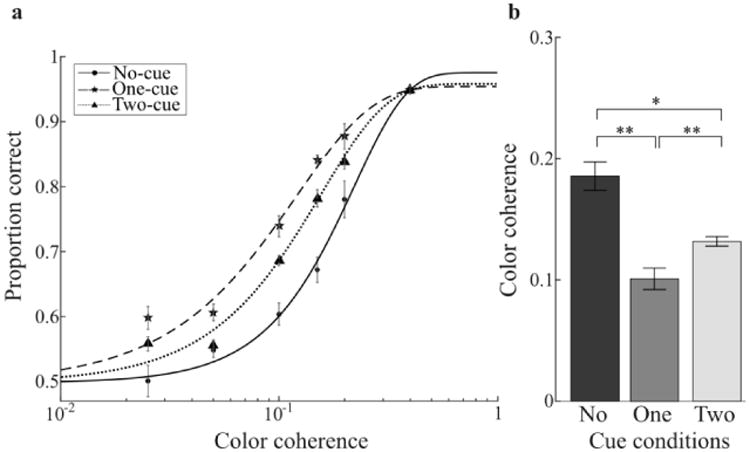
Experiment 1 results. a) Group-average psychometric function for each cueing condition. b) Color coherence thresholds evaluated at a proportion correct of 0.75. Error bars are ± s.e.m within subjects following the method of Cousineau (2005). Asterisks indicate the significance level in paired t-tests (**: p<0.01; *: p<0.05).
The reduced cueing effect in the two-cue condition demonstrates a limit in the ability to attend multiple colors, which is similar to our previous finding on attention to motion directions (Liu et al., 2013). We note that two colors are well within the storage capacity of working memory, which is estimated to be 3-4 items (Cowan, 2001; Luck & Vogel, 1997). In addition, we also queried observers after the experiment and none reported any confusion about which colors they needed to attend. Thus, the weaker cueing effect cannot be attributed to a failure in memory.
Another important consideration is whether the cue simply reduced decisional uncertainty (Lawrence & Coles, 1954; Shiu & Pashler, 1994), and in particular, our observed effects could be attributed to a greater uncertainty reduction in one-cue vs. two-cue conditions. Here, we highlight that our experimental design minimized such contributions of variable uncertainty reduction across cueing conditions. Importantly, in the two-cue condition, one of the cued colors was the target color while the other color was never presented in either stimulus on that trial. This should prevent observers from basing their decision on the cued non-target color. Had we presented both cued colors in the stimuli in the two-cue condition, for example, by presenting both red and green dots when “red” and “green” were cued and red was the target color, the presence of green dots could cause confusion and bias observers to choose the noise (incorrect) interval. However, because green was never presented in either interval, such uncertainty should have been greatly reduced.
Nevertheless, the 2-IFC task does require an explicit comparison between the two stimuli and it also requires consistent attentional deployment across both intervals. The temporally extended nature of the task is somewhat atypical in feature cueing studies, which tend to contain a single interval of stimuli. Therefore, we simplified the task demand in the next experiment and assessed whether our results can be generalized to a single-interval detection task. Because the single-interval task does not require comparison between two stimuli, this should further reduce the impact of decisional uncertainty on performance.
Experiment 2
Here, we used a single-interval detection task to examine the limit of attention to colors. Observers viewed a single stimulus whose color coherence varied on a trial-by-trial basis and were instructed to report whether or not a target was present.
Methods
Observers
Six observers (2 male and 4 female; mean age = 22 years; SD = 3) participated in the experiment, five of which participated in Experiment 1. All observers had normal or corrected-to-normal acuity and their color vision were assessed with the Dvorine Pseudo--Isochromatic Plates (Dvorine, 1953). Observers gave written informed consent under the study protocol approved by the Institutional Review Board at Michigan State University and were remunerated at a rate of $10/hour (except the author).
