Abstract
This post hoc analysis examined cruciferous vegetable intake on urinary oxidative metabolites in postmenopausal women. Intervention participants (n = 69) received cruciferous vegetables (≥14 cups/week) during a 3-week period. First morning urine measured 8-isoprostane and 8-hydroxy- 2′-deoxyguanosine. Dietary intake was estimated using 24-h recalls. When stratified by history of breast cancer, those with breast cancer had significantly lower post-intervention urinary 8-hydroxy-2′-deoxyguanosine values in the intervention arm versus. the control arm (1.1 ng/mL vs. 3.2 ng/mL, p=.01) after adjustment for baseline 8-hydroxy-2′-deoxyguanosine. This was not observed in those without breast cancer. Further work is needed to understand the role of breast cancer in these relationships.
Keywords: Cruciferous vegetables, urine lipoprotein oxidation, breast cancer, diet
Introduction
Lifestyle factors, including dietary choices, are known to affect rates of oxidative stress. In particular, evidence shows that dietary antioxidants can significantly lower the incidence of cardiovascular disease (CVD) and, to a smaller extent, cancer (1–4).
Lipid peroxidation-associated deoxyribonucleic acid (DNA) damage has been shown to be elevated in several age-related diseases, including atherosclerosis, diabetes, Alzheimer’s disease, and cancer (5–9). In 1990, a frequently used marker of lipid peroxidation belonging to a series of ring-containing prostaglandin-like F-type compounds, 8-isoprostane (8-iso-PGFα) was discovered to be produced in humans (10). Although not well understood, the formation of lipid peroxidation products is catalyzed by free radicals that also inflict DNA damage, suggesting their involvement early in the process of carcinogenesis (11, 12).
A complementary product to assess DNA damage is the tissue and urinary marker of 8-hydroxy- 2′-deoxyguanosine (8oxodG), the nucleoside of 8- hydroxyguanine. Levels of this marker in tissues reflect a dynamic equilibrium between rates of oxidative damage and rates of repair. Excretion of urinary 8oxodG is the preferred indicator of “total body” damage (13–15), even though values for this damage are not consistent across studies, which range from<0.1 to 100 damaged bases per 105 unmodified guanines (13, 16– 19), with a conservative estimate for all DNA damage to be approximately 1/105 unmodified DNA bases (20).
Although both oxidative stress markers have been favorably validated using markers of mutagenicity, 8oxodG has been subjected to extensive methodological comparisons (21–23). Case-control studies have shown increased levels of 8oxodG in women with breast cancer (BC) and one study has demonstrated increased levels of 8-iso-PGFα (24–27).
The Nutrition and Breast Health Study of women with a family history of breast cancer (26) demonstrated a significant decline in urinary 8-iso-PGFα with a 12-month low-fat diet in participants who lowered their body mass index (BMI). At baseline, in a large randomized controlled trial (RCT), there were lower levels of 8oxodG with increasing fruit and vegetable servings/day among those consuming 3–4 servings/day (median 8oxodG: 16.7 vs. 20.5, p = .05) or ≥5 servings per day (15.6 vs. 20.5, p = .02) compared with those consuming ≤2 servings per day. However, this RCT indicated that just supplementing with vitamins C and E will not lower 8oxodG levels (28). Lastly, two large cross-sectional studies of adolescents (29) and healthy older adults (30) demonstrated an inverse correlation of urinary 8-iso-PGFα with higher daily fruit and vegetable intake.
Few studies have examined the effectiveness of cruciferous vegetables (Brassicaceae family) to lower the levels of 8-iso-PGFα or 8oxodG (31–33). In a small cross-sectional study (n = 71), Giovannelli et al. found lower adjusted levels of 8oxodG among participants in the highest tertile of cruciferous vegetable consumption compared with participants in the lowest tertile (3.86% vs. 5.47%, respectively, p = .09) in healthy Mediterranean adults (32). A small RCT of non-smoking men (n = 10) looked at the effect of cruciferous vegetables on 8oxodG (33) and found significantly lower (p = .04) levels of 8oxodG after eating 300 g of Brussels sprouts daily over a 3- week period. A small intervention study in healthy Japanese adults (n = 12) observed lower levels of 8- iso-PGFα after a week of consuming 100-g broccoli sprouts (31).
In light of this noticeable gap in knowledge of the effect of cruciferous vegetable intake on 8oxodG and 8-iso-PGFα, a RCT was conducted to determine the influence of increasing the overall number of cruciferous vegetable servings on these markers of oxidative stress in a population of postmenopausal women. Access to data and samples for this investigation was made available through a study that was originally designed to examine the effect of an intensive cruciferous vegetable-rich dietary intervention on aryl hydrocarbon receptor activation, its protein products, and estrogen metabolites in women undergoing a biopsy following a suspicious mammogram. Study participants were recruited from a breast clinic serving a large African-American population, and included both women who had completed breast cancer treatment and those who were disease-free. For this post hoc analysis, we tested whether women in the dietary intervention arm would experience a mean decrease in the two oxidative stress metabolites compared with women in the non-intervention arm. To be consistent with the original design of this intervention study, a secondary analysis stratified the main analyses by breast cancer status.
Material and methods
Subjects
In accordance with the original purpose of this study, participants were recruited between April 2002 and September 2003 in-person at one of two clinical breast centers (Palmetto Health, Columbia, SC, USA) with a local press release. Eligibility requirements included the following: (1) Postmenopausal (>1 year since last menstrual period); (2) not using hormone replacement therapy, or on fixed dose; (3) willing to be randomized; (4) willing to provide post-intervention data, including a needle biopsy from the contra-lateral breast (if history of breast cancer); (5) no health condition that would limit participation; (6) no malignancy in the past 5 years (except non-malignant melanoma of the skin and breast cancer) and having completed all treatments for breast cancer at least 1 year before enrolling in the study; (7) not currently taking thyroid medication, antibiotics, diuretics, or steroids; (8) not following a rigorous diet and no weight change exceeding 5 pounds within the past year; (9) alcohol consumption of <2 drinks/day; and (10) not diabetic. Since it was impossible to ban medications in the study participants in this age group, medication regimens were documented and required to be followed consistently during the intervention. Women were excluded if they did not attend two clinic visits or were unable to provide two urine samples. A total of 69 women were enrolled into the study. The current, post hoc analysis is based on these 69 women.
