Abstract
Neuroinflammation is recognised as one of the potential mechanisms mediating the onset of a broad range of psychiatric disorders and may contribute to nonresponsiveness to current therapies. Both preclinical and clinical studies have indicated that aberrant inflammatory responses can result in altered behavioral responses and cognitive deficits. In this review, we discuss the role of inflammation in the pathogenesis of neuropsychiatric disorders and ask the question if certain genetic copy-number variants (CNVs) associated with psychiatric disorders might play a role in modulating inflammation. Furthermore, we detail some of the potential treatment strategies for psychiatric disorders that may operate by altering inflammatory responses.
1. Introduction
Neuropsychiatric disorders including devastating diseases such as schizophrenia, major depressive disorder, and bipolar disorder are generally considered to have a multifactorial pathophysiology including both genetic and environmental factors [1]. Neuroinflammation could be one of the potential mechanisms contributing to pathogenesis, anchored in the interplay of environmental factors such as hypoxia or infections and genetic susceptibility of the immune system.
In fact, increasing amount of evidence suggests that inflammatory processes have an important role in the pathophysiology of psychiatric disorders. The significantly higher level of different inflammatory markers such as cytokines, chemokines, and chemokine receptors in patients suffering from various forms of psychiatric disorder has laid molecular foundation for the important role of inflammation in the pathogenesis of neuropsychiatric disorder [2–6]. Furthermore, there is increasing evidence from genetic studies that these altered immune processes may play a primary role in the development of neuropsychiatric disorders, rather than simply being a consequence of associated brain pathology. Assuming inflammation plays a key role in the pathogenesis of psychiatric disorders, anti-inflammatory treatments may play a critical role in the treatment of these disorders.
To what extent and in which case has this anti-inflammatory therapy already been applied successfully is far from clear. The efficacy of prescribing anti-inflammatory drugs to treat depression and other psychiatric diseases, either alone or in conjunction with traditional medication, remains to be elucidated. In this review, we will try to summarize the answers to these questions and sum up treatment recommendations where available.
2. Relationship between Inflammation and Mental Illness
Recent studies on preclinical, genetics, and bioinformatics data have shown the activation of immune system molecules and pathways that can contribute to pathogenesis of psychiatric disorders [7]. Several lines of the evidence that support a role for inflammation as a contributing factor in psychiatric disorders include the following.
(I) It has been established that cytokines that are found typically during an ongoing inflammatory process are found to be elevated in blood samples of patients with various types of psychiatric disorders. This, depending on the study, includes both generally considered proinflammatory (i.e., interleukin- (IL-) 1-3, IL-5-9, IL-11-18, interferons (IFN), tumor necrosis factor (TNF), and chemokines) as well as anti-inflammatory (i.e., IL-4, IL-10, IL-11, and IL-13) cytokines and complement factors. Though the activation cascade of this elevated cytokine production is not yet understood, the findings may point to a significant role of peripheral inflammatory processes in psychiatric conditions. Following examination of blood samples from patients with schizophrenia [8, 9], depression [10, 11], anxiety [12], bipolar disorder [13–15], obsessive-compulsive disorder (OCD) [16, 17], posttraumatic stress disorder (PTSD) [18, 19], and autism spectrum disorder [20, 21] significantly elevated levels of all major kinds of cytokines were detected. This also included soluble interleukin receptors, interleukin antagonists, TNF, soluble TNF receptor IFN-γ, chemokines, and matrix metalloproteinases (MMP) (see Figure 1 and Table 1). The levels of some of these cytokines have also been correlated in some studies to the severity of disease symptoms [12, 17]. Work on the Whitehall II cohort shows that individuals with increased IL-6 levels over a prolonged period of time (one, two, or three measurements over a 5-year period) have an elevated subsequent 10-year risk for development of cognitive symptoms of depression [22]. There are multiple studies showing elevated high sensitivity- (hs-) C-reactive protein (CRP) levels in blood samples from patients with psychiatric disorders (schizophrenia, depression, PTSD, anxiety, and autism). Elevated levels of proinflammatory cytokines like IL-6 and IL-1β can lead to the production of this acute phase protein in the liver [14, 23–28]. Importantly, a meta-analysis of 8 longitudinal studies showed that increased CRP levels are significantly associated with the risk for later development of depressive symptoms. This result was independent of age and a range of other risk factors of depression [24]. Interestingly, higher levels of CRP and IL-6 during childhood (age of nine years) were shown to be a predictor of higher risk of depression and psychosis in later life [29]. Increased CRP was even suggested as a diagnostic tool of autism spectrum disorder due to a positive correlation with symptom severity [27].
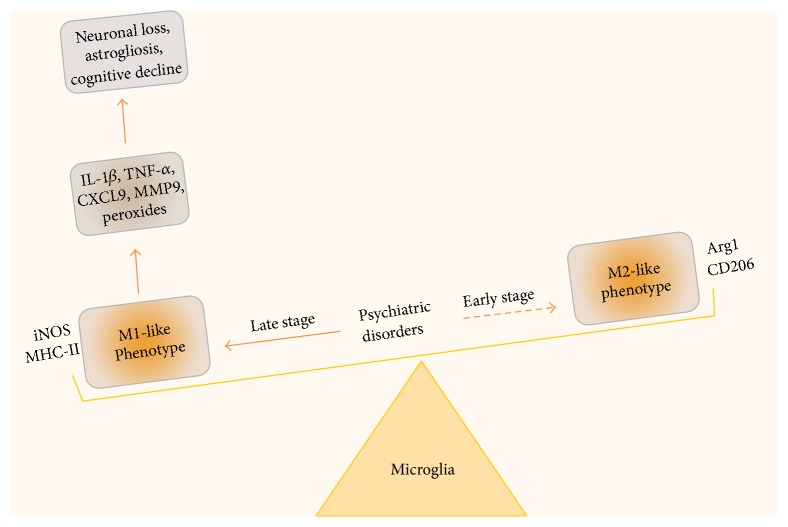
Figure 1.
Potential mechanism of microglia activation in psychiatric disorders. Neuroinflammation is one of the key components of the pathogenic mechanisms underlying several psychiatric disorders and is often associated with microglial activation/dysfunction. Accumulating evidence indicate that M1-like microglia (proinflammatory) are significantly increased in comparison to ramified microglia (resting) and elongated M2-like microglia (anti-inflammatory) phenotypes in disease states. The levels of M1-like microglia in brain predominate and potentially can be associated with the severity of the disease, suggesting an imbalance in M1/M2 phenotype. M1-like microglia are characterized by the expression of MHC class II antigens and by the production of proinflammatory cytokines and nitric oxide synthase (iNOS). Continued production of proinflammatory cytokines can lead to neuronal damage, astrogliosis, plasticity, and cognitive decline. Peripherally derived macrophages and monocytes also participate in the inflammatory response. It is likely that during early stage of disease onset microglia can have phenotypic switch to an alternative state knows as M2-like phenotype, which are characterized by presence of surface markers like Arginase 1 and mannose receptor CD206, leading to resolution of inflammatory response by secretion of anti-inflammatory cytokines. The efficacy of the anti-inflammatory drug targeting M1/M2 balance will significantly depend on therapeutic time window and severity of symptoms associated with the diseases.
Table 1.
Circulating cytokines and acute phase proteins that are found elevated in different psychiatric disorders.
| Psychiatric disorder | Cytokines/acute phase proteins elevated |
|---|---|
| Schizophrenia | IL-1β, IL-6, IL12-p70, sIL-2R, TNF-α, IFN-γ [8, 9] |
| Depression | IL-1, IL-6, sIL-1R, sIL-2R, sIL-6, TNF-α, CRP [10, 11] |
| Bipolar disorder | IL-4, IL-6, IL-10, sIL-2R, sIL-6R, TNF-α, sTNFR1, IL-1 receptor antagonist, INF-γ, hs-CRP [13–15] |
| Anxiety disorder | IL-6, IL-8, IL-10, IL-18, MCP-1, MMP2, TNF-β [12]∗ |
| OCD | IL-2, IL-4, IL-6, IL-10, TNF-α, sTNFR1, sTNFR2 [16, 17] |
| PTSD | IL-1α, IL-1β, IL-2, IL-4, IL-6, IL7, IL-8, IL-10 and, IL12p40, IL12p70, IL-13, IL-15, TNF-α, MIP-1α, GM-CSF, IP-10, eotaxin [18, 19]∗∗ |
| Autism | IL-1β, IL-1RA, IL-5, IL-8, IL12p40, IL-12(p70), IL-13, IL-17, GRO-α [20, 21] |
∗After stimulation with LPS; ∗∗in PTSD and panic disorder patients.
It is difficult to examine cytokine and acute phase protein levels in the brain directly, hence findings from the peripheral blood therefore have to be interpreted with caution. This is why it is worth looking, among other aspects, into gene expression when reviewing results from postmortem samples of the brain.
(II) In schizophrenia, transcriptome analysis of brain tissue from autopsy of schizophrenic patients showed an increased expression of IFITM2, IFITM3, SERPINA3, and GBP1 was found. These genes are directly regulated by TNF-α, IFN-α, and IFN-γ, suggesting a proinflammatory state [30]. A recent study examining gene expression of the dorsolateral prefrontal cortex from patients with mood disorders demonstrate an elevated expression of inflammatory agents (IL-1α, IL-2, IL-3, IL-5, IL-8, IL-9, IL-10, IL-12A, IL-13, IL-15, IL-18, IFN-γ, and lymphotoxin-α) compared to control subjects [31]. Therefore, these results suggest that the findings seen using blood samples may be recapitulated in gene expression of key inflammatory agents in the brain, albeit in postmortem samples. A systematic review of 119 publications on postmortem tissue investigating further evidence of inflammation in schizophrenia concluded that there is high variability between the results found in different studies. For astrocyte or microglia markers no consistent increase or decrease was found across different studies. These investigations varied in patient age, diagnosis, and brain region examined. Out of 33 studies, 6 showed an increase in the astrocyte marker, glia fibrillary acidic protein (GFAP), while 6 showed a decrease. An increase in microglial markers (CD68, human leukocyte antigens (HLA), MHC antigens, CD11b, calprotectin, and quinolinic acid) was found in 11 studies and a decrease in 3 studies. In studies on general glia cell density, 7 of 34 studies showed a decrease in glial cell density and 2 studies an increase. Furthermore, changes, mostly increases, in mRNA expression levels of chemokines and cytokines (interleukins, TNF-α, TNF-α receptor, and IFN-γ) were frequently found in postmortem studies of schizophrenic brains. Other proinflammatory markers such as substance P, NF-κB, SERPINA3, and interferon-induced transmembrane protein (IFITM) were found elevated in concentration and/or expression in certain brain regions in schizophrenia patients in some, though not all, studies [32]. A similar meta-analysis of the literature on postmortems in mood disorders was somewhat more conclusive: in addition to findings of changes in the cytokine profile in the brain, it is argued that microglia and perivascular macrophages are found at increased density in the postmortem tissue. Meanwhile, oligodendroglia and GABAergic interneurons are found at a diminished number. One hypothesis that could explain this observation is that activated microglia release glutamate, which might damage neurons. The loss of oligodendroglia might also be a direct consequence of inflammation. So, pathways of neuroexcitation may be highly changed through neuroinflammatory mechanisms [33].
(III) In multiple studies elevations of CRP and/or proinflammatory cytokine levels were shown to return to normal when the psychiatric disorder was treated. For example, the level of IL-1β and IL-2 in depression patients returned to a similar level to that seen in controls after treatment with serotonin reuptake inhibitors over a 20-week period [34]. Accordingly, bipolar disorder patients treated with mood-stabilizers and antipsychotics responded with a significant decrease in high sensitivity CRP in the aforementioned study [14]. IL-1RA and IL-10 have also been found to be increased in drug-naïve schizophrenia patients decreased after 6 weeks of treatment with atypical antipsychotics. A strong correlation between IL-10 level and a score of negative, general, and total symptoms was found. Plasma levels of IL-2 and IL-6 were found to be decreased after antipsychotic treatment of schizophrenic patients in a meta-analysis of 8 similar studies [35]. Though little is said in the above studies on the correlation of patient outcome and the effects of pharmaceutics on cytokine levels, psychotherapy alone showed a decrease of IL-1Ra, IL-5,-6,-8,-10, G-CSF, IFN-γ, and TNF-α levels parallel to improvement of symptoms in major depression patients [36].
(IV) There is increasing evidence that some primary genetic risk factors for psychiatric disorders impact on immune functions. Most common psychiatric disorders are highly polygenic. Genome-wide association analysis of 5 common psychiatric disorders including schizophrenia, bipolar disorder, depression, ADHD, and autism identified genes in immune pathways as significantly contributing to risk [37]. The strongest and consistent association of SNPs for immune response and synaptic plasticity is associated with HLA region [38]. Recently, the most significant GWAS hit for schizophrenia risk (rs13194053), a SNP in the major histocompatibility complex region [39], was shown to exert risk in part through impacting on the gene encoding complement 4 (C4) [40]. Some rare but recurrent copy-number variants (CNVs) associated with mental illness also impact on immune functions, including the 22q11.2 deletion associated with velo-cardio facial syndrome, a topic we will return to below.