Stimuli, task, and procedure
The experiment was identical to Experiment 1 with the following exceptions (Figure 3). Each trial contained a single interval of the dot stimulus. The stimulus contained a target on half the trials and contained noise on the other half. Targets could have one of six color coherence levels: 0.025, 0.05, 0.1, 0.175, 0.3, or 0.6; these values met the criteria mentioned in Experiment 1. Each observer completed a total of 42 blocks of 48 trials, with 14 blocks per cueing condition. Block order was pseudo-randomized as in Experiment 1.
Figure 3.
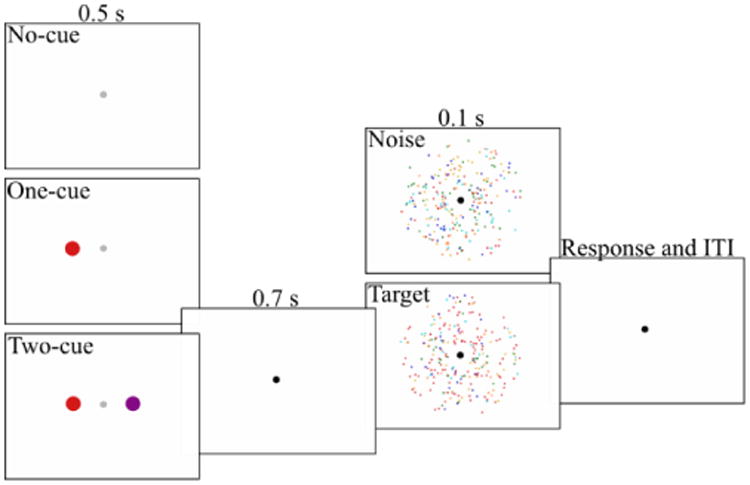
Trial sequence for Experiment 2.
The one naïve observer in this experiment underwent a separate practice session during which 4 blocks of each cueing condition were performed (12 blocks total). We also obtained the observer's isoluminance threshold before proceeding to the main experiment.
Analysis
Performance (P(c)) in this task was evaluated as the hit minus false alarm rate. To obtain an observer's psychometric function, their hit rate was fit with the Weibull function and their false alarm rate was subtracted from the computed model. This was done because hit-false alarm occasionally yielded small negative values at low coherence levels that cannot be fit with a Weibull, and also because false alarm rate did not vary with coherence level. Specifically, false alarms could only occur when noise (i.e., 0% color coherence) was presented, which was interleaved with all coherence conditions. Thus, only a single false alarm rate could be computed for each cueing condition. When fitting the hit rate, γ was constrained between 0 and 0.1. Color coherence threshold was evaluated at a hit-false alarm of 0.5.
Results and Discussion
Figure 4 shows the fits to the aggregate data across observers for each cueing condition. Performance differences between cueing conditions were identical to those observed in Experiment 1: one and two cues shifted the psychometric function to the left (Figure 4a) and reduced color coherence thresholds relative to baseline (Figure 4b; both t(5)>5.7, p<0.01); and one cue produced a lower threshold than two cues (t(5)=8.0, p<0.01). We note that the psychometric functions in Figure 4a reached different levels of asymptote. This was mainly due to the fact that the no-cue condition produced larger false alarm rates (mean: 0.37) relative to the one- (mean: 0.23) and two-cue conditions (mean: 0.24; both t(5)>3.0, p<0.05). The hit rates reached asymptotic levels close to 1 for all conditions and the threshold analysis based on hit rate showed similar, although smaller, effects compared to the hit minus false alarm rate. Importantly, the false alarm rates between one- and two-cue conditions were equivalent (t(5)=0.5, p>0.6), suggesting that the observed difference in thresholds cannot be attributed to different levels of false alarms. Importantly, the pattern of results was consistent between the single-interval and two-interval experiments: attending to either a single color or two colors lead to behavioral benefits compared to the baseline, although the behavioral benefit was diminished when attending to two colors. Thus, Experiment 2 provides further support for a severe limit in the number of colors that can be simultaneously attended.
Figure 4.