Randomization and informed consent
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Department of Defense Institutional Review Board, University of South Carolina Institutional Review Board and Palmetto Health Institutional Review Board. Before recruitment, participants provided informed written consent. Participants were randomly assigned by the status of breast cancer to the intervention or control group to ensure similarity of participants by study arm and to minimize the influence of background factors.
Study design
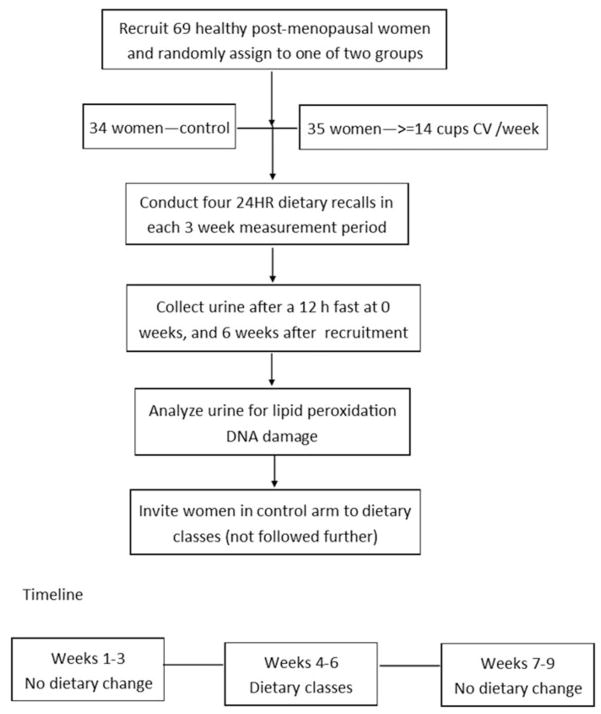
The intervention was based on participation in cooking classes. Women randomized to the dietary arm were invited to nine cooking classes conducted by a registered dietitian at one of the two local heart centers (South Carolina Heart Center or Providence Hospital). In addition, the intervention women received an adequate amount of fresh, locally grown cruciferous vegetables to incorporate into their weekly diet outside of cooking class attendance. A 10-point daily system was used to encourage consumption of less commonly consumed foods (e.g., 10 points for a single Brussels sprout compared with two cups of raw cabbage or broccoli). Women in the non-intervention arm were asked to follow usual eating patterns, and they were invited to attend cooking classes after completing their second clinical visit (Figure 1).
Figure 1.
Graphical representation of the study timeline and design.
Classes were participatory, consisting of individualized dietary counseling and “hands-on” cooking classes with menus based on the United States Department of Agriculture (USDA) Food Pyramid. Participants were encouraged to change food intake patterns, advised on how to overcome barriers (i.e., to improve self-efficacy) by substituting food choices, and informed on how to modify serving sizes. Most classes featured vegetables in the form of slaws, salads, and dips to encourage eating raw vegetables. Participants were provided a cruciferous vegetable cookbook and written instructions on how to read labels, incorporate “alternative” foods (e.g., soy products), and use herbs and spices. No advice was given to reduce total food intake or to count calories.
Data collection
Body mass, height, body circumferences (waist, abdomen, and hip), and percentage of body fat were measured by trained study personnel at clinical visits. Waist circumference was taken at the lower rib margin after removing heavy outerwear. Similarly, abdominal circumference was taken midway between the lower rib and iliac crest, and hip circumference was measured at the crotch level. Percentage of body fat was measured with a bioelectrical impedance analyzer (Quantum II Model, RJL Systems, Clinton Twp., MI, USA) with total body resistance (R) and reactance (Xc) recorded to the nearest Ohm. Electrode contact areas were wiped with alcohol before electrodes were placed between the distal prominences of the radius and ulna of the right wrist and between the medial and lateral malleoli of the right ankle. The resistance index (height2 divided by total body resistance) was calculated.
At baseline, a self-report questionnaire battery was used to collect data on demographics, personal health history, and lifestyle factors, including alcohol intake, reproductive history, breast cancer screening, past week physical activity, dietary knowledge, and personal food preferences.
Dietary data collection and analysis
Trained registered dietitians conducted unannounced telephone-administered 24-h recall interviews (24HR) (two on weekdays and two on weekend days) on randomly selected non-consecutive days during a 3-week sampling window. The Nutrition Data System Version 34 interactive software (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN, USA), which employs the multi-pass interview protocol, was used to collect all dietary data. Prior to the interviews, participants were provided a two-dimensional food portion poster and briefly trained to use this visual in the estimation of food portion sizes (34). Fruit and vegetable and cruciferous vegetable intakes were calculated based on gram weights of foods consumed using the USDA Pyramid equivalences from the Food and Nutrient Database for Dietary Studies. Data from the 24HRfromeach 3-week measurement period (i.e., pre and during-intervention) were averaged to provide an estimate of current dietary intake.
Urine collection and oxidative biomarker analyses
First-morning urine samples were collected from each woman in the study at the first and second clinical visits (cups contained 125-mg ascorbic acid to 100 mL of urine to prevent oxidation). The volume of urine was recorded as 100 mL of the first catch. Participants were asked to refrain from using dietary supplements or engaging in vigorous physical activity 12 h before urine collection. Urine was kept on ice (4°C) until aliquoted for long-term storage at −80°C.
For all analyses, baseline and follow-up urine samples for each participant were analyzed by a single technician in duplicate and on the same plate, and averaged to control for intra-assay variability. The technician was blinded as to the order of urine collection and identity of participants.