(V) Conversely, patients with CNS inflammation caused by infection or brain injury can develop symptoms similar to those seen in psychiatric patients. In a study of 83 patients with viral encephalitis 67% of them were presented with psychomotor agitation, 55% with drowsiness, 47% with disorientation, and 43% with visual hallucinations and 34% showed aggressiveness as a symptom. One year after hospital treatment in 70 of the same patients, 16% had memory disorders, 9% showed signs of aggressiveness, and 8% suffered from aphasia, 8% from visual hallucinations, and 7% from auditory hallucinations [41]. Brucellosis, typhoid fever, Lyme disease, Leptospirosis, and Whipple's disease are all examples of inflammatory diseases caused by infection that can be accompanied by psychiatric symptoms ranging from depression, mania, personality trait, and behavioral changes to manifest psychosis [42]. Regarding inflammation during healing after trauma, a survey of 722 outpatients after brain injury with an average of 10 days' unconsciousness after injury found a rate of DSM-IV valuable diagnosis of depression in 42% of patients [43]. While it is not possible to fully disentangle the causal direction in these associations, they are consistent with the view that infection and brain injury may lead to psychiatric symptoms via the triggering of inflammatory processes.
(VI) Increasing number of studies support the hypothesis that autoantibodies that bind mainly to neuronal membrane protein or synaptic proteins result in psychiatric disorders. To date, the clear causative role of autoantibodies on psychotic symptoms has been most clearly shown for the N-methyl-D-aspartate receptor (NMDA-R), although further studies are still needed [44–46]. Similarly autoantibodies against dopamine-2 receptor, which regulates movement and behavior, have been associated with paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection and subjects with Tourette's syndrome [47]. Autoantibodies against antiopioid receptor, 5-hydroxytryptamine receptor 1A, and muscarinic cholinergic receptor 1 are thought to induce a depressive state in psychiatric illness [48, 49].
(VII) Activation of inflammation in animal models leads to behavioral abnormalities in a range of experiments. In rodent models, peripheral injection of inflammation-provoking lipopolysaccharide (LPS, 0.83 mg/kg) has been shown to generate sickness/depression-like symptoms, that is, prolonged period of immobility in forced swim test and tail suspension test. This effect was ascribed to an activation of the tryptophan-degrading enzyme indoleamine 2,3-dioxygenase [50]. Injecting animals with proinflammatory cytokines like IL-1a or TNF-α has also been shown to lead to sickness behavior in a dose- and time-related manner [51]. LPS injection to pregnant rats could be demonstrated to lead to pronounced changes of the offspring's brain development: cortical and hippocampus thickness were found increased and markers for neural and glia progenitors decreased and abnormally distributed over the brain regions. This was accompanied by an inflammation reaction within the brain, as indicated by the elevated presence of cytokines [52]. It has been suggested that microglia activation through LPS-stimulation may lead to a damage of oligodendrocyte progenitor cells in a way that ultimately leads to severe losses of oligodendrocytes and myelination during development [53]. It is remarkable in this context that immune system stimulating drugs like β-interferon have been found to induce psychiatric symptoms, for instance, in hepatitis C treatment [54, 55].
All the above points support the hypothesis that inflammation plays a significant role in the pathophysiology of major psychiatric disorders. However it remains unclear to what extent immune changes are primary in the causation of these conditions and to what extent they are a consequence of brain pathology.
Finding pathways that can initiate neuroinflammation may help us find mechanistic key links between prolonged stress exposure or traumatic life events in relation to the development of a psychiatric disease. For instance, overexcitation of neurons in epilepsy has been demonstrated to activate microglia and inflammation [56]. If the same observation holds true for situations of sensory overload, experienced as stress and emotionally overwhelming feelings, remains to be elucidated. Similarly, it is unclear if metabolic changes, like changes in circulation or hormone levels and receptors in straining situations, lead to oxidative stress in the brain, which would in turn provoke neuroinflammation.
The identification of the molecular mechanisms that connect inflammation and altered behavior will be important in the development of new treatments for neuropsychiatric disorders. Some emerging studies hypothesize that synaptic damage seen in psychiatric disorders may in part be a consequence of neuroinflammation, reflecting the situation seen in other conditions such as multiple sclerosis. Importantly, dysfunctional synaptic activity is associated with high activity of glutamatergic synapses and loss of GABAergic synapse function in schizophrenia and autism spectrum disorders. This imbalance seems to be provoked through cytokines that are upregulated during inflammation, very much like the ones we see in psychiatric disorders, supposedly released by activated microglia [57]. In most cases, inflammation within the CNS can activate an array of cellular and molecular changes including activation of astrocytes, oligodendrocyte dysfunction, and mitochondrial damage, changes in neurogenesis and neural circuits, and a general imbalance of neurotransmitters with a surplus of dopaminergic and a loss of glutamatergic neurotransmission, and it is also crucial for synaptic plasticity and pruning. Arguably, in concert these alterations can then lead to an impairment of behavioral functioning like cognition or emotion regulation [58].
3. Microglial Pathology in Psychiatric Disorders
At a cellular level, microglia and astrocytes regulate the central nervous immune response. During the early embryonic brain development, microglia are the first glial cells to develop alongside neurons [59] and they constitute ≅10% of total glial cells in the adult brain [60]. Besides contributing to neuronal function [61, 62] microglia regulate the CNS response to antigens and inflammation [63], accompanied by cytokine and chemokine expression [64–67]. Following damage or stress, microglia located in intact areas elicit an immune response on the affected area by extending their processes. Microglial processes are highly dynamic and can sense the released ATP secreted from astrocytes and neurons in the damaged area [68]. Following activation microglia secrete two proinflammatory cytokines (IL-1β and TNFα), chemokines (CCL3, CCL5), and reactive oxygen/nitrogen species (nitric oxide, peroxides, superoxide, and peroxynitrite) (Figure 1) [69–71]. In turn, this neuroinflammation may cause damage to neurons and other glial cells. Aging is another key factor that influences the status of microglial state and the associated inflammatory condition. The gene expression profile in the aged brain indicates a clear upregulation of immune-related genes, including enhanced expression of IL-1β, TNFα, and IL-6 [72], suggesting that age related changes also lead to enhanced activation of microglial cells and supposedly inflammation, in the absence of external damage.
Besides being the first line of defense in the mammalian brain, microglia are important for synaptic formation and synaptic pruning [62, 73]. Microglia derived IL-10 has been shown to promote synapse formation. Reduction in the level of microglia during the postnatal stage or young adult stage of development results in reduced spine formation in the motor cortex. This reduction in synapse formation is associated with reduced motor performance and reduced levels of synaptic proteins like GluN2B and vGlut1 [74]. These studies suggest that microglia are important for learning and memory by promoting learning associated synapse formation [75]. A recent study has demonstrated early life infection can lead to an increase in microglial derived IL-1β level, which in turn modulates hippocampus-dependent learning and memory [76]. This observation is further supported by animal studies, where neonatal bacterial infection of rats induces hippocampus-dependent memory deficits in adulthood [77]. Microglia also eliminate nonfunctional synapses through the fractalkine receptor (CX3CR1) in the hippocampus [78, 79]. In retinal ganglion cells they regulate pruning through the C3 complement pathway [80]. In a recent study it was noted that increased expression of the C4 gene, encoding complement 4, was associated with high risk of schizophrenia. In C4-deficient mice, C4 promotes C3 activation which targets subsets of synapse elimination by microglia, suggesting its involvement in synaptic pruning [40].
Furthermore, TGF-beta signaling has been demonstrated to regulate microglial mediated pruning [81]. Microglia also modulate the sleep-wake cycle through extraction and retraction of the process [82]. It has been hypothesized that the microglial circadian clock might play a causative role in cognitive deficits and depression [83]. Exposure to environmental factors, especially during development, has been demonstrated to have profound effects on microglial state, inflammatory status of the brain, and cognition [84–86]. Although the underlying mechanism behind this in the context of neuropsychiatric disorders is poorly understood, fractalkine signaling at least in part seems to mediate microglial response to environmental risk factors [87].
Given the important role that microglia play in normal brain development and neuronal homeostasis, it is not surprising their dysfunction leads to neuropsychiatric disorders. Recent postmortem studies and PET imaging of peripheral benzodiazepine receptors provide evidence for microglial activation in the brains of patients with neuropsychiatric disorders such as schizophrenia, depression, and autism [88–95]. In schizophrenia, a significant increase in microglial density along with signs of activation in prefrontal and visual cortex has been reported [96, 97]. These results are highly promising, even though more specific markers of microglial activation are still needed to confirm these findings. One possible candidate for that might be translocator protein 18 kDa (TSPO), as decreased radiotracing was associated with schizophrenia-like behavior and inflammatory cytokine elevation in a mouse model [98]. Furthermore, in animal models of Rett syndrome (RTT), a devastating neurodevelopmental disorder, which is caused by a mutation in the X-linked MECP2 gene encoding methyl-CpG-binding protein 2, microglia release an abnormally high level of glutamate, causing excitotoxicity that may contribute to dendritic and synaptic abnormalities in RTT [99]. Furthermore, in Mecp2-null mice a reduced number of microglia, which fail to phagocytose debris as effectively as those in wild-type mice, were found. These finding suggest that microglia may also be responsible for the disorder [100]. Stress-induced depressive-like condition in rodents has shown to be caused by reduced levels of microglia within the hippocampus and reduced expression of activation markers, which in turn might contribute to reduced hippocampal neurogenesis and increased depressive-like behavior [101]. Microglia expressing glucocorticoid receptors have also been shown to undergo extreme alterations when exposed to prolonged stress, like cell body shrinkage and debris accumulation in lysosomes [102].
Sustained microglial activation has been shown to contribute to the alleviation of symptoms associated with neurological disorders including psychiatric disorders (Figure 1) [103]. Enhanced proinflammatory cytokine response in concert with microglia activation occurs in response to external immune challenges and has been associated with cognitive and behavioral deficits in rodents [104–106]. As described earlier, such associations between proinflammatory cytokine levels in blood and psychiatric disorders are numerous in humans. It is feasible that microglia, the most important proinflammatory secreting cells in the brain, might be responsible for or at least involved in this increase in blood cytokine levels. It is interesting in this context that microglia also are very important postnatal development defining cells [107]. It is plausible to speculate that microglial state and functionality in the context of inflammation may be altered in patients with a genetic susceptibility to psychiatric disorder tighter with environmental factors? With induced pluripotent stem cell based disease modeling, one way to further investigate this question would be to derive microglia with a known genetic high-risk profile for psychiatric disorders, which will help to understand the mechanisms and triggers involved behind pathological inflammation. The potential mechanism of microglial activation that occurs during the course of psychiatric disorders is illustrated in Figure 1.
4. High-Risk Copy-Number Variants (CNVs): A Link between Genetic Alteration, Psychiatric Disorders, and Inflammation?
A strong association has been found between specific rare but recurrent chromosomal CNVs and psychiatric disorders, including schizophrenia, autism spectrum disorder, and mood and anxiety disorders [108]. Microarray experiments have now revealed abundant copy-number variations, a type of genetic variations in which stretches of DNA are duplicated or deleted [109, 110]. These structural alterations can range from 1 kilobase to several megabases and include either a single gene or contiguous sets of genes. CNVs can control the underlying psychiatric phenotype in multiple ways. They can affect the gene expression level by gene dosage effects or act as a faulty regulatory element to gene transcription cascades affecting other gene loci. They can also lead to frame shifts resulting in an abnormal genetic fusion product [111]. The mechanisms by which CNVs can lead to neuropsychiatric disorders and underlying complex behavioral traits are perhaps most clearly shown by rare and highly penetrant CNVs, involving the loss, gain, or disruption of a dosage-sensitive gene(s).
Some of the most common CNVs associated with neuropsychiatric disorder include chromosomal regions 1q21.1, 3q29, 15q11.2, 15q13.3, 16p11.2, 16p13.1, and 22q11 [112]. The locus 1q21.1 is associated with duplications in autism and with deletions or duplications in mental retardation [113–115]. The 15q13.3 locus is associated with deletions in mental retardation with seizures and deletions or duplications in autism and other neuropsychiatric disorders [116–118]. The most common microdeletion syndrome in humans, 22q11.2, results in an increased rate of a range of psychiatric disorders in children with other developmental and intellectual disabilities [119–122]. A 15q13.3 microdeletion in humans results in a range of neurodevelopmental/psychiatric disorders, including autism spectrum disorder, schizophrenia, epilepsy, and intellectual disability [116–118, 123–131]. Microduplication or deletion at the 16p11.2 loci is associated with schizophrenia, bipolar disorder, mental retardation, autism, seizure disorders, and psychosis in Alzheimer's disease [132–135]. De novo CNV analysis has resulted in enrichment of genes that are associated with the NMDAR network, GABAA receptor, abnormal CNS synaptic transmission, abnormal learning/memory/conditioning, and abnormal cued conditioning behavior [112, 136–139]. However, these studies have not picked up genes that can influence inflammatory condition underlying psychiatric symptoms.
There is strong evidence that deletions at 22q11.2 directly impact immune function. Thymic hypoplasia is a common feature associated with 22q11.2 deletions resulting in CD4+CD45RA+ T cell counts, which can result in immunodeficiency [140]. A recent study looking at a wide range of cytokines in patients with 22q11.2 deletions demonstrates a significant increase in the serum levels of proinflammatory and angiostatic chemokine IP-10 in patients with 22q11.2 deletions compared to healthy individuals. The increased IP-10 profile was associated with conotruncal congenital heart defects [141]. It is less certain how these immune changes might relate to the psychiatric symptoms seen in the disorder, but a positive association between severity of autism-related behaviors and level of serum concentrations of inflammatory cytokines IL-1β, interferon gamma, and IL-12p70 in individuals with 22q11.2 deletions has been reported [142]. Furthermore, toddlers with 22q11.2 deletion have an elevated level of CD3+, CD4+, and IL-4-IFN-γ+ lymphocytes as compared to healthy control, suggesting that 22q11.2 deletion is associated with dysregulated Th1 cytokine production such as IL-4 and IFN-γ [143]. However, if the dysregulated Th1 cytokine production is also present in adulthood needs to be investigated. A recent study looking at cellular modeling of neurodevelopment by differentiating of hiPSCs carrying 22q11.2 deletions into neuronal lineage reports a reduction in the level of neural differentiation propensity and neurite outgrowth and migration as compared to controls [144]. This phenotype was further associated with reduced expression level of miR-17/92 cluster and miR-106a/b cluster. A number of studies have established direct linkage of these clusters with dysregulated inflammation [145–147], suggesting that the underlying psychiatric symptoms associated with 22q11.2 deletions is at least in part associated with altered inflammatory state and the subjects are prone to an increased risk for a variety of autoimmune diseases [148].