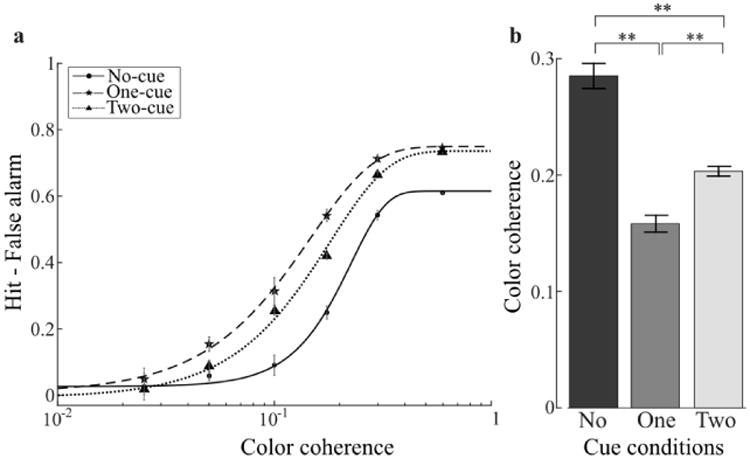
Experiment 2 results. a) Group-average psychometric function for each cueing condition. b) Color coherence thresholds evaluated at a hit-false alarm rate of 0.5. Plotting conventions are identical to Figure 2.
There seems to be at least two possibilities that could account for the diminished cueing effect for the two-cue condition. First, perhaps observers were able to attend to two colors simultaneously, but at a reduced efficiency. In other words, the amount of attention deployed to each cued color is reduced in the two-cue condition relative to the one-cue condition. Second, perhaps observers were only able to attend to a single color on two-cue trials. Assuming random selection, then they would attend to the target color on half of the trials, and a non-target color (indeed, a color absent in the stimulus) on the other half of the trials. Although behaviorally it seems difficult to dissociate these two explanations, we tested the feasibility of the second explanation (attending to a single color) by simulating such a scenario in the next experiment.
Experiment 3
In this experiment, we tested the possibility that the observed limit in attending to two colors is due to an attentional limitation of selecting a single color at a time. We presented observers with a single 50% valid cue while they performed the detection task. The cue indicated the target color only on half of the trials, thus simulating the scenario where observers randomly select a cued color to attend in the two-cue condition. We refer to this new cue manipulation as the partial one-cue condition.
Methods
Observers
Six observers (3 male and 3 female; mean age = 21 years; SD = 2) participated in the experiment, three of which participated in both Experiments 1 and 2, and one participated in Experiment 1. All observers had normal or corrected-to-normal acuity and their color vision were assessed with the Dvorine Pseudo-Isochromatic Plates (Dvorine, 1953). Observers gave written informed consent under the study protocol approved by the Institutional Review Board at Michigan State University and were remunerated at a rate of $10/hour (except the author).
Stimuli, task, procedure, and analysis
The experiment and data analysis were identical to Experiment 2 with a modification of the one-cue condition. To simulate selecting one cue during the two-cue condition, a 50% valid cue (instead of the 100% valid cue) was presented, which we refer to as the partial one-cue condition. When the cue was invalid, the cued color was not present in the dot stimulus. Observers were instructed to always attend to the cue. Observers completed 42 blocks of 48 trials, with 14 blocks per cueing condition. Block order was pseudo-randomized as in Experiment 1. Note an alternative method would be to present two color cues and instruct observers to choose one color to attend. However, it would be impossible to verify observers' strategy with such a protocol as they might still attempt to attend to both colors. Thus, we opted to present a single 50% valid cue.
The two naïve observers in this experiment underwent a separate practice session and performed 2.5 blocks of each cueing condition, on average (7.5 blocks total; SD=2.1). We also obtained each observer's isoluminance threshold before proceeding to the main experiment.