Creatinine was measured using a colorimetric assay kit (catalog No. KGE005; R & D Systems Minneapolis, MN) and according to manufacturer’s instructions. All samples were run in duplicate and the coefficient of variation was found to be<2%. In order to standardize urinary metabolite levels, 8oxodG concentration was expressed as ng/mg of creatinine and 8-iso-PGFα concentration was expressed as pg/mg of creatinine.
Urinary metabolites of oxidative stress were measured using commercially available ELISA kits and according to manufacturer’s instructions. For 8oxodG, all samples were run in duplicate using a kit (catalog No. KOG-200SE) from Genox Corp (Baltimore, MD, USA) (35). The coefficient of variation between duplicates was found to be <5%. For the 8-iso-PGFα assay, we used a kit (catalog #516351 from Cayman Chemicals, Ann Arbor, MI) that has been validated for measurement of this metabolite in urine (36). All samples were run in duplicate and the coefficient of variation was found to be <2%.
Statistical analysis
Differences at baseline in categorical variables (i.e., age, race, marital and employment status, education, participation in sports, and no history of breast cancer) were assessed using the Chi-square or Fisher’s Exact test for independence of proportions. Mean values for age, BMI, percentage of body fat, total cruciferous vegetable and fruit and vegetable intake, 8oxodG, and 8-iso-PGFα were compared between intervention and control arms at baseline using t-tests. In addition, using paired t-tests, differences in baseline and post-intervention 8oxodG and 8-iso-PGFα by treatment group were examined. All of the following were considered as potential confounders during analysis: physical activity status, cruciferous vegetable intake, fruit intake, vegetable intake, and fruit and vegetable intake. Through a series of multivariable analyses (i.e., treatment group, baseline outcome measure, and plus confounder) each potential confounder was individually substituted into this model. Variables with a p value of ≤.20 were added to a “full” model. A backward confounder selection procedure was then used to develop final models that included all variables that were statistically significant (α ≤ 0.05) or, when removed from the model, changed the beta coefficient of the primary independent variable (i.e., treatment group) by at least 10%.
To test for normality, models were run using the linear regression options to output normality statistics, including the model’s residuals. Using the Shapiro–Wilk normality test, both measures of oxidative stress were found to have non-normal distributions and therefore were log-transformed to obtain normally distributed model residuals that adhered to the normality assumption of linear regression. Mean values and confidence intervals were back-transformed for presentation. General linear models were used to compute least square (LS) mean values of each dependent variable (i.e., 8oxodG and 8-iso-PGFα) to test the difference in post-intervention 8oxodG and 8-iso-PGFα values between control and intervention arms after adjustment for baseline 8oxodG and 8-iso-PGFα values. Differences between the control and intervention arms were evaluated using the least significant difference statistics with the control arm as the referent. Secondary analyses stratified by breast cancer status. Statistical analyses were conducted using SAS® statistical software version 9.3 (Cary, NC, USA) and all statistical tests were 2-tailed with a critical α = 0.05.
Results
Characteristics of the study population
At baseline, women randomized to the treatment arm did not significantly differ from those in the control arm by relevant anthropometric, demographic, or fruit and/or vegetable categories. In addition, no differences in 8oxodG and 8-iso-PGFα were observed at baseline (Table 1). Overall, women were 61 ± 8.6 years of age (range 46–83); were, on average, overweight (BMI (kg/m2): 28.9±5.64; had moderate percentage of body fat: 39.6%±5.6%; and consumed an average of 5.6±2.4 fruit and vegetable servings per day. A high percentage of participants (78.3%) had at least completed high school. A majority of women were European-American (71%). An equal number of women (29%) with prior breast cancer (i.e., completed all treatment >1 year before entering study) were in the treatment and control arms.
Table 1.
Demographic, anthropometric, and fruit and vegetable consumption by treatment group at baseline.
| Group
|
p value | ||
|---|---|---|---|
| Intervention (n = 35) | Control (n = 34) | ||
| Racea | |||
| European-American | 24 (69%) | 25 (74%) | .65 |
| African-American | 11 (31%) | 9 (26%) | |
| Married/living with partnera | |||
| Yes | 25 (71%) | 18 (56%) | .20 |
| No | 10 (29%) | 14 (44%) | |
| Educationa | |||
| ≤High school | 7 (20%) | 8 (24%) | .72 |
| >High school | 28 (80%) | 26 (76%) | |
| Employment statusa | |||
| Full time | 13 (37%) | 14 (45%) | .78 |
| Part-time | 6 (17%) | 4 (13%) | |
| Unemployed | 16 (46%) | 13 (42%) | |
| Participation in sportsa | |||
| Yes | 31 (89%) | 29 (91%) | .99 |
| No | 4 (11%) | 3 (9%) | |
| Breast cancer statusa | |||
| History of breast cancer | 10 (29%) | 10 (29%) | .94 |
| No history of breast cancer | 25 (71%) | 24 (71%) | |
| Ageb | 60.3 ± 8.7 | 61.9 ± 8.7 | .44 |
| Body Mass Indexb | 29.3 ± 5.0 | 28.8 ± 6.3 | .69 |
| Percentage of body fatb | 40.1 ± 4.9 | 38.9 ± 6.2 | .41 |
| Total cruciferous vegetable intake (srv/d)b | 0.42 ± 0.5 | 0.64 ± 0.7 | .24 |
| Total vegetables intake (srv/d)b | 2.79 ± 1.0 | 3.32 ± 1.6 | .10 |
| Total fruit intake (srv/d)b | 1.67 ± 1.1 | 1.61 ± 1.3 | .83 |
| Total vegetable and fruit intake (srv/d)b | 4.47 ± 1.6 | 4.94 ± 2.4 | .33 |
| 8-iso-PGFα (pg/mL)c | 1394 ± 1491 | 1006 ± 799 | .66 |
| 8oxodG (ng/mL)c | 4.78 ± 4.56 | 4.96 ± 4.84 | .86 |
Frequency (%), p value based on Chi-square or Fisher’s Exact test.