TNF-α was found to be downregulated in expression in cells carrying the 15q13.3 microdeletion. TNF-α is known as a potent activator of inflammation [149]. It is plausible to speculate that genes that are involved in the 15q13.3 duplication are capable of altering TNF-α expression leading to an altered inflammatory state of the central nervous system. The CHRNA7 gene is one of the genes in 15q13.3 deletions. It encodes the nicotinic acetylcholine receptor alpha 7 subunit (α7nAChR), which is associated with schizophrenia in clinical studies and rodent models. A recent study using immortalized lymphoblastoid cell lines with 15q13.3 homozygous deletion has shown the CHRNA7 gene to modulate the production of TNFα. Furthermore, they identified STAT3 as one of the four genes that are differentially expressed following 15q13.3 deletions [149]. It has been demonstrated that STAT4 null mice showed impaired IL12-mediated functions [150]. IL-2 is an activator of the nitric oxide synthase [89], which has been demonstrated to be important for synaptic plasticity [151] suggesting that 15q13.3 deletion might modulate the synaptic plasticity through the STAT4/IL12/NOS pathway.
5. Modulating Neuroinflammation to Treat Psychiatric Disease
5.1. Theories on Pharmacological Effects of Anti-Inflammatory Drugs in Psychiatric Disorder
A number of drugs with anti-inflammatory features have been investigated either retrospectively or prospectively in terms of potential impacts on psychiatric symptoms including nonsteroidal anti-inflammatory drugs (NSAIDs) and the tetracycline related antibiotic minocycline. Aspirin, celecoxib, and the NSAID group of anti-inflammatory drugs, encompassing commonly prescribed drugs such as diclofenac, ibuprofen, or indomethacin, work by blocking the immune cascade enzyme cyclooxygenase (COX) [118], which result in reduced inflammation. COX is responsible for the translation of arachidonic acid into prostaglandins. COX-1, which is antagonized by classical NSAIDs, including diclofenac, ibuprofen and aspirin, is expressed ubiquitously in the body in addition to its role in inflammation. Celecoxib is a selective COX-2 inhibitor. COX-2 is mainly expressed during inflammation, but also in the brain and kidney [152]. In a rat model of neuroinflammation, created by injection of LPS, treatment with celecoxib significantly decreased the rate of microglia and astrocyte coactivation together with levels of IL-1β. Dopaminergic neurons and astrocytes with an upregulation of COX-2 were found in diminished numbers in treated individuals as compared to controls [153]. Inhibition of COX-2 has also been shown to lead to decreased production of kynurenic acid, which is a glycine antagonist at the NMDA-receptor and a nicotinic receptor antagonist. It has been found in elevated concentrations in schizophrenia patients' cerebrospinal fluid [154–156]. For minocycline, multiple effects seem to make up its anti-inflammatory character. In models of neuroinflammation, minocycline has been shown to inhibit activation and proliferation of microglia, as well as antagonizing important proinflammatory enzymes like COX-2, iNOS, NAPDH-oxidase, and P38 MAPK. It also seems to reduce T-leukocyte migration to the CNS by inactivation of MMP-9 (the enzyme rendering the brain-blood barrier permissive during inflammation).
In models of neurodegenerative disease, survival of neurons and glia cells can be increased through minocycline, through caspase dependent and independent mechanisms [157] (Figure 2). Little is known on why these drugs also work in psychiatric disorder, but one might speculate that the same cascades and factors of inflammation, notably microglia, are affected. We have summarized the clinical trials of compounds that aim to reduce the proinflammatory mediators in psychiatric disorders (Table 2).
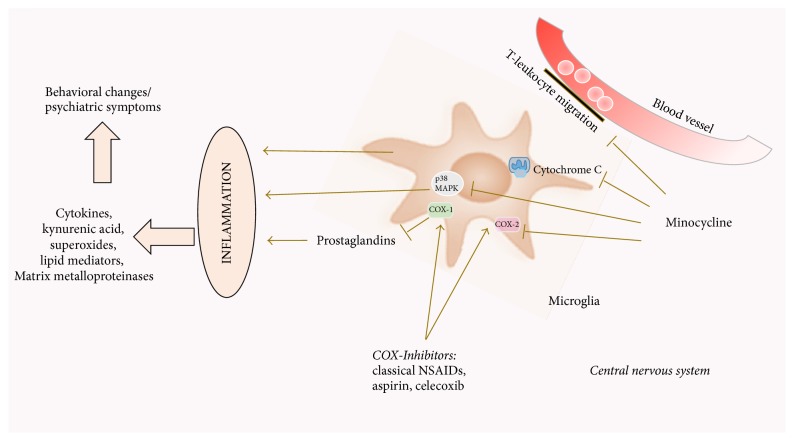
Figure 2.
Pictorial diagram summarizing the effects of anti-inflammatory medication on neuroinflammation mediated by microglia. Inflammation, amplified through the activation of microglia, leads to the release of cytokines, kynurenic acid, superoxides, lipid mediators, and matrix metalloproteinases. These molecules may contribute to the pathological changes seen in neuropsychiatric conditions. Anti-inflammatory drugs like cyclooxygenase inhibitors block the prostaglandin-mediated cascade of this inflammation. Minocycline acts via (i) inhibition of T-leucocyte migration over the blood-brain barrier, (ii) cytochrome c inhibition in mitochondria, (iii) antagonism of MAPK p38 and subsequent products, and (iv) inhibition of COX-2 (COX: cyclooxygenase, MAPK: mitogen activated protein kinase).
Table 2.
Clinical studies on use of anti-inflammatory drugs in treatment of psychiatric disorder. N: proband number, T: time frame, PANSS: Positive and Negative Symptom Score, SANS: Schedule for the Assessment of Negative Symptoms, and CGI score: clinical global impression score.
| Treatment | Psychiatric disorder | Probands | Trial design | Outcome | Authors |
|---|---|---|---|---|---|
| Aspirin 1000 mg/day plus 40 mg/day pantoprazole, in addition to antipsychotic treatment | Schizophrenia | Patients with at least moderate symptom burden and a DSM-IV diagnosis for schizophrenia spectrum disorder for under 10 years N = 70 |
Randomized controlled trial T = 3 months |
Mixed model improvement in overall psychopathology and in positive symptoms as measured by PANSS No differences in negative symptom or general scores |
Laan et al., 2010 [158] |
|
| |||||
| Aspirin 1000 mg/day plus 40 mg/day pantoprazole, in addition to antipsychotic treatment | Schizophrenia | Patients with moderate or above on CGI score, moderate score on two of PANSS items: delusions, hallucinatory behaviors, conceptual disorganization or suspiciousness/persecution, and/or a total PANSS negative symptoms score above 18 N = 400 |
Post hoc analysis of randomized controlled trial T = 16 weeks |
Improvements in patients with high CRP levels | Weiser et al., 2014 [159] |
|
| |||||
| Celecoxib 200 mg bid in addition to 2–6 mg/day risperidone | Schizophrenia | Patients with acute exacerbation of schizophrenia N = 50 |
Double-blind, Randomized Placebo-controlled T = 5 weeks |
Significant improvement of positive symptoms | Müller et al., 2002 [160] |
|
| |||||
| Celecoxib in addition to olanzapine | Schizophrenia | Patients with acute exacerbation of schizophrenia N = 94 |
Open-label, prospective, controlled T = 6 weeks |
Improvement of positive, negative, and general psychopathology and total scores as measured by PANSS | Baheti et al., 2013 [161] |
|
| |||||
| Celecoxib (200 mg bid) in addition to risperidone (200 mg/day) | Schizophrenia | inpatients diagnosed with active phase schizophrenia N = 60 |
Double-blind randomized placebo-controlled T = 8 weeks |
Significant improvement regarding positive symptoms as measured by PANSS | Akhondzadeh et al., 2007 [162] |
|
| |||||
| Celecoxib | Schizophrenia | Patients with a first manifestation of schizophrenia N = 49 |
Double-blind randomized placebo-controlled T = 6 weeks |
Significant improvement regarding positive and negative symptoms as measured by PANSS | Müller et al., 2010 [163] |
|
| |||||
| Celecoxib (400 mg/day) in addition to antipsychotic treatment | Schizophrenia | Outpatients with a DSM-IV diagnosis of schizophrenia, experiencing persistent symptoms despite treatment for 3 months N = 38 |
Double-blind, randomized, placebo-controlled, T = 8 weeks |
No difference in outcome, as measured by PANSS | Rapaport et al. 2005 [164] |
|
| |||||
| Concomitant intake of NSAID or paracetamol | Schizophrenia | Schizophrenia patients N = 2000 |
Retrospective investigation | Higher risk for relapse to active psychosis | Köhler et al., 2016 [165] |
|
| |||||
| Previous NSAID use | Schizophrenia | Patients prescribed antipsychotics 82 cases, 359 controls |
Case-control trial of antipsychotics prescription | Significant reduction of risk to develop psychosis when NSAID had been taken, but only in male individuals | Laan et al., 2007 [166] |
|
| |||||
| Use of COX-2 inhibitors | Schizophrenia | Case events indicating schizophrenia exacerbation in patients using antipsychotics N = 1443 |
Nested case-control study T = previous 93 days |
No increase of risk for schizophrenia exacerbation | Stolk et al., 2007 [167] |
|
| |||||
| Minocycline in addition to usual treatment | Schizophrenia | Patients with early-stage schizophrenia N = 94 |
Double-blind, randomized, placebo-controlled T = 1 year |
Significant improvement of negative symptoms as measured by PANSS | Chaudhry et al., 2012 [168] |
|
| |||||
| Minocycline (200 mg/day) in addition to risperidone | Schizophrenia | Patients with DSM-IV diagnosis of early-phase (less than 5 years) schizophrenia who had been on a steady dosage of risperidone N = 93 |
Double-blind, randomized, placebo-controlled T = 16 weeks |
Significant improvement in treatment response in the Scale for the Assessment of Negative Symptoms (SANS) total scores and PANSS for negative symptoms | Liu et al., 2014 [169] |
|
| |||||
| Minocycline (200 mg/day) | Schizophrenia | Patients with DSM-IV diagnosis of schizophrenia, no treatment with therapeutics for one week before start of trial N = 43 |
Randomized, placebo-controlled T = 8 weeks |
Significant decrease of SANS score after 8 weeks (though not 4 weeks) of treatment | Ghanizadeh et al., 2014 [170] |
|
| |||||
| Minocycline (up to 200 mg/day) in addition to risperidone (up to 6 mg/day) | Schizophrenia | Chronic schizophrenia patients N = 40 |
Double-blind, randomized, placebo-controlled T = 8 weeks |
Significant improvement of negative symptom as measured by PANSS | Khodaie-Ardakani et al., 2014 [171] |
|
| |||||
| Minocycline | Schizophrenia | Early-phase schizophrenia patients N = 54 |
Double-blind, randomized, placebo-controlled T = 6 months |
Significant improvement of negative symptoms and general outcome measured by PANSS, Clinical Global Impression (CGI) and insight score, and improvements of cognitive executive functions | Levkovitz et al., 2009 [172] |
|
| |||||
| NSAID and paracetamol | Bipolar disorder | DSM-IV-TR diagnosis of bipolar disorder I or II and at least mild symptoms N = 482 |
Secondary analysis from the Bipolar CHOICE study T = 6 months |
No difference as measured by CGI-BP | Köhler-Forsberg et al., 2017 [173] |
|
| |||||
| Aspirin | Bipolar disorder | Patients taking lithium with erectile dysfunction N = 32 |
Double-blind, randomized, placebo-controlled T = 6 weeks |
No differences in mania or depressive symptoms, significant improvement of erectile dysfunction | Saroukhani et al., 2013 [174] |
|
| |||||
| Aspirin, low-dose of 30 or 80 mg per day and for an unspecified time or for more than 1, 45, 90, or 180 days, in addition to lithium | Bipolar disorder | Patients with at least five previous prescriptions for lithium and at least 1-year drug history N = 5145 |
Pharmacoepidemiological study on the PHARMO Record Linkage System (RLS) in the Netherlands T = 10 year period |
Significantly fewer events of mood-stabilizer treatment alteration | Stolk et al., 2010 [175] |
|
| |||||
| Celecoxib (400 mg/day) | Bipolar disorder | DSM-IV diagnosis of bipolar disorder during a depressive or mixed episode of the disease N = 28 |
Double-blind, randomized, placebo-controlled T = 6 weeks |
Improvement of depressive symptoms (as measured by Hamilton Depression Rating Scale) found in the first week | Nery et al., 2008 [176] |
|
| |||||
| Celecoxib in addition to sodium valproate | Bipolar disorder | Patients with diagnosis of acute bipolar mania with manic episode without psychotic features N = 46 |
Double-blind, randomized, placebo-controlled T = 6 weeks |
Significantly higher remission rates as measured by young mania rating scale | Arabzadeh et al., 2015 [177] |
|
| |||||
| Concomitant NSAID use in addition to escitalopram or nortriptyline | Depression | Patients with major depression disorder treated in the GENDEP study N = 811 |
Retrospective on the GENDEP study T = up to 12 weeks |
No change of serotonin reuptake inhibitor treatment | Uher et al., 2012 [178] |
|
| |||||
| Previous aspirin use as anti-platelet treatment | Depression | Men of older age (69–87 years) N = 5273 |
Retrospective analysis of aspirin use after assessment of mood disorder symptoms T = last 5 years |
Significantly higher risk for depression in individuals who had used aspirin as antiplatelet agent drug in the past, but had stopped the treatment before the mood assessment (measured by Geriatric Depression scale) | Almeida et al., 2010 [179] |
|
| |||||
| Regular aspirin or statin use in the past | Depression | Depression patients N = 1631 |
Population-based study T = last 6 months |
No higher risk for development of depression | Glaus et al., 2015 [180] |
|
| |||||
| Celecoxib in addition to antidepressants | Depression | Patients with depressive episodes N = 150 |
Meta-analysis of 4 randomized controlled trials | Improvements in Hamilton Rating Scale for Depression scores, remission and response rate | Na et al., 2014 [181] |
|
| |||||
| Diclofenac (50 mg bid) versus celecoxib (200 mg bid) | Depression | Outpatients with breast cancer and concomitant depression N = 52 |
T = 6 weeks | Significant improvements by HDSR score in both, celecoxib significantly more successful than diclofenac, analgesic effect comparable | Mohammadinejad et al., 2015 [182] |
|
| |||||
| Celecoxib (200 mg bid) | Depression | Patients with depression due to brucellosis N = 40 |
Double-blind, randomized, placebo-controlled T = 8 weeks |