Results and Discussion
The change in performance between no-cue and two-cue conditions replicated the results from Experiments 1 and 2. Two cues shifted the psychometric function to the left (Figure 5a) and reduced the threshold relative to baseline (Figure 5b; t(5)=4.1, p<0.01). Critically, the partial one-cue condition shifted the psychometric function to the left, reduced the threshold relative to baseline (t(5)=4.7, p<0.01), and was indistinguishable from the two-cue condition (p=0.8).
Figure 5.
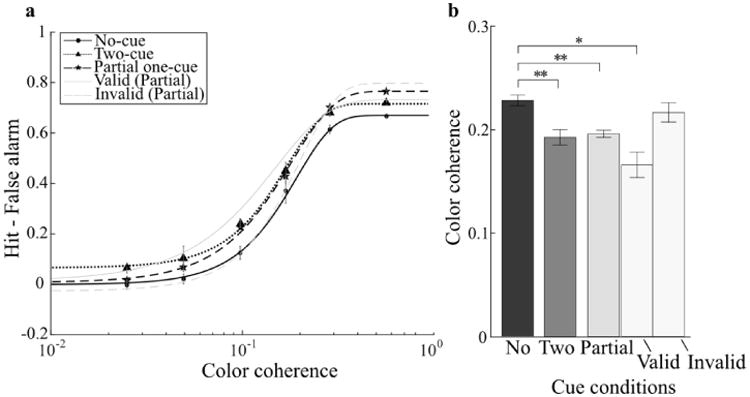
Experiment 3 results. a) Group-average psychometric function for each cueing condition. b) Color coherence thresholds evaluated at a hit-false alarm rate of 0.5. Plotting conventions are identical to Figure 2.
To verify that observers indeed attended the cued color, performance on valid and invalid trials were separately examined. As expected, performance with invalid cues was indistinguishable from baseline (p=0.3) while valid cues shifted the psychometric function to the left and reduced the threshold relative to baseline (t(5)=4.0, p<0.05), indicating that subjects successfully attended the cued color. Valid-cue threshold was numerically lower than that for two cues (Figure 5b), mimicking the benefit of a 100% valid cue as used in Experiments 1 and 2. We note that the psychometric functions in Figure 5a reached different levels of asymptote. This was due to the larger false alarm rate for the no-cue condition (mean: 0.33) relative to the partial one-cue (mean: 0.22), and two-cue conditions (mean: 0.28; both t(5)>2.8, p<0.05), with the latter two not significantly different from each other (t(5)=1.7, p>0.16).
The results of this experiment demonstrate that the behavioral benefits of attending to two colors are identical to attending one of the two colors. Thus, it is plausible that observers only effectively attended a single color at a time during two-cue trials. These data thus give credibility to the idea that feature-based attention is limited to a single feature at a time.
General Discussion
We measured the detection threshold for a weak color signal in a noisy stimulus while manipulating the number of color cues to direct attention. Over three experiments employing both single-interval and two-interval tasks, results showed a consistent pattern: relative to a neutral baseline without any pre-cue, attending to one color and two colors both improved performance, but the improvement when attending to two colors was diminished compared to attending to one color. These results suggest that observers cannot effectively attend to two colors, indicating a severe capacity limit of feature-based attention to color.
Limits in feature-based attentional modulation
There are several design features of our experiments that allowed us to investigate feature-based attentional modulation of sensory representations. First, we measured detection threshold in a psychophysical task to index the sensitivity to color. Second, we presented all stimuli at fixation, thus eliminating the contribution of spatial attention. Third, we reduced decision uncertainty by not presenting the cued, non-target color (see Experiment 1 Discussion). The experimental design is similar to our previous work on attention to motion direction (Liu et al., 2013). The color coherence manipulation is also analogous to the motion coherence manipulation in the well-studied random dot motion task (Britten, Shadlen, Newsome, & Movshon, 1992; Britten et al., 1992). Both tasks require the detection of a coherent feature among noise features near threshold. In general, our color results parallel those obtained with motion, in that attending to two features is less effective than attending to one feature, with both conditions yielding better performance than a neutral baseline. Results obtained in the current experiments thus suggest that attentional capacity is similar for different feature dimensions. Thus, our previous results obtained for motion seem to characterize the general property of the attention system.