Mean ± standard deviation, p value based on t-tests.
Mean ± standard deviation, p value based on the Wilcoxon rank sums test.
Differences between 8oxodG and 8-iso-PGFα
Consumption of cruciferous vegetables is significantly increased by 2.6 ± 1.5 srv/d in the intervention group but decreased slightly by −0.15 ± 0.6 srv/d (p < .01) in controls. Paired t-tests indicated an increase of 117 pg/mL and a decrease of 193 pg/mL in control and intervention groups, respectively, for 8-iso-PGFα at post-intervention compared with baseline. However, neither of these differences was statistically significant. As for 8oxodG, at post-intervention compared with the baseline, there was a 0.52 increase in controls but a 0.74 decrease in the intervention group; neither change was statistically significant (data not tabulated). Table 2 displays post-intervention 8-iso- PGFα and 8oxodG after adjustment for selected confounders and the baseline levels of those outcomes. There was no statistically significant difference in 8- iso-PGFα in the treatment group at post-intervention after adjusting for baseline levels of 8-iso-PGFα. There was a suggestion of a difference between intervention and control arms in post-intervention 8oxodG after adjustment for baseline levels (2.0 ng/mL vs. 2.8 ng/mL, respectively, p = .11), although not different statistically (Table 2).
Table 2.
Mean post-intervention adjusted 8-iso-PGFα and 8oxodG by treatment groups.
| Variable/treatment | LS Mean | LS Mean CI | p value |
|---|---|---|---|
| 8-iso-PGFα (pg/mL)a | |||
| Intervention (n = 35) | 696 | 524–923 | .39 |
| Control (n = 34) | 826 | 626–1091 | Referent |
| 8oxodG (ng/mL)a | |||
| Intervention (n = 35) | 2.0 | 1.4–2.8 | .11 |
| Control (n = 34) | 2.8 | 2.0–4.0 | Referent |
From analysis, using log values, mean values were back-transformed for presentation.
Adjustments: 8-iso-PGFα = baseline 8-iso-PGFα, age, and body mass index.
To be consistent with the design and original intention of the study, we performed a secondary analysis by stratifying the main results by breast cancer status. The breast cancer status-by-treatment group interaction was not statistically significant for 8-iso-PGFα (p = .63), but it was for 8oxodG (p = .03). When comparing the intervention with the control arm at follow-up after adjustment for baseline values, there were no statistically significant differences in 8oxodG or 8-iso-PGFα in women without a history of breast cancer, or in 8-iso-PGFα in women with a history of breast cancer. However, women with a history of breast cancer in the intervention arm showed statistically significantly lower 8oxodG levels than women with a history of breast cancer in the control arm (1.1 ng/mL vs. 3.2 ng/mL, respectively, p = 0.01, Table 3). In an attempt to determine whether there was a differential increase in cruciferous vegetable intake, we found that women with a history of breast cancer (0.1 to 3.2 servings/day, p < .01) and women without history of breast cancer (0.5 to 3.1 servings/day, p < .01) undergoing the intervention had similar increases in cruciferous vegetable consumption.
Table 3.
Mean post-intervention-adjusted 8-iso-PGFα and 8oxodG by treatment groups stratified by breast cancer status.
| Variable/treatment | LS Mean | LS mean CI | p value |
|---|---|---|---|
| BC history | |||
| 8-iso-PGFα (pg/mL)a | |||
| Intervention (n = 10) | 729 | 445–1197 | .37 |
| Control (n = 25) | 997 | 608–1637 | Referent |
| 8oxodG (ng/mL)a | |||
| Intervention (n = 10) | 1.1 | 1.0–1.9 | .01 |
| Control (n = 25) | 3.2 | 1.8–5.7 | Referent |
| No BC history | |||
| 8-iso-PGFα (pg/mL)a | |||
| Intervention (n = 10) | 670 | 478–966 | .67 |
| Control (n = 24) | 755 | 536–1064 | Referent |
| 8oxodG (ng/mL)a | |||
| Intervention (n = 10) | 2.5 | 1.7–3.7 | .81 |
| Control (n = 24) | 2.6 | 1.8–4.0 | Referent |
From analysis using log values, mean values were back-transformed for presentation.
Adjustments: 8-iso-PGFα = baseline 8-iso-PGFα, age, and body mass index; 8oxodG = baseline 8oxodG and education status.
Discussion
In this study, we observed no significant differences in either marker of oxidative stress as a result of a 3- week cruciferous vegetable dietary intervention among all women. However, we did find significantly lower levels of 8oxodG (but not for 8-iso-PGFα) in women with a history of breast cancer. It is not clear exactly why we observed this finding, given that all women had a significant increase in cruciferous vegetable consumption. It is possible that another underlying mechanism is driving the differences among women with breast cancer. Those in the intervention arm previously diagnosed with breast cancer (and who completed treatment>1 year before entering the study) had significantly lower (p = .03) mean values of 8oxodG than those without a prior diagnosis of breast cancer after the 3-week dietary intervention, which was not observed in women without a history of breast cancer. Regardless of breast cancer history, cruciferous vegetable consumption increased similarly in all women undergoing the intervention; this limits the possibility of differential increases in cruciferous vegetable intake between women with a history of breast cancer and women without a history of breast cancer undergoing the intervention as an explanation for the observed findings. On average, cruciferous vegetable consumption increased by 2.6 ± 1.5 srv/d among women in the intervention arm and decreased among those in the control arm by −0.15 ± 0.6 srv/d (p < .01).