Significant improvements on HDRS | Jafari et al., 2015 [183] |
|
| |||||
| Celecoxib (200 mg bid) or sodium naproxen (220 mg bid) | Depression | Individuals older than 70 years and with a family history of Alzheimer's disease (though without any cognitive dysfunction) N = 2312 |
Alzheimer's Disease Anti-Inflammatory Prevention Trial Yearly follow-up |
No significant change in individual or overall depression score measured by 30-item Geriatric Depression Scale | Fields et al., 2012 [184] |
|
| |||||
| Minocycline (150 mg/day) as adjunctive therapy to fluvoxamine, paroxetine, or sertraline | Depression | Inpatients with a DSM-IV diagnosis of major depression with psychotic features N = 25 |
Open-label study T = 6 weeks |
Significant improvement of depressive and psychotic symptoms as measured by Hamilton Depression Rating Scale and Brief Psychiatric Rating Scale | Miyaoka et al., 2012 [185] |
|
| |||||
| 100 mg bid minocycline | Depression | HIV positive patients with mild-to-moderate scores of depression N = 46 |
Double-blind, randomized, placebo-controlled T = 6 weeks |
Significant reduction of depression score (Hamilton Depression Rating Scale) | Emadi-Kouchak et al., 2016 [186] |
|
| |||||
| Celecoxib 200 mg bid in addition to fluvoxamine | OCD | OCD outpatient N = 50 |
Double-blind, randomized, placebo-controlled T = 10 weeks |
Significant improvement of symptoms | Shalbafan et al., 2015 [187] |
|
| |||||
| N-Acetylcysteine (up to 2400 mg/day) | OCD | OCD patients, nonresponsive to serotonin reuptake inhibitors N = 48 |
Double-blind, randomized, placebo-controlled T = 12 weeks |
15% higher response rate | Afshar et al., 2012 [188] |
|
| |||||
| Minocycline (100 mg/day) in addition to serotonin reuptake inhibitor | OCD | Treatment-resistant outpatients with OCD N = 9 |
Open-label study T = 12 weeks |
No significant improvements in outcome in cohort as a whole, more than 30% reduction of the Yale–Brown Obsessive Compulsive Scale | Rodriguez et al., 2010 [189] |
|
| |||||
| Minocycline (100 mg bid) as add-on to fluvoxamine (100 mg/day for the first two 200 mg/day for the remaining 6 weeks of the trial) | OCD | OCD patients N = 102 |
Double-blind, randomized, placebo-controlled T = 10 weeks |
Significantly higher treatment response rate (either complete or partial) measured by the Yale-Brown Obsessive compulsive scale. Specifically patients scored significantly lower in total and on the obsessive subscale | Esalatmanesh et al., 2016 [190] |
|
| |||||
| Celecoxib (up to 300 mg/day) in addition to risperidone | Autism | Children with autistic disorder N = 40 |
Double-blind, randomized, placebo-controlled T = 10 weeks |
Significant improvements of scores in irritability, social withdrawal, and stereotypy | Asadabadi et al., 2013 [191] |
|
| |||||
| Minocycline (1.4 mg/kg body weight) | Autism | Children with autism spectrum disorder N = 11 |
Open-label T = 6 months |
No clinical improvements (Clinical Global Impression Severity Scale, Clinical Global Impression Severity Scale Improvement, and Vineland Adaptive Behavior Scales) | Pardo et al., 2013 [192] |
5.2. Schizophrenia
In schizophrenic patients, several clinical studies exist on adjunctive treatment with drugs with anti-inflammatory features.
When 70 patients with at least a moderate symptom burden and a DSM-IV diagnosis for schizophrenia for under 10 years were treated with aspirin or placebo in addition to antipsychotic treatment, measurements by Positive and Negative Symptom Score (PANSS) showed an improvement in overall psychopathology and in positive symptoms. However, no differences in negative symptom or general scores were found [158]. In a similar study add-on aspirin given to patients improved symptoms, however only in patients with high CRP serum levels [159]. In patients with an acute exacerbation of schizophrenia treatment with cyclooxygenase-2 inhibitor, celecoxib in addition to risperidone led to a significant improvement of positive and negative symptoms in excess of the relief assignable to the antipsychotic [154, 161–163]. On the other hand, when patients who had suffered from symptoms for at least three months despite treatment, rather than patients with an acute exacerbation of psychosis, were treated with celecoxib in a similar study design, no difference in outcome was found [164]. So it might be that the effect of celecoxib add-on treatment in schizophrenia patients is dependent on the duration of the disorder. Looking at anti-inflammatory drugs as a possible risk or protective factor for psychiatric disorder, in a retrospective investigation looking at more than 2000 schizophrenic patients who had concomitantly taken nonsteroidal antirheumatic drugs or paracetamol, researchers found a higher risk for relapse to active psychosis. It is not clear whether this may be simply assignable to the parallel comorbidity treated with nonsteroidal anti-inflammatory drugs (NSAIDs) or the drug use per se [165]. Then, again, previous NSAID use was examined in a case-control trial (82 cases, 359 controls) of antipsychotics prescription. This showed a significant reduction of risk to develop psychosis when NSAID had been taken, but only in male individuals [166]. A nested case-control study of 1443 case events indicating schizophrenia exacerbation in patients using antipsychotics looked back at recent use of COX-2 inhibitors. The risk for schizophrenia exacerbation was not increased when the anti-inflammatory drug had been taken beforehand (for 0–93 days) [167].
Double-blind, randomized placebo-controlled studies showed that adjunctive treatment with the tetracycline minocycline to usual treatment could significantly improve negative symptoms [156, 168, 170]. Though unchanged by the treatment with minocycline, positive symptom score seems to be predictor for that improvement of negative symptoms [171]. Furthermore, minocycline seems to have positive effects on cognitive executive functions, that is, working memory, cognitive shifting, and planning [172]. A proportion of schizophrenic patient respond to clozapine. In a recent study positive outcome along with improved cognitive outcome has been reported when patient received minocycline together with clozapine in a 10-week double-blind, placebo-controlled trial involving 52 patients [193]. So minocycline add-on therapy seems especially interesting in negative and cognitive symptom treatment of schizophrenia.
Although the above data are interesting, it can only reflect implication of inflammation in pathogenesis of schizophrenia. Therefore, randomized prospective trials with a larger sample size and longer follow-up duration are crucial to establish anti-inflammatory therapy as an effective, safe, and tolerable add-on treatment in schizophrenia.
5.3. Bipolar Disorder
In bipolar disorder, investigations of proinflammatory cytokines, namely, TNF-α, INF-γ, IL-6, and high sensitivity CRP, found elevated levels in a cohort of 30 patients in acute mania as compared to healthy controls. Most significantly, only levels of CRP were decreased after treatment with antipsychotic drugs (± electroconvulsive therapy) while patient showed good outcomes [14]. In a study originally aiming at the examination of a potential drug blocking effect of NSAID on treatment with mood-stabilizers no difference in outcome for bipolar patients was found with and without NSAID [173].
A trial on the effect of aspirin on lithium-using patients with erectile dysfunction found no differences in depressive or mania symptoms before and with the add-on treatment [174]. Nevertheless, aspirin had beneficial effects in a pharmacoepidemiological studies looking at mood-stabilizer substance change or dosage increase in patients on lithium (interpreted as worsening of bipolar disorder) in correlation to the use of further drugs. Prescribed at a low-dose of 30 or 80 mg per day and for an unspecified time or for more than 1, 45, 90, or 180 days, the patients, picked from the nationwide Netherlands PHARMO Record Linkage System, had significantly fewer events of mood-stabilizer treatment alteration [175].
In 2008 Nery et al. investigated the effect of celecoxib treatment in 28 patients with a DSM-IV diagnosis of bipolar disorder during a depressive or mixed episode of the disease. The study was randomized, double-blind, and placebo-controlled and celecoxib was given out at a dosage of 400 mg per day over 6 weeks. This was in addition to a stable treatment with mood-stabilizers or atypical antipsychotics. Among the patients who finished the complete trial, a significant improvement of depressive symptoms (as measured by Hamilton Depression Rating Scale) could be found in the first week of celecoxib treatment [176]. Similarly, a study with celecoxib or placebo in 46 patients, in addition to sodium valproate treatment, showed that remission rates were significantly higher with the adjunctive treatment than without assessed by the young mania rating scale (YMRS) following treatment of 6 weeks. At day 42, YMRS scores were significantly lower in the celecoxib group [177]. Trials over multiple centres for treatment of psychiatric disease with add-on minocycline and/or celecoxib/aspirin are currently ongoing [194, 195].
The above adjunctive anti-inflammatory therapy has more efficacy and comparable tolerability compared with control and future prospective studies with a longer study duration which are based on larger sample sizes are needed to comprehensively evaluate the efficacy and tolerability of anti-inflammatory therapy in bipolar disorder.
5.4. Major Depression Disorder
Treatment of major depression with NSAID has been tried reluctantly so far because of reports in which parallel NSAID or COX-2 inhibitor treatment of comorbidity lead to supposed drug interaction and higher resistance level to antidepressant treatment [196]. However, there is a number of noteworthy, even if sometimes contradictory, clinical trials that frequently come from investigations of anti-inflammatory comorbidity treatment. In a study on the effect of NSAID use in addition to escitalopram or nortriptyline treatment in a cohort of 811 major depression patients no significant effect of NSAID use on the outcome of the antidepressant therapy was found [178]. An Australian study in over 5000 men of older age (69–87 years) investigated the correlation between aspirin use and development of depressive symptoms. Individuals who had used aspirin as antiplatelet agent drug in the past but had stopped the treatment before the mood assessment were at a significantly higher risk for higher scores of depression [179]. However, a population-based study of 1631 patients could not find any lower rates of depression in patients who had taken aspirin on a regular basis [180].
A meta-analysis of 4 randomized controlled trials with celecoxib adjunctive to treatment with antidepressants was able to show consistent improvements in depression scores and remission and response rate [181]. Similarly, in 52 outpatients with breast cancer and concomitant depression a trial compared outcome after treatment with diclofenac versus celecoxib. At the endpoint, both treatments showed significant improvement as indicated by HDSR score. The celecoxib treatment was significantly more successful than the diclofenac treatment, while the analgesic effect was comparable [182]. Patients with depression due to Brucellosis showed significant response to treatment with celecoxib (200 mg bid) as measured by Hamilton Depression Rating Scale in a study on 40 patients over 8 weeks [183].
Then, again, in the Alzheimer's Disease Anti-inflammatory Prevention Trial, a study in more than 2000 individuals older than 70 years and with a family history of Alzheimer's disease (though without any cognitive dysfunction), the application of celecoxib or sodium naproxen was tested against placebo. In the beginning of the trial, around 20% of the test persons had an increased score for depression as measured by 30-item Geriatric Depression Scale. However, there was no significant change in that percentage over the trial, or in scores of individual probands [184]. Thus, one could argue that the effect of celecoxib might be age-dependent.
A 6-week open-label study in 25 inpatients with a DSM-IV diagnosis of major depression with psychotic features showed that minocycline (150 mg/day) as adjunctive therapy to fluvoxamine, paroxetine, or sertraline was beneficial. Both depressive and psychotic symptoms were significantly improved [185]. Similarly, HIV positive patients (n = 46) with mild-to-moderate scores of depression (Hamilton Depression Rating Scale) were treated with 100 mg bid minocycline or placebo in a double-blind randomized trial. A significant reduction of depression score was observed by the end of six weeks [186].
5.5. Anxiety/Anxiety Disorder
Even though to our knowledge there are no clinical trials with celecoxib, other NSAIDs, or tetracyclines in anxiety disorder patients to date, there are some interesting observations on anti-inflammatory manipulation of the immune system in animal models of anxiety symptoms. One trial in experimental autoimmune encephalitis in a rat model of multiple sclerosis showed that gene treatment with IL-10 diminished anxiety and depression symptoms, indicated by voluntary wheel running frequency [197]. In mouse models of anxiety after traumatic brain injury [198] and of anxiety following neonatal immune activation by infection treatment with minocycline led to a significantly positive effect on psychopathologic outcome [199].