What could account for the smaller cueing effect for attending to two colors vs. one color? There are at least two possibilities: either observers attended to only one color on a given trial, or observers attended to two colors but with less efficiency. While it is difficult to directly assess these possibilities, we explored the first possibility in Experiment 3 by explicitly emulating a situation where observers only attended to one color on a two-cue trial. We found that performance in this partial one-cue condition was indistinguishable from a standard two-cue condition. This result thus suggests that observers might have attended to only one color on two-cue trials. Note that we do not wish to suggest that observers adopted an explicit strategy of voluntarily choosing one of the two cues to attend. Indeed, we queried observers about their strategy at the end of all of our experiments and none reported using this strategy. Instead, all observers reported trying to attend to both colors. Thus, results from Experiment 3 suggest that although observers attempted to attend to both colors, their effective attentional deployment was only restricted to one color at a time.
Although these results are consistent with the notion that observers can only attend to one color at a time, we cannot rule out the possibility that they could attend to both colors, albeit with a weaker perceptual modulation. Further research using physiological methods might be able to shed light on this issue. However, we note the first interpretation (attend to one color at a time) is consistent with an influential model in the research on active working memory templates, discussed below. Regardless of the exact mechanism underlying the observed attentional limitation, our overall results demonstrate a highly limited attentional capacity in attending to multiple features. Thus, although subjectively it seems we are able to attend to multiple, or at least two, simple features, psychophysical performance showed a reduced benefit compared to attending to a single feature. These results demonstrate a severe limit in the size of the attentional focus for visual features, and could inform further development of theories of visual attention (J. Lee & Maunsell, 2009; Reynolds & Heeger, 2009; Tsotsos et al., 1995; Wolfe, 1994).
Implications for research on visual search and working memory
As stated earlier, visual search studies that vary the number of search targets have usually found a decline in search performance as the number of targets increased (Dombrowe et al., 2011; Stroud et al., 2011; Wolfe, 1994). These results are consistent with ours where we found an elevated detection threshold for the two-cue than the one-cue condition. However, we also noted several studies, in particular ones employing the attentional capture paradigm (Folk et al., 1992), that reported that attention can be captured when searching for two target colors, suggesting that attentional templates can contain two colors (Adamo, Pun, Pratt, & Ferber, 2008; Becker et al., 2015; Irons et al., 2012; Moore & Weissman, 2010, see also Beck et al., 2012). Our results of a significant cueing effect for two cues relative to baseline can be consistent with these studies. However, our results would suggest that maintaining two color templates leads to weaker representation of each color compared to maintaining one color alone. While these studies tend not to explicitly compare attentional capture effects between maintaining one vs. two colors, one study made such a comparison and found no reliable differences (Moore & Weissman, 2010). However, they did observe a numeric trend toward larger attentional capture for attending to one vs. two colors (see their Fig. 3), which is potentially consistent with our findings. The differences in the methodological and analytical procedures make it difficult to directly compare results across studies. In addition, we note that these different paradigms could well be tapping into different processes. We measured how actively attending to colors improved detection sensitivity in a psychophysical procedure, whereas studies on attentional capture measured how task-irrelevant colors impaired performance, often in terms of a slowdown in response latency. Our task thus provided a more direct measure of the attentional modulation of early sensory representations, and how maintaining multiple attentional templates affects such modulations.
Another related area of research concerns the interaction between working memory and attention, which has shown that items held in working memory can bias attentional selection (Downing, 2000; Soto, Heinke, Humphreys, & Blanco, 2005). Because it is generally accepted that top-down attentional selection is mediated by the content of active working memory (Desimone & Duncan, 1995; Hamker, 2004; Wolfe, 1994), it is reasonable to hypothesize that performance in our task should be related to the number of active items in working memory. An influential theory proposed that the active working memory state contains only one item, which serves as an attentional template that guides selection (Olivers, Peters, Houtkamp, & Roelfsema, 2011). This view could fit well with our results. In particular, results from our Experiment 3 suggest that it is plausible that observers only effectively attended to a single color on two-cue trials. It is worth noting that the working memory studies used a very different paradigm where the memory items were irrelevant to the search task and a distractor cost was measured. Our paradigm required active attention to the features and we measured how detection sensitivity was improved by attention. Thus our results could provide converging evidence for the view that there is only one active attention template at a time (Houtkamp & Roelfsema, 2009).