Recent meta-analyses based on case-control studies have concluded that cruciferous vegetable consumption is associated with a lower risk of several cancer types (37–40). It also related to lower levels of markers of inflammation and oxidative stress (41, 42). However, few studies have examined the specific effect of cruciferous vegetables on oxidative damage in cancer patients. Of the larger intervention studies that have used fruit and vegetable intake to decrease levels of 8oxodG and 8-iso-PGFα in cancer patients, the Women’s Healthy Eating and Living (WHEL) RCT of women previously treated for breast cancer (43) found statistically significant decline in the levels of 8oxodG and 8-iso-PGFα after a 12-month low fat, high-fruit and vegetable dietary intervention. However, this analysis was based on a sub-sample of participants; no women without breast cancer were included as a control group, and the design used measured serum cholesterol rather than cruciferous vegetables to assess dietary adherence. In contrast to our results, two studies have shown decrease in 8-iso- PGF2α, including a small (n = 12) 1-week feeding study using broccoli sprouts (100 g/d) (31), as well as a more recent randomized cross-over intervention (n = 20) that asked participants to eat at least two cups (>160 g) of these vegetables daily (44). Lowering levels of oxidative damage as a result of cruciferous vegetable intake have been confirmed in small intervention trials, regardless of participants’ smoking status (45–47).
There is a wide range of effects observed as a result of using different markers of oxidative damage in epidemiological studies. Djuric and colleagues found significant differences by gender using a marker of oxidative damage (5-hydroxymethyl-2′-deoxyuridine [5-OHmdU] in DNA from nucleated blood cells) in a small isoflavone supplementation study (n = 12) (25). It took longer to observe a decrease inoxidative damage in men (>3 weeks) than in women (~1 week) possibly due to including premenopausal women who may exhibit lower levels of reactive oxygen species due to significant variability by phase of the menstrual cycle (48, 49) and because the small level of supplementation (50 mg) was not enough to impact the typically higher weight of men. However, no difference was observed using isoprostane biomarkers (25).
Isoprostane concentrations vary widely in healthy adults due to dietary intake differences and endogenous antioxidant defenses. Some healthy humans appear to have higher rates of lipid peroxidation, even when consuming comparable diets (50–53). These individuals could be at greater risk of diseases involving lipid peroxidation, such as atherosclerosis and cancer. The evidence suggests that variations in oxidative damage, as measured by isoprostanes, may be modified by random individual variation in lipid peroxidation as well as the type of dietary intervention (i.e., use of supplements rather than whole foods).
This dietary intervention successfully increased total vegetable servings as well as cruciferous vegetables regardless of race or breast cancer status. Our results are in agreement with the WHEL study on women with a history of breast cancer (43) as well as another large clinical trial (28), both of which demonstrated decrease in 8oxodG levels with increasing intake of fruits and vegetables. It is possible that the decreases observed in previous studies may be partly due to larger effects in men than in women rather than generally higher intake of vegetables (44, 54).
Many factors could explain why a reduction in oxidative stress is not observed due to dietary interventions. These include inherent biological variations in absorbing essential phytochemicals and rapid degradation, inactivity, or clearance of these chemicals, as well as differences in study methodology. Study design may further contribute by not accounting for individual genotypic differences in processing cruciferous vegetable metabolites (55–57) and requiring a larger quantity of cruciferous vegetables to observe metabolic changes.
Our study had several limitations that could be addressed in the future trials. It was limited by a small sample size (n = 69) compared to much larger sample sizes (i.e., n > 200) in more recent studies examining circulating levels of markers of inflammation and oxidative stress in women (41, 58). We know that self-reports of dietary intake are subject to biases of various types, including social desirability bias (59). So, it is conceivable that measurement error could explain some of these null results. For analysis of 8-iso-PGFα and 8oxodG in urine samples, although we used immunoassays, mass spectrometry analysis generally provides better results (60, 61). The exact amount of cruciferous vegetables consumed and its bioavailable functional components could not be determined during the class sessions or from foods consumed outside class sessions. The lack of information on the nutritional composition of cruciferous vegetables introduced (especially for bioactive constituents such as isothiocyanates) or a circulating marker of intake make it difficult to evaluate the strict relationship between the cruciferous treatment and the oxidative stress markers analyzed. Providing specific meal plans in addition to providing vegetables has been previously suggested to improve consumption of cruciferous vegetables and enhances the probability of differences during the study in the dietary behavior between participants (62–64). The future analyses may consider excluding women exposed to tobacco smoke (either active or passive) because of its well-known role in promoting oxidative damage (65–67). In addition, it may be useful to examine the role of caffeine, alcohol, and supplements in explaining the racial differences in oxidative damage over time.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article. This study was done in conjunction with a larger study funded by an IDEA award through the US Army Medical Research and Materiel Command under the Department of Defense Broad Agency Announcement (BAA) for Breast Cancer Research (#DAMD17- 99-1-9279). Dr. Wirth’s participation was supported through an ASPIRE-II Grant from the University of South Carolina Office of Research and by the South Carolina Cancer Prevention and Control Research Network funded under Cooperative Agreement Number 3U48DP001936-01 from the Centers for Disease Control and Prevention and the National Cancer Institute. Dr. Hébert was supported by an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute (K05 CA136975). The funding sources had no involvement in this research.