A recent study provides evidence of high levels of IFN-γ and TNF-α but low IL-10 serum levels in anxiety patients when compared to healthy control subjects [200]. Furthermore, a large cohort study looking at the association between anxiety disorders and inflammation identified an elevated level of CRP in male patients with anxiety disorders [201]. In another study which included patients with rheumatoid arthritis receiving anti-TNF-α therapy had less frequent prevalence of any mood or anxiety disorders [202] demonstrating a beneficial role of anti-inflammatory therapy in anxiety disorders. Coumarin-derivate esculetin [203] as well as phenolic-rich Aronia melanocarpa berry juice attenuated anxiety and depressive symptoms in mice and rats, as measured during LPS-inducement and stress test, respectively. Both substances are known for their antioxidative power and it is thus feasible that the effect is assignable to an anti-inflammatory impact [204].
A synthetic derivative (4-phenylselenyl-7-chloroquinoline) with anti-inflammatory and antioxidative potential was shown to have anxiolytic effects in mice, as measured by elevated-plus maze and light dark tests. The quinolone was applied 0.5 hours prior to onset and anxiety-related behavior seemed avoided for up to 72 h. However, the authors of this study assigned the effect primarily to a change in glutamate levels provoked by the drug rather than anti-inflammatory effect [205]. The polyphenol Honokiol is primarily known as a drug used in traditional Chinese medicine. In mice, application of Honokiol (for 2 days at either 2 or 5 mg/kg i.p.) prior to induction of inflammation by LPS injection led to a decrease of anxiety levels, as measured by behavioral testing using the elevated-plus maze and open field test. Furthermore, a significant decrease in plasma levels of IL-1β, IL-6, TNF-α, and BDNF was found in those mice pretreated with Honokiol as well as a liver-protective effect [206]. Of note, physical exertion is shown by several lines of evidence to have anti-inflammatory effects and at the same time is one of the most effective nonpharmaceutical remedies of anxiety [207].
5.6. Obsessive-Compulsive Disorder
In a double-blind randomized trial in 50 OCD outpatients, fluvoxamine in combination with anti-inflammatory COX-2 inhibitor celecoxib (200 mg bid) showed a significant improvement of symptoms in comparison with fluvoxamine alone and placebo control [187]. In a similarly designed study 48 OCD patients who were nonresponders to serotonin reuptake inhibitors were add-on treated with N-acetylcysteine (up to 2400 mg/d) or placebo over 12 weeks. At the end of this period 15% more than in the placebo group were responsive to therapy in the N-acetylcysteine group (52.6%) [188].
In an open-label study, 9 treatment-resistant outpatients with OCD were given 100 mg/day of minocycline adjunctive to their treatment with serotonin reuptake inhibitors. In diagnostic testing by Yale–Brown Obsessive-Compulsive Scale every 2 weeks over 12 weeks, no significant improvements in outcome could be found in the cohort as a whole. However, in 2 patients with early-onset OCD, more than 30% reduction of the Yale–Brown Obsessive-Compulsive Scale was reached, which was initially defined as a marker for treatment response [189]. Indeed, in a double-blind randomized trial on 102 OCD patients, therapy with minocycline (100 mg bid) as add-on to fluvoxamine (100 mg/day for the first two days and then 200 mg/day for the remaining 6 weeks of the trial) was associated with a significantly higher rate of patients responding completely or partially to the treatment. Measured by the Yale–Brown Obsessive-Compulsive Scale, total score and obsession subscale score were significantly lower in the minocycline than in the placebo group [190].
5.7. Autism Spectrum Disorder
Little is found on anti-inflammatory treatment in autism spectrum disorder so far. Add-on of celecoxib (up to 300 mg/day) to risperidone treatment in a study on 40 children suffering from autism leads to significant improvements of scores in irritability, social withdrawal, and stereotypy [191]. However, an open-label study in 11 children on treatment with 1.4 mg/kg body weight of minocycline found no clinical improvements after 6 months (measured using Clinical Global Impression Severity Scale, Clinical Global Impression Severity Scale Improvement, and Vineland Adaptive Behavior Scales). IL-8 was found significantly decreased in serum and cerebrospinal fluid, while other cytokines, that is, TNF-α, CD40L, IL-6, IFN-γ, and IL-1β, were unchanged [192].
6. Conclusions
A close examination of the literature demonstrates that the increased understanding of the microglia driven inflammatory effect on psychiatric diseases has not yet been translated into the clinical treatment for these disorders. The results from experimental animal models as well as from patients cohort studies are however quite promising. Adjunctive therapy with anti-inflammatory medication, especially in cases where conventional therapy has failed to bring complete recovery, may provide a novel route to treatment of these conditions.
Acknowledgments
Franziska A. Radtke is supported by a CMU studentship. Gareth Chapman is supported by the Neuroscience and Mental Health Research Institute and Waterloo Foundation studentship. Jeremy Hall is supported by Wellcome (DEFINE Strategic Award), Hodge Foundation (Centre Grant), MRC Centre Grant, and TWF Changing Minds Programme. Yasir A. Syed is supported by CMU fellowship and Hodge Foundation project grant.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Northoff G., Stanghellini G. How to link brain and experience? spatiotemporal psychopathology of the lived body. Frontiers in Human Neuroscience. 2016;10 doi: 10.3389/fnhum.2016.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bechter K., Reiber H., Herzog S., Fuchs D., Tumani H., Maxeiner H. G. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. Journal of Psychiatric Research. 2010;44(5):321–330. doi: 10.1016/j.jpsychires.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Benros M. E., Nielsen P. R., Nordentoft M., Eaton W. W., Dalton S. O., Mortensen P. B. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. The American Journal of Psychiatry. 2011;168(12):1303–1310. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- 4.Henje Blom E., Lekander M., Ingvar M., Åsberg M., Mobarrez F., Serlachius E. Pro-inflammatory cytokines are elevated in adolescent females with emotional disorders not treated with SSRIs. Journal of Affective Disorders. 2012;136(3):716–723. doi: 10.1016/j.jad.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Pfau M. L., Russo S. J. Neuroinflammation Regulates Cognitive Impairment in Socially Defeated Mice. Trends in Neurosciences. 2016;39(6):353–355. doi: 10.1016/j.tins.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates P., Kaletsky R. L., Francica J. R., Agrawal-Gamse C. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proceedings of the National Acadamy of Sciences of the United States of America. 2009;106(8):2886–2891. doi: 10.1073/pnas.0811014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Réus G. Z., Fries G. R., Stertz L., et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–154. doi: 10.1016/j.neuroscience.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Al-Asmari A. K., Khan M. W. Inflammation and schizophrenia: Alterations in cytokine levels and perturbation in antioxidative defense systems. Human & Experimental Toxicology. 2014;33(2):115–122. doi: 10.1177/0960327113493305. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Gras I., Garcia-Sanchez F., Guaza C., et al. P-1270 - Cytokines levels in schizophrenia patients and in theirs first- degree biological relatives. European Psychiatry. 2012;27:p. 1. doi: 10.1016/S0924-9338(12)75437-8. [DOI] [PubMed] [Google Scholar]
- 10.Sluzewska A., Rybakowski J., Bosmans E., et al. Indicators of immune activation in major depression. Psychiatry Research. 1996;64(3):161–167. doi: 10.1016/S0165-1781(96)02783-7. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y., Ho R. C., Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. Journal of Affective Disorders. 2012;139(3):230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Vogelzangs N., de Jonge P., Smit J. H., Bahn S., Penninx B. W. Cytokine production capacity in depression and anxiety. Translational Psychiatry. 2016;6(5):p. e825. doi: 10.1038/tp.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remlinger-Molenda A., Wojciak P., Michalak M., Karczewski J., Rybakowski J. K. Selected cytokine profiles during remission in bipolar patients. Neuropsychobiology. 2012;66(3):193–198. doi: 10.1159/000339949. [DOI] [PubMed] [Google Scholar]
- 14.Uyanik V., Tuglu C., Gorgulu Y., Kunduracilar H., Uyanik M. Assessment of cytokine levels and hs-CRP in bipolar I disorder before and after treatment. Psychiatry Research. 2015;228(3):386–392. doi: 10.1016/j.psychres.2015.05.078. [DOI] [PubMed] [Google Scholar]
- 15.Modabbernia A., Taslimi S., Brietzke E., Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biological Psychiatry. 2013;74(1):15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Rao N. P., Venkatasubramanian G., Ravi V., Kalmady S., Cherian A., Yc J. R. Plasma cytokine abnormalities in drug-naïve, comorbidity-free obsessive-compulsive disorder. Psychiatry Research. 2015;229(3):949–952. doi: 10.1016/j.psychres.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Fontenelle L. F., Barbosa I. G., Luna J. V., de Sousa L. P., Abreu M. N. S., Teixeira A. L. A cytokine study of adult patients with obsessive-compulsive disorder. Comprehensive Psychiatry. 2012;53(6):797–804. doi: 10.1016/j.comppsych.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Guo M., Liu T., Guo J.-C., Jiang X.-L., Chen F., Gao Y.-S. Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pacific Journal of Tropical Medicine. 2012;5(4):323–325. doi: 10.1016/S1995-7645(12)60048-0. [DOI] [PubMed] [Google Scholar]
- 19.Hoge E. A., Brandstetter K., Moshier S., Pollack M. H., Wong K. K., Simon N. M. Broad spectrum of cytokine abnormalities in Panic disorder and posttraumatic stress disorder. Depression and Anxiety. 2009;26(5):447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- 20.Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I., Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, Behavior, and Immunity. 2011;25(1):40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki K., Matsuzaki H., Iwata K., et al. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS ONE. 2011;6(5, article e20470) doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gimeno D., Kivimäki M., Brunner E. J., et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychological Medicine. 2009;39(3):413–423. doi: 10.1017/s0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fond G., Godin O., Llorca P., Leboyer M. Abnormal C-reactive protein (CRP) levels in schizophrenia and schizoaffective disorders. Results from the FACE-SZ dataset. European Psychiatry. 2015;30(8):p. S112. doi: 10.1016/j.eurpsy.2015.09.213. [DOI] [Google Scholar]
- 24.Valkanova V., Ebmeier K. P., Allan C. L. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. Journal of Affective Disorders. 2013;150(3):736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Spitzer C., Barnow S., Völzke H., et al. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. Journal of Psychiatric Research. 2010;44(1):15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Liukkonen T., Räsänen P., Jokelainen J., et al. The association between anxiety and C-reactive protein (CRP) levels: Results from the Northern Finland 1966 Birth Cohort Study. European Psychiatry. 2011;26(6):363–369. doi: 10.1016/j.eurpsy.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Khakzad M. R., Javanbakht M., Shayegan M. R., et al. The complementary role of high sensitivity C-reactive protein in the diagnosis and severity assessment of autism. Research in Autism Spectrum Disorders. 2012;6(3):1032–1037. doi: 10.1016/j.rasd.2011.10.002. [DOI] [Google Scholar]
- 28.Ridker P. M. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circulation Research. 2016;118(1):145–156. doi: 10.1161/circresaha.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khandaker G. M., Pearson R. M., Zammit S., Lewis G., Jones P. B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life a population-based longitudinal study. JAMA Psychiatry. 2014;71(10):1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saetre P., Emilsson L., Axelsson E., Kreuger J., Lindholm E., Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7, article no. 46 doi: 10.1186/1471-244X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ketharanathan T., Pereira A., Everall I., Sundram S. 165. Differing Expression Pattern of Epidermal Growth Factor (EGF) and Immune System Markers between Schizophrenia and Mood Disorder in the Dorsolateral Prefrontal Cortex. Biological Psychiatry. 2017;81(10):S68–S69. doi: 10.1016/j.biopsych.2017.02.178. [DOI] [Google Scholar]
- 32.Trépanier M. O., Hopperton K. E., Mizrahi R., Mechawar N., Bazinet R. P. Postmortem evidence of cerebral inflammation in schizophrenia: A systematic review. Molecular Psychiatry. 2016;21(8):1009–1026. doi: 10.1038/mp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mechawar N., Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Translational Psychiatry. 2016;6(11):p. e946. doi: 10.1038/tp.2016.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernández M. E., Mendieta D., Martínez-Fong D., et al. Variations in circulating cytokine levels during 52 week course of treatment with SSRI for major depressive disorder. European Neuropsychopharmacology. 2008;18(12):917–924. doi: 10.1016/j.euroneuro.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Capuzzi E., Bartoli F., Crocamo C., Clerici M., Carrà G. Acute variations of cytokine levels after antipsychotic treatment in drug-naïve subjects with a first-episode psychosis: A meta-analysis. Neuroscience & Biobehavioral Reviews. 2017;77:122–128. doi: 10.1016/j.neubiorev.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Dahl J., Ormstad H., Aass H. C. D., Sandvik L., Malt U. F., Andreassen O. A. Recovery from major depressive disorder episode after non-pharmacological treatment is associated with normalized cytokine levels. Acta Psychiatrica Scandinavica. 2016;134(1):40–47. doi: 10.1111/acps.12576. [DOI] [PubMed] [Google Scholar]