Conclusions
Our results demonstrate that people cannot effectively pay attention to more than one color during a threshold detection task. These results are probably due to an inability to simultaneously maintain multiple attentional templates to modulate sensory representations. This view is consistent with studies on different states of working memory and their influence on attention. Overall, these studies reveal a severe bottleneck in our ability to attend to multiple features and inform ways to optimize performance in visual tasks.
Acknowledgments
We would like to thank Rebecca Francis for assistance in data collection and Dr. Mark Becker for helpful suggestions on the manuscript. This work is supported by a grant from NIH (R01EY022727)
Footnotes
As another example, a number of studies have investigated the capacity of visual short-term (VSTM) memory consolidation. These studies briefly presented a variable number of items without distracters or pre-cues (Huang, Treisman, & Pashler, 2007; Mance, Becker, & Liu, 2012), thus did not explicitly manipulate selective attention. It remains to be seen how the capacity (or bandwidth) of VSTM consolidation relates to the attentional capacity measured here.
References
- Adamo M, Pun C, Ferber S. Multiple attentional control settings influence late attentional selection but do not provide an early attentional filter. Cognitive Neuroscience. 2010;1(2):102–110. doi: 10.1080/17588921003646149. [DOI] [PubMed] [Google Scholar]
- Adamo M, Pun C, Pratt J, Ferber S. Your divided attention, please! The maintenance of multiple attentional control sets over distinct regions in space. Cognition. 2008;107(1):295–303. doi: 10.1016/j.cognition.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Ball K, Sekuler R. Cues reduce direction uncertainty and enhance motion detection. Attention, Perception, & Psychophysics. 1981;30(2):119–128. doi: 10.3758/bf03204469. [DOI] [PubMed] [Google Scholar]
- Beck VM, Hollingworth A, Luck SJ. Simultaneous Control of Attention by Multiple Working Memory Representations. Psychological Science. 2012;23(8):887–898. doi: 10.1177/0956797612439068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MW, Ravizza SM, Peltier C. An inability to set independent attentional control settings by hemifield. Attention, Perception & Psychophysics. 2015;77(8):2640–2652. doi: 10.3758/s13414-015-0964-8. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Ciaramitaro VM, Arman AC. Effects of feature-based attention on the motion aftereffect at remote locations. Vision Research. 2006;46(18):2968–2976. doi: 10.1016/j.visres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. Journal of Neuroscience. 1992;12(12):4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. Covert attention increases contrast sensitivity: Psychophysical, neurophysiological and neuroimaging studies. Progress in Brain Research. 2006;154:33–70. doi: 10.1016/S0079-6123(06)54003-8. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. Using neuronal populations to study the mechanisms underlying spatial and feature attention. Neuron. 2011;70(6):1192–1204. doi: 10.1016/j.neuron.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. The Behavioral and Brain Sciences. 2001;24(1):87–114-185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18(1):193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dombrowe I, Donk M, Olivers CN. The costs of switching attentional sets. Attention, Perception, & Psychophysics. 2011;73(8):2481–2488. doi: 10.3758/s13414-011-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing PE. Interactions between visual working memory and selective attention. Psychological Science. 2000;11(6):467–473. doi: 10.1111/1467-9280.00290. [DOI] [PubMed] [Google Scholar]
- Duncan J, Humphreys G. Beyond the Search Surface. Journal of Experimental Psychology: Human Perception and Performance. 1992;18(2):578–588. doi: 10.1037//0096-1523.18.2.578. [DOI] [PubMed] [Google Scholar]
- Dvorine I. Dvorine pseudo-isochromatic plates. Harcourt; Brace and world, Incorporated; 1953. [Google Scholar]
- Egeth HE, Yantis S. Visual attention: Control, representation, and time course. Annual Review of Psychology. 1997;48(1):269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology Human Perception and Performance. 1992;18:1030–1030. [PubMed] [Google Scholar]