References
- 1.Prior RL. Fruits and vegetables in the prevention of cellular oxidative damage. Am J Clin Nutr. 2003;78:570S–578S. doi: 10.1093/ajcn/78.3.570S. [DOI] [PubMed] [Google Scholar]
- 2.Rautiainen S, Larsson S, Virtamo J, Wolk A. Total antioxidant capacity of diet and risk of stroke: a population-based prospective cohort of women. Stroke. 2012;43:335–340. doi: 10.1161/STROKEAHA.111.635557. [DOI] [PubMed] [Google Scholar]
- 3.Rautiainen S, Levitan EB, Orsini N, Akesson A, Morgen-stern R, Mittleman MA, et al. Total antioxidant capacity from diet and risk of myocardial infarction: a prospective cohort of women. Am J Med. 2012;125:974–980. doi: 10.1016/j.amjmed.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Lee SA, Shu XO, Li H, Yang G, Cai H, Wen W, et al. Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women’s Health Study. Am J Clin Nutr. 2009;89:1920–1926. doi: 10.3945/ajcn.2008.27361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic Biol Med. 2007;43:1109–1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Mahmoudi M, Mercer J, Bennett M. DNA damage and repair in atherosclerosis. Cardiovasc Res. 2006;71:259–268. doi: 10.1016/j.cardiores.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Tabak O, Gelisgen R, Erman H, Erdenen F, Muderrisoglu C, Aral H, et al. Oxidative lipid, protein, and DNA damage as oxidative stress markers in vascular complications of diabetes mellitus. Clin Invest Med. 2006;34:E163–E171. doi: 10.25011/cim.v34i3.15189. [DOI] [PubMed] [Google Scholar]
- 8.Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 9.Gago-Dominguez M, Castelao JE, Pike MC, Sevanian A, Haile RW. Role of lipid peroxidation in the epidemiology and prevention of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2829–2839. doi: 10.1158/1055-9965.EPI-05-0015. [DOI] [PubMed] [Google Scholar]
- 10.Morrow JD, Harris TM, Roberts LJ., 2nd Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal Biochem. 1990;184:1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- 11.Ames BN, Gold LS. Endogenous mutagens and the causes of aging and cancer. Mutat Res. 1991;250:3–16. doi: 10.1016/0027-5107(91)90157-j. [DOI] [PubMed] [Google Scholar]
- 12.Vaca CE, Nilsson JA, Fang JL, Grafstrom RC. Formation of DNA adducts in human buccal epithelial cells exposed to acetaldehyde and methylglyoxal in vitro. Chem Biol Interact. 1998;108:197–208. doi: 10.1016/s0009-2797(97)00107-5. [DOI] [PubMed] [Google Scholar]
- 13.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loft S, Deng XS, Tuo J, Wellejus A, Sorensen M, Poulsen HE. Experimental study of oxidative DNA damage. Free Radic Res. 1998;29:525–539. doi: 10.1080/10715769800300571. [DOI] [PubMed] [Google Scholar]
- 15.Cooke MS, Evans MD, Herbert KE, Lunec J. Urinary 8- oxo-2′-deoxyguanosine—source, significance and supplements. Free Radic Res. 2000;32:381–397. doi: 10.1080/10715760000300391. [DOI] [PubMed] [Google Scholar]
- 16.Kasai H. Analysis of a form of oxidative DNA damage, 8- hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res. 1997;387:147– 163. doi: 10.1016/s1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 17.Helbock HJ, Beckman KB, Shigenaga MK, Walter PB, Woodall AA, Yeo HC, et al. DNA oxidation matters: the HPLC-electrochemical detection assay of 8-oxodeoxyguanosine and 8-oxo-guanine. Proc Natl Acad Sci USA. 1998;95:288–293. doi: 10.1073/pnas.95.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senturker S, Dizdaroglu M. The effect of experimental conditions on the levels of oxidatively modified bases in DNA as measured by gas chromatography-mass spectrometry: how many modified bases are involved? Prepurification or not? Free Radic Biol Med. 1999;27:370–380. doi: 10.1016/s0891-5849(99)00069-6. [DOI] [PubMed] [Google Scholar]
- 19.Helbock HJ, Beckman KB, Ames BN. 8- Hydroxydeoxyguanosine and 8-hydroxyguanine as biomarkers of oxidative DNA damage. Methods Enzymol. 1999;300:156–166. doi: 10.1016/s0076-6879(99)00123-8. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B. Effect of diet on cancer development: is oxidative DNA damage a biomarker? Free Radic Biol Med. 2002;32:968–974. doi: 10.1016/s0891-5849(02)00808-0. [DOI] [PubMed] [Google Scholar]
- 21.Arashidani K, Iwamoto-Tanaka N, Muraoka M, Kasai H. Genotoxicity of ribo- and deoxyribonucleosides of 8-hydroxyguanine, 5-hydroxycytosine, and 2- hydroxyadenine: induction of SCE in human lymphocytes and mutagenicity in Salmonella typhimurium TA 100. Mutat Res. 1998;403:223–227. doi: 10.1016/s0027-5107(98)00086-4. [DOI] [PubMed] [Google Scholar]
- 22.Fujikawa K, Kamiya H, Kasai H. The mutations induced by oxidatively damaged nucleotides, 5-formyl-dUTP and 5-hydroxy-dCTP, in Escherichia coli. Nucleic Acids Res. 1998;26:4582–4587. doi: 10.1093/nar/26.20.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D, Kreutzer DA, Essigmann JM. Mutagenicity and repair of oxidative DNA damage: insights from studies using defined lesions. Mutat Res. 1998;400:99–115. doi: 10.1016/s0027-5107(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 24.Musarrat J, Arezina-Wilson J, Wani AA. Prognostic and a etiological relevance of 8-hydroxyguanosine in human breast carcinogenesis. Eur J Cancer. 1996;32A:1209–1214. doi: 10.1016/0959-8049(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 25.Djuric Z, Chen G, Doerge DR, Heilbrun LK, Kucuk O. Effect of soy isoflavone supplementation on markers of oxidative stress in men and women. Cancer Lett. 2001;172:1–6. doi: 10.1016/s0304-3835(01)00627-9. [DOI] [PubMed] [Google Scholar]