- 37.Cross-Disorder C., Cross-Disorder Group C. Group. Nature Genetics. Sep 2013;45:984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pribiag H., Peng H., Shah W. A., Stellwagen D., Carbonetto S. Dystroglycan mediates homeostatic synaptic plasticity at GABAergic synapses. Proceedings of the National Acadamy of Sciences of the United States of America. 2014;111(18):6810–6815. doi: 10.1073/pnas.1321774111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purcell S. M., Wray N. R., Stone J. L., et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sekar A., Bialas A. R., De Rivera H., et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530(7589):177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramirez-Bermudez J., Soto-Hernandez J. L., Lopez-Gomez M., Mendoza-Silva M., Colin-Piana R., Campillo-Serrano C. [Frequency of neuropsychiatric signs and symptoms in patients with viral encephalitis] Rev Neurol. 2005;41:p. 140. [PubMed] [Google Scholar]
- 42.Mufaddel A., Omer A. A., Salem M. O. Psychiatric Aspects of Infectious Diseases. Open Journal of Psychiatry. 2014;04(03):202–217. doi: 10.4236/ojpsych.2014.43027. [DOI] [Google Scholar]
- 43.Kreutzer J. S., Seel R. T., Gourley E. The prevalence and symptom rates of depression after traumatic brain injury: a comprehensive examination. Brain Injury. 2001;15(7):563–576. doi: 10.1080/02699050116884. [DOI] [PubMed] [Google Scholar]
- 44.Lennox B. R., Palmer-Cooper E. C., Pollak T., et al. Prevalence and clinical characteristics of serum neuronal cell surface antibodies in first-episode psychosis: a case-control study. The Lancet Psychiatry. 2017;4(1):42–48. doi: 10.1016/S2215-0366(16)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castillo-Gomez E., Kästner A., Steiner J., et al. The brain as immunoprecipitator of serum autoantibodies against N-Methyl-D-aspartate receptor subunit NR1. Annals of Neurology. 2016;79(1):144–151. doi: 10.1002/ana.24545. [DOI] [PubMed] [Google Scholar]
- 46.Choe C.-U., Karamatskos E., Schattling B., et al. A clinical and neurobiological case of IgM NMDA receptor antibody associated encephalitis mimicking bipolar disorder. Psychiatry Research. 2013;208(2):194–196. doi: 10.1016/j.psychres.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 47.Dale R. C., Merheb V., Pillai S., et al. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain. 2012;135(11):3453–3468. doi: 10.1093/brain/aws256. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka S., Matsunaga H., Kimura M., et al. Erratum: Autoantibodies against four kinds of neurotransmitter receptors in psychiatric disorders (Journal of Neuroimmunology (2003) 141 (155-164)) Journal of Neuroimmunology. 2003;144(1-2):p. 148. doi: 10.1016/j.jneuroim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka S., Kuratsune H., Hidaka Y., et al. Autoantibodies against muscarinic cholinergic receptor in chronic fatigue syndrome. International Journal of Molecular Medicine. 2003;12(2):225–230. [PubMed] [Google Scholar]
- 50.O'Connor J. C., Lawson M. A., André C., et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Molecular Psychiatry. 2009;14(5):511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dantzer R., O'Connor J. C., Freund G. G., Johnson R. W., Kelley K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghiani C. A., Mattan N. S., Nobuta H., et al. Early effects of lipopolysaccharideinduced inflammation on foetal brain development in rat. ASN Neuro. 2011;3(4):233–245. doi: 10.1042/AN20110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pang Y., Campbell L., Zheng B., Fan L., Cai Z., Rhodes P. Lipopolysaccharide-activated microglia induce death of oligodendrocyte progenitor cells and impede their development. Neuroscience. 2010;166(2):464–475. doi: 10.1016/j.neuroscience.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 54.Hosoda S., Takimura H., Shibayama M., Kanamura H., Ikeda K., Kumada H. Psychiatric symptoms related to interferon therapy for chronic hepatitis C: Clinical features and prognosis. Psychiatry and Clinical Neurosciences. 2000;54(5):565–572. doi: 10.1046/j.1440-1819.2000.00754.x. [DOI] [PubMed] [Google Scholar]
- 55.Nozaki O., Takagi C., Takaoka K., Takata T., Yoshida M. Psychiatric manifestations accompanying interferon therapy for patients with chronic hepatitis C: An overview of cases in Japan. Psychiatry and Clinical Neurosciences. 1997;51(4):175–180. doi: 10.1111/j.1440-1819.1997.tb02579.x. [DOI] [PubMed] [Google Scholar]
- 56.Devinsky O., Vezzani A., Najjar S., De Lanerolle N. C., Rogawski M. A. Glia and epilepsy: Excitability and inflammation. Trends in Neurosciences. 2013;36(3):174–184. doi: 10.1016/j.tins.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Mandolesi G., Gentile A., Musella A., et al. Synaptopathy connects inflammation and neurodegeneration in multiple sclerosis. Nature Reviews Neurology. 2015;11(12):711–724. doi: 10.1038/nrneurol.2015.222. [DOI] [PubMed] [Google Scholar]
- 58.Hagihara H., Takao K., Walton N. M., Matsumoto M., Miyakawa T. Immature dentate gyrus: An endophenotype of neuropsychiatric disorders. Neural Plasticity. 2013;2013 doi: 10.1155/2013/318596.318596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pont-Lezica L., Béchade C., Belarif-Cantaut Y., Pascual O., Bessis A. Physiological roles of microglia during development. Journal of Neurochemistry. 2011;119(5):901–908. doi: 10.1111/j.1471-4159.2011.07504.x. [DOI] [PubMed] [Google Scholar]
- 60.Perry E., Court J., Goodchild R., et al. Clinical neurochemistry: Developments in dementia research based on brain bank material. Journal of Neural Transmission. 1998;105(8-9):915–933. doi: 10.1007/s007020050102. [DOI] [PubMed] [Google Scholar]
- 61.Heppner F. L., Ransohoff R. M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nature Reviews Neuroscience. 2015;16(6):358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 62.Hong S., Dissing-Olesen L., Stevens B. New insights on the role of microglia in synaptic pruning in health and disease. Current Opinion in Neurobiology. 2016;36:128–134. doi: 10.1016/j.conb.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norden D. M., Godbout J. P. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathology and Applied Neurobiology. 2013;39(1):19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pousset F. Developmental expression of cytokine genes in the cortex and hippocampus of the rat central nervous system. Developmental Brain Research. 1994;81(1):143–146. doi: 10.1016/0165-3806(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 65.Pousset F. Cytokines as mediators in the central nervous system. Biomedicine & Pharmacotherapy. 1994;48(10):425–431. doi: 10.1016/0753-3322(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 66.Bilbo S. D., Schwarz J. M. Early-life programming of later-life brain and behavior: a critical role for the immune system. Frontiers in Behavioral Neuroscience. 2009;3, article no. 14 doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yeo Y. A., Martínez Gómez J. M., Croxford J. L., Gasser S., Ling E.-A., Schwarz H. CD137 ligand activated microglia induces oligodendrocyte apoptosis via reactive oxygen species. Journal of Neuroinflammation. 2012;9, article 173 doi: 10.1186/1742-2094-9-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davalos D., Grutzendler J., Yang G., et al. ATP mediates rapid microglial response to local brain injury in vivo. Nature Neuroscience. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 69.Appel K., Honegger P., Gebicke-haerter P. J. Expression of interleukin-3 and tumor necrosis factor-β mRNAs in cultured microglia. Journal of Neuroimmunology. 1995;60(1-2):83–91. doi: 10.1016/0165-5728(95)00057-9. [DOI] [PubMed] [Google Scholar]
- 70.Buttini M., Boddeke H. Peripheral lipopolysaccharide stimulation induces interleukin-1β messenger RNA in rat brain microglial cells. Neuroscience. 1995;65(2):523–530. doi: 10.1016/0306-4522(94)00525-A. [DOI] [PubMed] [Google Scholar]
- 71.Prinz M., Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nature Reviews Neuroscience. 2014;15(5):300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 72.Cribbs D. H., Berchtold N. C., Perreau V., et al. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: A microarray study. Journal of Neuroinflammation. 2012;9, article no. 179 doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bilimoria P. M., Stevens B. Microglia function during brain development: new insights from animal models. Brain Research. 2014 doi: 10.1016/j.brainres.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 74.Lim S.-H., Park E., You B., et al. Neuronal synapse formation induced by microglia and interleukin 10. PLoS ONE. 2013;8(11) doi: 10.1371/journal.pone.0081218.e81218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parkhurst C. N., Yang G., Ninan I. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williamson L. L., Sholar P. W., Mistry R. S., Smith S. H., Bilbo S. D. Microglia and memory: Modulation by early-life infection. The Journal of Neuroscience. 2011;31(43):15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bilbo S. D., Biedenkapp J. C., Der-Avakian A., Watkins L. R., Rudy J. W., Maier S. F. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. The Journal of Neuroscience. 2005;25(35):8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paolicelli R. C., Bolasco G., Pagani F., et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 79.Paolicelli R. C., Gross C. T. Microglia in development: linking brain wiring to brain environment. Neuron Glia Biology. 2011;7(1):77–83. doi: 10.1017/s1740925x12000105. [DOI] [PubMed] [Google Scholar]
- 80.Schafer D., Lehrman E. K., Kautzman A. G., et al. Microglia and the complement cascade: shaping neural circuits in the developing brain. Schizophrenia Research. 2012;136:S11–S12. doi: 10.1016/S0920-9964(12)70039-7. [DOI] [Google Scholar]
- 81.Bialas A. R., Stevens B. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nature Neuroscience. 2013;16(12):1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Mataga N., Mizuguchi Y., Hensch T. K. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron. 2004;44(6):1031–1041. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 83.Bhattacharjee Y. Is internal timing key to mental health? Science. 2007;317(5844):1488–1490. doi: 10.1126/science.317.5844.1488. [DOI] [PubMed] [Google Scholar]
- 84.Bilbo S. Early Life Infection. Microglia, and Cognition Throughout the Lifespan., Glia. Jul 2013;61:S28–S29. [Google Scholar]
- 85.Castanon N., Luheshi G., Layé S. Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Frontiers in Neuroscience. 2015;9, article 229 doi: 10.3389/fnins.2015.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knuesel I., Chicha L., Britschgi M., et al. Maternal immune activation and abnormal brain development across CNS disorders. Nature Reviews Neurology. 2014;10(11):643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- 87.Milior G., Lecours C., Samson L., et al. Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain, Behavior, and Immunity. 2015 doi: 10.1016/j.bbi.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 88.Steiner J., Mawrin C., Ziegeler A., et al. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathologica. 2006;112(3):305–316. doi: 10.1007/s00401-006-0090-8. [DOI] [PubMed] [Google Scholar]
- 89.Steiner J., Bielau H., Brisch R., et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. Journal of Psychiatric Research. 2008;42(2):151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 90.van Berckel B. N., Bossong M. G., Boellaard R., et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biological Psychiatry. 2008;64(9):820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 91.Doorduin J., de Vries E. F. J., Willemsen A. T. M., de Groot J. C., Dierckx R. A., Klein H. C. Neuroinflammation in schizophrenia-related psychosis: a PET study. Journal of Nuclear Medicine. 2009;50(11):1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- 92.Morgan J. T., Chana G., Pardo C. A., et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biological Psychiatry. 2010;68(4):368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 93.Takano D., Yamazaki T., Masaki H., Miyata M., Nakase T., Maeda T. Regional cerebral blood flow correlates of apathy and depression in demented patients. European Journal of Neurology. Sep 2010;17:275–275. [Google Scholar]
- 94.Tetreault N. A., Hakeem A. Y., Jiang S., et al. Microglia in the cerebral cortex in autism. Journal of Autism and Developmental Disorders. 2012;42(12):2569–2584. doi: 10.1007/s10803-012-1513-0. [DOI] [PubMed] [Google Scholar]
- 95.Suzuki T., Shiga T., Nishimura K., Ishigooka J., Hagiwara N. PHQ-9 screening for depression in hospitalized patients with heart failure. European Journal of Heart Failure. May 2013;12:S242–S242. [Google Scholar]
- 96.Garey L. When cortical development goes wrong: Schizophrenia as a neurodevelopmental disease of microcircuits. Journal of Anatomy. 2010;217(4):324–333. doi: 10.1111/j.1469-7580.2010.01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Foster R., Kandanearatchi A., Beasley C., et al. Calprotectin in microglia from frontal cortex is up-regulated in schizophrenia: Evidence for an inflammatory process? European Journal of Neuroscience. 2006;24(12):3561–3566. doi: 10.1111/j.1460-9568.2006.05219.x. [DOI] [PubMed] [Google Scholar]
- 98.Notter T., Coughlin J. M., Gschwind T., et al. Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Molecular Psychiatry. 2017 doi: 10.1038/mp.2016.248. [DOI] [PubMed] [Google Scholar]