- Hamker FH. The reentry hypothesis: the putative interaction of the frontal eye field, ventrolateral prefrontal cortex, and areas V4, IT for attention and eye movement. Cerebral Cortex. 2004;15(4):431–447. doi: 10.1093/cercor/bhh146. [DOI] [PubMed] [Google Scholar]
- Herrmann K, Heeger DJ, Carrasco M. Feature-based attention enhances performance by increasing response gain. Vision Research. 2012;74:10–20. doi: 10.1016/j.visres.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkamp R, Roelfsema PR. Matching of visual input to only one item at any one time. Psychological Research. 2009;73(3):317–326. doi: 10.1007/s00426-008-0157-3. [DOI] [PubMed] [Google Scholar]
- Huang L, Treisman A, Pashler H. Characterizing the limits of human visual awareness. Science. 2007;317(5839):823–825. doi: 10.1126/science.1143515. [DOI] [PubMed] [Google Scholar]
- Irons JL, Folk CL, Remington RW. All set! Evidence of simultaneous attentional control settings for multiple target colors. Journal of Experimental Psychology Human Perception and Performance. 2012;38(3):758–775. doi: 10.1037/a0026578. [DOI] [PubMed] [Google Scholar]
- Kaiser PK. Flicker as a function of wavelength and heterochromatic flicker photometry. In: Kulikowski JJ, Walsh V, Murray IJ, editors. Limits of Vision. 1991. pp. 171–190. [Google Scholar]
- Lawrence DH, Coles GR. Accuracy of recognition with alternatives before and after the stimulus. Journal of Experimental Psychology. 1954;47(3):208. doi: 10.1037/h0049877. [DOI] [PubMed] [Google Scholar]
- Lee BB, Martin PR, Valberg A. The physiological basis of heterochromatic flicker photometry demonstrated in the ganglion cells of the macaque retina. The Journal of Physiology. 1988;404(1):323–347. doi: 10.1113/jphysiol.1988.sp017292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Maunsell JH. A normalization model of attentional modulation of single unit responses. PLoS One. 2009;4(2):e4651. doi: 10.1371/journal.pone.0004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Becker MW, Jigo M. Limited featured-based attention to multiple features. Vision Research. 2013;85:36–44. doi: 10.1016/j.visres.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Hou Y. Global feature-based attention to orientation. Journal of Vision. 2011;11(10) doi: 10.1167/11.10.8. [DOI] [PubMed] [Google Scholar]
- Liu T, Larsson J, Carrasco M. Feature-Based Attention Modulates Orientation-Selective Responses in Human Visual Cortex. Neuron. 2007;55(2):313–323. doi: 10.1016/j.neuron.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Mance I. Constant spread of feature-based attention across the visual field. Vision Research. 2011;51(1):26–33. doi: 10.1016/j.visres.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390(6657):279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Mance I, Becker MW, Liu T. Parallel consolidation of simple features into visual short-term memory. Journal of Experimental Psychology: Human Perception and Performance. 2012;38(2):429. doi: 10.1037/a0023925. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Treue S. Feature-based attention increases the selectivity of population responses in primate visual cortex. Current Biology. 2004;14(9):744–751. doi: 10.1016/j.cub.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Treue S. Feature-based attention in visual cortex. Trends in Neurosciences. 2006;29(6):317–322. doi: 10.1016/j.tins.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Moore KS, Weissman DH. Involuntary transfer of a top-down attentional set into the focus of attention: evidence from a contingent attentional capture paradigm. Attention, Perception & Psychophysics. 2010;72(6):1495–1509. doi: 10.3758/APP.72.6.1495. [DOI] [PubMed] [Google Scholar]
- Motter BC, Belky EJ. The guidance of eye movements during active visual search. Vision Research. 1998;38(12):1805–1815. doi: 10.1016/s0042-6989(97)00349-0. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) The Journal of Neuroscience. 1988;8(6):2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivers CN, Peters J, Houtkamp R, Roelfsema PR. Different states in visual working memory: When it guides attention and when it does not. Trends in Cognitive Sciences. 2011;15(7):327–334. doi: 10.1016/j.tics.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Prins N, Kingdon FAA. Palamedes: Matlab routines for analyzing psychophysical data. 2009 Retrieved from http://www.palamedestoolbox.org.