- 26.Chen G, Heilbrun LK, Venkatramanamoorthy R, Maranci V, Redd JN, Klurfeld DM, et al. Effects of low-fat and/or high-fruit-and-vegetable diets on plasma levels of 8- isoprostane-F2alpha in the Nutrition and Breast Health study. Nutr Cancer. 2004;50:155–160. doi: 10.1207/s15327914nc5002_4. [DOI] [PubMed] [Google Scholar]
- 27.Soliman AS, Vulimiri SV, Kleiner HE, Shen J, Eissa S, Morad M, et al. High levels of oxidative DNA damage in lymphocyte DNA of premenopausal breast cancer patients from Egypt. Int J Environ Health Res. 2004;14:121–134. doi: 10.1080/0960312042000209534. [DOI] [PubMed] [Google Scholar]
- 28.Huang HY, Helzlsouer KJ, Appel LJ. The effects of vitamin C and vitamin E on oxidative DNA damage: results from a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2000;9:647–652. [PubMed] [Google Scholar]
- 29.Holt EM, Steffen LM, Moran A, Basu S, Steinberger J, Ross JA, et al. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. 2009;109:414–421. doi: 10.1016/j.jada.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polidori MC, Carrillo JC, Verde PE, Sies H, Siegrist J, Stahl W. Plasma micronutrient status is improved after a 3- month dietary intervention with 5 daily portions of fruits and vegetables: implications for optimal antioxidant levels. Nutr J. 2009;8:10. doi: 10.1186/1475-2891-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murashima M, Watanabe S, Zhuo XG, Uehara M, Kurashige A. Phase 1 study of multiple biomarkers for metabolism and oxidative stress after one-week intake of broccoli sprouts. Biofactors. 2004;22:271–275. doi: 10.1002/biof.5520220154. [DOI] [PubMed] [Google Scholar]
- 32.Giovannelli L, Saieva C, Masala G, Testa G, Salvini S, Pitozzi V, et al. Nutritional and lifestyle determinants of DNA oxidative damage: a study in a Mediterranean population. Carcinogenesis. 2002;23:1483–1489. doi: 10.1093/carcin/23.9.1483. [DOI] [PubMed] [Google Scholar]
- 33.Verhagen H, Poulsen HE, Loft S, van Poppel G, Willems MI, van Bladeren PJ. Reduction of oxidative DNA-damage in humans by brussels sprouts. Carcinogenesis. 1995;16:969–970. doi: 10.1093/carcin/16.4.969. [DOI] [PubMed] [Google Scholar]
- 34.Posner BM, Smigelski C, Duggal A, Morgan JL, Cobb J, Cupples LA. Validation of two-dimensional models for estimation of portion size in nutrition research. J Am Diet Assoc. 1992;92:738–741. [PubMed] [Google Scholar]
- 35.Erhola M, Toyokuni S, Okada K, Tanaka T, Hiai H, Ochi H, et al. Biomarker evidence of DNA oxidation in lung cancer patients: association of urinary 8-hydroxy-2′-deoxyguanosine excretion with radiotherapy, chemotherapy, and response to treatment. FEBS Lett. 1997;409:287– 291. doi: 10.1016/s0014-5793(97)00523-1. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Ciabattoni G, Creminon C, Lawson J, Fitzgerald GA, Patrono C, et al. Immunological characterization of urinary 8-epi-prostaglandin F2 alpha excretion in man. J Pharmacol Exp Ther. 1995;275:94–100. [PubMed] [Google Scholar]
- 37.Tang L, Zirpoli GR, Jayaprakash V, Reid ME, McCann SE, Nwogu CE, et al. Cruciferous vegetable intake is inversely associated with lung cancer risk among smokers: a case-control study. BMC Cancer. 2010;10:162. doi: 10.1186/1471-2407-10-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosetti C, Filomeno M, Riso P, Polesel J, Levi F, Talamini R, et al. Cruciferous vegetables and cancer risk in a network of case-control studies. Ann Oncol. 2012;23:2198–2203. doi: 10.1093/annonc/mdr604. [DOI] [PubMed] [Google Scholar]
- 39.Liu B, Mao Q, Lin Y, Zhou F, Xie L. The association of cruciferous vegetables intake and risk of bladder cancer: a meta-analysis. World J Urol. 2013;31(1):127–133. doi: 10.1007/s00345-012-0850-0. [DOI] [PubMed] [Google Scholar]
- 40.Liu B, Mao Q, Cao M, Xie L. Cruciferous vegetables intake and risk of prostate cancer: a meta-analysis. Int J Urol. 2012;19:134–141. doi: 10.1111/j.1442-2042.2011.02906.x. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Y, Wu SH, Shu XO, Xiang YB, Ji BT, Milne GL, et al. Cruciferous vegetable intake is inversely correlated with circulating levels of proinflammatory markers in women. J Acad Nutr Dietet. 2014;114:700 e2–708 e2. doi: 10.1016/j.jand.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riso P, Martini D, Visioli F, Martinetti A, Porrini M. Effect of broccoli intake on markers related to oxidative stress and cancer risk in healthy smokers and nonsmokers. Nutr Cancer. 2009;61:232–237. doi: 10.1080/01635580802425688. [DOI] [PubMed] [Google Scholar]
- 43.Thomson CA, Giuliano AR, Shaw JW, Rock CL, Ritenbaugh CK, Hakim IA, et al. Diet and biomarkers of oxidative damage in women previously treated for breast cancer. Nutr Cancer. 2005;51:146–154. doi: 10.1207/s15327914nc5102_4. [DOI] [PubMed] [Google Scholar]
- 44.Fowke JH, Morrow JD, Motley S, Bostick RM, Ness RM. Brassica vegetable consumption reduces urinary F2- isoprostane levels independent of micronutrient intake. Carcinogenesis. 2006;27:2096–2102. doi: 10.1093/carcin/bgl065. [DOI] [PubMed] [Google Scholar]
- 45.GillCI, Haldar S, Porter S, Matthews S, Sullivan S, Coulter J, et al. The effect of cruciferous and leguminous sprouts on genotoxicity, in vitro and in vivo. Cancer Epidemiol Biomarkers Prev. 2004;13:1199–1205. [PubMed] [Google Scholar]