- 99.Maezawa I., Jin L.-W. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. The Journal of Neuroscience. 2010;30(15):5346–5356. doi: 10.1523/JNEUROSCI.5966-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Derecki N. C., Cronk J. C., Lu Z., et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484(7392):105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reshef R., Kreisel T., Beroukhim Kay D., Yirmiya R. Microglia and their CX3CR1 signaling are involved in hippocampal- but not olfactory bulb-related memory and neurogenesis. Brain, Behavior, and Immunity. 2014;41(1):239–250. doi: 10.1016/j.bbi.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 102.Tanaka J., Fujita H., Matsuda S., Toku K., Sakanaka M., Maeda N. Glucocorticoid- and mineralocorticoid receptors in microglial cells: The two receptors mediate differential effects of corticosteroids. Glia. 1997;20(1):23–37. doi: 10.1002/(SICI)1098-1136(199705)20:1<23::AID-GLIA3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 103.Streit W. J., Kincaid-Colton C. A. The brain's immune system. Scientific American. 1995;273(5):54–61. doi: 10.1038/scientificamerican1195-54. [DOI] [PubMed] [Google Scholar]
- 104.Barrientos R. M., Frank M. G., Hein A. M., et al. Time course of hippocampal IL-1 β and memory consolidation impairments in aging rats following peripheral infection. Brain, Behavior, and Immunity. 2009;23(1):46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Henry S., Bigler S., Wang J. High throughput analysis of neural progenitor cell proliferation in adult rodent hippocampus. Biosci Trends. Dec 2009;3:p. 233. [PMC free article] [PubMed] [Google Scholar]
- 106.Püntener U., Booth S. G., Perry V. H., Teeling J. L. Long-term impact of systemic bacterial infection on the cerebral vasculature and microglia. Journal of Neuroinflammation. 2012;9, article 146 doi: 10.1186/1742-2094-9-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salter M. W., Beggs S. Sublime microglia: expanding roles for the guardians of the CNS. Cell. 2014;158:15–24. doi: 10.1016/j.cell.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 108.Burmeister M., Scott L. J., Li J. Z., Absher D., Thompson R. C., Meng F. Identifying genes associated with bipolar disorder in the NIMII genetic study intiative samples; Jul 2008; pp. 24–24. [Google Scholar]
- 109.Iafrate A. J., Feuk L., Rivera M. N., et al. Detection of large-scale variation in the human genome. Nature Genetics. 2004;36(9):949–951. doi: 10.1038/ng1416. [DOI] [PubMed] [Google Scholar]
- 110.Redon R., Ishikawa S., Fitch K. R., et al. Global variation in copy number in the human genome. Nature. 2006;444(7118):444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cook E. H., Jr., Scherer S. W. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455(7215):919–923. doi: 10.1038/nature07458. [DOI] [PubMed] [Google Scholar]
- 112.Kirov G. CNVs in neuropsychiatric disorders. Human Molecular Genetics. 2015;24(1):R45–R49. doi: 10.1093/hmg/ddv253.ddv253 [DOI] [PubMed] [Google Scholar]
- 113.Corrigendum: Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nature Genetics. 2007;39(10):1285–1285. doi: 10.1038/ng1007-1285a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sharp A. J., Hansen S., Selzer R. R., et al. Discovery of previously unidentified genomic disorders from the duplication architecture of the human genome. Nature Genetics. 2006;38(9):1038–1042. doi: 10.1038/ng1862. [DOI] [PubMed] [Google Scholar]
- 115.Stockman J. Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. Yearbook of Pediatrics. 2010;2010:300–302. doi: 10.1016/S0084-3954(09)79511-9. [DOI] [Google Scholar]
- 116.Ben-Shachar S., Lanpher B., German J. R., et al. Microdeletion 15q13.3: A locus with incomplete penetrance for autism, mental retardation, and psychiatric disorders. Journal of Medical Genetics. 2009;46(6):382–388. doi: 10.1136/jmg.2008.064378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sharp A. J., Mefford H. C., Li K., et al. A recurrent 15q13.3 microdeletion syndrome associated with mental retardation and seizures. Nature Genetics. 2008;40(3):322–328. doi: 10.1038/ng.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miller D. T., Shen Y., Weiss L. A., et al. Microdeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disorders. Journal of Medical Genetics. 2009;46(4):242–248. doi: 10.1136/jmg.2008.059907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Feinstein C., Eliez S., Blasey C., Reiss A. L. Psychiatric disorders and behavioral problems in children with velocardiofacial syndrome: Usefulness as phenotypic indicators of schizophrenia risk. Biological Psychiatry. 2002;51(4):312–318. doi: 10.1016/S0006-3223(01)01231-8. [DOI] [PubMed] [Google Scholar]
- 120.Baker A. S., Chung T. H., Pidgeon T. S., Mancini C., Price-Troska T., Van Wier S. A comparative analysis of chromosome 13 gene expression and copy number changes in multiple myeloma using array-based methods. Blood. Nov;106:447a–447a. [Google Scholar]
- 121.Maughan B. Depression in adolescence, early adulthood and mid-life: Developmental variations in risk. Journal of Affective Disorders. Mar 2004;78:S26–S26. [Google Scholar]
- 122.Maxeiner H.-G., Marion Schneider E., Kurfiss S.-T., Brettschneider J., Tumani H., Bechter K. Cerebrospinal fluid and serum cytokine profiling to detect immune control of infectious and inflammatory neurological and psychiatric diseases. Cytokine. 2014;69(1):62–67. doi: 10.1016/j.cyto.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 123.Mulley J. C., Dibbens L. M. Chipping away at the common epilepsies with complex genetics: The 15q13.3 microdeletion shows the way. Genome Medicine. 2009;1(3, article no. gm33) doi: 10.1186/gm33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Helbig I., Mefford H. C., Sharp A. J., et al. 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nature Genetics. 2009;41(2):160–162. doi: 10.1038/ng.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Muhle H., Mefford H. C., Obermeier T., et al. Absence seizures with intellectual disability as a phenotype of the 15q13.3 microdeletion syndrome. Epilepsia. 2011;52(12):e194–e198. doi: 10.1111/j.1528-1167.2011.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stefansson H., Rujescu D., Cichon S., Pietilainen O. P. H., Ingason A., Steinberg S. Large recurrent microdeletions associated with schizophrenia. Nature. Sep;455:232–61. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vassos E., Collier D. A., Holden S., et al. Penetrance for copy number variants associated with schizophrenia. Human Molecular Genetics. 2010;19(17):3477–3481. doi: 10.1093/hmg/ddq259.ddq259 [DOI] [PubMed] [Google Scholar]
- 128.Levinson D. F., Duan J., Oh S., et al. Copy number variants in schizophrenia: Confirmation of five previous finding sand new evidence for 3q29 microdeletions and VIPR2 duplications. The American Journal of Psychiatry. 2011;168(3):302–316. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vacic V., McCarthy S., Malhotra D., et al. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia (Nature (2011) 471 (499-501)) Nature. 2011;474(7349):p. 114. doi: 10.1038/nature10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shinawi M., Schaaf C. P., Bhatt S. S., et al. A small recurrent deletion within 15q13.3 is associated with a range of neurodevelopmental phenotypes. Nature Genetics. 2009;41(12):1269–1271. doi: 10.1038/ng.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pagnamenta A. T., Wing K., Akha E. S., et al. A 15q13.3 microdeletion segregating with autism. European Journal of Human Genetics. 2009;17(5):687–692. doi: 10.1038/ejhg.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Szatkiewicz J. P., O'Dushlaine C., Chen G. Copy number variation in schizophrenia in Sweden. Molecular Psychiatry. 2014;19:762–773. doi: 10.1038/mp.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee Y., Mattai A., Long R., Rapoport J. L., Gogtay N., Addington A. M. Microduplications disrupting the MYT1L gene (2p25.3) are associated with schizophrenia. Psychiatric Genetics. 2012;22(4):206–209. doi: 10.1097/YPG.0b013e328353ae3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weiss L. A., Shen Y., Korn J. M. Association between microdeletion and microduplication at 16p11.2 and autism. The New England Journal of Medicine. 2008;358(7):667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 135.Zheng X., Demirci F. Y., Barmada M. M., et al. A rare duplication on chromosome 16p11.2 is identified in patients with psychosis in Alzheimer's disease. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0111462.e111462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pocklington A. J., Rees E., Walters J. T. R., et al. Novel Findings from CNVs Implicate Inhibitory and Excitatory Signaling Complexes in Schizophrenia. Neuron. 2015;86(5):1203–1214. doi: 10.1016/j.neuron.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fromer M., Pocklington A. J., Kavanagh D. H., et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506(7487):179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Haines J. L., Korf B. R., Morton C. C., Seidman C. E., Seidman J., Smith D. R. Current Protocols in Human Genetics. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2001. [Google Scholar]
- 139.Kirov G., Pocklington A. J., Holmans P., et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Molecular Psychiatry. 2012;17(2):142–153. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Piliero L. M., Sanford A. N., McDonald-McGinn D. M., Zackai E. H., Sullivan K. E. T-cell homeostasis in humans with thymic hypoplasia due to chromosome 22q11.2 deletion syndrome. Blood. 2004;103(3):1020–1025. doi: 10.1182/blood-2003-08-2824. [DOI] [PubMed] [Google Scholar]
- 141.Aresvik D. M., Lima K., Øverland T., Mollnes T. E., Abrahamsen T. G. Increased Levels of Interferon-Inducible Protein 10 (IP-10) in 22q11.2 Deletion Syndrome. Scandinavian Journal of Immunology. 2016;83(3):188–194. doi: 10.1111/sji.12406. [DOI] [PubMed] [Google Scholar]
- 142.Ross H. E., Guo Y., Coleman K., Ousley O., Miller A. H. Association of IL-12p70 and IL-6: IL-10 ratio with autism-related behaviors in 22q11.2 deletion syndrome: A preliminary report. Brain, Behavior, and Immunity. 2013;31:76–81. doi: 10.1016/j.bbi.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zemble R., Luning Prak E., McDonald K., McDonald-McGinn D., Zackai E., Sullivan K. Secondary immunologic consequences in chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) Clinical Immunology. 2010;136(3):409–418. doi: 10.1016/j.clim.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Toyoshima M., Akamatsu W., Okada Y., et al. Analysis of induced pluripotent stem cells carrying 22q11.2 deletion. Translational Psychiatry. 2016;6(11, article no. e934) doi: 10.1038/tp.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Philippe L., Alsaleh G., Bahram S., Pfeffer S., Georgel P. The miR-17 ~ 92 cluster: A key player in the control of inflammation during rheumatoid arthritis. Frontiers in Immunology. 2013;4 doi: 10.3389/fimmu.2013.00070.Article 70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Qin H. H., Zhu X. H., Liang J., Wu J. F., Yang Y. S., Xu J. H. The expression and significance of miR-17-92 cluster miRs in CD4(+) T cells from patients with systemic lupus erythernatosus. Clinical and Experimental Rheumatology. 2013;31:472–473. [PubMed] [Google Scholar]
- 147.Zhai Z., Wu F., Chuang A. Y., Kwon J. H. MiR-106b fine tunes ATG16L1 expression and autophagic activity in intestinal epithelial HCT116 cells. Inflammatory Bowel Diseases. 2013;19(11):2295–2301. doi: 10.1097/MIB.0b013e31829e71cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shprintzen R. J., Higgins A. M., Antshel K., Fremont W., Roizen N., Kates W. Velo-cardio-facial syndrome. Current Opinion in Pediatrics. 2005;17(6):725–730. doi: 10.1097/01.mop.0000184465.73833.0b. [DOI] [PubMed] [Google Scholar]
- 149.Le Pichon J.-B., Yu S., Kibiryeva N., Graf W. D., Bittel D. C. Genome-wide gene expression in a patient with 15q13.3 homozygous microdeletion syndrome. European Journal of Human Genetics. 2013;21(10):1093–1099. doi: 10.1038/ejhg.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hong M. S., Song J. Y., Yun D. H., Cho J.-J., Chung J.-H. Increase of NADPH-diaphorase expression in hypothalamus of Stat4 knockout mice. Korean Journal of Physiology & Pharmacology. 2009;13(5):337–341. doi: 10.4196/kjpp.2009.13.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D. A., Stella A. M. G. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nature Reviews Neuroscience. 2007;8(10):766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 152.Capone M. L., Tacconelli S., Sciulli M. G., Patrignani P. Clinical pharmacology of selective COX-2 inhibitors. International Journal of Immunopathology and Pharmacology. 2003;16(2):49–58. doi: 10.1177/039463200301600107. [DOI] [PubMed] [Google Scholar]
- 153.Kaizaki A., Tien L.-T., Pang Y., et al. Celecoxib reduces brain dopaminergic neuronaldysfunction, and improves sensorimotor behavioral performance in neonatal rats exposed to systemic lipopolysaccharide. Journal of Neuroinflammation. 2013;10, article no. 45 doi: 10.1186/1742-2094-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Müller N., Schwarz M. J. The immunological basis of glutamatergic disturbance in schizophrenia: Towards an integrated view. Journal of Neural Transmission. Supplementa. 2007;(72):269–280. doi: 10.1007/978-3-211-73574-9-33. [DOI] [PubMed] [Google Scholar]
- 155.Erhardt S., Schwieler L., Engberg G. Developments in Tryptophan and Serotonin Metabolism. Vol. 527. Boston, MA: Springer US; 2003. Kynurenic Acid And Schizophrenia; pp. 155–165. (Advances in Experimental Medicine and Biology). [DOI] [PubMed] [Google Scholar]
- 156.Chaves C., Marque C. R., Wichert-Ana L., Maia-de-Oliveira J. P., Itikawa E. N., Crippa J. A. D. Minocycline and psychoneuroimmunology in schizophrenia, Progress in Neuro-Psychopharmacology Biological Psychiatry. Minocycline and psychoneuroimmunology in schizophrenia, Progress in Neuro-Psychopharmacology Biological Psychiatry. Aug;34:1133–1134. [Google Scholar]
- 157.Kim H., Suh Y. Minocycline and neurodegenerative diseases. Behavioural Brain Research. 2009;196(2):168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 158.Laan W., Grobbee D. E., Selten J.-P., Heijnen C. J., Kahn R. S., Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry. 2010;71(5):520–527. doi: 10.4088/jcp.09m05117yel. [DOI] [PubMed] [Google Scholar]