- Reynolds JH, Heeger DJ. The Normalization Model of Attention. Neuron. 2009;61(2):168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz M, Buracas GT, Boynton GM. Global effects of feature-based attention in human visual cortex. Nature Neuroscience. 2002;5(7):631–632. doi: 10.1038/nn876. [DOI] [PubMed] [Google Scholar]
- Saenz M, Buraĉas GT, Boynton GM. Global feature-based attention for motion and color. Vision Research. 2003;43(6):629–637. doi: 10.1016/s0042-6989(02)00595-3. [DOI] [PubMed] [Google Scholar]
- Scolari M, Ester EF, Serences JT. Feature-and object-based attentional modulation in the human visual system. The Oxford Handbook of Attention 2014 [Google Scholar]
- Shiffrin RM, Gardner GT. Visual processing capacity and attentional control. Journal of Experimental Psychology. 1972;93(1):72. doi: 10.1037/h0032453. [DOI] [PubMed] [Google Scholar]
- Shiu L, Pashler H. Negligible effect of spatial precuing on identification of single digits. Journal of Experimental Psychology: Human Perception and Performance. 1994;20(5):1037. [Google Scholar]
- Soto D, Heinke D, Humphreys GW, Blanco MJ. Early, involuntary top-down guidance of attention from working memory. Journal of Experimental Psychology: Human Perception and Performance. 2005;31(2):248. doi: 10.1037/0096-1523.31.2.248. [DOI] [PubMed] [Google Scholar]
- Stroud MJ, Menneer T, Cave KR, Donnelly N, Rayner K. Search for multiple targets of different colours: Misguided eye movements reveal a reduction of colour selectivity. Applied Cognitive Psychology. 2011;25(6):971–982. [Google Scholar]
- Theeuwes J. Top–down and bottom–up control of visual selection. Acta Psychologica. 2010;135(2):77–99. doi: 10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Townsend JT. Serial vs. parallel processing: Sometimes they look like Tweedledum and Tweedledee but they can (and should) be distinguished. Psychological Science. 1990;1(1):46–54. [Google Scholar]
- Tsotsos JK, Culhane SM, Wai WYK, Lai Y, Davis N, Nuflo F. Modeling visual attention via selective tuning. Artificial Intelligence. 1995;78(1–2):507–545. [Google Scholar]
- Wang Y, Miller J, Liu T. Suppression effects in feature-based attention. Journal of Vision. 2015;15(5) doi: 10.1167/15.5.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AL, Carrasco M. Feature-based attention involuntarily and simultaneously improves visual performance across locations. Journal of Vision. 2011;11(6):15–15. doi: 10.1167/11.6.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LG. The effect of target specification on objects fixated during visual search. Perception & Psychophysics. 1966;1(9):315–318. doi: 10.1016/0001-6918(67)90080-7. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided search 2.0 a revised model of visual search. Psychonomic Bulletin & Review. 1994;1(2):202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Guided search 4.0: Current progress with a model of visual search. In: Gray W, editor. Integrated models of cognitive systems. New York: Oxford; 2007. pp. 99–119. [Google Scholar]
- Wolfe JM. Saved by a log how do humans perform hybrid visual and memory search? Psychological Science. 2012;23(7):698–703. doi: 10.1177/0956797612443968. [DOI] [PMC free article] [PubMed] [Google Scholar]