- 46.Gill CI, Haldar S, Boyd LA, Bennett R, Whiteford J, Butler M, et al. Watercress supplementation in diet reduces lymphocyte DNA damage and alters blood antioxidant status in healthy adults. Am J Clin Nutr. 2007;85:504–510. doi: 10.1093/ajcn/85.2.504. [DOI] [PubMed] [Google Scholar]
- 47.Polidori MC, Pratico D, Mangialasche F, Mariani E, Aust O, Anlasik T, et al. High fruit and vegetable intake is positively correlated with antioxidant status and cognitive performance in healthy subjects. J Alzheimers Dis. 2009;17:921–927. doi: 10.3233/JAD-2009-1114. [DOI] [PubMed] [Google Scholar]
- 48.Zitnanova I, Rakovan M, Paduchova Z, Dvorakova M, Andrezalova L, Muchova J, et al. Oxidative stress in women with perimenopausal symptoms. Menopause. 2011;18:1249–1255. doi: 10.1097/gme.0b013e318224fa3d. [DOI] [PubMed] [Google Scholar]
- 49.Schisterman EF, Gaskins AJ, Mumford SL, Browne RW, Yeung E, Trevisan M, et al. Influence of endogenous reproductive hormones on F2-isoprostane levels in pre-menopausal women: the BioCycle study. Am J Epidemiol. 2010;172:430–439. doi: 10.1093/aje/kwq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 51.Roberts LJ, 2nd, Morrow JD. The generation and actions of isoprostanes. Biochim Biophys Acta. 1997;1345:121–135. doi: 10.1016/s0005-2760(96)00162-2. [DOI] [PubMed] [Google Scholar]
- 52.Basu S. Metabolism of 8-iso-prostaglandin F2alpha. FEBS Lett. 1998;428:32–36. doi: 10.1016/s0014-5793(98)00481-5. [DOI] [PubMed] [Google Scholar]
- 53.Davi G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 54.Verhagen H, de Vries A, Nijhoff WA, Schouten A, van Poppel G, Peters WH, et al. Effect of Brussels sprouts on oxidative DNA-damage in man. Cancer Lett. 1997;114:127–130. doi: 10.1016/s0304-3835(97)04641-7. [DOI] [PubMed] [Google Scholar]
- 55.Steck SE, Gaudet MM, Britton JA, Teitelbaum SL, Terry MB, Neugut AI, et al. Interactions among GSTM1, GSTT1 and GSTP1 polymorphisms, cruciferous vegetable intake and breast cancer risk. Carcinogenesis. 2007;28:1954–1959. doi: 10.1093/carcin/bgm141. [DOI] [PubMed] [Google Scholar]
- 56.Lee SA, Fowke JH, Lu W, Ye C, Zheng Y, Cai Q, et al. Cruciferous vegetables, the GSTP1 Ile105Val genetic polymorphism, and breast cancer risk. Am J Clin Nutr. 2008;87:753–760. doi: 10.1093/ajcn/87.3.753. [DOI] [PubMed] [Google Scholar]
- 57.Fowke JH, Gao YT, Chow WH, Cai Q, Shu XO, Li HL, et al. Urinary isothiocyanate levels and lung cancer risk among non-smoking women: a prospective investigation. Lung Cancer. 2011;73:18–24. doi: 10.1016/j.lungcan.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rink SM, Mendola P, Mumford SL, Poudrier JK, Browne RW, Wactawski-Wende J, et al. Self-report of fruit and vegetable intake that meets the 5 a day recommendation is associated with reduced levels of oxidative stress biomarkers and increased levels of antioxidant defense in premenopausal women. J Acad Nutr Diet. 2013;113:776–785. doi: 10.1016/j.jand.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol. 1995;24:389–398. doi: 10.1093/ije/24.2.389. [DOI] [PubMed] [Google Scholar]
- 60.European Standards Committee on Urinary Lesion A. Evans MD, Olinski R, Loft S, Cooke MS. Toward consensus in the analysis of urinary 8-oxo- 7,8-dihydro-2′-deoxyguanosine as a noninvasive biomarker of oxidative stress. FASEB J. 2010;24:1249– 1260. doi: 10.1096/fj.09-147124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klawitter J, Haschke M, Shokati T, Klawitter J, Christians U. Quantification of 15-F2t-isoprostane in human plasma and urine: results from enzyme-linked immunoassay and liquid chromatography/tandem mass spectrometry cannot be compared. Rapid Commun Mass Spectrom. 2011;25:463–468. doi: 10.1002/rcm.4871. [DOI] [PubMed] [Google Scholar]
- 62.Jeffery RW, Wing RR, Thorson C, Burton LR, Raether C, Harvey J, et al. Strengthening behavioral interventions for weight loss: a randomized trial of food provision and monetary incentives. J Consult Clin Psychol. 1993;61:1038–1045. doi: 10.1037//0022-006x.61.6.1038. [DOI] [PubMed] [Google Scholar]
- 63.Metz JA, Kris-Etherton PM, Morris CD, Mustad VA, Stern JS, Oparil S, et al. Dietary compliance and cardiovascular risk reduction with a prepared meal plan compared with a self-selected diet. Am J Clin Nutr. 1997;66:373–385. doi: 10.1093/ajcn/66.2.373. [DOI] [PubMed] [Google Scholar]
- 64.Wing RR, Jeffery RW, Burton LR, Thorson C, Nissinoff KS, Baxter JE. Food provision vs structured meal plans in the behavioral treatment of obesity. Int J Obes Relat Metab Disord. 1996;20:56–62. [PubMed] [Google Scholar]
- 65.Lam TK, Gallicchio L, Lindsley K, Shiels M, Ham-mond E, Tao XG, et al. Cruciferous vegetable consumption and lung cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev. 2010;18:184–195. doi: 10.1158/1055-9965.EPI-08-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bohn SK, Myhrstad MC, Thoresen M, Holden M, Karlsen A, Tunheim SH, et al. Blood cell gene expression associated with cellular stress defense is modulated by antioxidant-rich food in a randomised controlled clinical trial of male smokers. BMC Med. 2010;8:54. doi: 10.1186/1741-7015-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, et al. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol Biomarkers Prev. 2010;19:1806–1811. doi: 10.1158/1055-9965.EPI-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]