- 159.Weiser M., Burstein S., Fodoreanu L., et al. Positive symptoms respond to add-on aspirin in schizophrenia patients with high sera CRP levels: a post-hoc analysis of an RCT. Schizophrenia Research. 2014;153:p. S79. doi: 10.1016/S0920-9964(14)70256-7. [DOI] [Google Scholar]
- 160.Müller N., Riedel M., Scheppach C., et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. The American Journal of Psychiatry. 2002;159(6):1029–1034. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- 161.Baheti T., Nischal A., Nischal A., et al. A study to evaluate the effect of celecoxib as add-on to olanzapine therapy in schizophrenia. Schizophrenia Research. 2013;147(1):201–202. doi: 10.1016/j.schres.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 162.Akhondzadeh S., Tabatabaee M., Amini H., Ahmadi Abhari S. A., Abbasi S. H., Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophrenia Research. 2007;90(1–3):179–185. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 163.Müller N., Krause D., Dehning S., et al. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophrenia Research. 2010;121(1–3):118–124. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 164.Rapaport M. H., Delrahim K. K., Bresee C. J., Maddux R. E., Ahmadpour O., Dolnak D. Celecoxib augmentation of continuously Ill patients with schizophrenia. Biological Psychiatry. 2005;57(12):1594–1596. doi: 10.1016/j.biopsych.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 165.Köhler O., Petersen L., Benros M. E., Mors O., Gasse C. Concomitant NSAID use during antipsychotic treatment and risk of 2-year relapse – a population-based study of 16,253 incident patients with schizophrenia. Expert Opinion on Pharmacotherapy. 2016;17(8):1055–1062. doi: 10.1517/14656566.2016.1168400. [DOI] [PubMed] [Google Scholar]
- 166.Laan W., Selten J.-P., Grobbee D. E., Smeets H., Kahn R. S., Burger H. Non-steroidal anti-inflammatory drugs and the risk of psychosis. European Neuropsychopharmacology. 2007;17(4):309–311. doi: 10.1016/j.euroneuro.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 167.Stolk P., Souverein P. C., Leufkens H. G. M., Weil J. G., Egberts A. C. G., Heerdink E. R. The association between exposure to COX-2 inhibitors and schizophrenia deterioration. A nested case-control study. Pharmacopsychiatry. 2007;40(3):111–115. doi: 10.1055/s-2007-977714. [DOI] [PubMed] [Google Scholar]
- 168.Chaudhry I. B., Hallak J., Husain N., et al. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. Journal of Psychopharmacology. 2012;26(9):1185–1193. doi: 10.1177/0269881112444941. [DOI] [PubMed] [Google Scholar]
- 169.Liu F., Guo X., Wu R., et al. Minocycline supplementation for treatment of negative symptoms in early-phase schizophrenia: A double blind, randomized, controlled trial. Schizophrenia Research. 2014;153(1-3):169–176. doi: 10.1016/j.schres.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 170.Ghanizadeh A., Dehbozorgi S., OmraniSigaroodi M., Rezaei Z. Minocycline as add-on treatment decreases the negative symptoms of schizophrenia; a randomized placebo-controlled clinical trial. Recent Patents on Inflammation & Allergy Drug Discovery. 2014;8(3):211–215. doi: 10.2174/1872213X08666141029123524. [DOI] [PubMed] [Google Scholar]
- 171.Khodaie-Ardakani M.-R., Mirshafiee O., Farokhnia M., et al. Minocycline add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: Randomized double-blind placebo-controlled study. Psychiatry Research. 2014;215(3):540–546. doi: 10.1016/j.psychres.2013.12.051. [DOI] [PubMed] [Google Scholar]
- 172.Levkovitz Y., Mendlovich S., Riwkes S., et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. Journal of Clinical Psychiatry. 2010;71(2):138–149. doi: 10.4088/jcp.08m04666yel. [DOI] [PubMed] [Google Scholar]
- 173.Köhler-Forsberg O., Sylvia L., Thase M., et al. Nonsteroidal anti-inflammatory drugs (NSAIDs) and paracetamol do not affect 6-month mood-stabilizing treatment outcome among 482 patients with bipolar disorder. Depression and Anxiety. 2017;34(3):281–290. doi: 10.1002/da.22601. [DOI] [PubMed] [Google Scholar]
- 174.Saroukhani S., Emami-Parsa M., Modabbernia A., et al. Aspirin for treatment of lithium-associated sexual dysfunction in men: Randomized double-blind placebo-controlled study. Bipolar Disorder. 2013;15(6):650–656. doi: 10.1111/bdi.12108. [DOI] [PubMed] [Google Scholar]
- 175.Stolk P., Souverein P. C., Wilting I., et al. Is aspirin useful in patients on lithium? A pharmacoepidemiological study related to bipolar disorder. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2010;82(1):9–14. doi: 10.1016/j.plefa.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nery F. G., Monkul E. S., Hatch J. P., et al. Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Journal of Psychopharmacology. 2008;23(2):87–94. doi: 10.1002/hup.912. [DOI] [PubMed] [Google Scholar]
- 177.Arabzadeh S., Ameli N., Zeinoddini A., et al. Celecoxib adjunctive therapy for acute bipolar mania: A randomized, double-blind, placebo-controlled trial. Bipolar Disorder. 2015;17(6):606–614. doi: 10.1111/bdi.12324. [DOI] [PubMed] [Google Scholar]
- 178.Uher R., Carver S., Power R. A., et al. Non-steroidal anti-inflammatory drugs and efficacy of antidepressants in major depressive disorder. Psychological Medicine. 2012;42(10):2027–2035. doi: 10.1017/S0033291712000190. [DOI] [PubMed] [Google Scholar]
- 179.Almeida O. P., Alfonso H., Jamrozik K., Hankey G. J., Flicker L. Aspirin use, depression, and cognitive impairment in later life: The health in men study. Journal of the American Geriatrics Society. 2010;58(5):990–992. doi: 10.1111/j.1532-5415.2010.02827.x. [DOI] [PubMed] [Google Scholar]
- 180.Glaus J., Vandeleur C. L., Lasserre A. M., et al. Aspirin and statin use and the subsequent development of depression in men and women: Results from a longitudinal population-based study. Journal of Affective Disorders. 2015;182:126–131. doi: 10.1016/j.jad.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 181.Na K. S., Lee K. J., Lee J. S., Cho Y. S., Jung H. Y. Progress in Neuro-Psychopharmacology Biological Psychiatry. Vol. 48. A meta-analysis: Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder; 2014. Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: A meta-analysis; pp. 79–85. [DOI] [PubMed] [Google Scholar]
- 182.Mohammadinejad P., Arya P., Esfandbod M., et al. Celecoxib Versus Diclofenac in Mild to Moderate Depression Management Among Breast Cancer Patients: A Double-Blind, Placebo-Controlled, Randomized Trial. Annals of Pharmacotherapy. 2015;49(9):953–961. doi: 10.1177/1060028015592215. [DOI] [PubMed] [Google Scholar]
- 183.Jafari S., Ashrafizadeh S.-G., Zeinoddini A., et al. Celecoxib for the treatment of mild-to-moderate depression due to acute brucellosis: a double-blind, placebo-controlled, randomized trial. Journal of Clinical Pharmacy and Therapeutics. 2015;40(4):441–446. doi: 10.1111/jcpt.12287. [DOI] [PubMed] [Google Scholar]
- 184.Fields C., Drye L., Vaidya V., Lyketsos C. Celecoxib or naproxen treatment does not benefit depressive symptoms in persons age 70 and older: Findings from a randomized controlled trial. The American Journal of Geriatric Psychiatry. 2012;20(6):505–513. doi: 10.1097/JGP.0b013e318227f4da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Miyaoka T., Wake R., Furuya M., et al. Minocycline as adjunctive therapy for patients with unipolar psychotic depression: An open-label study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2012;37(2):222–226. doi: 10.1016/j.pnpbp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 186.Emadi-Kouchak H., Mohammadinejad P., Asadollahi-Amin A., et al. Therapeutic effects of minocycline on mild-to-moderate depression in HIV patients: A double-blind, placebo-controlled, randomized trial. International Clinical Psychopharmacology. 2016;31(1):20–26. doi: 10.1097/YIC.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 187.Shalbafan M., Mohammadinejad P., Shariat S.-V., et al. Celecoxib as an Adjuvant to Fluvoxamine in Moderate to Severe Obsessive-compulsive Disorder: A Double-blind, Placebo-controlled, Randomized Trial. Pharmacopsychiatry. 2015;48(4-5):136–140. doi: 10.1055/s-0035-1549929. [DOI] [PubMed] [Google Scholar]
- 188.Afshar H., Roohafza H., Mohammad-Beigi H., et al. N-acetylcysteine add-on treatment in refractory obsessive-compulsive disorder: A randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychopharmacology. 2012;32(6):797–803. doi: 10.1097/JCP.0b013e318272677d. [DOI] [PubMed] [Google Scholar]
- 189.Rodriguez C. I., Bender J., Jr., Marcus S. M., Snape M., Rynn M., Simpson H. B. Minocycline augmentation of pharmacotherapy in obsessive-compulsive disorder: An open-label trial. Journal of Clinical Psychiatry. 2010;71(9):1247–1249. doi: 10.4088/JCP.09l05805blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Esalatmanesh S., Abrishami Z., Zeinoddini A., et al. Minocycline combination therapy with fluvoxamine in moderate-to-severe obsessive–compulsive disorder: A placebo-controlled, double-blind, randomized trial. Psychiatry and Clinical Neurosciences. 2016;70(11):517–526. doi: 10.1111/pcn.12430. [DOI] [PubMed] [Google Scholar]
- 191.Asadabadi M., Mohammadi M.-R., Ghanizadeh A., et al. Celecoxib as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind, placebo-controlled trial. Psychopharmacology. 2013;225(1):51–59. doi: 10.1007/s00213-012-2796-8. [DOI] [PubMed] [Google Scholar]
- 192.Pardo C. A., Buckley A., Thurm A., et al. A pilot open-label trial of minocycline in patients with autism and regressive features. Journal of Neurodevelopmental Disorders. 2013;5(1):1–9. doi: 10.1186/1866-1955-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kelly D. L., Sullivan K. M., McEvoy J. P., et al. Adjunctive Minocycline in Clozapine-Treated Schizophrenia Patients with Persistent Symptoms. Journal of Clinical Psychopharmacology. 2015;35(4):374–381. doi: 10.1097/JCP.0000000000000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Husain M. I., Chaudhry I. B., Hamirani M. M., et al. Minocycline and celecoxib as adjunctive treatments for bipolar depression: A study protocol for a multicenter factorial design randomized controlled trial. Neuropsychiatric Disease and Treatment. 2017;13 doi: 10.2147/NDT.S115002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Savitz J., Preskorn S., Teague T. K., Drevets D., Yates W., Drevets W. Minocycline and aspirin in the treatment of bipolar depression: a protocol for a proof-of-concept, randomised, doubleblind, placebo-controlled, 232 clinical trial. BMJ Open. 2012;2(1) doi: 10.1136/bmjopen-2011-000643.000643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gallagher P. J., Castro V., Fava M., et al. Antidepressant response in patients with major depression exposed to NSAIDs: A pharmacovigilance study. The American Journal of Psychiatry. 2012;169(10):1065–1072. doi: 10.1176/appi.ajp.2012.11091325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Grace P. M., Loram L. C., Christianson J. P., et al. Behavioral assessment of neuropathic pain, fatigue, and anxiety in experimental autoimmune encephalomyelitis (EAE) and attenuation by interleukin-10 gene therapy. Brain, Behavior, and Immunity. 2017;59:49–54. doi: 10.1016/j.bbi.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Siopi E., Llufriu-Dabén G., Fanucchi F., Plotkine M., Marchand-Leroux C., Jafarian-Tehrani M. Evaluation of late cognitive impairment and anxiety states following traumatic brain injury in mice: the effect of minocycline. Neuroscience Letters. 2012;511(2):110–115. doi: 10.1016/j.neulet.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 199.Majidi J., Kosari-Nasab M., Salari A.-A. Developmental minocycline treatment reverses the effects of neonatal immune activation on anxiety- and depression-like behaviors, hippocampal inflammation, and HPA axis activity in adult mice. Brain Research Bulletin. 2016;120:1–13. doi: 10.1016/j.brainresbull.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 200.Hou R., Garner M., Holmes C., et al. Peripheral inflammatory cytokines and immune balance in Generalised Anxiety Disorder: Case-controlled study. Brain, Behavior, and Immunity. 2017;62:212–218. doi: 10.1016/j.bbi.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vogelzangs N., Beekman A. T. F., De Jonge P., Penninx B. W. J. H. Anxiety disorders and inflammation in a large adult cohort. Translational Psychiatry. 2013;3, article e249 doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Uguz F., Akman C., Kucuksarac S., Tufekci O. Anti-tumor necrosis factor-alpha therapy is associated with less frequent mood and anxiety disorders in patients with rheumatoid arthritis. Psychiatry and Clinical Neurosciences. 2009;63(1):50–55. doi: 10.1111/j.1440-1819.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- 203.Sulakhiya K., Keshavlal G. P., Bezbaruah B. B., et al. Lipopolysaccharide induced anxiety- and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin. Neuroscience Letters. 2016;611:106–111. doi: 10.1016/j.neulet.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 204.Valcheva-Kuzmanova S., Zhelyazkova-Savova M. Anxiolytic-like effect of Aronia melanocarpa fruit juice in rats. Methods and Findings in Experimental and Clinical Pharmacology. 2009;31(10):651–654. doi: 10.1358/mf.2009.31.10.1423884. [DOI] [PubMed] [Google Scholar]
- 205.Reis A. S., Pinz M., Duarte L. F. B., et al. 4-phenylselenyl-7-chloroquinoline, a novel multitarget compound with anxiolytic activity: Contribution of the glutamatergic system. Journal of Psychiatric Research. 2017;84:191–199. doi: 10.1016/j.jpsychires.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 206.Sulakhiya K., Kumar P., Gurjar S. S., Barua C. C., Hazarika N. K. Beneficial effect of honokiol on lipopolysaccharide induced anxiety-like behavior and liver damage in mice. Pharmacology Biochemistry & Behavior. 2015;132:79–87. doi: 10.1016/j.pbb.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 207.Moylan S., Eyre H. A., Maes M., Baune B. T., Jacka F. N., Berk M. Exercising the worry away: how inflammation, oxidative and nitrogen stress mediates the beneficial effect of physical activity on anxiety disorder symptoms and behaviours. Neuroscience & Biobehavioral Reviews. 2013;37(4):573–584. doi: 10.1016/j.neubiorev.2013.02.003. [DOI] [PubMed] [Google Scholar]